Abstract
Background: It remains unclear whether structural brain abnormalities in schizophrenia are caused by genetic and/or disease-related factors. Structural brain abnormalities have been found in nonpsychotic first-degree relatives of patients with schizophrenia, but results are inconclusive. This large magnetic resonance imaging study examined brain structures in patients with schizophrenia, their nonpsychotic siblings, and healthy control subjects using global and focal brain measurements. Methods: From 155 patients with schizophrenia, their 186 nonpsychotic siblings, and 122 healthy controls (including 25 sibling pairs), whole-brain scans were obtained. Segmentations of total brain, gray matter (GM), and white matter of the cerebrum, lateral and third ventricle, and cerebellum volumes were obtained. For each subject, measures of cortical thickness and GM density maps were estimated. Group differences in volumes, cortical thickness, and GM density were analyzed using Structural Equation Modeling, hence controlling for familial dependency of the data. Results: Patients with schizophrenia, but not their nonpsychotic siblings, showed volumetric differences, cortical thinning, and reduced GM density as compared with control subjects. Conclusions: This study did not reveal structural brain abnormalities in nonpsychotic siblings of patients with schizophrenia compared with healthy control subjects using multiple imaging methods. Therefore, the structural brain abnormalities observed in patients with schizophrenia are for the largest part explained by disease-related factors.
Keywords: structural magnetic resonance imaging, schizophrenia, siblings, family study, cortical thickness, voxel-based morphometry
Schizophrenia is characterized by gray matter (GM) reductions in cortical and subcortical regions, but the underlying mechanisms causing these abnormalities are largely unknown. Twin studies suggest that genetic influences play a role,1–3 but there is also convincing evidence that environmental influences, such as antipsychotic medication,4–7 obstetric complications,8–10 and cannabis use11–13 are involved. Furthermore, brain abnormalities appear to be related to clinical features such as duration of (untreated) psychosis14–16 and outcome.17,18
As the heritability to develop schizophrenia is estimated to be 81%,19 it is thought that the brain abnormalities reported in schizophrenia may also be present in unaffected relatives of patients with this illness. Indeed, a meta-analysis, including 23 studies, reported volumetric decreases in the hippocampus and GM, as well as increases in third ventricle volume in relatives of patients with schizophrenia compared with healthy control subjects.20 This meta-analysis pooled data from neuroimaging studies (largest study: n = 183) that examined various groups of relatives (ie, twins, parents, offspring, and siblings), all carrying their own specific genetic and environmental risk factors. The studies included in this meta-analysis did not provide enough data to examine the effects of age, which is relevant as offspring and young siblings are still at risk to develop the illness, while older siblings and parents are most likely beyond the age of risk. In addition, structural brain abnormalities seem progressive, even in unaffected relatives.21
Magnetic resonance imaging (MRI) studies that were included in the meta-analysis and focused on siblings of patients with schizophrenia reported GM reductions, most pronounced in the temporal areas8,22 (but not23) and hippocampus.24 Studies that were published after this meta-analysis came out reported GM reductions in the posterior cingulate cortex25 and the inferior frontal gyrus.26 In addition, larger orbitofrontal white matter (WM) was found,27 but when cortical thickness was examined, no differences were found in siblings of patients as compared with healthy control subjects.25 The largest sibling study to date, including 115 patients with schizophrenia, 192 nonpsychotic siblings, and 196 healthy control subjects, failed to find differences in global brain volumes,28 cortical thickness,29 and GM density30 between siblings and healthy control subjects. Interestingly, a study including siblings of patients with childhood-onset schizophrenia found no differences in GM volume and cortical thickness in siblings of 20 years and older, while in the younger siblings decreased (parietal) GM volume, as well as cortical thinning were reported in the prefrontal and temporal cortices.31
In summary, while smaller studies report reduced volumes in siblings of patients with schizophrenia compared with healthy control subjects, the largest study to date failed to find structural brain differences between these 2 groups. We therefore designed this large study of 155 patients with schizophrenia, 186 of their related (relatively young) nonpsychotic siblings, and 122 age-matched healthy control subjects (including 25 sibling pairs). Cortical and subcortical brain structures were examined by applying volumetric measurements, cortical thickness, and voxel-based morphometry (VBM). We hypothesized that nonpsychotic siblings show a similar but less pronounced pattern of structural brain differences relative to patients with schizophrenia as compared with healthy control subjects. As earlier studies reported that schizotypy was found to a much higher degree in first-degree relatives compared with control subjects,32,33 we hypothesized that these brain differences are related to (sub)clinical characteristics present in the siblings.
Materials and Methods
Participants
A total of 155 patients with schizophrenia, 186 related nonpsychotic siblings, and 122 healthy control subjects (including 25 sibling pairs) participated in this study. The recruitment was part of the baseline measurement of an ongoing longitudinal study in the Netherlands (Genetic Risk and Outcome of Psychosis; GROUP). From this study, subjects were recruited at the University Medical Center Utrecht, Utrecht, the Netherlands.
Eligible patients had to fulfill the following criteria: (1) age between 16 and 50 years, (2) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for a nonaffective psychotic disorder (including schizophrenia, schizophreniform disorder, and schizoaffective disorder), (3) fluent in Dutch, and (4) able and willing to give written informed consent. Eligible siblings (brothers and/or sisters) of participating probands had to fulfill the criteria of (1) age between 16 and 50 years, (2) fluent in Dutch, and (3) able and willing to give written informed consent. Eligible healthy control subjects had to fulfill the criteria of (1) age between 16 and 50 years, (2) no lifetime psychotic disorder and/or use of lithium medication (in the past), (3) no first- or second-degree family member with a lifetime psychotic disorder, (4) fluent in Dutch, and (5) able and willing to give written informed consent.
Patients and controls identified as potentially eligible were asked to provide consent for assessment and for contacting their siblings. Control subjects were selected through a system of random mailings to addresses in the catchment areas. Presence or absence of psychopathology was established by using Comprehensive Assessment of Symptoms and History interview (CASH34), performed by at least 1 independent rater who was trained to assess this interview. Diagnosis was based on the DSM-IV criteria. Of all subjects, urine was screened for cocaine, amphetamines, and for cannabis. Subjects with substance dependence/abuse (based on the criteria of the Composite International Diagnostic Interview35 [sections B, J, and L]) and a major medical or neurological illness were excluded.
Written informed consent was obtained from all subjects, and the study was approved by the Medical Ethics Committee for Research in Humans (METC) of the University Medical Center Utrecht.
Clinical And Neuropsychological Assessments
To evaluate severity of symptoms in patients with schizophrenia, the Positive and Negative Syndrome Scale (PANSS36) was performed. In siblings and healthy control subjects, the Structured Interview for Schizotypy-Revised (SIS-R37,38) was administered. The SIS-R is a semistructured interview containing 20 schizotypal symptoms and 11 schizotypal signs, rated on a 4-point scale. Scores were subdivided into positive, negative, and total schizotypal features. Furthermore, the Dutch translation of the Family Interview for Genetic Studies (FIGS) was used to estimate the presence of a psychiatric illness in first- and/or second-degree family members.
Imaging And Preprocessing
Structural MRI scans of the whole brain were obtained on a 1.5-T Achieva scanner (Philips, Best, the Netherlands). A 3-dimensional (3D), T1-weighted coronal spoiled-gradient echo scan of the whole head (256 × 256 matrix, echo time [TE] = 4.6 ms, repetition time [TR] = 30 ms, flip angle = 30°, 160–180 contiguous slices; 1 × 1 × 1.2 mm3 voxels, field of view [FOV] = 256 mm/70%) was acquired. Furthermore, a single-shot echo planar imaging scan was made as part of a diffusion tensor imaging series (SENSE factor 2.5; flip angle = 90°; 60 transverse slices of 2.5 mm; no gap; 128 × 96 acquisition matrix; FOV = 240 mm; TE = 78 ms) together with a magnetization transfer imaging scan (60 transverse slices of 2.5 mm; no gap; 128 × 96 acquisition matrix; FOV = 240 mm; flip angle = 8°; TE = 4.5 ms; TR = 37.5 ms).
Processing was done on the computer network of the Department of Psychiatry at the University Medical Center Utrecht. All images were coded to ensure investigator blindness to subject identification and diagnosis.
Volumetric Processing
The T1-weighted images were automatically put into Talairach orientation39 without scaling, by registering them to a model brain. The 2 other scans were registered to the T1-weighted image by minimizing a mutual information joint entropy function.40 The coregistered scans were used for automatic segmentation of the intracranial volume, based on histogram analysis and morphology operations. The intracranial segment served as a mask for all further segmentation steps. The T1-weighted images were corrected for field inhomogeneities using the N3 algorithm.41 Our automatic processing pipeline was used for segmentation of total brain (TB), GM, and WM of the cerebrum.48 In short, pure GM and WM intensities were directly estimated from the image. The amounts of pure and partial volume voxels were modeled in a nonuniform partial volume density, which is fitted to the intensity histogram. Expected tissue fractions, based on the pure intensities and the partial volume density, were subsequently computed in each voxel within the cerebrum. Total brain volume was calculated by adding the GM and WM segments. Binary images of GM and WM were created using 0.5 as a threshold: ie, voxls in the GM partial volume map with a fraction >0.5 were considered as GM, and similarly, voxels in the WM partial volume map with fractions >0.5 were classified as WM.
Lateral and third ventricle and cerebellum volumes were also assessed. The software included histogram analysis, mathematical morphology operations, and anatomical knowledge-based rules to connect all voxels of interest, as was validated before.43 The intracranial mask, ventricle, and cerebellum segments were all visually checked and edited if necessary.
Cortical Thickness
To compute cortical thickness, the binarized GM and WM segments were used as input for the CLASP algorithm designed at the McConnell Brain Imaging Center of the Montreal Neurological Institute.44–46 A 3D surface comprising 81 920 polygons per hemisphere was fitted to the WM/GM interface, which created the inner surface of the cortex which was then expanded to fit the GM/cerebrospinal fluid interface, creating the outer cortical surface. Cortical thickness was estimated by taking the distance between the 2 surfaces such that each polygon vertex on the outer surface had a counterpart vertex on the inner surface. Each subject's thickness measurements were smoothed across the surface using a 20-mm full-width at half maximum (FWHM) surface-based blurring kernel, as was done before.42 This method of blurring improves the chances of detecting population differences but also follows the curvature of the surface to preserve any anatomical boundaries within the cortex. The surfaces of each subject were registered to an average surface created from 152 healthy subjects aged 18–40 years (ICBM: International Consortium for Brain Mapping),49 allowing comparison of cortical thickness locally between subjects.
Voxel-Based Morphometry
Regional measures of GM and WM concentration (“density”) were generated using VBM in a similar manner as was done previously.3 VBM involved the following steps. First, a model brain was created on the total sample, similar to the method used by Grabner et al.50 After creation of the model brain, the partial volume GM and WM segments with voxels of 1 × 1 × 1.2 mm3 were blurred by a 3D Gaussian kernel (FWHM = 8 mm) to gain statistical power. The voxel values of these blurred partial volume GM and WM maps (between 0 and 1) reflect the local presence, or density, of GM or WM, respectively. These images are referred to as “density maps.” To compare brain tissue at the same anatomical location in all subjects, the GM and WM segments were transformed into a standardized coordinate system (the model space). These transformations were calculated in 2 steps. First, the T1-weighted images were linearly transformed to the model brain. In this linear step, a mutual information metric was optimized.40 In the second step, nonlinear (elastic) transformations were calculated to register the linearly transformed images to the model brain up to a scale of 4 mm (FWHM), thus removing global shape differences between brains but retaining local differences. For this step, the program ANIMAL51 was used. The GM and WM density maps were now transformed to the model space by applying the concatenated linear and nonlinear transformations. Finally, the maps were resampled to voxels of size 2 × 2 × 2.4 mm3. Voxels with an average GM density below 0.1 were excluded from the GM density voxel-based analysis. Using “nonmodulated” VBM analyses allow for direct investigation of regional differences in brain areas without being confounded by overall brain size, ie, these individual differences in brain size and shape have been removed by linear and nonlinear transformations.
Statistical Analysis
Demographic And Diagnostic Data.
Data were examined for outliers and normality of the distribution, using the Kolmogorov-Smirnov test for significance.
To assess whether the groups differed on demographic variables, univariate analyses of variance were conducted for noncategorical variables and χ2 tests for categorical variables.
SPSS 15.0 statistical package for Windows (SPSS Inc., Chicago, Illinois) was employed for analyses of demographic data.
Group Differences in Brain Volumes, Cortical Thickness, And GM Density Maps.
In the full model, TB, GM, WM, lateral ventricle, third ventricle, and cerebellum volumes were regressed on intracranial volume, gender, age, handedness, and group status (patients vs siblings vs healthy control subjects). Cortical thickness and GM density (VBM) were regressed on gender, age, handedness, and group status. Relatedness in the patient-sibling pairs and control pairs was accounted for in the covariance structure by allowing dependencies between the residuals in the regression analyses. Group effects were tested by comparing the −2 log-likelihoods of 2 nested models: a model that does allow for group effects on structural brain measures (the full model) and a model that does not allow for such an effect. The difference in −2 log-likelihood between these models is χ2 distributed. A χ2 >3.84 (1 df) indicates a significant difference at α = .05 and depicts that the discarded effect (ie, group effect) cannot be left out of the model without seriously reducing the goodness of fit.
For group effects in volumes, cortical thickness, and VBM, mixed model analysis was implemented using Structural Equation Modeling (SEM) with Mx software for Windows (Department of Psychiatry, Virginia Commonwealth University Richmond, Virginia). The present study aimed to examine a large group of families and variables. A distinction was made between mutual correlations between siblings and correlations between healthy control subjects. SEM is a useful design for such studies. To evaluate the differences in cortical thickness, a vertex-by-vertex analysis was carried out. In each vertex, group differences in cortical thickness were calculated using regression analyses with group, age, gender, and handedness as covariates. This produced χ2 statistics at each vertex, one for the effect of group, one for the effect of age, one for the effect of gender, and one for the effect of handedness. Statistical maps were created showing significant differences in cortical thickness between groups. For those cortical areas that showed significant differences, the most significant vertex was identified visually using the cortical surface viewer brain-view developed at the Montreal Neurological Institute.
To evaluate differences in GM density, regression analysis was done through all brains for each voxel separately in the GM and WM density maps. Similar to the cortical thickness analysis, this produced χ2 statistics at each voxel.
In all statistical analyses, a correction for multiple comparisons was carried out according to the false discovery rate (FDR).
Associations With Severity of Illness.
To address whether in patients, structural brain differences depend on severity of illness, post hoc analyses were performed. Brain measures were regressed on PANSS-positive symptoms scores, PANSS-negative symptoms scores, and PANSS total scores. For 8 patients, PANSS scores were missing. These were excluded from the analysis.
Associations With Schizotypy.
For the combined sample of siblings and control subjects, post hoc analyses were performed to address whether there is an association between positive or negative schizotypal features as measured with the SIS-R and brain measures. For 3 siblings and 1 healthy control subject, SIS-R scores were missing. These were excluded from the analysis.
Results
Demographic And Diagnostic Data
For demographic analyses, see table 1. No differences between groups were found for age (siblings: 27.54 years [SD = 6.75]; patients with schizophrenia: mean age = 26.91 years [SD = 5.58]; and healthy control subjects: 27.53 years [SD = 8.24]), parental educational level (defined as the total number of years of education), and handedness. Groups differed significantly in gender distribution; male and female subjects being equally divided within the siblings (45.7% male) and control subjects (50.0%) but not in the patient group (80.6% male). Groups differed significantly in Wechsler Adult Intelligence Scale IQ (siblings: mean IQ = 100.9 [SD = 15.10]; patients with schizophrenia: mean IQ = 93.3 [SD = 15.70]; healthy control subjects: mean IQ = 110.9 [SD = 14.60]). The majority of patients (90%) were taking antipsychotic medication at the time of scan, with olanzapine and risperdone being most often prescribed (N = 55 and N = 27, respectively). In patients, mean duration of illness was 4.02 years (SD = 3.63).
Table 1.
Demographic Information
| n or Mean (SD) [Range] | |||
| Patients (N = 155) | Siblings (N = 186) | Healthy Control Subjects (N = 122) | |
| Age (y) | 26.91 (5.6) [18.5–43.3] | 27.5 (6.8) [16.6–50.5] | 27.5 (8.2) [17.1–49.4] |
| Sex (M/F) | 125/30 (80.6% male) | 85/101 (45.7% male) | 61/61 (50.0% male) |
| Handedness (R/L) | 143/12 (92.3% right) | 166/20 (89.2% right) | 108/14 (88.5% right) |
| Parental education level (completed in y) | 13.04 (3.6) | 13.39 (3.1) | 13.5 (3.1) |
| Subject education level (completed in y) | 12.04 (2.3)a | 13.30 (2.4)a | 14.02 (1.9)a |
| WAIS IQ | 93.32 (15.7) [63–136]a | 100.9 (15.10) [68–155]a | 110.9 (14.6) [73–144]a |
| Paranoid type (%) | 100 (64.5) | 0 | 0 |
| Schizoaffective disorder (%) | 20 (12.9) | 0 | 0 |
| Undifferentiated type (%) | 17 (11.0) | 0 | 0 |
| Disorganized type (%) | 7 (4.5) | 0 | 0 |
| Catatonic type (%) | 1 (0.6) | 0 | 0 |
| Schizophreniform disorder (%) | 9 (5.8) | 0 | 0 |
| Residual type (%) | 1 (0.6) | 0 | 0 |
| Bipolar disorder (%) | 0 | 7 (3.8) | 0 |
| Major depression (%) | 0 | 36 (19.4) | 0 |
| Schizotypal personality disorder (%) | 0 | 1 (0.5) | 0 |
| Other disorders (%) | 0 | 6 (3.2) | 0 |
| No psychiatric disorder (%) | 0 | 136 (73.1) | 122 (100) |
| PANSS-positive symptoms scoreb | 15.34 (5.7) [7–35] | ||
| PANSS-negative symptoms scoreb | 15.41 (5.5) [6–31] | ||
| PANSS total symptoms scoreb | 62.22 (17.17) [30–133] | ||
| SIS-R-positive subscalec | 0.19 (0.4) [0–2] | 0.18 (0.24) [0–1.3] | |
| SIS-R-negative subscalec | 0.20 (0.3) [0–1] | 0.18 (0.21) [0–0.9] | |
Note: M/F, male/female; R/L, right/left; WAIS, Wechsler Adult Intelligence Scale; PANSS, Positive and Negative Syndrome Scale; SIS-R, Structured Interview for Schizotypy-Revised.
Significantly differed from both other groups.
For 8 cases, information was missing.
For 4 cases, information was missing.
Global Brain Volumes
After controlling for age, gender, intracranial volume, and handedness, nonpsychotic siblings did not differ from healthy control subjects in brain volumes. Patients with schizophrenia showed significant reductions in TB (χ2 = 23.72, P < .01), GM (χ2 = 10.82, P < .01), and WM volumes (χ2 = 6.62, P = .01) compared with healthy control subjects (see table 2). In addition, increased lateral (χ2 = 14.65, P < .01) and third ventricle (χ2 = 6.94, P < .01) volumes were found in patients relative to healthy control subjects. We have performed additional analyses in which we compared patients with their related siblings. The results of these analyses were similar to the results of the comparison of patients with control subjects. Post hoc analyses showed no association between brain volumes and dose or type of medication at inclusion nor did cannabis use (lifetime or past year) affect our results in patients with schizophrenia. In urine screening, 19 patients, 18 siblings, and 6 healthy control subjects were positive for cannabis, cocaine, or amphetamines at inclusion. Excluding these subjects from the analyses did not alter the results.
Table 2.
Brain Volumes ml: Uncorrected mean (SD)
| Brain Area | Patients | Siblings | Controls | χ2 (Patients vs Siblings) | χ2 (Siblings vs Controls) | χ2 (Patients vs Controls) |
| Intracranial volume | 1550.66 (145.54) | 1504.87 (137.38) | 1528.53 (141.09) | 2.34 | 1.26 | 1.27 |
| Whole brain | 1303.20 (128.90) | 1286.24 (123.94) | 1304.69 (133.59) | 44.58* | 0.50 | 23.72* |
| Cerebral gray matter | 622.79 (62.08) | 613.52 (59.70) | 622.59 (66.61) | 17.00* | 0.00 | 10.82* |
| Cerebral white matter | 510.32 (63.21) | 507.65 (60.76) | 512.66 (62.83) | 20.87* | 1.65 | 6.62* |
| Lateral ventricle | 16.89 (9.10) | 13.69 (7.95) | 13.16 (5.83) | 17.24* | 0.05 | 14.65* |
| Third ventricle | 0.91 (0.35) | 0.71 (0.30) | 0.78 (0.33) | 33.07* | 3.84 | 6.94* |
| Cerebellum | 157.42 (15.56) | 152.69 (15.88) | 156.59 (15.86) | 2.23 | 0.84 | 3.09 |
Note: In the analyses, means were corrected for intracranial volume, age, gender, and handedness.
*Significant differences (P < .01).
After controlling for IQ or parental education level (including age, gender, intracranial volume, and handedness), results were similar to those described above.
Furthermore, because there were more male than female patients, a separate analysis was performed for only male subjects (n = 113 patients; n = 84 siblings; n = 60 healthy control subjects). The results of this analysis were similar to the results described above.
Cortical Thickness
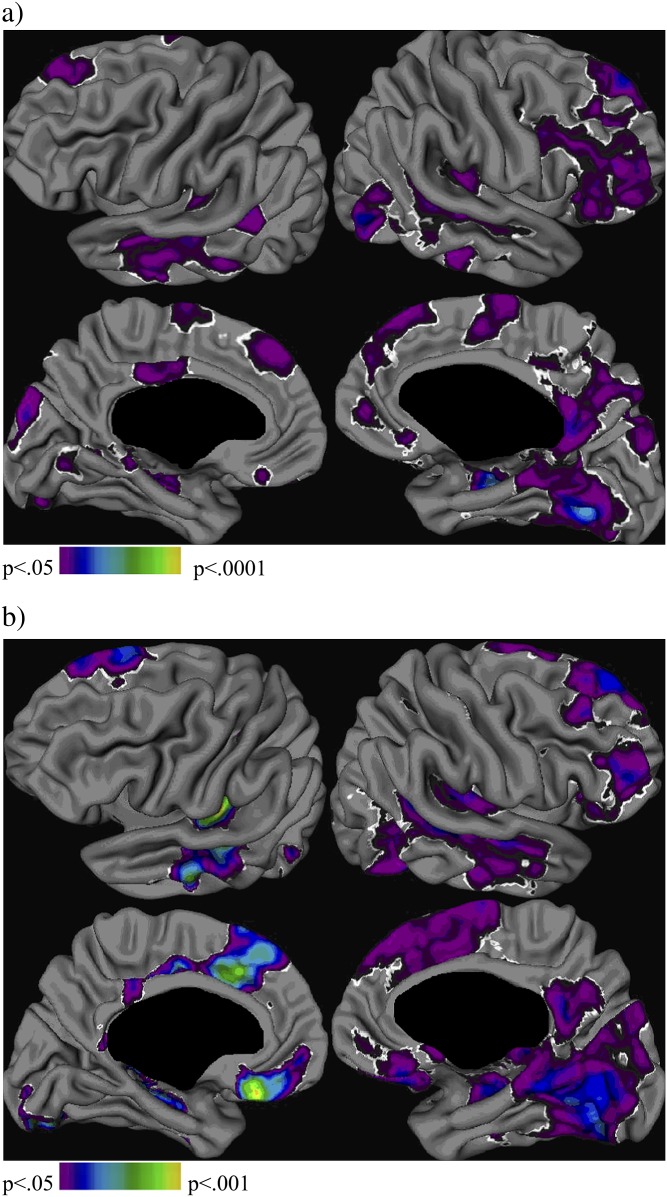
In focal cortical thickness analyses, nonpsychotic siblings did not show differences in cortical thickness compared with the healthy control subjects. Patients with schizophrenia showed cortical thinning compared with healthy control subjects. Figure 1 shows the statistical difference map of this analysis, corrected for the effect of age, gender, and handedness, at a corrected threshold of χ2 >7.50 for left and χ2 >5.82 for right hemisphere (FDR; α = .05, df = 1). As shown in figure 1 and table 3, cortical thinning in patients was most apparent bilaterally in the frontal and temporal cortex, with patients also showing cortical thinning bilaterally in the occipital cortex, Wernicke's area, left parahippocampal and posterior cingulate gyrus, and right parietal and precentral cortex. Cortical thinning was found also in patients as compared with nonpsychotic siblings (at a corrected threshold of χ2 >7.52 for left and χ2 >5.64 for right hemisphere), being most pronounced in the bilateral frontal and temporal cortex, but also in the Wernicke's area, the left parahippocampal and occipital gyrus, and the right parietal cortex. Patients did not show increased cortical thickness compared with healthy control subjects or nonpsychotic siblings.
Fig. 1.
Group Differences in Cortical Thickness. Difference maps (χ2), corrected for age, gender, and handedness: (a) patients compared with healthy control subjects; (b) patients compared with nonpsychotic siblings. Results were thresholded at P = .05, false discovery rate corrected.
Table 3.
Significant Differences in Cortical Thickness: Areas Showing Cortical Thinning in (a) Patients Compared With Healthy Control Subjects And (b) Patients Compared With Their Nonpsychotic Siblings
| Brain Area | Talairach Coords; x, y, z | BA | Mean Patients | Mean Siblings | Mean Controls | χ2 |
| a) | ||||||
| Bilateral middle temporal | 49, −32, 0 | 3.20 (0.24) | 3.27 (0.25) | 3.27 (0.21) | 19.86 | |
| Bilateral inferior occipital | 47, −78, −2 | 19 | 2.77 (0.27) | 2.81 (0.22) | 2.87 (0.25) | 19.85 |
| Bilateral superior frontal | 10, 53, 42 | 8 | 3.51 (0.29 | 3.61 (0.29) | 3.63 (0.29) | 19.13 |
| Bilateral Wernicke's area | −44, −29, 10 | 41 | 3.03 (0.20) | 3.13 (0.21) | 3.11 (0.22) | 13.12 |
| Bilateral orbitofrontal | 7, 48, −14 | 11 | 3.05 (0.22) | 3.12 (0.21) | 3.15 (0.23) | 12.19 |
| Left superior occipital | −6, −88, 23 | 18 | 2.59 (0.17) | 2.62 (0.19) | 2.71 (0.20) | 18.38 |
| Left parahippocampal | −37, −30, −11 | 36 | 2.84 (0.19) | 2.97 (0.20) | 3.01 (0.20) | 16.08 |
| Left posterior cingulate | −3, −18, 31 | 23 | 3.10 (0.21) | 3.16 (0.21) | 3.19 (0.21) | 12.95 |
| Right hippocampal | 34, −20, −13 | 2.99 (0.19) | 3.14 (0.22) | 3.19 (0.21) | 34.72 | |
| Right inferior occipital | 27, −68, −7 | 19 | 2.85 (0.17) | 2.95 (0.17) | 2.97 (0.17) | 33.40 |
| Right middle frontal | 39, 26, −8 | 47 | 3.25 (0.29) | 3.33 (0.29) | 3.37 (0.29) | 18.35 |
| Right posterior cingulate | 4, −50, 18 | 30 | 3.23 (0.29) | 3.29 (0.29) | 3.31 (0.29) | 18.06 |
| Right parietal | 7, −75, 44 | 7 | 2.63 (0.19) | 2.67 (0.19) | 2.71 (0.19) | 12.01 |
| Right superior frontal | 4, 10, 49 | 6 | 3.51 (0.29) | 3.57 (0.29) | 3.58 (0.29) | 12.14 |
| All significant with critical χ2 (α = .05) = 7.52 (left hemisphere) and χ2 (α = .05) = 5.64 (right hemisphere). | ||||||
| b) | ||||||
| Bilateral frontal pole | 18, 25, −24 | 47 | 2.78 (0.19) | 2.93 (0.20) | 2.87 (0.19) | 26.05 |
| Bilateral middle temporal | 48, −34, 0 | 3.20 (0.28) | 3.28 (0.28) | 3.27 (0.28) | 25.02 | |
| Bilateral Wernicke's area | −43, −29, 10 | 41 | 3.03 (0.21) | 3.13 (0.21) | 3.11 (0.21) | 23.63 |
| Bilateral lateral superior frontal | 12, 53, 40 | 8 | 3.29 (0.29) | 3.60 (0.29) | 3.61 (0.29) | 22.47 |
| Left parahippocampal | −9, −36, 4 | 27 | 2.21 (0.25) | 2.31 (0.25) | 2.31 (0.25) | 14.55 |
| Left occipital | −47, −75, 3 | 19 | 2.78 (0.19) | 2.85 (0.22) | 2.83 (0.21) | 11.19 |
| Right occipital | 33, −80, −12 | 19 | 2.73 (0.21) | 2.88 (0.21) | 2.84 (0.21) | 30.73 |
| Right posterior cingulate | 7, −51, 22 | 23 | 3.21 (0.21) | 3.28 (0.21) | 3.28 (0.21) | 18.88 |
| Right inferior frontal | 44, 47, 2 | 10 | 3.02 (0.21) | 3.09 (0.21) | 3.08 (0.21) | 18.37 |
| All significant with critical χ2 (α = .05) = 7.52 (left hemisphere) and χ2 (α = .05) = 5.64 (right hemisphere). | ||||||
Note: The table shows the anatomical location (brain area). Talairach coordinates (Talairach coords) and Brodman coordinates (BA). Mean (standard deviation) for each group is given with the statistics (χ2).
Table 4.
Focal Decreases in Gray Matter Density in (a) Patients With Schizophrenia As Compared With Healthy Control Subjects And (b) Patient As Compared With Nonpsychotic Siblings
| Brain Area | Talairach coords; x, y, z | BA | χ2 |
| a) | |||
| Anterior cingulate | −2, −15, 28 | 23 | 52.94 |
| Insula | 46, −12, 12 | 13 | 45.1 |
| Temporal cortex | 41, 9, −24 | 38 | 28.92 |
| Occipital cortex | 17, −96, 10 | 18 | 22.06 |
| Parietal cortex | −31, −80, 31 | 19 | 20.1 |
| Frontal cortex | 38, 55, 4 | 10 | 19.12 |
| Thalamus | −14, 23, 3 | 18.63 | |
| All significant with critical χ2 (α = .05) = 9.43 | |||
| b) | |||
| Frontal cortex | 3, 40, 18 | 32 | 42.16 |
| Insula | 41, 1, 5 | 13 | 42.16 |
| Anterior cingulate | 6, −20, 35 | 31 | 34.8 |
| Temporal cortex | −40, 2, −14 | 38 | 32.84 |
| Parietal cortex | −28, −67, 44 | 7 | 16.67 |
| Occipital cortex | 25, −91, 13 | 18 | 11.76 |
| All significant with critical χ2 (α = .05) = 9.13 | |||
Note: The table shows the anatomical location (Brain area) with its corresponding Talairach coordinates (Talairach coords) and Brodmann area (BA).
Voxel-Based Morphometry
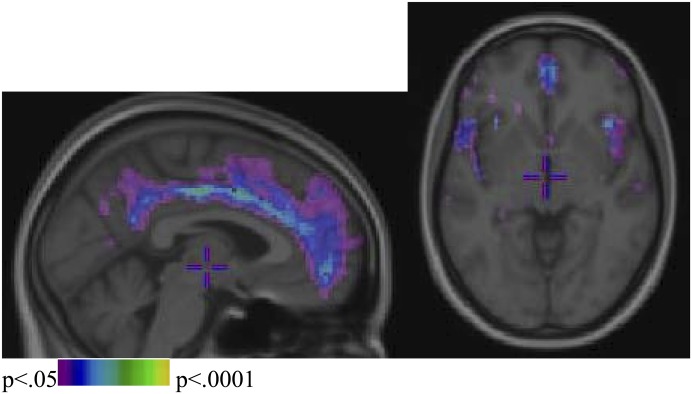
For GM density maps, nonpsychotic siblings did not reveal differences compared with healthy control subjects. Patients with schizophrenia were significantly different from healthy control subjects as shown in figure 2. The critical χ2 value of significance, corrected for multiple comparisons (FDR, α = .05) was 9.34. After correction for age, gender, and handedness, patients showed decreased GM density, most pronounced in the anterior cingulate gyrus and the insula as compared with healthy control subjects but also in the temporal, occipital, parietal, and frontal cortex; the thalamus; and the head of caudate. Similar to results in the comparison of patients vs controls, patients were different from siblings. The critical χ2 value of significance, corrected for multiple comparisons (FDR, α = .05), was 9.13. After correction, patients showed decreased GM density as compared with siblings most pronounced in the frontal cortex and the insula but also in the anterior cingulate, temporal, and parietal cortex; the head of caudate; and the occipital cortex.
Fig. 2.
Focal Decreases (Difference Maps Produced in χ2 Statistics) in Gray Matter Density in Patients With Schizophrenia as Compared With Healthy Control Subjects From a Given Section From The Sagittal And Axial Plane, Respectively.
Associations With PANSS
PANSS total symptom score was associated with TB (χ2 = 4.32, P < .05) and GM volume (χ2 = 7.30, P < .01), with decreased volumes related to higher scores. PANSS total positive symptom score was negatively associated with GM volume (χ2 = 8.22, P < .01) and positively with lateral ventricle volume (χ2 = 5.49, P < .05).
Associations With Schizotypy
In siblings and healthy control subjects, SIS-R total, positive, or negative scores were not related with brain volumes nor with cortical thickness and GM density maps.
Discussion
This cross-sectional imaging study including 463 subjects examined brain structures in a relatively young sample of patients with schizophrenia (n = 155), their nonpsychotic siblings (n = 186), and healthy control subjects (n = 122, including 25 sibling pairs), using various imaging techniques. Global brain volumes of nonpsychotic siblings were not different from those of healthy control subjects, nor did siblings and healthy control subjects differ in cortical thickness or GM density measured using a VBM approach. The paucity of cortical and subcortical brain differences in the siblings of patients is consistent with the findings from another large study in nonpsychotic siblings.28–30 The siblings in our study were about 10 years younger compared with the sample of Goldman et al,28 and in these analyses, we were able to take into account relatedness as we included patient-sibling pairs, as well as healthy control sibling pairs. Our findings contrast with those of smaller imaging studies in nonpsychotic siblings (largest study; total n = 155).8,22,23,52
Our study did find robust structural brain differences in patients with schizophrenia as compared with healthy control subjects. Indeed, we replicate the global volumetric abnormalities in patients with schizophrenia in TB, GM, WM, lateral ventricle, and third ventricle53,54. Furthermore, the decreases in cortical thickness and GM density, particularly in the frontal and temporal cortex, as well as in the anterior cingulate cortex, are consistent with earlier studies55–57 and with those studies using a VBM approach53,54,58. These most replicated findings in the inferior frontal, middle temporal, and the cingulate regions have been found to be associated with speech59.
Thus, our findings that brain abnormalities are expressed in patients with schizophrenia but not in nonpsychotic siblings suggest that brain abnormalities in schizophrenia mainly reflect processes related to the manifestation and/or treatment of the illness.
That the illness itself causes brain changes in schizophrenia is corroborated by the findings in ultra–high risk subjects and the association between brain changes and illness-related factors in schizophrenia. Only in those subjects who later converted to psychosis cortical GM deficits were found at baseline, but deficits were not found in those subjects who did not become psychotic over time.60,61 Furthermore, a longitudinal study in adolescents at ultra-high risk for psychosis showed that the development of psychosis was associated with progressive abnormalities around time of onset (which was not attributed to antipsychotic medication).62 In addition, studies that examined symptomatology in relation to brain imaging findings reported that reduced GM volume was related to duration of untreated psychosis14 and duration of psychosis.15,16 In addition, various other studies,6,47,63 but not all,64 reported a relationship between clinical outcome and reduced GM volume. Indeed, in the present study, we found that severity of illness (total and positive symptoms) was associated with reduced GM and increased lateral ventricle volume.
There is also evidence that brain abnormalities reported in schizophrenia are related to the effects of antipsychotic treatment. While post hoc analyses failed to show an association, in cross-sectional non-randomized studies such as ours, it is not possible to rule out medication effects on brain structure completely. A study in macaque monkeys treated long term with olanzapine or haloperidol reported that cortical volume was reduced by both these agents.65 In contrast, in a prospective study of Lieberman et al,66 obtaining MRI scans at multiple intervals, brain morphology was found to be differentially affected by olanzapine and haloperidol over time. In addition, other studies in patients with schizophrenia showed that decrements in GM volume over time, particularly in prefrontal regions, were associated with the (cumulative) intake of typical but not of atypical antipsychotic medication7,17.
Other nonshared environmental factors, such as obstetric complications, can result in brain abnormalities in patients with schizophrenia.9,67 Unfortunately, in our study, there was not sufficient information of obstetric complications to investigate its effects on structural brain abnormalities.
To date, the neurobiological processes that underlie the brain abnormalities in patients with schizophrenia remain unclear but may reflect anomalies of synaptic plasticity and abnormal brain maturation. Early (prenatal and perinatal) neurodevelopmental trauma may render the brain vulnerable to aberrant late neurodevelopmental processes, which may further interact with other causative factors associated with the onset of psychosis (eg, substance use, stress, and dysregulation of the hypothalamic-pituitary-adrenal axis function).68 Around transition to psychosis, these processes together may disrupt further brain development. Indeed, it has been suggested that the brain changes in the early state of schizophrenia are the result of the “toxic” effect of the psychotic state.63 Another theory was raised which guide neuroimmunology/virology studies of schizophrenia and derives from a general theoretical focus on central nervous system viral reactivation-induced immunological changes leading to psychosis.69
That the structural brain differences are under genetic control cannot be dismissed by the negative findings of our study. MRI studies in twins do report volume decreases in whole brain, GM and WM, or hippocampus in unaffected twins who are discordant for schizophrenia,1,21,70,71 but not all.72 These studies included monozygotic twins, sharing 100% of the genes with their sibling, and dizygotic twins, sharing 50% of the genes. Interestingly, brain volume differences in twins discordant for schizophrenia were more pronounced in the monozygotic than in the dizygotic twins, compared with healthy control twins.1–3,70 This suggests that the genetic contribution to brain volume reductions in schizophrenia may be subtle and is primarily detectable in subjects with high genetic loading, ie, monozygotic discordant twins and not in the healthy siblings of patients with schizophrenia.
The presence of brain volume differences in unaffected twins but not siblings could also be explained by the contribution of environmental factors that are specific for twins, such as intrauterine viral infections,73 prenatal environment,74 and delivery complications.75 These are common environmental factors that patients share with their (monozygotic) co-twins, while they are not shared with a nontwin sibling.8,76 Stress may also be such a common environmental factor.9 The emotional burden of the disease can be considerable in siblings of patients with schizophrenia.77 For twins, who often have a close emotional relationship with each other, the experience of having a co-twin with a severe psychiatric disease like schizophrenia may represent a more pronounced burden.
Furthermore, the heterogeneity produced by the broadly recruited sample of unaffected siblings in our study may have undermined the apparently high statistical power. Some previous computational neuroanatomical studies assessed relatively homogenous groups of unaffected relatives of patients with schizophrenia, which were chosen deliberately to maximize power through “genetic enrichment,” including high-risk familial subject78 and relatives from multiply affected families.79–82
Some limitations need to be addressed. First, a selection bias may have affected our results. This is reflected in that we included only siblings of patients who were willing to participate. Those siblings whom we were not able to include in the study may be of particular interest as they might share more (sub)clinical features with their ill proband. However, based on the FIGS, the included siblings were not different from those who were not included. Earlier studies reported that schizotypy was found to a much higher degree in first-degree relatives compared with healthy control subjects.32,33 As suggested by Diwadkar et al,83 relatives with high levels of schizotypy may define a hypervulnerable subsample among these relatives of patients with schizophrenia. Interestingly, in the present study, siblings and healthy control subjects were similar in schizotypal scores as measured with the SIS-R, suggesting that these siblings were possibly not vulnerable to develop schizophrenia. Second, there was a preponderance of men in the sample of patients compared with siblings and healthy control subjects. The epidemiological design of this study explains these differences. To minimize the effect of gender on brain structures, we controlled for this variable in all analyses. Male gender has been shown to be associated with larger cerebral volumes84,85 that disappears with head size correction.86 Greater decline of GM volume with age in males has also been reported in some87–89 but not other90 studies. Females have also been shown to have thicker cortex across many regions of the brain.85,91 As we know that gender but also age and handedness may influence brain structures, we have included these as covariates in our analyses. Third, it may be that cross-sectional MRI measurement might not be informative enough to find structural brain abnormalities in siblings of patients with schizophrenia. Fourth, it should be noted that the significant areas found in this study are indicative of locations of effects; their spatial extent is influenced by the smoothing of the data.92
In conclusion, our study did not find structural brain abnormalities in nonpsychotic siblings of patients with schizophrenia compared with healthy control subjects, using multiple imaging methods. This suggests that the structural brain abnormalities found in patients are most likely related to the illness itself.
Acknowledgments
All authors report no competing interest.
References
- 1.Baare WF, van Oel CJ, Hulshoff Pol HE, et al. Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry. 2001;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Rijsdijk FV, van Haren NE, Picchioni MM, et al. Brain MRI abnormalities in schizophrenia: same genes or same environment? Psychol Med. 2005;35:1399–1409. doi: 10.1017/S0033291705005167. [DOI] [PubMed] [Google Scholar]
- 3.Hulshoff Pol HE, Schnack HG, Mandl RC, et al. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. Neuroimage. 2006;31:482–488. doi: 10.1016/j.neuroimage.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 4.Madsen AL, Karle A, Rubin P, Cortsen M, Andersen HS, Hemmingsen R. Progressive atrophy of the frontal lobes in first-episode schizophrenia: interaction with clinical course and neuroleptic treatment. Acta Psychiatr Scand. 2000;101:338–339. doi: 10.1111/j.1600-0447.1999.tb10880.x. [DOI] [PubMed] [Google Scholar]
- 5.Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS. Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry. 2001;158:644–646. doi: 10.1176/appi.ajp.158.4.644. [DOI] [PubMed] [Google Scholar]
- 6.Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 7.Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010:1–14. doi: 10.1017/S0033291709992297. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TD, van Erp TG, Rosso IM, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- 9.McDonald C, Grech A, Toulopoulou T, et al. Brain volumes in familial and non-familial schizophrenic probands and their unaffected relatives. Am J Med Genet. 2002;114:616–625. doi: 10.1002/ajmg.10604. [DOI] [PubMed] [Google Scholar]
- 10.Falkai P, Schneider-Axmann T, Honer WG, et al. Influence of genetic loading, obstetric complications and premorbid adjustment on brain morphology in schizophrenia: a MRI study. Eur Arch Psychiatry Clin Neurosci. 2003;253:92–99. doi: 10.1007/s00406-003-0414-9. [DOI] [PubMed] [Google Scholar]
- 11.Szeszko PR, Robinson DG, Sevy S, et al. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry. 2007;190:230–236. doi: 10.1192/bjp.bp.106.024521. [DOI] [PubMed] [Google Scholar]
- 12.Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia—a region of interest, voxel based morphometric study. Schizophr Res. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Rais M, Cahn W, Van Haren NEM, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165:490–496. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- 14.Lappin JM, Morgan K, Morgan C, et al. Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res. 2006;83:145–153. doi: 10.1016/j.schres.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Premkumar P, Fannon D, Kuipers E, Cooke MA, Simmons A, Kumari V. Association between a longer duration of illness, age and lower frontal lobe grey matter volume in schizophrenia. Behav Brain Res. 2008;193:132–139. doi: 10.1016/j.bbr.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Cahn W, Rais M, Stigter FP, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19:147–151. doi: 10.1016/j.euroneuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 17.van Haren NE, Hulshoff Pol HE, Schnack HG, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 18.van Haren NE, Cahn W, Hulshoff Pol HE, Kahn RS. Schizophrenia as a progressive brain disease. Eur Psychiatry. 2008;23:245–254. doi: 10.1016/j.eurpsy.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 20.Boos HB, Aleman A, Cahn W, Hulshoff PH, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 21.Brans RG, van Haren NE, Van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008;65:1259–1268. doi: 10.1001/archpsyc.65.11.1259. [DOI] [PubMed] [Google Scholar]
- 22.Cannon TD, van Erp TG, Huttunen M, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 23.Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157:416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- 24.van Erp TG, Saleh PA, Rosso IM, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese DR, Wang L, Harms MP, et al. Cingulate gyrus neuroanatomy in schizophrenia subjects and their non-psychotic siblings. Schizophr Res. 2008;104:61–70. doi: 10.1016/j.schres.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harms MP, Wang L, Campanella C, et al. Structural abnormalities in gyri of the prefrontal cortex in individuals with schizophrenia and their unaffected siblings. Br J Psychiatry. 2010;196:150–157. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Gur RE, Gur RC, et al. Unaffected family members and schizophrenia patients share brain structure patterns: a high-dimensional pattern classification study. Biol Psychiatry. 2008;63:118–124. doi: 10.1016/j.biopsych.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman AL, Pezawas L, Mattay VS, et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63:475–483. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honea RA, Meyer-Lindenberg A, Hobbs KB, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogtay N, Greenstein D, Lenane M, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 32.Calkins ME, Curtis CE, Grove WM, Iacono WG. Multiple dimensions of schizotypy in first degree biological relatives of schizophrenia patients. Schizophr Bull. 2004;30:317–325. doi: 10.1093/oxfordjournals.schbul.a007081. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS, McGuire M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry. 1995;52:296–303. doi: 10.1001/archpsyc.1995.03950160046009. [DOI] [PubMed] [Google Scholar]
- 34.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 35.Smitten MHT, Smeets RMW, van de Brink W. Composite International Diagnostic Interview (CIDI). Dutch Basic Version 2.1. Lifetime. Amsterdam, The Netherlands: Academic Medical Center; 1998 [Google Scholar]
- 36.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 37.Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- 38.Vollema MG, Ormel J. The reliability of the structured interview for schizotypy-revised. Schizophr Bull. 2000;26:619–629. doi: 10.1093/oxfordjournals.schbul.a033482. [DOI] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. New York; NY: Thieme Medical Publishers, Inc; 1988 [Google Scholar]
- 40.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 41.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 42.Brouwer RM, Hulshoff Pol HE, Schnack HG. Segmentation of MRI brain scans using non-uniform partial volume densities. Neuroimage. 2010;49:467–477. doi: 10.1016/j.neuroimage.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 43.Schnack HG, Hulshoff HE, Baare WF, Viergever MA, Kahn RS. Automatic segmentation of the ventricular system from MR images of the human brain. Neuroimage. 2001;14:95–104. doi: 10.1006/nimg.2001.0800. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 45.Kabani N, Le GG, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13:375–380. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- 46.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Chung MK, Worsley KJ, Robbins S, et al. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003;18:198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- 48.Brans RG, Kahn RS, Schnack HG, et al. Brain plasticity and intellectual ability are influenced by shared genes. J Neurosci. 2010;30:5519–5524. doi: 10.1523/JNEUROSCI.5841-09.2010. http://www.ncbi.nlm.nih.gov/pubmed/20410105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 50.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9:58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- 51.Collins DL. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 52.Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–1240. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- 53.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 54.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 55.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 56.Janssen J, Reig S, Alemán Y, et al. Gyral and sulcal cortical thinning in adolescents with first episode early-onset psychosis. Biol Psychiatry. 2009;66:1047–1054. doi: 10.1016/j.biopsych.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 57.Gutiérrez-Galve L, Wheeler-Kingshott CA, Altmann DR, et al. Changes in the frontotemporal cortex and cognitive correlates in first-episode psychosis. Biol Psychiatry. 2010;68:51–60. doi: 10.1016/j.biopsych.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 59.Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2009;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 60.Lawrie SM, Whalley HC, Abukmeil SS, et al. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry. 2002;181:138–143. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- 61.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in 'at risk' individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ziermans TB, Schothorst PF, Schnack HG. Progressive structural brain changes during development of psychosis. Schizophr Bull. October 7, 2010; doi:10.1093/schbul/sbq113. [Google Scholar]
- 63.Lieberman J, Chakos M, Wu H, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 64.DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 66.Lieberman JA, Tollefson GD, Charles C, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 67.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 68.Pantelis C, Yucel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 69.Waltrip RW, II, Carrigan DR, Carpenter WT., Jr Immunopathology and viral reactivation. A general theory of schizophrenia. J Nerv Ment Dis. 1990;178:729–738. doi: 10.1097/00005053-199012000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Hulshoff Pol HE, Brans RG, van Haren NE, et al. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biol Psychiatry. 2004;55:126–130. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- 71.van Haren NE, Picchioni MM, McDonald C, et al. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry. 2004;56:454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 72.Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 73.Davis JO, Phelps JA. Twins with schizophrenia: genes or germs? Schizophr Bull. 1995;21:13–18. doi: 10.1093/schbul/21.1.13. [DOI] [PubMed] [Google Scholar]
- 74.Brown AS, Bottiglieri T, Schaefer CA, et al. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64:31–39. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- 75.McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- 76.van Erp TG, Saleh PA, Huttunen M, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- 77.Schmid R, Mobus C, Spiessl H. Paranoid schizophrenia in monozygotic twins—from caregiver to patient affected. Psychiatr Prax. 2006;33:395–397. doi: 10.1055/s-2004-834755. [DOI] [PubMed] [Google Scholar]
- 78.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 79.McIntosh AM, Job DE, Moorhead TW, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56:544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 80.McIntosh AM, Job DE, Moorhead WJ, et al. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141:76–81. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- 81.McDonald C, Bullmore ET, Sham PC, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 82.McDonald C, Marshall N, Sham PC, Iacono WG, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- 83.Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 84.Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci U S A. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 87.Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- 88.Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 89.Taki Y, Goto R, Evans A, et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004;25:455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 90.Lemaître H, Crivello F, Grassiot B, Alpérovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 91.Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006;17:1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- 92.Bernal-Rusiel JL, Atienza M, Cantero JL. Determining the optimal level of smoothing in cortical thickness analysis: a hierarchical approach based on sequential statistical thresholding. Neuroimage. 2010;52:158–171. doi: 10.1016/j.neuroimage.2010.03.074. [DOI] [PubMed] [Google Scholar]