Abstract
The purpose of the present study was to investigate the association of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9) expression with the histopathological grading of tumors in cerebral glioma. A total of 45 patients with pathologically confirmed cerebral glioma were divided into two groups: a low-grade group (grades I and II, 21 cases) and a high-grade group (grades III and IV, 24 cases). Immunohistochemical staining of tumor samples showed the percentages of tumors expressing VEGF and MMP-9 in the high-grade group to be 95.83 and 75%, respectively, significantly higher than those of the low-grade group (66.67 and 23.81%, P<0.05 and P<0.01, respectively). The magnetic resonance imaging (MRI) results indicated that the peripheral edema index (EI), enhancement percentage (EP), and the maximum diameter of the tumor in the high-grade group were significantly higher than those in the low-grade group (P<0.05, P<0.01, and P<0.05). Moreover, the expression of VEGF and MMP-9 was positively correlated with EI, EP and the maximum diameter of the tumor (P<0.05). Therefore, VEGF and MMP-9 expression were correlated to the invasion of glioma. The association of their expression levels with EI, EP and the maximum tumor diameter indicates that these markers may be used to estimate tumor malignancy for future clinical diagnosis and treatment.
Keywords: neuroglioma, vascular endothelial growth factor, matrix metalloproteinase-9, magnetic resonance imaging
Introduction
Neuroglioma, or glioma, is the most common primary tumor of the central nervous system, accounting for approximately 40–50% of all intracranial tumors (1). These tumors are characterized by a high invasive potential and a wide diversity of histological appearance. As with other tumors, one of the crucial steps in invasion is angiogenesis of the peritumoral tissues (2,3). Two primary factors that mediate tumor angiogenesis, vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9), have been researched in various tumor types (1–3). These studies show that VEGF and MMP-9 expression varies in the majority of tumor cells and is directly associated with tumor invasion.
Recent advances in imaging techniques have increased the use of non-invasive examination in the diagnosis and treatment of glioma. Magnetic resonance imaging (MRI) is one such valuable imaging technique, which has the advantages of non-traumatic, non-ionizing radiation and multiple planar imaging. In combination with other approaches, MRI is capable of visualizing various intracranial lesions (both structural and functional) and detecting the correlation between the major white matter fiber bundle and glioma lesions. Additionally, the complexity of these tumors has generated interest in identifying biomarkers that are capable of aiding in the diagnosis and treatment of gliomas (4). The current study used MRI to determine the correlation of expression of VEGF and MMP-9 with MRI characteristics and clinical pathological grades of cerebral gliomas to aid in clinical treatment and prognosis assessment.
Materials and methods
Study subjects
The study involved 45 cerebral glioma patients from the Department of Neurosurgery, the Third Affiliated Hospital of Harbin Medical University, China, between September 2008 and July 2010. The population included 26 males and 19 females aged 18–65 years, with a mean age of 52.6 years. The patients were divided into two groups: a low-grade group (grades I and II, n=21) and a high-grade group (grades III and IV, n=24), according to diagnostic gradation criteria for gliomas from the 2007 World Health Organization (WHO) classification of tumors of the central nervous system (5).
MRI scan
MRI scans were performed by a Philips Gyroscan Intra 1.5T superconductor MRI scanner. A standard quadrature head coil was used. SE sequences of MR scans included axial and sagittal T1WI and axial T2WI. A contrast agent (Gd-DAPA, 0.1 mM/kg) was administered by fast injection (1–2 min for completion) at a dose of 0.1 mM/kg via the elbow vein to enhance axial and sagittal T1WI scans. Capture settings were as follows: TlWI, TR/TE 431 ms/11 ms; T2WI, TR/TE 4850 ms/120 ms; FOV 24×24 cm; matrix 256×256; NEX 2; band width 12.5 kHz; slice thickness, 5 mm; interval, 1 mm. Enhanced scan parameters were identical to T1WI.
Immunohistochemical examination of VEGF and MMP-9
Fresh tumor specimens were obtained during surgery and fixed with 10% formalin solution. Tumor tissues were embedded in paraffin, cut into 5 μm serial sections, and placed onto glass slides. One slide was routinely stained with hematoxylin and eosin (H&E) to confirm the pathological results. Other sections were used to perform immunohistochemical staining with mouse anti-human VEGF monoclonal antibody and mouse anti-human MMP-9 monoclonal antibody (Wuhan Boster Biological Technology Co., Ltd., China) detected by SP or DAB kits, according to the manufacturer’s instructions (Wuhan Boster Biological Technology Co., Ltd.). VEGF was visualized by brown or dark brown staining in the cytoplasm or cell membrane of tumor cells; MMP-9 appeared as brown or brown-yellow particles in the cytoplasm of tumor cells. We assessed the distribution, intensity and location of staining in positive cells. Some sections were incubated with phosphate-buffered saline (PBS) instead of primary antibody as a negative control and known positive breast cancer sections were used as a positive control.
MRI detection for intracranial edema index (EI)
The edge clarity and uniformity of the tumor signal were evaluated by two highly qualified imaging physicians. Specifically, the largest lesion entity area on the enhanced horizontal axis TIWI was used to measure the maximum diameter of the tumors. Additionally, vertical diameter line length was measured on the enhanced horizontal axis of the MRI, and the maximum height of the tumor body was measured on the sagittal MRI. Thus, the volume of the tumor body was determined by multiplying these three parameters. Edema volume (including tumor body) and maximum edema height were measured according to standard methods (6) on T2WI to calculate the layer number occupied by T2WI edema. The layer number was also multiplied by the layer thickness and interval to obtain the edema volume/tumor body volume (EI) ratio.
MRI detection for the enhancement percentage (EP) of the intracranial lesions
On MRI contrast images, a region of interest (ROI) within the lesion entity area of the T1WI scan was selected, and the signal intensity was measured. The EP, which is used to reflect the degree of contrast enhancement in lesions, was calculated as EP = difference value of unenhanced and enhanced signal intensity/unenhanced signal intensity × 100.
Statistical analysis
Values are expressed as the mean ± standard deviation (x±s). Data were analyzed with SPSS 12.0 software. Comparisons of parameters among different groups were performed using t-tests and chi-square tests. Analysis was performed using the Pearson’s correlation method. P<0.05 was considered to be statistically significant.
Results
Immunohistochemical examination of VEGF
VEGF expression was detected in the cytoplasm of tumor cells or occasionally in cell membranes. Cell nucleus staining was also occasionally observed as yellow or yellow-brown in color, mostly in tumor cells and in a few interstitial cells. VEGF was detected in 82.2% of the cells (37/45). However, expression varied by tumor grade, with a positive expression in 66.67% (14/21) of the low-grade tumors and 95.83% (23/24) of the high-grade tumors. This expression difference was significant between the two groups (P<0.05, Table I and Fig. 1).
Table I.
Correlation between the expression of VEGF and MMP-9 in cerebral gliomas and clinical pathological grade of the tumor.
| Groups | Cases | VEGF | MMP-9 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value | ||
| Low-grade | 21 | 14 | 7 | 6.98 | <0.05 | 5 | 16 | 11.24 | <0.01 |
| High-grade | 24 | 23 | 1 | 18 | 6 | ||||
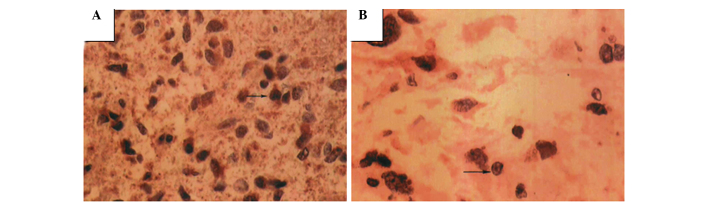
Figure 1.
VEGF expression in high- and low-grade gliomas (magnification, ×400). (A) High-grade glioma showing numerous tumor cells with darkly-stained nuclei and a different shape. Brown-stained particles are shown in the cytoplasm and intercellular space. (B) Low-grade glioma with few VEGF-positive tumor cells. Arrow indicates a positive cell; brown particles are shown in the cytoplasm (magnification, ×400).
Immunohistochemical examination of MMP-9
MMP-9 was detected in the cytoplasm of tumor cells, vascular endothelial cells and basal membranes. The percentage of tumors expressing MMP-9 was 51.1% (23/45), but, similar to VEGF, this expression varied by tumor grade: 23.81% (5/21) of low-grade tumors expressed MMP-9 versus 75% (18/24) of high-grade tumors. These differences were statistically significant (P<0.01; Table I and Fig. 2).
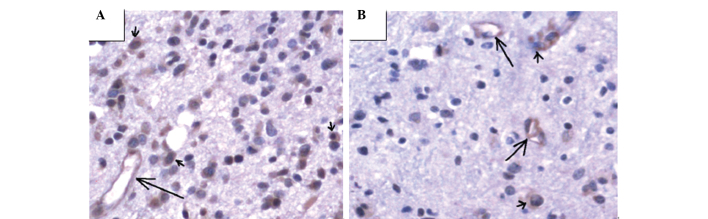
Figure 2.
MMP-9 staining of gliomas. MMP-9 was detected in the cytoplasm of tumor cells (brown, short arrow), as well as the cytoplasm of vascular endothelial cells (brown particles, long arrow). (A) High-grade and (B) low-grade glioma.
MRI scanning results
Standard T1WI scans of high-grade gliomas showed low signals or equal mixed signals; only a few showed low, equal and high mixed signals. T2WI scans of high-grade gliomas showed equal and high mixed signals, with clear boundaries and observable necrosis or bleeding, and the majority of the gliomas showed uneven and marked enhancement. By contrast, low-grade gliomas showed low or slightly low signals in T1WI. The majority of signals were relatively uniform, but a few signals were mixed. T2WI scans of low-grade gliomas showed high or slightly high, signals, and the majority of the signals were extremely uniform, with clear boundaries, usually not enhanced or slightly enhanced, with rare bleeding. Gliomas were observed invading the corpus callosum to the contralateral hemisphere through the midline, and a few of the gliomas affected multiple sites, showing multiple lesions. EI, EP and the maximum tumor diameter were (1.23±0.78), (4.57±3.79)% and (36.2±11.17) mm, respectively, for low-grade tumors, and (7.75±6.61), (35.46±18.78)% and (56.22±10.12) mm, respectively, for high-grade tumors.
Correlation between VEGF and MMP-9 expression in gliomas and peritumoral EI, EP and tumor size
The correlation between EI, EP and the maximum tumor diameter are shown in Table II. EI, EP and the maximum diameter were all significantly higher in the high-grade tumor patients than in the low-grade patients. Pearson’s correlation analysis showed that the expression of VEGF and MMP-9 was positively correlated with EI (r=0.516, r=0.626, P<0.05), EP (r=0.567, r=0.549, P<0.05) and maximum tumor diameter (r=0.655, r=0.506, P<0.05).
Table II.
Correlation between EI, EP, and maximum tumor diameter.
| Groups | EI | EP (%) | Max tumor diameter (mm) |
|---|---|---|---|
| Low-grade | 1.23±0.78 | 4.57±3.79 | 36.2±11.17 |
| High-grade | 7.75±6.61 | 35.46±18.78 | 56.22±10.12 |
| t | 6.32 | 9.65 | 5.23 |
| P-value | <0.05 | <0.01 | <0.05 |
Discussion
Glioblastoma is the most common cancer in the central nervous system, and preoperative examinations depend mainly on CT and MRI (7,8). The properties of tumors are usually determined by gross specimen observation and microscopic observation of their shape and arrangement. The type and degree of differentiation of the tumor tissues may be determined by immunohistochemical markers (9,10).
Prompt and accurate diagnosis and treatment of cerebral neoplasms are critical for decreasing morbidity and mortality. New therapeutic modalities, such as image-guided surgery and anti-angiogenic agents, are becoming increasingly reliant on high-quality imaging for diagnostic evaluation, treatment planning and post-treatment follow-up. CT and MRI are the mainstays of imaging in current practice. MRI, with its multiplanar abilities and superior contrast resolution, is now the modality of choice. MRI is accurate in preoperative diagnosis and assessing the characteristics of primary intra-axial brain tumors. This technique is extremely accurate in assessing the grade of gliomas. Tumor necrosis, irregular margins and peritumoral edema are the most significant indicators of tumor grade (11,12).
The major biological characteristics of tumors include uncontrollable growth, metastatic potential, serious harm to the body and mortality. Spreading of tumors involves early growth of the primary tumor, angiogenesis, tumor cell detachment and invasion of matrix, invasion of the vascular system, thrombus formation and secondary growth location in tissues and organs (13). Throughout this process, tumor angiogenesis and matrix destruction are key events and have been central to oncology research in recent years.
During tumor growth, angiogenesis factors, including VEGF, are secreted to promote tumor vascularization, which leads to the continuous growth of tumor cells and invasion into adjacent tissues and structures (14). Additionally, the interaction between tumor cells and the extracellular matrix is a critical step of invasion and metastasis. Matrix metalloproteinases cause degradation of the extracellular matrix and regulate cell adhesion, aiding these steps (15,16). MMP-9, in particular, degrades and destroys the adjacent matrix and creates tumor vascular endothelial barrier damage, leading to a decreased steric hindrance, an increased vascular permeability and the extravasation of nutrients (17). These changes provide matrix and space for the neovascularization of gliomas and promote tumor growth and invasion.
Results of our study show that glioma tissues with a higher degree of malignancy are more likely to express VEGF and MMP-9, indicating that VEGF and MMP-9 play significant roles in the progression of gliomas. Thus, the expression of VEGF and MMP-9 shows the malignant phenotype of gliomas, and serves as an indicator of glioma invasion.
CT and MRI have great significance in localization diagnosis and qualitative diagnosis of gliomas (18). CT is extremely accurate and reliable in the localization of diagnosis of gliomas with a certain reference value for qualitative diagnosis. However, MRI is not affected by the artifact of posterior cranial fossa, has a more vivid black and white contrast, is capable of simultaneously running transverse, coronal and sagittal scans, and thus is better than pure transverse CT. The biggest advantage of a brain MRI is that it provides a good anatomical background without bone artifact and is also capable of exhibiting the panorama and three-dimensional location of the tumor. This feature is an improvement on the CT for tumors of the cerebellum, brain stem, saddle area and craniocervical junction. Information provided by conventional MRI includes enhancement degree, enhancement form, peritumoral edema scope, tumor size, clarity of the boundaries, uniformity of the signals, mass effect, midline shift, lesion sites, necrotic cyst and bleeding, flowing void effect and T1WI and T2WI signal strength. MRI features correlate with glioma pathological stages (19). In particular, the EP, peritumoral edema scope, tumor diameter, unclear boundaries, signal asymmetry, mass effect and midline shift are positively correlated with pathological grade; in particular, peritumoral edema and EP were highly correlated (20). MRI studies show unclear enhancement in brain-tumor interfaces and partial gliosis, indicating low-grade glioma. Notably, with increasing pathological grade the brain-tumor interfaces become irregular, with uneven enhancement in wall thickness, and the enhancement gradually becomes clear. Since VEGF and MMP-9 were capable of promoting tumor angiogenesis, and high-grade gliomas have significant angiogenesis, EP may be a significant reference index for determining tumor malignancy through imaging (21). In this study, the expression of VEGF and MMP-9 were positively correlated with EI, EP and maximum tumor diameter. These findings suggest that the expression of VEGF and MMP-9 may be used as a reference factor in judging tumor malignancy in combination with intracranial MRI examination.
In conclusion, the expression of VEGF and MMP-9 in tumor tissues, in combination with the peritumoral EI, EP and tumor size, as well as uniformity of the signal and other characteristics of MRI, may be used as indicators of malignant behavior, key to diagnosing brain gliomas early. Thus, patients are able to receive timely surgical treatment, in combination with radiation therapy and chemotherapy, which is significant in improving the quality of life of patients.
References
- 1.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–50. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1 alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 3.Zheng SQ, Huang RQ, Zhang YJ. Role of matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial growth factor C in lymph node metastasis of breast cancer [J] Zhonghua Bing Li Xue Za Zhi. 2010;39:240–244. [PubMed] [Google Scholar]
- 4.Sulman EP, Aldape K. The use of global profiling in biomarker development of gliomas. Brain Pathol. 2011;21:88–95. doi: 10.1111/j.1750-3639.2010.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inamura T, Nishio S, Takeshita I, Fujiwara S, Fukui M. Peritumoral brain edema in meningiomas influence of vascular supply on its development. Neurosurgery. 1992;31:179–185. doi: 10.1227/00006123-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Piroth MD, Pinkawa M, Holy R, Stoffels G, Demirel C, Attieh C, Kaiser HJ, Langen KJ, Eble MJ. Integrated-boost IMRT or 3-D-CRT using PET-PET based auto-contoured target volume delineation for glioblastoma multiforme - a dosimetric comparison. Radiat Oncol. 2009;4:57. doi: 10.1186/1748-717X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67:139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Chan ES, Yeh MM. The use of immunohistochemistry in liver tumors. Clin Liver Dis. 2010;14:687–703. doi: 10.1016/j.cld.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Sethi K, Sarkar S, Das S, Mohanty B, Mandal M. Biomarkers for the diagnosis of thyroid cancer. J Exp Ther Oncol. 2010;8:341–352. [PubMed] [Google Scholar]
- 11.Chen W, Silverman DH. Advances in evaluation of primary brain tumors. Semin Nucl Med. 2008;38:240–250. doi: 10.1053/j.semnuclmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Costello JF. DNA methylation in brain development and gliomagenesis. Front Biosci. 2003;8:s175–s184. doi: 10.2741/1027. [DOI] [PubMed] [Google Scholar]
- 13.Diamandis EP, Fritsche HA, Lilja H, Daniel WC, Schwartz MK, editors. Tumor Markers: Physiology, Pathobiology, Technology, and Clinical Applications. AACC press; Washington DC: 2002. p. 17. [Google Scholar]
- 14.Puduvalli VK, Sawaga R. Antiangiogenesis therapeutic stragegios and clinical implications for brain tumors. J Neurooncol. 2000;50:189–200. doi: 10.1023/a:1006469830739. [DOI] [PubMed] [Google Scholar]
- 15.Amãlinei C, Cãruntu ID, Giuscã SE, Bãlan RA. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51:215–228. [PubMed] [Google Scholar]
- 16.Savinov AY, Strongin AY. Matrix metalloproteinases, T cell homing and beta-cell mass in type 1 diabetes. Vitam Horm. 2009;80:541–562. doi: 10.1016/S0083-6729(08)00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 18.Langer A. A systematic review of PET and PET/CT in oncology: a way to personalize cancer treatment in a cost-effective manner? BMC Health Serv Res. 2010;10:283–299. doi: 10.1186/1472-6963-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CQ, Liao WH, Chen CY. Correlation between peritumoral edema on MRI, VEGF expression, MVD and pathological grading in astrocytomas. Chin J Mod Med. 2004;14:38–43. [Google Scholar]
- 20.Deng GX, Zhang H, Qian LX. Ordinal logistic regression analysis of MRl and pathological grade in astrocytoma. Chin Remed Clin. 2005;5:680–682. [Google Scholar]
- 21.Anderson JC, Grammer JR, Wang W, Nabors LB, Henkin J, Stewart JE, Jr, Gladson CL. ABT-510, a modified type 1 repeat peptide of thrombospondin, inhibits malignant glioma growth in vivo by inhibiting angiogenesis. Cancer Biol Ther. 2007;6:454–462. doi: 10.4161/cbt.6.3.3630. [DOI] [PubMed] [Google Scholar]