We live in an era of rapidly changing global landscapes and local environments. Viruses with RNA as their genetic material can quickly adapt to and exploit these varying conditions because of the high error rates of the virus enzymes (polymerases) that replicate their genomes. It comes as no surprise, then, that several prominent recent examples of emerging or re-emerging diseases are caused by RNA viruses. However, a complex interplay of factors can influence disease emergence. In addition to virus genetic variation (mutation, recombination, and reassortment), environmental factors (including ecological, social, health care, and behavioral influences) can play important roles. These can include (i) changing weather patterns (e.g., El Niño effects) and damming of rivers, which alters potential virus vector or host abundance and distribution, and (ii) tropical deforestation, which brings humans in close contact with these species-rich (hosts and their parasites) environments. Such factors, coupled with enormous increases in the human population during the last 50 years and urbanization in many developing countries, have greatly expanded the number of sampling events testing the fitness of RNA virus variants in different human cell backgrounds and potential transmission modes. This change, together with the advances in the speed and volume of global transportation, combines to create increased opportunity for emergence and re-emergence of viral diseases. The purpose of this review is to present some prominent recent examples of emerging and re-emerging RNA virus diseases (influenza, hantaviruses, Ebola virus, and Nipah virus) to try to convey a sense of the excitement within this field and the important advances coming about as new technologies are being applied to research the basic question of how new disease outbreaks occur and whether we can gain predictive capability.
Influenza Virus
Influenza virus strains that cause worldwide outbreaks (pandemics) are classic examples of emerging viruses that are maintained in other animal hosts before transmission to humans. Influenza viruses are isolated from a variety of animals, including humans, pigs, horses, wild and domestic birds, and even sea mammals. The most devastating viral infection in this century was not caused by HIV, but by Spanish influenza, which killed more than 20 million people worldwide. Genetic studies suggest that the Spanish influenza virus originally was derived from birds. Furthermore, the causative viruses for the 1957 and 1968 influenza pandemics were hybrids between human and avian influenza viruses. Because humans did not have immunity to avian influenza viruses, the hybrid viruses produced devastating consequences (70,000 and 46,500 deaths globally in the 1957 and 1968 pandemics, respectively). Thus, it is critical to understand the mechanisms by which new influenza strains capable of causing pandemics emerge.
Possible Mechanisms for the Generation of Pandemic Influenza Viruses.
Future influenza pandemics will likely be caused by an avian virus possessing a hemaggluttinin surface protein to which humans lack immunity (1). Whether this virus will be introduced into the human population directly or indirectly is uncertain, but at least two mechanisms seem plausible.
Role of Pigs.
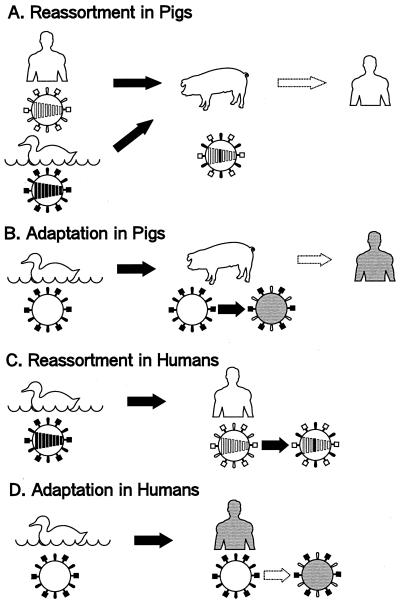
If the next pandemic strain proves to be a reassortant virus like those in 1957 and 1968, a single animal will have to be infected with two different viruses, one avian and one human. Because of host-range restrictions governing the transmission of the majority of avian influenza viruses in humans, but not in pigs, the latter animal has become the principal candidate for the role of intermediary, or “mixing vessel,” in genetic reassortment steps, leading to efficient replication of viruses with avian-like hemagluttinins in humans (Fig. 1A).
Figure 1.
Possible mechanisms for generation of pandemic influenza viruses. [Reproduced with permission from ref. 9 (Copyright 1998, American Society for Microbiology).]
Evidence that the receptor specificity of an H1N1 avian influenza virus changed during replication of the virus in pigs toward one recognizing receptors in humans suggests a mechanism by which pigs could serve as intermediate hosts for the adaptation of avian viruses to humans (Fig. 1B). It should be stressed that the models in Fig. 1 A and B are not mutually exclusive; indeed, alteration of receptor specificity during replication of an avian virus in pigs may occur both before and after reassortment with a human virus. Alternatively, an avian virus may become adapted in pigs to the extent that it would not require reassortment with a human virus for efficient replication in humans.
Direct Transmission.
The virus that caused an outbreak in Hong Kong in 1997 was unique in that it suggested additional models for the generation of pandemic strains from avian viruses: direct transmission and reassortment (Fig. 1C) or adaption (Fig. 1D) in humans. Whether these mechanisms operate with only a limited number of avian viruses or apply more widely than previously thought remains in question.
Hantavirus
Hantaviruses are segmented RNA viruses belonging to the genus Hantavirus in the family Bunyaviridae. Hantaviruses are maintained in various rodent reservoirs, in which the hosts are persistently infected without disease symptoms. Specific hantaviruses transmitted from the contaminated urine and feces of infected rodents cause two important human diseases, hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). Annually, hundreds of thousands of cases of HFRS are reported throughout Euro-Asia, whereas hundreds of cases of HPS are reported in North and South American countries. Because rodents act as the natural reservoir for hantaviruses and human-to-human infections are rare, understanding the ecology of hantaviruses within their natural reservoir is important for preventing and controlling the emergence of such diseases (2).
The relatively recent application of the PCR technique has allowed amplification of viral genomes from small amounts of rodent or human tissues without isolating the virus by classic culture methods. In this technique, many copies of a piece of DNA or RNA are synthesized after repeating a series of chemical reactions. The comparison of many hantavirus genomes from different rodent species has shown a clear correlation between the rodent species and the virus genotype, suggesting that hantaviruses have coevolved with their natural hosts for >20 million years, since before the first humans evolved (3). However, it remains unclear how hantaviruses exist within a rodent reservoir, particularly how they establish a persistent infection. In experiments with laboratory rats and mice, several groups have shown that an experimentally infected newborn animal readily develops a persistent infection, whereas an adult animal only develops a transient infection and recovers completely. On the other hand, epizootiological investigations have demonstrated that virus is transmitted between adult animals through wounds, and the adults develop a persistent infection. This discrepancy may be explained by the suppression of the immune system of adult rodents in nature leading to virus persistence, compared with immunologically intact adult laboratory rodents in which virus infection is cleared (4). The effect of a mixed infection with several organisms, which contributes to maintaining a balance between the host and parasite in nature, needs to be examined in more detail.
Ebola Virus
Ebola virus is a nonsegmented RNA virus, which, together with Marburg virus, makes up the filovirus family. This now notorious group of viruses was discovered in 1967 when Marburg virus was identified as the etiologic agent of a hemorrhagic fever outbreak in research facilities in Europe, which handled tissues from African green monkeys imported from Uganda. Subsequently, Ebola viruses were shown to be the cause of simultaneously occurring hemorrhagic fever outbreaks in 1976 in the Democratic Republic of Congo (DRC, formerly Zaire) and Sudan. These outbreaks were shown to be caused by two different subtypes of Ebola virus, which became known as the Zaire and Sudan subtypes. Mortality rates of up to 80% were recorded in these and more recent outbreaks in DRC and Gabon in 1995–1996. Epidemiologic data from recent outbreaks indicate that close contact is necessary for efficient transmission of Ebola virus from one individual to another, and little evidence can be found for aerosol transmission of the virus (5). Despite considerable efforts to identify the natural reservoir for Ebola and Marburg viruses, the host species remains an enigma. Although nonhuman primates have been implicated as the source of introduction of the virus into humans during several of the identified outbreaks, they are not considered likely to represent reservoir species because of their susceptibility to high-mortality hemorrhagic disease similar to that seen in humans. Little genetic difference has been detected between Ebola-Zaire viruses isolated 20 years apart and from locations over 1,000 km from one another, suggesting that ecological rather than genetic factors may play the dominant role in initiation of Ebola hemorrhagic fever outbreaks (6).
Nipah Virus
Nipah virus is a newly discovered member of the paramyxovirus family of nonsegmented RNA viruses. This virus was responsible for a viral encephalitis outbreak in Malaysia that was first recognized in October 1998 and ended in midsummer 1999. This outbreak resulted in almost 300 confirmed infections, and the mortality rate for hospitalized cases was approximately 35%. Initially, Malaysian authorities thought the outbreak was caused by Japanese encephalitis (JE) virus, a mosquito-borne RNA virus. However, JE vaccination and mosquito control efforts failed to halt the epidemic. In addition, several features of the disease epidemiology were inconsistent with past JE outbreaks, most notably the absence of illness in children, and a concurrent predominantly respiratory disease in pigs. Laboratory investigations by Dr. K. B. Chua at the University of Malaysia, uncovered the culprit, the newly discovered Nipah virus (7). Epidemic control efforts resulted in the culling of over 1 million pigs at affected farms. Detection of Nipah virus neutralizing antibodies in fruit bats of the genus Pteropus has implicated them as the likely virus reservoir. The virus appeared to be first introduced into pigs, where close contact caused by intensive farming practices led to efficient pig-to-pig transmission, and subsequently pig-to-human transmission. Virtually all human cases were in close proximity to the infected pigs (8). Genetic analysis showed Nipah virus to be closely related to Hendra virus, which recently was discovered in Australia as a cause of disease in horses and humans and also is maintained in Pteropus species fruit bats. These fruit bat-associated viruses appear to constitute a new genus in the paramyxovirus family. Little genetic diversity has been detected within the Nipah virus and Hendra virus groups, suggesting that ecological factors, rather than genetic factors, are likely playing a more important role in determining these disease emergences.
These examples highlight the subtle balance of environmental and genetic factors that can mold the diverse evolutionary patterns observed for RNA viruses and illustrate the complexity of these systems, which makes it difficult to predict future viral disease emergences.
Acknowledgments
We thank Dr. Tom Ksiazek for sharing his insights into the recent Nipah virus outbreak in Malaysia and Drs. Paul Ahlquist and Norio Ishida for their organization of this session.
Footnotes
This paper is a summary of a session presented at the second annual Japanese–American Frontiers of Science symposium, held October 1–3, 1999, at the International Conference Center, Tsukuba, Japan.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210382297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210382297
References
- 1.Horimoto, T. & Kawaoka, Y. (2000) Clin. Microbiol. Rev., in press. [DOI] [PMC free article] [PubMed]
- 2.Khan A S, Ksiazek T G, Peters C J. Lancet. 1996;347:739–741. doi: 10.1016/s0140-6736(96)90082-3. [DOI] [PubMed] [Google Scholar]
- 3.Marshall E. Science. 1993;262:832–836. doi: 10.1126/science.8235602. [DOI] [PubMed] [Google Scholar]
- 4.Meyer B J, Schmaljohn C S. Trends Microbiol. 2000;8:61–67. doi: 10.1016/s0966-842x(99)01658-3. [DOI] [PubMed] [Google Scholar]
- 5.Peters C J, LeDuc J W. J Infect Dis. 1999;179:S1–S288. [Google Scholar]
- 6.Sanchez A, Trappier S, Mahy B W J, Peters C J, Nichol S T. Proc Natl Acad Sci USA. 1996;93:3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua K B, Goh K J, Wong K T, Kamarulzaman A, Tan P S W, Ksiazek T G, Zaki S R, Paul G, Lam S K, Tan C T. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Morbid Mortal Wkly Rep. 1999;48:335–337. [Google Scholar]
- 9.Ito T, Couceiro J N S S, Kelm S, Baum L G, Krauss S, Castrucci M R, Donatelli I, Kida H, Paulson J C, Webster R G, Kawaoka Y. J Virol. 1998;72:7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]