Abstract
Pathophysiological changes in basal ganglia-thalamo-cortical circuits are well established in idiopathic Parkinson's disease (PD). However, it remains open whether such alterations already occur at early stages representing a characteristic neurophysiological marker of PD. Therefore, the present study aims at elucidating changes of synchronised oscillatory activity in early PD patients. In this study, we performed whole-head magnetoencephalography (MEG) in a resting condition and during steady state contraction of the more severely affected forearm in 10 drug–naive, de novo patients, in 10 early-stage patients with chronic medication and in 10 age-matched control subjects. While cortico-muscular coherence (CMC) did not differ between groups, patients showed increased sensori-motor cortical power at beta frequency (13–30 Hz) during rest as well as during isometric contraction compared to controls. In healthy control subjects the power of the contralateral hemisphere was significantly suppressed during isometric contraction. By contrast, both hemispheres were activated equally strongly in de novo patients. In medicated patients, the pattern was found to be reversed. Contralateral beta power was significantly correlated with motor impairment during isometric contraction but not during rest. The present results suggest that the reduced ability of the primary motor cortex to disengage from increased beta band oscillations during the execution of movements is an early marker of PD.
Key points
Parkinson's disease (PD) is a common movement disorder due to dopaminergic denervation of the basal ganglia. It is characterized by exaggerated oscillatory activity within central motor-control loops, while cerebro-muscular coherence is reduced at frequencies below 30 Hz.
So far, studies investigating the neurophysiological alterations of PD have focused on patients with advanced PD. It remains open to what extent changes of oscillatory activity might occur at early disease stages, representing a characteristic feature of the disease.
Using magnetoencephalography we show that cerebro-muscular coherence is unaffected in early PD while beta band oscillations of bilateral primary sensori-motor cortices are already increased at the earliest stages of PD and, as the disease progresses, evolve a hemispheric imbalance associated with movement execution.
Introduction
PD is a common movement disorder owing to dopaminergic denervation of the striatum (reviewed in Hutchison et al. 2004; Schnitzler & Gross, 2005; Hammond et al. 2007). Exaggerated oscillatory activity at lower frequencies (4–30 Hz) is thought to represent an important pathophysiological mechanism affecting basal ganglia–thalamo-cortical, cortico-cortical and cerebro-muscular loops (Salenius et al. 2002; Stoffers et al. 2008a; Pollok et al. 2009; for reviews see Bergman et al. 1998; Brown, 2003; Hutchison et al. 2004; Schnitzler & Gross, 2005; Hammond et al. 2007; Weinberger et al. 2009). Synchronised oscillatory activity at beta frequency (13–30 Hz), in particular, is assumed to be anti-kinetic in nature and pathophysiologically relevant for the origin of bradykinesia (Brown, 2006). Clinical improvement is associated with the decrease of beta-band oscillatory activity, evidence of the latter's relevance for clinical impairment in PD (Cassidy & Brown, 2001; Williams et al. 2002; Brown, 2003; Priori et al. 2004; Silberstein et al. 2005; Marceglia et al. 2006; Stoffers et al. 2008b; Kuhn et al. 2009). This assumption is also supported by the fact that experimentally augmented oscillations at lower frequencies have been shown to disrupt motor functions (Timmermann et al. 2004; Fogelson et al. 2005; Chen et al. 2007; Eusebio et al. 2008).
Most neurophysiological studies so far have focused on patients at advanced PD. The question remains to what extent such changes represent a characteristic neurophysiological marker of PD. Leblois and co-workers have proposed a model which accounts for the relation between motor symptoms and exaggerated oscillatory activity in PD. This model predicts that abnormally increased oscillations are not directly related to motor impairment since they do not occur at early stages of dopamine depletion (Leblois et al. 2006). Similarly, a recent study suggests that changes of oscillatory patterns could evolve during the course of PD affecting alpha frequency (8–12 Hz) oscillations in early stages without medication and spread to the 4–30 Hz frequency range in moderately affected patients with medication (Stoffers et al. 2008a).
Cortico-muscular coherence is a well-established measure of functional connectivity between the primary motor cortex (M1) and muscles, reflecting the integrity of the pyramidal motor system (Hari & Salenius, 1999). During weak to moderate isometric contraction, CMC is particularly evident at beta frequency (Conway et al. 1995; Salenius et al. 1997; Halliday et al. 1998; Hari & Salenius, 1999; Jabre & Salzsieder, 1999; Kilner et al. 1999; Gross et al. 2000; Mima et al. 2000, 2001; Ohara et al. 2000; Salenius & Hari, 2003; Kristeva et al. 2007). Its amplitude is reduced in non-medicated PD patients but can be remedied pharmacologically (Salenius et al. 2002) or by deep brain stimulation (Brown et al. 2001; Marsden et al. 2001a). Additionally, an increase in CMC was shown to vary with the decrease of bradykinesia (Brown et al. 2001), which suggests that the impaired ability of the pyramidal system to engage in oscillatory activity at beta frequency might contribute to bradykinesia.
The present study investigates CMC and oscillatory activity of M1 in early stages of PD in order to clarify the functional relevance of altered oscillatory patterns for the origin of PD symptoms. Since cortical and basal ganglia oscillatory activity is largely coherent (Marsden et al. 2001b; Cassidy et al. 2002; Williams et al. 2002; Fogelson et al. 2005; Hammond et al. 2007; Hirschmann et al. 2011; Litvak et al. 2011), synchronised oscillatory activity within M1 is assumed to exemplify a feature of the entire basal ganglia–cortical loop (Hammond et al. 2007).
Methods
Patients
A consecutive series of 20 mildly impaired akineto-rigid PD patients (Hoehn and Yahr stage I–II) attending the Movement Disorders Center of the University Hospital Düsseldorf were recruited. Ten of them received anti-parkinsonian medication with a mean daily l-Dopa equivalent dosage (Baas, 2006) of 190 ± 27 mg and were measured ON medication. Another 10 patients were drug-naïve and had received no medication at all before the MEG recording. We labelled this group de novo. They were measured OFF medication. In none of the patients tremor prevailed. We measured one group ON and another group OFF medication since previous studies revealed an effect of medication on oscillatory activity within M1 (Silberstein et al. 2005) as well as on CMC (Salenius et al. 2002) in PD patients at advanced stages. Thus, this study intended to ascertain whether comparable alterations are evident in early PD.
The diagnosis of PD requires the presence of a slowly progressive asymmetric parkinsonian syndrome responsive to l-Dopa and the absence of atypical features like early dementia, supranuclear gaze palsy, apraxia or early autonomic disturbance during clinical follow-up examinations. Prior to MEG recordings, each patient underwent a detailed neurological examination including neuropsychological testing, routine laboratory tests, presynaptic striatal DAT scintigraphy with FP-CIT to confirm a neurodegenerative parkinsonian syndrome and diagnostic magnetic resonance brain imaging to exclude symptomatic Parkinsonism. l-Dopa responsiveness was assessed by oral administration of 200–250 mg l-Dopa. The test was considered positive if patients showed improvement of at least 30% over baseline. The patients’ characteristics are summarized in Table 1. Additionally, 10 healthy subjects matched with respect to age, sex, handedness and performing hand served as control group.
Table 1.
The patients' characteristics
| Patient | Age | Sex | Duration (months) | H & Y | Side | UPDRS III | MMSE | Medication | cMRI | FP-CIT | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | M | 30 | I–II | Ri | 17 | 30 | Rasagiline, pramipexole | Normal | + | 28 |
| 2 | 64 | M | 30 | II | Ri | 11 | 30 | Pramipexole | Normal | + | 29 |
| 3 | 69 | M | 36 | I | Le | 16 | 30 | Rasagiline | Normal | + | 29 |
| 4 | 54 | M | 12 | I | Le | 12 | 30 | Rasagiline | Normal | + | 21 |
| 5 | 47 | F | 36 | I–II | Ri | 16 | 30 | Rasagiline | Normal | n.d. | 27 |
| 6 | 59 | M | 24 | I | Le | 17 | 30 | Pramipexole | Normal | n.d. | 21 |
| 7 | 60 | F | 15 | I | Ri | 11 | 30 | Rasagiline, pramipexole | Normal | + | 22 |
| 8 | 45 | F | 24 | I–II | Ri | 10 | 30 | Pramipexole, l-Dopa | Normal | + | 31 |
| 9 | 52 | M | 42 | I–II | Ri | 14 | 30 | Rasagiline | Normal | + | 44 |
| 10 | 44 | M | 36 | I | Le | 7 | 30 | Rasagiline, pramipexole | Normal | + | 53 |
| 11 | 72 | M | 11 | I | Ri | 6 | 30 | None | Normal | + | 21 |
| 12 | 42 | M | 18 | I | Ri | 10 | 30 | None | Normal | n.d. | 21 |
| 13 | 56 | M | 6 | I | Ri | 5 | 30 | None | Normal | + | 18 |
| 14 | 62 | M | 5 | I | Le | 6 | 30 | None | Normal | + | 18 |
| 15 | 63 | F | 12 | I | Ri | 12 | 30 | None | Normal | + | 24 |
| 16 | 56 | M | 6 | I | Le | 4 | 30 | None | Normal | + | n.d. |
| 17 | 71 | F | 6 | I | Ri | 5 | 30 | None | Normal | + | 20 |
| 18 | 51 | M | 24 | I–II | Le | 8 | 30 | None | Normal | + | n.d. |
| 19 | 71 | F | 24 | I–II | Le | 12 | 30 | None | Normal | + | 15 |
| 20 | 43 | M | 30 | I | Le | 13 | 30 | None | Normal | + | 27 |
Le = Left; Ri = Right; cMRI = cranial magnetic resonance imaging; H&Y = Hoehn & Yahr; n.d. = not determined.
Motor performance in all participants was assessed by means of the motor part of the Unified Parkinson Disease Rating Scale (UPDRS III) administered by an experienced neurologist (MS) on the day of MEG recording. All participants gave their written informed consent prior to the study, which was in accordance with the Declaration of Helsinki and approved by the ethics committee of the University Hospital Düsseldorf.
MEG recordings
Neuromagnetic activity was recorded using a 306-channel whole-head neuromagnetometer (Elekta Neuromag) consisting of 204 planar gradiometers and 102 magnetometers. Simultaneously, electromyography (EMG) was recorded from extensor forearm muscles using bipolar surface electrodes. Vertical electrooculogram (EOG) was additionally recorded to control for eye-blink artifacts. During measurement, subjects were comfortably seated in a magnetically shielded room and performed an isometric contraction of the forearm of the more severely affected side at about 20% of maximal contraction strength. To this end, participants kept their forearm elevated at about 30 deg with hand outstretched and fingers slightly abducted. After 60 s of contraction, participants put down their arm on a table fixed in front of them for a 60 s resting period. Contraction strength was controlled online with respect to the EMG signal during MEG recording. A total of 4 min of isometric contraction and rest, respectively, were collected for each participant. Control subjects performed the task with the same forearm as the matching patient. All signals were measured with a band-pass filter of 0.03 to 330 Hz, digitized at 1000 Hz, and stored digitally for off-line analysis. For MEG source localization, T1 weighted magnetic resonance images (MRI) were acquired for each participant with a 3 T Magnetom (Siemens, Germany) with a slice thickness of 1 mm. Alignment of MRI and MEG data was achieved by measuring magnetic signals from four indicator coils placed on the scalp of each individual. Anatomical landmarks – nasion and left and right preauricular points – were identified using a three-dimensional digitizer (Polhemus, Germany) and used to calculate the positioning of the coils.
Data analysis
The data were analysed with respect to time periods of isometric contraction and rest, respectively. Epochs were determined according to the EMG signal. The first 5 s of each epoch was discarded from the analysis in order to remove movement-related time periods. The EMG signal was baseline corrected and rectified. Data were transformed from the time into the frequency domain by fast Fourier transformation (FFT). FFT size was 1024 samples. For the analysis of oscillatory patterns below 30 Hz, cross-spectral density was calculated with a frequency resolution of 0.98 Hz after applying a discrete prolate spheroidal sequence (dpss) taper. Smoothing of 4 Hz was applied for the analysis of oscillatory activity between 30 and 90 Hz frequency. MEG data were bandpass-filtered at 50 Hz. Spectral power of bilateral S1/M1 and EMG as well as coherence between MEG and EMG signals were calculated in individual datasets at alpha (8–12 Hz), beta (13–30 Hz) and gamma (30–90 Hz) frequencies. While spectral power provides information on synchronised oscillatory activity within local brain areas, coherence is an established measure of functional connectivity between spatially distributed sites and resembles the amount of linear correlation in the frequency domain. It is defined as the absolute value of the cross spectrum normalized by the square root of the auto spectra of two signals and varies between 1, indicating a perfect linear correlation, and 0 suggesting absolute linear independence. Power and CMC were localized using the dynamic imaging of coherent sources (DICS) method. To reduce inter-individual variance, power was estimated by logarithmic transformation (Halliday et al. 1995). All analyses were performed using the Fieldtrip toolbox (Oostenveld et al. 2011). In each frequency range the largest peak of power and coherence were determined without any further considerations.
Statistics
Statistics were calculated using IBM SPSS Statistics 19. Gaussian distribution of the data was ascertained by means of the Kolmogorov–Smirnov test for one sample. Analyses of variance (ANOVA) and Student's t test were used for group comparisons. Correlation between UPDRS III and neurophysiological measures was calculated using Pearson's correlation. P values were corrected for multiple testing.
Results
Participants’ characteristics
Mean age was 54.6 ± 2.6 years (medicated patients), 58.7 ± 3.5 years (de novo patients) and 56.1 ± 3.3 years (controls; F(2,27) = 0.42, P= 0.66).
Mean disease duration as determined by the time of firm diagnosis was 28.5 ± 3.1 months in medicated and 14.2 ± 2.9 months in de novo patients. Statistical analysis rendered this difference to be significant (t(18) = 3.38, P < 0.01). Analysis of UPDRS III motor score revealed a main effect of factor group (F(2,27) = 34.45, P < 0.00; Fig. 1).
Figure 1. Mean UPDRS III scores of participating patients and control subjects as a measure of motor impairment.
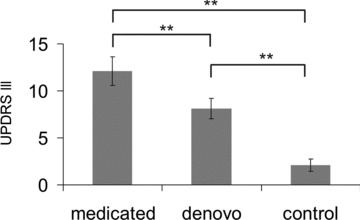
Error bars indicate standard error of the mean (SEM).
In order to confirm the diagnosis of PD, DAT scintigraphy was obtained for both patient groups. The analysis revealed significantly reduced values ipsilateral to the more severely affected hand (2.3 ± 0.2) as compared to the contralateral hemisphere (2.6 ± 0.2; t(16) = 4.7, P < 0.00) suggesting unilaterally predominant neuro-degeneration. Comparison between both patient groups did not result in significant group differences (t(15) = 0.9, P= 0.37).
S1/M1 Power
During isometric contraction no significant group differences of amplitude (F(2,27) = 2.51, P= 0.10) or frequency (F(2,27) = 0.43, P= 0.65) were found at alpha frequency. At beta frequency a discernible peak was determined around 18 Hz in each subject. While groups did not differ with respect to frequency (F(2,27) = 0.10, P= 0.90), analysis of amplitude revealed a significant main effect of group (F(2,27) = 21.02, P < 0.00) and a significant hemisphere×group interaction (F(2,27) = 10.70, P < 0.00; Fig. 2).
Figure 2. Analysis of MEG spectral power during isometric contraction.
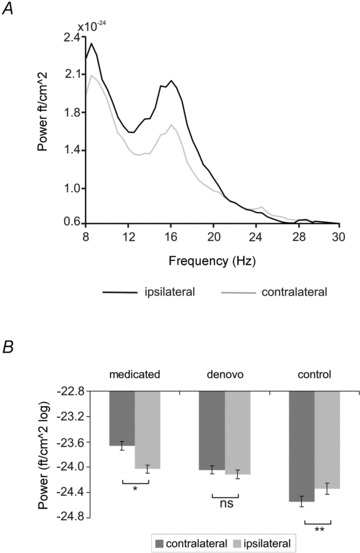
A, power averaged across sensors covering S1/M1 contra- and ipsilateral to isometric contraction in one control subject. Note that power of the hemisphere contralateral to isometric contraction is suppressed at frequencies below 30 Hz as compared to the ipsilateral side. B, mean power of ipsi- and contralateral S1/M1 in medicated and de novo patients and control subjects at beta frequency. Power was estimated by logarithmic transformation. Error bars indicate SEM.
The latter result suggests that in control subjects, power contralateral to isometric contraction was significantly lower as compared to the ipsilateral side (t(9) = 3.60, P= 0.01), while this pattern appeared to be attenuated in de novo patients (t(9) = 0.90, P= 0.39) and reversed in medicated patients (t(9) =–2.66, P= 0.03). To test whether the lack of suppression shown in de novo patients differs significantly from controls, a ratio between both hemispheres was calculated (i.e. power amplitude ipsilateral/power amplitude contralateral). Values above 1 indicate higher amplitudes of S1/M1 ipsilateral to isometric contraction, while values below 1 indicate higher amplitudes of S1/M1 contralateral to isometric contraction. The analysis revealed mean ratios of 1.00 ± 0.01 (de novo) and 0.99 ± 0.003 (controls). Statistical analysis showed this difference to be significant (t(18) = 2.48, P= 0.02).
Since disease duration, UPDRS scores and treatment were confounded, we tried to determine which of these factors prevailed in affecting S1/M1 power amplitudes. To this end (1), all patients – independent of medication – were categorized according to disease duration (i.e. short <20 months (10.1 ± 1.5; range 5–18 months) and long >20 months (31.3 ± 2.0; range 24–42 months)) and (2) the data were analysed with respect to UPDRS III. The patients were divided into two groups with low (i.e. scores <10 (6.7 ± 0.7)) and high scores (>10 (14.0 ± 0.8)). Analysis of S1/M1 power amplitude regarding UPDRS III differences revealed a significant hemisphere×group interaction (F(1,18) = 5.1; P= 0.04) while this was not the case for groups differing with respect to disease duration (F(1,18) = 1.7; P= 0.21).
In order to determine to what extent S1/M1 power changes are related to clinical symptoms, we conducted a correlation analysis between S1/M1 power at beta frequency and UPDRS III. The analysis revealed a significant correlation between S1/M1 power contralateral to isometric contraction and UPDRS III (R= 0.7, P < 0.00; Fig. 3) whereas no significant correlation between ipsilateral S1/M1 power and clinical measures was found (R= 0.33, P= 0.15).
Figure 3. Correlation between S1/M1 power contralateral to isometric contraction at beta frequency and UPDRS III.
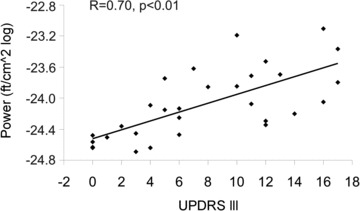
During isometric contraction S1/M1 power at beta frequency is significantly correlated with UPDRS III as a measure of motor impairment. Please note that power values from two control subjects (UPDRS III = 0) show a strong overlap. The lowest data point actually represents data from two control subjects.
At gamma frequency a discernible power peak between 55 and 70 Hz was found in each individual. S1/M1 power amplitude did not differ between groups (F(2,27) = 0.08, P= 0.99).
In order to determine whether there is evidence of changes at beta frequency during rest, the same analysis was performed for the resting period. Power frequency did not differ between groups (F(2,27) = 0.22, P= 0.80). Analysis of power amplitude revealed a significant main effect of factor group (F(2,27) = 5.33, P= 0.01), while neither a main effect of hemisphere (F(1,27) = 1.68, P= 0.21) nor a hemisphere×group interaction (F(2,27) = 0.59, P= 0.56) was found to be significant. A post hoc Scheffé test revealed that power was generally higher in both patient groups as compared to controls (P < 0.04) while there was no evidence of significant difference between patient groups (Fig. 4). Accordingly, S1/M1 power was not significantly correlated with UPDRS III (R= 0.36, P= 0.15).
Figure 4. Mean S1/M1 power amplitudes at beta frequency during rest.
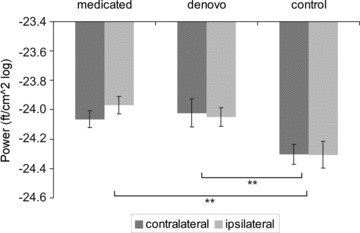
Power was estimated by logarithmic transformation. Note that the terms ipsi- and contralateral refer to the more severely affected hand. Error bars indicate SEM. In contrast to isometric contraction, no significant hemisphere×group interaction was evident.
Cerebro-muscular coherence
A main CMC peak was found at beta frequency in each individual. Sources were localized within the primary motor cortex corresponding to Brodmann area 4 (Fig. 5A). Main peaks were found at 16.2 ± 1.0 Hz in medicated patients, at 18.3 ± 1.7 Hz in de novo patients and at 19.15 ± 1.0 Hz in control subjects. ANOVA revealed no significant main effect regarding amplitude (F(2,27) = 0.23, P= 0.79; Fig. 5B) or of frequency (F(2,27 = 1.27, P= 0.29).
Figure 5. Cortico-muscular coherence at beta frequency.
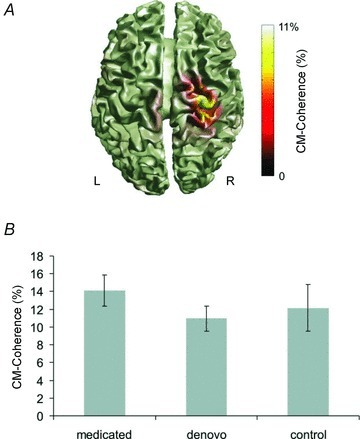
A, example of localization of CMC in one control subject during isometric contraction with the left hand in the contralateral primary motor cortex (Brodmann area 4). B, group statistics did not reveal significant differences between groups. Presented are mean values. Error bars indicate SEM.
EMG power
The analysis of the EMG during isometric contraction revealed discernible peaks at alpha, beta and low gamma (30–60 Hz) frequencies. No significant frequency or amplitude differences either at the alpha (frequency: (F(2,27) = 0.68, P= 0.52); amplitude: (F(2, 27) = 0.83, P= 0.45)) or at the gamma range (frequency: (F(2,27) = 1.48, P= 0.25); amplitude: (F(2,27) = 1.03, P= 0.37)) were observed. At beta frequency discernible peaks occurred at 16.0 ± 0.6 Hz (medicated patients), at 19.0 ± 1.3 Hz (de novo patients) and at 18.5 ± 0.5 Hz (control subjects; F(2,27) = 4.53, P= 0.02). A post hoc Scheffé test suggested slowing in medicated as compared to de novo patients (P= 0.03; Fig. 6). Analysis of the EMG amplitude at beta frequency did not result in significant group effects (F(2,27) = 0.32, P= 0.73). In order to clarify the effect of treatment, disease duration and severity on EMG frequency, an additional ANOVA with disease duration and UPDRS as covariates was calculated. The analysis revealed that neither duration (F(1,16) = 0.79, P= 0.38) nor UPDRS (F(1,16) = 1.39, P= 0.25) has a significant effect on EMG frequency.
Figure 6. Analysis of EMG spectral power during isometric contraction.
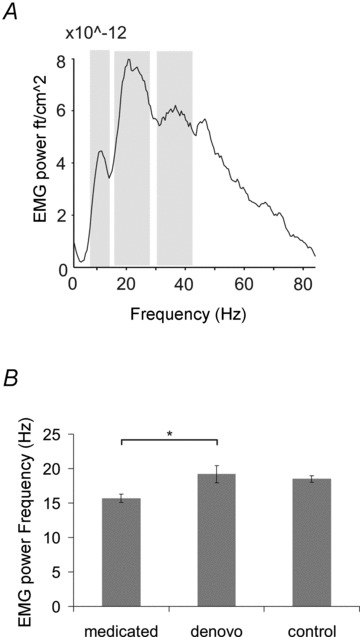
A, the spectrum of one characteristic control subject. Discernible peaks at alpha (8–12 Hz), beta (13–30 Hz) and low gamma (30–60 Hz) frequencies were evident. B, while power amplitude did not differ between groups at any of these frequencies, a shift towards lower frequencies was found in medicated patients in the beta range. Shown are mean frequency values. Error bars indicate SEM.
Discussion
The present study investigates synchronised oscillatory activity at early PD stages to explore whether well-established alterations at advanced stages might evolve during disease progression. To this end, we examined CMC, EMG and sensori-motor cortical power during isometric contraction of the more severely affected forearm and during rest in PD patients at early stages ON and OFF medication. Data were compared with those from a healthy control group matched with respect to age, sex and performing hand. As a main result, the balance between left and right motor cortex activity was found to vary with clinical severity. The more patients are impaired, the less suppressed are beta band oscillations of contralateral S1/M1 as compared to the ipsilateral hemisphere during isometric contraction. In contrast to this, CMC as a measure of functional interaction between the primary motor cortex and muscles did not differ between patients and controls, which suggests that the well-established decrement of CMC during isometric contraction in advanced PD (i.e. Salenius et al. 2002) does not represent a characteristic feature of early PD. In addition, the results support the assumption that motor cortical oscillations and CMC are independent characteristics (Baker & Baker, 2003). Taken together, the data support the hypothesis that the pattern of synchronised oscillatory activity changes with disease severity affecting the pyramidal system at advanced but not at early stages of PD.
S1/M1 Power and cerebro-muscular coherence
As the main finding, the present data suggest reduced suppression of motor cortical oscillations at beta frequency in early PD that develops a hemispheric imbalance as the disease increases in severity. In addition, the amplitude of S1/M1 power contralateral to isometric contraction was significantly correlated with UPDRS III as a measure of disease severity. This would suggest that particular movement-related modulation of S1/M1 oscillations varies with disease progression. Unfortunately, the two patient groups differed not only with respect to medication but also as regards disease duration and severity. Since UPDRS III scores and S1/M1 power amplitudes were significantly correlated and motor impairment increases with disease duration, it did not appear to be suitable to use a covariate approach to clarify the effect of each single variable on S1/M1 power amplitude. Rather, we additionally compared the effects of duration and severity on M1 power amplitude independent of medication. The analysis revealed evidence in support of the theory that disease severity, in particular, affects changes of motor cortical beta oscillations – a result corroborated by the significant correlation between contralateral M1 power amplitude and UPDRS III scores. Nevertheless, we cannot definitely exclude the possibility that medication might also have influenced present results.
Although increased beta oscillations within the basal ganglia are well established in advanced PD (for reviews see Hutchison et al. 2004; Schnitzler & Gross, 2005) there is no clear evidence that the degree of increased synchronized oscillatory activity directly accounts for motor deficits in PD. Rather, changes of STN oscillations following dopamine replacement (Weinberger et al. 2006; Stoffers et al. 2008b; Kuhn et al. 2009) and deep brain stimulation (Ray et al. 2008) have been shown to be correlated with clinical improvement. Alterations of local oscillatory activity of the sensori-motor cortex have been evidenced in PD by means of event-related synchronisation (ERS) and desynchronisation (ERD) during the execution of voluntary movements. In addition to a delay of the ERD (Magnani et al. 2002), attenuated ERS after movement termination has been shown in PD patients at advaced stages (Pfurtscheller et al. 1998) as well as in early stage PD patients (Devos et al. 2003). Accordingly, cortical power was found to be attenuated after deep brain stimulation of the STN (Silberstein et al. 2005) and following medical treatment (Brown & Marsden, 1999) in humans at advanced stages and following l-Dopa substitution in rodents (Sharott et al. 2005), but no direct relation between clinical measures and motor cortical alterations has been shown so far. Extending these findings, the present data suggest a direct relation between disease severity and S1/M1 power amplitude in early PD stages. Particularly, in more severely affected patients, suppression of motor cortical activation associated with movements was not evident. This result suggests as an early marker of PD the disability of the primary motor cortex to disengage from synchronised beta band activity.
The present data support previous findings indicating that depending on disease stage, synchronization likelihood (SL) as a measure of functional connectivity within and between cortical areas differs during rest (Stoffers et al. 2008b). Interestingly enough, SL in moderately affected patients was increased within local networks but did not affect intra- or inter-hemispheric connectivity measures (Stoffers et al. 2008b). The latter result agrees well with the present data suggesting increased oscillatory activity within S1/M1 that did not affect CMC shown in patients with advanced PD (Salenius et al. 2002). In favour of the hypothesis that the pattern of oscillatory activity varies with disease stage, animal studies suggest that synchronous oscillatory firing within the internal segment of the globus pallidus (Leblois et al. 2007) and STN on the one hand and cerebral cortex on the other hand (Mallet et al. 2008) represents a delayed consequence rather than an immediate result of acute dopamine depletion. Similarly, exaggerated oscillations occurred after the manifestation of akinesia (Leblois et al. 2007; Degos et al. 2009) which would suggest that motor cortical oscillatory patterns vary with disease severity, a hypothesis corroborated by the present findings.
A comparable effect of disease severity on oscillatory activity at the gamma range was not obtained. Although the amplitude of oscillations at frequencies above 50 Hz is generally small, a discernible peak in this frequency range was found in each individual. It has been argued that gamma band synchronization within the basal ganglia might reflect physiological motor functions since an increase was shown following l-Dopa (Androulidakis et al. 2007) and higher gamma synchronization is associated with improvement of motor functions (Kuhn et al. 2006). But, over the motor cortex gamma oscillations were not found in advanced PD even ON medication (Devos et al. 2006). According to these results, one might speculate that in early PD, physiological gamma band synchronization might be preserved while local beta band synchronization is already affected. But, since synchronised gamma band oscillations between basal ganglia and cortex have been specifically observed before and during voluntary movements (Cassidy et al. 2002), we would like to interpret this result with caution. It should be merely seen as a piece of evidence for the hypothesis that synchronised oscillatory activity at different frequencies might be differentially affected depending on disease stage.
In the present study synchronised oscillatory activity of S1/M1 was analysed since it is assumed to exemplify a feature of the entire basal ganglia–cortical loop (Hammond et al. 2007). Nevertheless, it should be stressed that functional connectivity between basal ganglia and the cortex is usually below 30% (Hirschmann et al. 2011; Litvak et al. 2011) indicating that most of the signal variance within S1/M1 seems to be independent of the basal ganglia.
EMG
The analysis of the EMG revealed discernible peaks at about 10, 20 and 40 Hz. The 40 Hz component – the so-called Piper-rhythm – was found in all patients while it is not detectable in advanced PD (Brown, 1997) suggesting that its loss does not represent a characteristic feature of early disease stages. The analysis of peak frequency, which was determined at the beta range, revealed a shift towards lower frequencies in medicated patients as compared to de novo patients. The analysis with duration and severity as covariates supports the hypothesis that this result is an effect of, in particular, chronic medication.
Conclusion
The present results support the hypothesis that reduced CMC observed in advanced stages of PD is not a characteristic initial feature of PD, but evolves during disease progression. While prominent changes of CMC and gamma band oscillations do not occur at early stages, motor cortical beta band synchronization is increased already in the earliest stages of PD and evolves a hemishperic imbalance as the disease progresses.
Acknowledgments
B.P. is grateful for support from the Deutsche Forschungsgemeinschaft (PO 806/3-1) and from the Research Commission of the Medical Faculty of the Heinrich-Heine University (9772440). V.K. is supported by the Research Commission of the Medical Faculty of the Heinrich-Heine University (9772467, 9772440).
Glossary
- CMC
cortico-muscular coherence
- DICS
dynamic imaging of coherent sources
- dpss
discrete prolate spheroidal sequence
- EMG
electromyography
- EOG
electrooculogram
- ERD
Event-related desynchronisation
- ERS
event-related synchronisation
- FFT
fast Fourier transformation
- M1
primary motor cortex
- MEG
magnetoencephalography
- MRI
magnetic resonance images
- PD
Parkinson's disease
- S1/M1
primary sensori-motor cortex
- SL
synchronization likelihood
- UPDRS
Unified Parkinson's Disease Rating Scale
Author contributions
B.P.: conception and design of the experiment, collection, analysis and interpretation of the data, drafting the article. V.K.: collection and interpretation of the data, critical revision of the article. W.M.: collection, analysis and interpretation of the data. C.W.: interpretation of the data and critical revision of the article. A.S.: interpretation of the data and critical revision of the article. M.S.: conception and design of the experiment, collecting and interpretation of the data, critical revision of the article. All authors approved the final version for publication.
References
- Androulidakis AG, Kuhn AA, Chen CC, Blomstedt P, Kempf F, Kupsch A, Schneider GH, Doyle L, Dowsey-Limousin P, Hariz MI, Brown P. Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson's disease. Brain. 2007;130:457–468. doi: 10.1093/brain/awl358. [DOI] [PubMed] [Google Scholar]
- Baas H. [Pharmacotherapy and guidelines] Pharm Unserer Zeit. 2006;35:242–248. doi: 10.1002/pauz.200600172. [DOI] [PubMed] [Google Scholar]
- Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol. 2003;546:931–942. doi: 10.1113/jphysiol.2002.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Brown P. Muscle sounds in Parkinson's disease. Lancet. 1997;349:533–535. doi: 10.1016/S0140-6736(97)80086-4. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P. Bad oscillations in Parkinson's disease. J Neural Transm Suppl. 2006:27–30. doi: 10.1007/978-3-211-45295-0_6. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. Bradykinesia and impairment of EEG desynchronization in Parkinson's disease. Mov Disord. 1999;14:423–429. doi: 10.1002/1531-8257(199905)14:3<423::aid-mds1006>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden J, Defebvre L, Cassim F, Mazzone P, Oliviero A, Altibrandi MG, Di Lazzaro V, Limousin-Dowsey P, Fraix V, Odin P, Pollak P. Intermuscular coherence in Parkinson's disease: relationship to bradykinesia. Neuroreport. 2001;12:2577–2581. doi: 10.1097/00001756-200108080-00057. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Brown P. Task-related EEG-EEG coherence depends on dopaminergic activity in Parkinson's disease. Neuroreport. 2001;12:703–707. doi: 10.1097/00001756-200103260-00018. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Chen CC, Litvak V, Gilbertson T, Kuhn A, Lu CS, Lee ST, Tsai CH, Tisch S, Limousin P, Hariz M, Brown P. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson's disease. Exp Neurol. 2007;205:214–221. doi: 10.1016/j.expneurol.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Conway B, Halliday D, Farmer S, Shahani U, Maas P, Weir A, Rosenberg J. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489:917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos B, Deniau JM, Chavez M, Maurice N. Chronic but not acute dopaminergic transmission interruption promotes a progressive increase in cortical beta frequency synchronization: relationships to vigilance state and akinesia. Cereb Cortex. 2009;19:1616–1630. doi: 10.1093/cercor/bhn199. [DOI] [PubMed] [Google Scholar]
- Devos D, Labyt E, Derambure P, Bourriez JL, Cassim F, Guieu JD, Destee A, Defebvre L. Effect of L-Dopa on the pattern of movement-related (de)synchronisation in advanced Parkinson's disease. Neurophysiol Clin. 2003;33:203–212. doi: 10.1016/j.neucli.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Devos D, Szurhaj W, Reyns N, Labyt E, Houdayer E, Bourriez JL, Cassim F, Krystkowiak P, Blond S, Destee A, Derambure P, Defebvre L. Predominance of the contralateral movement-related activity in the subthalamo-cortical loop. Clin Neurophysiol. 2006;117:2315–2327. doi: 10.1016/j.clinph.2006.06.719. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Chen CC, Lu CS, Lee ST, Tsai CH, Limousin P, Hariz M, Brown P. Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson's disease. Exp Neurol. 2008;209:125–130. doi: 10.1016/j.expneurol.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson N, Kuhn AA, Silberstein P, Limousin PD, Hariz M, Trottenberg T, Kupsch A, Brown P. Frequency dependent effects of subthalamic nucleus stimulation in Parkinson's disease. Neurosci Lett. 2005;382:5–9. doi: 10.1016/j.neulet.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Gross J, Tass P, Salenius S, Hari R, Freund HJ, Schnitzler A. Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol. 2000;527:623–631. doi: 10.1111/j.1469-7793.2000.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data-theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Molec Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hari R, Salenius S. Rhythmical corticomotor communication. Neuroreport. 1999;10:R1–10. [PubMed] [Google Scholar]
- Hirschmann J, Ozkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson's disease. Neuroimage. 2011;55:1159–1168. doi: 10.1016/j.neuroimage.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 2004;24:9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabre JF, Salzsieder BT. An EMG study of functional cortico-motoneuronal connections in humans. J Physiol Paris. 1999;93:147–154. doi: 10.1016/s0928-4257(99)80145-4. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516:559–570. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage. 2007;36:785–792. doi: 10.1016/j.neuroimage.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J Neurosci. 2006;26:3567–3583. doi: 10.1523/JNEUROSCI.5050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur J Neurosci. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Magnani G, Cursi M, Leocani L, Volonte MA, Comi G. Acute effects of L-dopa on event-related desynchronization in Parkinson's disease. Neurol Sci. 2002;23:91–97. doi: 10.1007/s100720200033. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceglia S, Foffani G, Bianchi AM, Baselli G, Tamma F, Egidi M, Priori A. Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson's disease. J Physiol. 2006;571:579–591. doi: 10.1113/jphysiol.2005.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden J, Limousin-Dowsey P, Fraix V, Pollak P, Odin P, Brown P. Intermuscular coherence in Parkinson's disease: effects of subthalamic nucleus stimulation. Neuroreport. 2001a;12:1113–1117. doi: 10.1097/00001756-200105080-00013. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Limousin-Dowsey P, Ashby P, Pollak P, Brown P. Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson's disease. Brain. 2001b;124:378–388. doi: 10.1093/brain/124.2.378. [DOI] [PubMed] [Google Scholar]
- Mima T, Matsuoka T, Hallett M. Information flow from the sensorimotor cortex to muscle in humans. Clin Neurophysiol. 2001;112:122–126. doi: 10.1016/s1388-2457(00)00515-0. [DOI] [PubMed] [Google Scholar]
- Mima T, Steger J, Schulman AE, Gerloff C, Hallett M. Electroencephalo-graphic measurement of motor cortex control of muscle activity in humans. Clin Neurophysiol. 2000;111:326–337. doi: 10.1016/s1388-2457(99)00229-1. [DOI] [PubMed] [Google Scholar]
- Ohara S, Nagamine T, Ikeda A, Kunieda T, Matsumoto R, Taki W, Hashimoto N, Baba K, Mihara T, Salenius S, Shibasaki H. Electrocorticogram-electromyogram coherence during isometric contraction of hand muscle in human. Clin Neurophysiol. 2000;111:2014–2024. doi: 10.1016/s1388-2457(00)00448-x. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok B, Makhloufi H, Butz M, Gross J, Timmermann L, Wojtecki L, Schnitzler A. Levodopa affects functional brain networks in Parkinsonian resting tremor. Mov Disord. 2009;24:91–98. doi: 10.1002/mds.22318. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Pichler-Zalaudek K, Ortmayr B, Diez J, Reisecker F. Postmovement beta synchronization in patients with Parkinson's disease. J Clin Neurophysiol. 1998;15:243–250. doi: 10.1097/00004691-199805000-00008. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp Neurol. 2004;189:369–379. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Salenius S, Avikainen S, Kaakola S, Hari R, Brown P. Defective cortical drive to muscle in Parkinson's disease and its improvement with levodopa. Brain. 2002;125:491–500. doi: 10.1093/brain/awf042. [DOI] [PubMed] [Google Scholar]
- Salenius S, Hari R. Synchronous cortical oscillatory activity during motor action. Curr Opin Neurobiol. 2003;13:678–684. doi: 10.1016/j.conb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Neurosci Rev. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Pogosyan A, Kuhn AA, Hotton G, Tisch S, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P. Cortico-cortical coupling in Parkinson's disease and its modulation by therapy. Brain. 2005;128:1277–1291. doi: 10.1093/brain/awh480. [DOI] [PubMed] [Google Scholar]
- Stoffers D, Bosboom JL, Deijen JB, Wolters E, Stam CJ, Berendse HW. Increased cortico-cortical functional connectivity in early-stage Parkinson's disease: an MEG study. Neuroimage. 2008a;41:212–222. doi: 10.1016/j.neuroimage.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Stoffers D, Bosboom JL, Wolters E, Stam CJ, Berendse HW. Dopaminergic modulation of cortico-cortical functional connectivity in Parkinson's disease: an MEG study. Exp Neurol. 2008b;213:191–195. doi: 10.1016/j.expneurol.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, Treuer H, Sturm V, Schnitzler A. Ten-Hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson's disease. Mov Disord. 2004;19:1328–1333. doi: 10.1002/mds.20198. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Dostrovsky JO. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp Neurol. 2009;219:58–61. doi: 10.1016/j.expneurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol. 2006;96:3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]


