Abstract
During the antigen-dependant activation process several subsets CD8+ T cells appear with different phenotypic and functional characteristics. Recent studies indicate that the state of T cell differentiation radically affects their ability to effectively respond to tumor challenge, with early effector CD8+ T (CD62Lhigh) cells having better anti-tumor activity. Thus strategies aimed at optimizing the generation of such subpopulations could significantly enhance the effectiveness of adoptive cell therapy (ACT) for cancer. In this study, we show that priming of naïve CD8+ T cells in the presence of IL-12 selectively rescued early CD8+ CD62Lhi from activation induced cell death and resulted in the increased accumulation of this subset of CD8+ T cells. Furthermore, we demonstrated that IL-12 directly modulated the expression of CD62L on activated CD8+ T cells. When used for ACT, naïve CD8+ T cells primed in vitro in the presence of IL-12 showed superior anti-tumor activity toward B16 melanoma. Importantly, using the Pmel-1 model, priming pmel-1 cells in vitro with IL-12 reduced the state of functional tolerance associated with the non-mutated “self” tumor antigen gp100, as demonstrated by significant tumor responses in the absence of vaccination. Together, our results suggest that in vitro conditioning of naïve CD8+ T cells with IL-12 prior to ACT could significantly enhance their anti-tumor activity.
Keywords: IL-12, L-selectin, CD62L, CD8+ T cells, OT-1, Pmel-1
Introduction
The success of adoptive cell transfer (ACT) as anti-cancer treatment depends on the functional quality of the cells transferred [11]. Ideal cells for ACT must show high activity and tumor specificity. Because cytokines are known to play fundamental roles in the differentiation and maturation of antigen reactive T cells, cytokine conditioning prior to cell transfer has been considered as a means to increase the proliferation and function of tumor reactive T cells. Perhaps the most studied cytokines are those that share the receptors of the common cytokine-receptor γ-chain family, such as interleukin-2 (IL-2), IL-4, IL-7, IL-15 and IL-21, given their requirement for the development or maintenance of memory T cells [28]. Of significant interest has also been the role of inflammatory cytokines such as IL-12. In addition to its role in polarizing Th1 responses and in the maturation of cytotoxic functions of CTLs and NK cells [38], IL-12 acts as a third signal along with antigen and IL-2 on naive CD8+ T cells increasing the pool of effector and memory cells [6, 35, 36]: two crucial components of a successful ACT approach. Evidence for this has been provided in recent reports demonstrating increased anti-tumor capabilities of CD8+ T cells after ex vivo culture in the presence of IL-12 [8, 18]. However, the phenotypic and functional characteristics of the resulting population have not been described. Understanding this is of particular importance since the intrinsic characteristics associated with each stage of CD8+ T cell differentiation ultimately determine their anti-tumor activity. It has been suggested that the more differentiated effector CD8+ T cells are less efficient at killing tumors after adoptive transfer [10]. Early effector CD8+ T cells are characterized by the expression of high levels of the lymphoid homing molecule CD62L or l-selectin. Expression of CD62L decreases as activation progresses, with re-expression almost restricted to central memory cells (TCM) [14, 26]. It is believed that the enhanced anti-tumor activity associated with T cells expressing high levels of CD62L relates to their ability to home to secondary lymph nodes, where effective stimulation can take place [31]. Thus, approaches aimed at the generation and maintenance of early effector populations could prove valuable in the development of effective ACT-based immunotherapies for cancer.
In this study, we report that in vitro priming of naïve cells in the presence of IL-12 results in decreased activation induced cell death, and that such IL-12-mediated anti-apoptotic effects resulted in the accumulation of a subpopulation of CD8+ T cells expressing high levels of CD62L with superior anti-tumor capabilities. This represents the first report to link enhanced anti-tumor activity to increased expression of CD62L in an IL-12 preconditioned CD8+ T cell subpopulation.
Materials and methods
Mice and cell lines
B6.SJL (Ly5.1), and C57BL/6 (Ly5.2) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). OT-1 TCR transgenic (Vα2/Vα5) mice (Jackson) were bred with B6.SJL mice to generate Ly5.1+/Ly5.1+ mice heterozygous for the OT-1 TCR transgene. Transgene status was confirmed by flow cytometry with monoclonal antibodies (mAbs) specific for Vα2 after 7 rounds of back crosses. Pmel-1 transgenic (Ly5.2) mice (Jackson) were bred with Ly5.2 wild type mice (both are on C57BL/6 background) to generate Ly5.2+/Ly5.2+ mice heterozygous for the pmel-1 TCR (Vα1/Vβ13) transgene. Transgene status was confirmed by PCR analysis. All animals were housed under specific pathogen-free conditions in accordance with institutional and federal guidelines at the Medical University of South Carolina. B16 (H-2Kb) cells, derived from a gp100+ spontaneous murine melanoma cell line, were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The OVA-transfected B16 cell line (B16–OVA) was kindly provided by Dr. R. Dutton (Trudeau Institute, Saranab Lake, NY, USA). Cells were cultured in complete RPMI medium (RPMI 1640, 0.1% penicillin/streptomycin, 0.2% l-glutamine, 0.05% 2-mercaptoethanol, 0.01% sodium pyruvate, 0.1% HEPES, and 0.1% nonessential amino acids). B16-OVA cells were cultured in complete RPMI medium containing 0.5 mg/ml G418 antibiotic during in vitro propagation.
Monoclonal antibodies and reagents
Anti-CD16/CD32, and FITC, PE, APC, and cychrome-conjugated monoclonal antibodies were purchased from BD Pharmingen (San Diego, CA, USA). Gp10025–33 (EGSRNQDWL and KVPRNQDWL) melanoma peptides and SIINFEKL OVA peptide (all are from American Peptide Company, Inc., Sunnyvale, CA, USA) were dissolved in 10% DMSO (Sigma, St. Louis, MO, USA) and diluted in PBS. Recombinant mouse IL-12 and IL-2 were purchased from R and D Systems (Minneapolis, MN, USA). Cytokines were stored as a lyophilized powder at −20°C, and reconstituted immediately prior to use in 0.1% bovine serum albumin in PBS. CTX (Sigma) was reconstituted in PBS and stored at 4°C until used.
In vitro T cell activation
Spleen, peripheral and mesenteric lymph nodes (LNs) from OT-1 or Pmel-1 transgenic mice were harvested and homogenized. Single cell suspensions of OT-1 or pmel-1 cells were cultured at a density of 1 × 106 cells/ml with 1 μg/ml SIINFEKL or gp10025–33, respectively, for 3 days (unless specified otherwise) in the presence or absence of 10 ng/ml IL-12 or IL-2. When indicated, cells were washed and recultured in fresh complete RPMI at a density of 1 × 106 cells/ml with or without addition of 10 ng/ml IL-12.
Surface staining, flow cytometry and cell sorting
At the indicated time points, cells were harvested and washed in FACS buffer (0.5% BSA, 0.02% sodium azide in PBS). Cultured cells were incubated with the indicated flurochrome-conjugated mAbs for 30 min. Stained cells were washed and resuspended in 300 μl of FACS buffer. Cells were acquired and analyzed by flow cytometry using CellQuest™ software (BD Biosciences). For sorting CD62Llow and CD62Lhigh OT-1 cells, cells were harvested in 0.1% BSA in PBS and stained with mAbs against Va2, CD8, and CD62L. After staining cells were washed twice with 0.1% BSA in PBS and passed through a cell strainer (40 μM diameter). Cells were acquired and sorted using a FACSAria™ instrument (BD Biosciences).
Detection of apoptotic death
Apoptosis of in vitro activated OT-1 cells was determined by annexin-V binding following manufacture’s instructions (BD Pharmingen). At the indicated time points, cells were harvested and stained for surface markers Vα2 and CD62L. After staining, cells were washed in annexin-V binding buffer (BD Pharmingen) and resuspended in 100 μl of annexin buffer containing 5 μl of annexin V-FITC. Cells were incubated at room temperature for 15 min. After incubation, 200 μl of annexin binding buffer was added to each sample. Cells were analyzed by flow cytometry and data were analyzed using CellQuest™ software (BD Biosciences).
Adoptive transfer and in vivo anti-tumor activity
OT-1 or Pmel-1 cells were activated in vitro with the relevant peptide and treated with or without 10 ng/ml IL-12 as described above. Naïve C57BL/6 Ly5.2 mice received 1 × 106 or when indicated 5 × 106 cells by lateral tail-vein injection. In vivo survival of the transferred OT-1 cells was determined by flow cytometry analysis of Vα2 and Ly5.1 expression on OT-1 cells in PBL samples at the indicated time points. For anti-tumor activity mice were challenged with 5 × 105 B16-OVA or 2.5 × 105 B16 cells by subcutaneous injection. One week after tumor challenge, mice received 1 or 5 × 106 OT-1 or Pmel-1 cells treated as indicated. Mice were monitored frequently for tumor growth. Mice with tumors exceeding 300 mm2 were humanely euthanized.
Results
Antiapoptotic effects of in vitro priming with IL-12 resulted in an increased frequency of OT-1+CD62Lhi T-cells
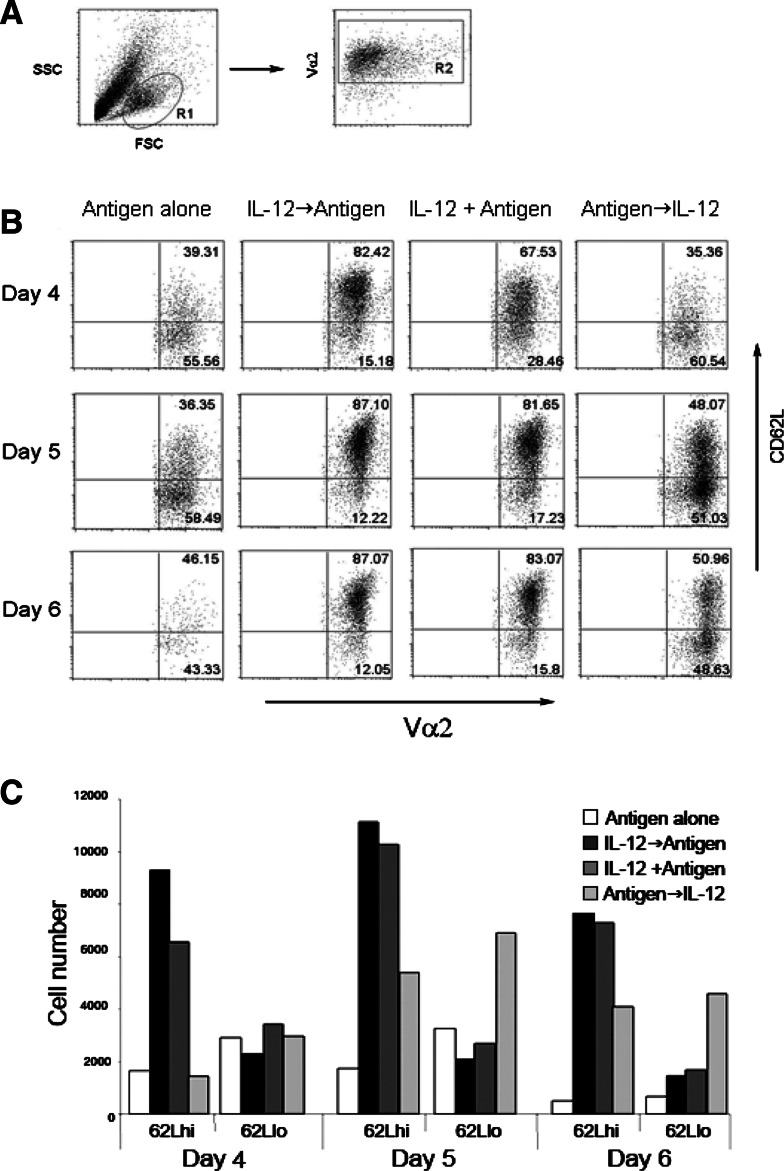
It has been reported that addition of IL-12 during antigen priming rescues cells from activation induced cell death [4, 15, 30, 36]. Because anti-tumor efficacy varies significantly with the state of T cell differentiation [10, 13, 17], it is important to determine the phenotypic and functional characteristics of the resultant population. Since expression of CD62L on activated CD8+ T cells has been associated with superior anti-tumor efficacy [10, 13], we determined the sate of differentiation of the resulting population based on the expression of CD62L. Spleen and LN suspensions from OT-1 mice were primed with SIINFEKL. To determine the optimal timing of IL-12 effect on naïve CD8+ T cells, cell suspensions were either: (1) pre-treated overnight with 10 ng/ml of IL-12 then washed and primed with antigen (IL-12→antigen), (2) primed with antigen and IL-12 simultaneously (IL-12 + antigen), (3) primed with antigen overnight and then treated with IL-12 (antigen→IL-12), or (4) primed with antigen alone. On day 3 after priming, approximately 95% of cells were both CD8+ and OVA specific as determined by the expression of Vα2 (data not shown). Cells were washed to remove other factors that might influence the resulting population generated in response to IL-12 treatment. Expression of CD62L was determined by flow cytometry gating on viable antigen experienced CD8+ T cells (Fig. 1a, upper panel) on day 4, 5 and 6 post-priming (Fig. 1b). As previously described, antigen priming resulted in the gradual down-regulation of CD62L as cells became more activated. In contrast, addition of IL-12, either before or during antigen priming, resulted in the accumulation (approximately 80%) of cells expressing high levels of CD62L (Fig. 1a, lower panel). This accumulation of CD62LhiOT-1+ T cells was sustained for at least 6 days after priming. By sharp contrast, there was a marked decrease in both CD62Llow and CD62Lhi OT-1 cells primed in the absence of IL-12, presumably secondary to activation induced cell death (Fig. 1b). As far as timing is concerned, addition of IL-12 either before or during antigen priming, and to a lesser extent after priming, resulted in significant increases in the percentages of viable CD62LhiOT-1+ T cells when compared with the cells primed with antigen alone. Thus, the influence of IL-12 on activated CD8+ T cells resulted in the accumulation of cells expressing high levels of CD62L.
Fig. 1.
Expression of CD62L on CD8+ T cells primed in the presence of IL-12. Spleen and LN suspensions (1 × 106 cells/ml) from OT-1 mice were primed with 1 ug/ml SIINFEKL. Cell suspensions were either: (1) pretreated overnight with 10 ng/ml of IL-12 and then washed and primed with antigen (IL-12→antigen), (2) primed with antigen and IL-12 simultaneously (IL-12 + antigen), (3) primed with antigen overnight and then treated with IL-12 (antigen→IL-12), or (4) primed with antigen alone. Three days after priming cells were re-cultured at a density of 1 × 106 cells/ml. Cells were harvested and expression of CD62L was determined by flow cytometry on Vα2 (antigen specific transgenic cells) on days 4, 5, and 6 after priming (a). Absolute numbers were calculated for each experimental group (b) and were expressed as total cell number
IL-12 selectively rescued OT-1+CD62Lhi cells from activation induced apoptosis
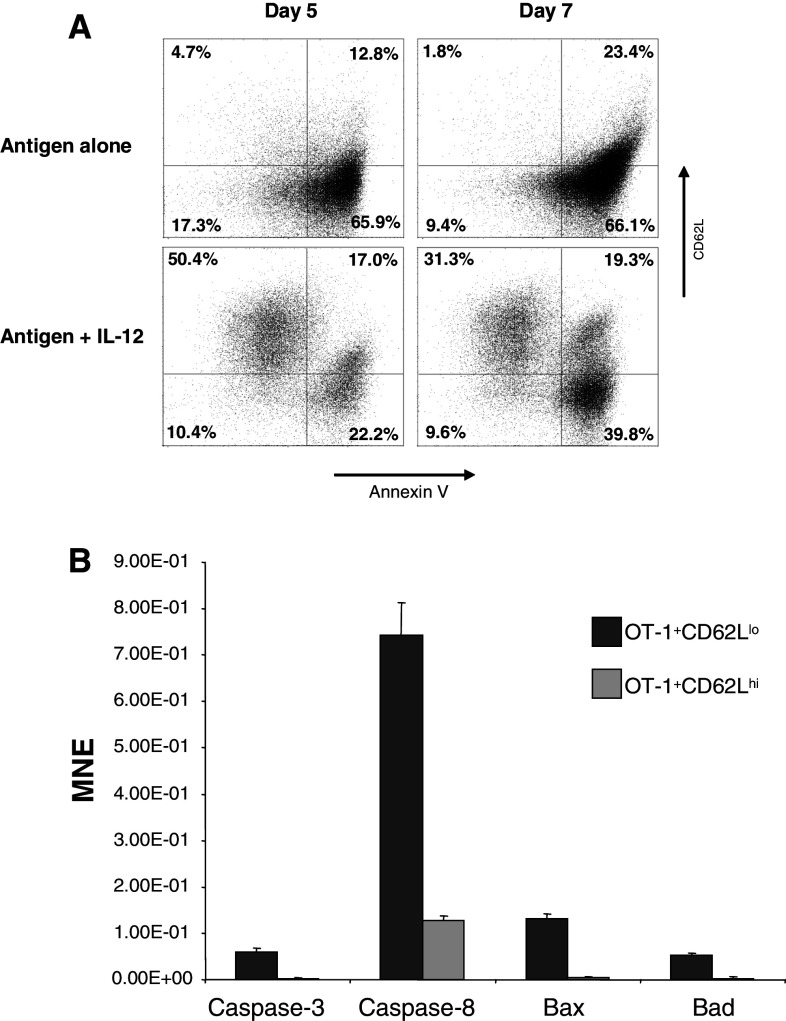
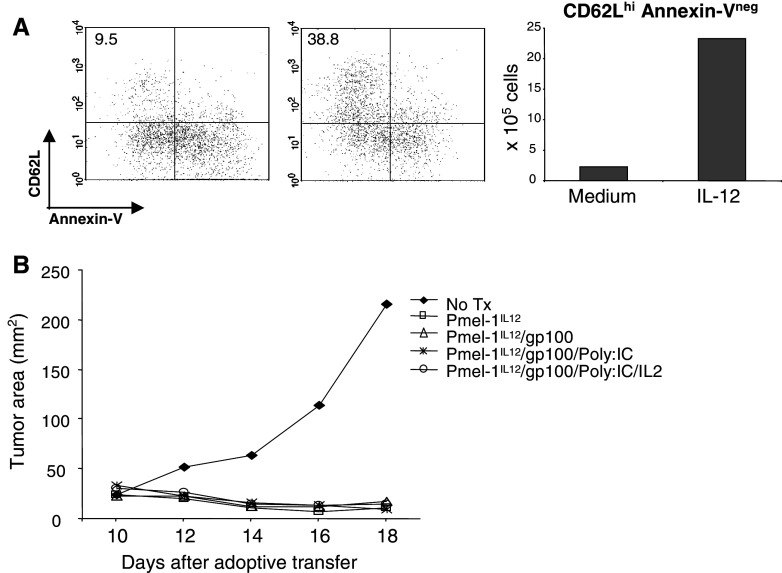
It has been reported that IL-12 enhances CD8+ T cell primary expansion by reducing death rather than by promoting cell division [4]. To determine whether the anti-apoptotic effect of IL-12 was selective for CD8+ T cells expressing high levels of CD62L spleen and LN cell suspensions from OT-1 mice were primed with 1 μg/ml of SIINFEKL with or without 1 ng/ml of IL-12. Cells were harvested on days 5 and 7 after priming and stained with a mixture of mAbs against Vα2, CD62L and then cell death was analyzed by annexin-V staining. Cells positive for Vα2 were assayed for expression of CD62L and annexin-V binding. As expected, the vast majority of cells activated with antigen only were annexin V positive, indicative of activation induced cell death (Fig. 2a upper panels). By sharp contrast, activated cells in the presence of IL-12 were more resistant to activation induced cell death (Fig. 2a lower panels). The surviving population after 5 (61%) or 7 (41%) days of priming was mainly composed of cells expressing high levels of CD62L. Approximately 50% of total cells by day 5 and 30% by day 7, which in both cases represent about 80% of the surviving population. To confirm these findings, OT-1 spleen suspensions primed in the presence of IL-12 were sorted on day 3 post-priming into two populations: Vα2+ CD62Llo and Vα2+CD62Lhi (Fig. 2a). Cells were re-cultured at a density of 1 × 106 cells/ml and harvested 5 days after priming. Expression of pro-apoptotic mediators such as caspase 3 and 8, Bax and Bad was determined by real time RTPCR. Levels of expression of all four mediators tested was found to be increased in OT-1 cells expressing low levels of CD62L (Fig. 2b). Thus, confirming that the anti-apoptotic effect of IL-12 is specific for activated CD8+ T cells expressing high levels of CD62L and is associated with down-regulation of pro-apoptotic mediators.
Fig. 2.
In vitro anti-apoptotic effect of IL-12 on CD8+ CD62Lhi cells. OT-1 cells were primed with or without 10 ng/ml of IL-12. Cells were harvested at day 5 and 7 after priming and stained with a mixture of mAbs against Vα2, CD62L and Annexin-V. Cells positive for Vα2 were assayed for CD62L and annexin-V binding (a). Expression of caspase-3, caspase-8, Bax, and Bad was determined on sorted OT-1+CD62Llo/IL-12 or OT-1+CD62Lhi/IL-12 at day-5 after priming by real-time PCR (b). Results are presented as the mean normalized expression which is the number of transcripts per 103 copies of β-actin ± SEM
IL-12 modulated the expression of CD62L in antigen primed CD8 T cells
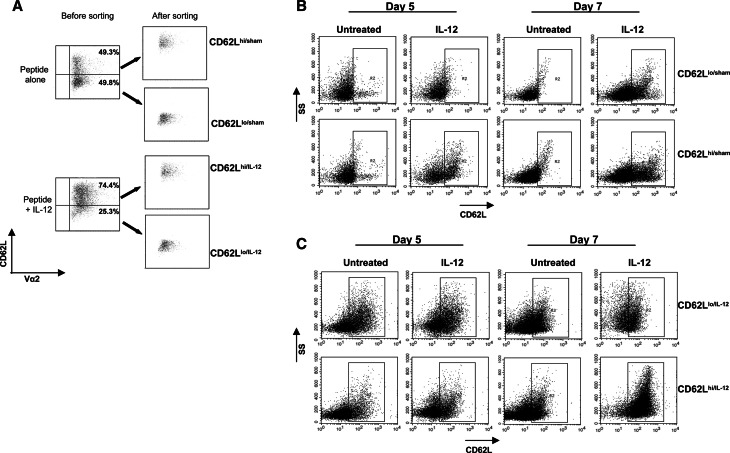
It is known that IL-12 modulates the expression of CD62L on CD4+ T cells [37]. To determine whether IL-12 also regulates the expression of CD62L on activated CD8+ T cells, spleen and LN cell suspensions from OT-1 mice were primed with SIINFEKL with or without IL-12. Three days after priming, IL-12 or sham treated cells were sorted into CD62LloVα2+ or CD62LhiVα2+ populations and recultured in the presence or absence of 10 ng/ml IL-12 and harvested 2 and 4 days later. Consistent with the results shown in Fig. 1, in vitro priming in the presence of IL-12 resulted in an increased frequency of OT-1+ CD62Lhi cells when compared with cells primed with antigen alone (Fig. 3a). Sorted OT-1+ CD62Llo/sham or OT-1+ CD62Lhi/sham (Fig. 3a) were re-cultured in the presence or absence of IL-12. Re-culture of OT-1+ CD62Llo/sham in the presence of IL-12 resulted in enhanced expression of CD62L that occurred at day 7 after priming rather than at day 5 (Fig. 3b upper panels). OT-1+ CD62Lhi/sham re-cultured in the absence of IL-12 resulted in the loss of CD62L expression. Interestingly addition of IL-12 to the same cell fraction was able to maintain a higher frequency of OT-1+ CD62Lhi cells (Fig. 3b lower panels). These findings suggest that IL-12 not only selectively rescues CD8+ CD62Lhi cells from apoptosis, but is also able to induce the expression of CD62L on CD8+ CD62Llo cells. Interestingly, the timing of IL-12 exposure affected the timing of CD62L upregulation, perhaps as the result of the kinetics of CD62L expression during the differentiation of CD8+ cells to a memory phenotype. Because the sorted population was very pure (≈90%), these findings also suggest that the effects of IL-12 are likely exerted directly on CD8+ cells. To corroborate these findings OT-1 cells primed in the presence of IL-12 were also sorted into CD62LloVα2+ or CD62LhiVα2+ populations. As in Fig. 3b, sorted populations were re-cultured with or without IL-12, and expression of CD62L was analyzed at days 5 and 7 after priming. Surprisingly, a significant fraction of OT-1+ CD62Llo/IL-12 cells regained expression of CD62L, which was maintained until day 7 regardless of the addition of IL-12 during re-culturing (Fig. 3c upper panels). In contrast, only 25% of OT-1+ CD62Lhi/IL-12 cells maintained high levels of CD62L expression when recultured without IL-12. Interestingly, the addition of IL-12 at the time of re-culture induced CD62L expression in all OT-1 cells (Fig. 3c lower panels). Together, these findings suggest that that IL-12 modulates the expression of CD62L.
Fig. 3.
IL-12 modulated the expression of CD62L on in vitro activated OT-1 cells. OT-1 cells primed with or without 10 ng/ml of IL-12 were cultured for 3 days and sorted into CD8+ CD62Llo/sham, CD8+ CD62Lhi/sham, CD8+ CD62Llo/IL-12 or CD8+ CD62Lhi/IL-12 (a). Sham-treated (b) or IL-12-treated (c) cells were re-cultured with or without IL-12 at a cell density of 1 × 106/ml. Expression of CD62L was analyzed by flow cytometry at days 5 and 7 after priming (days 2 and 4 after reculture)
OT-1+CD62Lhi/IL-12 showed an “early effector/TCM-like” phenotype
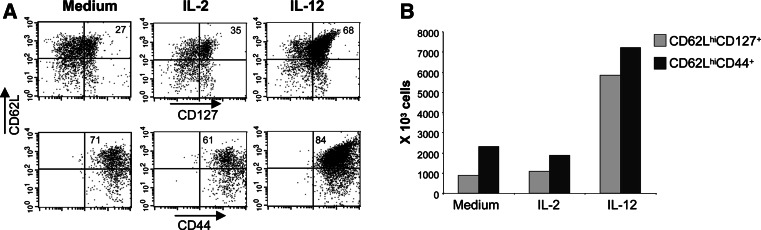
To further understand the effect of antigen priming in the presence of IL-12 on the phenotypic characteristics of the resulting population, OT-1IL-12 or OT-1sham cells were generated as described above, and on day 7 after antigen priming the expression of CD127 (IL-7R α-chain) and CD44 was determined by flow cytometry. CD127 expression has been described as a marker for memory cells because of the requirement for IL-7 in the homoeostatic maintenance of memory cells in the absence of antigen [27]. Although CD127 is also expressed in naïve CD8+ T cells, the kinetics of its expression follows that of CD62L, with that being a downregulation as activation progresses, with the subsequent upregulation only in memory cells [2]. Phenotypic polarization by IL-12 was compared to untreated and CD8+ T cells primed in the presence of IL-2. Culture of activated CD8+ T cells in the presence of IL-2 is known to result in polarization to an effector memory (TEM)-like phenotype, which is characterized by low expression levels of CD62L[16, 39]. Figure 4a shows that all OT-1 splenocytes expressed high levels of CD44, indicating that the cells had become antigen experienced (Fig. 4a). OT-1 cells primed in the presence of IL-12 showed greater frequency of cells expressing both CD62L and CD127 than cells primed in the presence of IL-2 (Fig. 4a). This polarization towards “early effector/TCM-like” phenotype was more evident after determining the absolute number of cells. Priming in the presence of IL-12, resulted in a 6 and 7 fold increase in CD62LhiCD127+ and in CD62LhiCD44+, respectively, relative to priming in the presence of IL-2 (Fig. 4b).
Fig. 4.
In vitro IL-12 conditioning induces “Early effector/TCM-like” phenotype of OT-1+CD62Lhi/IL-12 cells. OT-1 cells were primed in vitro with 1 μg/ml SIINFEKL. Ten ng/ml of IL-2 or IL-12 was added at the time of priming. Cells were cultured for 3 days, harvested, washed and recultured at a density of 1 × 106 cells/ml. At day-7 after priming cells were harvested and stained with mAbs against Vα2, CD62L, CD127 and CD44. Percentages (a) and absolute number (b) of CD62LhiCD127+ and CD62LhiCD44+ cells were determined
OT-1+CD62Lhi/IL-12 cells had greater in vivo survival and recall response than OT-1+CD62Lhi/sham
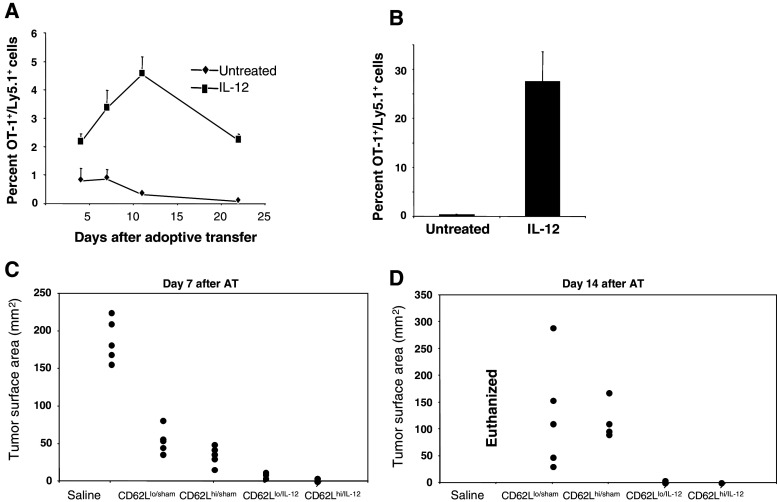
To determine whether expression of CD62L was the only requirement for in vivo survival, a model for homeostatic proliferation was utilized. Sorted OT-1+CD62Lhi/IL-12 and OT-1+CD62Lhi/sham cells were adoptively transferred to mice previously treated with 100 μg of cyclophosphamide. The presence of donor cells (Ly5.1+) was monitored in the peripheral blood at several time points. Frequencies of OT-1+CD62Lhi/sham cells were less that 1% at day 5 after adoptive transfer, and were almost undetectable by day 22 (Fig. 5a). By sharp contrast, the frequencies of OT-1+CD62Lhi/IL-12 cells were significantly higher, reaching a maximum level by day 10 after adoptive transfer (Fig. 5a). To determine whether the recall capabilities were also affected by priming in the presence of IL-12, the same mice were vaccinated with 100 μg of SIINFEKL at day 22 post-adoptive transfer. The percentages of donor cells in peripheral blood were determined 3 days after vaccination. Figure 5b shows a striking difference in the recall capability between OT-1+CD62Lhi/IL-12 and OT-1+CD62Lhi/sham cells. About 25% of the total circulating lymphocytes were donor OT-1+CD62Lhi/IL-12 cells, in contrast to about 1% of OT-1+CD62Lhi/sham cells. These results suggest that expression of CD62L alone at the time of transfer is not sufficient to ensure survival and hence Ag recall responses.
Fig. 5.
In vivo survival, recall potential and anti-tumor activity of OT-1 cells primed in vitro in the presence of IL-12. OT-1+Ly5.1+ cells were primed with 1 μg/ml of SIINFEKL with or without 10 ng/ml of IL-12 and cultured for 3 days. After culture cells were sorted into CD62Lhi/sham or CD62Lhi/IL-12 cells and adoptively transferred (1 × 106 cells/mice) into recipient wild type Ly5.2 mice (n = 4 mice) previously treated with CTX. The percentage of Ly5.1+ cells in the peripheral blood was determined at the indicated time points (a). The same mice were vaccinated at day-22 post-transfer and frequency of donor cells (Ly5.1+) in the periphery was determined 3 days after vaccination (b). Wild type C57BL/6 mice were injected s.c with 5 × 105 B16-OVA cells. Tumors were allowed to grow for 7 days. Mice received sorted CD8+CD62Lhi/IL-12, CD8+CD62Llo/IL-12, CD8+CD62Lhi/sham, CD8+CD62Llo/sham or saline. Tumor size was determined at days 7 (C) and 14 (D) after transfer. Tumor size is shown as the surface area in mm2
Antigen priming in the presence of IL-12 resulted in enhanced eradication of previously established solid tumors
To better understand which subpopulation had better anti-tumor activity in vivo, sorted OT-1+CD62Lhi/IL-12, OT-1+CD62Llo/IL-12, OT-1+CD62Lhi/sham, OT-1+CD62Llo/sham cells were adoptively transferred to recipient mice bearing tumors from B16-OVA cells. At day 7 after transfer, partial responses were observed in mice injected with both CD62Lhi or CD62Llo sham treated OT-1 cells. Near complete and complete tumor responses were observed in the groups that received CD62Llo/IL-12 or CD62Lhi/IL-12 OT-1 cells, respectively (Fig. 5c). At day 14 after transfer, disease progression was observed in both groups that received OT-1 sham treated cells. In contrast, groups that received OT-1 cells primed in the presence of IL-12 maintained their complete response (Fig. 5d). Interestingly, the complete responses observed after OT-1IL-12 cell transfer were independent of the expression of CD62L at the time of transfer. However, this does not diminish the importance of CD62L expression, since treatment with IL-12 at the time of priming results in late upregulation (day 5 after priming) of CD62L among OT-1+CD62Llo/IL-12 cells (Fig. 3c, upper panels). Furthermore, the fact that disease progression was observed in mice that received OT-1+CD62Lhi/sham corroborates that increased levels of CD62L expression are not sufficient to confer long-lasting anti-tumor protection.
Adoptively transferred Pmel-1IL-12 cells exhibited anti-tumor activity in the absence of vaccination
To determine whether the enhanced anti-tumor activity of OT-1IL-12 cells was also observed in a self tumor antigen model, Pmel-1IL-12 cells were generated. CD8+ T cells from Pmel-1 T cell receptor transgenic mice show specificity for a Db-restricted epitope from the non-mutated self tumor antigen gp10025–33 [20]. Pmel-1IL-12 cells were generated by incubating spleen and lymph node suspensions with 1 μg/ml gp10025–23 peptide and 10 ng/ml of IL-12. On day 3 after priming, cells were washed and recultured at a density of 1 × 106 cells/ml. Expression of CD62L and Annexin-V binding of antigen primed Pmel-1sham or Pmel-1IL-12 was performed on re-cultured cells at day 5 after antigen priming. As seen in OT-1 cells, antigen priming of Pmel-1 cells in the presence of IL-12 resulted in decreased activation induced apoptosis of Pmel-1 cells expressing high levels of CD62L (Fig. 6a). To determine the effect on in vivo anti-tumor responses, 5 × 106 in vitro-generated Pmel-1IL-12 cells were harvested on day 3 and adoptively transferred into mice bearing 7-day old B16 melanoma tumors. Untreated group did not receive adoptive transfer. As indicated, adoptive transfer was followed by different vaccination regimens. Complete responses were observed in all animals treated regardless whether vaccination took place (Fig. 6b). These findings are remarkable because of the self-nature of the gp100 antigen. Objective responses seen with the Pmel-1 model require a vaccination regimen that is usually comprised of peptide and systemic IL-2. Our findings suggest that in vitro preconditioning with IL-12 at the time of antigen priming can significantly enhance the reversal of functional tolerance associated with non-mutated “self antigens” resulting in efficient anti-tumor activity.
Fig. 6.
Anti-tumor activity of Pmel-1IL-12 cells in the absence of vaccination. Spleen and LN suspensions from Pmel-1 mice were cultured for 3 days with 1 μg/ml gp10025–33 peptide with or without 10 ng/ml of IL-12. After culture cells were washed and recultured at a density of 1 × 106 cells/ml (a) or transfused i.v. (1 or 5 × 106 cells/mouse) to mice bearing 10-day old B16 tumors (b). Recultured cells were harvested at day 5 after priming and stained with antibodies against Vβ13 and CD62L and Annexin-V. Annexin binding and CD62L expression of Vβ13+ cells was analyzed by flow cytometry. Percentages and absolute number of non-apoptotic cells expressing high levels of CD62L was determined (a). B16-bearing mice were adoptively transferred (7 days after tumor challenge) with 1 × 106 of Pmel-1IL12 cells and the tumor size was determined at the indicated time points, and it is shown as the surface area in mm2 (b)
Discussion
In this study, we demonstrate that priming of naïve CD8+ T cells in the presence of IL-12 results in the persistence of a subpopulation that exhibits superior anti-tumor capabilities when adoptively transferred to tumor-bearing hosts. IL-12 is well documented as a potent modulator of immune responses [22, 29, 38]. In vitro or in vivo treatment with IL-12 results in enhanced activity of T and NK cells which is characterized by increased secretion of IFN-γ [34]. Such enhanced immune responses are in part the result of the direct stimulation of antigen presenting cells. A previous study by our group has shown that paracrine administration of IL-12 was capable of increasing the frequencies of dendritic cells in both spleen and peripheral blood, and enhanced the functional capability of spleen dendritic cells to present peptide to naïve CD8+ T cells [25]. IL-12 can also directly affect the development of CD8+ T cell-mediated responses. It is known that optimal clonal expansion and acquisition of effector function requires a third signal that can be provided by IL-12, and that in the absence of third signal, tolerance can occur [6, 7, 29, 30, 35, 36]. In support of these findings, Chang et al. reported that IL-12 priming at the time of antigenic stimulation increased the primary expansion of CD8+ T cells by reducing cell death, rather that by increasing cell proliferation, resulting in a larger CD8+ T cell memory pool [4]. The ability of IL-12 to enhance proliferation of activated CD8+ T cells has been recently associated with enhanced expression of IL-2Rα in response to IL-12R engagement [35]. IL-12R expression is upregulated upon TCR activation [9], however low levels of expression have been reported in resting NK cells, dendritic cells and B cell lines [1, 12]. Historically, the expression of IL-12R was confined to activated Th1 cells [23, 32], however the pivotal role of IL-12 in providing third signal for the clonal expansion of naïve CD8+ T cells, demonstrates a similar pattern of expression on activated CD8+ T cells [15, 19, 35, 36].
Altogether, it appears that the anti-tumor effects of IL-12 directly stem from its immune-enhancing effects. However, the clinical application of systemic IL-12 has been hindered by considerable toxicities [3, 5]. Several approaches to circumvent IL-12-related toxicities include the use of viral, plasmid, polymer or liposome-based delivery systems [24]. In vitro conditioning of isolated T cells with IL-12 would potentially offer an additional alternative approach. In fact, it has been reported that culture of CD8+ T cells in the presence of IL-12 improved their anti-tumor capability in murine models for leukemia and CEA-expressing malignancies [8, 18]. However the phenotypic characteristics of the resulting populations were not addressed. To our knowledge, this is the first report to link the enhanced antitumor activity of IL-12-preconditioned CD8+ T cell subpopulation to increased levels of CD62L expression. A similar effect has been reported for IL-15 in which a polarization towards a less differentiated phenotype expressing high levels of CD62L occurs after in vitro priming, such polarization resulted in superior anti-tumor activity [10].
We also showed evidence for a direct effect of IL-12 on the expression of CD62L on activated CD8+ T cells. Sorted OT-1+ CD62Llo/sham cells re-cultured in the presence of IL-12 upregulated the expression of CD62L, and sorted OT-1+ CD62Llo/IL-12 re-cultured in the absence of IL-12 regained CD62L expression (Fig. 1). Thus it seems that the programming signaling triggered by IL-12 at the time of priming affects the expression of CD62L. Although higher levels of CD62L expression can be obtained by continuous exposure to IL-12, initial exposure during priming or even after priming is sufficient to enhance the expression of CD62L among activated CD8+ T cells (Fig. 1). Thus direct modulation of CD62L could represent a mechanism by which IL-12 enhances the activity of CD8+ T cells.
The physiological contributions of CD62L expression on enhanced anti-tumor activity are thought to relate to the ability of T cells to migrate to lymph nodes, where further stimulation can take place. Tumor studies in mice lacking peripheral lymphoid structures (LTα−/− mice) or CD62L showed sub-optimal anti-tumor responses after ACT [10, 17]. Interestingly, in our study, adoptive transfer of sorted OT-1sham cells expressing high levels of CD62L resulted in tumor relapse, suggesting that the mere capability of CD8+ T cells to home to lymph nodes is not sufficient for long-lasting T-cell mediated anti-tumor responses. As such, there must be additional phenotypic and functional characteristics induced by IL-12 priming which positively affect the functional capability of this T cell subset such as the modulation of regulators of apoptosis. Thus, effective strategies to generate competent CD8+ T cells for adoptive transfer should promote not only the generation of key subpopulations with superior anti-tumor activity, but also the maintenance of their viability in vivo. Our results suggest that antigen priming in the presence of IL-12 accomplishes both ends. It not only directly modulates the expression of CD62L on activated CD8+ T cells, but also selectively promotes the survival of activated CD8+ T cells expressing high levels of CD62L.
Enhancing the activity of anti-tumor CD8+ T cells could contribute to the reversal of functional tolerance. Under functional tolerance, both tumor-specific T cells and tumor cells coexist. The Pmel-1 model closely mimics functional tolerance to antigens from the gp100 enzyme [21]. In this model, the mere presence of an overwhelming frequency of gp100 specific CD8+ T cells (>95% of all CD8+ T cells) is not sufficient to delay tumor growth [21]. Furthermore, in order to reverse functional tolerance and obtain significant anti-tumor activity, adoptively transferred Pmel-1 cells, even in high numbers, need to be further stimulated with vaccination and systemic IL-2 [20]. Our results suggest that priming in the presence of IL-12 significantly reverses the functional tolerance of Pmel-1 cells (Fig. 6b). Pmel-1IL-12 cells were able to prevent the growth of B16 tumors even in the absence of vaccination and systemic IL-2. These findings would suggest that functional tolerance could be reversed ex vivo, obviating the need for systemic interventions that can result in adverse toxicities. Teague, et al., have shown that in vitro IL-15-induced proliferation abrogated tolerance of CD8+ T cells, and that the rescued cells became effective in treating leukemia [33].
In conclusion, in vitro conditioning with cytokines prior to adoptive transfer could prove effective in the programming of CD8+ T cells for effective immunotherapy and warrant further investigation in human subjects. Particularly for IL-12, in vitro conditioning prior to ACT is an attractive strategy to exploit its potent immune-enhancing capabilities without the detrimental toxic side effects.
Acknowledgments
This work was supported by the National Institutes of Health Grant 1 R01 CA94856-01. We thank Dr. Yian Chen and Elizabeth Little for technical assistance.
References
- 1.Airoldi I, Gri G, Marshall JD, Corcione A, Facchetti P, Guglielmino R, Trinchieri G, Pistoia V. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J Immunol. 2000;165:6880. doi: 10.4049/jimmunol.165.12.6880. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 3.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27:58. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- 4.Chang J, Cho JH, Lee SW, Choi SY, Ha SJ, Sung YC. IL-12 priming during in vitro antigenic stimulation changes properties of CD8 T cells and increases generation of effector and memory cells. J Immunol. 2004;172:2818. doi: 10.4049/jimmunol.172.5.2818. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 6.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 7.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emtage PC, Clarke D, Gonzalo-Daganzo R, Junghans RP. Generating potent Th1/Tc1 T cell adoptive immunotherapy doses using human IL-12: harnessing the immunomodulatory potential of IL-12 without the in vivo-associated toxicity. J Immunother. 2003;26:97. doi: 10.1097/00002371-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 10.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev. 2006;6:383. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315. doi: 10.1016/S1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 13.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down-regulation of homing receptors after T cell activation. J Immunol. 1988;141:4110. [PubMed] [Google Scholar]
- 15.Kieper WC, Prlic M, Schmidt CS, Mescher MF, Jameson SC. Il-12 enhances CD8 T cell homeostatic expansion. J Immunol. 2001;166:5515. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macgregor JN, Li Q, Chang AE, Braun TM, Hughes DP, McDonagh KT. Ex vivo culture with interleukin (IL)-12 improves CD8(+) T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-gamma but requires perforin. Cancer Res. 2006;66:4913. doi: 10.1158/0008-5472.CAN-05-3507. [DOI] [PubMed] [Google Scholar]
- 19.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 20.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portielje JE, Gratama JW, van Ojik HH, Stoter G, Kruit WH. IL-12: a promising adjuvant for cancer vaccination. Cancer Immunol Immunother. 2003;52:133. doi: 10.1007/s00262-002-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem ML, Gillanders WE, Kadima AN, El-Naggar S, Rubinstein MP, Demcheva M, Vournakis JN, Cole DJ. Review: novel nonviral delivery approaches for interleukin-12 protein and gene systems: curbing toxicity and enhancing adjuvant activity. J Interferon Cytokine Res. 2006;26:593. doi: 10.1089/jir.2006.26.593. [DOI] [PubMed] [Google Scholar]
- 25.Salem ML, Kadima AN, Zhou Y, Nguyen CL, Rubinstein MP, Demcheva M, Vournakis JN, Cole DJ, Gillanders WE. Paracrine release of IL-12 stimulates IFN-gamma production and dramatically enhances the antigen-specific T cell response after vaccination with a novel peptide-based cancer vaccine. J Immunol. 2004;172:5159. doi: 10.4049/jimmunol.172.9.5159. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 27.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 28.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev. 2003;3:269. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J Immunol. 1999;163:2561. [PubMed] [Google Scholar]
- 30.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168:5521. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 31.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 32.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Rev. 2003;3:133. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 35.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002;169:6842. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 36.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol. 2005;174:600. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- 37.van Wely CA, Beverley PC, Brett SJ, Britten CJ, Tite JP. Expression of l-selectin on Th1 cells is regulated by IL-12. J Immunol. 1999;163:1214. [PubMed] [Google Scholar]
- 38.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361. doi: 10.1016/S1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 39.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]