Abstract
The metabolic syndrome is common after liver transplant being present in approximately half of recipients. It has been associated with adverse outcomes such as progression of hepatitis C and major vascular events. As the United States population ages and the rate of obesity increases, prevention of the metabolic syndrome in the post-transplant population deserves special consideration. Currently, the metabolic syndrome after transplant appears at least two times more common than observed rates in the general population. Specific guidelines for patients after transplant does not exist, therefore prevention rests upon knowledge of risk factors and the presence of modifiable elements. The current article will focus on risk factors for the development of the metabolic syndrome after transplant, will highlight potentially modifiable factors and propose potential areas for intervention. As in the non-transplant population, behavioral choices might have a major role. Opportunities exist in this regard for health prevention studies incorporating lifestyle changes. Other factors such as the need for immunosuppression, and the changing characteristics of wait listed patients are not modifiable, but are important to know in order to identify persons at higher risk. Although immunosuppression after transplant is unavoidable, the contribution of different agents to the development of components of the metabolic syndrome is also discussed. Ultimately, an increased risk of the metabolic syndrome after transplant is likely unavoidable, however, there are many opportunities to reduce the prevalence.
Keywords: Liver transplantation, Diabetes mellitus, Dyslipidemias, Hypertension, Metabolic syndrome X, Obesity, Hypertension, Immunosuppression
INTRODUCTION
Liver transplantation is a life saving and life changing procedure for patients with advanced chronic liver disease, hepatocellular carcinoma and acute liver failure. The exceptional liver transplant recipient has normal fitness[1] and can literally climb mountains[2]. However, the more typical patient has impaired fitness[3], gains weight[4-6] and develops the metabolic syndrome[7-10]. In fact, the prevalence of the posttransplant metabolic syndrome (PTMS) ranges from 44%-58%[7-10], much higher than the 23% observed in the United States population[11]. Is the high prevalence of the metabolic syndrome after liver transplant an unavoidable consequence driven by factors such as immunosuppression? Or is it a reflection of behavioral tendencies in liver transplant recipients that can be modified to prevent illness? Although a definite conclusion is not likely, data can be found to support both possibilities. After a brief overview of the scope of the problem, the current manuscript will examine the factors which contribute to the PTMS and potential means of prevention.
PROBLEM DEFINED
The National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III)[12] defined the components of the metabolic syndrome is as follows; (1) impaired fasting glucose (≥ 100 mg/dL); (2) Abdominal obesity (> 102 cm in men, > 88 cm in women); (3) hypertriglyceridemia (≥ 150 mg/dL or drug therapy for triglycerides); (4) low levels of high density lipoprotein (HDL) (< 40 mg/dL in men, < 50 mg/dL in women or drug treatment for low HDL); and (5) elevated blood pressure (≥ 130/85 mmHg or drug treatment for hypertension). The presence of 3 or more of these components defines the metabolic syndrome. Complications of advanced liver disease can confound the diagnosis of the metabolic syndrome in the pretransplant setting. The presence of ascites alters waist circumference. Vasodilation and decreased effective circulating volume found with portal hypertension results in lowered systemic blood pressure. Synthetic dysfunction observed with end stages of liver disease immediately prior to transplant can result in lowered serum glucose and lipid values. A study evaluating the metabolic syndrome prior to liver transplantation found a rate of 5.4%[10]. Pretransplant rates for hypertension were 9%-19%[8-10,13], diabetes, 10%-22%[7-10,13], dyslipidemia, 3%-43%[9,10] and obesity, 11%-38%[7,10].
Studies of the metabolic syndrome after transplant are summarized in Table 1. The rate of the metabolic syndrome may be underestimated in some reports as diabetes is substituted for impaired fasting glucose, hypertension is substituted for elevated blood pressure and body mass index (BMI) is substituted for elevated waist circumference. The presence of the metabolic syndrome after transplant was associated with more rapid progression of hepatitis C[8] and an increased rates of major vascular events[9,10]. Although no prospective data are available, it would be reasonable to expect that the metabolic syndrome might contribute to graft loss and patient death.
Table 1.
Studies examining the prevalence of the metabolic syndrome after liver transplant
| Study | Number subjects (n) | Metabolic syndrome (%) | Elevated glucose (%) | Elevated blood pressure (%) | Increased waist circumference (%) | Dyslipidemia (%) | Adverse outcome reported with metabolic syndrome |
| Bianchi et al[7] | 296 | 45 | 60 | 53 | 32 | 37 increased TG | Not studied |
| 50 low HDL | |||||||
| Laryea et al[9] | 118 | 58 | 61 (diabetes) | 62 (HTN) | 36 (BMI > 30 kg/m2) | 45 increased TG | More cardiovascular events |
| 48 low HDL | |||||||
| Hanouneh et al[8] | 82 | 50 | 52 (diabetes) | 64 (HTN) | 45 (BMI > 28 kg/m2) | Not reported | Increased fibrosis with HCV |
| Kallwitz et al[88] | 172 | 65 | 68 | Not reported | 53 | 42 increased TG | More cardiovascular events |
| Laish et al[10] | 252 | 52 | 40 (diabetes) | 58 (HTN) | 31 | 47 increased TG | More cardiovascular events |
| 49 increased HDL |
HTN: Hypertension; TG: Triglycerides; HDL: High density lipoprotein; BMI: Body mass index; HCV: Hepatitis C virus.
PRETRANSPLANT RISK FACTORS
The transplant population has been changing with regard to indication and recipient characteristics. Hepatitis C is declining as an indication for transplant while non-alcoholic fatty liver disease (NAFLD) is increasing and the average age of transplant candidates is rising. With these changes, risk factors for the metabolic syndrome are becoming more common in liver transplant candidates and are important predictors of PTMS development. Although studies evaluating risk factors to predict PTMS are relatively small, certain factors identified were consistent across multiple series. A summary of risk factors is presented in Table 2. Obesity before transplant is a key factor in predicting the metabolic syndrome after transplant. Both pretransplant weight[9] and BMI[7,10,14] were correlated with post transplant metabolic syndrome. Not surprisingly, persons who were obese prior to transplant were often obese after transplant[7]. The rate of obesity in wait-listed patients varies by transplant indication. Persons awaiting transplant with cryptogenic cirrhosis, which often results from NAFLD, were found to be more commonly obese than age and gender matched controls[15]. NAFLD has increased annually as an indication for liver transplantation and is projected to become the most common indication for transplantation over the next 10-20 years[16,17]. Review of Scientific Registry of Transplant Recipients data showed recipients characterized as other/unknown causes, which includes NAFLD, has steadily increased in the past 10 years currently representing 23.6% of recipients, almost equaling the percentage of recipients with hepatitis C[18]. The proportion of obese persons awaiting transplant will presumably increase with the indication for transplant changing to a higher proportion of recipients with NAFLD. Compounding the effect of obesity, transplant for cryptogenic cirrhosis was found to be a risk factor for PTMS when controlling for other factors including pretransplant BMI[9,10].
Table 2.
Factors associated with the metabolic syndrome after transplant
| Pretransplant risk factors | Posttransplant risk factors |
| Weight[9] | Change in BMI[7] |
| Body mass index[7,10,14] | |
| Cryptogenic cirrhosis[9,10] | |
| Alcohol cirrhosis[9] | |
| Hepatitis C cirrhosis[9] | |
| Pretransplant diabetes[7,10,14] | |
| Age[9,10] | |
| Triglycerides[10] | |
| High density lipoprotein[10] |
BMI: Body mass index.
Other risk factors for the PTMS have been identified. Pretransplant diabetes was found to predict PTMS in multiple series[7,10,14]. In fact, in one study, persons with pretransplant diabetes had nearly a 6 fold higher odds of having the metabolic syndrome after transplant[10]. Age was additionally predictive of the metabolic syndrome after transplant[9,10]. This is particularly important as the recipient population in the United States is aging. In 2009, nearly 75% of transplant recipients were above the age of 50, compared to 1993 where only 42% of recipients were 50 years of age or greater[18]. Other pretransplant factors that were associated with the development of the metabolic syndrome after transplant in at least one series include hypertriglyceridemia[10], low HDL[10] and transplantation for hepatitis C or alcohol cirrhosis[9].
A ROLE FOR PREVENTION PRIOR TO TRANSPLANT?
A preventive intervention could target persons with chronic liver disease, before they require transplant. The long term benefit of weight loss in an obese patient with cirrhosis is not known. In limited series from weight loss surgery, fibrosis improvement in cirrhotic patients after weight loss was inconsistent[19-21]. However, in selected persons with compensated cirrhosis, diet and weight loss are reasonable recommendations. The potential for a weight loss intervention in reducing future need for transplant in obese persons with cirrhosis is an area in need of study. An exercise program in persons with advanced cirrhosis awaiting transplant may have additional benefits. Sarcopenia[22] and decreased functional capacity measured by a six-minute walk test[23] was associated with mortality in persons with cirrhosis. Clearly, increasing physical activity in cirrhotic patients at various degrees of synthetic dyfunction has potential utility and deserves further study.
Existing data indicate that as liver disease progresses to the point of needing transplant, participation in lifestyle modification becomes increasingly limited by malnutrition, muscle loss and reduced exercise capacity. Protein calorie malnutrition is common, and occurs more frequently with advanced liver disease[24]. In cirrhosis, a shift toward a catabolic state with utilization of stored fat as a primary energy source during rest and exercise has been observed[25,26]. The catabolic state induced by an overnight fast in a cirrhotic patient is roughly equivalent to a 36 h fast in a healthy subject[27]. In a state of protein malnutrition, there is no rationale for a calorie restricted diet in a patient that is potentially requiring transplant. Alterations in physical activity are additionally vital for clinically significant weight loss, with a recent recommendation of greater than 250 min/wk[28]. There are limitations for cirrhotic patients to achieve this goal. In health-related quality of life measures, persons awaiting liver transplant reported decreased scores relating to physical functioning[29]. Oxygen consumption during peak exercise is often severely impaired in liver transplant candidates, and is correlated with disease severity[30]. Additionally, cirrhotic patients develop anaerobic metabolism at low work loads, an effect that also becomes more pronounced as liver disease progresses[31]. Studies have shown decreased muscle mass[22] and strength[31] in cirrhotic patients which may further limit the ability to exercise. The increase in portal pressure which can occur during exercise could potentially increase the risk of complications such as variceal bleeding is another barrier to exercise[32].
POST TRANSPLANT RISK FACTORS FOR METABOLIC SYNDROME
As obesity prior to transplant is a risk factor for PTMS, it is intuitive that weight gain after transplant might predict the metabolic syndrome. However, data are mixed. The overall change in BMI after transplant was associated with the metabolic syndrome in one series[7] but not in another[9]. It is possible that ascites pre transplant may have resulted in the underestimation of weight gain post transplant in the later series. Weight gain after transplant is well described, however. In one series, the proportion of overweight and obese persons after transplant was 57% compared to 38% prior to transplant[7]. In a large series of almost 600 liver transplant recipients, the median weight gain at 1 years and 3 years was 5.1 kg and 9.5 kg, respectively[6]. In studies with longitudinal data, it appears that most weight gain occurs within the first year after liver transplant[5,33,34]. Data from our center echoes these findings and is shown in Figure 1[35]. Weight gain after transplant should be viewed in the context of persons returning to health after illness. Aggregate data from three studies which measured the rate of an elevated wait circumference after transplant totaled 36.9%[7,10,35]. Although the populations are not matched, the absolute number is strikingly similar to the 38.6% rate reported for the United States population[11]. In a study comparing rates of obesity, the standard prevalence ratio was not significantly higher in persons after liver transplant compared to the general United States population[36].
Figure 1.
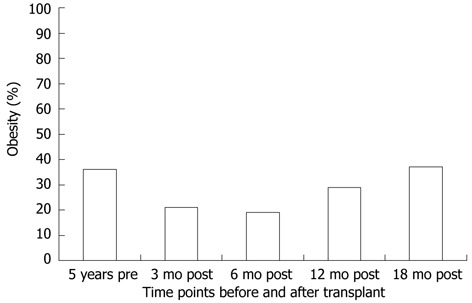
The prevalence of obesity at time points before and after liver transplant.
Despite the weight gain over time after transplant, time since transplant was not associated with the prevalence of PTMS[7,9,10]. This finding suggests factors resulting in PTMS develop soon after transplant and factors in addition to obesity require further scrutiny. Immunosuppression is one such factor. The overall contribution of immunosuppression to the development of the metabolic syndrome after transplant is difficult to measure, since immunosuppression is unavoidable. Although, the choice of calcineurin inhibitor (CNI) was not associated with the development of the metabolic syndrome[7,8], different immunosuppressive agents have been shown to increase risk for various components of the metabolic syndrome. With regard to weight gain after transplant, one series found more weight gain with cyclosporine in the first year[6]. However, the effect was not seen at 2 years. Another series found a higher overall BMI in cyclosporine treated patients, but no difference in rates of elevated waist circumference[7]. Weight loss occurred in a majority of liver transplant recipients after switching to tacrolimus from cyclosporine[37]. Although corticosteroids are often associated with weight gain, this effect after transplant was found in some[5,38], but not all series[6,34].
Post transplant diabetes
When compared to the general population, a higher prevalence of posttransplant diabetes mellitus (PTDM) has been observed in liver transplant recipients[36]. Much of this risk can be related to immunosuppression with both calcineurin inhibitors and steroids playing a major role. Both CNIs have been associated with decreased insulin sensitivity and reduced insulin release[39]. The reduced insulin release might result from CNI induced damage to pancreatic beta cells[40]. Comparing the CNIs, most studies show higher rates of PTDM with tacrolimus use compared to cyclosporine[41-46]. New onset PTDM was found in 27% of recipients on tacrolimus compared to 22% on cyclosporine [hazard ratio (HR) 1.24, 95% confidence interval: 1.07-1.43] in an analysis of over 15 000 recipients from United Network for Organ Sharing data[45]. Switching tacrolimus to cyclosporine was found to improve PTDM[47]. However, the absolute rates may not tell the entire story. Lowered serum levels of tacrolimus were associated with better glycemic control[48]. Corticosteroid use with CNIs has an influence on PTDM rate. Corticosteroid use increases the risk of diabetes through decreasing insulin production and peripheral sensitivity and increasing hepatic gluconeogenesis[49]. Avoidance of corticosteroids and the use of induction therapy played an important role in decreasing rates of PTDM[45,50-57]. In steroid free regimens using tacrolimus and an induction agent, PTDM was seen in less than 10% of recipients[50,53]. Other series showed that patients receiving cyclosporine are more likely to require corticosteroids[38,58]. Tacrolimus was associated with less graft loss and rejection[43] potentially lessening the need for concomitant immunosuppressive agents such as steroids. Further studies are needed to compare rates of PTDM between the two CNIs used with induction therapy.
There are multiple studies examining risk factors in addition to immunosuppression for PTDM. Impaired glucose tolerance prior to transplant consisting of abnormal glucose levels[59,60] or pretransplant diabetes[7,44,61], was shown to be associated with post transplant diabetes. Hepatitis C as the transplant indication was linked to higher rates of PTDM in multiple series[7,41,45,60-63]. Furthermore, eradication of hepatitis C led to improved glycemic control[62]. In addition to hepatitis C, alcohol cirrhosis was additionally associated with PTDM[7,44]. The number of episodes of acute cellular rejection[64,65] and the number of steroid boluses[62] were tied to higher rates of PTDM. Other factors correlated with PTDM include male gender[44,61,66], older age[41,45,67], African American race[45] and a BMI > 25 kg/m2[45,60]. Donor factors including age and diabetes were additional risk factors for PTDM[45]. Insulin resistance after transplant was associated with a pretransplant BMI > 30 kg/m2, older age, increased triglycerides, HCV infection and steroid boluses[10].
Post transplant hypertension
Hypertension is another metabolic component with a higher standardized prevalence ratio in liver transplant recipients compared to the general population[36]. Immunosuppression appears to have a profound effect in this setting. CNIs are known to increase sympathetic tone, result in vasoconstriction and cause sodium dependent volume expansion[68]. Hypertension was observed less often with tacrolimus than with cyclosporine[37,38,56,58,69]. Additionally, changing CNIs from cyclosporine to tacrolimus resulted in decreased rates of hypertension[70,71]. Hypertension is observed with corticosteroid use[49]. However, decreasing rates of hypertension with steroid sparing immunosuppression regimens were seen in a series using cyclosporine[54,57] but not observed in series using tacrolimus[50,51].
Post transplant dyslipidemia
Unlike diabetes and hypertension, the rate of hyperlipidemia was not found to be higher in persons after liver transplant compared to the general population[36]. The definition of hyperlipidemia varied widely in studies and few used NCEP-ATP III definitions for dyslipidemia. This is important as one study found the rate of hypertriglyceridemia to be higher than that of elevated cholesterol after transplant[72]. Although the absolute rate of hyperlipidemia after transplant may not be elevated compared to the general population, various risk factors and choice of immunosuppressive agent may influence the prevalence of hyperlipidemia. Pretransplant hepatocellular disease and posttransplant renal dysfunction were found to be associated with hypertriglyceridemia after transplant[72]. With regard to immunosuppressive agent, cyclosporine was associated with more hyperlipidemia[38,56,69,73,74] and hypertriglyceridemia[38] compared to tacrolimus. Additionally, changing from cyclosporine to tacrolimus improved hyperlipidemia in multiple series[37,70,71]. The reason for this effect with cyclosporine could be related to inhibition of bile salt synthesis resulting in hyperlipidemia[75]. Long term corticosteroid use may additionally contribute to hyperlipidemia[57,76]. Steroid free or sparing regimens were associated with improved lipid levels[52,53], including less hypertriglyceridemia in one series[51]. Sirolimus, an agent used either in conjunction or in place of a CNI, is associated with high rates of dyslipidemia. With sirolimus, dyslipidemia was observed in 55% of patients, the majority of which required therapy[77]. This finding observed with sirolimus use might result from changes in insulin signaling pathways resulting in excess triglyceride production and secretion[78]. Rates of dyslipidemia were less when sirolimus was combined with tacrolimus compared to cyclosporine[79].
In summary, it appears that higher rates of the metabolic syndrome after transplant compared to the general population might be attributed to increased rates of PTDM and hypertension. Although obesity does not appear more prevalent in post transplant populations, weight gain after transplant is pervasive. These findings could lead to a two-fold plan to reduce the metabolic syndrome after transplant. The first would be prevention of excess weight gain through behavioral changes that could start before transplant and continue afterward. The second would be focusing on immunosuppressive strategies to lower rates of diabetes, hypertension and hyperlipidemia after transplant, especially in persons with multiple risk factors present prior to transplant.
PREVENTION AFTER TRANSPLANT
After transplant, patients have an improved functional capacity and can perform tasks independently[80]. The use of a structured exercise program increased exercise capacity and fitness for the first six months after transplant followed by a plateau[81]. The improvement in fitness was three times greater than expected in a sedentary person undergoing a similar training regimen. Multiple studies have shown that although exercise performance improved after transplant, it remained lower than predicted values for age matched controls[3,81,82]. Despite the improvement in fitness and quality of life, many persons after transplant remain sedentary[81,83]. Only a quarter of persons were found to be physically active after transplant, and those that were physically active had less hypertension and decreased BMI[83]. Clearly there is a role for programs dedicated to increase physical activity after transplant. There are little data regarding nutritional composition and caloric intake after transplantation. Up to two thirds of subjects were found to have more than recommended energy intake[84]. Overall, more data is required to assess the contribution of caloric intake to weight gain after transplant.
There is very limited data regarding behavioral changes and exercise therapy after liver transplantation. A single randomized trial evaluated the effects of exercise and dietary counseling after transplantation. An improvement in cardiorespiratory fitness and quality of life was reported in the intervention group, but no changes were noted in body composition or muscle strength[82]. There was a trend toward improved body composition in the 37% of subjects that adhered to the intervention[82]. There are no data regarding the impact of an exercise program on the prevalence of the metabolic syndrome or singular components after transplant. Behavioral therapy after transplant represents an area with vast potential for important research. It is additionally important to note that the primary goal of intervention in this setting should be the prevention of weight gain immediately after transplant as opposed to the treatment of the metabolic syndrome once present.
Additional therapies for weight loss have been tried after transplant. A study using orlistat found a reduction in waist circumference, but not body mass index in a small group of patients[85]. Concern over interference with the absorption of immunosuppression limited the use of this agent. Bariatric surgery at the time of transplant and in the post transplant period was described in case reports[86,87]. However, bariatric surgery should be done in a way that preserves access to the biliary system and to minimize interference with the absorption of immunosuppression. As a result of these obvious pitfalls, bariatric surgery during and after liver transplant has not been widely performed.
CONCLUSION
The transplant population is aging and, consistent with the overall population, becoming more often overweight or obese. Immunosuppression likely contributes to an increased prevalence of the metabolic syndrome in liver transplant recipients, especially through increased rates of diabetes and hypertension. These features make it likely that the metabolic syndrome after transplant will become more prevalent. A number of preventative measures could be considered to reduce the burden of the metabolic syndrome in liver transplant recipients.
Patients should be screened for risk factors for post transplant metabolic syndrome at the time of transplant evaluation. Consistent risk factors include diabetes, obesity and cirrhosis from NAFLD. When assessing obesity, screening for the highest lifetime BMI may be beneficial. Identification of at risk patients will allow special focus on preventive effort after transplant.
Although there are no data supporting a definitive increase in risk resulting from the choice in calcineurin inhibitor, immunosuppression is modifiable. Minimization of corticosteroid use, possibly in conjunction with induction agents, appears to be beneficial in reducing components of the metabolic syndrome. The choice of CNI could be individualized based on the presence of components of the metabolic syndrome prior to transplant. For example, tacrolimus may be a better agent for someone with long standing pretransplant hypertension.
Consideration should be given for early eradication of hepatitis C, especially in those persons at risk for posttransplant diabetes.
Preventative efforts through behavioral changes should be made before and after transplant. At minimum, improved fitness prior to transplant is associated with better survival. After transplant, a multidisciplinary approach with increased activity, diet modifications and behavioral therapy should be explored. Since the metabolic syndrome develops early after transplant, intervention should begin as soon as medically feasible.
Footnotes
Peer reviewers: Dr. Yasuhiko Sugawara, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113, Japan; Salvatore Gruttadauria, Professor, Abdominal Transplant Surgery, Mediterranean Institute for Transplantation and Advanced Specialized Therapies, Via E Tricomi, 90127 Palermo, Italy
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Painter PL, Luetkemeier MJ, Moore GE, Dibble SL, Green GA, Myll JO, Carlson LL. Health-related fitness and quality of life in organ transplant recipients. Transplantation. 1997;64:1795–1800. doi: 10.1097/00007890-199712270-00029. [DOI] [PubMed] [Google Scholar]
- 2.Pirenne J, Van Gelder F, Kharkevitch T, Nevens F, Verslype C, Peetermans WE, Kitade H, Vanhees L, Devos Y, Hauser M, et al. Tolerance of liver transplant patients to strenuous physical activity in high-altitude. Am J Transplant. 2004;4:554–560. doi: 10.1111/j.1600-6143.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson AL, Yoshida EM, Abboud RT, Fradet G, Levy RD. Impaired exercise performance after successful liver transplantation. Transplantation. 2001;72:1161–1164. doi: 10.1097/00007890-200109270-00032. [DOI] [PubMed] [Google Scholar]
- 4.Stegall MD, Everson G, Schroter G, Bilir B, Karrer F, Kam I. Metabolic complications after liver transplantation. Diabetes, hypercholesterolemia, hypertension, and obesity. Transplantation. 1995;60:1057–1060. [PubMed] [Google Scholar]
- 5.Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: incidence and risk factors. Liver Transpl Surg. 1998;4:285–296. doi: 10.1002/lt.500040402. [DOI] [PubMed] [Google Scholar]
- 6.Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461–466. doi: 10.1111/j.1432-2277.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648–1654. doi: 10.1002/lt.21588. [DOI] [PubMed] [Google Scholar]
- 8.Hanouneh IA, Feldstein AE, McCullough AJ, Miller C, Aucejo F, Yerian L, Lopez R, Zein NN. The significance of metabolic syndrome in the setting of recurrent hepatitis C after liver transplantation. Liver Transpl. 2008;14:1287–1293. doi: 10.1002/lt.21524. [DOI] [PubMed] [Google Scholar]
- 9.Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 10.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 12.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 13.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Rebollo ML, Sánchez-Antolín G, García-Pajares F, Fernández-Orcajo P, González-Sagrado M, Cítores-Pascual MA, Velicia-Llames R, Caro-Patón A. Risk of development of the metabolic syndrome after orthotopic liver transplantation. Transplant Proc. 2010;42:663–665. doi: 10.1016/j.transproceed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 16.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12:523–534. doi: 10.1002/lt.20738. [DOI] [PubMed] [Google Scholar]
- 17.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 18.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2010 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation;; 2011. [Google Scholar]
- 19.Kral JG, Thung SN, Biron S, Hould FS, Lebel S, Marceau S, Simard S, Marceau P. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135:48–58. doi: 10.1016/j.surg.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 21.Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, Eid GM, Ramanathan R, Taylor DS, Schauer PR. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610–617; discussion 617-620. doi: 10.1097/01.sla.0000179652.07502.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173, 173.e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, Vargas HE, Douglas DD. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 24.Lautz HU, Selberg O, Körber J, Bürger M, Müller MJ. Protein-calorie malnutrition in liver cirrhosis. Clin Investig. 1992;70:478–486. doi: 10.1007/BF00210228. [DOI] [PubMed] [Google Scholar]
- 25.DeLissio M, Goodyear LJ, Fuller S, Krawitt EL, Devlin JT. Effects of treadmill exercise on fuel metabolism in hepatic cirrhosis. J Appl Physiol. 1991;70:210–215. doi: 10.1152/jappl.1991.70.1.210. [DOI] [PubMed] [Google Scholar]
- 26.Merli M, Riggio O, Romiti A, Ariosto F, Mango L, Pinto G, Savioli M, Capocaccia L. Basal energy production rate and substrate use in stable cirrhotic patients. Hepatology. 1990;12:106–112. doi: 10.1002/hep.1840120117. [DOI] [PubMed] [Google Scholar]
- 27.Owen OE, Trapp VE, Reichard GA, Mozzoli MA, Moctezuma J, Paul P, Skutches CL, Boden G. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72:1821–1832. doi: 10.1172/JCI111142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 29.Pieber K, Crevenna R, Nuhr MJ, Quittan M, Peck-Radosavljevic M, Fialka-Moser V, Wiesinger GF. Aerobic capacity, muscle strength and health-related quality of life before and after orthotopic liver transplantation: preliminary data of an Austrian transplantation centre. J Rehabil Med. 2006;38:322–328. doi: 10.1080/16501970600680288. [DOI] [PubMed] [Google Scholar]
- 30.Dharancy S, Lemyze M, Boleslawski E, Neviere R, Declerck N, Canva V, Wallaert B, Mathurin P, Pruvot FR. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86:1077–1083. doi: 10.1097/TP.0b013e318187758b. [DOI] [PubMed] [Google Scholar]
- 31.Wiesinger GF, Quittan M, Zimmermann K, Nuhr M, Wichlas M, Bodingbauer M, Asari R, Berlakovich G, Crevenna R, Fialka-Moser V, et al. Physical performance and health-related quality of life in men on a liver transplantation waiting list. J Rehabil Med. 2001;33:260–265. doi: 10.1080/165019701753236446. [DOI] [PubMed] [Google Scholar]
- 32.García-Pagàn JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, Bosch J, Rodés J. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300–1306. doi: 10.1053/gast.1996.v111.pm8898644. [DOI] [PubMed] [Google Scholar]
- 33.Palmer M, Schaffner F, Thung SN. Excessive weight gain after liver transplantation. Transplantation. 1991;51:797–800. doi: 10.1097/00007890-199104000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Wawrzynowicz-Syczewska M, Karpińska E, Jurczyk K, Laurans L, Boroń-Kaczmarska A. Risk factors and dynamics of weight gain in patients after liver transplantation. Ann Transplant. 2009;14:45–50. [PubMed] [Google Scholar]
- 35.TenCate V, Kallwitz ER, Ovrahim K, Mettu PS, Huang Y, Berkes JL, Walzer N, Layden TJ, Cotler SJ. Timing and Correlates of Obesity After Liver Transplantation. Hepatology. 2011;54:A604. [Google Scholar]
- 36.Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, Emre S, Fishbein TM, Guy SR, Schwartz ME, et al. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation. 2000;69:781–789. doi: 10.1097/00007890-200003150-00018. [DOI] [PubMed] [Google Scholar]
- 37.Neal DA, Gimson AE, Gibbs P, Alexander GJ. Beneficial effects of converting liver transplant recipients from cyclosporine to tacrolimus on blood pressure, serum lipids, and weight. Liver Transpl. 2001;7:533–539. doi: 10.1053/jlts.2001.24637. [DOI] [PubMed] [Google Scholar]
- 38.Canzanello VJ, Schwartz L, Taler SJ, Textor SC, Wiesner RH, Porayko MK, Krom RA. Evolution of cardiovascular risk after liver transplantation: a comparison of cyclosporine A and tacrolimus (FK506) Liver Transpl Surg. 1997;3:1–9. doi: 10.1002/lt.500030101. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez LA, Lehmann R, Luzi L, Battezzati A, Angelico MC, Ricordi C, Tzakis A, Alejandro R. The effects of maintenance doses of FK506 versus cyclosporin A on glucose and lipid metabolism after orthotopic liver transplantation. Transplantation. 1999;68:1532–1541. doi: 10.1097/00007890-199911270-00017. [DOI] [PubMed] [Google Scholar]
- 40.Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68:396–402. doi: 10.1097/00007890-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 41.Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004;10:349–355. doi: 10.1002/lt.20092. [DOI] [PubMed] [Google Scholar]
- 42.Levy G, Villamil F, Samuel D, Sanjuan F, Grazi GL, Wu Y, Marotta P, Boillot O, Muehlbacher F, Klintmalm G. Results of lis2t, a multicenter, randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus with C0 monitoring in de novo liver transplantation. Transplantation. 2004;77:1632–1638. doi: 10.1097/01.tp.0000129095.51031.42. [DOI] [PubMed] [Google Scholar]
- 43.McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6:1578–1585. doi: 10.1111/j.1600-6143.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 44.Tueche SG. Diabetes mellitus after liver transplant new etiologic clues and cornerstones for understanding. Transplant Proc. 2003;35:1466–1468. doi: 10.1016/s0041-1345(03)00528-1. [DOI] [PubMed] [Google Scholar]
- 45.Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation. 2010;89:1134–1140. doi: 10.1097/TP.0b013e3181d2fec1. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-Pérez B, Aranda Narváez JM, Santoyo Santoyo J, Fernández-Aguilar JL, Suárez Muñoz MA, González-Sánchez AJ, Pérez Daga JA, Ramírez Plaza CP, Carrasco Campos J, Jiménez Mazure C, et al. Influence of immunosuppression and effect of hepatitis C virus on new onset of diabetes mellitus in liver transplant recipients. Transplant Proc. 2008;40:2994–2996. doi: 10.1016/j.transproceed.2008.08.116. [DOI] [PubMed] [Google Scholar]
- 47.Emre S, Genyk Y, Schluger LK, Fishbein TM, Guy SR, Sheiner PA, Schwartz ME, Miller CM. Treatment of tacrolimus-related adverse effects by conversion to cyclosporine in liver transplant recipients. Transpl Int. 2000;13:73–78. doi: 10.1007/s001470050012. [DOI] [PubMed] [Google Scholar]
- 48.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25:583–592. doi: 10.2337/diacare.25.3.583. [DOI] [PubMed] [Google Scholar]
- 49.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 50.Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, Madariaga J, Selvaggi G, Levi D, Ruiz P, et al. Steroid-free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Transplant Proc. 2005;37:1217–1219. doi: 10.1016/j.transproceed.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 51.Moench C, Barreiros AP, Schuchmann M, Bittinger F, Thiesen J, Hommel G, Kraemer I, Otto G. Tacrolimus monotherapy without steroids after liver transplantation--a prospective randomized double-blinded placebo-controlled trial. Am J Transplant. 2007;7:1616–1623. doi: 10.1111/j.1600-6143.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- 52.Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, Montgomery RA, Cameron AM, Maley WR. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]
- 53.Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, Trunecka P, Krawczyk M, Clavien PA, Ducerf C, et al. Corticosteroid-free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transpl. 2005;11:61–67. doi: 10.1002/lt.20307. [DOI] [PubMed] [Google Scholar]
- 54.Lladó L, Xiol X, Figueras J, Ramos E, Memba R, Serrano T, Torras J, Garcia-Gil A, Gonzalez-Pinto I, Castellote J, et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: results from a prospective multicenter randomized study. J Hepatol. 2006;44:710–716. doi: 10.1016/j.jhep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Tisone G, Angelico M, Vennarecci G, Palmieri G, Buonomo O, Negrini S, Casciani CU. Metabolic findings after liver transplantation within a randomised trial with or without steroids. Transplant Proc. 1998;30:1447–1448. doi: 10.1016/s0041-1345(98)00310-8. [DOI] [PubMed] [Google Scholar]
- 56.Stegall MD, Wachs ME, Everson G, Steinberg T, Bilir B, Shrestha R, Karrer F, Kam I. Prednisone withdrawal 14 days after liver transplantation with mycophenolate: a prospective trial of cyclosporine and tacrolimus. Transplantation. 1997;64:1755–1760. doi: 10.1097/00007890-199712270-00023. [DOI] [PubMed] [Google Scholar]
- 57.Stegall MD, Everson GT, Schroter G, Karrer F, Bilir B, Sternberg T, Shrestha R, Wachs M, Kam I. Prednisone withdrawal late after adult liver transplantation reduces diabetes, hypertension, and hypercholesterolemia without causing graft loss. Hepatology. 1997;25:173–177. doi: 10.1002/hep.510250132. [DOI] [PubMed] [Google Scholar]
- 58.Williams R, Neuhaus P, Bismuth H, McMaster P, Pichlmayr R, Calne R, Otto G, Groth C. Two-year data from the European multicentre tacrolimus (FK506) liver study. Transpl Int. 1996;9 Suppl 1:S144–S150. doi: 10.1007/978-3-662-00818-8_36. [DOI] [PubMed] [Google Scholar]
- 59.Trail KC, McCashland TM, Larsen JL, Heffron TG, Stratta RJ, Langnas AN, Fox IJ, Zetterman RK, Donovan JP, Sorrell MF, et al. Morbidity in patients with posttransplant diabetes mellitus following orthotopic liver transplantation. Liver Transpl Surg. 1996;2:276–283. doi: 10.1002/lt.500020405. [DOI] [PubMed] [Google Scholar]
- 60.Saliba F, Lakehal M, Pageaux GP, Roche B, Vanlemmens C, Duvoux C, Dumortier J, Salamé E, Calmus Y, Maugendre D. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection: an observational multicenter study. Liver Transpl. 2007;13:136–144. doi: 10.1002/lt.21010. [DOI] [PubMed] [Google Scholar]
- 61.Bigam DL, Pennington JJ, Carpentier A, Wanless IR, Hemming AW, Croxford R, Greig PD, Lilly LB, Heathcote JE, Levy GA, et al. Hepatitis C-related cirrhosis: a predictor of diabetes after liver transplantation. Hepatology. 2000;32:87–90. doi: 10.1053/jhep.2000.8270. [DOI] [PubMed] [Google Scholar]
- 62.Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, Tolkoff-Rubin N, Pascual M. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066–1072. doi: 10.1097/00007890-200109270-00015. [DOI] [PubMed] [Google Scholar]
- 63.AlDosary AA, Ramji AS, Elliott TG, Sirrs SM, Thompson DM, Erb SR, Steinbrecher UP, Yoshida EM. Post-liver transplantation diabetes mellitus: an association with hepatitis C. Liver Transpl. 2002;8:356–361. doi: 10.1053/jlts.2002.31745. [DOI] [PubMed] [Google Scholar]
- 64.Navasa M, Bustamante J, Marroni C, González E, Andreu H, Esmatjes E, García-Valdecasas JC, Grande L, Cirera I, Rimola A, et al. Diabetes mellitus after liver transplantation: prevalence and predictive factors. J Hepatol. 1996;25:64–71. doi: 10.1016/s0168-8278(96)80329-1. [DOI] [PubMed] [Google Scholar]
- 65.John PR, Thuluvath PJ. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl. 2002;8:708–713. doi: 10.1053/jlts.2002.34638. [DOI] [PubMed] [Google Scholar]
- 66.Saab S, Shpaner A, Zhao Y, Brito I, Durazo F, Han S, Farmer DG, Ghobrial RM, Yersiz H, Goldstein LI, et al. Prevalence and risk factors for diabetes mellitus in moderate term survivors of liver transplantation. Am J Transplant. 2006;6:1890–1895. doi: 10.1111/j.1600-6143.2006.01385.x. [DOI] [PubMed] [Google Scholar]
- 67.Samuelson AL, Lee M, Kamal A, Keeffe EB, Ahmed A. Diabetes mellitus increases the risk of mortality following liver transplantation independent of MELD score. Dig Dis Sci. 2010;55:2089–2094. doi: 10.1007/s10620-010-1267-5. [DOI] [PubMed] [Google Scholar]
- 68.Curtis JJ. Hypertensinogenic mechanism of the calcineurin inhibitors. Curr Hypertens Rep. 2002;4:377–380. doi: 10.1007/s11906-002-0067-5. [DOI] [PubMed] [Google Scholar]
- 69.Rabkin JM, Corless CL, Rosen HR, Olyaei AJ. Immunosuppression impact on long-term cardiovascular complications after liver transplantation. Am J Surg. 2002;183:595–599. doi: 10.1016/s0002-9610(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 70.Manzarbeitia C, Reich DJ, Rothstein KD, Braitman LE, Levin S, Munoz SJ. Tacrolimus conversion improves hyperlipidemic states in stable liver transplant recipients. Liver Transpl. 2001;7:93–99. doi: 10.1053/jlts.2001.21289. [DOI] [PubMed] [Google Scholar]
- 71.Pratschke J, Neuhaus R, Tullius SG, Haller GW, Jonas S, Steinmueller T, Bechstein WO, Neuhaus P. Treatment of cyclosporine-related adverse effects by conversion to tacrolimus after liver transplantation. Transplantation. 1997;64:938–940. doi: 10.1097/00007890-199709270-00029. [DOI] [PubMed] [Google Scholar]
- 72.Gisbert C, Prieto M, Berenguer M, Bretó M, Carrasco D, de Juan M, Mir J, Berenguer J. Hyperlipidemia in liver transplant recipients: prevalence and risk factors. Liver Transpl Surg. 1997;3:416–422. doi: 10.1002/lt.500030409. [DOI] [PubMed] [Google Scholar]
- 73.Charco R, Cantarell C, Vargas V, Capdevila L, Lázaro JL, Hidalgo E, Murio E, Margarit C. Serum cholesterol changes in long-term survivors of liver transplantation: a comparison between cyclosporine and tacrolimus therapy. Liver Transpl Surg. 1999;5:204–208. doi: 10.1002/lt.500050303. [DOI] [PubMed] [Google Scholar]
- 74.Guckelberger O, Bechstein WO, Neuhaus R, Luesebrink R, Lemmens HP, Kratschmer B, Jonas S, Neuhaus PL. Cardiovascular risk factors in long-term follow-up after orthotopic liver transplantation. Clin Transplant. 1997;11:60–65. [PubMed] [Google Scholar]
- 75.Hulzebos CV, Bijleveld CM, Stellaard F, Kuipers F, Fidler V, Slooff MJ, Peeters PM, Sauer PJ, Verkade HJ. Cyclosporine A-induced reduction of bile salt synthesis associated with increased plasma lipids in children after liver transplantation. Liver Transpl. 2004;10:872–880. doi: 10.1002/lt.20168. [DOI] [PubMed] [Google Scholar]
- 76.Fernández-Miranda C, de la Calle A, Morales JM, Guijarro C, Aranda JL, Gómez-Sanz R, Gómez-Izquierdo T, Larumbe S, Moreno E, Rodicio JL, et al. Lipoprotein abnormalities in long-term stable liver and renal transplanted patients. A comparative study. Clin Transplant. 1998;12:136–141. [PubMed] [Google Scholar]
- 77.Neff GW, Montalbano M, Tzakis AG. Ten years of sirolimus therapy in orthotopic liver transplant recipients. Transplant Proc. 2003;35:209S–216S. doi: 10.1016/s0041-1345(03)00217-3. [DOI] [PubMed] [Google Scholar]
- 78.Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S, Kahan BD. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170–1180. [PubMed] [Google Scholar]
- 79.Trotter JF, Wachs ME, Trouillot TE, Bak T, Kugelmas M, Kam I, Everson G. Dyslipidemia during sirolimus therapy in liver transplant recipients occurs with concomitant cyclosporine but not tacrolimus. Liver Transpl. 2001;7:401–408. doi: 10.1053/jlts.2001.23916. [DOI] [PubMed] [Google Scholar]
- 80.Robinson LR, Switala J, Tarter RE, Nicholas JJ. Functional outcome after liver transplantation: a preliminary report. Arch Phys Med Rehabil. 1990;71:426–427. [PubMed] [Google Scholar]
- 81.Beyer N, Aadahl M, Strange B, Kirkegaard P, Hansen BA, Mohr T, Kjaer M. Improved physical performance after orthotopic liver transplantation. Liver Transpl Surg. 1999;5:301–309. doi: 10.1002/lt.500050406. [DOI] [PubMed] [Google Scholar]
- 82.Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, Painter PL. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6:1896–1905. doi: 10.1111/j.1600-6143.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 83.Painter P, Krasnoff J, Paul SM, Ascher NL. Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl. 2001;7:213–219. doi: 10.1053/jlts.2001.22184. [DOI] [PubMed] [Google Scholar]
- 84.Roske AE, Plauth M. Liver transplantation, body composition, and substrate utilization: does organ transplantation normalize the metabolic situation of the patient? Nutrition. 1999;15:504–505. doi: 10.1016/s0899-9007(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 85.Cassiman D, Roelants M, Vandenplas G, Van der Merwe SW, Mertens A, Libbrecht L, Verslype C, Fevery J, Aerts R, Pirenne J, et al. Orlistat treatment is safe in overweight and obese liver transplant recipients: a prospective, open label trial. Transpl Int. 2006;19:1000–1005. doi: 10.1111/j.1432-2277.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 86.Butte JM, Devaud N, Jarufe NP, Boza C, Pérez G, Torres J, Pérez-Ayuso RM, Arrese M, Martínez J. Sleeve gastrectomy as treatment for severe obesity after orthotopic liver transplantation. Obes Surg. 2007;17:1517–1519. doi: 10.1007/s11695-008-9432-z. [DOI] [PubMed] [Google Scholar]
- 87.Campsen J, Zimmerman M, Shoen J, Wachs M, Bak T, Mandell MS, Kam I. Adjustable gastric banding in a morbidly obese patient during liver transplantation. Obes Surg. 2008;18:1625–1627. doi: 10.1007/s11695-008-9633-5. [DOI] [PubMed] [Google Scholar]
- 88.Kallwitz ER, TenCate V, Mettu PS, Koyhnw N, Berkes JL, Layden TJ, Cotler SJ. Elevated Serum Creatinine and the Metabolic Syndrome are Associated with Major Vascular Events After Liver Transplantation. Hepatology. 2011;54:A574. [Google Scholar]


