Abstract
The parallel processing of information forms an important organisational principle of the primate visual system. Here we describe experiments which use a novel chromatic–achromatic temporal compound stimulus to simultaneously identify colour and luminance specific signals in the human electroretinogram (ERG). Luminance and chromatic components are separated in the stimulus; the luminance modulation has twice the temporal frequency of the chromatic modulation. ERGs were recorded from four trichromatic and two dichromatic subjects (1 deuteranope and 1 protanope). At isoluminance, the fundamental (first harmonic) response was elicited by the chromatic component in the stimulus. The trichromatic ERGs possessed low-pass temporal tuning characteristics, reflecting the activity of parvocellular post-receptoral mechanisms. There was very little first harmonic response in the dichromats’ ERGs. The second harmonic response was elicited by the luminance modulation in the compound stimulus and showed, in all subjects, band-pass temporal tuning characteristic of magnocellular activity. Thus it is possible to concurrently elicit ERG responses from the human retina which reflect processing in both chromatic and luminance pathways. As well as providing a clear demonstration of the parallel nature of chromatic and luminance processing in the human retina, the differences that exist between ERGs from trichromatic and dichromatic subjects point to the existence of interactions between afferent post-receptoral pathways that are in operation from the earliest stages of visual processing.
Key points
It has recently been shown that, with careful control over stimulation, the electroretinogram (ERG) can reflect the properties of the post-receptoral parvocellular (P) and magnocellular (M) pathways, which are thought of as the respective substrates for red–green colour and luminance vision.
Using a novel compound stimulus, which contains red–green colour information at the fundamental frequency and luminance information at the second harmonic, we were able to simultaneously record colour and luminance ERGs.
In trichromats, the temporal tuning curves of these components reflected the known properties of the P and M systems, whereas the dichromats showed negligible chromatic response but a normal achromatic response.
Parallel retinal processing that is relevant for vision can be reflected in the ERG.
Introduction
The electroretinogram (ERG), a mass electrical potential that can be recorded from the eye in response to visual stimulation, reflects the activity of different cell types in the retina. It can be recorded non-invasively from corneal electrodes and has proved to be an important tool in improving our understanding of both normal and pathological human retinal function. In this role the ERG has traditionally been viewed as providing an index of outer or distal retinal function. For example, the selective stimulation of rods and of long- (L), middle- (M) and short- (S) wavelength-sensitive cones has enabled characterisation of the spectral properties of these photoreceptors, as well as their relative distributions in the human retina (Jacobs & Neitz, 1993; Usui et al. 1998; Kremers & Meierkord, 1999; Kremers et al. 2000; Carroll et al. 2002; Kremers, 2003; Murray et al. 2004; Hofer et al. 2005; Challa et al. 2010). However, the ERG has also been shown to reflect the function of inner retinal layers (Bush & Sieving, 1996; Viswanathan et al. 1999, 2000, 2002). Importantly, the inner retinal layers contain many different types of bipolar, amacrine and ganglion cells which form the physiological substrates for different parallel visual processing pathways (Dacey, 2000; Wassle, 2004; Nassi & Callaway, 2009). Thus, in addition to providing information about the earliest stages of visual processing, the ERG has the potential to provide an assay of function in the different post-receptoral, afferent, processing pathways that carry visual information from the retina to the brain.
In the primate visual system, two major post-receptoral pathways have been particularly well described: the parvocellular and magnocellular systems. Crucially, these two systems form the substrates for key functional aspects of vision. The parvocellular pathway is based upon midget ganglion cells, which account for approximately 70% of the total retinal ganglion cell population. These cells respond in a sustained fashion, have low-pass temporal frequency response characteristics and receive opponent input from L- and M-cones. As a result of this cone opponent input, the parvocellular pathway forms the main substrate for red–green (L-M cone opponent) colour vision (De Monasterio & Gouras, 1975; Dreher et al. 1976; Hicks et al. 1983; Derrington et al. 1984; Derrington & Lennie, 1984; Shapley & Perry, 1986; Lee et al. 1987, 1989c; Schiller et al. 1990). The magnocellular pathway, on the other hand, is based upon activity in the less numerous (∼10%) parasol ganglion cell population. The parasol ganglion cells respond transiently and exhibit band-pass temporal response characteristics. They receive additive input from the L- and M-cones and evidence suggests that the magnocellular pathway forms the main physiological substrate for luminance (achromatic) vision (Wiesel & Hubel, 1966; De Monasterio & Gouras, 1975; Dreher et al. 1976; Hicks et al. 1983; Derrington & Lennie, 1984; Shapley & Perry, 1986; Lee et al. 1988; Kaiser et al. 1990; Valberg et al. 1992; Dacey, 2000). A third major processing stream, the koniocellular pathway, has also been well-characterised. This pathway contains bi-stratified ganglion cells and is thought to be the substrate for S-cone mediated colour vision (Dacey & Lee, 1994; Dacey, 2000).
The parvo- and magnocellular functional subdivisions are primarily a feature of retinal ganglion cells. However, this dichotomy also ocurrs at the level of the bipolar cells, which provide the main inputs to these respective pathways (Dacey, 1999). Thus the bipolar cells are the precursors of the main functional pathways that originate in the ganglion cell layer of the primate retina. Midget bipolar cells, for example, provide input to the midget ganglion cells of the parvocellular pathway and they connect selectively with either L- or M-cones in the photoreceptor layer. They have thus been viewed as playing a major role in the generation of L-M opponency in the primate retina (Dacey, 1999). Diffuse bipolar cells, on the other hand, connect with the parasol ganglion cells of the magnocellular pathway. These neurons synapse in a non-selective and additive fashion with L- and M-cones, making them ideal substrates for non-opponent, luminance processing (Boycott & Wassle, 1991; Wassle et al. 1994; Dacey, 1999). Studies of the non-primate mammalian retina have also clearly demonstrated the existence of separate and distinct bipolar cell populations that are involved in the encoding of chromatic and luminance signals (Li & DeVries, 2006; Breuninger et al. 2011).
The existence of such functional segregation at the level of the bipolar cells raises the possibility that the ERG might also reflect this post-receptoral separation of colour and luminance processing. As stated previously, the ERG provides an index of inner retinal function and the ERG b-wave in particular has been shown to be highly dependent on bipolar cell activity (Stockton & Slaughter, 1989; Bush & Sieving, 1996). So far there have been few attempts at correlating ERG response behaviour with the known properties of the major post-receptoral processing pathways described above. However, two recent studies have demonstrated that careful manipulation of the temporal frequency of spatially homogeneous flickering stimuli can result in the generation of ERGs that reflect either parvocellular (red–green chromatic) or magnocellular (luminance) processing (Kremers & Link, 2008; Kremers et al. 2010). It was shown that, unlike the responses elicited by 30 Hz stimulation, ERGs generated by slower temporal rates (∼12 Hz) exhibit ratios of L- and M-cone driven activity that are close to unity. In addition, the L- and M-cone driven responses are 180 deg out of phase and the signal is quite robust to small changes in cone adaptation. All of these observations strongly suggest that the signals elicited by low temporal frequency flicker are derived from L-M cone-opponent, parvocellular neurons, whilst the faster responses (∼30 Hz) correlate more closely with non-opponent, magnocellular response properties (Kremers & Link, 2008).
Obtaining the physiological signatures of the parvo- and magnocellular pathways (or their immediate precursors) from the ERG relies on stimulating at different temporal frequencies in separate recordings (Kremers & Link, 2008; Kremers et al. 2010). As described above, these pathways operate in parallel and therefore respond simultaneously. As has been pointed out in numerous reports (e.g. Shevell & Kingdom, 2008; Lee et al. 2011), there is rarely a situation in the natural environment where chromatic and luminance information are completely separated. If the distinct temporal frequency characteristics of these pathways could be retrieved from simultaneous human ERG recordings, this would indicate that the signals were representing parallel processing in the normal retina. To this end, a compound grating stimulus composed of spatially interleaved red and green raised cosines has been described which tests the notion that the achromatic and chromatic channels can be activated simultaneously, whilst their responses can be separated in the primate retina (Lee et al. 2011). In this stimulus, the luminance component has twice the spatial frequency of the chromatic component. When used to stimulate retinal ganglion cells in the non-human primate retina, chromatic and luminance responses were readily discriminated, providing compelling evidence that information about these two features is transmitted concurrently by parvocellular and magnocellular retinal mechanisms, respectively.
The present study is based on observations that heterochromatic flicker ERGs reflect magnocellular, luminance-based activity at high temporal frequencies (Jacobs et al. 1996; Carroll et al. 2002) and parvocellular, chromatic activity at low temporal frequencies (Kremers & Link, 2008; Kremers et al. 2010). We sought to separate luminance and chromatic signals in the human ERG using a temporal compound stimulus that is closely related to the spatial compound described above. The temporal profile of the chromatic–achromatic temporal compound stimulus comprises a series of alternating red and green raised cosines. The luminance and chromatic components are separated because the luminance modulation has twice the temporal frequency of the chromatic modulation. The purpose of the study was to use these chromatic–achromatic temporal compound stimuli in order to simultaneously identify chromatic (parvocellular-like) and luminance (magnocellular-like) responses in the human ERG.
As stated above, key properties of the parvocellular and magnocellular systems are that their constituent neurons display respectively low-pass and band-pass temporal frequency response characteristics (Lee et al. 1990). Thus the first stage of testing the compound stimulus involved varying its temporal frequency. If the two components are separable by this stimulus then the prediction would be that the ERG responses generated by the chromatic modulation should have a low-pass temporal response function whilst those generated by the luminance modulation should exhibit band-pass temporal tuning. As a further control, the chromatic–achromatic compound stimulus was also used to generate ERGs in dichromatic observers, who lack a red–green chromatic system. In these observers, we would expect the putative chromatic response to be absent. The results are consistent with our predictions, demonstrating that it is possible to simultaneously elicit responses from the human retina that reflect the operation of colour and luminance post-receptoral processing pathways.
Methods
Ethical approval
Full informed consent was obtained and the study was conducted in agreement with the tenets of the Declaration of Helsinki. Approval was granted by the Research Ethics committee of the Faculty of Life Sciences, University of Manchester.
Stimuli
Stimuli were presented using a commercial ganzfeld stimulator (ColorDome, Diagnosys LLC, Lowell, MA, USA), controlled by the manufacturer's scripting language. The stimulator contains four types of LED with different emission spectra, although only the red and green ones were used in these studies. Their peak wavelengths (±half-bandwidth at half-height) were 635 (±10) nm and 514 (±20) nm. Each LED class was modulated in turn with a raised cosinusoidal function so that, in a single temporal cycle, the green luminance increased from black to a preset maximum, then reduced to black again, after which the red luminance followed a similar time course (see Fig. 1). Temporal resolution was 1 ms. Luminance of each LED class was calibrated using a PR650 spectrophotometer (Photo Research Inc., Chatsworth, CA, USA).
Figure 1.
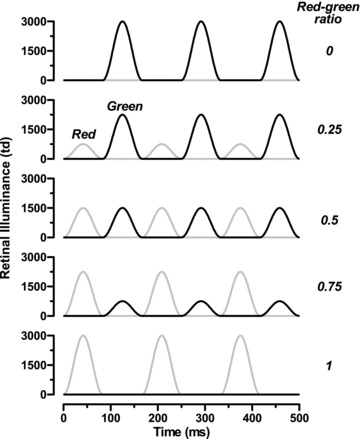
Stimulus profile for red:green ratios between 0 and 1 and a temporal frequency of 6 Hz
To determine individual isoluminance of the red and green stimuli, the red and green maximum luminances were varied in a reciprocal manner whilst mean luminance was kept constant. The red:green ratio (RGR) was defined as:
| (1) |
where LR and LG are the peak luminances of the red and green half-cycles. Thus, when RGR was 0, the stimulus was a series of green raised co-sinusoids with black intervals; when RGR was 1, the stimulus was red and black. At photometric isoluminance (RGR = 0.5), red and green luminance output profiles were equal (see Fig. 1).
Figure 2 displays the chromatic (upper plot) and luminance (lower plot) profiles of the stimuli when RGR = 0.5. The nominal frequency of the stimulus was defined to be that of the chromatic component. The chromatic and luminance profiles are obtained by subtraction and summation, respectively, of the luminance output of two LED types. Observe that the luminance output modulates at twice the temporal frequency of the chromatic output. As shown, the chromatic component is not purely sinusoidal but the harmonics are relatively small compared to the fundamental.
Figure 2. Chromatic (top) and luminance (bottom) modulation for RGR = 0.5 and temporal frequency of 6 Hz.
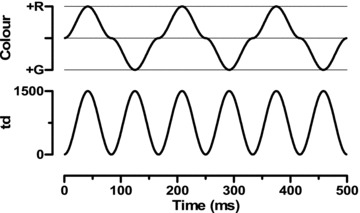
Note that the red–green alternation is at this frequency, but the luminance modulation occurs at twice the frequency (12 Hz).
ERG recording
Electroretinograms were recorded from each subject's right eye using a corneal fibre electrode (DTL, Dept of Physics and Clinical Engineering, Royal Liverpool University Hospital, UK). The reference electrode was a 9 mm Ag–AgCl electrode (Biosense Medical, Chelmsford, UK) attached to the outer canthus and a similar electrode was affixed to the forehead to serve as ground. Impedance was maintained below 5 kΩ. Signals were amplified and filtered using a Grass Model 15A94 neurophysiological amplifier (Astro-Med Inc., W. Warwick, RI, USA). Gain was 20,000× and the bandwidth was 3–100 Hz. The analogue signals were fed to a CED1401plus interface (Cambridge Electronic Design Ltd, Cambridge, UK) for storage and further analysis. Acquisition was triggered by the beginning of a single red–green cycle, starting with a red raised cosine. The signals were digitised at 1024 Hz and each recording epoch was 4 s long. Each recording was repeated 5 times. Any epochs containing significant blinks or eye-movement artefacts were manually rejected and the recording repeated. The subject was instructed to avoid blinking during each epoch. The ERGs were then submitted to discrete Fourier transform in order to extract amplitude and phase of the 1st, 2nd and 4th harmonics of the fundamental frequency, which was defined as the number of red–green cycles per second.
Subjects
Four colour-normal trichromats and two dichromats (a deuteranope and a protanope) participated in the study. Colour vision was measured using the Nagel Model I Anomaloscope (Schmidt & Haensch, Berlin, Germany). All subjects were male and their ages were 53 (N.R.A.P.), 48 (J.K.), 57 (I.J.M.) and 29 (N.C.). The deuteranope (S.D.) was 28 and the protanope (I.P.) was 41. N.R.A.P., J.K. and I.J.M. were all authors. Subjects’ pupils were dilated using 1% tropicamide and the pupil diameter was used to calculate the luminance required to give mean retinal illumination of 750 td. Pupil diameter was monitored throughout the experiment to ensure that (1) it was fully exposed during a sampling epoch and (2) dilatation did not wear off.
Procedure
Isoluminance
To be able to distinguish the responses elicited by the chromatic and the luminance component in the stimulus, we needed to ensure that the outputs of the red and green LEDs were isoluminant for each subject. As can be seen in Fig. 1, and assuming an isoluminant RGR of 0.5, any deviation from isoluminance would result in luminance signals at two temporal frequencies, one corresponding to the constant luminance signal (second harmonic) and one to the unbalanced red–green alternation (fundamental). To establish each subject's individual isoluminant point, the temporal frequency was set to 18 Hz, so that the luminance frequency was 36 Hz. A range of red:green ratios (RGR) were then presented in order to establish the ratio that generated the minimum signal to the red–green (18 Hz) alternations. This was done in two runs, firstly sampling RGR coarsely (in steps of 0.1 from 0 to 1) and then re-sampling with higher resolution around the approximate minimum. We established that this minimum was not dependent upon whether we started with a RGR of 0 or 1. In control studies we confirmed that this procedure produces the same values for isoluminance as conventional heterochromatic flicker photometry.
Temporal frequency response
Using each subject's isoluminant RGR, a range of between 15 and 25 temporal frequencies from 6 to 30 Hz were then presented. Thus the luminance modulation was between 12 and 60 Hz. Each temporal frequency was presented 5 times. For the main experiments, temporal frequency was sampled closely (in 2 Hz increments) between 9 and 20 Hz with additional recordings at 6, 24 and 30 Hz. In control experiments, the entire temporal frequency range was sampled with high resolution (every 2 Hz) and three additional runs were conducted using red, yellow and green sinusoidal modulation over the range 12–60 Hz.
Results
Isoluminance
Figure 3 shows the 18 Hz ERGs for selected RGRs in a normal trichromat (N.R.A.P.), the deuteranope (S.D.) and the protanope (I.P.). In order to clearly depict the changing shape of the ERG, and for illustrative purposes only, the waveforms have been re-sampled and averaged over a 500 ms epoch, so that they are averages of 40 repetitions. Only the first 250 ms are shown. At the extremes of red:green ratio, where the stimulus is a series of raised cosinusoidal pulses of one hue, with intervening black intervals of the same duration, the response is a prominent positivity (equating possibly to the b-wave) and a small secondary positivity which may be the equivalent of the d-wave (‘off’ response). Note that, when RGR was 0, the first green pulse commenced 28 ms after the trigger and reached its peak at 42 ms. When RGR was 1, the red pulse occurred half a cycle earlier and the data clearly show this abrupt 180 deg phase shift (see Fig. 4). Intermediate values show asymmetrical responses to the red and green pulses with one exception: at a single value of RGR, the response takes the form of a simple sinusoid and all the response is at the second harmonic. As described below, the ratio that minimised the first harmonic response was assumed to represent individual isoluminance. In Fig. 3, these ratios are depicted with an asterisk and the waveform is plotted with a darker line.
Figure 3. Averaged 18 Hz ERGs for extreme red–green ratios (0 = green only, 1 = red only), equal luminance (RGR = 0.5) and two intermediate values for a normal trichromat (N.R.A.P., left), a deuteranope (S.D., centre) and a protanope (I.P., right).
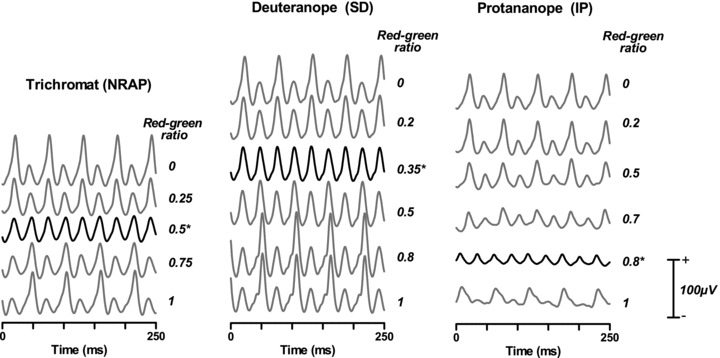
In addition, the isoluminant ERG (i.e. that showing minimum 1st harmonic response) is shown in bold, with an asterisk denoting the isoluminant ratio. For the trichromat this coincided with RGR = 0.5, for the deuteranope it was 0.35 and for the protanope it was 0.8. The waveforms are derived from averages of 5 repetitions of 4 s each which have then been re-sampled over an epoch of 500 ms and re-averaged. Thus each waveform is an average of 40 sweeps. Only the first 250 ms are shown.
Figure 4.
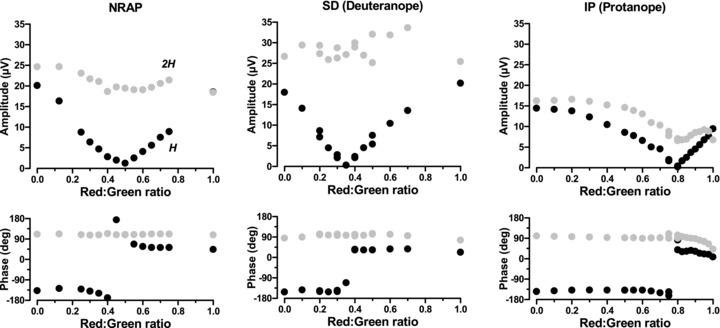
ERG amplitude and phase of the first harmonic (black circles) and second harmonic (grey circles) responses as a function of red–green ratio in a trichromat (N.R.A.P., left), a deuteranope (S.D., centre) and a protanope (I.P., right)
In Fig. 4, the amplitude and phase of the the same subjects’ ERGs are shown as functions of red:green ratio. Each data point is the vector average of the Fourier-transformed ERG signals for each of the five stimulus presentations. Clearly the first and second harmonics behave quite differently. The first harmonic is related to the difference in response to the red and green raised cosines. In all subjects, a minimum was reached at a given RGR. This is reminiscent of heterochromatic flicker photometry (HFP), where the percept of flicker is lowest when the luminance content is minimised. There is, however, an important difference in comparison with the HFP procedure, since there is still significant luminance activity at the second harmonic at every red–green ratio. Our interpretation is that the additional, first harmonic, luminance activity caused by the red–green imbalance is minimised. The fact that there is no residual signal in the dichromat's ERG is evidence that there is no chromatic response here either. In both the trichromat and the deuteranope, the amplitude of the 2nd harmonic remains more or less constant, since the luminance modulation is always present. The protanope shows a reduction in amplitude as the red end of the ratio is reached. As the amplitude of the first harmonic reaches zero, its phase changes sharply by 180 deg, reflecting the change in dominance from the red to the green luminance signal. The phase of the 2nd harmonic remains constant at all ratios and for all subjects except the protanope, where the signal slows in line with the reduction in amplitude.
With one exception (I.J.M.), the normal subjects’ isoluminant points were at photometric isoluminance (RGR = 0.5). Although colour vision is normal in his case, I.J.M. is known to have an unusually high proportion of long-wavelength cones (see Kremers et al. 2011) and it is therefore unsurprising that his isoluminant red:green ratio is shifted towards green (RGR = 0.4). As would be expected, the dichromats also show a displaced isoluminant value (0.35 for the deuteranope, 0.8 for the protanope), indicating the need for a higher than normal proportion of one colour to balance the other. In the following experiments, each subject's individual isoluminant ratio was used for the temporal frequency runs.
Temporal frequency
Figure 5 shows ERGs for all six subjects at selected temporal frequencies. Again they have been re-averaged for illustrative purposes. The figure also depicts the stimulus profile for the slowest (6 Hz) stimulus so that the relationship between stimulus and response can clearly be seen. At 6 Hz, all four trichromats show a similar pattern, with asymmetrical red and green responses. On the other hand, the dichromats show no difference in the response to the green or the red pulse, and the data resemble those shown by Pangeni et al. (2010) to a luminance sinusoid.
Figure 5. Averaged isoluminant ERGs for a range of temporal frequencies in four normal trichromats (columns 1 to 4), a deuteranope (S.D., column 5) and a protanope (I.P., column 6).
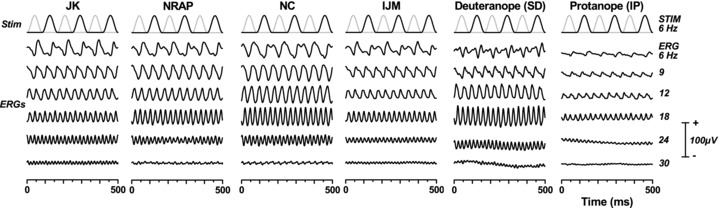
The waveforms are re-sampled and re-averaged from the original 4 s sweeps, as in Fig. 3, although here we show the full 500 ms. At the top of each column is the red and green luminance profile for the slowest (6 Hz) stimulus.
Harmonic analysis
The raw data were submitted to discrete Fourier transform and 1st, 2nd and 4th harmonics were extracted. Amplitude (Fig. 6) and phase (Fig. 7) are shown here as a function of temporal frequency. To aid interpretation, the x-axes are labelled with the harmonic frequency (i.e. once, twice and four times the stimulus frequency). Although these data are vector averages of five repeats, they are remarkably consistent, the error bars being smaller than the data points. There is also minimal between-subject variation in the trichromatic data. In each of the four trichromats, there is a low-pass first harmonic response (to the red–green alternations) which descends to noise levels between 15 and 20 Hz. The second harmonic, which is related to the luminance signal, has a much more band-bass shape, peaking between 30 and 40 Hz. There is an additional lobe on the low frequency branch peaking at around 18–20 Hz for all the trichromats, suggesting there may be two separate response components.
Figure 6. ERG amplitude as a function of harmonic frequency for fundamental, 2nd and 4th harmonics.
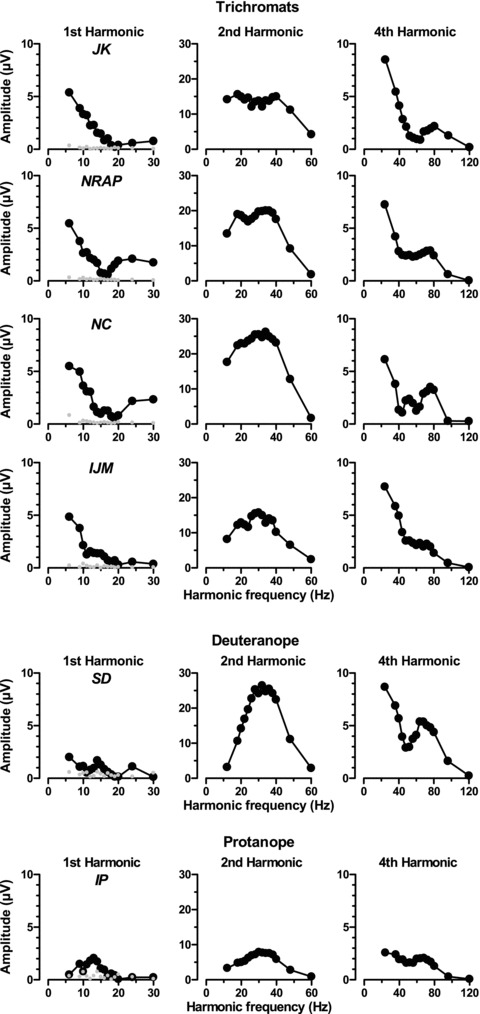
The top 4 rows are the 4 trichromats’ data and the bottom 2 rows are the the dichromats’ data. The leftmost column also depicts noise (at H-1 Hz) as grey symbols. Vector averages of 5 repetitions. The error bars are smaller than the data points.
Figure 7. ERG phase as a function of harmonic frequency for fundamental, 2nd and 4th harmonics.
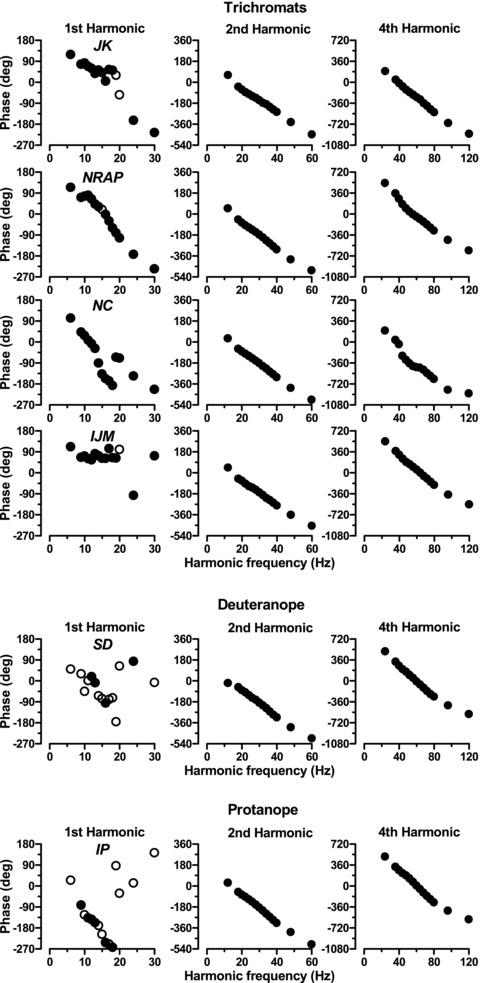
The top 4 rows are the 4 trichromats’ data and the bottom 2 rows are the dichromats’ data. For the 1st harmonic data, filled circles depict phase where signal:noise ratio exceeded 4, and open circles depict the remainder.
The dichromats’ data are dramatically different: there is minimal response at the first harmonic and the second harmonic has a much simpler band-pass characteristic, again peaking between 30 and 40 Hz. The low frequency lobe seen in the trichromatic responses appears to be absent. All six subjects show similar shaped 4th harmonic responses in the form of a deeply notched low-pass function with the notch occurring at a harmonic frequency around 48 Hz (i.e. when the red–green or fundamental frequency was about 12 Hz). In this study, the 4th harmonic can be regarded as the second harmonic of the luminance component.
Phase of the 2nd and 4th harmonics was highly linear throughout the frequency range, indicating that the responses have a constant time delay. The phase of the first harmonic is less easy to interpret. We have plotted with filled circles the data where signal:noise ratio exceed 4, and open circles for the rest. One clear point is that most of the deuteranope's and a good deal of the protanope's responses fell short of this criterion. Nevertheless the protanope's data seem to show a more systematic variation of phase with temporal frequency. Clearly though this is quite different from the trichromat's 1st harmonic response. Virtually all of the trichromats’ data exceeded the criterion value, but showed a more complex relationship with temporal frequency. We have the impression that the overall slope is shallower, but this is a feature that we will be examining in more detail in a future study.
Simple vs compound sinusoidal stimulation
In order to investigate the relationship between the luminance component of the compound responses and more conventional methods of obtaining temporal tuning functions, we also measured ERGs to simple sinusoidal luminance flicker stimuli. Responses were recorded to luminance flicker stimuli using only the red or green LEDs or a photometrically equal mixture of the two. This yellow stimulus coincided with the neutral point between red and green, in an analogy with the Sloan notch seen in spectral sensitivity functions (Sloan, 1928). In this region, spectral increments are signalled largely by non-opponent (i.e. luminance) mechanisms (King-Smith & Carden, 1976; Foster et al. 1986; Calkins et al. 1992; Schwartz et al. 1995). Our red and green stimuli, on the other hand, are located either side of neutral yellow and are thus more likely to contain contributions from cone-opponent chromatic mechanisms (Sperling & Harwerth, 1971; King-Smith & Carden, 1976; Foster & Snelgar, 1983). The temporal frequency of these stimuli was closely sampled in steps of 2 Hz throughout the range (12–60 Hz).
The results shown in Fig. 8A compare the ERGs obtained from a trichromat (J.K.) in response to a simple yellow flickering stimulus with the 2nd harmonic response to the compound stimulus obtained from the dichromatic subject (S.D.). Although there is a scaling difference, both temporal frequency functions are similar in shape, exhibiting band-pass temporal tuning (see the upper panel of Fig. 8A, noting the different y-axes). In addition, the phase variations (lower panel Fig. 8A) for the two responses are virtually identical as a function of temporal frequency. In Fig. 8B (upper plot), the trichromat's ERG response amplitudes to the yellow, red and green simple flicker stimuli are plotted as a function of temporal frequency. In addition to these responses to simple sinusoidal luminance modulation, the second harmonic response to the compound stimulus is also shown. Unlike the temporal frequency response curve for the yellow luminance flicker stimulus, the response functions to the red and, to a much lesser extent, the green simple stimuli exhibit an additional lobe in the low temporal frequency region which has a local maximum between 15 and 20 Hz. The similarity between the responses is also observed in the phase plots (Fig. 8B, lower panel).
Figure 8. Simple vs compound sinusoidal stimulation.

A, a trichromat's achromatic temporal frequency function, recorded using a luminance-modulated yellow temporal sine-wave, plotted on top of a dichromat's 2nd harmonic response to the red–green compound stimulus. Subjects J.K. (trichromat) and S.D. (deuteranope). The top panel shows amplitude and bottom panel shows phase. B, J.K.'s (trichromatic) response to yellow-only, red-only and green-only luminance modulation, together with the same subject's 2nd harmonic response to the red–green compound stimulus.
Discussion
In this study we have used a novel temporal compound stimulus, composed of separate chromatic and achromatic components, to elicit ERGs across a range of temporal frequencies from trichromatic and dichromatic human retinae. The stimulus we used is the temporal analogue of the spatial compound described recently by Lee et al. (2011). The idea behind these types of stimuli is that separate post-receptoral retinal pathways are simultaneously stimulated by their chromatic and luminance content. So, for compound colour and luminance stimulation, the responses should reflect the inner retinal precursors of both parvocellular and magnocellular activity. This hypothesis is supported by our demonstration that, in the trichromatic retina, there are different harmonic components in the ERGs elicited by these compound stimuli. These show different temporal frequency characteristics and seem to reflect the activity of two of the major post-receptoral processing pathways that are involved in the transfer of red–green chromatic and luminance information from the retina to the cortex. The 1st harmonic component is specific to the isoluminant red–green alternations that occur in the stimulus and has a low-pass temporal frequency response function, consistent with its mediation by the midget bipolar/parvocellular pathway. The second harmonic component reflects activity in the retina generated in response to the luminance modulation within the compound stimulus. It has a band-pass temporal frequency response function which peaks around 30–40 Hz, a property which suggests that the magnocellular pathway (or its precursor stage – the diffuse bipolars) is a likely substrate for this ERG component. These findings, derived from the human ERG, are consistent with the results of Lee et al. (2011), which were obtained from single unit recordings in macaque parvocellular and magnocellular ganglion cells. Therefore, in both the human and non-human primate retina we have the same effect. A single stimulus can simultaneously reveal the operation of two parallel post-receptoral processing mechanisms, one responding to the luminance frequency and the other to the frequency of the chromatic modulation.
As to the origin of these postreceptoral signals, previous studies have shown that the flicker ERG probably reflects the activity of bipolar cells (Bush & Sieving, 1996). Our data demonstrate quite clearly that it is possible to differentiate simultaneously between colour- and luminance-related activity in the ERG response. Thus we might speculate that the colour and luminance ERG components have the midget and diffuse bipolars as their respective retinal substrates. Certainly, the cone selective connectivity of the midget bipolars has led to their being proposed as key elements in the generation of L-M opponency in the primate retina (Dacey, 1999). By the same token, the non-selective, additive combination of cone inputs has led to diffuse bipolars being implicated in luminance processing (Boycott & Wassle, 1991). From a functional perspective, data from the (non-primate) ground squirrel retina also demonstrate a clear functional dichotomy within the bipolar cell population for the signalling of colour and luminance information (Li & DeVries, 2006). Interestingly, the differences in temporal response properties that are apparent between the bipolars that signal colour compared to those that signal luminance (Li & DeVries, 2006), echo those found for the colour and luminance ERG reponses here. Ultimately they are consistent with those of the parvo- and magnocellular ganglion cells (De Monasterio & Gouras, 1975; Dreher et al. 1976; Hicks et al. 1983; Derrington et al. 1984; Derrington & Lennie, 1984; Shapley & Perry, 1986; Lee et al. 1989a, c; Schiller et al. 1990).
A central question is whether the ERG can signal red–green modulation and therefore chromatic activity. We propose that the low-pass fundamental response is specific to the isoluminant red–green alternation in the stimulus; certainly its low-pass nature reflects what is known about the temporal characteristics of chromatic processing (DeLange, 1958; Brindley et al. 1966; Kelly, 1974, 1983; McKeefry et al. 2001). However, an alternative explanation should be considered. Is it possible that low-pass rod and/or S-cone driven responses are intruding into this response component? After all, the red–green stimuli used in this study are not photoreceptor-isolating (cf. Kremers, 2003). As a result, each luminance pulse will be potentially stimulating all four classes of photoreceptor: L-cones (except in the protanope), M-cones (except in the deuteranope), S-cones and rods. The initial experiments ensured that red and green luminances were balanced. However, based on photoreceptor spectral sensitivity (e.g. Smith & Pokorny, 1972), the rods and the S-cones will have been more sensitive to modulation of the green than to the red LEDs. In this respect, the data obtained with the dichromats serve as useful controls as they showed a very impoverished fundamental response. The dichromats lack a functional red–green chromatic system but possess functional rods and S-cones. If they played a significant part, rod- and S-cone driven responses at the first harmonic would also be revealed in the dichromatic data. However, we saw no such systematic response to the chromatic (1st harmonic) component. This is strong evidence that the signals generated by the chromatic and achromatic components of the stimulus originate in the pathways involving parvocellular and magnocellular retinal ganglion cells and not in the activity of rods or S-cones.
Another major difference which can be found between ERGs recorded from trichromatic and dichromatic subjects lies in the temporal response characteristics of the second harmonic elicited by the compound stimulus. In the trichromats, the second harmonic clearly exhibits an additional lobe in the low frequency region that has a small local maximum between 15 and 20 Hz (see Fig. 6, middle column). This low frequency lobe is absent in the dichromats’ responses. We advance the view that the second harmonic is mediated by the diffuse bipolar/magnocellular luminance post-receptoral processing pathway, in line with single-unit studies (Lee et al. 2011). Note also its similarity to the first harmonic of a simple luminance signal and the similarity of the 4th harmonic to the simple luminance second harmonic seen in our own data and, for example, in Pangeni et al. (2010). The presence of the additional lobe is clearly suggestive of a contribution to the response from a different mechanism. In order to establish the nature of this contribution we used simple sinusoidal luminance flicker stimuli of different peak wavelengths (red, green and yellow) to elicit ERGs and compared these responses to those obtained using the compound stimulus in trichromatic and dichromatic observers. These comparisons lead us to conclude that these intrusions at lower temporal frequencies into the second harmonic response are related to the chromatic properties of the compound stimulus, rather than its luminance properties. This conclusion is based on three main observations: firstly, the low frequency lobe is absent from the temporal response function of the compound second harmonic response recorded from the dichromatic subjects, who have no functional red–green colour vision. Here the amplitude and the phase of the second harmonic response are similar to the yellow luminance flicker responses recorded form the trichromats (Fig. 8A). This indicates that, for the dichromats, the compound stimulus is identical to a luminance stimulus; the red–green colour change is not detected in the retina and therefore cannot be discriminated in the ERG. Secondly, the low frequency lobe of the 2nd harmonic response is similar to lobes found in simple red and green luminance flicker stimuli which, by virtue of their position relative to neutral yellow, stimulate chromatic as well as luminance mechanisms. For the yellow flicker stimulus, which lies in the neutral zone between red and green, the low frequency lobe is minimal or absent. Thirdly, in Fig. 9 we have plotted the difference between red and yellow temporal frequency functions and between the red–green compound's second harmonic and the yellow function. They both show the same low-pass characteristic, which is qualitatively similar to the red–green compound's fundamental response (also shown). Thus we have good evidence to support the idea that the additional low temporal frequency lobe that is found in the ostensibly luminance/magnocellular based 2nd harmonic response is in fact generated by chromatic as opposed to luminance attributes of the compound stimulus. The basis for this intrusion might be non-linear responses from the red–green chromatic system which would introduce higher harmonics. However, two observations argue against this possibility. Firstly, the second harmonic component would generally be smaller than the first harmonic component unless there is a frequency doubled response. This is not the case. Secondly, deviations from a sine-wave like response would also introduce a fourth and higher harmonic components. The fourth harmonic, however, is similar to that generated by the simple luminance flicker stimuli (see also Pangeni et al. (2010)), suggesting that there are no additional fourth harmonic components to the chromatic stimulation in the compound stimulus.
Figure 9. The difference between yellow and red luminance modulated responses, superimposed on the fundamental response to the red–green compound.
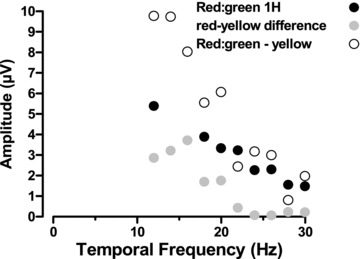
Subject: J.K.
An alternative explanation for these chromatically based intrusions into the second harmonic of the response to the compound stimulus might lie in the fact that the low frequency lobe reflects a frequency-doubled response of the magnocellular luminance system to the chromatic component in the stimulus (Schiller & Colby, 1983; Lee et al. 1989b; Kaiser et al. 1990). Because the chromatic component has half the temporal frequency of the luminance component, the response frequency would therefore be indistinguishable from the luminance response. Furthermore, the single cell data tell us that it is difficult to distinguish between magnocellular responses caused by the luminance and chromatic component in the stimulus (Lee et al. 2011). But the data suggest that the cells can display substantial frequency doubled responses to the chromatic component.
To summarise, we have demonstrated that it is possible to simultaneously record ERGs from the human retina that reflect the operation of chromatic (parvocellular) and luminance (magnocellular) post-receptoral processing pathways. This was achieved using a novel chromatic–achromatic temporal compound stimulus. The first harmonic of the retinal response reflects chromatic processing, whilst the second harmonic is mediated by luminance mechanisms. In addition to providing a clear demonstration of the parallel nature of chromatic and luminance processing in the human retina, differences found between trichromatic and dichromatic responses point to the existence of interactions between post-receptoral pathways that are in operation from the earliest stages of the visual pathway.
Acknowledgments
We would like to acknowledge support from the following organisations: NIHR Manchester Biomedical Research Centre (N.R.A.P.), the Excellence Program of the Hertie Foundation (J.K.), the Vision Science Fund, Manchester (A.P.), and the National Institutes of Health (Grant EY 13112 to B.B.L.).
Glossary
- DTL
Dawson–Trick–Litzkow
- ERG
electroretinogram
- HFP
heterochromatic flicker photometry
- L (-cone)
long-wavelength
- LED
light-emitting diode
- M (-cone)
medium-wavelength
- RGR
red:green ratio
- S (-cone)
short-wavelength
Author contributions
The studies were carried out in Dr Parry's laboratory in the Vision Science Centre, Manchester Royal Eye Hospital. N.R.A.P. and J.K. conceived and designed the experiments, based on the original idea by B.B.L. A.P. conducted the control experiments with I.J.M., D.J.M., J.K. and N.R.A.P. N.R.A.P. wrote the software and analysed the data and, with J.K., interpreted them. All six authors contributed substantially to writing and revision of the manuscript and jointly approved the final version.
References
- Boycott BB, Wassle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cells in the mouse retina. J Neurosci. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley GS, DuCroz J, Rushton WAH. The flicker fusion frequency of the blue-sensitive mechanism of colour vision. J Physiol. 1966;183:497–500. doi: 10.1113/jphysiol.1966.sp007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush RA, Sieving PA. Inner retinal contributions to the primate photopic fast flicker electroretinogram. J Opt Soc Am A Opt Image Sci Vis. 1996;13:557–565. doi: 10.1364/josaa.13.000557. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Thornton JE, Pugh EN. Monochromatism determined at a long-wavelength middle-wavelength cone-antagonistic locus. Vision Res. 1992;32:2349–2367. doi: 10.1016/0042-6989(92)90098-4. [DOI] [PubMed] [Google Scholar]
- Carroll J, Neitz J, Neitz M. Estimates of L:M cone ratio from ERG flicker photometry and genetics. J Vis. 2002;2:531–542. doi: 10.1167/2.8.1. [DOI] [PubMed] [Google Scholar]
- Challa NK, McKeefry D, Parry NRA, Kremers J, Murray IJ, Panorgias A. L- and M-cone input to 12 Hz and 30 Hz flicker ERGs across the human retina. Ophthalmic Physiol Opt. 2010;30:503–510. doi: 10.1111/j.1475-1313.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Primate retina: Cell types, circuits and color opponency. Prog Retin Eye Res. 1999;18:737–763. doi: 10.1016/s1350-9462(98)00013-5. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annu Rev Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The blue-on opponent pathway in primate retina originates from a distinct bistratified ganglion-cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- De Monasterio FM, Gouras P. Functional properites of ganglion cells of the rhesus monkey retina. J Physiol. 1975;251:167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange H. Research into the dynamic nature of the human fovea-cortex systems with intermittent and modulated light. I Attenuation characteristics with white and coloured light. J Opt Soc Am. 1958;48:784–789. doi: 10.1364/josa.48.000784. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B, Fukada Y, Rodieck RW. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976;258:433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DH, Snelgar RS. Test and field spectral sensitivites of colour mechanisms obtained on small white backgrounds: action of unitary opponent-colour processes? Vision Res. 1983;23:787–797. doi: 10.1016/0042-6989(83)90201-8. [DOI] [PubMed] [Google Scholar]
- Foster DM, Scase MO, Snelgar RS. Functional isolation of normal human opponent colour processes at increment thresholds. J Physiol. 1986;377:44P. [Google Scholar]
- Hicks TP, Lee BB, Vidyasagar TR. The response of cells in macaque lateral geniculate nucleus to sinusoidal gratings. J Physiol. 1983;337:183–200. doi: 10.1113/jphysiol.1983.sp014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. J Neurosci. 2005;25:9669–9679. doi: 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J. Electrophysiological estimates of individual variation in the L/M cone ratio. In: Drum B, editor. Colour Vision Deficiencies XI. Dordrecht: Kluwer Academic Publishers; 1993. pp. 107–112. [Google Scholar]
- Jacobs GH, Neitz J, Krogh K. Electroretinogram flicker photometry and its applications. J Opt Soc Am A Opt Image Sci Vis. 1996;13:641–648. doi: 10.1364/josaa.13.000641. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Lee BB, Martin PR, Valberg A. The physiological basis of the minimally distinct border demonstrated in the ganglion cells of the macaque retina. J Physiol. 1990;422:153–183. doi: 10.1113/jphysiol.1990.sp017978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DH. Spatio-temporal frequency characteristics of color-vision mechanisms. J Opt Soc Am. 1974;64:983–990. doi: 10.1364/josa.64.000983. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Spatiotemporal variation of chromatic and achromatic contrast thresholds. J Opt Soc Am. 1983;73:742–750. doi: 10.1364/josa.73.000742. [DOI] [PubMed] [Google Scholar]
- King-Smith PE, Carden D. Luminance and opponent-color contributions to visual detection and adaptation and to temporal and spatial integration. J Opt Soc Am. 1976;66:709–717. doi: 10.1364/josa.66.000709. [DOI] [PubMed] [Google Scholar]
- Kremers J. The assessment of L- and M-cone specific electroretinographical signals in the normal and abnormal human retina. Prog Ret Eye Res. 2003;22:579–605. doi: 10.1016/s1350-9462(03)00049-1. [DOI] [PubMed] [Google Scholar]
- Kremers J, Link B. Electroretinographic responses that may reflect activity of parvo- and magnocellular post-receptoral visual pathways. J Vis. 2008;8:11.1–14. doi: 10.1167/8.15.11. [DOI] [PubMed] [Google Scholar]
- Kremers J, Meierkord S. Rod-cone-interactions in deuteranopic observers: models and dynamics. Vision Res. 1999;39:3372–3385. doi: 10.1016/s0042-6989(99)00027-9. [DOI] [PubMed] [Google Scholar]
- Kremers J, Parry NRA, Panorgias A, Murray IJ. The influence of retinal illuminance on L- and M-cone driven electroretinograms. Vis Neurosci. 2011;28:129–135. doi: 10.1017/S0952523810000556. [DOI] [PubMed] [Google Scholar]
- Kremers J, Rodrigues AR, de Lima Silveira LC, da Silva Filho M. Flicker ERGs representing chromaticity and luminance signals. Invest Ophthalmol Vis Sci. 2010;51:577–587. doi: 10.1167/iovs.09-3899. [DOI] [PubMed] [Google Scholar]
- Kremers J, Scholl HPN, Knau H, Berendschot T, Usui T, Sharpe LT. L/M cone ratios in human trichromats assessed by psychophysics, electroretinography, and retinal densitometry. J Opt Soc Am A Opt Image Sci Vis. 2000;17:517–526. doi: 10.1364/josaa.17.000517. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Kulikowski JJ. Chromatic and luminance channels in the primate visual pathway. In: Kulikowski JJ, Dickinson CM, Murray IJ, editors. Seeing Contour and Colour. Oxford: Pergamon; 1989a. pp. 54–56. [Google Scholar]
- Lee BB, Martin PR, Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. J Physiol. 1988;404:323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Nonlinear summation of M and L cone inputs to phasic retinal ganglion cells of the macaque. J Neurosci. 1989b;9:1433–1442. doi: 10.1523/JNEUROSCI.09-04-01433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989c;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am A Opt Image Sci Vis. 1990;7:2223–2236. doi: 10.1364/josaa.7.002223. [DOI] [PubMed] [Google Scholar]
- Lee BB, Sun H, Valberg A. Segregation of chromatic and luminance signals using a novel grating stimulus. J Physiol. 2011;589:59–73. doi: 10.1113/jphysiol.2010.188862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Valberg A, Tigwell DA, Tryti J. An account of responses of spectrally opponent neurons in macaque lateral geniculate-nucleus to successive contrast. Proc R Soc Lond B Biol Sci. 1987;230:293–314. doi: 10.1098/rspb.1987.0021. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries H. Bipolar cells pathways for color and luminance visison in a dichroamtic mammalian retina. Nat Neurosci. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- McKeefry DJ, Murray IJ, Kulikowski JJ. Red-green and blue-yellow mechanisms are matched in sensitivity for temporal and spatial modulation. Vision Res. 2001;41:245–255. doi: 10.1016/s0042-6989(00)00247-9. [DOI] [PubMed] [Google Scholar]
- Murray IJ, Parry NRA, Kremers J, Stepien M, Schild A. Photoreceptor topography and cone-specific electroretinograms. Vis Neurosci. 2004;21:231–235. doi: 10.1017/s0952523804213268. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangeni G, Horn FK, Kremers J. A new interpretation of components in the ERG signals to sine wave luminance stimuli at different temporal frequencies and contrasts. Vis Neurosci. 2010;27:79–90. doi: 10.1017/S0952523810000179. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Colby CL. The responses of single cells in the lateral geniculate nucleus of the rhesus monkey to colour and luminance contrast. Vision Res. 1983;23:1631–1641. doi: 10.1016/0042-6989(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK, Charles ER. Functions of the colour-opponent and broad-band channels of the visual system. Nature. 1990;343:68–70. doi: 10.1038/343068a0. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Blalock J, Mallory L, Rowley D. Backward-masking effects on achromatic and chromatic spectral sensitivity. Invest Ophthalmol Vis Sci. 1995;36:S907. [Google Scholar]
- Shapley R, Perry VH. Cat and monkey retinal ganglion cells and their visual functional roles. Trends Neurosci. 1986;9:229–235. [Google Scholar]
- Shevell SK, Kingdom FAA. Color in complex scenes. Annu Rev Psychol. 2008;59:143–166. doi: 10.1146/annurev.psych.59.103006.093619. [DOI] [PubMed] [Google Scholar]
- Sloan LL. The effect of intensity of light, state of adaptation of the eye, and size of photometric field on the visibility curve. Psychol Monogr. 1928;38:1–87. [Google Scholar]
- Smith VC, Pokorny J. Spectral sensitivity of color-blind observers and cone photopigments. Vision Res. 1972;12:2059–2071. doi: 10.1016/0042-6989(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Sperling HG, Harwerth RS. Red-green cone interaction in the incremental threshold spectral sensitivity of primates. Science. 1971;172:180–184. doi: 10.1126/science.172.3979.180. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Slaughter MM. The b-wave of the electroretinogram; a reflection of ON-bipolar cell activity. J Gen Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Kremers J, Sharpe LT, Zrenner E. Flicker cone electroretinogram in dichromats and trichromats. Vision Res. 1998;38:3391–3396. doi: 10.1016/s0042-6989(97)00466-5. [DOI] [PubMed] [Google Scholar]
- Valberg A, Lee BB, Kaiser PK, Kremers J. Responses of macaque ganglion-cells to movement of chromatic borders. J Physiol. 1992;458:579–602. doi: 10.1113/jphysiol.1992.sp019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Frishman LJ, Robson JG. The uniform field and pattern ERG in macaques with experimental glaucoma: Removal of spiking activity. Invest Ophthalmol Vis Sci. 2000;41:2797–2810. [PubMed] [Google Scholar]
- Viswanathan S, Frishman LJ, Robson JG. Inner-retinal contributions to the photopic sinusoidal flicker electroretinogram of macaques: Macaque photopic sinusoidal flicker ERG. Doc Ophthalmol. 2002;105:223–242. doi: 10.1023/a:1020505104334. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL. The photopic negative response of the macaque electroretinogram: Reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1124–1136. [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wassle H, Gruenert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Res. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]


