Abstract
AIM: To evaluate the efficacy and safety of a hybrid bioartificial liver (HBAL) system in the treatment of acute liver failure.
METHODS: Canine models with acute liver failure were introduced with intravenous administration of D-galactosamine. The animals were divided into: the HBAL treatment group (n = 8), in which the canines received a 3-h treatment of HBAL; the bioartificial liver (BAL) treatment group (n = 8), in which the canines received a 3-h treatment of BAL; the non-bioartificial liver (NBAL) treatment group (n = 8), in which the canines received a 3-h treatment of NBAL; the control group (n = 8), in which the canines received no additional treatment. Biochemical parameters and survival time were determined. Levels of xenoantibodies, RNA of porcine endogenous retrovirus (PERV) and reverse transcriptase (RT) activity in the plasma were detected.
RESULTS: Biochemical parameters were significantly decreased in all treatment groups. The TBIL level in the HBAL group was lower than that in other groups (2.19 ± 0.55 μmol/L vs 24.2 ± 6.45 μmol/L, 12.47 ± 3.62 μmol/L, 3.77 ± 1.83 μmol/L, P < 0.05). The prothrombin time (PT) in the BAL and HBAL groups was significantly shorter than the NBAL and control groups (18.47 ± 4.41 s, 15.5 ± 1.56 s vs 28.67 ± 5.71 s, 21.71 ± 3.4 s, P < 0.05), and the PT in the HBAL group was shortest of all the groups. The albumin in the BAL and HBAL groups significantly increased and a significantly higher level was observed in the HBAL group compared with the BAL group (27.7 ± 1.7 g/L vs 25.24 ± 1.93 g/L). In the HBAL group, the ammonia levels significantly decreased from 54.37 ± 6.86 to 37.75 ± 6.09 after treatment (P < 0.05); there were significant difference in ammonia levels between other the groups (P < 0.05). The levels of antibodies were similar before and after treatment. The PERV RNA and the RT activity in the canine plasma were all negative.
CONCLUSION: The HBAL showed great efficiency and safety in the treatment of acute liver failure.
Keywords: Hybrid bioartificial liver, Acute liver failure, Flat plate bioreactor, Co-culture, Nanofiber scaffold
INTRODUCTION
The mortality associated with acute liver failure or acute-on-chronic liver failure remains dismally high[1]. Orthotropic liver transplantation is a unique and effective treatment for acute liver failure[2]. However, many patients still die while waiting for liver transplantation due to a persistent scarcity of donors. Therefore, many investigators have attempted to develop diversified extracorporeal liver assist devices as a bridge for patients until recovery or liver transplantation[3-5]. Extracorporeal liver assist devices can be classified into artificial systems and bioartificial systems. An effective liver assist device should include three primary functions: detoxification, biosynthesis and regulation. The main functions of artificial systems include the removal of toxins (e.g., aromatic amino acids and ammonia), but does not include the synthesis of products that take place in the liver. Bioartificial systems could be the ideal replacement therapy in the long run. However, bioartificial liver systems have not reached their full efficiency yet, since enhancing the viability and functions of hepatocytes in the bioreactor remains a difficult problem, and the function of detoxification is inadequate[6-8]. For these reasons, we consider the application of artificial systems in combination with bioartificial liver systems presents a potential method for aiding patients waiting for liver transplantation.
In this study, we first developed a newly bioartificial liver system based on multi-layer flat-plate bioreactor with co-cultured pig hepatocytes and bone marrow mesenchymal stem cells, so as to mimic the microenvironment in vivo. We then combined this newly bioartificial liver system with an anionic resin adsorption column to form a novel hybrid bioartificial liver (HBAL) system. The efficacy and safety of this novel HBAL system was evaluated via treatment of canines with acute liver failure.
MATERIALS AND METHODS
Animals and reagents
Outbred white pigs with a weight of 15-20 kg, as well as dogs with a weight of 10-15 kg, received humane care. All animal procedures were performed according to institutional and national guidelines and approved by the Animal Care Ethics Committee of Nanjing University and Nanjing Drum Tower Hospital. RPMI 1640 were purchased from GIBCO (United States). Lactobionic acid and chitosan (low molecular weight, Brookfield viscosity 20 000 cps, 85% deacetylation) were purchased from Sigma-Aldrich (Saint Louis, United States). N-Hydroxysuccinimide was purchased from Thermo-Pierce (Rockford, United States). 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N, N, N0, N0-tetramethylethylenediamine were obtained from TCI (Tokyo, Japan). Polyethylenoxid (MW ≈ 106) was supplied by Guoren Chemical Co. (Beijing, China). All other reagents were of analytical reagent grade.
Cell isolation and culture
Porcine mesenchymal stem cells were isolated by bone marrow aspirates from the iliac crest of pigs, as described previously, with slight modification[9]. Briefly, mononuclear cells were collected by gradient centrifugation over a Ficoll Histopaque layer (20 min, 400 g, density 1.077 g/mL) and seeded at a density of 1 × 106 cells/cm2 in growth medium containing low-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (100 IU/mL) and streptomycin (100 μg/mL). The non-adherent cells were removed after the first 24 h and changed every 3 d to 4 d thereafter. The primary pig hepatocytes were then harvested by a two-step in situ collagenase perfusion technique[10]. The viability of the isolated primary hepatocytes, determined by trypan blue exclusion, was more than 95%.
Non-bioartificial liver system
Whole blood was removed at a rate of 30 mL/min from the jugular vein of the canine and separated to plasma by a plasma separator (Bellco, Italy) at a rate of 30 mL/min. The separated plasma was pumped into an anionic resin adsorption column (Aier, China) where the toxic substances were absorbed, and then reconstituted with red blood cells and returned to the canine via the venous cannula (Figure 1A).
Figure 1.
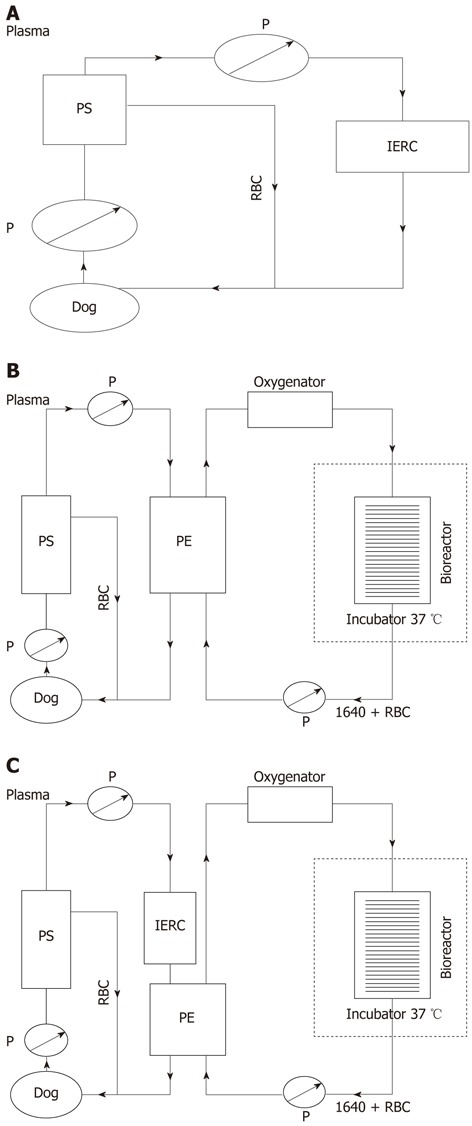
Schematic assembly of three artificial liver systems. A: Schematic assembly of non-bioartificial liver; B: Schematic assembly of bioartificial liver; C: Schematic assembly of hybrid bioartificial liver. P: Pump; RBC: Red blood cells; PS: Plasma separator; PE: Plasma component exchange column; IERC: Anionic resin adsorption column.
Bioartificial liver system
Bioreactor configuration: The multi-layer bioreactor consisted of housing, a hollow column stent, and stacked flat plates, all of which were made of polycarbonate. The fully assembled bioreactor contained a stack of 65-layer round flat plates, on which galactosylated chitosan nanofiber scaffolds were electrospun for hepatocyte immobilization and aggregation. The diameter and thickness of each plate were 10.4 cm and 1 mmol, respectively. There was a hole with a diameter of 1 cm in the center of each plate, which was used to fix them onto the stent. The channel height between every two neighboring plates was maintained at 0.5 mmol with the spacers attached to the bottom of each plate. The stent was open on the top and closed at the bottom, with four vertical-side holes on the lateral wall, which were broken up into eyelets between every two plates. The last component was housing, which was a cylindrical container with an outlet on the bottom. When the stacked plates were fixed onto the stent, they were put into the housing, and the lid with an O-ring was screwed onto the housing in order to provide a water tight seal. The culture medium entered the bioreactor from the top opening of the stent, then flowed onto the surface of each flat plate though the eyelets, and finally flowed out from the outlet of housing. The height of this bioreactor was about 10 cm, and the effective volume was 480 mL.
The multi-layer flat-plate bioartificial liver consists of a cell circuit and a blood circuit. Freshly isolated porcine hepatocytes (approximately 1010) were pre-mixed in 480 mL RPMI 1640 culture medium containing 10% BSA, 0.5 mg/mL insulin, 10 mmol NaHCO3, 50 mg/mL penicillin and streptomycin, and 100 mg/ML neomycin. The medium was then filled into the bioreactor by a peristaltic pump (JHBP-2000B, Guangzhou, China). The whole bioreactor was incubated for 1 h at 37 °C and 5% CO2 until the cells were adhered onto the surface of plates. The culture medium was pumped though an oxygenator (Affinity, United States) via Fresenius silicone tubing (Fresenius, Germany) by a peristaltic pump before flowing into the bioreactor, and then circulated into a blood component exchange column (Asahi Kasei Corporation, Japan) at 25 mL/min, which constitute the cell circuit. As for the blood circuit, whole blood was removed at a rate of 30 mL/min from the jugular vein of the canine and separated to plasma by a plasma separator (Bellco, Italy) at a rate of 15 mL/min. The separated plasma was pumped into the blood component exchange column where the plasma component and culture medium component exchanged, and then reconstituted with red blood cells and returned to the canine via the venous cannula (Figure 1B).
HBAL system
We combined the bioartificial liver system with an anionic resin absorption column to form a novel HBAL system. This HBAL system differs from the bioartificial liver system in that the separated plasma was pumped into an anionic resin adsorption column where the toxic substances were absorbed, and then passed though the blood component exchange column (Figure 1C).
Canine models of acute liver failure and treatment groups
The canines were injected intravenously with D-galactosamine to induce acute liver failure and then randomly divided into 4 groups: the HBAL system treatment group (n = 8), in which the canines received a 3 h treatment of HBAL system (as described above) 24 h after the administration of the drug; the bioartificial liver system treatment group (n = 8), in which the canines received a 3 h treatment of bioartificial liver system; the non-bioartificial liver system (artificial support system) treatment group (n = 8), in which the canines received a 3 h treatment of artificial support system; the control group (n = 8), in which the canines received no additional treatment.
Blood biochemistry and survival rate
Blood samples were measured with an automatic analyzer (Hitachi 7600, Tokyo, Japan) for alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), total bilirubin (TBIL), albumin (ALB), ammonia, and prothrombin time (PT). Survival was observed for 7 d.
Safety evaluation
Levels of xenoantibodies (IgG, IgM) were detected by enzyme linked immunosorbent assay (ELISA) kit (Alpha Diagnostic International Inc., United States). To identify the transmission of porcine endogenous retroviruses (PERVs), the PERV RNA and reverse transcriptase (RT) activity in the plasma was detected with reverse transcription-polymerase chain reaction (RT-PCR) and RT activity assay kits (Cavidi-Tech, Uppsala, Sweden), respectively.
Statistical analysis
All values were expressed as the mean ± SD. The two-tailed unpaired Student’s t-test or one-way analysis of variance was used to evaluate the statistical significance of differences, which were set with a P value < 0.05. The survival rates were analyzed using the Kaplan-Maier method and compared using a log-rank test.
RESULTS
Common conditions
After treatment, hepatic encephalopathy was significantly improved in all treatment groups; most of the canines could stand, eat and drink by themselves. However, the canines in the control group became gradually worse, progressing into a coma and eventually death. Body temperature was stable in all treatment groups (38 ± 0.5 °C in non-bioartificial liver (NBAL) group; 37 ± 1 °C in bioartificial liver (BAL) group; 38 ± 0.8 °C in HBAL group); however, the canines in the control group became lower than other groups.
Liver function parameters
Several laboratory parameters have been determined, such as ALT, AST, LDH, TBIL, ALB, PT and ammonia. As Table 1 and Figure 2 show, there was no significant difference between any of the groups before treatment. In all treatment groups, ALT, AST, LDH, TNIL and ammonia were shown to be statistically decreased compared to the control group. Moreover, the TBIL level in the HBAL group was lower than that in the other groups (2.19 ± 0.55 μmol/L vs 24.2 ± 6.45 μmol/L, 12.47 ± 3.62 μmol/L, 3.77 ± 1.83 μmol/L, P < 0.05). There was no significant difference in PT and ALB between the NBAL and control groups. On the contrary, the PT in the BAL and HBAL groups was significantly shorter than in the NBAL and control groups (18.47 ± 4.41 s, 15.5 ± 1.56 s vs 28.67 ± 5.71 s, 21.71 ± 3.4 s, P < 0.05), and the PT in the HBAL group was the shortest of all the groups. The ALB in the BAL and HBAL groups significantly increased and a significantly higher level was observed in the HBAL group compared with the BAL group (27.7 ± 1.7 g/L vs 25.24 ± 1.93 g/L). In the HBAL group, ammonia levels significantly decreased from 54.37 ± 6.86 to 37.75 ± 6.09 after treatment (P < 0.05); there were significant differences in ammonia levels between the other groups (P < 0.05).
Table 1.
Comparison of biochemical index between different groups in different time points
| Groups | Time points | ALT | AST | LDH | TBIL | ALB | Ammonia | PT |
| Control (n = 8) | ||||||||
| Before modeling | 64 ± 22.67 | 44.87 ± 11.6 | 210.14 ± 48.32 | 1.67 ± 0.57 | 30 ± 5 | 46.5 ± 11.98 | 8.4 ± 1.7 | |
| After modeling | 485.28 ± 140.2 | 651.35 ± 125.9 | 694.14 ± 232.8 | 28.45 ± 9.8 | 26.6 ± 5 | 62.25 ± 21.56 | 16.4 ± 5.2 | |
| 1 d after treatment | 2438.64 ± 720.3 | 3201.9 ± 903 | 2433 ± 838.47 | 49 ± 15.77 | 24.8 ± 6.5 | 98.67 ± 21.7 | 28.67 ± 5.71 | |
| 3 d after treatment | 860 ± 237.96 | 230.37 ± 92.37 | 867.25 ± 86.57 | 56.1 ± 5.3 | 22.3 ± 3.6 | 66.5 ± 8.54 | 24.83 ± 2.55 | |
| 5 d after treatment | 144.6 ± 55.4 | 172.9 ± 36.57 | 639 ± 117.88 | 24.2 ± 6.45 | 21.4 ± 3.8 | 39.3 ± 9.5 | 10.5 ± 4.1 | |
| Bioartificial liver system (n = 8) | ||||||||
| Before modeling | 51.78 ± 13.1 | 58.77 ± 13.4 | 285.5 ± 83.18 | 1.1 ± 0.4 | 29.42 ± 2.48 | 50.28 ± 14.28 | 8.2 ± 1.43 | |
| After modeling | 439.7 ± 180.98 | 811.7 ± 209.85 | 438.5 ± 138.2 | 18.12 ± 4.85 | 24.46 ± 1.97 | 55.5 ± 15.4 | 13.5 ± 4.1 | |
| 1 d after treatment | 1511.54 ± 183.4 | 1472.46 ± 365 | 463 ± 76 | 25.92 ± 6.2 | 24.76 ± 4.46 | 46.4 ± 18.1 | 18.47 ± 4.41 | |
| 3 d after treatment | 331.8 ± 31 | 51.2 ± 15.1 | 278.8 ± 67.2 | 28.84 ± 6.23 | 25.44 ± 3.52 | 38.2 ± 10.2 | 15.2 ± 7.6 | |
| 5 d after treatment | 86.2 ± 24.5 | 46.3 ± 10.54 | 311.5 ± 84.35 | 12.47 ± 3.62 | 25.24 ± 1.93 | 34 ± 9.46 | 8.8 ± 2.7 | |
| Non-bioartificial liver system (n = 8) | ||||||||
| Before modeling | 43.11 ± 19.44 | 41.77 ± 14.32 | 216.87 ± 46.2 | 2.44 ± 1 | 29.35 ± 3.08 | 50.25 ± 17.07 | 7.67 ± 0.315 | |
| After modeling | 447.5 ± 99.44 | 660.15 ± 152.13 | 536 ± 110 | 24.65 ± 2.57 | 24.36 ± 5.56 | 60.25 ± 15.12 | 14.6 ± 2.17 | |
| 1 d after treatment | 1164.5 ± 332.5 | 1531.31 ± 294.96 | 485.5 ± 119.41 | 28.07 ± 9 | 23.61 ± 3.77 | 46 ± 13.6 | 21.71 ± 3.4 | |
| 3 d after treatment | 286.2 ± 99.8 | 190.62 ± 30.9 | 379.8 ± 67.36 | 7.21 ± 3.6 | 23.94 ± 2.13 | 42.6 ± 8.17 | 19.48 ± 3.19 | |
| 5 d after treatment | 89.15 ± 5.32 | 85.9 ± 11.98 | 530.25 ± 136.55 | 3.77 ± 1.83 | 23.92 ± 0.82 | 39.5 ± 7.59 | 7.95 ± 0.64 | |
| Hybrid bioartificial liver system (n = 8) | ||||||||
| Before modeling | 56.5 ± 12.62 | 45.9 ± 10.56 | 233.25 ± 39 | 1.71 ± 0.28 | 29.4 ± 2.6 | 51.5 ± 8.94 | 7.62 ± 0.47 | |
| After modeling | 459.05 ± 80.57 | 681.95 ± 101 | 520.75 ± 101.22 | 18.8 ± 3.49 | 25.8 ± 3.2 | 54.37 ± 6.86 | 13.67 ± 2.66 | |
| 1 d after treatment | 655.45 ± 97 | 1010.67 ± 118.95 | 301.12 ± 69.26 | 20.35 ± 2.68 | 28 ± 1.7 | 37.75 ± 6.09 | 15.5 ± 1.56 | |
| 3 d after treatment | 117.97 ± 30.61 | 44.52 ± 9.7 | 222.5 ± 53.19 | 4.44 ± 1.02 | 28.3 ± 1.4 | 173.7 ± 8 | 8.36 ± 0.99 | |
| 5 d after treatment | 55 ± 10.73 | 39.81 ± 8.72 | 334.25 ± 76.99 | 2.19 ± 0.55 | 27.7 ± 1.7 | 17.75 ± 3.77 | 8.3 ± 1.22 | |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; LDH: Lactate dehydrogenase; TBIL: Total bilirubin; ALB: Albumin; PT: Prothrombin time.
Figure 2.
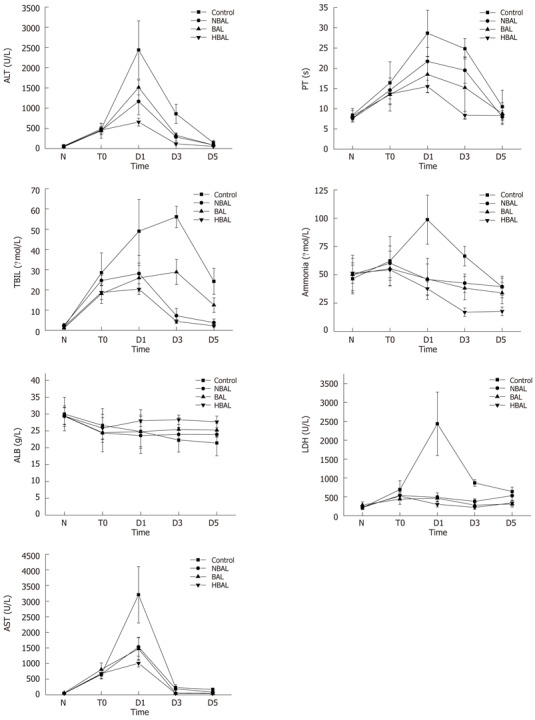
Comparisons of biochemical index between the treatment groups and the control group. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; LDH: Lactate dehydrogenase; TBIL: Total bilirubin; ALB: Albumin; PT: Prothrombin time; HBAL: Hybrid bioartificial liver; BAL: Bioartificial liver; NBAL: Non-bioartificial liver; N: Normal; T0: Before treatment; D1: Day 1 after treatment; D3: Day 3 after treatment; D5: Day 5 after treatment.
Animal survival
Analysis of survival curves (Figure 3) showed that the 7 day survival rate was 37.5% (3/8) in the control group, 50% (4/8) in the NBAL group, 62.5% (5/8) in the BAL group and 87.5% (7/8) in the HBAL group. Survival time was significantly prolonged by HBAL treatment (P = 0.028). However, the results showed there was no significant difference between the other groups.
Figure 3.
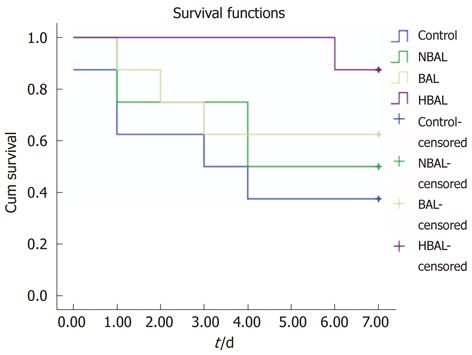
Kaplan-Meier analysis of survival of two groups. HBAL: Hybrid bioartificial liver; BAL: Bioartificial liver; NBAL: Non-bioartificial liver.
Safety evaluation
To evaluate the humoral immune response in canine models after treatment with BAL, we examined the levels of antibodies. The levels of antibodies were measured by ELISA in canine plasma obtained before and after BAL. As shown in Figure 4A, immediately after the first BAL treatment, the levels of IgG and IgM were suitable before and after treatment. All results of RT-PCR with the RNA from collected plasma were negative (Figure 4B). In addition, the RT activity in the canine plasma was negative as well.
Figure 4.
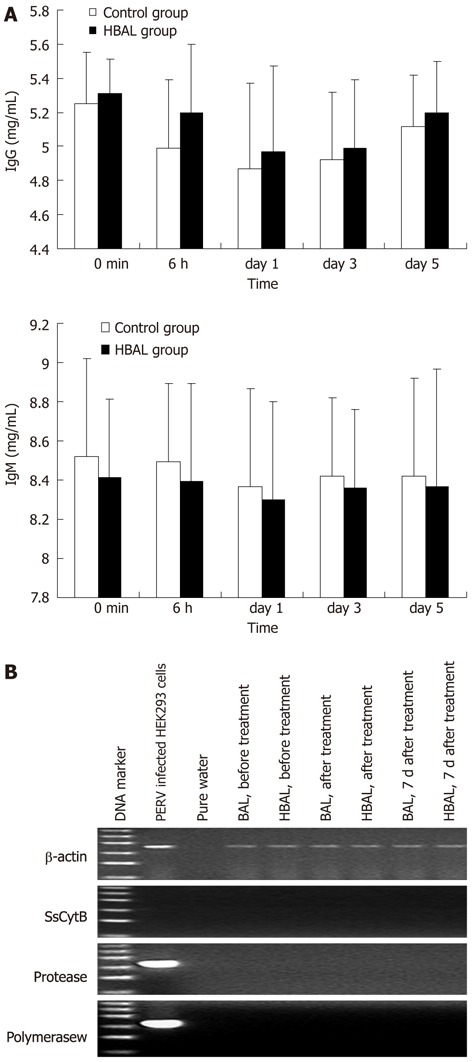
Safety evaluation. A: Xenoreactive antibodies levels during bioartificial liver treatments; B: Representative results of reverse transcription-polymerase chain reaction electrophoresis with the RNA extracted from the plasma. The ladder ranged from 100 bp to 600 bp. NBAL: Hybrid bioartificial liver; BAL: Bioartificial liver; PERV: Porcine endogenous retrovirus.
DISCUSSION
In recent years, extracorporeal liver assist devices have been developed and widely used to bridge the gap between for patients until liver transplantation or a spontaneous recovery of liver function. There are currently two types of liver assist devices: artificial and bioartificial livers. Artificial liver systems include conventional plasmapheresis with plasma exchange, hemodialysis using large pore membranes and hemoperfusion using exchange resin columns or activated charcoal[11-13]. These systems could remove toxic substances (e.g., ammonia, endotoxin, mercaptans and endogenous inhibitor neurotransmitters) but also some helpful cytokines, such as interleukin-6, hepatocyte growth factor. Moreover, these devices could not replace the synthetic and metabolic functions, nor could they prolong patient survival time. Bioartificial liver systems could replace the primary and most important liver functions, such as oxidative detoxification, biotransformation, excretion and synthesis. However, there are still many problems holding back the development of bioartificial livers in a clinical setting. How to maintain the functions of hepatocytes and mimic cell microenvironment in vitro remains a difficult problem. In addition, plasma from patients with acute liver failure have some toxic factors, which are harmful to the hepatocytes in the bioreactor. In the present study, to resolve these problems, we first developed a new bioartificial liver system based on a multi-layer flat-plate bioreactor with co-cultured pig hepatocytes and bone marrow mesenchymal stem cells.
Due to the short-term viability and rapid phenotypic de-differentiation of primary hepatocytes in vitro, mimicking the three-dimensional microenvironment of liver sinusoid in vitro should be important for reconstruction of tissue architectures and restoration of isolated hepatocyte functions. Recent studies have shown that functions of primary hepatocytes can be maintained by co-culturing with non-parenchymal cells[14,15]. The interaction between cells and the extracellular matrix (ECM) provides direct regulatory signals to cells though adhesion. In addition, various soluble factors secreted by mesenchymal cells have been shown to be important in maintaining hepatocyte phenotypes. In our previous study, we confirmed that the morphology and functionality of co-culturing such heterotypic cells were well-maintained and improved over hepatocytes homo-culture[16,17]. Therefore, in this study, we adopted co-cultured porcine hepatocytes and bone marrow mesenchymal stem cells as the cell resource.
Hepatocytes in vivo are exposed to sinusoidal endothelial cells mediated by Disse space filled with ECM, which consists of a complex topography in the nanometer range and functional proteins that provide signals regulating cellular functions though cell surface receptors. In order to mimic the topography of ECM, various materials have been fabricated into nanometer materials and proven to affect cell migration, adhesion, proliferation and other cellular behaviors[18]. In the past decade, nanometer scaffolds have been widely applied in various areas of tissue engineering. In our previous study, galactosylated chitosan nanofiber scaffolds could adequately mimic the topography of ECMs in vitro and increase the contact sites between cells and materials, thus enhancing the signal transduction between cells, promoting cell functions, and the pseudopod of hepatocytes could be formatted on the scaffolds, which further facilitated cells to adhere tightly to the nanofiber surface, and enhance cell adhesion and viability. Moreover, the galactose group grafted onto the scaffold could selectively adhere to the asialoglycoprotein receptors on the surface of hepatocytes, which could then induce the formation of hepatocyte aggregates and exhibit higher levels of liver-specific functions[19,20]. Based on these results, we introduced galactosylated chitosan nanofiber scaffolds into the bioreactor to mimic the topography and biochemical environment of ECMs.
Bioreactors can be divided into four types: (1) hollow fibers (e.g., the HepatAssist system and the Extracorporeal Liver Assist Device, which suffer from oxygen substrate limitations[21-23], and so a large proportion of hepatocytes may be anoxic and lose their functions); (2) beds or scaffolds (the major disadvantage of which is the direct contact between plasma and porcine liver cells, and so there is no barrier for porcine pathogens[24,25]); (3) suspension and encapsulation chambers; and (4) flat plate and monolayer cultures. As one classical type of bioreactor, flat-plate bioreactors have many advantages[26-28]. Firstly, the cells can adhere to the plates evenly and therefore making the microenvironment homogenous. Secondly, the culture medium/plasma can make contact with seeded cells adequately, which allows sufficient material exchange. Thirdly, the bioreactor can be easily scaled up by adjusting the flat plates to meet the clinical requirements. Finally, the structure of multi-layer flat plates is similar to that of hepatic plates of a normal liver, which is crucial for the long-term maintenance of liver functions.
A successful liver support system is likely to depend on the viability and functions of the hepatocytes in the bioreactor. Previous research has shown that the toxic substances of acute liver failure plasma can damage the hepatocytes[29]. In our study, in order to reduce the damage to the hepatocytes caused by the toxic substances in acute liver failure plasma and improve the efficacy of the treatment, we combined bioartificial liver system with an anionic resin absorption column to form a novel HBAL system. An anionic resin absorption column was used before the bioreactor, which may not only protect the co-culture cells in the bioreactor from toxic effect of acute liver failure plasma, but also improve the effect of plasma detoxification.
In this study, we found that the ALT, AST, LDH, TBIL and ammonia in HBAL group appeared to be statistically decreased compared to the control group. Furthermore, the TBIL level in the HBAL group was lower than that in other treatment groups. In addition, the ALB in the BAL and HBAL groups significantly increased, and a significantly higher level was observed in the HBAL group as compared with the BAL group. The survival rate of the HBAL group was higher than in the other treatment groups and the control group. This result suggests that HBAL significantly prolonged the survival time of the canine models with acute liver failure. These data indicate that the performance of this novel HABL system may be superior to that of the simple BAL systems.
The main recognized disadvantages of the use of porcine hepatocytes are the risk of infection of PERV and immunological rejection. Previous reports have shown that PERV successfully infected a variety of human cells in vitro, such as endothelial cells, fibroblasts and bone marrow stromal cells[30-32]. Moreover, Wilson found that implanted porcine islets may lead to PERV infection in non-obese diabetic severe combined immunodeficiency mice[33]. On the other hand, elevated titers of xenoantibodies have been found after treatment with porcine-based BAL systems, indicating that host exposure to porcine antigens occurs[34]. In our study, the PERV RNA and RT activity in the canine plasma were all negative. No direct evidence of severe immunological complications were been observed. Our results were similar to other reports[35,36].
In conclusion, This novel HBAL system showed great efficiency and safety in the treatment of canines with acute liver failure and seemed to be applied in a clinical setting.
COMMENTS
Background
Orthotropic liver transplantation is a unique and effective treatment for acute liver failure. However, due to severe donor-liver shortage, high cost and exacerbation of disease, many patients die before they can receive the operation. Therefore, bioartificial livers have been proposed as temporary liver support for patients awaiting liver transplantation. However, bioartificial liver systems have not reached their full efficiency yet, since a method to enhance the viability and functions of hepatocytes in the bioreactor remains a difficult problem, and the function of detoxification is inadequate.
Research frontiers
Nowadays, various types of bioartificial liver are applied in clinic, and obtain satisfactory curative effects. However, in a bioartificial liver, the problem remains as to how it would be possible to enhance the viability and functions of hepatocytes in the bioreactor.
Innovations and breakthroughs
In this study, the authors first developed a new bioartificial liver system based on a multi-layer flat-plate bioreactor with co-cultured pig hepatocytes and bone marrow mesenchymal stem cells so as to mimic the microenvironment in vivo. They then combined this new bioartificial liver system with an anionic resin absorption column to form a novel hybrid bioartificial liver (HBAL) system. An anionic resin absorption column was used before the bioreactor, which may not only protect the co-culture cells in the bioreactor from toxic effect of acute liver failure plasma, but also improve the effect of plasma detoxification.
Applications
The study results suggest that the HBAL showed great efficiency and safety in the treatment of acute liver failure.
Terminology
BAL: An artificial extracorporeal supportive device in which patient plasma is circulated extracorporeally though a bioreactor. The bioreactor houses metabolically active liver cells (hepatocytes) between artificial plates or capillaries. The functions of the liver are principally carried out by hepatocytes. The goal is to develop bioartificial liver devices in which hepatocytes are optimally maintained so that they carry out as many activities as possible.
Peer review
This is a good descriptive study in which authors evaluate the efficacy and safety of a HBAL system in the treatment of acute liver failure. The results are interesting and suggest that the HBAL could be used in the treatment of acute liver failure.
Footnotes
Supported by A grant from the National Natural Science Foundation of China, No. 30772129
Peer reviewer: Dr. Chih-Chi Wang, Department of Surgery, Kaohsiung Chang Gung Memorial Hospital, 123, Ta-Pei Road, Niao Sung, Kaohsiung 833, Taiwan, China
S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN
References
- 1.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51:1869–1884. doi: 10.1002/hep.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinasiewicz A, Gautier A, Lewiska D, Smietanka A, Legallais C, Weryński A. Three-dimensional growth of human hepatoma C3A cells within alginate beads for fluidized bioartificial liver. Int J Artif Organs. 2008;31:340–347. doi: 10.1177/039139880803100411. [DOI] [PubMed] [Google Scholar]
- 4.Poyck PP, van Wijk AC, van der Hoeven TV, de Waart DR, Chamuleau RA, van Gulik TM, Oude Elferink RP, Hoekstra R. Evaluation of a new immortalized human fetal liver cell line (cBAL111) for application in bioartificial liver. J Hepatol. 2008;48:266–275. doi: 10.1016/j.jhep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Poyck PP, Hoekstra R, van Wijk AC, Attanasio C, Calise F, Chamuleau RA, van Gulik TM. Functional and morphological comparison of three primary liver cell types cultured in the AMC bioartificial liver. Liver Transpl. 2007;13:589–598. doi: 10.1002/lt.21090. [DOI] [PubMed] [Google Scholar]
- 6.Zinchenko YS, Coger RN. Engineering micropatterned surfaces for the coculture of hepatocytes and Kupffer cells. J Biomed Mater Res A. 2005;75:242–248. doi: 10.1002/jbm.a.30399. [DOI] [PubMed] [Google Scholar]
- 7.Seo SJ, Kim IY, Choi YJ, Akaike T, Cho CS. Enhanced liver functions of hepatocytes cocultured with NIH 3T3 in the alginate/galactosylated chitosan scaffold. Biomaterials. 2006;27:1487–1495. doi: 10.1016/j.biomaterials.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa M, Kojima N, Komori K, Yamamoto T, Fujii T, Sakai Y. Enhanced maintenance and functions of rat hepatocytes induced by combination of on-site oxygenation and coculture with fibroblasts. J Biotechnol. 2008;133:253–260. doi: 10.1016/j.jbiotec.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Gu J, Shi X, Chu X, Zhang Y, Ding Y. Contribution of bone marrow mesenchymal stem cells to porcine hepatocyte culture in vitro. Biochem Cell Biol. 2009;87:595–604. doi: 10.1139/o09-017. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 11.Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl. 2004;10:1099–1106. doi: 10.1002/lt.20139. [DOI] [PubMed] [Google Scholar]
- 12.Skwarek A, Grodzicki M, Nyckowski P, Kotulski M, Zieniewicz K, Michalowicz B, Patkowski W, Grzelak I, Paczkowska A, Giercuszkiewicz D, et al. The use Prometheus FPSA system in the treatment of acute liver failure: preliminary results. Transplant Proc. 2006;38:209–211. doi: 10.1016/j.transproceed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Evenepoel P, Laleman W, Wilmer A, Claes K, Kuypers D, Bammens B, Nevens F, Vanrenterghem Y. Prometheus versus molecular adsorbents recirculating system: comparison of efficiency in two different liver detoxification devices. Artif Organs. 2006;30:276–284. doi: 10.1111/j.1525-1594.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 14.Thomas RJ, Bhandari R, Barrett DA, Bennett AJ, Fry JR, Powe D, Thomson BJ, Shakesheff KM. The effect of three-dimensional co-culture of hepatocytes and hepatic stellate cells on key hepatocyte functions in vitro. Cells Tissues Organs. 2005;181:67–79. doi: 10.1159/000091096. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda Y, Kikuchi A, Yamato M, Chen G, Okano T. Heterotypic cell interactions on a dually patterned surface. Biochem Biophys Res Commun. 2006;348:937–944. doi: 10.1016/j.bbrc.2006.07.138. [DOI] [PubMed] [Google Scholar]
- 16.Gu J, Shi X, Zhang Y, Chu X, Hang H, Ding Y. Establishment of a three-dimensional co-culture system by porcine hepatocytes and bone marrow mesenchymal stem cells in vitro. Hepatol Res. 2009;39:398–407. doi: 10.1111/j.1872-034X.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Shi X, Zhang Y, Ding Y. Heterotypic interactions in the preservation of morphology and functionality of porcine hepatocytes by bone marrow mesenchymal stem cells in vitro. J Cell Physiol. 2009;219:100–108. doi: 10.1002/jcp.21651. [DOI] [PubMed] [Google Scholar]
- 18.Gu HY, Chen Z, Sa RX, Yuan SS, Chen HY, Ding YT, Yu AM. The immobilization of hepatocytes on 24 nm-sized gold colloid for enhanced hepatocytes proliferation. Biomaterials. 2004;25:3445–3451. doi: 10.1016/j.biomaterials.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Chu XH, Shi XL, Feng ZQ, Gu ZZ, Ding YT. Chitosan nanofiber scaffold enhances hepatocyte adhesion and function. Biotechnol Lett. 2009;31:347–352. doi: 10.1007/s10529-008-9892-1. [DOI] [PubMed] [Google Scholar]
- 20.Feng ZQ, Chu X, Huang NP, Wang T, Wang Y, Shi X, Ding Y, Gu ZZ. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials. 2009;30:2753–2763. doi: 10.1016/j.biomaterials.2009.01.053. [DOI] [PubMed] [Google Scholar]
- 21.Hay PD, Veitch AR, Smith MD, Cousins RB, Gaylor JD. Oxygen transfer in a diffusion-limited hollow fiber bioartificial liver. Artif Organs. 2000;24:278–288. doi: 10.1046/j.1525-1594.2000.06499.x. [DOI] [PubMed] [Google Scholar]
- 22.Hay PD, Veitch AR, Gaylor JD. Oxygen transfer in a convection-enhanced hollow fiber bioartificial liver. Artif Organs. 2001;25:119–130. doi: 10.1046/j.1525-1594.2001.025002119.x. [DOI] [PubMed] [Google Scholar]
- 23.Strain AJ, Neuberger JM. A bioartificial liver--state of the art. Science. 2002;295:1005–1009. doi: 10.1126/science.1068660. [DOI] [PubMed] [Google Scholar]
- 24.Shi XL, Gu JY, Zhang Y, Han B, Xiao JQ, Yuan XW, Zhang N, Ding YT. Protective effects of ACLF sera on metabolic functions and proliferation of hepatocytes co-cultured with bone marrow MSCs in vitro. World J Gastroenterol. 2011;17:2397–2406. doi: 10.3748/wjg.v17.i19.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Li Y, Berthiaume F, Toner M, Yarmush ML, Tilles AW. Radial flow hepatocyte bioreactor using stacked microfabricated grooved substrates. Biotechnol Bioeng. 2008;99:455–467. doi: 10.1002/bit.21572. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Ding YT. Functional evaluation of a new bioartificial liver system in vitro and in vitro. World J Gastroenterol. 2006;12:1312–1316. doi: 10.3748/wjg.v12.i8.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding YT, Qiu YD, Chen Z, Xu QX, Zhang HY, Tang Q, Yu DC. The development of a new bioartificial liver and its application in 12 acute liver failure patients. World J Gastroenterol. 2003;9:829–832. doi: 10.3748/wjg.v9.i4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Bartolo L, Bader A. Review of a flat membrane bioreactor as a bioartificial liver. Ann Transplant. 2001;6:40–46. [PubMed] [Google Scholar]
- 29.Chen XP, Xue YL, Li XJ, Zhang ZY, Li YL, Huang ZQ. Experimental research on TECA-I bioartificial liver support system to treat canines with acute liver failure. World J Gastroenterol. 2001;7:706–709. doi: 10.3748/wjg.v7.i5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 31.Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681–682. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 32.Han B, Shi XL, Xiao JQ, Zhang Y, Chu XH, Gu JY, Tan JJ, Gu ZZ, Ding YT. Influence of chitosan nanofiber scaffold on porcine endogenous retroviral expression and infectivity in pig hepatocytes. World J Gastroenterol. 2011;17:2774–2780. doi: 10.3748/wjg.v17.i22.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson CA. Porcine endogenous retroviruses and xenotransplantation. Cell Mol Life Sci. 2008;65:3399–3412. doi: 10.1007/s00018-008-8498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baquerizo A, Mhoyan A, Shirwan H, Swensson J, Busuttil RW, Demetriou AA, Cramer DV. Xenoantibody response of patients with severe acute liver failure exposed to porcine antigens following treatment with a bioartificial liver. Transplant Proc. 1997;29:964–965. doi: 10.1016/s0041-1345(96)00330-2. [DOI] [PubMed] [Google Scholar]
- 35.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 36.van de Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, Scala D, Scala S, Zeuli L, Di Nicuolo G, et al. Bridging a patient with acute liver failure to liver transplantation by the AMC-bioartificial liver. Cell Transplant. 2003;12:563–568. [PubMed] [Google Scholar]


