Abstract
AIM: To assess vitamin D (Vit D) abnormalities in hepatitis C infected patients and their relationship with interleukin (IL)-17, IL-23 and N-terminal propeptide of type III pro-collagen (PIIINP) as immune response mediators.
METHODS: The study was conducted on 50 Egyptian patients (36 male, 14 female) with hepatitis C virus (HCV) infection, who visited the Hepatology Outpatient Clinic in the Endemic Disease Hospital at Cairo University. Patients were compared with 25 age- and sex-matched healthy individuals. Inclusion criteria were based on a history of liver disease with HCV genotype 4 (HCV-4) infection (as new patients or under follow-up). Based on ultrasonography, patients were classified into four subgroups; 14 with bright hepatomegaly; 11 with perihepatic fibrosis; 11 with hepatic cirrhosis; and 14 with cirrhosis and hepatocellular carcinoma (HCC). Total Vit D (i.e., 25-OH-Vit D) and active Vit D [i.e., 1,25-(OH)2-Vit D] assays were carried out using commercial kits. IL-17, IL-23 and PIIINP levels were assayed using enzyme linked immunosorbent assay kits, while HCV virus was measured by quantitative and qualitative polymerase chain reaction.
RESULTS: Levels of Vit D and its active form were significantly lower in advanced liver disease (hepatic cirrhosis and/or carcinoma) patients, compared to those with bright hepatomegaly and perihepatic fibrosis. IL-17, IL-23 and PIIINP levels were markedly increased in HCV patients and correlated with the progression of hepatic damage. The decrease in Vit D and active Vit D was concomitant with an increase in viral load, as well as levels of IL-17, IL-23 and PIIINP among all subgroups of HCV-infected patients, compared to normal healthy controls. A significant negative correlation was evident between active Vit D and each of IL-17, IL-23 and PIIINP (r = -0.679, -0.801 and -0.920 at P < 0.001, respectively). HCV-infected men and women showed no differences with respect to Vit D levels. The viral load was negatively correlated with Vit D and active Vit D (r = -0.084 and -0.846 at P < 0.001, respectively), and positively correlated with IL-17, IL-23 and PIIINP (r = 0.951, 0.922 and 0.94 at P < 0.001, respectively). Whether the deficiency in Vit D was related to HCV-induced chronic liver disease or was a predisposing factor for a higher viral load remains to be elucidated.
CONCLUSION: The negative correlations between Vit D and IL-17, IL-23 and PIIINP highlight their involvement in the immune response in patients with HCV-4-related liver diseases in Egypt.
Keywords: Vitamin D, Interleukin-17, Interleukin-23, N-terminal propeptide of type III pro-collagen, Hepatitis genotype 4
INTRODUCTION
Hepatitis C virus genotype 4 (HCV-4) is the most common variant of hepatitis C virus (HCV) in the Middle East and Africa, particularly Egypt. This region has the highest prevalence of HCV worldwide, with > 90% of infections due to HCV-4, which is considered a major cause of chronic hepatitis, liver cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation in the country[1]. HCV-4 has recently spread beyond its strongholds in Africa and the Middle East to several western countries, particularly in Europe, due to variations in population structure, immigration and routes of transmission. However, the features of this genotype and management strategies for patients infected with this genotype are not well developed[2].
HCV is remarkably efficient at establishing persistent infections. This suggests that HCV has evolved one or more strategies to evade the host immune response, among which are effects upon T-lymphocyte responses, including interferon (IFN)-γ production; documented by the severely suppressed T-lymphocyte responses in patients with chronic HCV infections[3].
Vitamin D (Vit D) is a critical regulator of immunity, playing a role in both innate and cell-mediated immune responses[4]. Vit D suppresses production of T-helper (Th)-1 lymphocyte type cytokines, such as IFN-γ and interleukin (IL)-2, and consequently leads to an enhanced production of Th-2 cytokines, such as IL-4 and IL-5, thereby promoting humoral immune responses. Vit D also endorses innate immunity by directly inducing gene expression of antimicrobial peptides, such as cathelicidin and β-defensin 2, in various human cell types[5-8]. Vit D deficiency has been shown to associate several immune-mediated diseases, as well as to increase susceptibility to both infections and cancer. Specifically, a 25(OH)-Vit D concentration < 50 nmol/L (i.e., 20 ng/mL) is accepted as a marker of deficiency, whereas a concentration of 51-74 nmol/L (21-29 ng/mL) indicates insufficiency[8,9].
Chronic hepatic cirrhotic patients with HCV genotype 1 have low serum 25(OH)-Vit D levels, and a low Vit D status is linked to severe fibrosis and a low sustained virological response (SVR) during IFN-α-based therapy[10,11]. Moreover, there is interesting preliminary data that indicate that 1,25-(OH)2 Vit D suppresses Th-17 driven cytokine responses and the differentiation and maturation of B lymphocytes, while it induces formation/activation of T-regulatory lymphocytes, stimulates IL-4 production (Th-2), and enhances natural-killer-T-cell functions[12,13].
It has also been shown that treatment with Vit D receptor (VDR) agonists inhibits T-lymphocyte production of IL-17, which is a potent mediator of delayed-type reactions. It achieves this effect (in a manner similar to IFNγ) by elevating chemokine production in various tissues that, in turn, leads to recruitment of monocytes and neutrophils to the site of inflammation. Furthermore, IL-17 acts synergistically with tumor necrosis factor (TNF)-α and IL-1[14] to stimulate immune functions, and its production is sustained by IL-23, an IL-12 family member, the latter of which is strongly inhibited by VDR agonists[15]. IL-23, in conjunction with IL-6 and transforming growth factor (TGF)-β, also stimulate the differentiation of Th-17 cells with the subsequent production of IL-17[16].
The aim of the work was to assess Vit D status in HCV-4-infected patients and its relationship to levels of IL-17 and IL-23, as well as the N-terminal propeptide of type III pro-collagen (PIIINP), the direct serologic marker of collagen turnover, as immuno-inflammatory mediators.
MATERIALS AND METHODS
Subjects
Prior to initiation, this study received approval by the Ethical Committee of the Faculty of Medicine, Cairo University. The study recruited 50 patients with HCV-related chronic liver disease (minimum duration of 7 years; group I) who visited the Hepatology Outpatient Clinic in the Endemic Disease Hospital at Cairo University. Inclusion criteria were based on a history of liver disease with HCV-4 infection (as new patients or under follow-up). Patients with hepatitis B virus (HBV) or co-infection with HBV and human immunodeficiency virus were excluded. All included patients underwent tests for liver function and abdominal ultrasonography, and were tested for the presence of HCV antibodies. When fulfilled, the investigated patients included 36 men and 14 women, ranging in age from 30 to 55 years (mean age = 42.5 years). Twenty-five age- and sex-matched healthy individuals were then recruited as a control group (group II). The controls had normal liver functions and abdominal ultrasonography and negligible HCV antibody levels. Informed consent was obtained from the patients and controls regarding all the procedures. All patients were subjected to a thorough history taking.
Based on ultrasonography results, patients were classified into four subgroups: 14 with bright hepatomegaly; 11 with perihepatic fibrosis; 11 with hepatic cirrhosis; and 14 with cirrhosis and HCC. After subclassification, venous blood samples (5 mL) were obtained (after overnight fasting) from all patients/controls. Samples were allowed to clot and sera were then separated by centrifugation (3500 rpm, 20 min, 25 °C) and then stored at -20 °C until used for analysis of the various parameters outlined below.
Assessment of Vit D, active Vit D, IL-17, IL-23 and PIIINP levels
Total Vit D (i.e., 25-OH-Vit D) assay was carried out using a commercial solid phase radioimmunoassay kit (Medgenix Diagnostics SA Zoning Industrial, Fleurus, Belgium) according to the method of Mawer[17]. The active Vit D (i.e., 1,25-[OH]2-Vit D) assay was carried out according to Hollis[18] using a commercial kit from Incstar Corporation (Stillwater, MN, United States). IL-17, IL-23 and PIIINP levels were assayed using enzyme linked immunosorbent assay kits obtained from Biosource Europe SA (Nivelles, Belgium). The sensitivity of the 25-OH-Vit D, 1,25-(OH)2-Vit D, IL-17, IL-23 and PIIINP kits were 2.4 ng/mL, 5.5 pg/mL, 10 pg/mL, 5 pg/mL, and 10 pg/mL, respectively.
Assessment of HCV levels
Quantitative reverse transcription polymerase chain reaction (RT-PCR) for HCV was done using TaqMan technology according to the method of Scott and Gretch[19], and only HCV-4-infected patients were included in the study. Typically, an RT-PCR has a limit of quantification (LOQ) of 25 IU/mL and a limit of detection (LOD) of 10-15 IU/mL; in the assays used here for HCV-RNA testing, the LOQ was 24 IU/mL and the LOD 12 IU/mL.
For genotyping, HCV type-specific primers designed by Okamoto et al[20] were utilized. Assessments of genotype burdens required three steps: (1) RNA virus was extracted from patient samples using a Tripure Method (Roche, Mannheim, Germany); (2) isolated RNA was converted to cDNA using random hexamers and Moloney Murine Leukemia Virus Reverse Transcriptase enzymes from Promega (Madison, WI, United States); and (3) the product cDNA was amplified using an allele-specific PCR method. The PCR program was set for 1 cycle at 96 °C for 6 min, then for 40 cycles at 95 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min, and a final extension cycle of 72 °C for 10 min. For each patient, two vials containing primer specific for virus types 1a/1b and for 2a and 3a were used. A positive control for each genotype (supplied by the kit manufacturer) was also run in parallel with each set of samples. In addition, HCV viral load was also determined using an Artus Real Art PCR Kit (Qiagen, Valencia, CA, United States). For both assays, a LightCycler® 480 real-time PCR System (Roche) was used. The slope of each reaction was between 3.2 and 3.4, and the error < 0.002. All results of the quantitative HCV analyses were expressed as IU/mL.
Statistical analysis
All data are expressed as mean ± SD. All analyses utilized SPSS for Windows version 15.0 (SPSS, Chicago, IL, United States). Analysis of variance was employed for comparisons of means of the different parameters, with Tukey-Kramer test as the post hoc test. P < 0.05 was accepted as statistically significant. Correlation analyses were done using Pearson’s correlation.
RESULTS
Table 1 demonstrates significantly decreasing levels of Vit D and active Vit D [1,25-(OH)2-Vit D], along with the progressive hepatic state induced by chronic HCV infection, ranging from bright hepatomegaly and perihepatic fibrosis to hepatic cirrhosis and further HCC. Levels of Vit D and active 1,25-(OH)2-Vit D were significantly lower in advanced liver disease (i.e., hepatic cirrhosis and/or carcinoma), compared to bright hepatomegaly and perihepatic fibrosis. A similar pattern was seen for the levels of IL-17, IL-23 and PIIINP and the viral load, for which the levels were significantly elevated in hepatic cirrhosis and/or carcinoma, compared to bright hepatomegaly and perihepatic fibrosis. Only the IL-17, PIIINP and the viral load of patients with HCC were significantly higher than in cirrhotic patients. The decrease in Vit D and active Vit D was concomitant with increases in viral load, as well as levels of IL-17, IL-23 and PIIINP among all subgroups of HCV-infected patients, compared to normal healthy controls.
Table 1.
Levels of vitamin D, its active form, interleukin-17, interleukin-23, N-terminal propeptide of type III pro-collagen and viral load in the four subgroups of hepatitis C virus-infected patients
| Item | Group (Ia) Bright hepatomegaly (n = 14) | Group (Ib) Perihepatic fibrosis (n = 11) | Group (Ic) Liver cirrhosis (n =11) | Group (Id) HCC (n = 14) | Group II Normal (n = 25) |
| Vit D (ng/mL) | 19.80 ± 3.33h | 19.40 ± 3.52h | 10.90 ± 3.74bdh | 9.70 ± 3.88bdh | 39.70 ± 10.80 |
| Active VitD (ng/mL) | 20.60 ± 3.50h | 21.00 ± 3.44h | 13.00 ± 2.10bdh | 11.70 ± 2.52bdh | 41.90 ± 7.90 |
| IL-17 (ng/mL) | 7.60 ± 2.66h | 5.10 ± 2.44h | 115.90 ± 38.70bdh | 150.30 ± 46.80bdfh | 1.20 ± 0.40 |
| IL-23 (ng/mL) | 76.80 ± 14.51h | 51.20 ± 14.60h | 259.30 ± 49.4bdh | 225.90 ± 42.10bdh | 6.70 ± 2.17 |
| Viral load (IU/mL) | 66.30 ± 23.55h | 42.40 ± 9.66h | 165.10 ± 31.40bdh | 231.10 ± 44.60bdfh | 0 ± 0 |
| PIIINP (μg/L) | 91.03 ± 18.99h | 83.88 ± 28.77h | 209.09 ± 31.3bdh | 244.80 ± 34.10bdfh | 22.61 ± 0.54 |
Values shown are means ± SD of data in four subgroups of hepatitis C virus-infected patients and normal controls. b, d, f, h means within lines with no common superscripts differ significantly. As compared with
group (Ia),
group (Ib),
group (Ic),
control (group II) (one-way analysis of variance followed by Tukey-Kramer test), P < 0.01. HCC: Hepatocellular carcinoma; IL: Interleukin.
Vit D insufficiency (21-29 ng/mL) was detected in 14 (28%) HCV patients and three (12%) controls, while Vit D deficiency, defined as serum level < 20 ng/mL, was present in 36 (72%) of the HCV-infected patients and none of the controls. Lastly, Vit D deficiency was seen in all 25 cirrhotic patients and 10 (40%) of the non-cirrhotic HCV-infected patients.
The parameters studied in HCV-infected patients when classified into two subgroups according to sex are shown in Table 2. HCV-infected men and women showed no differences with respect to Vit D levels. There were significant correlations between different parameters in HCV-infected patients and controls. There was a significant negative correlation between Vit D and IL-17, IL-23 and PIIINP. Viral load was negatively correlated with Vit D and active Vit D levels (r = -0.084 and -0.846 at P < 0.001, respectively), and positively correlated with IL-17, IL-23 and PIIINP (r = 0.951, 0.922 and 0.94 at P < 0.001). The significant negative correlations between active Vit D and IL-17 (r = -0.679), IL-23 (r = -0.801), and viral load (r = -0.84), are illustrated in Figure 1.
Table 2.
Vitamin D, its active form, interleukin-17, interleukin-23, N-terminal propeptide of type III pro-collagen and viral load in male and female subgroups of hepatitis C virus-infected patients
| Item | Male group (n = 36) | Female group (n = 14) | P value |
| Vit D (ng/mL) | 10.30 ± 2.12 | 10.00 ± 2.60 | 1.00 |
| Active Vit D (ng/mL) | 12.10 ± 2.80 | 12.40 ± 2.60 | 0.96 |
| Viral load (IU/mL) | 198.00 ± 38.10 | 199.00 ± 31.10 | 0.93 |
| IL-17 (ng/mL) | 133.80 ± 32.50 | 134.90 ± 32.60 | 0.90 |
| IL-23 (ng/mL) | 241.00 ± 28.10 | 243.00 ± 26.50 | 0.92 |
| PIIINP (μg/L) | 226.57 ± 346.54 | 227.94 ± 36.45 | 0.90 |
Values shown are means ± SD of data for both male and female hepatitis C virus-infected subjects. Values shown include group Ic (liver cirrhosis) and Id (hepatocellular carcinoma) subjects. Significant difference is determined at P < 0.05. IL: Interleukin; Vit: Vitamin.
Figure 1.
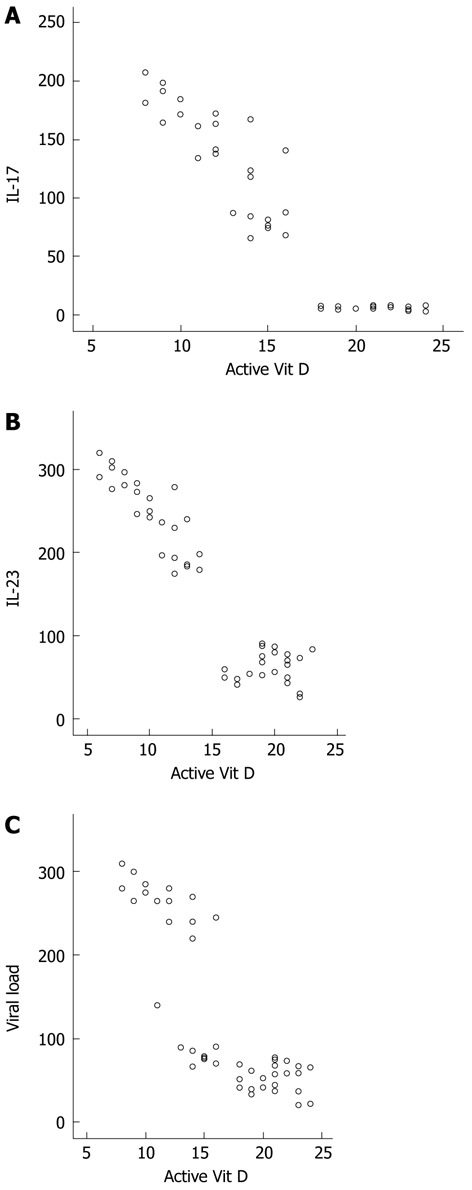
Correlation between active vitamin D levels and interleukin-17, interleukin-23 and viral load. A: Correlation between active Vit D levels and interleukin (IL)-17, revealing a negative correlation (r = -0.679); B: Correlation between active Vit D levels and IL-23, revealing a negative correlation (r = -0.801); C: Correlation between active Vit D levels and viral load, revealing a negative correlation (r = -0.846). Vit: Vitamin.
DISCUSSION
The liver plays a central role in Vit D metabolism, and its inadequacy is common in non-cholestatic chronic liver diseases and correlates with disease severity[21]. The current study showed a significant reduction of Vit D and its active metabolite in HCV-4-infected patients compared to healthy controls. HCV-infected patients were classified according to sonar finding into four groups with progressive hepatic states; bright hepatomegaly and perihepatic fibrosis to hepatic cirrhosis and further HCC. The reduction of the levels of Vit D and its active form was more prevalent and severe in cirrhotic patients (versus non-cirrhotic patients), and much lower in patients with HCC; these differences were each highly significant. This is consistent with previous studies in patients with HCV genotype I, which showed that Vit D deficiency is universal (92%) among patients with chronic liver disease, and at least one-third of the patients have severe Vit D deficiency[21-23].
A significant negative correlation was reported between viral load and Vit D and active Vit D. Vit D is an important immune modulator and preliminary data have indicated associations between Vit D deficiency and failure to achieve SVR rates in HCV patients[24]. It has been reported that the reduced (25-OH Vit D) levels and CYPB27-1260 promoter polymorphism lead to reduced [1,25-(OH)2 Vit D] levels, and are associated with failure to achieve an SVR in patients infected with HCV genotypes 1, 2 or 3[14,25]. The patients in the present study with HCV-4 need further follow-up to confirm the effect of Vit D deficiency upon their responses to treatment.
Furthermore, the present study showed that IL-17 and IL-23 were markedly increased in HCV-infected patients in comparison to controls. The difference in viral load among these groups may explain, in part, the differences noted in levels of inflammatory cytokines in the patients in the current study. Regulation of Th1 and Th17 responses in HCV-infected individuals has previously been studied[25], and it has been reported that TGF-β and IL-6 promote differentiation of naïve murine CD4+ T lymphocytes into IL-17-secreting Th17 lymphocytes. In addition, it has been reported that other innate cytokines, including IL-1, IL-23, TNF-α and IL-21, in different combinations or with TGF-β, are also involved in the differentiation, amplification, or stabilization of the Th17 phenotype[26]. A significant negative correlation between Vit D and both IL-17 and IL-23 was a prominent finding in the current investigation. Previous studies in mice have shown that Vit D is a strong inhibitor of Th17 polarization and Th17 cytokine expression by splenic CD4+ T lymphocytes. Furthermore, Th17 differentiation from naïve T lymphocytes is affected by Vit D. These data imply a regulatory effect on Th17 cells by Vit D, through the reduction of retinoic acid-related orphan receptor (ROR)γt expression[22]. The effect of Vit D on the behavior of Th17 cells has been investigated in different diseases and Vit D suppresses the expression of IL-17 and IL-23[27-32], as documented in the current study.
We reported a positive correlation between IL-17 and IL-23 and viral load; a finding that supports our hypothesis regarding a link between Vit D and both IL-17 and IL-23 in immunoregulation of HCV-4-related chronic liver disease, and may explain how Vit D deficiency plays a role in increasing liver fibrosis. Our results also revealed no significant differences between HCV-infected men and women with respect to Vit D status. In contrast, Arteh et al[33] have reported that African American women with chronic liver disease are at higher risk of Vit D deficiency.
During the process of liver fibrosis, type III procollagen is converted to type III collagen by cleavage of its amino terminal and carboxy terminal propeptides. Serum levels of PIIINP, which are direct serologic markers of collagen turnover in liver fibrosis, are elevated in both acute and chronic liver diseases; and further reflect the histologic stage of hepatic fibrosis in various chronic liver diseases[34]. There was a significant increase (in comparison to controls) in the serum levels of PIIINP in patients with all grades of hepatic disease; results that are in agreement with Walsh et al[34]. However, Panasiuk et al[35] have reported a decrease in PIIINP levels in cirrhotic patients in comparison to controls, and have not shown any inflammatory process in the cirrhosis, hence more studies are needed to resolve this point of controversy. The results of the current study also revealed a significant negative correlation between Vit D and PIIINP levels, supporting a role for decreased Vit D in inflammation and fibrosis; a relationship that has not previously been investigated in patients with hepatic disease. Interestingly, Zehnder et al[36] have reported that reduction of the Vit D hormonal system in kidney diseases is associated with increased renal inflammation and fibrosis. These investigators have also reported a significant negative correlation between Vit D and PIIINP levels. Logistic regression analysis with urinary PIIINP, as a binary outcome, has shown that a 10-U increase in serum 1,25(OH)2-D or 25-OH-Vit D resulted in lower renal inflammation[37].
In conclusion, Vit D deficiency is prevalent in HCV-4-infected patients and the viral load is negatively correlated with Vit D status. In view of the role of Vit D in maintaining optimal immune function, Vit D status may be assessed and supplements may be considered to achieve an SVR during IFN-α-based therapy. The negative correlation between Vit D and IL-17, IL-23 and PIIINP levels appears to highlight, at least in part, how these cytokines are involved with Vit D in immune responses in HCV-4-related liver disease, and could explain how Vit D deficiency plays a role in liver fibrosis.
ACKNOWLEDGMENTS
The authors extend special thanks to the workers at the Hepatology Clinic in the Endemic Disease Hospital, Cairo University for their help during this research.
COMMENTS
Background
Hepatitis C virus (HCV) infects primarily the hepatocytes, leads to the development of fibrosis or cirrhosis of the liver, and is a significant risk factor for the development of hepatocellular carcinoma (HCC). The cell-mediated immune response plays a central role in hepatocellular necrosis and in the immunopathogenic mechanisms involved in viral clearance and persistence in liver disease of viral etiology, such as HCV-related chronic liver disease. In this context, cytokines modulate the immune system and exert direct antiviral activity by cytopathic and non-cytopathic mechanisms. T-cell immunoregulatory cytokines influence the persistence of HCV chronic infection and the extent of liver damage.
Research frontiers
Vitamin (Vit D) abnormalities are well documented in patients with chronic liver disease, but the association of the degree of Vit D abnormality with the progressive hepatic necroinflammatory state has not been thoroughly investigated. Furthermore, the proinflammatory cytokine profile in patients infected with HCV genotype 4 (HCV-4) needs further study. The research hotspot is how to determine/assess the extent of Vit D abnormality in HCV-4-infected patients and determine its relationship with proinflammatory markers, namely, interleukin (IL)-17, IL-23, and the N-terminal propeptide of type III pro-collagen (PIIINP) as immune response mediators.
Innovations and breakthroughs
A plethora of studies has investigated the association of Vit D abnormalities with individual liver diseases, including hepatitis B, alcoholic hepatitis, autoimmune hepatitis, and HCC. Nevertheless, the role of Vit D abnormalities in the progression of HCV to cirrhosis and then to HCC remains to be elucidated. To this end, this study sought to investigate the potential association between levels of Vit D and IL-17, IL-23 and exacerbation of hepatic damage in chronic HCV patients. The results showed that levels of Vit D and its active form were significantly lower in patients with advanced liver disease (hepatic cirrhosis and/or carcinoma), compared to those with bright hepatomegaly and perihepatic fibrosis. IL-17, IL-23 and PIIINP levels were markedly increased in HCV patients and correlated with progression of hepatic damage. The decrease in Vit D and its active form was concomitant with increases in viral load, as well as levels of IL-17, IL-23 and PIIINP among all subgroups of HCV-infected patients, compared to normal healthy controls. The negative correlation between Vit D and IL-17, IL-23 and PIIINP may highlight, at least in part, how these cytokines are involved with Vit D in the immune response to HCV-4-related liver disease, and may explain how Vit D deficiency plays a role in the progression of liver fibrosis. In view of the role of Vit D in maintenance of immune function, its status may be assessed and supplements should be considered to achieve a sustained virological response during therapy.
Applications
The actual role of Vit D in the context of hepatic inflammatory process is still not fully elucidated. Given the major significance of the inflammatory response in mediating HCV clearance, as well as the anti-inflammatory actions displayed by Vit D in vitro, Vit D could have a positive influence on HCV infection. Further studies are needed to explain which stages of HCV infection require higher levels of Vit D and the mechanism of Vit D supplementation for these patients.
Terminology
Hepatitis C is a chronic liver infection that can be complicated by liver failure and liver cancer. In the liver, cytokines coordinate physiological and pathological processes such as liver growth and regeneration, inflammatory processes including viral liver disease, liver fibrosis and cirrhosis. T-cell immunoregulatory cytokines may play a key role in influencing the persistence of HCV infection and the extent of liver damage. IL-6 and transforming growth factor β, as the differentiation factors for Th17 cells, both cytokines together induce massive amounts of IL-17 from naïve T cells. Vit D has an important role in the treatment of different bacterial and viral infections; this vitamin is synthesized in the skin by absorption of ultraviolet light from the sun. The mechanism of action of this vitamin is unknown, but it may improve the activities of immune cells that are important in the eradication of HCV.
Peer review
This article reveals the importance of Vit D and/or its active form in HCV-4 infection, which is the most common form of hepatitis C in Egypt. The study also showed that this deficiency progresses with disease deterioration. This may indicate that Vit D supplements could be efficient in early stages of the disease.
Footnotes
Peer reviewers: Hanan S El-Abhar, Professor, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Cairo University, Cairo 11562, Egypt; Dr. Sahar Mohammad Abdelraouf, Department of Biochemistry, Misr International University, Cairo 00202, Egypt; Murat Sayan, Associate Professor, PCR Unit, Kocaeli University Hospital, Umuttepe Campus, Izmit, Kocaeli 41380, Turkey
S- Editor Gou SX L- Editor Kerr C E- Editor Zhang DN
References
- 1.Kamal SM, Nasser IA. Hepatitis C genotype 4: What we know and what we don’t yet know. Hepatology. 2008;47:1371–1383. doi: 10.1002/hep.22127. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis C virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol. 2005;3:S97–S101. doi: 10.1016/s1542-3565(05)00711-1. [DOI] [PubMed] [Google Scholar]
- 3.Eisen-Vandervelde AL, Waggoner SN, Yao ZQ, Cale EM, Hahn CS, Hahn YS. Hepatitis C virus core selectively suppresses interleukin-12 synthesis in human macrophages by interfering with AP-1 activation. J Biol Chem. 2004;279:43479–43486. doi: 10.1074/jbc.M407640200. [DOI] [PubMed] [Google Scholar]
- 4.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 5.Muller K, Svenson M, Bendtzen K. 1 alpha,25-Dihydroxyvitamin D3 and a novel vitamin D analogue MC 903 are potent inhibitors of human interleukin 1 in vitro. Immunol Lett. 1988;17:361–365. doi: 10.1016/0165-2478(88)90012-0. [DOI] [PubMed] [Google Scholar]
- 6.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 7.Shirakawa AK, Nagakubo D, Hieshima K, Nakayama T, Jin Z, Yoshie O. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J Immunol. 2008;180:2786–2795. doi: 10.4049/jimmunol.180.5.2786. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 9.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert Rev Clin Immunol. 2009;5:693–702. doi: 10.1586/eci.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 12.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA. 2010;107:22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yusupov E, Li-Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF. Vitamin d and serum cytokines in a randomized clinical trial. Int J Endocrinol. 2010;2010:305054. doi: 10.1155/2010/305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 15.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 16.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Mawer EB. Clinical implications of measurements of circulating vitamin D metabolites. Clin Endocrinol Metab. 1980;9:63–79. doi: 10.1016/s0300-595x(80)80021-1. [DOI] [PubMed] [Google Scholar]
- 18.Hollis BW. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification procedure. Clin Chem. 1986;32:2060–2063. [PubMed] [Google Scholar]
- 19.Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA. 2007;297:724–732. doi: 10.1001/jama.297.7.724. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto H, Sugiyama Y, Okada S, Kurai K, Akahane Y, Sugai Y, Tanaka T, Sato K, Tsuda F, Miyakawa Y. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73(Pt 3):673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 21.Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5:513–520. doi: 10.1016/j.cgh.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Bouillon R, Auwerx J, Dekeyser L, Fevery J, Lissens W, De Moor P. Serum vitamin D metabolites and their binding protein in patients with liver cirrhosis. J Clin Endocrinol Metab. 1984;59:86–89. doi: 10.1210/jcem-59-1-86. [DOI] [PubMed] [Google Scholar]
- 23.Duarte MP, Farias ML, Coelho HS, Mendonça LM, Stabnov LM, do Carmo d Oliveira M, Lamy RA, Oliveira DS. Calcium-parathyroid hormone-vitamin D axis and metabolic bone disease in chronic viral liver disease. J Gastroenterol Hepatol. 2001;16:1022–1027. doi: 10.1046/j.1440-1746.2001.02561.x. [DOI] [PubMed] [Google Scholar]
- 24.Lange CM, Bojunga J, Ramos-Lopez E, von Wagner M, Hassler A, Vermehren J, Herrmann E, Badenhoop K, Zeuzem S, Sarrazin C. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol. 2011;54:887–893. doi: 10.1016/j.jhep.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 25.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 26.Rowan AG, Fletcher JM, Ryan EJ, Moran B, Hegarty JE, O’Farrelly C, Mills KH. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J Immunol. 2008;181:4485–4494. doi: 10.4049/jimmunol.181.7.4485. [DOI] [PubMed] [Google Scholar]
- 27.Mus AM, van Hamburg JP, Asmawidjaja P, Hazes JMW, van Leeuwen H, Boon L, Colin E. Vitamin D Suppresses Th17 Cytokines Via Down Regulation of RORgammat and NFATC2 and by Differential Regulation of GATA3. Arthritis and Rheumatism. 2010;62:38. [Google Scholar]
- 28.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramírez P, Martin-Loeches I, Varillas D, Gallegos MC, Serón C, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartosik-Psujek H, Tabarkiewicz J, Pocinska K, Stelmasiak Z, Rolinski J. Immunomodulatory effects of vitamin D on monocyte-derived dendritic cells in multiple sclerosis. Mult Scler. 2010;16:1513–1516. doi: 10.1177/1352458510379611. [DOI] [PubMed] [Google Scholar]
- 30.Krstić G. Th17 mediators and vitamin D status. Crit Care. 2010;14:410. doi: 10.1186/cc8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zold E, Szodoray P, Kappelmayer J, Gaal J, Csathy L, Barath S, Gyimesi E, Hajas A, Zeher M, Szegedi G, et al. Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scand J Rheumatol. 2010;39:490–497. doi: 10.3109/03009741003781951. [DOI] [PubMed] [Google Scholar]
- 32.van Hamburg JP, Asmawidjaja PS, Davelaar N, Cornelissen FC, Mus AM, Bakx PA, Colin EM, van Leeuwen H, Hazes JM, Dolhain RJ, et al. Vitamin D suppresses the pathogenic behaviour of primary TH17 cells from patients with early rheumatoid arthritis. Ann Rheum Dis. 2011;70(S2):A47. [Google Scholar]
- 33.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 34.Walsh KM, Fletcher A, MacSween RN, Morris AJ. Comparison of assays for N-amino terminal propeptide of type III procollagen in chronic hepatitis C by using receiver operating characteristic analysis. Eur J Gastroenterol Hepatol. 1999;11:827–831. doi: 10.1097/00042737-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Panasiuk A, Zak J, Kasprzycka E, Janicka K, Prokopowicz D. Blood platelet and monocyte activations and relation to stages of liver cirrhosis. World J Gastroenterol. 2005;11:2754–2758. doi: 10.3748/wjg.v11.i18.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, Raymond NT, Howie AJ, Cockwell P, Stewart PM, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74:1343–1353. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babbs C, Smith A, Hunt LP, Rowan BP, Haboubi NY, Warnes TW. Type III procollagen peptide: a marker of disease activity and prognosis in primary biliary cirrhosis. Lancet. 1988;1:1021–1024. doi: 10.1016/s0140-6736(88)91843-0. [DOI] [PubMed] [Google Scholar]


