Abstract
Caloric restriction attenuates the onset of a number of pathologies related to ageing. In mammals, circadian rhythms, controlled by the hypothalamic suprachiasmatic (SCN) clock, are altered with ageing. Although light is the main synchronizer for the clock, a daily hypocaloric feeding (HF) may also modulate the SCN activity in nocturnal rodents. Here we report that a HF also affects behavioural, physiological and molecular circadian rhythms of the diurnal rodent Arvicanthis ansorgei. Under constant darkness HF, but not normocaloric feeding (NF), entrains circadian behaviour. Under a light–dark cycle, HF at midnight led to phase delays of the rhythms of locomotor activity and plasma corticosterone. Furthermore, Per2 and vasopressin gene oscillations in the SCN were phase delayed in HF Arvicanthis compared with animals fed ad libitum. Moreover, light-induced expression of Per genes in the SCN was modified in HF Arvicanthis, despite a non-significant effect on light-induced behavioural phase delays. Together, our data show that HF affects the circadian system of the diurnal rodent Arvicanthis ansorgei differentially from nocturnal rodents. The Arvicanthis model has relevance for the potential use of HF to manipulate circadian rhythms in diurnal species including humans.
Key points
Timed hypocaloric feeding alters the main circadian clock, the suprachiasmatic nucleus (SCN), in nocturnal rodents.
The endogenous oscillatory mechanism in the SCN is similar between nocturnal and diurnal mammals.
In this study we report that in the diurnal rodent Arvicanthis ansorgei, a timed hypocaloric feeding (HF) entrains and shifts behavioural and molecular circadian rhythms in the SCN. Nevertheless, instead of phase advancing the clock as in nocturnal rodents, HF phase delays the Arvicanthis SCN pacemaker.
Thus, HF modifies the circadian system of the diurnal rodent Arvicanthis ansorgei differentially from nocturnal rodents.
The present results will help us to better understand the circadian system in diurnal species and how feeding cues can synchronize daily rhythms.
Introduction
Circadian clocks confer adaptive advantages to an organism, enabling it to anticipate daily environmental changes (Pittendrigh, 1993). In mammals, the principal circadian clock resides in the suprachiasmatic nucleus (SCN) of the hypothalamus (Moore & Eichler, 1972; Stephan & Zucker, 1972). Whereas photic stimuli entrain the SCN clock (Albrecht et al. 1997; Meijer & Schwartz, 2003), restricted feeding schedules in constant darkness conditions (DD) have been reported to affect behavioural rhythms and the molecular clockwork of the SCN in a few mammalian species (Kennedy et al. 1991; Mistlberger, 1994; Stephan, 2002; Castillo et al. 2004; Lamont et al. 2005; Mendoza et al. 2005a). Restricted feeding schedules are also able to synchronize behavioural and physiological rhythms independently of the SCN clock, and under the control of a circadian food-entrainable oscillator (FEO); however, the locus of FEO is still unidentified (Mistlberger, 1994; Stephan, 2002).
Otherwise, when feeding/fasting cycles comprise a hypocaloric feeding (HF), strong effects on the SCN molecular activity are observed (Caldelas et al. 2005; Mendoza et al. 2005b). Under DD, behavioural rhythms and clock gene oscillations in the SCN are entrained by a daily HF in rats (Caldelas et al. 2005). Moreover, in mice entrained to a light–dark (LD) cycle, HF presented during the day causes phase advances of behavioural and physiological rhythms as well as of clock gene expression in the SCN (Mendoza et al. 2005b). Despite a considerable literature on food entrainment in nocturnal rodents, relatively little is known about circadian phase resetting by feeding cues in diurnal mammalian species. In squirrel monkeys, 3 h of food access (without caloric restriction) has no effect on the locomotor activity rhythm under a LD cycle or in constant light (LL) conditions (Aschoff & von Goetz, 1986; Boulos et al. 1989). However, there is one report in the same species showing that in LL, 3 h of food access entrains the free-running rhythm of locomotor activity (Sulzman et al. 1977). In rodents, to our knowledge, there are very few reports showing that free-running behavioural rhythms of diurnal species are entrained to restricted feeding schedules (squirrels; Chandrashekaran, 1982). Recently it has been reported that the diurnal rodent Octodon degus may develop a nocturnal phenotype when scheduled feeding is imposed at night (Vivanco et al. 2010).
The small rodent Sudanian grass rat (Arvicanthis ansorgei) expresses a diurnal-like activity pattern in a crepuscular fashion (Challet et al. 2002). The phase responses to light and melatonin in Arvicanthis ansorgei do not differ from those in nocturnal rodents, with similar phase delays and advances in the early and late night, respectively, for light, and entrainment to daily melatonin pulses when they occur at the end of the subjective day (Caldelas et al. 2003; Slotten et al. 2005). However, the circadian times when serotonergic and dark cues can phase shift the Arvicanthis SCN clock are opposite to those of nocturnal rodents (Mendoza et al. 2007a; Cuesta et al. 2008). This suggests that, at least for these non-photic stimuli (serotonin and dark pulses), there are differences in the circadian windows of sensitivity in the SCN between diurnal and nocturnal species. Nevertheless, in other diurnal species (ground squirrels), phase advances to non-photic stimulation (activity-inducing stimulus) occur when stimulation coincide with the end of the (subjective) day, similarly to nocturnal rodents (Syrian hamsters). This study suggests that phase response to non-photic stimulation depends on the phase of the clock and not on the phase of the activity cycle (Hut et al. 1999). Therefore, it may be possible that phase responses to non-photic cues in diurnal rodents are species dependent.
To gain a better knowledge in non-photic entrainment in diurnal species, the aim of the present study was to examine the effect of restricted feeding schedules with and without a HF regimen on circadian behaviour and expression of clock genes in the SCN of Arvicanthis. Moreover, we investigated the modulation of the synchronization to the LD cycle and phase shifting responses to light in Arvicanthis subjected to a daily HF.
Methods
Animal housing
Adult male Sudanian grass rats (Arvicanthis ansorgei) from our breeding colony in Strasbourg, weighing 100–120 g at the beginning of the study, were maintained in a 12:12 h light–dark cycle (LD, lights on at 07.00 h; light intensity about 200 lux), regulated temperature (22 ± 1°C), free access to regular laboratory diet (Ref. 105, SAFE, 89290, Augy, France) and tap water unless otherwise stated. Animals were acclimated to environmental conditions for at least 2 weeks before starting the experimental procedures. Animal handling was conducted in accordance with The Journal of Physiology's standards and advice (Drummond, 2009) and the Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 86-23, revised 1985) and the French Department of Agriculture (licence no. 67–378 to J.M.).
Experimental design
Effects of a daily hypocaloric feeding in Arvicanthis under constant darkness conditions
At a first stage of the study, we explored the effect of a timed hypocaloric feeding (HF) in the wheel-running activity rhythms of Arvicanthis (n= 8) exposed to constant darkness (DD) conditions. As a control group we used animals exposed to daily normocaloric feeding (NF, n = 8). Free-running circadian rhythms of the animals were monitored at least 15 days in DD before the onset of feeding schedules. Since no reports exist about protocols of caloric restriction in this species, HF was induced by reducing progressively the time of food access. Therefore, HF animals were exposed first to 6 h of food access during 10 days (the amount of food eaten at this schedule was 8.9 ± 0.6 g), then to 4 h (8.2 ± 0.5 g) and finally to 2 h (6.6 ± 0.2 g) of food access during 7 and 10 days, respectively. In the control NF group, the time of daily food access was restricted to 6 h (8.8 ± 0.4 g) during the whole experiment. Since in this experiment animals were under DD conditions, mealtime was at 18.00 h geographical time for both NF and HF Arvicanthis. Animals were weighed weekly to verify body weight loss by feeding conditions.
Timed hypocaloric feeding in Arvicanthis entrained to a light–dark cycle
For this experiment, animals entrained to a LD cycle (12–12 h) received a NF or HF (n = 6–8 per group) at the zeitgeber time 18 (considering ZT-0 as lights on; ZT-18 corresponds to the middle of the Arvicanthis rest period) for 5 weeks. The caloric restriction protocol was different in this experiment compared to the previous one. For this first group of animals, the 100% (e.g. 9 g) of food intake was determined in the first 2 weeks of baseline under food ad libitum (AL) conditions, and was the amount of food given to the control NF group daily at ZT-18. The HF consisted of the 66% (e.g. 5.6 g) of the daily food intake for each animal. The order of these protocols was chosen to assess the global effect of caloric restriction on circadian behaviour with different methods. Finally animals were re-fed ad libitum and behaviour was recorded for at least 3 weeks.
To evaluate the effects of HF on the phase of wheel-running activity rhythms avoiding the masking effect of light, another group of Arvicanthis (n = 10–12 per group) was exposed, under a LD cycle, to AL and HF (66%) conditions during 3 weeks. On the last day of food restriction, animals were released in DD conditions (at least for 3 weeks) to evaluate behavioural phase shifts. Arvicanthis were weighed weekly.
Clock gene expression in the SCN of Arvicanthis: differences between ad libitum, normocaloric and hypocaloric feeding conditions
New animals under a 12–12 LD cycle were exposed to AL, or timed NF (100%) and HF (66%) conditions. Food was delivered at ZT-18 (6 h after lights off) similar to the behavioural experiments under LD cycle. Three weeks after the food restriction period, animals were killed at four different zeitgeber times (n = 4–6 per group and time point); ZT-0 (lights on), ZT-6, ZT-12 (lights off) and ZT-18 (mealtime) with an overdose of isoflurane and brains were rapidly removed and blood samples were obtained for in situ hybridization and hormonal assays, respectively.
Light resetting: behavioural phase shifts, genes and protein expression in the SCN clock induced by light
To evaluate the effect of HF in photic resetting, two groups of animals (ad libitum, AL vs. HF, n = 8 per group) under DD conditions were exposed to a 30 min light pulse (200 lux) at the circadian time 0 (CT-0; activity onset) and CT-12. At the end of baseline conditions (LD 12:12 for 2 weeks), animals were transferred to DD and fed ad libitum for 2 weeks. The control group remained under food ad libitum conditions and the HF group received food (66%) at 12.00h (geographical time) for 3 weeks. After the light pulse manipulation, all animals were fed ad libitum on the same day and remained on DD for 3 weeks. Another group of animals was used under the same feeding conditions (AL vs. HF) and was exposed to a light stimulation (30 min) only at CT-12. Animals were killed 1 h (CT-13; n = 8 per group) after light exposure. Dark control animals were killed at CT-13 (n = 8 per group) without light stimulation. Brains were removed for in situ hybridization and immunohistochemistry experiments.
Behavioural recordings and analysis
For behavioural recordings, animals were housed singly in plastic cages (35 × 22 × 40 cm) equipped with running wheels (30 cm diameter). Each running wheel was equipped with a magnetic microswitch connected to a PC. Wheel-running activity was continuously recorded with a data acquisition system (CAMS; Circadian Activity Monitoring System, University of Lyon, France) and analysed with ClockLab software (Actimetricts, Evanston, IL, USA). Activity data were displayed as actograms and average waveforms using ClockLab. The following parameters were quantified: 24 h mean of activity for animals under a LD cycle, and the endogenous period (τ) of behavioural rhythms for animals in DD. The endogenous period (τ) was assessed for each animal before, during and after food entrainment using a χ2 periodogram analysis and by a regression line fitted to activity onsets (ClockLab software, Actimetrics, Evanston, IL, USA). The duration of nocturnal and diurnal activity was calculated using the onset and offset of light. In addition, we measured the magnitude of food anticipatory activity (FAA) as the total daily activity occurring during the 3 h preceding mealtime in experiment 1. For light resetting experiment, the magnitude of phase shifts was determined by measuring the phase difference, based on the activity onset as a phase reference point, between regression lines fitted to activity onsets of the 10 days before the light pulse and at least 10 consecutive activity-onset times after the light pulse.
In situ hybridization
Animals were deeply anaesthetized with isoflurane and decapitated. Brains were rapidly excised and frozen on dry ice. Coronal sections of 18 μm were cut in a cryostat for in situ hybridization at the level of the SCN. Antisense RNA probes were transcribed in the presence of α35S-UTP (1250 Ci mmol−1, PerkinElmer, Milan, Italy) according to the manufacturer's protocol (MAXIscript, Ambion, Austin, TX, USA). Here we used riboprobes for rPer1 (bases 638–1618 from GenBank accession no. NM001034125), rPer2 (bases 1170–1930 from NM031678; Per1 and Per2 plasmids were kindly donated by H. Okamura, Department of Systems Biology, Kyoto University, Japan), rAvp (vasopressin, bases 68–539 from M25646) and rRev-erbα (bases 908–1890 GenBank accession NM145775). Brain sections were fixed in 4% phosphate-buffered paraformaldehyde, rinsed twice with phosphate-buffered saline (PBS) and then acetylated twice in 0.1 m triethanol-amine, washed again with PBS and dehydrated in a graded ethanol series. Sections were hybridized overnight with either denatured antisense riboprobes (1.107 c.p.m. per ml of hybridization medium buffer) in a humid chamber at 54°C with radiolabelled probe in a solution containing 50% deionized formamide, 10% sulfate dextran, 1× Denhardt's solution, 2× SSC (sodium citrate saline), 0.5 mg ml−1 salmon sperm DNA, 0.25 mg ml−1 transfer RNA, and 10 mm dithiothreitol. Sections were then rinsed with SSC, treated with ribonuclease A (Sigma), rinsed with stringency washes of SSC and dehydrated in a graded ethanol series. Slices and radioactive standards were exposed for 5 days to an autoradiographic film (Biomax MS-1 Kodak, Sigma). Standards were included in each cassette to verify that the measured values of optical densities were in the linear response range of the film. Densitometric analysis of hybridization signals was performed using ImageJ (NIH). The optical density of specific signal was calculated by subtracting the intensity of staining background area measured in surrounding (i.e. anterior) hypothalamic area where no hybridization signal was detected from that measured in the SCN. Measures were made bilaterally on three slices and averaged for a given brain. Data were expressed as relative optical density values.
Immunohistochemistry
For immunohistochemistry experiments, animals were killed with an isoflurane overdose and perfused transcardially with 100 ml of 0.9% saline followed by 100 ml of cold 4% paraformaldehyde (PAF) in 0.1 m phosphate buffer (PB; pH 7.4). Brains were removed, post-fixed (overnight in 4% PAF at 4°C) and transferred to a cryoprotectant buffered sucrose solution (30%) for 72 h at 4°C. Brains were then frozen in isopentane at −30°C and then stored at −80°C. Coronal cryosections through the SCN of 30 μm were prepared on a cryostat at −20°C, collected and stored in 0.1 m phosphate-buffered saline (PBS; pH 7.4; Sigma, St Louis, MO, USA) supplemented with 0.01% sodium azide. Free-floating sections were washed in cold PBS and incubated in a solution of 3% of H2O2 (Sigma) in PBS for 30 min at room temperature. Brain sections were then rinsed in PBS for 10 min three times, and incubated for 2 h in a blocking solution in 10% normal donkey serum (NDS) in PBS with 0.3% Triton X-100 (0.3% PBS-X), followed by an incubation in the primary antibody (in 0.3% PBS-X plus NDS) for 24 h at 4°C. We used a rabbit polyclonal anti-c-Fos antibody (1:10,000, raised against an epitope mapping the N-terminus of human c-Fos; SC-7724, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a rabbit polyclonal anti-p-ERK (rabbit polyclonal antibody, dilution: 1:20000; Cell Signaling Technologies, Danvers, MA, USA). Following incubation in the primary antibody, sections were rinsed in PBS-X and incubated for 2 h at 4°C with a biotinylated anti-rabbit IgG made in donkey (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA, diluted 1:2000 with 0.3% PBS-X. Following incubation with secondary antibody, sections were rinsed in PBS-X and incubated for 1 h at room temperature with an avidin–biotin–peroxidase complex (Vectastain Standard Elite ABC Kit; Vector Laboratories, Inc., Burlingame, CA, USA). Following incubation with the ABC reagents, sections were rinsed two times for 10 min in PBS, and incubated with 0.025% 3,3′-diaminobenzidine (Sigma) with 0.01% H2O2 in 50-mm Tris buffer. After this final incubation, sections were rinsed with PBS, wet-mounted onto gel-coated slides, dehydrated through a series of alcohols, soaked in xylene and coverslipped.
Microscopic quantification
Cells were visualized by using a Leica DMRB microscope (Leica Microsystems, Rueil-Malmaison, France) equipped with an Olympus DP50 digital camera (Olympus France, Rungis, France). Images were taken (5×) standardizing all lighting parameters on the microscope and the camera software (Viewfinder Lite, Olympus) to ensure consistent stable lighting throughout the image capture, and stored on a PC as described previously (Mendoza et al. 2008). Positive c-Fos cells (cells were considered positive when the signal represented more than 3 times the background value) were counted in the whole central SCN (2–3 sections/animal), based upon a series of Nissl stained Arvicanthis brain sections. The number of immunoreactive (ir) nuclei was expressed as the mean number of immunopositive cells per section. Since p-ERK-positive staining is present not only in the SCN cell bodies but also in fibres, expression of p-ERK was quantified by measuring staining density as described previously (Mendoza et al. 2008). The optical density (OD) of p-ERK-ir in the SCN was expressed in arbitrary units corresponding to grey levels. To calculate the OD, the background intensity of staining was subtracted from the intensity of staining in the middle SCN. The background intensity was measured in an area devoid of p-ERK cell bodies or fibres (lateral hypothalamic area) in the same coronal section as the SCN analysis.
Hormonal and metabolic assays
Blood glucose (mg dl−1) was determined on fresh blood using a digital glucometer (Glucotrend, Roche Diagnostics, Meylan, France). Plasma insulin was determined by an ELISA kit for rats (90060, Crystal Chem, Inc., Downers Grove, IL, USA). The limit of sensitivity of insulin assay was 0.1 ng ml−1. Plasma corticosterone was assayed with a commercial 125I RIA kit for mice and rats (ImmuChem Double Antibody, MP Biomedicals, Orangeburg, NY, USA). The limit of sensitivity of corticosterone assay was 7.7 ng ml−1.
Statistical analysis
The statistical analysis was performed by one- and two-way analyses of variance (ANOVA), for both independent and repeated measures followed by a Fisher's least significant difference (LSD) post hoc test. A critical value for significance in all experiments was P < 0.05. Statistical analysis was performed with the statistical package Statistica (version 8.0; StatSoft Inc., 2007, France). Values are means ± SEM.
Results
HF synchronizes circadian rhythms of wheel-running behaviour in Arvicanthis
All Arvicanthis recorded in DD displayed stable free-running rhythms of wheel-running behaviour (Fig. 1A and 1B). Differentially from NF animals, Arvicanthis fed with a daily HF did not show an increase of body weight. This difference was significant when animals had 2 h food access (Fig. 1C; P < 0.05). Moreover, food intake was also significantly lower in HF animals compared to NF Arvicanthis at the 2 h feeding schedule (Fig. 1C; P < 0.05). The amount of food ingested during the 2 h feeding schedule in Arvicanthis is quite similar to the amount of food intake in the protocol of the second experiment (66% of the daily food intake; 5.6 g vs. 6.6 g; P= 0.181). This suggests that the protocol used in this experiment is reflecting a hypocaloric state in animals and not only the strongest form of restricted feeding.
Figure 1. HF entrains behavioural circadian rhythms of Arvicanthis.
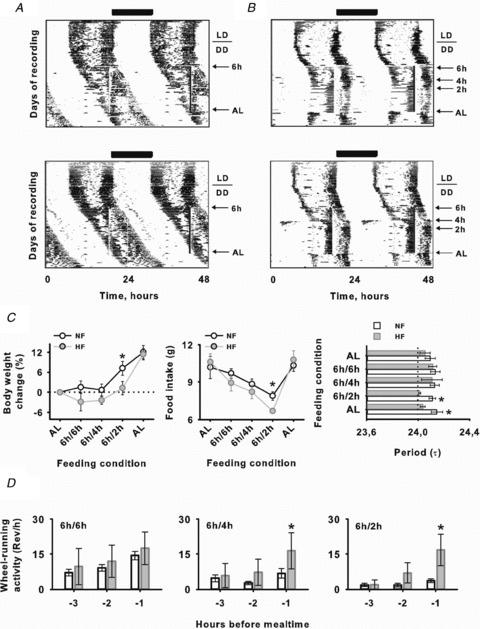
Circadian wheel-running activity of NF (A) and HF (B) fed Arvicanthis. In the representative actograms daily NF and HF feeding is indicated by vertical lines. C, left and middle, body weight (left) and food intake (middle) of Arvicanthis subjected to NF (fixed, 6 h food access) and HF (progressive, from 6 to 2 h food access) restricted feeding schedules. Right, endogenous period values (τ) in both NF and HF animals during previous food ad libitum conditions, during food entrainment and under ad libitum conditions after re-feeding. D, food anticipatory activity (FAA) from 3 to 1 h before mealtime in Arvicanthis subjected to NF (6 h food access) and HF (from 6 to 2 h food access) conditions. *P < 0.05, differences between groups (NF vs. HF).
In animals from the NF group (6 h of food access), no entrainment was observed (Fig. 1A and C). Consequently, the mealtime schedule was timed to overlap with the phase of both rest and activity in Arvicanthis. In animals that did not entrain, some slight changes in τ were present (data not shown) but not real entrainment. The meal schedule was continued until all circadian phases were sampled without entrainment (Fig. 1A). The lack of entrainment in NF Arvicanthis was confirmed by χ2 periodogram analysis (Fig. 1C). In all NF Arvicanthis free-running period was still longer than 24 h (24.16 ± 0.02 h) during synchronization and after the entraining stimulus was removed (24.26 ± 0.07 h; Fig. 1A and C), without significant differences (NS) between values before and during entrainment.
In HF animals, following a short time on the 4 and 2 h feeding schedules, the free-running component became entrained to the feeding time (Fig. 1B). In addition, χ2 periodogram analysis showed values of τ of almost 24 h for HF animals significantly different from those of NF Arvicanthis (Fig. 1C; P < 0.05). Furthermore, τ values of entrained rhythms remained of almost 24 h after food restriction in the HF group (Fig. 1B and C; P < 0.05). These data suggest that under hypocaloric conditions, the behavioural rhythms in Arvicanthis can be entrained by a daily feeding schedule.
During the period of food restriction, food anticipatory activity (FAA) was present in both NF and HF groups (Fig. 1D). However, FAA duration and magnitude in HF animals were greater than those in NF Arvicanthis (Fig. 1D; P < 0.05).
Behavioural and physiological circadian rhythms during food restriction in LD
In all Arvicanthis, the actograms and average waveforms revealed that wheel-running activity was exclusively diurnal (with a crepuscular pattern) in LD prior to food restriction (Fig. 2A). For the NF group, when food availability was limited to 100% of their daily food intake at 6 h after dark onset, all Arvicanthis showed some evidence of nocturnal wheel-running activity in anticipation to feeding time (Fig. 2A). The duration and magnitude (total number of daily wheel revolutions) of FAA was weak compared to the diurnal activity, which remains entrained to the LD cycle (Fig. 2A). At the end of restricted feeding, when NF animals returned to free access to food, no FAA was present and the diurnal rhythm of activity was not altered (Fig. 2A).
Figure 2. Behavioural rhythms entrained to a LD cycle are affected by HF.
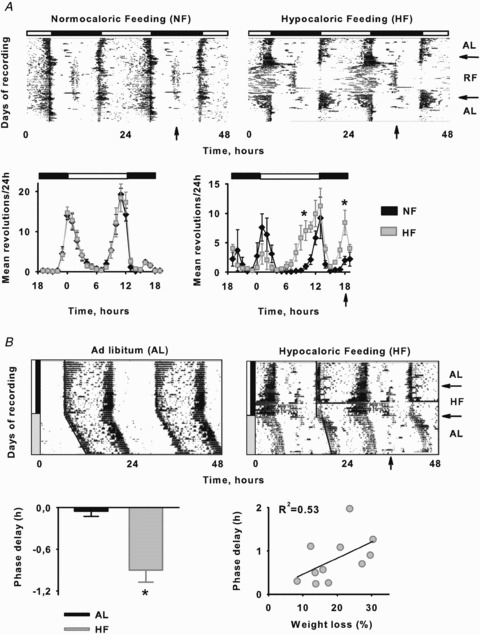
A, daily wheel-running activity of Arvicanthis entrained to a light–dark cycle (indicated by the top black–white bar) and to a NF or HF at the middle of the night period (vertical arrow). In actograms horizontal arrows on the right indicate transition ad libitum–restricted feeding–ad libitum feeding conditions. Activity profiles of wheel-running activity over the 24 h cycle of NF and HF Arvicanthis (means ± SEM) under food ad libitum conditions (left) and during the restricted feeding period (right). B, representative actograms of wheel-running behaviour in Arvicanthis under AL (left) and HF (right) conditions (ZT-18) during the entrainment to a LD cycle and then released in DD conditions. Arrows on the right indicate the time duration of HF. Group mean (±SEM) of phase delays in AL and HF fed animals after to be released in DD conditions. Linear regression of weight loss (%) plotted against phase delays in HF Arvicanthis (R2= 0.53). *P < 0.05, differences between groups.
In Arvicanthis fed with a HF, FAA was as evident as in NF animals; however, it was most robust during the last days of food restriction. Moreover, the diurnal pattern was altered in almost all Arvicanthis, which demonstrated more activity principally during offset of light period, leading to a higher day–night variation compared to NF animals (Fig. 2A). During the re-feeding period, the circadian rhythm of locomotion returned to being diurnal as in the previous food ad libitum conditions (Fig. 2A).
In a second protocol to evaluate modulation of light entrainment by caloric restriction, and avoid possible masking effects by light in phase resetting, animals exposed to HF under LD cycles were released into DD at the end of the feeding schedule. Differentially to AL animals, HF Arvicanthis showed significant phase delays in behaviour after to be released in DD conditions (Fig. 2B; P < 0.05). Moreover, a regression analysis revealed a strong correlation (R2= 0.53) between the body weight loss and the magnitude of phase delays (Fig. 2B; P < 0.05). Using comparable protocols previously in nocturnal rodents, we observed phase advances rather than delays in behavioural rhythms (Challet et al. 1998; Mendoza et al. 2005b).
During baseline, all Arvicanthis (AL, NF and HF) under food ad libitum conditions showed a daily pattern of food intake with a higher feeding behaviour during the day (day, 9.96 ± 0.6; night, 1.93 ± 0.3; Fig. 3A, Inset; P < 0.05). Nevertheless, when animals from NF and HF groups were given food at ZT-18, feeding behaviour showed some changes. In NF animals, the main amount of food was ingested between ZT-0 and ZT-6 (6.6 ± 0.7 g), and between ZT-18 (mealtime; 5.7 ± 0.4 g) and ZT-0 (Fig. 3A). HF Arvicanthis, however, ate almost the whole amount of food from ZT-18 to ZT-0 (5.9 ± 0.13 g) showing a nocturnal feeding behaviour (Fig. 3A; P < 0.05).
Figure 3. Physiological rhythms and Arvicanthis SCN clock gene expression are delayed by HF.
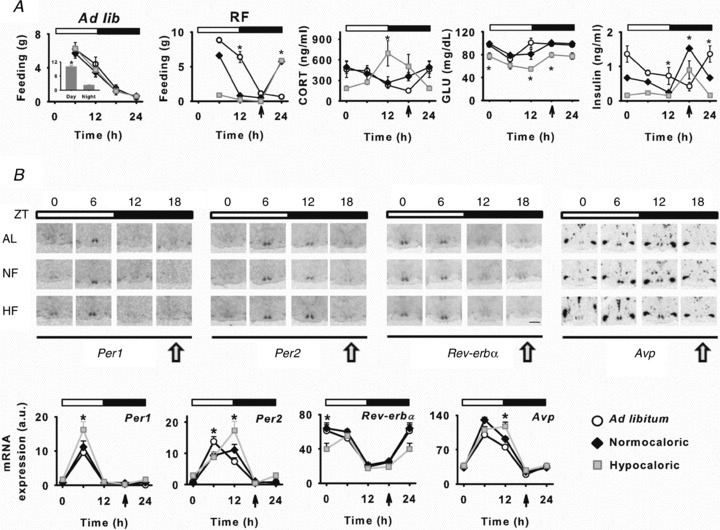
A, daily food intake, metabolic and hormonal profiles in AL, NF and HF fed Arvicanthis. Feeding behaviour under food ad libitum (Ad lib) conditions, previous to exposure to restricted feeding protocols was significantly diurnal in all animals (inset plot). Daily feeding behaviour patterns changed under restricted feeding schedules (RF), leading to a critical nocturnal feeding behaviour in HF fed Arvicanthis. Corticosterone (CORT), glucose and insulin daily profiles in AL, NF and HF fed animals. *P < 0.05, differences between groups. B, daily profiles of clock genes expression in the SCN of AL, NF and HF fed Arvicanthis. Top, representative photomicrographs show coronal sections through the SCN of Arvicanthis kept in LD conditions (indicated by white and black bars) and exposed to AL, NF or HF conditions (Scale bar = 1 mm). Animals were sampled every 6 h at different zeitgeber times (ZT; 0, 6, 12 and 18). Yellow arrows indicate feeding time. Bottom, daily profiles of Per1, Per2, Rev-erbα and Avp in the SCN of AL, NF and HF fed Arvicanthis. Data are presented as the mean ± SEM (a.u., arbitrary units) of 4–6 animals per group and time point. LD conditions are indicated by white and black bars. Time of feeding is indicated by vertical arrows, 6 h after lights off. *P < 0.05, differences between groups.
Corticosterone (CORT) daily profiles were not different between AL and NF animals; both showed a peak at ZT-0, corresponding to the night–day transition (Fig. 3A; P < 0.05). However, in HF animals, CORT daily profile was phase shifted with significantly higher levels and with an acrophase at the day–night transition as in nocturnal rodents (Fig. 3A; P < 0.05). Daily concentrations of plasma glucose were down-regulated in the HF group compared to those in AL and NF animals (Fig. 3A; P < 0.05). Finally, daily rhythms of plasma insulin were shifted in both NF and HF animals compared to AL Arvicanthis (Fig. 3A; P < 0.05).
SCN clock gene expression during food restriction in LD
In Arvicanthis exposed to AL food conditions, daily rhythms in the expression of Per1–2, Rev-erbα and vasopressin (Avp) genes were observed with an acrophase at ZT-6 for Per1–2 and Avp, and ZT-0 for Rev-erbα (Fig. 3B). Daily profiles of Per1 and Rev-erbα were similar between AL and NF animals (Fig. 3B). Interestingly, for HF animals whereas Per1 expression showed a significant increase of the acrophase at ZT-6 compared to the AL control group (Fig. 3B; P < 0.05), a significant phase delay of the rhythmic expression was detected for Per2 and Avp (Fig. 3B; P < 0.05). This effect is consistent with the delayed pattern of behaviour in HF Arvicanthis, and different from the phase advances in clock genes induced by HF in nocturnal rodents (Mendoza et al. 2005b). For Rev-erbα gene expression in HF animals, a down regulation was observed at ZT-0 leading to a phase delayed pattern (Fig. 3B; P < 0.05).
Light-resetting in Arvicanthis under hypocaloric food conditions
Light resetting is altered in mice under hypocaloric feeding conditions, showing changes principally in phase advances induced by light (Challet et al. 1998; Mendoza et al. 2005b). We asked whether a HF can affect light information input to the clock in the SCN of the diurnal rodent Arvicanthis in a similar manner as in nocturnal mice. Therefore, we applied light pulses to ad libitum and hypocaloric fed Arvicanthis that were kept under DD conditions. We found that a light pulse (30 min) applied at circadian time (CT) 0, which corresponds to onset of subjective day, did evoke a phase advance of the clock in both AL and HF animals with no difference in the magnitude of the shift between groups (NS; Fig. 4A). A light pulse applied at CT-12 resulted in a phase delay of the clock in AL animals, and although the phase shift in HF Arvicanthis was larger, it was not significantly different from the AL group (P= 0.08; Fig. 4A).
Figure 4. Photic resetting of the clock is changed in Arvicanthis fed with a HF.
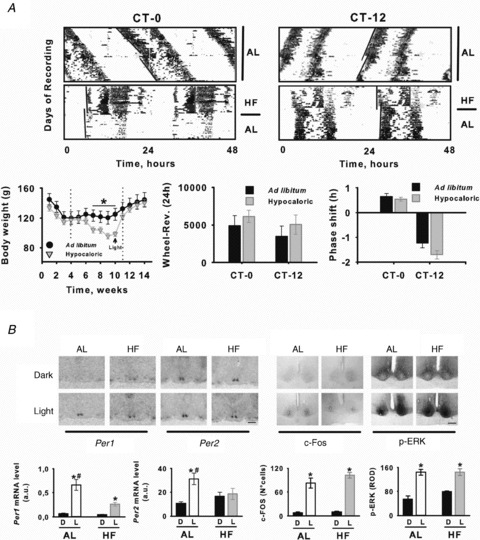
A, top, circadian wheel-running activity from AL and HF Arvicanthis receiving a 30 min light pulse at CT-0 and CT-12. In double-plotted actograms stars indicate the time of light stimulation. Bottom, body weight (left), total locomotion (middle) and group mean (±SEM) phase shifts to light at CT-0 and CT-12 (right) of AL and HF Arvicanthis. In the body weight plot, vertical dotted lines indicate the time in which animals were exposed to HF condition, and arrow indicates body weight values at the moment of light stimulation. B, light exposure induces differential changes on Per1, Per2, c-Fos and p-ERK in the SCN of AL and HF Arvicanthis. Top, representative photomicrographs show coronal sections through the SCN of Arvicanthis kept in constant darkness and exposed to a HF or AL feeding conditions and 30 min light pulse (Light) or a control dark pulse (Dark) at CT-12. Scale bars = 1 mm (Per1–2) and 200 μm (c-Fos, p-ERK). Bottom, semiquantitative analysis of Per1, Per2, c-Fos and p-ERK in the SCN of AL and HF Arvicanthis after 30 min light exposure (L) or after exposure to darkness (D) at CT-12. Data are presented as the mean ± SEM of 4 animals per group. *P < 0.05, differences between light conditions (Light vs. Dark). #P < 0.05, differences between groups (AL vs. HF).
We used in situ hybridization and immunohistochemistry analysis to examine whether a 30 min light pulse delivered during the onset of subjective night (CT-12) had an effect on Per1, Per2, c-Fos and p-ERK in the Arvicanthis SCN. In AL animals, a light pulse delivered at CT-12 led to a dramatic increase in Per1, Per2, c-Fos and p-ERK-IR 1 h after exposure different from dark control animals (Fig. 4B; P < 0.05). However, the increase of Per1 and Per2 expression was lower in HF Arvicanthis (Fig. 4B). Similarly to AL animals, c-Fos and p-ERK expression was enhanced after light stimulation in the SCN of HF Arvicanthis without significant differences (Fig. 4B; NS).
Discussion
The present study showed that a daily HF affects both the photic resetting and the endogenous mechanisms of the SCN clock in the diurnal rodent Arvicanthis ansorgei in a different manner from nocturnal rodents.
Effects of HF in animals under DD conditions
In contrast to a NF, a daily HF is capable of producing entrainment in Arvicanthis under DD conditions. These findings are in accordance with a previous study in rats (Caldelas et al. 2005). Entrainment was produced when timed HF fell in either the subjective night or the subjective day, indicating that the sensitivity of the SCN to HF is not dependent on the behavioural state of animals as for other non-photic cues (Mendoza et al. 2007a; Cuesta et al. 2008).
Behavioural synchronization by feeding (without caloric restriction) in nocturnal rodents under DD conditions has been previously observed (Abe & Rusak, 1992; Marchant & Mistlberger, 1997; Castillo et al. 2004). Lighting condition (DD or LL) is a critical variable in the SCN entrainment by feeding schedules (Caldelas et al. 2005; Lamont et al. 2005). Nevertheless, no entrainment to feeding schedules has previously been observed in diurnal mammals under LL conditions (Aschoff & von Goetz, 1986; Boulos et al. 1989), suggesting that HF is necessary to entrain behavioural rhythms.
Behavioural activation can entrain the SCN in diurnal and nocturnal rodents (Mrosovsky, 1996; Marchant et al. 1997; Hut et al. 1999; Kas & Edgar, 2001). HF Arvicanthis showed intense arousal previous to mealtime (food anticipatory activity; FAA). Thus, it is possible that FAA, higher in HF than in NF, would contribute to clock entrainment in Arvicanthis. Furthermore, serotonin (5-HT) afferents from the raphe nuclei to the SCN are strongly implicated in entrainment by arousal in nocturnal rodents (Pickard & Rea, 1997; Marchant et al. 1997). 5-HT release in the SCN of nocturnal rodents shows a daily rhythm in freely behaving animals, with an increase in release during late midday, peaking at the light–dark transition (Dudley et al. 1998). In the Arvicanthis SCN, in an opposite manner to nocturnal rodents, rhythmic 5-HT concentrations are higher during the day (Cuesta et al. 2009). That suggests that 5-HT (induced by high locomotion during mealtime anticipation) could be an important neurotransmitter implicated in the entrainment of behavioural rhythms in Arvicanthis by HF.
Behavioural and molecular changes in HF fed animals under a LD cycle
Arvicanthis exposed to a LD cycle and under food AL conditions showed a clear diurnal behaviour and in some cases a bimodal wheel-running activity pattern, with peaks of activity at the day–night and night–day transitions (Challet et al. 2002). Interestingly, CORT daily rhythms peaked with a single peak (ZT-0) in AL Arvicanthis, and previously in the same species a two-daily peak rhythm (ZT-23 and ZT-11) of CORT was reported (Verhagen et al. 2004). Here, animals were sampled every 6 h (ZT-0, ZT6, ZT-12 and ZT-18) and in the previous study animals sampling was every 2 h starting at ZT-9 (Verhagen et al. 2004). Thus, the closer time points of this study to the current data are ZT-1 and ZT-13. Therefore, it is possible that in the present experiments, our sampling just skipped the second peak (ZT-11) of CORT. Moreover, in the present study animals were exposed to a running-wheel access. The size of alpha (i.e. the portion of a daily rest–activity cycle corresponding to activity) is compressed in Arvicanthis with access to a running wheel (Challet et al. 2002; Cuesta et al. 2009). Thus, running-wheel activity could also influence the shape of the rhythm of CORT.
Differentially to AL animals, HF Arvicanthis spent more time being active during late day (i.e. last part of the activity period) and early night (i.e. before mealtime). The induction of activity is one of the main responses in organisms exposed to caloric restriction (Bordone & Guarente, 2005). Similarly, in mice exposed to some way of caloric restriction (‘work for food’ paradigm) there is an increase in activity during day time (Hut et al. 2011). This spontaneous diurnal behaviour in mice (animals are not submitted to a kind of feeding time) has been proposed to be important to minimize daily energy expenditure (Hut et al. 2011). In our study HF animals are submitted to timed feeding schedules, and therefore a different mechanism seems to increase night time activity in Arvicanthis. Increase of activity at night time in HF Arvicanthis could be related to the expression of FEO in this species (Mistlberger, 1994). Under food ad libitum conditions the SCN and FEO are strongly coupled, but under restricted feeding schedules (with or without caloric restriction), the FEO becomes uncoupled from the SCN clock (Mistlberger, 1994; Stephan, 2002).
Otherwise, a switch of the behavioural rhythms has been reported in another species as a function of wheel access (Kas & Edgar, 1999; Smale et al. 2003; Redlin & Mrosovsky, 2004). Comparable effects in behavioural timing, albeit in DD, have been observed in rats as a consequence of a HF (Caldelas et al. 2005). Recently a diurnal–nocturnal inversion was reported in the diurnal rodent Octodon degus as a function of restricted feeding (Vivanco et al. 2010). A two-process account of switching is advocated, involving both a change in clock-controlled outputs and a change in the direct response (i.e. masking) to food (Mrosovsky, 1999; Redlin & Mrosovsky, 2004). However, our molecular data indicate that the behavioural shift in HF Arvicanthis is not due only to masking mechanisms. Circadian rhythms of clock gene expression are similar within the SCN of diurnal and nocturnal species (Mrosovsky et al. 2001; Caldelas et al. 2003). Here we observed that circadian genes are shifted or affected in the SCN of HF fed Arvicanthis. In the SCN of control AL animals, Per genes peaked around midday (ZT-6; Caldelas et al. 2003). In HF animals, however, whereas Per1 expression was increased around midday at ZT-6, Per2 and Avp were phase delayed. Moreover, a decrease in Rev-erbα expression at ZT-0 in HF animals suggests a possible phase delay in the daily rhythm of this gene as well. Differential effects have also been observed in nocturnal rodents. In mice, a HF induces an increase in the expression of Per2 and a phase advance of Per1 and Avp expression in the SCN (Mendoza et al. 2005b). The differential responses to HF between diurnal and nocturnal species indeed support the proposition that Per1 and Per2 are more related to phase advances and phase delays, respectively (Albrecht et al. 2001). Together, this demonstrates that the internal phase relationships between circadian cycles of clock gene expression in the Arvicanthis SCN and their phasing relative to the light–dark cycle are phase delayed by HF conditions.
A possible factor influencing the phase delays in behaviour and clock genes in the SCN by HF is the free-running period. Due to the long free-running period in Arvicanthis, the circadian clock delays to synchronize to HF. In mice, which show a free-running period shorter than 24 h, the circadian clock advances in response to a HF (Mendoza et al. 2005b). However, in nocturnal rats with endogenous periods longer than 24 h, behavioural rhythms advance whatever the time of HF exposure (Caldelas et al. 2005). Thus, other mechanisms exclusive to the diurnal species could determine the direction of phase shifts of the Arvicanthis clock under HF conditions.
Light resetting in Arvicanthis fed with a HF
Light induces phase delays and advances in Arvicanthis, regardless of whether they were under AL or HF conditions, similarly to those of a wide variety of nocturnal and diurnal mammals (Daan & Pittendrigh, 1976; Mahoney et al. 2001; Caldelas et al. 2003). A tendency to larger phase delays was visible in Arvicanthis fed with a HF; however, this difference was not significant. Previous studies have indicated that low-caloric diet conditions modify the circadian responses to light in mice (Mendoza et al. 2005b).
Arousal can also influence the magnitude of photic phase shifts in mammals via 5-HT modulation in the SCN (Mrosovsky, 1996). 5-HT stimulation in Arvicanthis potentiates light-induced phase shifts (Cuesta et al. 2008). In nocturnal rodents, however, 5-HT stimulation decreases phase shifts produced by light (Weber et al. 1998; Challet et al. 2001). Thus, non-photic modulation of the SCN clock appears to differ between nocturnal and diurnal species, highlighting the potentiating effects of 5-HT and possibly of caloric restriction on light resetting in diurnal species.
Light resetting of the SCN involves an induction of Per1–2 expression (Albrecht et al. 1997; Shearman et al. 1997). Here we observed an increase in the expression of Per1 in the SCN of both AL and HF animals after light exposure at CT-12. In nocturnal rodents fed with HF, we previously reported a reduction in light-induced of Per1 transcription at CT-12 (Mendoza et al. 2005b). Despite a trend for larger phase delays in circadian behaviour, HF fed Arvicanthis also showed significant lower light-induced increases of Per1 in the SCN. The most likely scenario is that even if Per1 normally participates in light-induced resetting responses, in the absence of its induction by light in HF animals, other mechanisms may maintain SCN responsiveness. Alternatively, these may be due to a delayed photic response as observed in HF mice (Mendoza et al. 2007b).
In mice under HF conditions, light-induced expression of Per2 in the SCN (at CT-12) is not altered (Mendoza et al. 2005b). However, in HF Arvicanthis exposed to light at CT-12, no induction of Per2 in the SCN was observed. In Arvicanthis fed with HF under LD cycle, Per2 was phase delayed compared to AL animals. Therefore, it is possible that the absence of Per2 light-induction is the consequence of a change in the time course of Per2 sensitivity to light due to the SCN metabolic state. However, the role of other clock genes (e.g. Cry, Dec) in light resetting of HF fed Arvicanthis remains to be studied.
In conclusion, the present study indicates that feeding is a potent synchronizer for the main circadian clock in the diurnal rodent Arvicanthis ansorgei. Importantly, most of these effects are in a different direction from those found in nocturnal rodents.
The use of a diurnal rodent to study the effects of synchronizers in circadian (e.g. jet-lag, scheduled-work rotations) and non-circadian disruptions (e.g. obesity, ageing-related health problems) could be useful for future basic and biomedical research. Caloric restriction has been reported to induce molecular and cellular changes that reduce ageing and prevent metabolic diseases (Bordone & Guarente, 2005). Notwithstanding, the report of food-induced entrainment in a diurnal rodent could suggest that the use of timed feeding programmes in people (Aschoff et al. 1986) would be useful to ameliorate circadian and non-circadian pathologies and for disease prevention and human health.
Acknowledgments
Funding of the present study was provided by the Fondation pour la Recherche Médicale (J.M.), Institut Servier (J.M.), Agence Nationale de la Recherche ANR-07-JCJC-0111 (E.C. and J.M.) and the Centre National de la Recherche Scientifique (E.C., J.M., P.P.). Riboprobes used for Per1 and Per2 gene detection were generously provided by Prof. Hitoshi Okamura (Kyoto University, Japan). We thank Dr Hughes Dardente for the Avp and Rev-erbα clones. We thank Miss Clare Quigley for English revision.
Glossary
- AL
ad libitum
- Avp
vasopressin
- CORT
corticosterone
- CT
circadian time
- DD
constant darkness
- FAA
food anticipatory activity
- HF
hypocaloric feeding
- 5-HT
serotonin
- LD
light–dark cycle
- NF
normocaloric feeding
- SCN
suprachiasmatic nucleus
- ZT
zeitgeber time
Author contributions
All the experiments were conducted at the Institute of Cellular and Integrative Neurosciences, CNRS UPR-3212 and the Chronobiotron Platform (IFR37-CNRS) of the Neurosciences Federation of Strasbourg (IFR-37 France). Conception and design of the experiments: J.M., P.P. and E.C. Collection, analysis and interpretation of data: J.M., S.G., S.D. and D.S.-C. Drafting the article or revising it critically for important intellectual content: all the authors. All authors approved the final version of the manuscript and declare no conflict of interest.
References
- Abe H, Rusak B. Anticipatory activity and entrainment of circadian rhythms in Syrian hamsters exposed to restricted palatable diets. Am J Physiol Regul Integr Comp Physiol. 1992;263:R116–R124. doi: 10.1152/ajpregu.1992.263.1.R116. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- Aschoff J, von Goetz C. Effects of feeding cycles on circadian rhythms in squirrel monkeys. J Biol Rhythms. 1986;1:267–276. doi: 10.1177/074873048600100401. [DOI] [PubMed] [Google Scholar]
- Aschoff J, von Goetz C, Wildgruber C, Wever RA. Meal timing in humans during isolation without time cues. J Biol Rhythms. 1986;1:151–162. doi: 10.1177/074873048600100206. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Frim DM, Dewey LK, Moore-Ede MC. Effects of restricted feeding schedules on circadian organization in squirrel monkeys. Physiol Behav. 1989;45:507–515. doi: 10.1016/0031-9384(89)90066-8. [DOI] [PubMed] [Google Scholar]
- Caldelas I, Poirel VJ, Sicard B, Pévet P, Challet E. Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei. Neuroscience. 2003;116:583–591. doi: 10.1016/s0306-4522(02)00654-1. [DOI] [PubMed] [Google Scholar]
- Caldelas I, Feillet CA, Dardente H, Eclancher F, Malan A, Gourmelen S, Pévet P, Challet E. Timed hypocaloric feeding and melatonin synchronize the suprachiasmatic clockwork in rats, but with opposite timing of behavioral output. Eur J Neurosci. 2005;22:921–929. doi: 10.1111/j.1460-9568.2005.04284.x. [DOI] [PubMed] [Google Scholar]
- Castillo MR, Hochstetler KJ, Travernier RJ, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol. 2004;287:R551–R555. doi: 10.1152/ajpregu.00247.2004. [DOI] [PubMed] [Google Scholar]
- Challet E, Solberg LC, Turek FW. Entrainment in calorie-restricted mice: conflicting zeitgebers and free-running conditions. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1751–R1761. doi: 10.1152/ajpregu.1998.274.6.R1751. [DOI] [PubMed] [Google Scholar]
- Challet E, Turek FW, Laute M, Van Reeth O. Sleep deprivation decreases phase-shift responses of circadian rhythms to light in the mouse: role of serotonergic and metabolic signals. Brain Res. 2001;909:81–91. doi: 10.1016/s0006-8993(01)02625-7. [DOI] [PubMed] [Google Scholar]
- Challet E, Pitrosky B, Sicard B, Malan A, Pévet P. Circadian organization in a diurnal rodent, Arvicanthis ansorgei Thomas 1910: chronotypes, responses to constant lighting conditions, and photoperiodic changes. J Biol Rhythms. 2002;17:52–64. doi: 10.1177/074873002129002339. [DOI] [PubMed] [Google Scholar]
- Chandrashekaran MK. Social cues and circadian rhythms. Curr Sci. 1982;51:158–167. [Google Scholar]
- Cuesta M, Mendoza J, Clesse D, Pévet P, Challet E. Serotonergic activation potentiates light resetting of the main circadian clock and alters clock gene expression in a diurnal rodent. Exp Neurol. 2008;210:501–513. doi: 10.1016/j.expneurol.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Cuesta M, Clesse D, Pévet P, Challet E. From daily behavior to hormonal and neurotransmitters rhythms: comparison between diurnal and nocturnal rat species. Horm Behav. 2009;55:338–347. doi: 10.1016/j.yhbeh.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents II. The variability of phase response curves. J Comp Physiol [A] 1976;106:253–266. [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci. 1998;18:5045–5052. doi: 10.1523/JNEUROSCI.18-13-05045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Mrosovsky N, Daan S. Nonphotic entrainment in a diurnal mammal, the European ground squirrel (Spermophilus citellus. J Biol Rhythms. 1999;14:409–419. doi: 10.1177/074873099129000812. [DOI] [PubMed] [Google Scholar]
- Hut RA, Pilorz V, Boerema AS, Strijkstra AM, Daan S. Working for food shifts nocturnal mouse activity into the day. PLoS One. 2011;6:e17 527. doi: 10.1371/journal.pone.0017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, Edgar DM. A nonphotic stimulus inverts the diurnal-nocturnal phase preference in Octodon degus. J Neurosci. 1999;19:328–333. doi: 10.1523/JNEUROSCI.19-01-00328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, Edgar DM. Scheduled voluntary wheel running activity modulates free-running circadian body temperature rhythms in Octodon degus. J Biol Rhythms. 2001;16:66–75. doi: 10.1177/074873040101600108. [DOI] [PubMed] [Google Scholar]
- Kennedy GA, Coleman GJ, Armstrong SM. Restricted feeding entrains circadian wheel-running activity rhythms of the kowari. Am J Physiol Regul Integr Comp Physiol. 1991;261:R819–R827. doi: 10.1152/ajpregu.1991.261.4.R819. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Renteria Diaz L, Barry-Shaw J, Stewart J, Amir S. Daily restricted feeding rescues a rhythm of period2 expression in the arrhythmic suprachiasmatic nucleus. Neuroscience. 2005;132:245–248. doi: 10.1016/j.neuroscience.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Mahoney M, Bult A, Smale L. Phase response curve and light-induced fos expression in the suprachiasmatic nucleus and adjacent hypothalamus of Arvicanthis niloticus. J Biol Rhythms. 2001;16:149–162. doi: 10.1177/074873001129001854. [DOI] [PubMed] [Google Scholar]
- Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res. 1997;765:273–282. doi: 10.1016/s0006-8993(97)00571-4. [DOI] [PubMed] [Google Scholar]
- Marchant EG, Watson NV, Mistlberger RE. Both neuropeptide Y and serotonin are necessary for entrainment of circadian rhythms in mice by daily treadmill running schedules. J Neurosci. 1997;17:7974–7987. doi: 10.1523/JNEUROSCI.17-20-07974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci. 2005a;22:2855–2862. doi: 10.1111/j.1460-9568.2005.04461.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Graff C, Dardente H, Pévet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005b;25:1514–1522. doi: 10.1523/JNEUROSCI.4397-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Revel F, Pévet P, Challet E. Shedding light on circadian clock resetting by dark exposure: differential effects between diurnal and nocturnal rodents. Eur J Neurosci. 2007a;25:3080–3090. doi: 10.1111/j.1460-9568.2007.05548.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Pévet P, Challet E. Circadian and photic regulation of clock and clock-controlled proteins in the suprachiasmatic nuclei of calorie-restricted mice. Eur J Neurosci. 2007b;25:3691–3701. doi: 10.1111/j.1460-9568.2007.05626.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Pévet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586:5901–5910. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Edelstein K, Hastings MH, Maywood ES. Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): implications for nonphotic phase shifting. J Biol Rhythms. 2001;16:471–478. doi: 10.1177/074873001129002141. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Rea MA. Serotonergic innervation of the hypothalamic suprachiasmatic nucleus and photic regulation of circadian rhythms. Biol Cell. 1997;89:513–523. doi: 10.1016/s0248-4900(98)80007-5. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Nocturnal activity in a diurnal rodent (Arvicanthis niloticus): the importance of masking. J Biol Rhythms. 2004;19:58–67. doi: 10.1177/0748730403260371. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- Slotten HA, Krekling S, Pévet P. Photic and nonphotic effects on the circadian activity rhythm in the diurnal rodent Arvicanthis ansorgei. Behav Brain Res. 2005;165:91–97. doi: 10.1016/j.bbr.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The‘other’ circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzman FM, Fuller CA, Moore-Ede MC. Feeding time synchronizes primate circadian rhythms. Physiol Behav. 1977;18:775–779. doi: 10.1016/0031-9384(77)90182-2. [DOI] [PubMed] [Google Scholar]
- Verhagen LA, Pévet P, Saboureau M, Sicard B, Nesme B, Claustrat B, Buijs RM, Kalsbeek A. Temporal organization of the 24-h corticosterone rhythm in the diurnal murid rodent Arvicanthis ansorgei Thomas 1910. Brain Res. 2004;995:197–204. doi: 10.1016/j.brainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Vivanco P, López-Espinoza A, Madariaga AM, Rol MA, Madrid JA. Nocturnalism induced by scheduled feeding in diurnal Octodon degus. Chronobiol Int. 2010;27:233–250. doi: 10.3109/07420520903398575. [DOI] [PubMed] [Google Scholar]
- Weber ET, Gannon RL, Rea MA. Local administration of serotonin agonists blocks light-induced phase advances of the circadian activity rhythm in the hamster. J Biol Rhythms. 1998;13:209–218. doi: 10.1177/074873098129000057. [DOI] [PubMed] [Google Scholar]


