Abstract
The purpose of this study was to determine the efficacy of a peer-led illness self-management intervention called Wellness Recovery Action Planning (WRAP) by comparing it with usual care. The primary outcome was reduction of psychiatric symptoms, with secondary outcomes of increased hopefulness, and enhanced quality of life (QOL). A total of 519 adults with severe and persistent mental illness were recruited from outpatient community mental health settings in 6 Ohio communities and randomly assigned to the 8-week intervention or a wait-list control condition. Outcomes were assessed at end of treatment and at 6-month follow-up using an intent-to-treat mixed-effects random regression analysis. Compared to controls, at immediate postintervention and at 6-month follow-up, WRAP participants reported: (1) significantly greater reduction over time in Brief Symptom Inventory Global Symptom Severity and Positive Symptom Total, (2) significantly greater improvement over time in hopefulness as assessed by the Hope Scale total score and subscale for goal directed hopefulness, and (3) enhanced improvement over time in QOL as assessed by the World Health Organization Quality of Life-BREF environment subscale. These results indicate that peer-delivered mental illness self-management training reduces psychiatric symptoms, enhances participants’ hopefulness, and improves their QOL over time. This confirms the importance of peer-led wellness management interventions, such as WRAP, as part of a group of evidence-based recovery-oriented services.
Keywords: illness self-management, recovery, peer-led intervention
Introduction
Illness self-management programs for people with chronic medical conditions are an important part of patient-centered care as articulated by the Institute of Medicine.1 These programs produce positive changes in health outcomes, attitudes, and behaviors via acquisition of new information and skills to better manage troublesome symptoms, maintain higher levels of health and functioning, and enhance quality of life (QOL).2–7 Recently developed mental illness self-management programs have extended this approach to behavioral health by imparting information, teaching wellness skills, and providing emotional support to enhance recovery.8,9 One example is the Illness Management and Recovery (IMR) program, consisting of 3–6 months of weekly sessions delivered by mental health agency staff such as case managers or other clinicians.10 IMR helps participants learn structured problem solving, develop personalized strategies for managing symptoms, set personal goals, and develop social support systems.11 In a study of IMR delivered to 24 individuals,12 participants showed significant decreases in symptom severity, increases in recovery, improvement in functioning, and increased knowledge about mental illness at 3-month follow-up; moreover, satisfaction with the program was high. A study of IMR delivered to 324 community mental health center clients found significant increases in hope at 6-month and 12-month follow-up but no changes in satisfaction with services.13 IMR was also evaluated among 210 individuals with severe mental illness receiving community rehabilitation using a randomized controlled trial design comparing it with treatment as usual.14 At posttest immediately following the intervention, compared with controls, IMR participants showed increased knowledge of their illness and improved personal goal attainment but did not experience increased levels of social support.
The present study examined the efficacy of a behavioral health illness self-management intervention called Wellness Recovery Action Planning (WRAP). WRAP is typically taught by individuals in stable recovery from mental illness and is offered in 8–12 weekly sessions.15 WRAP participants create an individualized plan to achieve and maintain recovery by learning to utilize wellness maintenance strategies, identify and manage symptoms and crisis triggers, and cope with psychiatric crises during and following their occurrence.16 Instructional techniques promote peer modeling and support by using personal examples from peer facilitators’ and students’ lives to illustrate key concepts of self-management and recovery.
The process of illness self-management has its conceptual foundation in the psychological theory of self-determination.17 In this framework, lasting health behavior change occurs through autonomous motivation in which actors experience a sense of volition, self-initiation, and endorsement of their behavior.18 This type of motivation occurs in autonomy supportive environments defined as settings in which health care providers understand the actor’s perspective, acknowledge his/her feelings, offer choices, and provide information.19 Also integral to the change process is perceived competence because patients who feel more competent in carrying out a health-related behavior are more likely to engage in that behavior.20 WRAP is designed to create a safe, nonjudgmental autonomy supportive environment in which people feel motivated to manage their mental health issues, while their perceived competence for doing so is enhanced through development of a detailed and personalized WRAP plan.
The social support provided in illness self-management programs is viewed as a critical component to successful health behavior change,21,22 and support from peer instructors may enhance the efficacy of these interventions.23 Prior research has shown that peer support and education leads to health behavior change for patients with a number of chronic illnesses including HIV, diabetes, and asthma.24–26 Peers who are successfully managing physical health challenges may provide others with an incentive to develop their own self-management skills and a greater sense of optimism.27,28 Similarly, research has shown that peer support services are effective in promoting mental health recovery.29–31 Peer support, defined broadly as interpersonal interactions and activities facilitated by peers and aimed at achieving recovery goals in affirming environments,32 has been shown to decrease inpatient admissions, improve functioning, and reduce mental health treatment costs.33,34
The peer support component of WRAP has conceptual underpinnings in self-efficacy theory35 and social comparison theory.36 Self-efficacy or the belief that one is capable of successfully executing behaviors that produce desired outcomes is enhanced by seeing similar others (peers) achieve gains through sustained effort.37 In social comparison theory, upward social comparison with healthier peers provides actors with an incentive to develop their skills and a greater sense of optimism.27,28 Studies of people with serious mental illness (SMI) show that development of a more efficacious sense of self following exposure to peers is linked to recovery.38–40 When illness self-management is taught by peers, self-efficacy may be enhanced through positive social comparison, thereby generating hope and perceived competency for health behavior changes that promote recovery such as symptom management.
In the burgeoning field of mental illness self-management models, WRAP is probably the most widely disseminated program of its type in the United States.41 More than 10 000 copies of the WRAP curriculum have been distributed and over 2000 people have been trained as WRAP group facilitators by the nonprofit Copeland Center for Wellness and Recovery as of February 2011. There are 150 individuals trained as Advanced Level Facilitators who are qualified to teach others to facilitate WRAP groups. While every state in the United States has publicly funded WRAP programs, over half also have large-scale comprehensive and integrated WRAP initiatives. WRAP has also spread around the world, with extensive WRAP training and program development occurring in Canada, Japan, Hong Kong, New Zealand, Australia, England, Scotland, and Ireland.42
While the growth of WRAP has been impressive, it has been infrequently evaluated and reported on in the published literature.43–46 Therefore, the purpose of the present study was to conduct a randomized controlled trial of WRAP delivered to psychiatric outpatients by people in recovery from SMIs. The study tested the primary hypothesis that experimental group participants would experience greater symptom reduction than controls and that this effect would be maintained over time. Also tested were 2 secondary hypotheses that experimental group subjects would exhibit greater increases in hopefulness and enhanced QOL than controls and that these effects also would be maintained over time.
Methods
Participants
Subjects were adults with SMIs who were receiving publicly funded outpatient psychiatric services in 6 Ohio communities: Canton, Cleveland, Columbus, Dayton, Lorain, and Toledo. These areas were chosen because they contained a sufficient number of certified WRAP educators, and because WRAP had not already been offered extensively there. All subjects met federal criteria for having SMI based on diagnosis, duration, and level of disability stipulated in Public Law 102–321,47 requiring the person to have at least one 12-month disorder (other than a substance use disorder) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria48 and to have “serious impairment” defined in the state of Ohio as having “within the past 6 months … functional limitations on a continuing or intermittent basis in major life activities that would be appropriate for the individual’s developmental stage.”49 Additional inclusion criteria were being 18 years of age or older at the time of study enrollment, willing and able to provide informed consent, able to understand spoken English, and not previously exposed to WRAP education.
Recruitment was conducted with the assistance of Ohio Department of Mental Health (ODMH) administrators, the cooperation of the County Mental Health Boards in all 6 regions, and collaboration with the statewide consumer organization (Ohio Advocates for Mental Health) as well as another peer-run organization that administered state WRAP funds (Depression and Bipolar Support Alliance Ohio). From October 2006 through April 2008, individuals were recruited via clinician and peer referral, self-referral, newspaper advertisement, county mental health board Web sites, and word of mouth. The majority of recruitment activities occurred in mental health service delivery settings, including traditional treatment programs (eg, community mental health centers, outpatient clinics, residential programs) and self-help and peer support programs (eg, consumer-run recovery centers, mental health support groups). Local peer study coordinators made presentations at these programs about WRAP and the research study, and encouraged interested individuals to call the study’s toll-free number at the University of Illinois at Chicago (UIC) to enroll. All participants provided written informed consent to participate using procedures approved by the UIC Institutional Review Board. The study was registered at ClinicalTrials.gov under identifier NCT01024569, and all outcomes, hypotheses, and statistical analyses presented here were prespecified at the time the proposal was submitted to the federal government for funding consideration.
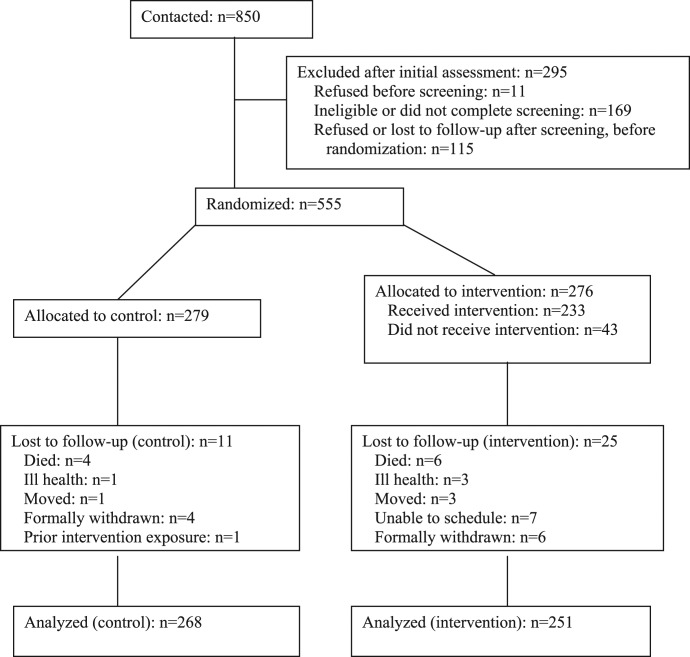
As shown in figure 1, 850 individuals were contacted. Of these, 295 were excluded due to refusal prior to screening, ineligibility, failure to complete the screening process, or refusal after screening but prior to randomization. A total of 555 were randomly assigned, 279 to the control (ie, waiting list) and 276 to the experimental (ie, intervention) conditions. Of the 276 experimental subjects, 233 (84%) received the intervention and 43 (16%) did not. Eleven control subjects and 25 intervention subjects were lost to follow-up with reasons including death or ill health, moving away from the area, and formal withdrawal from the study. The majority of the 25 experimental subjects lost to follow-up (68%) received no sessions of WRAP and only 1 (4%) attended 6 or more sessions. No other subjects were excluded from the analysis for any other reason given the “intent-to-treat” design.50 Thus, the analyzed sample consisted of 251 in the experimental and 268 in the control condition, for a total of 519 individuals.
Fig. 1.
Recruitment and flow of participants in a study of illness self-management for people with severe and persistent mental illness.
Intervention
The WRAP intervention was delivered in 8 weekly sessions of 2.5 hours that were cofacilitated by 2 peers, with a third backup educator available for emergencies. Classes were offered in accessible community settings, free of charge, with class sizes ranging from 5 to 12 participants. Coursework included lectures, group discussions, personal examples from the lives of the educators and participants, individual and group exercises, and voluntary homework assignments. An introductory session conveyed the key concepts of WRAP and recovery. Sessions 2 and 3 addressed development of personalized wellness strategies that can be used to maintain recovery and manage difficulties in functioning. Also included were special exercises to enhance self-esteem, build competence, and explore the benefits of peer support. The fourth session introduced a daily maintenance plan that comprised simple, inexpensive strategies to use every day to stay emotionally and physically healthy, including a plan for recognizing and responding to symptom triggers in order to prevent crises. The fifth session educated participants about early warning signs and how these signal a need for additional support. The sixth and seventh sessions involved creation of a crisis plan specifying signs of impending crisis, names of individuals willing to help, and types of assistance preferred. The final session covered postcrisis support and the benefits of retooling WRAP plans after a crisis to avoid relapse.
Model fidelity was assessed weekly by use of a detailed checklist to track adherence to prescribed topics, time frames, and instructional modalities. In addition, all educators were observed on multiple occasions by one or both of the local study coordinators for quality control purposes and provision of detailed feedback. Following the National Institutes of Health Behavior Change Consortium’s recommendations for enhancing treatment fidelity in health behavior research,51 we monitored fidelity throughout the entire period of service delivery, reviewed fidelity checklist scores weekly with instructors first individually and then in a group teleconference, and followed procedures ensuring that any missed material was covered in subsequent sessions. The weekly teleconference calls convened by UIC researchers and the local statewide WRAP coordinators included review of each site’s attendance and fidelity scores, discussion of the following week’s topics and instructional methods, and group problem-solving to deal with any difficulties that had emerged.
Control Condition
Control group participants were assigned to a course waiting list and guaranteed an opportunity to receive WRAP from the study once their third and final interview wave ended. Otherwise, they continued to receive services as usual. To assess the integrity of this no-treatment condition, we measured receipt of WRAP or other peer-support interventions at each assessment point.
Procedures
Researchers employed by the UIC Survey Research Laboratory (SRL) administered structured telephone interviews, and interviewers were blinded to respondents’ study condition. These 1-hour interviews occurred at: Time 1 (T1), 6 weeks before the start of WRAP classes; Time 2 (T2), 6 weeks following the end of WRAP classes; and Time 3 (T3), 6 months post-T2. Participants received a research stipend of $20 for the first interview, $25 for the second, and $30 for the third, with a $10 bonus for completing all 3. Interviews were conducted via Computer Assisted Personal Interviewing (CAPI) software, with data downloaded into the commercially available database system SPSS Inc.52 and analyzed using Mixed Effects Random Regression (MIXREG) software version 1.2.53
Randomization was performed by SRL staff at the end of each interview using a random allocation sequence programmed into CAPI administration software that allowed for complete allocation concealment up to the point of assignment.54 Thus, both interviewers and respondents had no way of knowing each subject’s study condition until after the assignment had occurred. To monitor the integrity of the blind, at the conclusion of each T2 and T3 interview, interviewers were asked whether subjects had explicitly or inadvertently revealed their actual study condition. This was found to have occurred in only 4% of all T2 and T3 interviews.
Measures
The primary outcome was reduction of psychiatric symptom severity, measured by the Brief Symptom Inventory (BSI), a patient self-report research instrument showing high concordance with clinician symptom assessment.55 This measure was chosen due to its frequent use as an index of clinical improvement and treatment outcome in randomized trials of a wide variety of mental health interventions.56–59 Respondents are asked how much they were bothered in the past week by 53 symptoms with a 5-point response scale ranging from “not at all” to “extremely.” The BSI’s Global Severity Index is designed to quantify a patient’s severity of illness and provides a single composite score for measuring the outcome of a treatment program based on reducing symptom severity.55 The BSI Positive Symptom Total captures the number of symptoms endorsed in a pathological direction, representing the total volume of different symptoms reported to be present to any degree.60
The second outcome was hopefulness, assessed with the Hope Scale (HS), an instrument designed to measure hope as a cross-situational long-term trait in general populations.61 Twelve items are rated on a 4-point response scale ranging from “definitely false” to “definitely true” and summed to produce a total score. Two subscales measure belief in one’s capacity to initiate and sustain actions (agency) and ability to generate routes by which goals may be reached (pathways). These 2 components of hope are assumed to be reciprocal, additive, and positively related to one another, but not synonymous, because individuals may believe in their ability to act without being aware of how to achieve a goal and vice versa.62 Research has found HS scores to be positively associated with goal-related activities and coping strategies.63
The final outcome was QOL, assessed by the World Health Organization Quality of Life Brief instrument (WHOQOL-BREF).64 We selected the environment subscale to measure this construct because of its suitability for use with people who have multiple needs65 and because it captures dimensions specific to the posited effects of WRAP such as acquiring new skills and information, enhanced leisure and recreation, and feelings of security and freedom.64 Respondents rate their experience of 8 quality indicators over the past 2 weeks using a 5-point Likert response scale, with higher scores indicating higher QOL.
Given that randomization was successful (described below), the only control variable used in the analysis was study site (also described below). Indicator variables were created for each of the sites with the Lorain site used as the contrast. The other model variables were time and the interaction of study condition by time.
Data Analysis
We began by testing the success of randomization and intercorrelations between study variables. Next, multivariate longitudinal random-effects linear regression analysis was conducted to test for differences between experimental and control subjects’ outcomes over time. A 2-level random intercepts model was fitted to the data, controlling for study site as a fixed effect. This approach was chosen given the superiority of random regression models in addressing issues commonly found in longitudinal multisite data, including: (1) state dependency or serial correlations among repeated observations within individual participants, (2) individual heterogeneity or varying propensities toward the outcomes of interest due to subjects’ predispositions and other unobserved influences, (3) missing observations due to the fact that not all subjects completed all assessments, and (4) inclusion of the both time-varying and fixed covariates.66
Results
Subject Characteristics
Demographics, clinical status, and employment status of study subjects are shown in table 1. A fifth (21%) reported diagnoses of schizophrenia or schizoaffective disorder, another 38% reported bipolar disorder, and another a quarter (25%) reported a depressive disorder. The high prevalence of Axis I diagnoses (85%) and the fact that most were not employed (85%) or married/cohabiting (88%) confirms SMI with considerable occupational and social role impairment. This is further supported by the fact that these subjects were recruited at publicly funded programs for individuals with SMI. The success of randomization was confirmed by the absence of statistically significant differences by study condition at baseline on all characteristics. We also found no significant differences (not shown) between experimental and control participants in prebaseline use of services including case management, medication management, individual therapy, group therapy, employment services, residential services, and substance abuse treatment.
Table 1.
Characteristics of Participants in Each Study Condition
| Total (N = 519) | Experimental (n = 251)a | Control (n = 268)a | |
| n (%) | n (%) | n (%) | |
| Sex | |||
| Male | 177 (34.1) | 83 (33.1) | 94 (35.1) |
| Female | 342 (65.9) | 168 (66.9) | 174 (64.9) |
| Mean (SD) age, years | 45.8 (9.88) | 45.7 (9.80) | 45.8 (9.97) |
| Race/ethnicity | |||
| Caucasian | 328 (63.2) | 156 (62.2) | 172 (64.2) |
| Black | 146 (28.1) | 76 (30.3) | 70 (26.1) |
| Hispanic/Latino | 25 (4.8) | 11 (4.4) | 14 (5.2) |
| Asian/Pacific Islander | 3 (0.6) | 2 (0.8) | 1 (0.4) |
| American Indian/Alaskan | 15 (2.9) | 6 (2.4) | 9 (3.4) |
| Other | 2 (0.4) | — | 2 (0.7) |
| Education | |||
| <High school | 95 (18.3) | 44 (17.5) | 51 (19.0) |
| High school/GED | 182 (35.1) | 95 (37.8) | 87 (32.5) |
| Some college or greater | 242 (46.6) | 112 (44.6) | 130 (48.5) |
| Marital status | |||
| Married or cohabiting | 62 (12.0) | 26 (10.4) | 36 (13.5) |
| All other | 455 (88.0) | 224 (89.6) | 231 (86.5) |
| One or more children | |||
| Yes | 294 (57.0) | 143 (57.4) | 151 (56.6) |
| No | 222 (43.0) | 106 (42.6) | 116 (43.4) |
| Lives in own home/apt. | |||
| Yes | 346 (66.7) | 167 (66.5) | 179 (66.8) |
| No | 173 (33.3) | 84 (33.5) | 89 (33.2) |
| Employed | |||
| Yes | 76 (14.7) | 44 (17.6) | 32 (11.9) |
| No | 442 (85.3) | 206 (82.4) | 236 (88.1) |
| Mean (SD) # in household | 2.3 (2.32) | 2.3 (2.28) | 2.4 (2.36) |
| Ever psychiatric inpatient treatment | |||
| Yes | 392 (75.8) | 195 (78.0) | 197 (73.8) |
| No | 125 (24.2) | 55 (22.0) | 70 (26.2) |
| DSM-IV diagnosis | |||
| Schizophrenia | 58 (11.7) | 29 (11.9) | 29 (11.6) |
| Schizoaffective | 47 (9.5) | 26 (10.7) | 21 (8.4) |
| Bipolar | 188 (38.1) | 95 (38.9) | 93 (37.2) |
| Depressive | 125 (25.3) | 60 (24.6) | 65 (26.0) |
| Other | 76 (15.4) | 34 (13.9) | 42 (16.8) |
| Services received T1–T2 | |||
| Case management | 333 (72.7) | 170 (75.9) | 163 (69.7) |
| Medication management | 343 (74.9) | 170 (75.9) | 173 (73.9) |
| Individual therapy | 344 (75.3) | 162 (72.3) | 182 (78.1) |
| Group psychotherapy | 108 (23.6) | 61 (27.4) | 47 (20.1) |
| Employment services | 87 (19.0) | 44 (19.6) | 43 (18.4) |
| Residential services | 77 (16.8) | 40 (17.9) | 37 (15.8) |
| Substance abuse treatment | 34 (7.4) | 11 (4.9) | 23 (9.8) |
| Study site | |||
| Canton | 81 (15.6) | 38 (15.1) | 43 (16.0) |
| Cleveland | 98 (18.9) | 51 (20.3) | 47 (17.5) |
| Columbus | 107 (20.6) | 52 (20.7) | 55 (20.5) |
| Dayton | 26 (5.0) | 12 (4.8) | 14 (5.2) |
| Lorain | 110 (21.2) | 53 (21.1) | 57 (21.3) |
| Toledo | 97 (18.7) | 45 (17.9) | 52 (19.4) |
Note: T1, Study baseline; T2, 2-month follow-up; GED, General Education Development. Variations in n due to missing data.
Chi-square and analysis of variance tests revealed no significant differences by study condition.
Intervention Implementation
The intervention was delivered simultaneously across study sites, with 5 waves of classes taught over a 3-year period. At each site, WRAP was codelivered by 2 lead facilitators, with 1 or more backup facilitators who were available in case of illness or emergencies. Of the 20 facilitators, 85% were female and 15% male, 90% were Caucasian and 10% African American, and their average age was 48 years. All facilitators were individuals in stable recovery from a mental illness, defined as living in the community and maintaining emotional wellness through use of a personalized WRAP plan. Facilitators were experienced WRAP educators with a Mental Health Recovery Educator certificate from the Copeland Center for Wellness and Recovery and were selected by the study’s local coordinators who had trained them and, in some cases, led WRAP groups with them. At all sites, one or both of the lead facilitators remained the same every time the intervention was offered. Four of the 6 sites delivered WRAP 5 times, a fifth site delivered it 4 times, and a sixth site delivered it once, during the final wave when the fifth site’s facilitators were unavailable. Prior to intervention implementation, all instructors attended a 2½-day training session convened by the researchers and the study’s local coordinators who are certified WRAP Advanced Level Facilitators. Training involved detailed review and practice of the 8-session curriculum, training on the fidelity assessment and attendance tracking procedures, and discussion of research procedures and related logistical issues.
The WRAP fidelity assessment tool was developed by one of WRAP’s authors (M.E.C) and UIC investigators (J.A.J. and J.A.C.) and administered following each class by the study’s local coordinators (C. B. F. and W. H.). Within 48 hours of each class session, local coordinators telephoned instructors and completed the assessment for that session to determine fidelity to the content prescribed for that module. Each curriculum component was scored as 1 if the prescribed element occurred and 0 otherwise. Fidelity scores were computed as the proportion of prescribed elements present for that module. Across all modules taught in all waves, total course fidelity ranged from 90.3% in wave 1 to 91.7% in wave 5, with a mean of 91.3% (SD = 0.01). There were no significant differences in course fidelity by wave (F 4,20 = 1.50, P = .24) or by study site (F 5,19 = 1.86, P = .15). Overall, results indicated excellent intervention fidelity.
Intervention Completion Rates
Instructors maintained attendance logs for each participant with attendance at each class coded as 1 if present (either in-person or by makeup over the telephone) and 0 otherwise. Total attendance was computed by summing attendance scores for each participant. On average, participants attended 5 of 8 classes (mean = 5.05, SD = 3.08), and there were no significant differences in attendance by wave (F 4,271 = 1.12, P = .34). However, there were significant differences in attendance by site (F 5,270 = 3.30, P = .007), with attendance ranging from a low of 4.43 classes at one site to a high of 6.35 classes at another. Because of this, site was used as a control variable in the next phase of the analysis. The most commonly reported reasons for nonattendance were physical illness, transportation problems, and schedule conflicts.
Services As Usual Control Condition
During the 2-month intervention period, control subjects continued with the same treatment they were receiving upon study entry. As shown in table 1, 70% reported receiving case management, 74% reported medication management, 78% individual therapy, 20% group therapy, 18% employment services, 16% residential services, and 10% substance abuse treatment. As shown in the second column of table 1, there were no significant differences between control and experimental subjects in receipt of any of these services. Throughout the intervention period and 6-month follow-up, no WRAP classes were offered outside of the study in any of the host counties and, thus, the intervention was not available locally to control subjects. However, control subjects could and did participate in peer-led mental health support groups. Between T1 and T2, 41.9% of control subjects (n = 98) reported attending such groups, and between T2 and T3, 44.9% (n = 97) reported doing so. Because of this, all models were rerun controlling for exposure to peer-led support groups.
Follow-up Rates and Attrition
Of the 519 subjects who completed T1 assessments, 458 subjects (88.2%) completed T2 interviews, and 448 (86.3%) completed T3 interviews, for a combined attrition rate of 6.6%. There were no statistically significant differences in follow-up rates between intervention and control conditions. At T2, interviews were completed by 224 (89.2%) of the intervention group and 234 (87.3%) of the control group (χ2 1,1 = 0.49, P = .29). At T3, assessments were completed by 220 (87.6%) of the intervention group and 228 (85.1%) of the control group (χ2 1,1 = 0.39, P = .23). Finally, there were no significant differences in completion of T2 or T3 interviews by study site.
Participant Outcomes
Table 2 shows unadjusted mean values over time for each of the 3 outcomes by study condition. Multivariable random-effects linear regression analysis (table 3) of all 3 outcomes showed significant interactions of study condition by time. Compared with controls, experimental group participants reported significantly greater symptom reduction over time in BSI Global Symptom Severity and Positive Symptom Total. Intervention participants also reported significantly greater improvement over time than controls in their hopefulness as measured by total HS scores. Those who received WRAP also reported significantly greater improvement than controls in the hopefulness subscale measuring belief in one’s capacity to initiate and sustain actions (agency), but not the subscale measuring belief in one’s ability to devise routes by which goals may be reached (pathways). Finally, intervention participants reported significantly greater improvement than controls in QOL regarding opportunities for acquiring new skills and information, enhanced leisure and recreation, and feelings of security and freedom.
Table 2.
Unadjusted Mean Scores and Standard Deviations for Outcome Measures
| Measure by Time Point | Intervention | Control | ||
| Mean (SD) | No. | Mean (SD) | No. | |
| BSI global severity index | ||||
| Baseline | 0.76 (0.72) | 251 | 0.73 (0.73) | 268 |
| Postintervention 1 | 0.72 (0.64) | 224 | 0.85 (0.70) | 234 |
| Postintervention 2 | 0.42 (0.61) | 220 | 0.47 (0.67) | 228 |
| BSI positive symptom total | ||||
| Baseline | 20.60 (14.67) | 251 | 19.29 (14.09) | 268 |
| Postintervention 1 | 19.52 (13.74) | 224 | 21.38 (13.68) | 234 |
| Postintervention 2 | 12.20 (220) | 220 | 12.65 (15.00) | 228 |
| Hope | ||||
| Baseline | 21.67 (4.66) | 248 | 21.87 (4.42) | 264 |
| Postintervention 1 | 22.47 (4.39) | 221 | 22.07 (4.06) | 228 |
| Postintervention 2 | 22.76 (4.68) | 212 | 22.16 (4.21) | 222 |
| Hope—agency | ||||
| Baseline | 10.62 (2.81) | 249 | 10.67 (2.64) | 266 |
| Postintervention 1 | 11.20 (2.50) | 223 | 10.88 (2.47) | 231 |
| Postintervention 2 | 11.33 (2.70) | 215 | 10.92 (2.59) | 223 |
| Hope—pathways | ||||
| Baseline | 11.06 (2.38) | 250 | 11.19 (2.29) | 265 |
| Postintervention 1 | 11.26 (2.34) | 222 | 11.19 (2.09) | 229 |
| Postintervention 2 | 11.44 (2.39) | 213 | 11.24 (2.06) | 225 |
| WHO quality of life—environment | ||||
| Baseline | 13.1 (2.94) | 251 | 13.1 (2.74) | 268 |
| Postintervention 1 | 13.7 (2.97) | 224 | 13.5 (2.79) | 234 |
| Postintervention 2 | 14.1 (2.83) | 212 | 13.4 (2.97) | 219 |
Note: BSI, Brief Symptom Inventory; WHO, World Health Organization.
Table 3.
Effects of Study Condition (Intervention vs Control) on Participant Outcomes, Mixed Effects Random Regression (MIXREG) Controlling for Study Site (N = 519)
| Outcome Variable | MIXREG Estimatea | SE | P Value |
| BSI global severity index | |||
| Intercept | 0.85 | 0.07 | .000 |
| Intervention condition | 0.06 | 0.07 | .360 |
| Time | −0.12 | 0.02 | .000 |
| Intervention × time | −0.05 | 0.02 | .023 |
| BSI positive symptom total | |||
| Intercept | 22.29 | 15.09 | .000 |
| Intervention condition | 1.98 | 1.33 | .182 |
| Time | −3.01 | −8.18 | .000 |
| Intervention × time | −1.16 | −2.21 | .027 |
| Hope total | |||
| Intercept | 21.79 | 46.68 | .000 |
| Intervention condition | −0.57 | −1.21 | .227 |
| Time | 0.15 | 1.25 | .213 |
| Intervention × time | 0.40 | 2.37 | .018 |
| Hope—agency | |||
| Intercept | 10.69 | 0.28 | .000 |
| Intervention condition | −0.26 | 0.28 | .355 |
| Time | 0.12 | 0.07 | .089 |
| Intervention × time | 0.24 | 0.20 | .020 |
| Hope—pathway | |||
| Intercept | 11.07 | 0.24 | .000 |
| Intervention condition | −0.27 | 0.25 | .276 |
| Time | 0.03 | 0.07 | .607 |
| Intervention × time | 0.14 | 0.10 | .140 |
| WHO quality of life—environment | |||
| Intercept | 13.29 | 0.30 | .000 |
| Intervention condition | −0.46 | 0.31 | .134 |
| Time | 0.09 | 0.08 | .219 |
| Intervention × time | 0.39 | 0.11 | .001 |
Note: Abbreviations are explained in the first footnote to table 2.
Estimates are unstandardized MIXREG coefficients and do not represent effect sizes; sign of coefficient indicates direction of effect.
Because a substantial minority of control-condition subjects reported exposure to peer support groups, all models were rerun with a time-varying variable controlling for exposure to such groups at each time point. Results did not differ substantially from those obtained in the original MIXREG analyses, with time by study condition remaining significant in all analyses.
Because MIXREG does not provide estimates of effect size, we calculated average proportional odds ratios67 by marginalizing the beta estimates from the MIXREG analysis to create odds ratios that have the advantage of adjusting for heterogeneity of study participants. SAS/IML software68 was used to perform these calculations on estimates from the original MIXREG models. The estimated odds ratio for the condition by time interaction in the model predicting BSI Global Symptom Severity was 0.95 (df = 8, 510; 95% CI = 0.91–0.98), and for HS the odds ratio was 1.49 (df = 8, 510; CI = 1.47–1.51). The estimated odds ratio for the model predicting QOL was 1.48 (df = 8, 510; 95% CI = 1.32–1.65).
Finally, to address whether degree of exposure to the WRAP intervention was related to study outcomes, we used ordinary linear regression to predict T3 outcome scores. In an analysis restricted to experimental subjects, we examined the effect of number of WRAP sessions attended (ranging from 0 to 8) calculating both unadjusted B and partial-B (ie, controlling for study site). Attendance was significant in 2 of the 3 models. For the GSI, B = −1.06 and partial-B = −1.16, indicating a 1-point decrease in symptom severity scores for each WRAP session attended. For the QOL, B = 0.19 and partial-B = 0.19, indicating a 0.2 unit increase in quality of life scores with each WRAP class attended.
Discussion
This is the first randomized trial of WRAP and results show that it is an effective treatment when compared with usual community care. Psychiatric symptom severity scores are significantly reduced among WRAP participants compared with those receiving services as usual, while hopefulness and QOL are significantly increased among WRAP vs usual care recipients. Thus, a major finding of this study was that, compared to services as usual, intervention participants reported significantly greater improvement in 3 outcome areas that are widely acknowledged to be indicators of recovery. This was the case controlling for the effects of time, showing that positive changes persisted for at least 6 months after the intervention’s conclusion. Results were also consistent across study site, confirming WRAP’s effectiveness in large- to midsize urban communities in diverse regions of a populous Midwestern state. We also found that the greater participants’ exposure to WRAP, the more they improved on psychiatric symptom severity and hopefulness for their futures. This supports the ongoing availability of this model to ensure that participants can obtain adequate exposure to impact life outcomes.
Study results point to somewhat divergent effects of WRAP on the different recovery outcomes studied. On psychosocial measures of hopefulness and QOL, WRAP recipients reported not only significantly greater improvement relative to controls, but this advantage appeared to grow over time. On the other hand, the experimental vs control differences in symptom severity were larger between T1 and T2 and seemed to attenuate over the long term, even though WRAP participants were still doing better at T3 in the multivariate analysis. Future research is needed to understand the differences between these outcomes and their relationship to other personal changes in areas such as functioning, empowerment, self-advocacy, and self-esteem. Data from the present study will be used in subsequent analyses to explore these questions and thus illuminate the subjective components of recovery.
Also noted in these results was improvement among control-condition subjects on all 3 outcomes. This may have been due to the high number of clinical services they were receiving and/or may have been due to an "anticipation effect" because controls were promised an opportunity to receive WRAP at the end of the study. The fact that noteworthy proportions of subjects in both conditions were receiving peer support at both follow-up time points may also account for both improvement among the control subjects and convergence of the symptom outcome between the 2 study conditions at T3.
Another finding inviting further explication is that regarding participants’ degree of hopefulness given that observed changes in raw scores were relatively modest. However, in research on hope interventions, it is widely acknowledged that “… a statistically small change in hope may be clinically meaningful,”69 and our intervention condition mean of 22.8 at follow-up compares well with Irving and colleagues’70 normative sample mean of 20.7 for low-hope college women. Relative to controls, WRAP participants reported greater feelings of hope related to “agency” or their views of their own ability to influence their lives and make sustained changes. However, there were no differences by study condition in subjects’ self-perceived ability to construct successful plans of action, as measured by the “pathways” subscale. This suggests that while WRAP improves confidence in one’s ability to take action, additional supports may be needed to help people make plans for rebuilding their lives in the community. These might include, eg, access to financial resources, social support, employment services, peer supports, and health care as well as traditional clinical psychiatric services.
Regarding QOL, again changes in raw scores were somewhat modest. However, research on the clinical meaning of the WHOQOL-BREF scores shows that a one-point difference between domain scores is actually quite significant. Analysis of data from 23 countries found that scores on the environment subscale discriminated significantly between those who were ill (mean = 13.8) and those who were well (mean = 14.1).64 Our results indicate that intervention recipients improved from a baseline mean of 13.1 (below the average for the “ill” group) to a posttest mean of 14.1 that compares favorably with the mean for the “well” group.
Another more anecdotal finding of the study was that WRAP could be delivered to a sizable population of people with SMI by their peers in successive waves with a high level of fidelity. The fact that WRAP was delivered every 3–4 months over a period of several years at greater than 90% fidelity with at least 1 educator teaching consistently at each site indicates that a well-supported peer workforce can deliver this intervention to high standards. Additional studies are needed to determine how best to develop and nurture a workforce of peer providers using models such as WRAP that support recovery on a large scale.
There are a number of study limitations that should be considered when interpreting these results. The first major caveat to our findings is that the study’s subjects were not drawn from a national probability sample of individuals with severe and persistent mental illness, which limits the generalizability of our results. A second caveat is the fact that all subjects came from a single Midwestern state, preventing an assessment of potential US regional variations in WRAP implementation and outcomes. A third caveat concerns the design of the study using a wait-list control condition. Use of an attention-control placebo would have allowed us to assess whether 8 weeks of peer interaction alone, and not the specific features of the WRAP intervention, caused the observed outcomes. A fourth caveat is that the study relied on participant self-report data that were uncorroborated by clinicians or objective observers such as research staff. Future studies using external raters and attention-control placebo interventions will offer a more rigorous evaluation of WRAP’s efficacy. A fifth caveat is that fidelity assessment was limited to WRAP facilitator self-report, while the additional use of direct observation to verify the validity of self-reports would have added credibility to fidelity assessment. Another potential confound is the high level of study subjects’ participation in peer-led programs and support groups, which may have exposed control-condition subjects to some of the same active ingredients as those contained in the WRAP intervention. As a result, the study may have underestimated the effects of WRAP relative to its impact in communities with low levels of peer support, as is typical in many areas of the United States. Finally, a longer time period of data collection might have revealed different findings than those attained at the end of the 8 months tracked in this study. All these limitations suggest that caution should be applied to interpretations from study results.
Study results build on prior evidence concerning the efficacy of self-management interventions taught by clinicians but go further in demonstrating the longitudinal effectiveness of these interventions when taught by peers. WRAP’s focus on planning, skill building, social support, and confidence enhancement may promote perceived competence and inculcate autonomous motivation for attitudinal and behavioral changes that lead to recovery. If these specific processes are confirmed in future studies, this intervention has the potential to work in a wide variety of regions and settings.
Given research cited earlier concerning the benefits of self-management for individuals with psychiatric disabilities, findings from this study can be used to create the next generation of evidence-based models71 that contribute to recovery and increased community integration. Additional research on WRAP and other peer-led programs can point us to the active ingredients in this type of intervention, and thereby inform the development of new ways for peers to promote self-determination and social participation.
Funding
US Department of Education, National Institute on Disability and Rehabilitation Research; and the Substance Abuse & Mental Health Services Administration, Center for Mental Health Services, Cooperative Agreement (H133B050003 and H133B100028).
Acknowledgments
The views expressed do not reflect the policy or position of any Federal agency. The authors gratefully acknowledge the cooperation and assistance of the following organizations: the ODMH; the Lorain County Board of Mental Health; the Mental Health and Recovery Services Board of Lucas County; the Alcohol, Drug and Mental Health Board of Franklin County; the Mental Health and Recovery Services Board of Stark County; the Alcohol, Drug Addiction, and Mental Health Services Board for Montgomery County; the Alcohol, Drug Addiction and Mental Health Services Board of Cuyahoga County; Ohio Advocates for Mental Health; Depression and Bipolar Support Alliance Ohio; and the UIC SRL. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. Institute of Medicine. [PubMed] [Google Scholar]
- 2.Lorig KR, Ritter PL, Stewart AL, et al. Chronic disease self-management program: two-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing utilization and costs: a randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Glasgow RE, Toobert DJ, Hampton SE. Effects of a brief office-based intervention to facilitate diabetes dietary self management. Diabetes Care. 1996;19:835–842. doi: 10.2337/diacare.19.8.835. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow RE, Toobert DJ, Hampton SE, Noell JW. A brief office-based intervention to facilitate diabetes dietary self management. Health Educ Res. 1995;10:467–478. doi: 10.1093/her/10.4.467. [DOI] [PubMed] [Google Scholar]
- 6.Brown JE, Glasgow RE, Toobert DJ. Integrating dietary self-management counseling into the regular office visit. Practical Diabetol. 1996:16–22. [Google Scholar]
- 7.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 8.Anzai N, Yoneda S, Kumagai N, Nakamura Y, Ikebuchi E, Liberman RP. Training persons with schizophrenia in illness self-management: a randomized controlled trial in Japan. Psychiatr Serv. 2002;53:545–547. doi: 10.1176/appi.ps.53.5.545. [DOI] [PubMed] [Google Scholar]
- 9.Lawn S, Battersby MW, Pols RG, Lawrence J, Parry T, Urukalo M. The Mental Health Expert Patient: findings from a pilot study of a generic chronic condition self-management programme for people with mental illness. Int J Soc Psychiatry. 2007;53:63–74. doi: 10.1177/0020764007075010. [DOI] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration. Illness Management and Recovery: Practitioner Guides and Handouts. HHS Pub. No. SMA-09-4462, Rockville, MD: Center for Mental Health Services, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services; 2009; [Google Scholar]
- 11.Gingerich S, Mueser K. Illness management and recovery. In: Drake R, Merrens M, Lyne D, editors. Evidence-based Mental Health Practice: a Textbook. New York, NY: WW Norton; 2005. pp. 395–424. [Google Scholar]
- 12.Mueser KT, Corrigan PW, Hilton DW, et al. Illness management and recovery: a review of the research. Psychiatr Serv. 2002;53:1272–1284. doi: 10.1176/appi.ps.53.10.1272. [DOI] [PubMed] [Google Scholar]
- 13.Salyers MP, Godfrey JL, McGuire AB, Gearhart T, Rollins AL, Boyle C. Implementing the illness management and recovery program for consumers with severe mental illness. Psychiatr Serv. 2009;60:483–490. doi: 10.1176/ps.2009.60.4.483. [DOI] [PubMed] [Google Scholar]
- 14.Hasson-Ohayon I, Roe D, Kravetz S. A randomized controlled trial of the effectiveness of the illness management and recovery program. Psychiatr Serv. 2007;58:1461–1466. doi: 10.1176/ps.2007.58.11.1461. [DOI] [PubMed] [Google Scholar]
- 15.Copeland ME. Wellness recovery action plan: a system for monitoring, reducing and eliminating uncomfortable or dangerous physical symptoms and emotional feelings. Occup Ther Ment Health. 2002;17:127–150. [Google Scholar]
- 16.Copeland ME. Wellness Recovery Action Plan. Dummerston, VT: Peach Press; 1997. [Google Scholar]
- 17.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 18.Reeve J, Nix G, Hamm D. Testing models of the experience of self-determination in intrinsic motivation and the conundrum of choice. J Educ Psychol. 2003;95:375–392. [Google Scholar]
- 19.Williams GC, Lynch MF, Glasgow RE. Computer-assisted intervention improves patient-centered diabetes care by increasing autonomy support. Health Psychol. 2007;26:728–734. doi: 10.1037/0278-6133.26.6.728. [DOI] [PubMed] [Google Scholar]
- 20.Williams GC, McGregor HA, Sharp D, et al. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol. 2006;25:91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 21.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav. 2003;30:170–195. doi: 10.1177/1090198102251030. [DOI] [PubMed] [Google Scholar]
- 22.van Dam HA, van der Horst FG, Knoops L, Ryckman RM, Crebolder HF, van den Borne BH. Social support in diabetes: a systematic review of controlled intervention studies. Patient Educ Couns. 2005;59:1–12. doi: 10.1016/j.pec.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Cook JA. Patient-Centered and Consumer-Directed Mental Health Services: A Report Prepared for the Institute of Medicine. Chicago, IL: University of Illinois at Chicago National Research & Training Center; 2005. [Google Scholar]
- 24.Bartlett EE. Educational self-help approaches in childhood asthma. J Allergy Clin Immunol. 1983;72:545–554. doi: 10.1016/0091-6749(83)90481-5. [DOI] [PubMed] [Google Scholar]
- 25.Hope KR. Promoting behavior change in Botswana: an assessment of the peer education HIV/AIDS prevention program at the workplace. J Health Commun. 2003;8:267–281. doi: 10.1080/10810730305685. [DOI] [PubMed] [Google Scholar]
- 26.Wilson W, Pratt C. The impact of diabetes education and peer support upon weight and gylcemic control of elderly persons with noninsulin dependent diabetes mellitus (NIDDM) Am J Public Health. 1987;77:634–635. doi: 10.2105/ajph.77.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suls J, Martin R, Wheeler L. Social comparison: why, with whom and with what effect? Curr Dir Psychol Sci. 2002;11:159–163. [Google Scholar]
- 28.Taylor CL, Kulikb J, Badra H, et al. A social comparison theory analysis of group composition and efficacy of cancer support programs. Soc Sci Med. 2007;65:262–273. doi: 10.1016/j.socscimed.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Davidson L, Chinman M, Kloos B, Weingarten R, Stayner DA, Tebes JK. Peer support among individuals with severe mental illness: a review of the evidence. Clin Psychol Sci Pract. 1998;6:165–187. [Google Scholar]
- 30.Davidson L, Chinman M, Sells D, Rowe M. Peer support among adults with serious mental illness: a report from the field. Schizophr Bull. 2006;32:443–450. doi: 10.1093/schbul/sbj043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon P. Peer support/peer provided services underlying processes, benefits, and critical ingredients. Psychiatr Rehabil J. 2006;27:392–401. doi: 10.2975/27.2004.392.401. [DOI] [PubMed] [Google Scholar]
- 32.Daniels A, Grant E, Filson B, Powell I, Fricks L, Goodale L, editors. Pillars of Peer Support: Transforming Mental Health Systems of Care Through Peer Support Services. http://www.pillarsofsupport.org. Accessed January 2010. [Google Scholar]
- 33.Clarke GN, Herinckx HA, Kinney RF, et al. Psychiatric hospitalizations, arrests, emergency room visits, and homelessness of clients with serious and persistent mental illness: findings from a randomized trial of two ACT programs vs. usual care. Ment Health Serv Res. 2000;2:155–164. doi: 10.1023/a:1010141826867. [DOI] [PubMed] [Google Scholar]
- 34.Eiken S, Campbell J. Medicaid coverage of peer support for people with mental illness: available research and state examples. http://www.peerspecialist.org/Services1.0/Pdf/HelpfulDocuments/Peer_Support_11608.pdf. Accessed November 22, 2010. [Google Scholar]
- 35.Bandura A. Self-efficacy: the Exercise of Control. New York, NY: W.H. Freeman and Company; 1997. [Google Scholar]
- 36.Festinger L. A theory of social comparison processes. Hum Relations. 1954;7:117–140. [Google Scholar]
- 37.Bandura A. A social cognitive theory of personality. In: Pervin L, John O, editors. Handbook of Personality. 2nd ed. New York, NY: Guilford Publications; 1999. pp. 154–194. [Google Scholar]
- 38.Solomon P, Draine J. One-year outcomes of a randomized trial of consumer case management. Eval Program Plann. 1995;18:117–127. [Google Scholar]
- 39.Solomon P, Draine J. The efficacy of consumer case management team: two year outcomes of a randomized trial. J Ment Health Adm. 1995;22:135–146. doi: 10.1007/BF02518754. [DOI] [PubMed] [Google Scholar]
- 40.Magura S, Cleland C, Vogel H, Knight E, Laudet A. Effects of “dual focus” mutual aid on self-efficacy for recovery and quality of life. Adm Policy Ment Health. 2007;34(1):1–12. doi: 10.1007/s10488-006-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts G, Wolfson P. The rediscovery of recovery. Adv Psychiatr Treat. 2004;10:37–49. [Google Scholar]
- 42.Copeland ME, Cook JA, Razzano LA. Wellness Recovery Action Plan: Application to the National Registry of Effective Programs and Practices. Chicago, IL: University of Illinois at Chicago Center on Mental Health Research and Policy; 2010. [Google Scholar]
- 43.Cook JA, Copeland ME, Hamilton M, et al. Initial outcomes of a mental illness self-management program based on wellness recovery action planning. Psychiatr Serv. 2009;60:246–249. doi: 10.1176/ps.2009.60.2.246. [DOI] [PubMed] [Google Scholar]
- 44.Cook JA, Copeland ME, Corey L, et al. Developing the evidence base for peer-led services: changes among participants following Wellness Recovery Action Plan (WRAP) education in two statewide initiatives. Psychiatr Rehab J. 2010;34:113–120. doi: 10.2975/34.2.2010.113.120. [DOI] [PubMed] [Google Scholar]
- 45.Starnino VR, Mariscal S, Holter M, et al. Outcomes of an illness self-management group using Wellness Recovery Action Planning. Psychiatr Rehabil J. 2010;34:57–60. doi: 10.2975/34.1.2010.57.60. [DOI] [PubMed] [Google Scholar]
- 46.Doughty C, Tse S, Duncan N, McIntyre L. The Wellness Recovery Action Plan (WRAP): workshop evaluation. Australas Psychiatry. 2008;20:1–7. doi: 10.1080/10398560802043705. [DOI] [PubMed] [Google Scholar]
- 47.Epstein J, Barker P, Vorburger M, Murtha C. Serious Mental Illness and Its Co-occurrence With Substance Use Disorders. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2002. (DHHS Publication No. SMA 04–3905, Analytic Series A-24) [Google Scholar]
- 48.Diagnostic and Statistical Manual of Mental Disorders. Revised 4th ed. Washington, DC: American Psychiatric Association; 2000. American Psychiatric Association. [Google Scholar]
- 49.State of Ohio. Pre-admission screening and resident review (PAS/RR): a review of changes to Ohio administrative code. Columbus, Ohio: State of Ohio; 2009. http://mentalhealth.ohio.gov/assets/licensure-certification/rules/5101-3-3-14-pasrr-definitions.pdf. Accessed November 1, 2010. [Google Scholar]
- 50.Gross D, Fogg L. A critical analysis of the intent-to-treat principle in prevention research. J Prim Prev. 2004;25:475–489. [Google Scholar]
- 51.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 52.SPSS for Windows, Rel. 15.0.0. Chicago, IL: SPSS Inc; 2006. [Google Scholar]
- 53.Hedeker D, Gibbons R. MIXREG: a computer program for mixed-effects regression analysis with autocorrelated errors. Comput Methods Programs Biomed. 1996;49:229–252. doi: 10.1016/0169-2607(96)01723-3. [DOI] [PubMed] [Google Scholar]
- 54.Gluud LL. Bias in clinical intervention research. Am J Epidemiol. 2006;163:493–501. doi: 10.1093/aje/kwj069. [DOI] [PubMed] [Google Scholar]
- 55.Derogatis LR, editor. Brief Symptom Inventory: Administration Scoring and Procedures Manual. 3rd ed. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 56.Piersma HL, Reaume WM, Boes JL. The Brief Symptom Inventory (BSI) as an outcome measure for adult psychiatric outpatients. J Clin Psychol. 1994;50:555–563. doi: 10.1002/1097-4679(199407)50:4<555::aid-jclp2270500410>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 57.Ward E, King M, Lloyd M, et al. Randomised controlled trial of non-directive counselling, cognitive-behaviour therapy, and usual general practitioner care for patients with depression. I: clinical effectiveness. Br J Psychiatry. 2000;321:1383–1388. doi: 10.1136/bmj.321.7273.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kissane DW, McKenzie M, Bloch S, Moskowitz C, McKenzie DP, O’Neill I. Family focused grief therapy: a randomized, controlled trial in palliative care and bereavement. Am J Psychiatry. 2006;163:1208–1218. doi: 10.1176/ajp.2006.163.7.1208. [DOI] [PubMed] [Google Scholar]
- 59.Kashner TM, Rosenheck R, Campinell AB, et al. Impact of work therapy on health status among homeless, substance dependent veterans: a randomized controlled trial. Arch Gen Psychiatry. 2002;59:938–944. doi: 10.1001/archpsyc.59.10.938. [DOI] [PubMed] [Google Scholar]
- 60.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 61.Snyder CR, Harris C, Anderson JR. The will and the ways: development and validation of an individual-differences measure of hope. J Pers Soc Psychol. 1991;60:570–585. doi: 10.1037//0022-3514.60.4.570. [DOI] [PubMed] [Google Scholar]
- 62.Lyndall G. A psychometric comparison of four measures of hope and optimism. Educ Psychol Meas. 2002;62:466–482. [Google Scholar]
- 63.Snyder CR, Sympson SC, Ybasco FC, Borders TF, Babyak MA, Higgins RL. Development and validation of the State Hope Scale. J Pers Soc Psychol. 1996;70:321–335. doi: 10.1037//0022-3514.70.2.321. [DOI] [PubMed] [Google Scholar]
- 64.Skevington SM, Lotfy M, O'Connell KA. The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Rea E, LePage JP. Reliability and validity of World Health Organization Quality of Life-100 in homeless substance-dependent veteran population. J Rehabil Res Dev. 2008;45:619–626. doi: 10.1682/jrrd.2007.03.0048. [DOI] [PubMed] [Google Scholar]
- 66.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: application of NIMH treatment of depression collaborative research program dataset. Arch Gen Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 67.Hedeker D, Mermelstein RJ. A multilevel thresholds of change model for analysis of stages of change data. Multivariate Behavioral Res. 1998;33:427–455. [Google Scholar]
- 68.SAS 9.1.3 SAS/IML software. Cary, NC: SAS Institute Inc.; 2004. SAS Institute Inc. [Google Scholar]
- 69.Berg CJ, Snyder CR, Hamilton N. The effectiveness of a hope intervention in coping with cold pressor pain. J Health Psychol. 2008;13:804–809. doi: 10.1177/1359105308093864. [DOI] [PubMed] [Google Scholar]
- 70.Irving LM, Snyder CR, Crowson JJ. Hope and coping with cancer by college women. J Pers. 1998;66:195–214. doi: 10.1111/1467-6494.00009. [DOI] [PubMed] [Google Scholar]
- 71.Anthony WA, Rogers ES, Farkas M. Research on evidence-based practices: future directions in an era of recovery. Community Ment Health J. 2003;39:101–114. doi: 10.1023/a:1022601619482. [DOI] [PubMed] [Google Scholar]