SUMMARY
The interleukin-17 (IL-17) family of cytokines phylogenetically pre-dates the evolution of T cells in jawed vertebrates, suggesting that the ontogeny of the Th17 cell lineage must have arisen to confer an evolutionary advantage to the host over innate sources of IL-17. Using a model of mucosal immunization with the encapsulated bacteria Klebsiella pneumoniae, B cells largely recognized polysaccharide capsular antigens, which limited protection to only the vaccine strain. In contrast, memory Th17 cells proliferated in response to conserved outer membrane proteins and conferred protection against several serotypes of K. pneumoniae, including the recently described multi-drug resistant New Dehli metallolactamase strain. Notably, this heterologous, clade specific protection was antibody-independent, demonstrating the Th17 cell lineage confers a host advantage by providing heterologous mucosal immunity independent of serotype specific antibody.
INTRODUCTION
The interleukin-17 (IL-17) family of cytokines is evolutionarily conserved with IL-17D having orthologs in Ciona intestinalis and mollusks (Roberts et al., 2008). IL-17A, often referred as IL-17, and IL-17F, the two isoforms expressed in memory Th17 cells (Weaver et al., 2007; Khader et al., 2009; Dong, 2008) arose concomitantly with T cells in jawed vertebrates and mammals (Guo et al., 2009). Two effector cytokines expressed by Th17 cells, IL-17 and IL-22, have been implicated to play key roles in mucosal immunity primarily to extracellular pathogens. IL-17 regulates the expression of G-CSF, granulopoiesis, and mucosal CXC chemokines important in neutrophil recruitment (Khader et al., 2009). IL-22 increases barrier function of the epithelium, synergizes with IL-17 in the expression of mucosal chemokines and induces the expression of antimicrobial peptides (Aujla et al., 2008; Zheng et al., 2008). However, this model does not clearly explain why memory Th17 cells evolved to be a critical source of these cytokines instead of local mucosal structural cells such as fibroblasts or epithelial cells. Theoretically, the advantage of T-cell encoded IL-17 and IL-22 could be threefold: 1) T cells can rapidly divide and undergo apoptosis, providing a mechanism for rapid amplification and termination of the Th17 response, 2) T cells can traffic to and from mucosal sites to provide immune reconnaissance, and 3) The generation of memory Th17 cells might confer an advantage to the host above and beyond what pathogen specific antibody can provide. This latter hypothesis was attractive since extracellular pathogens such as K. pneumoniae and S. pneumoniae have evolved to rapidly change their capsular polysaccharide to avoid host specific antibody (Weinberger et al., 2010; Malley, 2010; Kohler et al., 2007; Podschun and Ullmann, 1998).
To investigate the roles of Th17 cells in vaccine-induced immunity, we employed a model of pulmonary infection with the encapsulated gram-negative pathogen Klebsiella pneumoniae. Immunizing mice with heat-killed K. pneumoniae organisms generated a substantial pool of Th17 cells in lung mucosa, and also elicited a robust antibody response against capsular polysaccharides. While the antibody response was effective at reducing bacterial burden with the vaccine strain, it afforded little protection against heterologous K. pneumoniae isolates with distinct polysaccharide serotypes. Th17 cells were found to be the critical CD4 T cell population required for immunity to heterologous K. pneumoniae strains. We provide evidence that outer membrane proteins conserved across several serotypes of K. pneumoniae are responsible for antigen-specific Th17 cell priming in mediastinal lymph nodes during vaccination, resulting in long-term protection against strains that are not effectively neutralized by antibodies. Thus, our findings illustrate that Th17 cells can provide clade-specific, serotype-indendent immunity against bacteria, suggesting a possible evolutionary advantage for the acquisition of IL-17 expression by CD4 T cell subsets.
RESULTS
Intranasal immunization induces robust mucosal Th17 response
Figure 1A shows a radial Cladogram of IL-17A, IL-17D, IL-17F protein families from different organisms including mammal, bird, fish, frog, vase trunicate and oyster indicates that existence of the gene predates the development of adaptive T-cell immunity (Figer 1A). Orthologos of IL-17A and IL-17F arise with lower jawed vertebrates and tarck closely with the evolution of T-cells and recombinase activating genes suggesting an evolutionary advantage of T-cell encoded IL-17A and IL-17F (Figure 1A). To test the role of memory Th17 cells in mucosal immunity, we developed a method to generate robust memory Th17 cell responses. C57BL/6 mice were immunized intranasally with 20 µg of heat-killed K. pneumoniae-43816 (ATCC) on Day 0 and Day 7, and then sacrificed on Day14 to assess T cell responses in the lung. Intracellular cytokine staining showed that immunization substantially increased numbers of Th17 (IL-17+) and Th22 (IL-22+) cells in the lung, but only moderately increased Th1 cell numbers (IFN-γ+) (Figure 1B and 1C). Further analysis of IL-17+ [Au: Global change - superscript, if you mean “positive” cells.] cells revealed that while γδ-T cells were the major source of IL-17 during primary infection, CD4+ T cells were primarily responsible for the increased IL-17+ cell population. [Au: “level” is vague. Use amounts, conc, activity etc.] observed following immunization (Figure 1B, lower panel). These results were confirmed using IL-17F-Thy1.1 reporter mice (Figure 1D)(Lee et al., 2009) RT-PCR and flow cytometry analysis also identified Vγ4 (Heilig naming system) as the major subtype of IL-17 producing γδ-T cells (Figure S1A and S1B), consistent with previous findings in an autoimmune disease model (Martin et al., 2009; Sutton et al., 2009). To further characterize IL-17 producing cells induced by primary infection versus immunization, we sorted Thy1.1+ cells based on surface expression of γδ-TCR (naïve mice, termed GD17), or CD4 (immunized mice, termed Th17). In addition, we sorted Thy1.1− CD4+ cells (immunized mice, termed Th-non-IL17F). Real-time PCR analysis confirmed that both GD17 cell and Th17 cell expressed high amounts of Il17 and Il22 (Fig. 1E), while only Th17 cells expressed Il21. Genes that are critical for Th17 cell induction or function were highly expressed in both GD17 and Th17 cell lineages, such as Rorc, Ahr, Ccr6, Il1r1 and Il23r (Fig. S1D and Fig. S1E) (Weaver et al., 2007; Chung et al., 2009). When cultured with different species of heat-killed gram-positive or gram-negative bacteria, Th17 cells were only capable of responding to K. pneumoniae-43816 (ATCC) re-stimulation, producing microgram quantities of IL-17 in culture supernatants (Figure 1F and 1G). This demonstrated the immunization-induced Th17 cells were antigen specific. To further support this, equal numbers of sorted GD17 and Th17 cell were cultured with heat-killed K. pneumoniae-43816 (ATCC), Th17 cells, but not GD17 cells, cells responded to K. pneumoniae-43816 (ATCC) restimulation and produced IL-17 in the presence or absence of neutralizing antibodies against IL-1β and IL-23 (Figure S1F), indicating the antigen-specific memory Th17 cell recall response occurred independently of IL-1β and IL-23.
Figure 1.
Immunization with heat-killed K. pneumoniae induces antigen-specific Th17 responses. (A) Radial Cladogram of IL-17A(A), IL-17D, IL-17F protein families from different organisms including mammal (dark blue), bird (red), fish (sky blue), frog (pink), vase trunicate (lime) and oyster (green). Abbreviations: Hs: Homo Sapiens, Mm: Mus musculus, Bt: Bos Taurus, Rn: Rattus norvegicus, Ss: Sus scrofa, Ec: Equus caballus, Md: Monodelphis domestica, Ol: Oryzias latipes, Sr: Salmo salar, Tr: Takifugu rubripes, Dr: Danio rerio, Gg: Gallus gallus, Ci: Ciona intestinalis, Xt: Xenopus tropicalis, Cg: Crassostrea gigas (B) C57BL/6 mice were immunized intranasally with 20 µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7 and sacrificed on Day14. Naïve mice were sacrificed 24h after 104 live K. Pneumoniae-43816 (ATCC) (serotype K2) infection. IL-17 and IL-22 producing cells in the lungs were analyzed by intracellular cytokine staining (upper panel). Subsets of IL-17 producing cells were determined by analyzing CD4 and γδ-TCR on IL-17 gated cells (lower panel). (C) Frequencies (left panel) and total numbers (right panel) of CD4 gated IFN-γ, IL-17A and IL-22 producing cells. (D) IL-17F-Th1.1 reporter mice were treated the same as in (B) and mononuclear cells in the lung were analyzed by flow cytometry. flow cytometry sorted splenic CD4+CD44lo (naïve CD4), splenic CD3+GL3+ (spleen GD), nTh-non17F, GD17 and Th17 were lysed in TriZol and RNA were extracted. Transcripts of cytokines were measured by real-time RT-PCR. Data represents two independent experiments. flow cytometry sorted Th17 from reporter mice were cultured with heat-killed K. pneumoniae, E. coli and S. aures in the presence of congenic CD45.1 splenocytes as APC for 4 days and responding Th17 cells were analyzed by staining congenic marker CD45.2 and CD4 (F). IL-17A released into the medium was measured by Luminex (G left panel) and total numbers were graphed (G right panel).
To further characterize the T cell responses induced by immunization, we immunized and sacrificed mice at various time points and collected tissue from different organs to analyze Th1 (IFN-γ+CD4+) and Th17 (IL-17A+CD4+) cells by flow cytometry. Representative flow cytometry plots from the spleen (Figure 2A) showed that frequencies of Th17 (IL-17A+) cells increased at week 1 and 2 after heat-killed K. pneumoniae-43816 (ATCC) immunization and contracted at week 4 while frequencies of Th1 (IFN-γ+) cells remained constant following immunization. Representative flow cytometry plots from the lung and mediastinal lymph nodes showed similar staining pattern with very few IFN-γ-IL-17A double positive cells (Figure S1B). To further characterize these Th1 and Th17 cells, intracellular staining of T-bet and Rorγt was performed (Figure 2B). Th17 cells showed a transient increase in T-bet expression at week 1 and 2 after immunization, consistent with previous report showing Th17 cells can up-regulate T-bet when stimulated with IL-12 (Lee et al., 2009). However, Rorγt was strictly expressed by Th17 cells. Analysis of Th1 and Th17 cell numbers in the lung, spleen, mediastinal lymph nodes (mLN) (Figure 2C) and total numbers from all three organs (Figure 2D) revealed that Th17 cells activated and expanded at week1 and 2 and contracted at week 4 while the Th1 cell response did not follow this pattern. Immunization had a minimal effect on Th1 cells with significant increase only observed in mediastinal lymph nodes after one week, suggesting that Th17 but not Th1 cells are strongly induced and may be more important in this extracellular bacterial infection model.
Figure 2.
Characterization of immunization induced T cell responses. C57BL/6 mice were immunized intranasally with 20 µg of heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and sacrificed 1, 2 and 4 weeks after. Lung, spleen and mediastinal lymph nodes were collected and intracellular staining was performed. Each time point has a group of 3 mice. (A) Representative flow cytometry plots of IL-17A and IFN-γ staining on CD4 gated cells in the spleen. (B) Representative flow cytometry plots of T-bet (upper panel) and Rorγt (lower panel) staining on IL-17A+ (Th17 blue line) and IFN-γ+ (Th1 red line) from the spleen. Mean fluorescent intensity of T-bet or Rorγt is shown on each plot. (C) Total numbers of Th1 (upper panel) and Th17 (lower panel) in lung, spleen and mediastinal lymph nodes. (D) Total numbers of Th1 and Th17 in three organs together were graphed. *Denotes significant higher cell numbers compared to naïve group.
Protection in an autologous challenge model is mediated by antibody and Th17 cell effector cytokines
To evaluate the protective efficacy of immunization against subsequent pathogen challenge, C57BL/6 mice were immunized and then challenged with 104 CFUs of the autologous K. pneumoniae-43816 (ATCC) 4 weeks later. Immunized animals showed a two-log lower bacterial burden after challenge (Figure 3A). Almost half of the immunized animals had sterile spleens when challenged, showing that mucosal immunization is very effective in controlling local infection and systemic dissemination. To examine the contributions of IL-17A and IL-22 in the protection by immunization, we administered neutralizing antibodies against IL-17A (Fei et al., 2011) and/or IL-22 (Aujla et al., 2008) after immunization and prior to challenge with live K. pneumoniae-43816 (ATCC). In addition, some mice were treated with 1A8 antibody to deplete neutrophils. Despite the robust induction of memory Th17 cells, IL-17A, IL-22, or neutrophils were dispensable for vaccine-induced protection (Figure 3B), suggesting other mechanisms are involved in the autologous protection.
Figure 3.
Mucosal immunity to an autologous bacterial challenge is mediated by B-cells and Th17 cells. (A) C57BL/6 mice were immunized intranasally with 20 µg of heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7. 4 weeks after the 2nd immunization, immunized mice and naïve unimmunized mice were infected with 104 live K. Pneumoniae-43816 (ATCC) (serotype K2) and sacrificed 24h after. Lung burden and systemic dissemination were determined by lung (left panel) and spleen (right panel) CFUs. Each group has an N>20 from 4 independent experiments. (B) 4 week after 2nd immunization, immunized mice and naïve mice were infected with 104 live K. Pneumoniae-43816 (ATCC) (serotype K2) and sacrificed 24h after. A group of immunized mice also received i.p. rat IgG or 1A8 24 hours and 2 hours prior to infection. A group of immunized mice received neutralizing antibody against IL-17A and/or IL-22 i.t. immediately prior to infection. Bacterial burdens were determined by lung CFU (left panel) and spleen CFU (middle panel) and neutrophil recruitment was determined by flow cytometry on Bronchoalveolar lavage cells (right panel). Each group has an N=5. (C) Rag2−/−Il2rg−/− mice were immunized intranasally with 20 µg K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7. Four weeks after the 2nd immunization, immunized mice and naïve unimmunized mice were infected with 104 live K. Pneumoniae-43816 (ATCC) (serotype K2) and sacrificed 24h after. Lung burden and systemic dissemination were determined by lung (left panel) and spleen (right panel) CFUs. Each group has 4–5 mice. (D) C57BL/6 mice were immunized intranasally with 20 µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7. Four weeks after the 2nd immunization, immunized mice and naïve unimmunized mice were infected with 104 live K. Pneumoniae-43816 (ATCC) (serotype K2) and sacrificed 24h after. Anti- K. Pneumoniae-43816 (ATCC) (serotype K2) IgG and IgA in the serum and lung homogenate from naïve uninfected, infected naïve unimmunized and immunized mice were measured by ELISA. (E) C57BL/6 mice were immunized intranasally with 20 µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7 and sacrificed on Day14. flow cytometry sorted B220+ cells from immunized mice lung were adoptively transferred into Rag2−/− Il2rg−/− mice. Rag2−/−Il2rg−/− mice receiving PBS or B-cells from immunized mice were infected with 104 live K. Pneumoniae-43816 (ATCC) (serotype K2) and sacrificed 24h later. Lung burden and systemic dissemination were determined by lung (left panel) and spleen (right panel) CFUs. Each group has N=4 mice. (F) Ighm−/− mice were immunized intranasally with 20 µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7. 4 weeks after the 2nd immunization, immunized Ighm−/− mice and naïve-unimmunized Ighm−/− mice were infected with 104 live K. Pneumoniae-43816 (ATCC) (serotype K2) and sacrificed 24h after. A group of immunized mice also received i.p. rat IgG or 1A8 24h and 2h prior to infection. A group of immunized mice received neutralizing antibody against IL-17A i.t. immediately prior to infection. Bacterial burden were determined by lung CFU (left panel) and spleen CFU (right panel). Data are from two independent experiments with 2–4 mice in each group.
We then postulated that humoral immunity may be dominant in this model, as IL-17 has been shown to support antibody responses (Mitsdoerffer et al., 2010). To determine whether B-cells and/or adaptive immunity are required for vaccine-induced protection, we immunized Rag2−/−Il2rg−/− mice, which lack T, B and NK cells and infected them 4 weeks after. No protection was observed in these mice (Figure 3C), suggesting that B-cells might play a protective role in wild type mice. Measuring K. pneumoniae-43816 (ATCC) specific antibodies in serum and lung homogenate by ELISA in WT mice confirmed that immunized mice had substantial titers of K. pneumoniae-43816 (ATCC) specific IgG and IgA in comparison to naïve mice (Figure 3D). This antibody response induced by immunization was not IL-17 dependent, as immunized Il17ra−/− mice had similar amounts of anti-K. pneumoniae-43816 (ATCC) IgG in the lung as their wild type litter mate controls (Figure S2A). As a negative control, antibody concentrations from B-cell-deficient (Ighm−/−) mice were also measured (Figure S2A). CD4 depletion prior to immunization did not affect the protection against this autologous K. Pneumoniae-43816 (ATCC) challenge (Figure S2C). Moreover, the anti-K. pneumoanie-43816 (ATCC) antibody response was not affected in CD4-depleted mice demonstrating that the antibody response is T cell independent. (Figure S2D). Adoptive transfer of B-cells from immunized mice conferred protection in Rag2−/−Il2rg−/− mice (Figure 3E), confirming that B cells are capable of mediating the protection to a secondary autologous challenge.
To determine if the generated Th17 cells could confer protection independent of antibody, we immunized B-cell deficient (Ighm−/−) mice. Immunized (Ighm−/−) mice still demonstrated a one log protection in the lung and spleen against pulmonary bacterial challenge (Figure 3F and S2B). To examine if this protection required Th17 cell effector cytokines we administered anti-IL-17A or anti-Ly6g (1A8) immediately prior to the autologous bacterial challenge. In contrast to WT mice, immunization in Ighm−/− mice was dependent on IL-17A and neutrophils (compare Figs. 3B and 3F). These results demonstrate that in the autologous challenge model B cells or antibodies confer primary protection but IL-17A and neutrophils confer secondary protection in the absence of antibody.
Cross-serotype protection in heterologous challenge is Th17 cell dependent
Like many extracellular pathogens, K. pneumoniae species are clades of organisms that have multiple capsular serotypes (Petermann et al., 2010; Podschun and Ullmann, 1998). Thus, we hypothesized that although antibody responses are dominant in an autologous challenge model, an advantage of Th17 cells may be broader serotype-independent immunity. To investigate whether antibodies are protective against infection from heterologous strains, we immunized mice with the K. Pneumoniae-43816 (ATCC) (serotype K2) strain and tested antibody cross reactivity to clades of clinical isolates obtained from Southeast Asia, where K. pneumoniae liver and lung abscesses (Table S1) are endemic (Ho et al., 2010). The IgG antibody response in mice immunized with K. Pneumoniae-43816 (ATCC) only reacted with K2 serotypes, not K1 or K16 serotypes (Figure 4A). We observed no antibody cross reactivity to different serotypes of K. pneumoniae, and only 2/3 of the K2 strains showed cross reactivity (Figure 4A), indicating that antibodies were unlikely to confer protection against heterologous serotypes. Serum from immunized mice only killed the autologous K. Pneumoniae-43816 (ATCC) (serotype K2) but not the heterologous K. Pneumoniae-396 (serotype K1) isolate in vitro (Figure S3A) further supporting that the B-cell response is strain specific.
Figure 4.
Cross-serotype protection with heterologous bacterial challenge requires Th17 cells, not B-cells. (A) Various stains of heat-killed K. pneumoniae were coated on a ELISA plate and lung homogenate from naïve mice (labelled as N) and K. Pneumoniae-43816 (ATCC) (serotype K2) immunized mice (labelled as V) were tested for cross reactivity. K. Pneumoniae strain numbers and serotypes are also listed. (B) Mediastinal lymph nodes from immunized C57BL/6 mice were labelled with CFSE and cultured with different serotypes of heat-killed K. Pneumoniae, E. coli, S. aureus and S. pneumoniae for 3 days. Proliferation of IL-17+ cells was analyzed by intracellular IL-17 staining and CFSE dilution. C57BL/6 mice were immunized intranasally with 20 µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7. 10 days after the 2nd immunization, immunized mice and naïve unimmunized mice were infected with 104 K. Pneumoniae-303 (serotype K16) (C), K. Pneumoniae-396 (serotype K1) (D) or K. Pneumoniae-NDM1+(serotype unknown) (E) and sacrificed 24h after. Lung burden and systemic dissemination were determined by lung (left panel) and spleen (right panel) CFUs. Each groups has 4–5 mice. (F)Ighm−/− mice were immunized intranasally with 20 µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7. Four weeks after the 2nd immunization, immunized Ighm−/− mice and naïve unimmunized Ighm−/− mice were infected with 104 live K. pneumoanie-396 (serotype K1) and sacrificed 24h after. A group of immunized mice also received i.t. neutralizing antibody against IL-17A right before infection. Bacterial burden were determined by lung CFU (left panel) and spleen CFU (right panel). Th17 responses in the lung were analyzed by RT-PCR (G). Data are from two independent experiments with 3–4 mice each group. (H) IL-17F-Thy1.1 reporter mice were immunized with heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) and were sacrificed one week after. Lung single cells were prepared and stained with CD4 and Thy1.1. flow cytometry sorted 105 Th17 (CD4+Thy1.1+) per mouse or 105 B cells (CD19+CD4− Thy1.1−) per mouse were intravenously transferred to Rag2−/− mice. Four week after transfer, mice were infected with 104 K. pneumoanie-396 (serotype K1) and sacrificed at 24h. Lung burdens were assessed by CFU. Each group has an N=5.
Interestingly, local memory Th17 cells generated from K. Pneumoniae-43816 (ATCC) immunization demonstrated proliferative responses to all serotypes of K. pneumoniae tested, including the recently described New Delhi metallolactamase (NDM-1+) strain (Yong et al., 2009) (Figure 4B). However, neither S. pneumoniae nor S. aureus induced proliferation of memory Th17 cells (Figure 4B). In contrast to IL-17+ cells, we observed only minimal proliferation of Th1 (CD4+IFN-γ+) cells to K. pneumoniae serotypes, E. coli, S. pneumoniae or S. aureus, with no striking difference between bacterial species (Figure S3B). E. coli, another member of the Enterobacteriaceae family, induced a notable level of Th17 proliferation, but not as significant as the K. pneumoniae strains (Figure 4B).
To determine if this serotype-independent Th17 cell response induced with mucosal immunization confers protection against heterologous K. pneumoniae serotypes, we immunized mice with K. pneumoniae-43816 (ATCC) (serotype K2) and challenged with either K. pneumoniae-303 (serotype K16), K. pneumoniae-396 (serotype K1) or the recently isolated K. pneumoniae- New Delhi metallolactamase (NMD1+) strain. Despite lacking serotype-specific antibodies, all of the challenged mice were protected from heterologous serotypes (Figure 4C, 4D and 4E). To further exclude a possible role of antibodies in this heterologous challenge model, we performed immunization with the K. pneumoniae-43816 (ATCC) (serotype K2) and challenge with the K. pneumoniae-396 (serotype K1) in Ighm−/− mice. As shown in Figure 4F, immunization of Ighm−/− mice with this K2 serotype provided significant protection against the highly virulent K. pneumoniae-396 (serotype K1) strain. Expression of Il17a and Il17f mRNA as well as IL-17 protein were up regulated in the lung during this heterologous challenge (Figure 4G and S3C), suggesting a critical role for Th17 cells. Furthermore, IL-17 neutralizing antibody significantly attenuated vaccine efficacy (Figure 4F) in this heterologous challenge model. To confirm that Th17 cells, not B cells, confer cross protection, cells were sorted from IL-17F-Thy1.1 reporter mice immunized with K. pneumoniae-43816 (ATCC) (serotype K2) and adoptively transferred in to Rag1−/− hosts. One week after transfer, mice were challenged with the virulent K. pneumoniae-396 (serotype K1) strain. Notably, mice receiving the Th17 cell transfer, but not CD19+ B cells, had a significantly reduced lung burden (Figure 4H).
Cross-serotype protection in heterologous challenge is independent of Interferon-γ
To investigate the role of Th1 cells in cross-serotype protection, Ifng−/− and age matched WT mice were immunized with K. pneumoniae-43816 (ATCC) (serotype K2) and challenged with K. pneumoniae-396 (serotype K1). We observed equal protection in WT and Ifng−/− mice (Figure 5A) demonstrating that IFN-γ is dispensable for this response. To determine the role of Th17 and Th1 cells more specifically, flow cytometry sorted Th17 and Th-non-17F (which contains Th1 cells) were adoptively transferred into Rag2−/−Il2rg−/− mice. Only the transfer of Th17 cells provided protection when mice were challenged with the heterologous K. pneumoniae-396 (serotype K1) strain four weeks later (Figure 5B). To confirm the role of IL-17 signaling in the heterologous challenge, Rag2−/− mice receiving adoptively transferred Th17 cells were administered IL-17RC neutralizing antibody or IFN-γ neutralizing antibody after K. pneumoniae-396 (serotype K1) challenge. Blocking IL-17RC signaling by anti-IL17RC, but not blocking IFN-γ, abolished the protection conferred by the adoptively transferred cells (Figure 5C), demonstrating that IL-17 from Th17 cells are key for cross-serotype protection. To investigate the potential role of IFN-γ in the absence of IL-17RA signaling and B cell-mediated immunity, K. pneumoniae-43816 (ATCC) (serotype K2) immunized Il17ra−/− mice with B cell depletion were challenged with K. pneumoniae-396 (serotype K1) with or without anti-IFN-γ. Blockade of IFN-γ did not make the mice more susceptible to K. pneumoniae-396 (serotype K1) challenge even when IL-17RA signalling is absent (Figure 5D).
Figure 5.
Cross-serotype protection in heterologous challenge is mediated by Th17 not Th1. (A) C57BL/6 and Ifng−/− mice were immunized intranasally with 20 µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and Day 7. 28 days after the 2nd immunization, immunized mice and naïve-unimmunized mice were infected with 104 K. pneumoanie-396 (serotype K1) and sacrificed 24h after. Lung burden and systemic dissemination were determined by lung (left panel) and spleen (right panel) CFUs. Each group has 4–5 mice. (B) IL-17F-Thy1.1 reporter mice were immunized with heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) and were sacrificed one week after. Lung single cells were prepared and stained with CD4 and Thy1.1. flow cytometry sorted 105 Th17 (CD4+Thy1.1+) per mouse or 105 Th-non17F (CD4+Thy1.1−) per mouse were intravenously transferred to Rag2−/−Il2rg−/− mice. Four week after transfer, mice were infected with 104 K. Pneumoniae-396 (serotype K1) and sacrificed at 24h. Lung burdens were assessed by CFU. Each group has 3–4 mice. (C) IL-17F-Thy1.1 reporter mice were immunized with heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) and were sacrificed one week after. Lung single cells were prepared and stained with CD4 and Thy1.1. flow cytometry sorted 105 Th17 (CD4+Thy1.1+) per mouse were intravenously transferred to Rag2−/− mice. Four week after transfer, mice were infected with 104 K. Pneumoniae-396 (serotype K1). Control IgG, α-IL17RC or α-IFN-γ were given i.t. 12h after infection and mice were sacrificed at 24h after infection. Lung and spleen burdens were assessed by CFU. Each group has 4 mice. (D) Il17ra−/− mice with B-cell depletion (0.1mg anti-CD20 i.p. weekly) mice were immunized intranasally with 20µg heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) at Day 0 and challenge with 104 K. Pneumoniae-396 (serotype K1) on Day28. Control IgG or α-IFN-γ were given i.t. 12h after infection and mice were sacrificed at 24h after infection. Lung bacterial burdens were assessed by CFU. Each group has 4–5 mice.
Th17 cells recognize outer membrane proteins
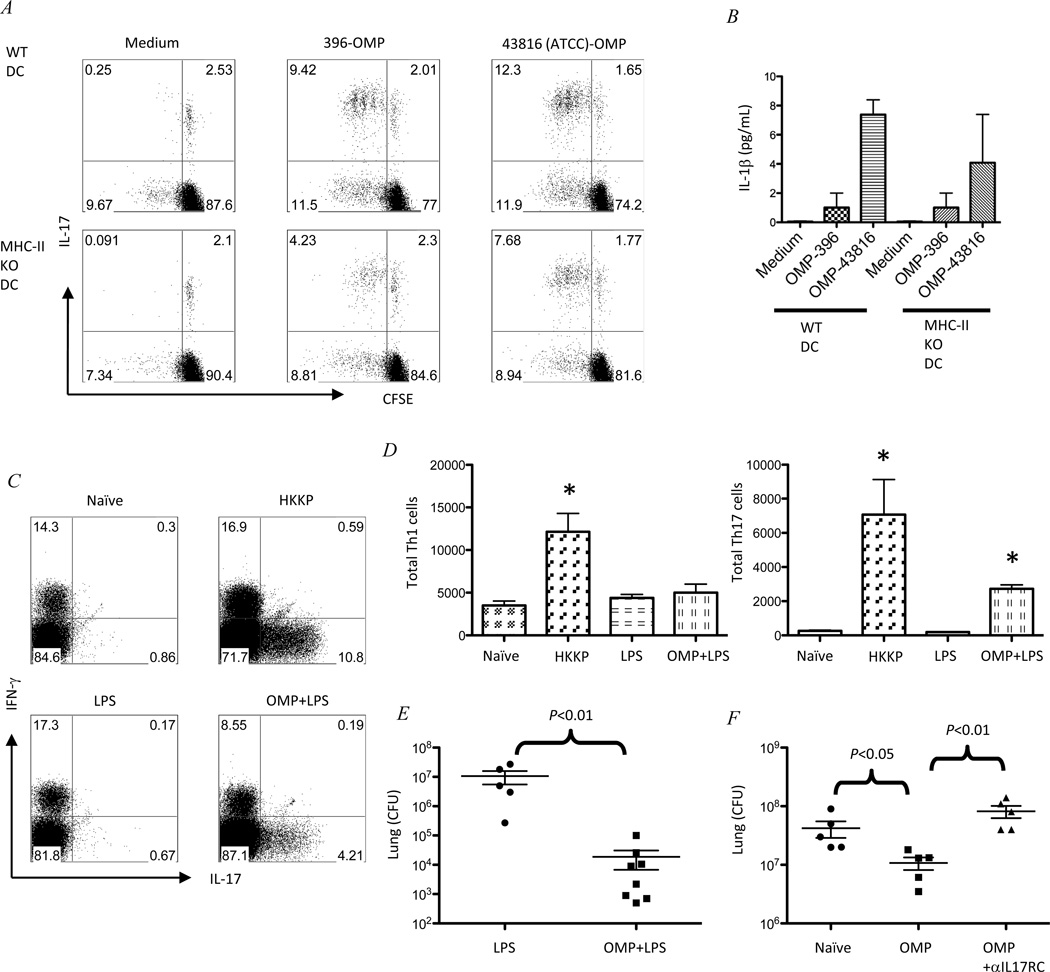
To explore the mechanism of this Th17 cell-mediated, clade specific, serotype independent protection, we sought to determine which antigen(s) these Th17 cells respond to. We hypothesized that outer membrane proteins (OMPs) might be a potential candidate and isolated OMPs by ultracentrifugation from the K. pneumoniae-43816 (ATCC) (serotype K2) vaccine strain to stimulate CFSE-labelled mediastinal lymph nodes cells from immunized mice. Th17 cells responded to K. pneumoniae-43816 (ATCC) (serotype K2)-OMP but not recombinant E. coli OmpA (EC-OMP), confirming the antigen specificity of these Th17 cells induced by immunization (Figure S4A). To test the cross reactivity of Th17 cells to K. pneumoniae strains with different serotypes, we isolated OMPs from various serotypes of K. pneumoniae including K. pneumoniae-43816 (ATCC) (serotype K2), K. pneumoniae-396 (serotype K1) and K. pneumoniae-NMD1+. Interestingly, when these OMPs were added to the culture of CFSE-labelled lymph nodes cells from immunized mice, they all induced robust Th17 proliferation comparable to the OMPs isolated from the immunization strain (K. pneumoniae-43816 (ATCC) (serotype K2) (Figure S4B), further supporting that immunization-induced Th17 cells are clade specific and serotype independent. To confirm the Th17 cell proliferation is OMP-specific, we restimulated the Th17 cells from mediastinal lymph nodes with OMPs in the presence of wild type or MHC-II-deficient dendritic cells. While Th17 cells cultured with wild type DCs responded to autologous and heterologous OMPs (Figure 6A, upper panel), Th17 cell cultured with MHC-II-deficient DCs, which are deficient in antigen presentation, had significantly reduced proliferative responses to OMP antigens (Figure 6A, lower panel), demonstrating that OMP induced Th17 cell proliferation is at least partially MHC-II dependent. Cytokine analysis of the tissue culture supernatant revealed that MHC-II independent Th17 cell proliferation could be a result of IL-1β produced by DCs regardless of MHC-II expression (Figure 6B). These observations led us to hypothesize that immunization with OMPs together with an adjuvant may confer the same level of protection as observed in the animals immunized with heat-killed bacteria. We immunized mice intranasally with LPS (as a TLR4 adjuvant) with or without OMPs isolated from K. Pneumoniae-43816 (ATCC) (serotype K2). 7 days later, OMPs with LPS adjuvant induced robust Th17 responses in the lung, but not Th1 responses (Figure 6C and 6D). Importantly, these animals were protected against a heterologous challenge with live K. pneumoniae-396 (serotype K1) (Figure 6E). To further clarify the specific role of Th17 cells, we did heterologous challenge in Ighm−/− mice. Again, IL-17RC blockade in K. pneumoniae-43816 (ATCC) (serotype K2) immunized Ighm−/− mice completely abrogated the protection to a K. pneumoniae-396 (serotype K1) heterologous challenge (Figure 6F), confirming the crucial role of IL-17RC signaling in OMP induced cross-serotype protection.
Figure 6.
The Th17 response to Klebsiella outer membrane proteins is partially MHC-II dependent. (A) flow cytometry sorted CD4 T cells from K. Pneumoniae-43816 (ATCC) (serotype K2) immunized mice mediastinal lymph nodes were labelled with CFSE and cultured with flow cytometry sorted CD11+ DCs from C57BL/6 or MHC-II KO spleen for 4 days in the presence or absence of 10µL/mL OMP from K. Pneumoniae-396 (serotype K1) or K. Pneumoniae-43816 (ATCC) (serotype K2). Th17 proliferation was measure by intracellular IL-17 staining and CFSE dilution. Data are representative from two independent experiments. (B) IL-1β levels in the culture supernatants were measured by Luminex. (C) C57BL/6 mice were immunized with heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) (labeled as HKKP) or 1µg LPS or 1µg LPS plus 10µg OMPs isolated from K. Pneumoniae-43816 (ATCC) (serotype K2). 7 days later, mice were sacrificed and lungs were harvested for intracellular IL-17 and IFN-γ analysis on gated CD4+ T cells by flow cytometry. (D) Total Th1 (CD4+IFN-γ+) and Th17 (CD4+IL-17+) cells were graphed. (E) 4 week after immunization, LPS or LPS+OMP immunized mice were challenged with 104 live K. Pneumoniae-396 (serotype K1), bacterial burden were determine by lung and spleen CFU. Data are from two independent experiments with 3–5 mice each group. (F) Ighm−/− mice were immunized with 10µg OMPs isolated from K. Pneumoniae-43816 (ATCC) (serotype K2) and challenged with K. Pneumoniae-396 (serotype K1) 4 weeks later. Control IgG or anti-IL17RC was given i.t.12h after infection. Mice were sacrificed at 24h and bacterial burden were determined by lung CFU. Each group has 5 mice.
DISSCUSSION
The involvement of Th17 cells in protective vaccination against intracellular pathogens has been previously documented (Khader et al., 2007). In this model, IL-17 was required for the induction of CXCR3 ligands that regulated the recruitment of Th1 cells which controlled the growth of the pathogen. However, there is a relative lack of evidence that memory Th17 cells mediate direct protection in an antigen-specific manner. Our data demonstrate that instead of recruiting other subsets of T helper cells by orchestrating chemokine gradients as in the Mycobacterium tuberculosis model, Th17 cells can be primed by mucosal immunization and respond to secondary stimulation with either whole bacterial lysates in an antigen-dependent manner or to purified outer membrane proteins from both vaccine and challenge strains of K. pneumoniae. Approximately 50% of this latter proliferative response was MHC II-dependent; however, there was also significant ex vivo proliferation when sorted CD4+ T-cells were cultured with class II MHC-deficient antigen presenting cells. The MHC-II independent Th17 proliferation may be be driven by IL-1β. We were also able to detect some soluble IL-1β in S. aureus and S. pneumoniae cultures (data not shown), yet we did not observe significant Th17 cell proliferation to these antigens after K. pneumoniae vaccination. However, in these cultures, we used bulk mediastinal LN cells and Th17 frequencies are lower compared to the OMP MHC-II-deficient DC experiments. These latter experiments used flow cytometry-sorted purified CD4+ T cells. Thus one possibility that we did not detect any proliferating Th17 cells in S. aureus and S. pneumonia is that the precursor frequency of IL-1β responsive cells was lower. Importantly, these proliferating Th17 cells were able to confer protection to several heterologous serotypes of K. pneumoniae including NDM-1, and that protection to the most virulent K. Pneumoniae-396 (serotype K1) strain was dependent on IL-17A and IL-17RC and was independent of IFN-γ.
Recently, Wuthrich et al have also shown that vaccine induced IL-17+ cells can also mediate protection against pulmonary infection with Coccidioides posadasii, Histoplasma capsulatum, and Blastomyces dermatitidis (Wuthrich et al., 2011). This same group has recently made a blastomyces specific CD4+ TCR receptor transgenic mouse line and interestingly the transgenic CD4+ T cells proliferate in response to several dimorphic fungi (Wuthrich et al., 2011b). These data suggest that T cells can have broad specificity and recognize clades of organisms. Consistent with this a recent analysis of Mycobacterium tuberculosis antigens showed that human T cell epitopes of Mycobacterium tuberculosis are strongly evolutionarily conserved (Comas et al., 2010). Moreover, Malley has shown that vaccination with S. pneumoniae cell wall polysaccharide elicits IL-17 from CD4+ T cells and this response is required to eradicate S. pneumoniae colonization in the mouse (Lu et al., 2008). In follow-up work, this group has identified a number of S. pneumoniae antigens that can induce Th17 responses in whole-cell antigen immunized mice (Moffitt et al., 2011). Our study shows that immunization-induced Th17 cells provide serotype-independent protection to another important encapsulated bacteria K. pneumoniae. Moreover protection still occurred in B cell deficient (Ighm−/−) mice. Taken together, these data indicate that one theoretical evolutionary advantage of Th17 cells as opposed to non-CD4+ T cell sources of IL-17 is they can provide protection to the host when antibody responses are compromised or restricted to the capsular serotype. These data also suggest that Th17 based vaccines can be designed for immunocompromised patients that may lack B cell responses or patients undergoing anti-CD20 treatment.
In the setting of K. pneumoniae, one T cell antigen that is sufficient to induce heterologous Th17 mediated protection independent of capsular serotype are outer membrane proteins (OMPs). Our data demonstrate that OMPs from Klebsiella in conjunction with LPS (an experimental adjuvant that activates TLR4) generated comparable Th17 response as the whole lysate. Safety is a paramount concern in vaccine development so to translate these studies to potential clinical use would require additional testing of adjuvants such as monophosphoryl lipid A.
Taken together, we provide strong evidence that an evolutionary advantage of Th17 cells is to provide clade-specific immunity that is independent of capsular serotype. Many important human bacterial pathogens are encapsulated organisms, such as S. pneumoniae and K. pneumoniae. Since polysaccharide capsules can avoid non-opsonic phagocytosis, successful vaccination against these bacteria requires polyvalent vaccines consisting of the most lethal serotypes (Weinberger et al., 2010; Malley, 2010; Ovodov, 2006). To successfully co-evolve with these pathogens, mammals needed to acquire host resistance mechanisms. A limitation of humoral immunity is its specificity to the serotype of the infecting strain. However, as demonstrated here, the induction of mucosal Th17 cells provides a broader resistance mechanism than antibodies. We postulate that this may be very important in the gastrointestinal tract, where mucosal Th17 responses must deal with diverse clades of organisms. Although memory Th17 cells have been shown to be short lived in response to an intracellular pathogen such as Listeria monocytogenes (Pepper et al., 2010), we observed significant Th17 memory populations 4 weeks after immunization. In the mediastinal lymph nodes, Th17 cells are CD127hiCD69loCD44hiCD25lo CD62Llo, resembling an effector memory phenotype. Moreover, many extracellular pathogens in humans also colonize mucosal surfaces and thus prolonged or recurrent antigen exposure may play a role in maintaining memory Th17 responses. Our data shows that approaches to induce mucosal Th17 cells will be an effective way of vaccination against organisms with multiple capsular serotypes. However, this broader specificity may also be why Th17 cells have been linked to autoimmunity, and one would need to screen vaccine antigens carefully to avoid this caveat. In closing, we believe these data provide an explanation of why IL-17 has evolved to be expressed in CD4 T cells, and this evolutionary advantage can be exploited for improved vaccination strategies.
Experimental Procedures
Immunization and infection
20µg Heat-killed K. Pneumoniae-43816 (ATCC) (serotype K2) was given to isoflurane anesthetized mice in sterile PBS (40µL) intranasally once or twice as indicated. Mice were then infected with 104 live K. pneumoniae by oropharyngeal aspiration-tongue pull technique (referred as i.t.) and sacrificed at 24h after infection. Bacterial burden in the lung and spleen were analyzed by CFU on the homogenates. Lung homogenates were then centrifuged, supernatant were used for Luminex analysis and pellets were lysed in TRIzol for RNA extraction.
Mice and intratrachel administrations
C57BL/6 (B6), Ifng−/− Ighm−/− mice were purchased from The Jackson Laboratory. Rag2−/− and Rag2−/−Il2rg−/− mice were purchased from Taconic farm. IL-17F Thy1.1 reporter mice have been previously described (Lee et al., 2009). 104 K. pneumoniae were given to isoflurane anesthetized mice in sterile PBS (50µL) by oropharyngeal aspiration-tongue pull technique (i.t.). Il17ra−/− mice on the B6 background were provided by Amgen (Seattle, WA).
Ethics Statement
All procedures were performed according to the LSU Health Sciences Center IACUC guidelines (protocol 2660).
Experimental Klebsiella pneumoniae Infection
Klebsiella pneumoniae ATCC strain 43816, serotype 2, BAA-2146 (NDM1+) (ATCC, Rockville, MD) or clinical isolates were grown in 100 mL of tryptic soy broth (Difco, Detroit, MI) for 18 h at 37°C. The quantity of 1 mL of the culture was added to 100 mL of fresh tryptic soy broth, grown for 2 h, allowing the culture to reach early log phase. The concentration of K. pneumoniae was determined by measuring the absorbance at 600 nm. A standard curve of absorbance units based on known colony-forming units (CFU) was used to calculate inoculum concentration. Bacteria were pelleted by centrifugation at 5,000 rpm for 15 min, washed twice in PBS, and resuspended at the desired concentration. 24h after infection, mice were sacrificed and left lungs were harvested in medium with serum for flow cytometry analysis or in TriZol for RNA extraction, right lungs were harvested in sterile PBS for CFU and Luminex (Millipore) on the Bioplex reader (Bio-Rad). In some experiments 0.1mg/mouse anti-IL17RC (R&D system) or 0.1mg/mouse anti-IFNγ XMG1.2 (eBioscience) were administered i.t. 12h after bacterial challenge.
Isolation of murine lung single cells for ICS
Lungs are isolated and minced with forceps and small scissors and digested with 1–2mg/mL collagenase (Sigma) for 90min at 37°C. Digested tissue were passed through a sterile 70 um filter (BD Falcon) to get a single cell suspension. After washing, the cells are ready for flow cytometry or in vitro stimulation.
Flow cytometric analysis
Single cells from mouse lung were stimulated for 5–6 h with 50 ng/ml PMA (Sigma) and 750 ng/ml ionomycin (Sigma). After 1 h, 1µL/mL GolgiStop (BD Pharmingen, San Diego, CA) was added to block cytokine secretion. Cells were surface stained for 15–30 min at 4°C with anti-Thy1.1, anti-CD4 mAb (RM45; BD Pharmingen, San Diego, CA) and GL3 (eBioscience) in PBS supplemented with 1% BSA and 0.2% sodium azide. Cells were then fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen, San Diego, CA) and stained intracellularly with anti-IL-17, anti-IL-22 and anti-IFNγ (XMG1.2) (eBioscience, San Diego, CA). Samples were acquired on a flow cytometryAria or LSR-II flow cytometer and data analysis was conducted using FlowJo software (Treestar).
Gene expression assays
Gene expression was measured by real-time RT-PCR using pre-made primers and probes from ABI Biosystems. Briefly, total lung tissue was harvested and total RNA was extracted using TRIzol (Invitrogen). First strand cDNA was synthesized using BioRad iScript kit. cDNA was then used for real-time PCR on a Bio-Rad CFX96 thermal cycler and fold changes were calculated by the delta-delta Ct method.
Statistical Analyses
Unpaired, two-tailed, Student's t tests, ∝ = 0.05, were used to assess whether the means of two normally distributed groups differed significantly. One-way ANOVA analysis was used to compare multiple means. Significance is indicated as P < 0.05, P < 0.01 and P < 0.001. All error bars represent the s.d.
HIGHLIGHTS.
Th17 cells generated by vaccination confer cross-serotype protection.
Memory Th17 cells mediate direct protection in an antigen specific manner.
Th17 cells protect against multi-drug resistant Klebsiella challenge.
Supplementary Material
Acknowledgements
The authors would like to thank Candice Fisher for technical assistance for Luminex analyses and Constance Porretta for assistance with flow cytometry. The authors would also like to thank Dr. Andrew Chan at Genentech for kindly providing the B-cell depletion antibodies. This work was supported by grant R37 HL079142 from the NIH.
The authors would like to acknowledge support from the following PHS grants: P50HL084932, 5R01HL061271, and R37 HL079142 to JKK and Dr. Nico Ghilardi at Genentech for providing the IL-23p19 −/− mice,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: K.C., and J.K.K. designed the experiments. K.C., J.P.M. and J.K.K. wrote the manuscript with input from the co-authors. Y.L. generated the IL-17 cladogram. M.Z. and J.F.A. provided reagents and read the paper. D.L.P. provided the clinical isolates of K. pneumoniae. C.T.W. provided the IL-17F-Thy1.1 reporter mice.
No authors have any competing interests.
References
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, Saijo S, Iwakura Y, Fallert Junecko BA, Reinhart TA, Foreman O, Ray P, Kolls J, Ray A. TNF-{alpha} from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5360–5365. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Tambyah PA, Paterson DL. Multiresistant Gram-negative infections: a global perspective. Curr. Opin. Infect. Dis. 2010;23:546–553. doi: 10.1097/QCO.0b013e32833f0d3e. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal. Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler JE, Hutchens MP, Sadow PM, Modi BP, Tavakkolizadeh A, Gates JD. Klebsiella pneumoniae necrotizing fasciitis and septic arthritis: an appearance in the Western hemisphere. Surg. Infect. (Larchmt.) 2007;8:227–232. doi: 10.1089/sur.2006.007. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. Interleukin-17A Mediates Acquired Immunity to Pneumococcal Colonization. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000159. e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol. Med. 2010;88:135–142. doi: 10.1007/s00109-009-0579-4. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host. Microbe. 2011;9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovodov YS. Bacterial capsular antigens. Structural patterns of capsular antigens. Biochemistry (Mosc.) 2006;71:937–954. doi: 10.1134/s000629790609001x. [DOI] [PubMed] [Google Scholar]
- Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Gueguen Y, de LJ, Goetz F. Rapid accumulation of an interleukin 17 homolog transcript in Crassostrea gigas hemocytes following bacterial exposure. Dev. Comp Immunol. 2008;32:1099–1104. doi: 10.1016/j.dci.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Ruckinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, O'Brien KL, Anthony SJ, Lipsitch M. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin. Infect. Dis. 2010;51:692–699. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin. Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, De Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.