Abstract
Background: Aged patients (>50 years old) with residual schizophrenic symptoms differ from young patients. They represent a subpopulation with a more unfavorable Kraepelinian course and have an increased risk (up to 30%) for dementia of unknown origin. However, our current understanding of age-related brain changes in schizophrenia is derived from studies that included less than 17% of patients who were older than 50 years of age. This study investigated the anatomical distribution of gray matter (GM) brain deficits in aged patients with ongoing schizophrenia. Methods: Voxel-based morphometry was applied to 3D-T1 magnetic resonance images obtained from 27 aged patients with schizophrenia (mean age of 60 years) and 40 age-matched normal controls. Results: Older patients with schizophrenia showed a bilateral reduction of GM volume in the thalamus, the prefrontal cortex, and in a large posterior region centered on the occipito-temporo-parietal junction. Only the latter region showed accelerated GM volume loss with increasing age. None of these results could be accounted for by institutionalization, antipsychotic medication, or cognitive scores. Conclusions: This study replicated most common findings in patients with schizophrenia with regard to thalamic and frontal GM deficits. However, it uncovered an unexpected large region of GM atrophy in the posterior tertiary cortices. The latter observation may be specific to this aged and chronically symptomatic subpopulation, as atrophy in this region is rarely reported in younger patients and is accelerated with age.
Keywords: aging/MRI/VBM, schizophrenia/gray matter/cross-sectional
Introduction
Early in the 20th century, Kraepelin1 and Bleuler2 and others3 reported non-specific brain atrophy with ventricular enlargement in patients with dementia praecox/schizophrenia. This observation was subsequently confirmed in vivo using gas pneumo-encephalography4, CT scanning5, and, most recently, magnetic resonance imaging (MRI)6. Although early studies suggested that brain volume reduction in schizophrenia was static over time7, there is now evidence for accelerated loss of gray matter (GM) during both the first episodes8 and in patients with chronic disease9. An accelerated fronto-temporal cortical GM decline at the onset of psychosis is the most frequent observation10.
But surprisingly, very little is currently known about brain changes in aged patients with a classical onset of schizophrenia. To our knowledge, patients over the age of 50 represent less than 17% of all patients according to cross-sectional and longitudinal studies investigating brain changes associated with aging in schizophrenia11–13. Moreover, most of these studies examined total GM volume rather than focal changes. As a result, data are sparse regarding the anatomy of GM changes in this specific subpopulation of older symptomatic patients.
Accordingly, the dynamic of brain changes in schizophrenia is not appropriately captured as it might change with increase in age. Therefore, recruiting older patients from psychiatric institutions might more closely match the Kraepelinian concept because they represent patients requiring ongoing psychiatric care due to ongoing symptoms. Indeed, 46%–65% of patients with an International Classification of Diseases /DSM diagnosis of schizophrenia are considered to be cured after 15 years or more and do not require further psychiatric care14, a fact confirmed by the recent World Health Organization 15 years follow-up study15. Interestingly enough, a fraction of this chronically disabled population has been reported to develop severe non-Alzheimer’s dementia16. Thus, old patients with schizophrenia may represent a particular subpopulation that deserves further investigation.
This study (1) describes GM deficits in aged patients with schizophrenia compared with data on young adults with schizophrenia available in the literature and (2) examines the correlation between decreases in GM and aging. In this cross-sectional study, we used voxel-based morphometry (VBM)17, an automated method allowing identification of anatomical differences in the whole brain without prespecification of a region of interest. We hypothesized that the same frontal and temporal GM reductions previously described in young patients would be more obvious in this aged population.
Methods
Study Participants
Fifty-six patients who met inclusion criteria, ie, DSM IV diagnosis of schizophrenia, stable for ≥3 months, were recruited from 2 European psychiatric centers (Strasbourg, France and Luxembourg, Luxembourg). The patients had no current or past substance abuse histories, and no medical condition that may have affected brain anatomy. From this population, 23 patients refused to give consent for the MRI (refusal and/or lack of understanding), 2 withdrew from the study before the MRI ended, and 4 were excluded from analysis due to movement-related imaging artefacts. Mean chlorpromazine equivalent were computed according to Woods18. Clinical symptoms were assessed using the Positive and Negative Assessment Syndrome Scale19.
Forty healthy normal controls were also recruited from both centers. They were matched with the patient group in terms of handedness (all right-handed), age, education, and gender. Controls were matched to patients independently in each center in order to avoid Group × Center interaction. Neither was taking medication nor had a significant neurological or psychiatric history. All participants gave written informed consent prior to taking part in the study, which was approved by the local ethics committee.
Each participant was evaluated with 3 cognitive tests: (1) the Mini Mental State Examination (MMSE)20 to screen for cognitive impairment, (2) the Mattis Dementia Rating Scale (MDRS)21 to assess the global cognitive functioning, and (3) the Frontal Assessment Battery (FAB)22 to explore functions related to the frontal lobe.
Brain Imaging
The MRI scans were collected in both centers (Strasbourg and Luxembourg). Strasbourg’s center is equipped with a Siemens 1.5-tesla scanner, and Luxembourg’s center has a GE 1.5-tesla scanner. Whole-brain 3D-T1 Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) sequences were obtained with the following parameters: MPRAGE (1 × 1 × 1 mm, RT = 9.7 ms, TE = 4 ms, FA = 12°; Strasbourg) and MPRAGE (0.5 × 0.5 × 0.7 mm, RT = 6.5 ms, TE = 1.448 ms, FA = 25°; Luxemburg).
The analysis followed the VBM methods17 using SPM2 (Statistical Parametric Mapping, developed by the Wellcome Institute, London, United Kingdom) running on Matlab version 6.5 (MathWorks, Inc., Natick, Massachusetts). Brain volumes were first normalized and density was corrected using Jacobian determinants derived from the deformation fields to preserve the regional amount of GM. All images were resampled to a voxel resolution of 1 × 1 × 1 mm and further segmented into GM, white matter (WM), and cerebral spinal fluid. Finally, GM images were smoothed using a 3D Gaussian kernel (8 mm full width at half maximum).
Statistical Analysis
We compared the socio-demographic, clinical, and cognitive characteristics of patients and controls using an ANOVA including the Group and the Center variable with a threshold of P < 0.05, except for gender, which was analyzed with a chi-square test without accounting for the center. Only GM-segmented images were processed. All SPM maps were obtained using multiple regression, modeling 3 factors of interest: Group (control vs patient), Age, and the interaction between Group and Age. Three additional covariates of non-interest were added to the statistical model by introducing the variables of Center (Luxembourg and Strasbourg), Gender (male or female), and Education (years). The GM probability volumes of all participants were averaged to build a mask for the analysis to display differences in GM only (threshold of 0.2 probability). Maps for the main effect of age and group were set at a threshold of P < 0.001 (t-test, unilateral) with an extent threshold of k = 1000 voxels (thus, 1 cm3).
For the Group × Age interaction and the Group × Center one, the volume of interest was limited to the regions already different in the group comparison (with a threshold of P < 0.002, F-test, thus bilateral) and a lenient threshold of P < 0.05 (F-test) was used with an extent threshold of k = 1000 voxels (combined significance of P < 10−4).
No correction for multiple testing was used for map display using the above mentioned thresholds, but the corrected P value is given in the related table using the false-discovery rate method.23
As complementary analysis, we compared demented (MMSE ≤ 23) vs non demented patients (MMSE > 23), institutionalized vs non-institutionalized patients, and patients receiving typical vs atypical antipsychotics using a 2 sample t-test on the patient population only. We also looked for a correlation between cognitive scores (MMSE, MDRS, and FAB) and chlorpromazine equivalent within the patient group. The methods were the same as previously described: same covariates of non-interest, uncorrected threshold of P < 0.05, extension of k = 1000 voxels.
Results
Socio-demographic, Clinical, and Cognitive Characteristics
The scores and MRI of 27 right-handed patients with schizophrenia were analyzed. None had a late onset of schizophrenia (ie, ≥50 years). All patients, except 2, were treated with antipsychotics (3 received an atypical, 14 received a typical, and 8 received a combination). Twelve patients received anticholinergic medication. Fourteen patients were institutionalized (table 1).
Table 1.
Demographic, Clinical and Cognitive Characteristics. Mean (Standard Deviation)
| Patients | Controls | Statistics (P values) | |||
| Sample size (n) | 27 | 40 | Group | Center | GxC |
| Age (y) | 59.9 (9.1) | 62.2 (7.8) | n.s. | 0.002 | n.s. |
| Range | 50–82 | 50–86 | |||
| Gender (% male) | 52 | 43 | n.s. | (Chi2 sta) | |
| Educational level (y) | 8.9 (2.3) | 10.6 (2.2) | 0.002 | n.s. | n.s. |
| Age at first episode (y) | 29.7 (9.94) | NA | n.s. | ||
| Illness duration (y) | 29.2 (9.6) | n.s. | |||
| PANSS | |||||
| Total score | 64.0 (24.6) | n.s. | |||
| Positive scale | 20.0 (8.7) | n.s. | |||
| Negative scale | 25.7 (8.0) | n.s. | |||
| Psychopathology | 48.3 (2.0) | n.s. | |||
| Institutionalized (n) | 14 | n.s. | |||
| Chlorpromazine equivalent (mg/d) | 187.2 (192.8) | n.s. | |||
| Cognition | |||||
| DRS (score/144) | 113.7 (30.9) | 141.2 (2.2) | <10−4 | n.s. | n.s. |
| FAB (score/18) | 11 (5.0) | 17.5 (1.1) | <10−4 | n.s. | n.s. |
| MMSE (score/30) | 22.2 (7.7) | 29.1 (1.0) | 8 × 10−4 | n.s. | n.s. |
There were no differences between the 2 groups for age or gender. Despite our effort to match patients with controls, the education level of the patients was significantly lower. Mean scores from the cognitive impairment scales were below or close to the pathological level in the patient group and differed very significantly from controls. However, this was mainly due to a small group of 7 patients (approximately one-quarter) that were severely impaired (MMSE ≤ 23), whereas the other patients were in the normal range. Participants in Luxembourg, ie, both patients and controls, were significantly older than the ones in France (64 ±9 and 58 ±6 y respectively). But for all the above-mentioned variables, there was no significant Group × Center interaction.
Group Effect
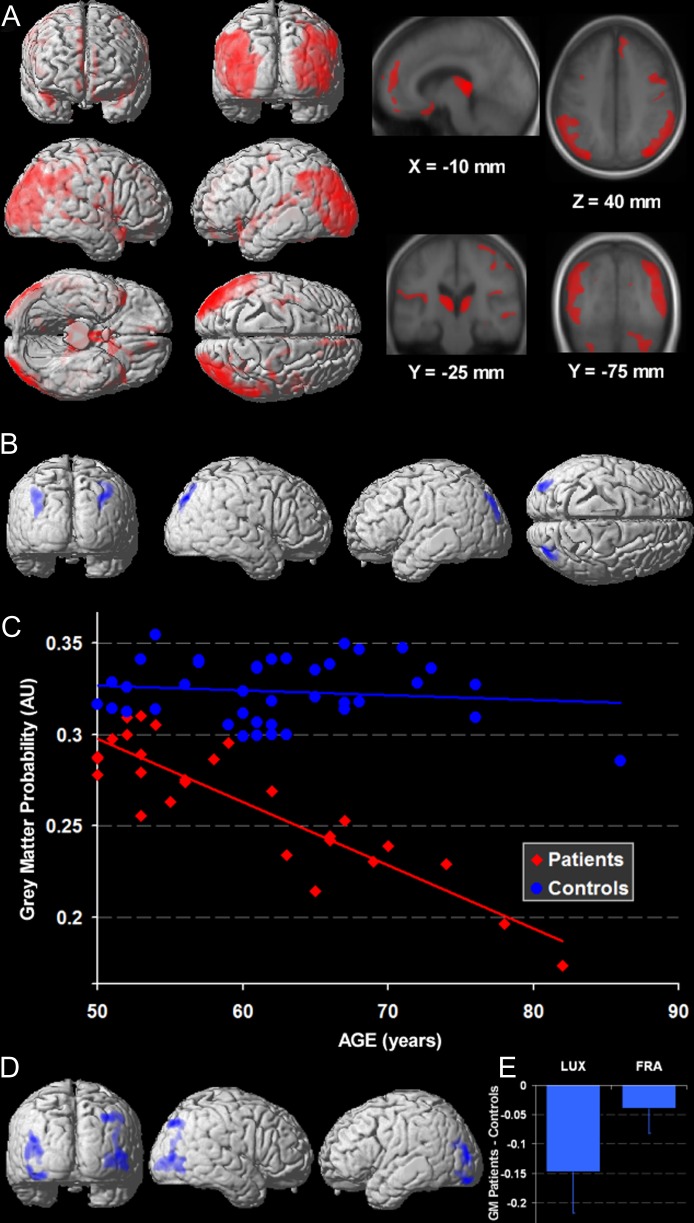
In a control vs patient contrast, patients had a lower GM volume in the thalamus and the region around the third ventricle in both hemispheres (figure 1A). A large cluster of GM atrophy bilaterally encompassed the superior temporal sulcus, the mid and superior occipital gyrus, and the inferior parietal lobule. The other structures showing significant GM reduction encompassed mainly the frontal lobes: the bilateral inferior frontal gyri, the right medial frontal gyrus, the right superior frontal gyrus, the bilateral calloso-marginal sulcus, and the left precentral sulcus. Finally, the left fusiform gyrus also showed a GM decrease in patients. The reverse contrast, ie, patients vs controls, showed no GM loss in controls relative to patients (table 2).
Fig. 1.
3D Rendering of Statistical Parametric Maps (SPM) for GM volume in patients and group interactions with age. (A) The GM decreases in patients with schizophrenia (NC-SCZ) are larger in the occipito-temporo-parietal region than in the anterior regions (P < 0.001 ext. 1 cc). (B) The Group × Age interaction was significant in the parieto-occipital junctions (P < 0.05, ext. 1 cc) and was accounted for by a steeper GM volume decrease with age in schizophrenia (C) (AU = arbitrary unit). (D) The Group × Center interaction was significant in lateral occipital cortices (P < 0.05, ext. 1 cc), but even in these regions, the Group effect, ie, patients vs controls GM decrease, was observed in both Centers (E) (main effect and standard deviation in AU).
Table 2.
SPM Results. The Z is given uncorrected, the P is corrected for multiple testing according to the false discovery rate method for the voxel with the highest value (x, y, z stands for Talairach coordinates). Cluster volume is given in cubic centimetre, ie, cc (equivalent to millilitre).
| Brain region | Brodmann’s area (BA) | P (FDR-cor) | Z score | x, y, z coordinates | Cluster volume (cc) | ||
| x | y | z | |||||
| Controls vs patients contrast | |||||||
| Thalamus (R/L) | <2 × 10−16 | 6.28 | −11 | −31 | 4 | 17.8 | |
| Medial temporal gyrus/medial and superior occipital gyrus/inferior parietal lobule (L) | 39/19 | <2 × 10−16 | 6.14 | −41 | −82 | 23 | 45 |
| Medial temporal gyrus/medial and superior occipital gyrus/inferior parietal lobule (R) | 19 | <2 × 10−16 | 5.87 | 37 | −78 | 39 | 42 |
| Inferior frontal gyrus (R) | 45 | <2 × 10−16 | 5.14 | 49 | 16 | 5 | 10.8 |
| Calloso-marginal sulcus (L) | 10 | <2 × 10−16 | 4.84 | −10 | 51 | 19 | 2.4 |
| Medial frontal gyrus (R) | 10 | <2 × 10−16 | 4.83 | 10 | 48 | −7 | 1.3 |
| Fusiform gyrus (L) | 19 | <2 × 10−16 | 4.8 | −23 | −59 | −25 | 5.7 |
| Precentral sulcus (L) | 6 | <2 × 10−16 | 4.54 | −34 | −12 | 62 | 1.6 |
| Superior frontal gyrus (R) | 6 | <2 × 10−16 | 4.42 | 16 | −6 | 69 | 2.4 |
| Fusiform gyrus (R) | 37 | 0.001 | 4.42 | 44 | −59 | −42 | 6.5 |
| Inferior frontal gyrus/superior temporal pole (L) | 47/38 | 0.001 | 4.28 | −38 | 21 | −17 | 2.8 |
| Calloso-marginal sulcus (R) | 9 | 0.001 | 4.19 | 8 | 52 | 28 | 3.7 |
| Group × Age interaction contrast | |||||||
| Parieto-occipital sulcus/cuneus (R) | 19 | 0.7 | 3.02 | 35 | −81 | 37 | 1.8 |
| Parieto-occipital sulcus/cuneus (L) | 19 | 0.7 | 2.5 | −33 | −89 | 35 | 2 |
| Group × Center interaction contrast | |||||||
| Middle occipital gyrus/Parieto-occipital sulcus (R) | 19 | 0.4 | 4.1 | 46 | −80 | 4 | 6.2 |
| Middle occipital gyrus (L) | 19 | 0.4 | 3.7 | −35 | −89 | 8 | 4.5 |
Note: R, right; L, left.
Age Effect
The regions of reduced GM volume as a function of age corresponded bilaterally to the thalamic area, the internal temporal region (hippocampus and parahippocampal gyrus), and the intermediate cerebellar lobule. In the right hemisphere, age-related GM decreases were found in the insula and the superior temporal sulcus. In the left hemisphere, decreases in GM were observed in the inferior parietal sulcus and the inferior frontal gyrus. There was no region in which GM volume increased with age (See online supplementary figure 1).
Group × Age Interaction
We observed a Group × Age interaction in the bilateral occipito-parietal area (figure 1B), corresponding to a steeper rate of GM decreases with age in patients with schizophrenia relative to controls (figure 1C, table 2).
Group × Center Interaction
At our lenient threshold, the Group × Center interaction was significant in the middle occipital gyrus bilaterally (figure 1D). In these regions, the main group effect was significant for both centers but was larger for the participants in Luxembourg than those in Strasbourg (figure 1E, table 2).
Comparison of Different Subgroups of Patients According to the Demented Status (n = 7/20), Institutionalization (n = 14/13), Typical/atypical Antipsychotic Medication (n = 14/3), and Correlations with Cognitive Scores and Chlorpromazine Equivalent Dose in the Patients Group
For all these contrasts, we did not find any differences between patients in any of these tests, including in the occipito-temporo-parietal region, even at a very lenient threshold of P < 0.05 (unilateral, uncorrected) with k > 1000 cm3.
Discussion
In short, a quarter of elderly patients had cognitive scores meeting the values observed in dementia. Older patients had expected GM volume deficits in the thalami, pre-motor regions, medial and inferio-lateral frontal lobes, and superior temporal sulci. Expected dorso-lateral prefrontal GM atrophy was not observed. In addition, older patients displayed unexpected extensive atrophy encompassing the bilateral occipital-parietal-temporal junctions. In the latter region, patients showed an accelerated decrease in GM with age relative to controls. The institutionalization status, demented status, cognitive scores, and treatment type and dosage were not significantly correlated with a decrease in GM volume. These results will first be confronted to the present literature for each region. Two alternative interpretations will then be discussed before stressing the limitation of the study.
Prefrontal Cortices
The fronto-temporal atrophy in these patients reasonably matched that reported in studies of young adult patients6,24. A noticeable exception was the absence of GM loss in the dorso-lateral prefrontal cortices. Significant differences were reported in 25% of previous VBM studies24 and in up to 50% of volumetric studies6. Another exception was the absence of a loss in the medio-temporal regions, where GM deficits were reported in 61%–70% of previous studies6,24. It is always difficult to interpret a non-significant result because, in a modest-sized study such as ours, the absence of a significant effect may only reflect a lack of power or sensitivity. However, considering that the above-mentioned group differences and age-related atrophy reasonably matched the data reported in the literature, the modest sample size might have been compensated for by a greater effect size in our sample. This greater effect size may have been expected from the large difference in cognitive scores between the 2 groups. Accordingly, the absence of dorsolateral prefrontal involvement is surprising.
Internal temporal structures
This is also true for the medial temporal regions, in which atrophy was significantly correlated with age but was not different between groups. Thus, it can be postulated that the effect of GM atrophy is smaller in the internal temporal regions and possibly in the dorsolateral prefrontal cortex relative to the effect of atrophy in the thalamus and the other frontal and temporal regions.
One possible reason for the absence of change in the internal temporal and dorsolateral prefrontal cortices may be that normal controls have an accelerated GM loss in these structures beyond a certain age. As a result, the GM volume in controls may tend to match the volume in patients. To date, this is suggested by 2 studies11,13 over 312. But, none of these studies specifically evaluated differences in aged patients and controls (>50 years), which accounted for less than 17% of total participants.
Thalami
A reduced GM volume was also noted in the thalami and can be explained by 2 non-exclusive interpretations. First, thalamic volume6 and MRI signal25 have both been reported to be reduced in schizophrenia. In contrast to cortical atrophy, this may not be only due to atrophy of the neuropil but also to neuronal loss26. Second, the reduced GM loss in the thalami may also reflect the enlargement of the third ventricle, which is incompletely corrected by the normalization process. Indeed, a significant enlargement of the third ventricle has been reported in 73% of previous studies6.
Occipito-tempo-parietal Atrophy
The large bilateral occipito-temporo-parietal GM atrophy was a more surprising result. Both the extent of atrophy and the level of significance were striking considering the paucity of previous positive reports in these regions (8% of studies which is close to chance level)24. This result also contrasts with the smaller differences in the frontal lobe, eg, the dorso-lateral prefrontal cortex was not significantly different between the patient and control groups. The involvement of these regions was strengthened by the Group × Age interaction, showing that patients with schizophrenia had accelerated GM decreases in the occipito-parietal junction. This suggests that these cortices probably atrophy only later in the course of the disease in accordance with the negative results of young patients’ studies.
Because long-institutionalized patients may be less stimulated than patients living on their own, one can postulate that differences in stimulation levels may explain the change in GM volume in the large occipito-parieto-temporal region27–32. However, the absence of significant differences between institutionalized and non-institutionalized patients does not support the possible role of institutionalization in the GM differences observed in the posterior regions.
Another interpretation is that demented patients accounted for most of the occipito-temporo-parietal GM atrophy. However, the absence of significant difference according to the demented status and the absence of correlation between the GM deficit and the cognitive scores, regardless of the scales that has been used, do not support this view. Correlation with a specific function cannot be ruled out, however, because the scales give a composite score reflecting multiple functions. Moreover, the scales mostly tested executive functions’ deficits, which, although present, might not account for the GM atrophy of posterior regions.
A large majority of the patients received antipsychotic medication, which could also account for our results. However, the absence of correlation with chlorpromazine equivalent does not support this interpretation. It has been suggested that only typical antipsychotics may lead to GM atrophy33,34. In this study, we observed no difference between typical and atypical antipsychotics regarding GM atrophy. Although this result should be interpreted with caution, given the small number of patients in each treatment group, it seems unlikely that the type of antipsychotic accounts for the large posterior GM atrophy.
Interpretation
Because none of the above factors accounted for the large GM atrophy in the occipito-temporo-parietal regions of aged patients with schizophrenia, 2 hypotheses remain. First, aged patients with schizophrenia may be impaired by an accelerated cerebral aging process in regions spared in younger patients. Considering controls, the atrophy slowly reaches that observed in patients in the prefrontal and internal temporal areas. As for the angular cortex, one of the regions we have found to be atrophied in aged patients with schizophrenia, it has been shown to have an accelerated GM decrease with normal aging35. GM change in schizophrenia might be viewed as a mere acceleration of the aging process.
Second, considering that we recruited patients in a psychiatric setting, we might have selected the 35%–54% of the patients that are not cured with increasing age14,15. This might have biased our population who might have developed a different type of psychosis, as suggested by the Wernicke-Kleist-Leonhard school36. According to the latter, a subgroup of psychoses, ie, the unsystematized schizophrenias, pursue their evolution even in the older age, which could account for our observations.
Limitations
Methodological limitations should be considered. First, the sample size was modest, largely because we included only aged patients and controls who did not present medical comorbidity that could affect brain anatomy unrelated to schizophrenia. Because such comorbidities are frequently observed in older patients with schizophrenia, we had to exclude many of these patients.
Second, performing the study on 2 different sites raised the issue of a center effect. Indeed participants in Luxembourg were slightly older than those in Strasbourg. But because both centers independently matched their patients with their controls, there were no group × center interaction for none of the variables under study. Between centers, although we chose equivalent sequences with close enough GM/WM contrast37, our resolution was different. We faced the issue in 2 ways. First, by down-sampling the resolution of all scans to the ones with the lowest resolution before segmentation. Second, by accounting for a “center effect” by a variable of no interest. As in inter-scanner reproducibility studies, we observed a between-platform bias38. We found a group × center interaction in the occipital cortices bilaterally, which did not survive correction for multiple testing. In these regions, although the group statistical effect was greater in Luxembourg, it was present in both centers. This might be partly explained by the older age of the Luxembourg participants. The limits of performing a study on 2 or more centers might be outweighed by the advantage of recruiting a more representative patient population.
Finally, because the design of our study was cross-sectional, we cannot be sure that there was a faster decline in GM volume on a patient-by-patient basis. Our observations should be confirmed using longitudinal studies.
Conclusions
Although replication and longitudinal studies are needed to draw stronger conclusions, specific cerebral GM decreases seem to be present in aged patients with schizophrenia. A large occipito-temporo-parietal region, generally not reported in younger patients and in normal aging, seems to be differentially affected by age in patients with schizophrenia. Our study raises the question of subgroups of patients with one with the most chronic course experiencing late atrophy in posterior regions.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org
Funding
This study was supported by a Projet Hospitalier de Recherche Clinique Régional 2002: Neuropsychologie cognitive du Vieillissement dans la Schizophrénie, a Luxemburger funding project number FNR/03/04/07, and a European fund INTERREG IIIB (Coopération hospitalière transnationale Strasbourg-Liège-Luxembourg-Metz-Thionville): Réinsertion socioprofessionnelle des patients schizophrènes : développement de méthodes d’évaluation et de prise en charge cognitive.
Supplementary Material
Acknowledgments
Financial disclosures: Dr Foucher and Pr Danion report no competing interests.
References
- 1.Kraepelin E. Dementia Praecox and Paraphrenia. Edinburgh, UK: E. & S. Livingstone; 1919. (from Ein Lehrbuch für Studierende und Ärzte. 8e ed. 1913, Johann Ambrosius Barth, Leipzig) (transl by Barclay RM, Robertson GM) [Google Scholar]
- 2.Bleuler E, Claude H. Congrès des médecins aliénistes et neurologistes de France et des pays de langue française. XXXe session, Genève-Lausanne (2–7 août 1926) Paris, France: Masson; 1926. La Schizophrénie; pp. 93–153. Comptes rendus par A. Répond. [Google Scholar]
- 3.Meduna L. Versuche über die biologische Beeinflussung des Ablaufes der Schizophrenie. Zeitschrift für die gesamte Neurologie und Psychiatrie. 1935;152:235–262. [Google Scholar]
- 4.Johnstone EC, Owens DG. Early studies of brain anatomy in schizophrenia. In: Lawrie, Johnstone E, Weinberger D., editors. Schizophrenia from Neuroimaging to Neuroscience. Oxford University Press; 2004. pp. 1–19. [Google Scholar]
- 5.Weinberger DR, Torrey EF, Neophytides AN, Wyatt RJ. Lateral cerebral ventricular enlargement in chronic schizophrenia. Arch Gen Psychiatry. 1979;36:735–739. doi: 10.1001/archpsyc.1979.01780070013001. [DOI] [PubMed] [Google Scholar]
- 6.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLisi LE, Stritzke P, Riordan H, et al. The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry. 1992;31:241–254. doi: 10.1016/0006-3223(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 8.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 9.Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 10.Pantelis C, Yücel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 11.van Haren NEM, Hulshoff Pol HE, Schnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Hulshoff Pol HE, Schnack HG, Bertens MGBC, van Haren NEM, van der Tweel I, et al. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159:244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- 13.Bose SK, Mackinnon T, Mehta MA, et al. The effect of ageing on grey and white matter reductions in schizophrenia. Schizophr Res. 2009;112:7–13. doi: 10.1016/j.schres.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry. 1994;151:1409–1416. doi: 10.1176/ajp.151.10.1409. [DOI] [PubMed] [Google Scholar]
- 15.Hopper K, Harrison G, Janca A, Sartorius N. Recovery from Schizophrenia. Oxford: Oxford University Press; 2007. [Google Scholar]
- 16.Harvey PD. Dementia and schizophrenia: similarities and differences. In: Harvey PD, editor. Schizophrenia In Late Life: Aging Effects On Symptoms And Course Of Illness. Washington: American Psychological Association; 2004. pp. 101–116. [Google Scholar]
- 17.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 18.Woods S. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 19.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Mattis S. Dementia Rating Scale: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 22.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 23.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 24.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen NC, Arndt S, Swayze V, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 26.Kreczmanski P, Heinsen H, Mantua V, et al. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130:678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- 27.Diamond MC, Rosenzweig MR, Bennett EL, Lindner B, Lyon L. Effects of environmental enrichment and impoverishment on rat cerebral cortex. J Neurobiol. 1972;3:47–64. doi: 10.1002/neu.480030105. [DOI] [PubMed] [Google Scholar]
- 28.Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaser C, Schlaug G. Gray matter differences between musicians and nonmusicians. Ann N Y Acad Sci. 2003;999:514–517. doi: 10.1196/annals.1284.062. [DOI] [PubMed] [Google Scholar]
- 30.Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 32.Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N. Voxel-based morphometry reveals increased gray matter density in Broca's area in male symphony orchestra musicians. NeuroImage. 2002;17:1613–1622. doi: 10.1006/nimg.2002.1288. [DOI] [PubMed] [Google Scholar]
- 33.van Haren NEM, Hulshoff Pol HE, Schnack HG, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 34.Thompson PM, Bartzokis G, Hayashi KM, et al. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 2009;19:1107–1123. doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 36.Leonhard K, Beckmann H. Classification of Endogenous Psychoses and Their Differential Etiology. Vienna, Australia: Springer Verlag; 1999. [Google Scholar]
- 37.Ashton E. Quantitative MR in multi-center clinical trials. J Magn Reson Imaging. 2010;31:279–288. doi: 10.1002/jmri.22022. [DOI] [PubMed] [Google Scholar]
- 38.Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.