An increase in cytosolic free calcium concentration is used as a key signaling messenger in virtually every cell throughout the phylogenetic tree. Calcium regulates a host of kinetically distinct processes ranging from neurotransmitter release and skeletal muscle contraction (which operate on a submillisecond time frame) to gene transcription and cell proliferation (which operate over a time course of hours to days). This impressive array of vital functions controlled by calcium nevertheless poses a fundamental problem in cell signaling: How can the same messenger selectively activate only one (or a handful) of calcium-dependent responses to best fit the requirements of the cell at that particular instant? Put another way, how does a cell decode the calcium signal? The calcium signaling system has obviously evolved specificity; however, it is not foolproof, and when calcium signaling goes awry, the consequences can be devastating. A particular striking example is described in this issue of PNAS by Raraty et al. (1). In an elegant series of experiments, Raraty et al. have now unraveled the link between calcium signaling and trypsin activation within acinar cells. Premature activation of trypsin within the granules is often fatal, because it results in autodigestion of the pancreas. It had been suspected for some time that aberrant calcium signaling plays a major role in the etiology of acute pancreatitis, and now that suspicion has been confirmed.
Before the work of Raraty et al. (1), an important clue as to how cytosolic free calcium discriminates between a wide array of specific responses was provided by technical advances that enabled intracellular calcium levels to be observed dynamically in single living cells. Rather than increasing in a gradual manner after stimulation, it turned out that activation of cell-surface receptors that hydrolyzed the minor membrane phospholipid phosphatidyl inositol 4,5,-bisphosphate (PIP2) generated rhythmic oscillations in cytosolic free calcium concentration (2–4). Strikingly, different PIP2-hydrolyzing receptors evoked distinct patterns of oscillation in the same cell (5). With a few notable exceptions, most cells can generate at least some calcium oscillations even when external calcium has been removed. This indicates that the underlying mechanism involves rhythmic calcium release from, and reuptake into, intracellular calcium storage compartments (mainly the sarcoplasmic/endoplasmic reticulum; ref. 6). The first messenger found to release calcium from these stores was calcium itself, through the regenerative process of calcium-induced calcium release that is particularly important in muscle (7). Since then, it has been established that one of the hydrolysis products of PIP2, inositol 1,4,5-trisphosphate (InsP3), is a ubiquitous calcium-releasing messenger (8). Other messengers include nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic adenosine 5′-diphosphate-ribose (cADPR) (9). These are particularly important in pancreatic acinar cells, although their roles in other mammalian cells are unclear at present.
Why would evolution select a complex oscillatory mechanism? This probably arises from the fact that chronic elevations of intracellular calcium are toxic to cells. By evoking only transient high-amplitude spikes, calcium is not sustained long enough for its undesirable effects to be manifested. Moreover, different proteins seem to respond to different features of the calcium transients (amplitude and frequency) and then translate these into specific responses. For example, the multisubunit enzyme calcium–calmodulin kinase apparently counts the calcium spikes (10) and then activates different calcium-dependent processes according to the number of spikes (frequency). Calcium oscillations have been found to control several physiological processes, including exocytosis, mitochondrial ATP production, and gene transcription (11–14).
The key step in acute pancreatitis is the premature intracellular activation of the broad protease trypsin (15, 16). Normally, as Raraty et al. show (1), trypsin is stored within the zymogen granules in the apical pole of the acinar cell in an inactive form (protrypsin). On stimulation (with the secretagogue cholecystokinin), the granules are exocytosed in a calcium-dependent manner, thereby releasing the proenzyme into the pancreatic ducts where it is subsequently carried into the gut by the secreted fluids. In the gut, protrypsin is cleaved to form active trypsin, which then helps digest foodstuffs into peptides and amino acids. These are then reabsorbed by the epithelial cells lining the gut wall. The results, in this issue of PNAS (1), are exciting on three counts. First, compelling evidence linking intracellular calcium to abnormal trypsin activation has been presented for the first time. Second, a novel target has been identified for the design of therapeutic agents aimed at combating this killer disease. Third, new insight is provided into the molecular mechanisms involved in calcium signaling in acinar cells, which may have far-reaching implications.
Raraty et al. (1) also developed a neat method that enabled them to measure simultaneously intracellular calcium levels and the activity of trypsin in single cells. After stimulation with physiological concentrations of cholecystokinin (10 pM), repetitive calcium transients were evoked. These oscillations, which drive exocytosis, consistently failed to activate trypsin stored within the granules. Raising the cholecytsokinin to 10 nM resulted in a sustained elevation of intracellular calcium. This prolonged component arises from calcium entry into the cell through specialized calcium channels in the plasma membrane. These channels are activated by the process of emptying the intracellular calcium stores (called store-operated or capacitative calcium entry; refs. 17, 18). After calcium influx for several minutes, trypsin was activated prematurely within the granules, and detailed electron micrograph analysis revealed the abundant presence of vacuoles in the apical region. These vacuoles represent trypsin-digested zymogen granules. Maneuvers to prevent either calcium entry or the subsequent increase in intracellular calcium suppressed the formation of the vacuoles. Hence it is the rise in calcium itself that engenders trypsin activation, and the rogue calcium enters through store-operated calcium channels. Strikingly, the oscillatory calcium signal had a much bigger amplitude than the sustained entry component, yet only the latter triggered trypsin activation. It would appear that high calcium is not dangerous in the apical pole of the pancreas, provided it is brief. What seems more important is the duration of the calcium signal. This is a beautiful illustration of the importance of an oscillatory signaling mechanism.
Little is known about the molecular properties of the store-operated calcium channels, and electrophysiological experiments indicate there may be a family of such channels with distinct biophysical features (18). Store-operated calcium entry has been linked to several disorders, including primary immunodeficiency (19) and possibly Alzheimer's disease (20). Acute pancreatitis can now be added to this burgeoning list. The results of Raraty et al. (1) imply that drugs aimed at interfering with store-operated calcium influx will be a very effective way to treat pancreatitis. Hopefully, selective inhibitors will be developed soon.
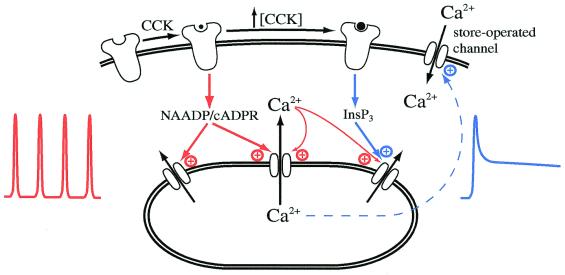
More fundamentally, why do low doses of cholecystokinin evoke calcium oscillations, whereas higher concentrations lead to calcium entry and hence a sustained cytosolic calcium plateau? The possibility that two distinct cholecystokinin receptors are simultaneously expressed in the plasma membrane of acinar cells, one with high sensitivity to cholecystokinin and directing an oscillatory response and the other with lower sensitivity and promoting calcium entry, is unlikely because only one type of cholecystokinin receptor (the A-subtype; ref. 21) is found in the mouse cells used by Raraty et al. (1). A key finding is that low concentrations of cholecystokinin evoke calcium oscillations, not through increases in the levels of InsP3, but by utilizing the NAADP/cADPR system instead (22). Under these conditions, InsP3 in fact plays a minor role. On the other hand, higher concentrations of cholecystokinin evoke calcium signals that depend mainly on InsP3. This means that the same receptor can link to two distinct calcium-mobilizing transduction pathways depending on the level of receptor occupancy and thereby evoke quite different spatial and temporal calcium signals (Fig. 1). This endows cholecystokinin with the ability to accommodate a range of processes having quite distinct calcium requirements. Processes that respond rapidly to calcium increases or that sense the rate of change of calcium or that are positioned close to calcium release sites may be preferentially activated by the oscillatory signals evoked by low levels of receptor occupancy. Calcium-dependent processes that respond slowly or that are located close to the plasma membrane (where the store-operated calcium channels are) may be more efficiently stimulated by higher concentrations of the same agonist. The findings of Raraty et al. therefore provide a new perspective to the old problem of how specific responses can be obtained by using a promiscuous signaling messenger.
Figure 1.
Model for calcium signaling after activation of cholecystokinin receptors. Low (physiological) levels of cholecystokinin utilize mainly the NAADP/cADPR pathway. Calcium release by cADPR acting on an open ryanodine-sensitive receptor exerts positive feedback on the receptor as well as adjacent ones, resulting in regenerative calcium-induced calcium release and thus oscillations in intracellular free calcium concentration. This pathway is shown in red. Calcium release by cADPR can also interact synergistically with InsP3 to facilitate further calcium release via open InsP3 receptors. However, physiological levels of cholecystokinin do not increase the levels of InsP3. Higher concentrations of cholecystokinin trigger a robust increase in InsP3, and this results in calcium mobilization from InsP3-sensitive calcium stores. The fall in calcium concentration within the stores activates store-operated calcium entry that underlies the sustained phase of the calcium signal under these conditions. This pathway is shown in blue. The calcium entry and not the oscillatory response results in the premature activation of trypsin within the zymogen granules.
Acknowledgments
I thank Daniel Bakowski for help with the figure and the Wellcome Trust for support.
Footnotes
See companion article on page 13126.
References
- 1.Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos J P, Sutton R, Petersen O H. Proc Natl Acad Sci USA. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods N M, Cuthbertson K S, Cobbold P H. Nature (London) 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- 3.Jacob R, Merritt J E, Hallam T J, Rink T J. Nature (London) 1988;335:40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- 4.Tsien R W, Tsien R Y. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- 5.Thomas A P, Bird G S J, Hajnoczky G, Robb-Gaspers L D, Putney J W. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- 6.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 7.Endo M. Physiol Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- 8.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 9.Petersen O H, Burdakov D, Tepikin A V. BioEssays. 1999;21:851–860. doi: 10.1002/(SICI)1521-1878(199910)21:10<851::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.De-Koninck P, Schulman H. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 11.Tse A, Tse F W, Almers W, Hille B. Science. 1993;260:82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- 12.Hajnoczky G, Robb-Gaspers L D, Seitz M B, Thomas A P. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 13.Dolmetsch R, Xu K, Lewis R S. Nature (London) 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 14.Li W-H, Llopis J, Whitney M, Zlokarnik G, Tsien R Y. Nature (London) 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 15.Neoptolemos J P. In: Bailliere's Clinical Gastroenterology. Neoptolemos J P, editor. Vol. 13. London: Bailliere Tindall; 1999. pp. 213–370. [Google Scholar]
- 16.Neoptolemos J P, Kemppainen E, Mayer J M, Fitzpatrick J M, Raraty M G T, Slavin J, Beger H-G, Hietaranta A J, Puolakkainen P A. Lancet. 2000;355:1955–1960. doi: 10.1016/s0140-6736(00)02327-8. [DOI] [PubMed] [Google Scholar]
- 17.Putney J W. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Parekh A B, Penner R. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 19.Partiseti M, Le D F, Hivroz C, Fischer A, Korn H, Choquet D. J Biol Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 20.Yoo A S, Cheng I, Chung S, Grenfell T Z, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, et al. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 21.Saillan-Bareau C, Clerc P, Adato M, Escrieut C, Vaysse N, Fourmy D, Dufresne M. Gasteroenterology. 1998;115:988–996. doi: 10.1016/s0016-5085(98)70271-9. [DOI] [PubMed] [Google Scholar]
- 22.Cancela J M, Gerasimenko O V, Gerasimenko J V, Tepikin A V, Petersen O H. EMBO J. 2000;19:2549–2557. doi: 10.1093/emboj/19.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]