Abstract
Light exposure in the early night induces phase delays of the circadian rhythm in melatonin in humans. Previous studies have investigated the effect of timing, intensity, wavelength, history and pattern of light stimuli on the human circadian timing system. We present results from a study of the duration–response relationship to phase-delaying bright light. Thirty-nine young healthy participants (16 female; 22.18 ± 3.62 years) completed a 9-day inpatient study. Following three baseline days, participants underwent an initial circadian phase assessment procedure in dim light (<3 lux), and were then randomized for exposure to a bright light pulse (∼10,000 lux) of 0.2 h, 1.0 h, 2.5 h or 4.0 h duration during a 4.5 h controlled-posture episode centred in a 16 h wake episode. After another 8 h sleep episode, participants completed a second circadian phase assessment. Phase shifts were calculated from the difference in the clock time of the dim light melatonin onset (DLMO) between the initial and final phase assessments. Exposure to varying durations of bright light reset the circadian pacemaker in a dose-dependent, non-linear manner. Per minute of exposure, the 0.2 h duration was over 5 times more effective at phase delaying the circadian pacemaker (1.07 ± 0.36 h) as compared with the 4.0 h duration (2.65 ± 0.24 h). Acute melatonin suppression and subjective sleepiness also had a dose-dependent response to light exposure duration. These results provide strong evidence for a non-linear resetting response of the human circadian pacemaker to light duration.
Key points
Light is the strongest time cue for entrainment and phase resetting of the circadian clock.
In humans, exposure to long-duration light (6.5 h) in the late evening/early night causes phase delays, suppresses melatonin and increases alertness.
Here we studied the effects of different durations of exposure to a single high-intensity (∼10,000 lux) light pulse (0.2 h, 1 h, 2.5 h and 4.0 h) on phase shifting, suppression of melatonin and self-reported sleepiness in young men and women.
Phase-resetting and melatonin-suppression responses were dose dependent and non-linear; shorter light exposures more efficiently phase-shift the clock, suppress melatonin and induce alertness.
Introduction
Light is the strongest time cue for entrainment and phase resetting of the circadian timing system. Previous studies have investigated the effect of timing (Honma & Honma, 1988; Minors et al. 1991; Dawson et al. 1993; Van Cauter et al. 1994; Khalsa et al. 2003; Rüger et al. 2003), intensity (Boivin et al. 1996; Zeitzer et al. 2000), wavelength (Lockley et al. 2003; Revell et al. 2005; Gooley et al. 2010), history (Chang et al. 2011) and pattern of light stimuli (Rimmer et al. 2000; Weimerskirch & Ernst, 2001; Burgess et al. 2003; Gronfier et al. 2004) on the phase-resetting response of the human circadian system. The timing of light exposure greatly impacts the resulting response of the circadian pacemaker. In humans, ocular light exposure in the evening and early night prior to the circadian phase of the core body temperature minimum induces phase delays of circadian rhythms, as opposed to light during the late night/early morning which results in phase advances (Khalsa et al. 2003).
The majority of human studies exploring the effect of light administered in the biological night have used light exposure durations on the order of several hours (Hastings & Sweeney, 1958). A single continuous 6.5 h light exposure administered in the biological night results in ∼3 h phase delay of the pacemaker (Zeitzer et al. 2000; Khalsa et al. 2003; Gronfier et al. 2004). Phase–response curves generated using single light exposure durations of 3–4 h (2,500–10,000 lux) have maximum delays of ∼2 h (Beersma et al. 2009). Minors and colleagues (Minors et al. 1991) studied in one participant the effect of a 1 h pulse timed 0.5 h before core body temperature minimum and obtained a phase delay of ∼1 h. A 2 h, 4000 lux light pulse administered in the biological night induced an average phase delay of 1.3 h (Canton et al. 2009). A recent study of increasing duration (1, 2 and 3 h) and increasing intensity (2000, 4000 and 8000 lux) of light found that longer duration exposures resulted in larger phase shifts than shorter exposures at higher intensity light (Dewan et al. 2011).
In addition to phase-shifting circadian rhythms, light exposure at night also suppresses plasma melatonin concentrations, which are at peak levels during the biological night. Suppression is both intensity- (McIntyre et al. 1989; Zeitzer et al. 2000) and wavelength-dependent (Brainard et al. 2001; Thapan et al. 2001; Gooley et al. 2010) and affected by prior light history (Hébert et al. 2002; Smith et al. 2004; Chang et al. 2011). Melatonin suppression has been observed for short light exposure durations, for example as short as 15 min (Gronfier et al. 2004; St Hilaire et al. 2007). Light at night also has direct alerting effects on objective performance and subjective alertness with demonstrated intensity- (Cajochen et al. 2000) and wavelength-dependent (Lockley et al. 2006) responses.
The aim of the current study was to construct a duration–response curve of circadian phase shifts to a ∼10,000 lux light exposure administered in the biological night at a time when maximal phase delays would be expected (Khalsa et al. 2003). Our goal was to systematically explore the effect of a single continuous light exposure over a wide range of durations (12 min to 6.5 h) under conditions in which all other factors known to affect circadian phase resetting (e.g. intensity, timing, pattern) were held constant. We also explored the duration responses of acute melatonin suppression and alerting effects of light which have not previously been reported.
Methods
Ethical approval
Screening and study procedures were approved by the Partners Human Research Committee and the study protocol was conducted according to the Declaration of Helsinki. Informed written consent was obtained from all study participants prior to enrollment and they were paid for their participation.
Subjects and study screening
A total of 39 healthy young adults (22.18 ± 3.62 years; 16 female) participated in the 9-day study. Prior to inpatient admission, study participants were screened for medical and psychological health including medical history and questionnaires, physical examination and EKG, ophthalmological examination, laboratory tests, psychological questionnaires and assessment by interview with a clinical psychologist. Participants with chronic medical or psychological conditions, sleep disorders, eye or vision abnormalities or those taking prescription medications were excluded from the study. Potential participants were also excluded for night/shift work in the past 3 years or travel across more than one time zone in the previous 3 months.
Participants were required to refrain from use of caffeine, nicotine, alcohol, medications, drugs or dietary supplements during the pre-study screening period and the inpatient study. This was verified by toxicological testing at admission. Participants were also required to maintain a regular, self-selected, 8 h sleep schedule and to call in to a time-stamped voicemail system at bedtime and wake time each day for 3 weeks prior to admission. Maintenance of this schedule was verified by wrist actigraphy (Actiwatch-L; Philips/Respironics, Bend, OR, USA) for a minimum of 1 week prior to admission.
Study protocol
Participants were admitted to the Intensive Physiological Monitoring Unit of the Center for Clinical Investigation of the Brigham and Women's Hospital for the 9-day inpatient protocol. During this time participants lived in a private room in an environment free of time cues. The study protocol (shown in Fig. 1) consisted of three baseline days during which time participants were scheduled to sleep for 8 h per night at their habitual times. On day 4, participants woke to a ∼50 h constant routine (CR) circadian phase estimation procedure, which was followed by an 8 h sleep episode. The following day included a 4.5 h controlled posture session beginning 6 h after wake time. During this session, participants were administered an experimental bright light exposure (LE). Following the subsequent 8 h sleep episode, participants completed a ∼30 h CR followed by the final 8 h sleep episode, after which they were discharged.
Figure 1. Raster plot of the 9-day study protocol.
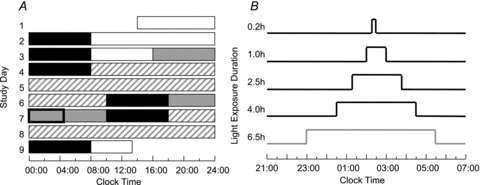
A, the representative raster is for a participant with a habitual sleep schedule of 12 am–8 am. Sleep episodes are denoted by the black bars. Open bars represent 90 lux levels, grey bars show <3 lux levels and striped bars represent CR conditions in 1 lux. The box on day 7 shows the timing of the 4.5 h episode of constant posture. B, light exposure was scheduled within the 4.5 h constant-posture procedure on day 7. The 4 h LE was scheduled to start 0.5 h after the start of constant posture. The 1 h and 2.5 h LEs were aligned by midpoint to the 4 h LE. The midpoint of the 0.2 h LE was shifted 12 min earlier compared with the 1 h and 2.5 h LE. The timing of the 6.5 h LE was conducted under a separate set of experiments (Gronfier et al. 2004) and is included for comparison.
Constant routine conditions
CR procedures were used before and after the experimental LE to determine the circadian phase of melatonin under constant conditions while minimizing the masking effects or influences of exogenous factors (Duffy & Dijk, 2002). During CR participants were asked to remain awake with minimal activity while sitting in a semi-recumbent position in bed. Room temperature and dim light remained constant and participants were given small equicaloric snacks and fluids at hourly intervals. A study staff member remained in the room with the participant during CR to monitor wakefulness and adherence to study procedures.
Lighting conditions and experimental light exposure
Lighting was generated using ceiling-mounted 4100K fluorescent lamps (F96T12/41U/HO/EW, 95 W; F32T8/ADV841/A, 32 W; F25T8/TL841, 25 W; Philips Lighting, the Netherlands) with digital ballasts (Lutron Electronics Co., Inc., PA, USA) transmitted through a UV-stable filter (Lextran 9030 with prismatic lens, GE Plastics, MA, USA). Ambient room lighting during scheduled wake episodes on baseline days was approximately 90 lux at the cornea (∼0.23 W m−2 at 137 cm from the floor in the vertical plane, with a maximum of 0.48 W m−2 (∼150 lux) at 187 cm from the floor in the horizontal plane anywhere in the room) (Fig. 1, white bars). From midway through the third baseline day, ambient lighting was reduced to ∼0.5 lux (0.001 W m−2) at 137 cm from the floor in the vertical plane with a maximum <3 lux (0.01 W m−2) at 187 cm from the floor in the horizontal plane anywhere in the room (Fig. 1, grey bars). Subjects were in darkness (<0.02 lux, <0.00006 W m−2) during scheduled bed-rest episodes.
During the experimental bright light exposure, all ceiling lamps were lit and at full brightness. The target corneal light illuminance during the experimental LE was >6000 lux (>8 W m−2). The controlled posture and light exposure session began 6 h after wake time on the LE day (day 7, Fig. 1) for 4.5 h. This timing was chosen so that the LE would occur 0.5–4.5 h after the melatonin onset, when plasma melatonin levels are high and when maximal phase delays of the endogenous circadian melatonin rhythm are expected (Khalsa et al. 2003). Participants remained seated in a specific location of the room for the entire 4.5 h constant posture. Participants wore Uvex glasses (Uvex Winter Optical, Smithfield, RI, USA) and were asked to maintain a ‘fixed gaze’ on a target on the wall directly in front of them for 6 min alternating with a 6 min ‘free gaze’ during which they were allowed to look elsewhere, as long as they did not close their eyes or shield them from the light. Light illuminance was measured using a IL1400 radiometer/powermeter with an SEL-033/Y/W detector (International Light, Inc., Peabody, MA, USA) at every change of gaze with the sensor placed next to the participant's eye and pointed at the target during ‘fixed gaze’ and in the angle of gaze during ‘free gaze’. A research technician was present during the LE session to administer the LE, measure and record light readings, and monitor adherence to study procedures.
Thirty participants were randomized to receive 1.0 h, 2.5 h or 4.0 h LE (n= 10 in each group). Subsequently, an additional group (n= 9) completed the study and were administered a 0.2 h LE. Demographic data and mean LE illuminance achieved for each group are shown in Table 1. The 4.0 h LE was scheduled to begin 0.5 h after the start of the 4.5 h constant posture session and end at the same time as the LE session. The 2.5 h and 1.0 h LEs were each scheduled so that the midpoint of the LE occurred at the same time of the midpoint for the 4.0 h LE (Fig. 1B). The timing of the 0.2 h LE occurred slightly earlier, with the midpoint 12 min earlier than the other groups.
Table 1.
Demographic data and light exposure illuminance for the four duration groups
| LE duration (h) | Age (years ± SD) | Sex (f/m) | LE illuminance (lux ± SD) |
|---|---|---|---|
| 0.2 | 21.78 ± 3.19 | 3/6 | 7669 ± 1321 |
| 1.0 | 21.70 ± 2.79 | 4/6 | 9040 ± 498 |
| 2.5 | 22.30 ± 4.52 | 4/6 | 8927 ± 666 |
| 4.0 | 22.90 ± 4.12 | 5/5 | 8396 ± 1604 |
Illuminance levels among the four groups were not significantly different (P= 0.06).
Historical control and 6.5 h LE duration participants
Data from 13 participants (23.31 ± 4.11 years; 4 female) who completed a previously published 10-day study (Gronfier et al. 2004) were included in the analysis of melatonin phase shifts, suppression, and subjective alertness in order to include a group that received a longer LE (6.5 h) and a control group (continuous dim background). The 10-day study protocol, described elsewhere (Gronfier et al. 2004), was conducted in the same facility using near-identical procedures. Any differences between protocols are specifically described here. Lighting conditions for day 3 were maintained at ∼90 lux in the 10-day study. The 10-day protocol included a 26.2 h CR1 and a 64 h CR2 and blood samples were collected every 10 min during the LE. The 13 participants completed a 6.5 h LE session which was centred 5.8 h before their habitual wake time. Six participants were randomized to a 6.5 h bright LE (∼9500 lux) and seven were randomized to a control condition (<3 lux).
Outcome measures of melatonin phase, suppression and subjective sleepiness
Blood samples were collected every 30–60 min throughout the protocol and assayed by radioimmunoassay for melatonin concentration (Pharmasan, Osceola, WI, USA). Sensitivity of the assay was 2.5 pg ml−1; inter-assay and intra-assay coefficients of variance were 9.2–13.2% and 9.8–12.1%, respectively. Dim light melatonin onset was calculated as the time at which levels of melatonin went above the 25% threshold (DLMO25%) of the first 3-harmonic fitted peak-to-trough amplitude of melatonin during CR1 (Wright & Lack, 2001; Klerman et al. 2002). Phase shifts were calculated as the difference in clock time between CR1 and CR2 phases. Due to the length of CR1 (50 h) in the 9-day study, there were two DLMO25% phase measures determined for each participant during this CR; however, phase shifts were calculated using the second phase which occurred temporally closer (∼24 h prior) to the LE.
Melatonin suppression was determined using the area under the curve (AUC) during the 4.5 h constant posture compared with the AUC during the corresponding 4.5 h time window 24 h earlier, during CR1, for data from the 9-day study. The AUC during the 6.5 h LE session and 6.5 h during CR1 were used to calculate melatonin suppression for data from the 10-day study. The resulting percent melatonin suppression due to the bright light was calculated using the formula:
Subjective ratings of sleepiness were collected using a computerized Karolinska sleepiness scale (KSS), which participants completed every 30–60 min throughout the protocol. The KSS is a 9-point Likert scale with all numbers having point values but descriptors on odd numbers only: 1 representing ‘extremely alert’, 3 representing ‘alert’, 5 representing ‘neither alert nor sleepy’, 7 representing ‘sleepy’ and 9 representing ‘extremely sleepy’ (Åkerstedt & Gillberg, 1990). Subjective sleepiness was assessed during the LE day beginning 2 h after waking.
Data management and statistical analysis
Data from 36 of 39 participants who participated in the 9-day protocol were included in the analysis of melatonin phase shifts. Two participants were excluded from analysis because post hoc analysis showed that the LE occurred outside the targeted range for inducing a phase delay (Khalsa et al. 2003). One participant had too many missing blood samples during CR2 which prevented an accurate determination of DLMO25% and was therefore excluded from phase analysis. Data from this participant were included in the analysis of melatonin suppression because there were no missing samples in the segments required for that analysis. Melatonin phase-shift and suppression data were compared among the six LE duration groups using a one-way ANOVA (SAS 9.2, SAS Institute Inc., Cary, NC, USA) with LE duration as the main factor.
Melatonin phase-shift and suppression data were fitted by a 4-parameter logistic model to characterize the relationship between phase shift or suppression and increasing duration. The 4-parameter logistic model was previously found to best fit the relationship between phase-shift or suppression data with increasing stimulus intensity (Zeitzer et al. 2000). The equation for the 4-parameter logistic model is
where a is the estimated response of the system to a light pulse of 0 h duration, b is the duration at which 50% of the maximal shift or suppression is observed, c is the asymptotic maximal responsiveness of the system and d is a measure of the steepness of the rising portion of the curve. Melatonin phase-shift and suppression data from the 0.2 h, 1.0 h, 2.5 h, 4.0 h and 6.5 h groups were fitted with a non-linear least-squares analysis using the Levenberg–Marquardt method in OriginPro 8.5 (Northamptom, MA, USA). The goodness of fit was assessed by computing the adjusted correlation coefficient R2. For melatonin phase shift, parameters were fit unconstrained; for melatonin suppression, the asymptotic maximal responsiveness was constrained to values less than or equal to 100% suppression.
KSS scores were transformed (z-score) in order to minimize inter-individual and inter-group baseline differences in subjective sleepiness. We compared KSS scores across the six LE duration groups during three intervals: (1) the 4.5 h constant posture, (2) the LE and (3) the 4.5 h following the end of the LE. For the 0.2 h LE duration group, no KSS was administered during the LE and for the dim-light control group, there was no LE; therefore these groups were excluded from analysis during LE (no. 2 above), but were included in other analyses. The first 4.5 h of the 6.5 h LE session for the 6.5 h group was used in analysis (no. 1 above). Since the midpoint of the LE was centred for each group, the end of the LE in each group occurred at a different phase and after a different duration of time awake. Likewise, the 4.5 h analysis window following the LE (no. 3 above) occurred at different times in each group. Given that light, time awake and circadian phase have effects on alertness/sleepiness (Cajochen et al. 1999, 2000), KSS scores were analysed using a mixed model ANCOVA with LE duration and time awake as main factors and circadian phase determined from CR2 as a covariate.
Results
Melatonin phase delays
Bright light exposure induced phase delays of the melatonin rhythm in all participants in all LE duration groups (see Figs 2 and 3, top panels). Mean phase delays (±SD) induced by the control (n= 7), 0.2 h (n= 9), 1.0 h (n= 8), 2.5 h (n= 10), 4.0 h (n= 9) and 6.5 h (n= 6) LE were: 0.40 ± 0.39 h, 1.07 ± 0.36 h, 1.55 ± 0.38 h, 2.29 ± 0.28 h, 2.65 ± 0.24 h and 3.05 ± 0.45 h, respectively. The magnitude of the phase delay differed significantly between groups (P < 0.0001). The 4-parameter logistic function had a goodness of fit of R2= 1.00 with a= 0.97, b= 2.69, c= 3.69 and d= 1.28. This fit estimates the half-maximum value at ∼2.7 h light duration (see Fig. 3, top right panel). Melatonin profiles from CR1 and CR2 in representative subjects from the 0.2 h, 1.0 h, 2.5 h and 4.0 h groups are shown in Fig. 2.
Figure 2. Representative CR1 and CR2 melatonin profiles.
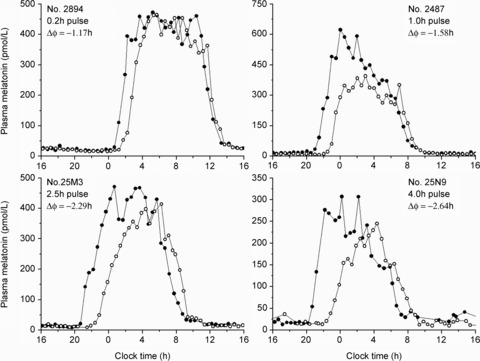
The 24 h melatonin profiles on CR1 (filled symbols) and CR2 (open symbols) from one representative individual in each of the 4 LE groups demonstrate the dose effect of light duration on the phase-resetting response.
Figure 3. Duration–response curves of melatonin phase shifts and suppression.
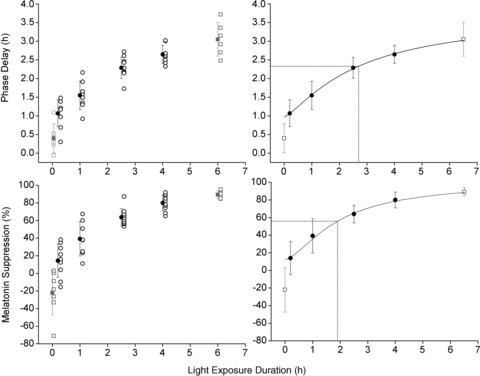
Individual and mean phase shifts and melatonin suppression for each of the 4 LE duration groups studied under the current set of experiments (black symbols) and the 6.5 h LE and dim background controls studied previously (grey symbols) (Gronfier et al. 2004). The panels on the left show the phase delay (top) and melatonin suppression (bottom) for the individual participants (open symbols) and mean ± SD (filled symbols) for the groups. The panels on the right again show the mean phase shift (top) and suppression (bottom). Data on the right are fitted using a 4-parameter logistic model and the predicted half-maximum values are shown. The dim background controls were excluded from the fit.
Melatonin suppression
Individual and group mean levels of melatonin suppression are shown in Fig. 3 (bottom panels). Mean percent suppression of melatonin induced by the control (n= 7), 0.2 h (n= 9), 1.0 h (n= 8), 2.5 h (n= 10), 4.0 h (n= 10) and 6.5 h (n= 6) LE were: −22 ± 25%, 14 ± 19%, 39 ± 19%, 64 ± 10%, 80 ± 9% and 89 ± 5% respectively. Some individuals had higher levels of melatonin during the LE session than during the same time window the day prior, resulting in a negative per cent suppression of melatonin. The magnitude of melatonin suppression increased as the duration of the LE increased and was significantly different between groups (P < 0.0001). As with the phase-shifting results, suppression data were fitted by a 4-parameter logistic function (R2= 0.99) with a= 12.44, b= 1.92, c= 100 and d= 1.55. This fit estimates the half-maximum value at ∼1.9 h light duration (Fig. 3, bottom right panel).
Subjective sleepiness
Mean KSS scores and mean melatonin profiles across the LE day for the six groups are shown in Fig. 4. Participants rated themselves as sleepier with longer time awake (P < 0.0001). Comparison of KSS z-scores during the 4.5 h constant posture showed a significant main effect of LE duration (P= 0.007) and time awake (P < 0.0001); and significant interaction of time awake and LE duration (P < 0.0001) with the dim-light control group having the most sleepy ratings and the 4.0 h group being the most alert. There was no difference in KSS score among the four LE duration groups (the control and 0.2 h groups not included) during the bright light administration (P= 0.922). There was a significant main effect of time awake (P < 0.0001) and interaction of time awake and LE duration (P= 0.004) during the 4.5 h following the LE. Ratings of sleepiness during this interval found the 0.2 h group to be the least sleepy followed by the 1.0 h, 4.0 h, 2.5 h, 6.5 h and control groups.
Figure 4. Subjective sleepiness and melatonin concentrations during LE day.
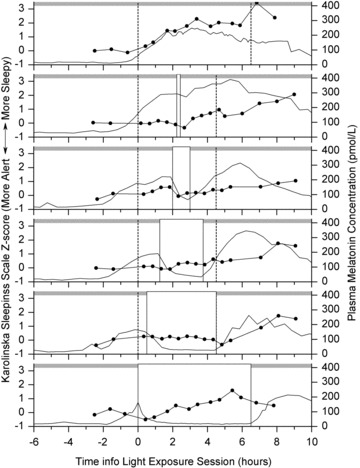
Mean KSS z-scores (symbols with line) and plasma melatonin levels (continuous line) are shown for the control, 0.2 h, 1.0 h, 2.5 h, 4.0 h and 6.5 h duration groups (top to bottom). The grey area represents the 1 lux level. The white boxes (continuous lines) represent the ∼10,000 lux LE. The dotted lines show the timing of the constant posture (0-4.5 in the current study; 0-6.5 in the groups studied previously (Gronfier et al. 2004).
Discussion
Our results demonstrate that the magnitude of the response of the circadian timing system to bright light increases with increasing duration of exposure. The present analysis included five different durations of light stimulus ranging from 0.2 to 6.5 h, and the resulting duration–response curves (DRC) for both phase shifts and melatonin suppression were described by a non-linear, 4-parameter logistic model. These results demonstrate that brief exposure (0.2 h) to bright light in humans can induce a substantial phase shift of the human circadian pacemaker, resetting circadian rhythms much more efficiently per minute of light exposure than longer durations of light. In the present analysis, a 12 min light exposure reset the pacemaker at a rate of 5.4 min per minute of light, while the 4 h light exposure resulted in <1 min phase shift per minute of exposure.
The 4-parameter logistic model used in the present study also best described the intensity–response curve (IRC) data to white light (Zeitzer et al. 2000). In that previous study, the relationship of the magnitude of phase shift and light intensity was approximately linear between ∼50 and 500 lux and asymptotic at light levels above ∼550 lux. In comparison, our results showed that the relationship of the magnitude of phase shift and light duration is approximately linear between 0.2 and 4 h with a rate of 0.41 h shift per 1 h of saturating light exposure. Our results suggest that the saturating duration of continuous light exposure is between 4 and 6.5 h, as has been suggested by previous studies (Beersma et al. 2009).
Dewan and colleagues (Dewan et al. 2011) recently investigated light exposure durations of 1, 2 and 3 h and found that longer durations, within this range, were more effective in phase-shifting the melatonin rhythm than increases in light intensity (2000, 4000 and 8000 lux). All light intensities were of sufficient intensity (>1000 lux) to saturate the circadian phase-resetting response (Zeitzer et al. 2000) and therefore it would be expected that longer durations of light exposure would induce larger phase shifts than shorter exposures to a lower intensity but still saturating light. The 1–3 h duration corresponds to the linear portion of our DRC which would predict larger phase delays with increasing stimulus duration. Analysis of ‘phase progression curves’ using phase–response curves constructed from light pulses ranging from 3 to 6.7 h duration demonstrated that the majority of a phase shift occurs at the beginning of a light exposure (Beersma et al. 2009). Our results, therefore, add to those findings and provide strong evidence that the duration response of the human circadian pacemaker is non-linear. Based on these findings, it could be argued that increasing the duration of light exposure is not comparable to increasing the total photons per unit of time to induce larger phase shifts.
Longer durations of light exposure (>3 h) may also have differential effects on resetting circadian phase whereby light in the fourth, fifth or later hours may not elicit the same phase shift that light in the first or second hour would, particularly if the phase has already been shifted to a later time (delay) when smaller phase delays or possibly even phase advances would be predicted by the light PRC (Khalsa et al. 2003). Animal studies have shown that resetting of the pacemaker occurs rapidly, within 1 to 2 h (Best et al. 1999), and that the response of the circadian system to phase delaying light pulses saturates at ∼6 h (Comas et al. 2006). In mice, PRCs constructed using single pulse LEs of different durations (Comas et al. 2006) and a double skeleton pulse 1 h LE with differing durations of the intervening dark period (Comas et al. 2007) showed a change in the amplitude and shape of the PRC at longer durations, including the disappearance of the dead zone; however, longer duration LEs did not change the PRC from Type 1 to Type 0 resetting (Comas et al. 2006).
Photic adaptation may be driving the differential response of photoreceptors to short versus long durations of light exposure. Saturation by longer durations of light stimuli has been shown (Nelson & Takahashi, 1999), as has a reduction in sensitivity (Kronauer et al. 1999; Vidal & Morin, 2007). We have previously shown photic adaptation of the human phase-resetting response to a non-saturating light stimulus (6.5 h 90 lux LE) (Chang et al. 2011) but more studies are needed. Examination of this adaptation response to shorter-duration light stimuli and PRCs of different LE durations in humans would greatly increase our understanding of phase-shifting responses to photic stimuli and the mechanisms underlying them.
To our knowledge, this is the first study to demonstrate significant responses of the human circadian pacemaker to a single, continuous light exposure duration of <1 h. Previous studies in humans have examined the effects of light durations as short as 5, 15 and 45 min (Rimmer et al. 2000; Gronfier et al. 2004, 2007), and a recent study tested the effect of millisecond flashes of light (Zeitzer et al. 2011) on the phase-resetting response, but in both the former and latter studies the short-duration light exposures were administered in a series over a total duration of 6.5 h, 2.5 h or 1 h, respectively. The 4-parameter logistic model fit to the five different LE durations in our present study predicts an intercept of ∼1 h for a 0 h duration light exposure. Although data from the dim background control suggest that exposure to continuous dim light in the biological night can achieve only ∼0.4 h phase delay, the fit nevertheless raises the possibility that light durations between 0 and 12 min may yield significant phase responses. Further experiments are necessary to characterize the response of the circadian pacemaker within this duration range.
Results from ratings of subjective sleepiness in the present study also indicate that short durations of light exposure are more efficient at inducing alertness for at least several hours after removal of the stimulus. The significant difference in subjective sleepiness among the groups during the 4.5 h constant posture session demonstrates the acute alerting effects of bright light, with the longer duration groups (4 h and 6.5 h), which received more light during this interval, self-reporting less sleepiness. Furthermore, when comparing the groups only during the portion of the session when the lights were switched on, we saw no difference in sleepiness scores. Interestingly, for 4.5 h following the end of the LE there were significant differences in subjective sleepiness but with the shorter-duration groups rating themselves as less sleepy. These findings suggest that shorter light exposures may be sufficient to induce not only acute but also sustained alerting effects.
Taken together, the present findings show that brief exposures to bright light are more efficient for phase-shifting, suppressing melatonin and inducing alertness than longer exposures. This has important clinical implications for the use of light in the treatment of circadian rhythm/sleep and mood disorders. A shorter-duration light exposure is likely to improve compliance and, based on evidence provided here, also likely to yield improved outcomes. Although our results demonstrate a non-linear duration response of the circadian system, further investigation is needed to better understand how this response changes at shorter durations. It would also be important to examine the duration response of the circadian system at different light intensities, spectra and times of day.
Acknowledgments
We thank the study participants, the staff of the General Clinical Research Center and Center for Clinical Investigation of the Brigham & Women's Hospital and the staff of the Chronobiology Core. We also thank the recruiters: Lisa McCaig, Sophie Delano, Elizabeth Lydon and David Klements. We would like to acknowledge Jessie Ricker for her contributions to data analysis. The work presented in this study was supported by the National Institutes of Health (NIH) grants R01MH045130 and R01HL077453; and the National Aeronautics and Space Administration grant NAG 5-3952. The inpatient studies were conducted in the General Clinical Research Center supported by M01-RR-02635 and the Harvard Clinical and Translational Science Center supported by UL1 RR025758 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the NIH.
Glossary
- AUC
area under the curve
- CR
constant routine
- DLMO
dim light melatonin onset
- DRC
duration–response curve
- IRC
intensity–response curve
- KSS
Karolinska sleepiness scale
- LE
light exposure
Author contributions
All authors have made substantial contributions to the work presented and have approved the final version of the manuscript. A.-M.C. conducted inpatient studies, analysed data and wrote the manuscript. N.S. contributed to data collection and conduct of inpatient studies and editing the manuscript. M.St.H. contributed to data analysis, writing and preparation of the manuscript. C.G. conducted the inpatient studies of the historical dataset and edited the manuscript. D.S.B. assisted with data analysis and preparation of the manuscript. J.F.D. contributed to the experimental design and editing of the manuscript. S.W.L. contributed to the concept and design of the study, interpretation of results, and edited the manuscript. R.E.K. made contributions to experimental design, data analysis, interpretation of results, and editing the manuscript. C.A.C. conceived and designed the study, edited the paper, and the experimental protocols were conducted in his laboratory.
References
- Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Beersma DG, Comas M, Hut RA, Gordijn MC, Rueger M, Daan S. The progression of circadian phase during light exposure in animals and humans. J Biol Rhythms. 2009;24:153–160. doi: 10.1177/0748730408330196. [DOI] [PubMed] [Google Scholar]
- Best JD, Maywood ES, Smith KL, Hastings MH. Rapid resetting of the mammalian circadian clock. J Neurosci. 1999;19:828–835. doi: 10.1523/JNEUROSCI.19-02-00828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Rollag MD, Greeson J, Byrne B, Glickman G, Gerner E, Sanford B. Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab. 2001;86:433–436. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol Regul Integr Comp Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Canton JL, Smith MR, Choi HS, Eastman CI. Phase delaying the human circadian clock with a single light pulse and moderate delay of the sleep/dark episode: no influence of iris color. J Circadian Rhythms. 2009;7:8. doi: 10.1186/1740-3391-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms. 2006;21:362–372. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Circadian response reduction in light and response restoration in darkness: a ‘skeleton’ light pulse prc study in mice (Mus musculus. J Biol Rhythms. 2007;22:432–444. doi: 10.1177/0748730407305728. [DOI] [PubMed] [Google Scholar]
- Dawson D, Lack L, Morris M. Phase resetting of the human circadian pacemaker with use of a single pulse of bright light. Chronobiol Int. 1993;10:94–102. doi: 10.3109/07420529309059697. [DOI] [PubMed] [Google Scholar]
- Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–599. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SMW, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24 h days. Proc Natl Acad Sci U S A. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JW, Sweeney BM. A persistent diurnal rhythm of luminescence in Gonyaulax polyedra. Biological Bulletin. 1958;115:440–458. [Google Scholar]
- Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42:167–168. [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Kronauer RE, Forger DB, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. J Biol Rhythms. 1999;14:500–515. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin suppression by light is intensity dependent. J Pineal Res. 1989;6:149–156. doi: 10.1111/j.1600-079x.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1351–R1361. doi: 10.1152/ajpregu.1999.277.5.R1351. [DOI] [PubMed] [Google Scholar]
- Revell VL, Arendt J, Terman M, Skene DJ. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms. 2005;20:270–272. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Acute and phase-shifting effects of ocular and extraocular light in human circadian physiology. J Biol Rhythms. 2003;18:409–419. doi: 10.1177/0748730403256650. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Gronfier C, Zeitzer JM, Klerman EB. A physiologically-based mathematical model of melatonin including ocular light suppression and interactions with the circadian pacemaker. J Pineal Res. 2007;43:294–304. doi: 10.1111/j.1600-079X.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Balériaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol Endocrinol Metab. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- Vidal L, Morin LP. Absence of normal photic integration in the circadian visual system: response to millisecond light flashes. J Neurosci. 2007;27:3375–3382. doi: 10.1523/JNEUROSCI.5496-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimerskirch PR, Ernst ME. Newer dopamine agonists in the treatment of restless legs syndrome. Ann Pharmacother. 2001;35:627–630. doi: 10.1345/aph.10271. [DOI] [PubMed] [Google Scholar]
- Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18:801–808. doi: 10.1081/cbi-100107515. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6:e22078. doi: 10.1371/journal.pone.0022078. [DOI] [PMC free article] [PubMed] [Google Scholar]


