Abstract
We discuss 3 neurophysiological approaches to study auditory verbal hallucinations (AVH). First, we describe “state” (or symptom capture) studies where periods with and without hallucinations are compared “within” a patient. These studies take 2 forms: passive studies, where brain activity during these states is compared, and probe studies, where brain responses to sounds during these states are compared. EEG (electroencephalography) and MEG (magnetoencephalography) data point to frontal and temporal lobe activity, the latter resulting in competition with external sounds for auditory resources. Second, we discuss “trait” studies where EEG and MEG responses to sounds are recorded from patients who hallucinate and those who do not. They suggest a tendency to hallucinate is associated with competition for auditory processing resources. Third, we discuss studies addressing possible mechanisms of AVH, including spontaneous neural activity, abnormal self-monitoring, and dysfunctional interregional communication. While most studies show differences in EEG and MEG responses between patients and controls, far fewer show symptom relationships. We conclude that efforts to understand the pathophysiology of AVH using EEG and MEG have been hindered by poor anatomical resolution of the EEG and MEG measures, poor assessment of symptoms, poor understanding of the phenomenon, poor models of the phenomenon, decoupling of the symptoms from the neurophysiology due to medications and comorbidites, and the possibility that the schizophrenia diagnosis breeds truer than the symptoms it comprises. These problems are common to studies of other psychiatric symptoms and should be considered when attempting to understand the basic neural mechanisms responsible for them.
Keywords: auditory hallucinations, EEG, MEG, ERPs
Neurophysiology of Auditory Verbal Hallucinations: Current State of Knowledge
In this section, we describe the current state of knowledge about the neurophysiology of auditory verbal hallucinations (AVH). First, we describe “state” studies in which periods of hallucinations and nonhallucinations are compared within a patient; these are also known as “symptom capture” studies. These studies take 2 forms: passive studies and probe studies. Second, we describe “trait” studies in which patients who hallucinate are compared with those who do not, with some studies using hallucination severity as a continuous variable. Third, we describe studies attempting to understand a basic neural mechanism using neurophysiological methods that may underlie AVH.
Assessments of State (Symptom Capture)
“Symptom capture” is a naturalistic approach where neurobiological data are collected as patients experience a hallucination. While this approach is conceptually simple, it is extremely difficult in practice because it relies not only on the timely occurrence of an illusive subjective experience but also on the ability of the patient to reliably report its initiation and completion. Symptom capture studies require patience from the research team and cooperation and insight from the patient. To control for the effects of attention and button pressing, ideally patients need to be able to indicate both when they hear voices and when they do not. Because it is difficult to satisfy all these criteria, only a small percentage of patients interviewed are enrolled in symptom capture studies.
Two main approaches have been used in symptom capture studies. The most obvious involves comparing “spontaneous” neural activity during periods with and without hallucinations. A slightly less direct approach involves comparing responses to external auditory “probes” during periods with and without hallucinations. Auditory cortex should be relatively active during periods of hallucinations, and thus, less responsive to external auditory probes. That is, if the brain is busy listening to an internal auditory stream, it should be less responsive to external sounds. The literature largely supports that prediction.
Symptom Capture.
As reviewed by Van Lutterveld et al,1 before the era of antipsychotic medications, depth electrocorticography studies were sometimes conducted in conjunction with neurosurgery for relief of severe psychotic symptoms. Other than providing a fascinating historical note, old electroencephalography (EEG) findings are not easy to incorporate into the contemporary literature with more sophisticated data collection and analysis. Using newer methods, one group reported an increase in alpha band (8–12 Hz) power in the left superior temporal cortex during AVH in 7 schizophrenia patients2 and an increase in synchronization between the left and right superior temporal cortices, suggesting an increase in functional coupling between these brain regions.2 Others using magnetoencephalography (MEG) showed increased theta (4–8 Hz)3 and beta band (12.5–30 Hz)4 activity in the left superior temporal cortex during AVH in a single subject. A third study included 5 patients with nonverbal auditory hallucinations (eg, noise, music) and 3 patients with verbal command hallucinations.5 In both groups, hallucinations were associated with an increase in beta-band activity in the left superior temporal cortex. In patients hearing voices, the activation pattern extended into left dorsolateral prefrontal cortex suggesting more complex mechanisms are involved in the generation of voices than music. Most recently, increased phase coupling in the alpha band, both inter and intrahemispherically between temporal and frontal lobes, was reported during AVH.6 Although it is difficult to understand the functional significance of the different neural frequency bands during AVH in these different reports, they are generally consistent with functional magnetic resonance imaging (fMRI) data,7 pointing to activity in both right and left temporal and frontal regions of the brain during AVH.
Recently, microstates (described below) have been used to study state changes in neural activity associated with periods of AVH. A microstate that correlates with a dorsal attention-reorientation resting-state network8 was observed to be shortened by several milliseconds during periods with AVH.9 Shortening of this microstate might indicate a premature termination of the delicate balance between goal-directed and salience-driven processes, compatible with the observed psychopathology. It should be noted that microstates are spatial maps of activity that are indifferent to EEG frequency bands; as such, microstate data are difficult to reconcile with the EEG data described above.
Symptom Capture With External Probes.
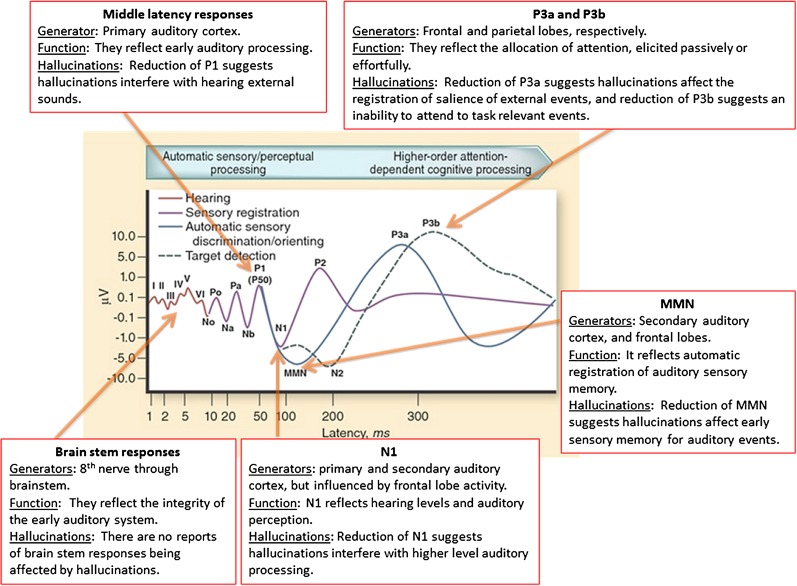
Activity elicited by external probes can be studied by assessing the various components of the event-related potential (ERP) or its MEG counter-part, the event-related field. The auditory ERP is illustrated in figure 1 where we show the earliest to the latest occurring components. We indicate the brain regions believed to be responsible for the generation of each component, what function the component might reflect, and how it is affected by the state or trait of hallucinations. While AVH involve a number of cortical and subcortical areas,7 ERPs are best able to assess activity in the cortical mantle, particularly auditory cortex. Thus, most probe studies focus on auditory cortex because that’s where the “light is best.”
Fig. 1.
A schematic representing the idealized components of the auditory ERP plotted on logarithmic scales to allow the visualization of the smallest and fastest early components emanating from the brain stem. Time in milliseconds is on the x-axis, and voltage in microvolts is on the y-axis. The components are labeled according to convention with N referring to a negative going potential and P referring to a positive potential. MMN refers to the mismatch negativity. The idealized event-related potential resembles waveforms recorded from the vertex and referred to the mastoids. The center image is taken from Rissling et al10 and adapted from Picton et al.11 It is reproduced here with permission from SpringerImages.com.
The N1component of the auditory ERP is the only component shown in figure 1 that has been reported during symptom capture studies. Although N1 is affected by activity in the frontal lobes and other areas of the brain, it primarily emanates from primary and secondary auditory cortex,12 and as such, it is an excellent probe of auditory cortical activity, albeit, as affected by activity in other areas of the brain.
Hubl and colleagues13 recorded ERPs to 1000 Hz tones while 7 schizophrenia subjects indicated by button press the beginning and ending of an AVH. Patients were instructed to listen to their voices and ignore the tones. In every patient, N1 amplitude was reduced during AVH; further, N1 reduction localized to left primary auditory cortex, consistent with an earlier study using both EEG and MEG methods.14 Finding diminished responses in the primary auditory cortex rather than in secondary auditory cortex or Wernicke’s area may have been due to the use of a pure-tone probe rather than a speech probe. Together, these findings indicate competition between auditory probes and hallucinations for auditory resources, with activation of the primary auditory cortex reflecting the physical acoustic image of verbal thoughts that are misperceived as voices.
Assessments of Trait
As illustrated in figure 1, other components of the auditory ERP reflect responsiveness of auditory cortex or other areas of the brain involved in auditory processing. Each is described briefly below.
P1.
P1 (also referred to as P50) is an early positive ERP component peaking at about 50 ms. Like N1, P1 also depends on auditory cortex for its generation and as such should be a valid probe of auditory cortical responses to external stimuli. (Smith D.M., Grant B., Fisher D.J., Borracci G., Labelle A., and Knott V.J., unpublished data) asked patients to listen to click pairs, followed by questions regarding the duration, loudness, and clarity of any hallucinations they had just experienced during the recording session. They found that P1 to the first click was significantly reduced in patients with AVH, with more severe hallucinations being associated with smaller P1. Additional significant correlations between P1 amplitude to the first click and individual items on the Psychotic Symptom Rating Scales, such as amount of negative content of voices, degree of negative control, amount of distress, and disruption to life caused by voices were also reported.
N1.
To our knowledge, there are no reports of relationships between N1 and the trait to hallucinate. This could be due to a failure to find a positive relationship or a failure to test for such a relationship. The history of ERPs in psychiatry started with an effort to find relationships between neurobiology and diagnosis, rather than symptoms, perhaps explaining the lack of data on this relationship.
Mismatch Negativity.
As can be seen in figure 1, mismatch negativity (MMN) occurs after N1 and is a measure of automatic auditory sensory memory. It is considered automatic because it does not require any behavioral response and can be elicited in the absence of explicit instructions to attend to the auditory stream.15 However, it is affected by concurrent auditory (but not visual) discrimination tasks, suggesting that sounds compete with ongoing processing of auditory information.16 MMN can be elicited by any auditory event (tones, clicks, phonemes, etc.) that is deviant from the preceding events in a sequence, such as a change of sound duration, intensity, frequency, pattern, rhythm, and so on. Its elicitation indicates that a sequence was learned and that an auditory change was detected.
Perhaps for the reasons given above for N1, few articles have reported relationships between MMN and AVH.17 Schizophrenia patients with clear persistent AVH have smaller MMNs elicited by duration18 and phoneme19 deviants than nonhallucinating patients and controls. Additionally, hallucinating patients show altered processing of across-phoneme change,19 as indexed by the MMN. Using hallucination severity as a continuous variable, others have reported decreased MMN amplitude with increase in hallucination severity.20–22 These findings support the suggestion that either the storage of auditory information in short-term (echoic) memory or the registration that a deviant occurred, or both, is altered in patients who have a predisposition to hallucinate. While MMN reduction is associated with a tendency to hallucinate, it is closely linked to schizophrenia-related changes in global function23 and gray matter volume,24 with reductions of left temporal gray matter being associated with increased frequency and duration of AVH.25
P300.
Task-relevant target stimuli elicit a P300 (see figure 1). While the target status of a stimulus is essential for eliciting the parietally maximal P300 (also called “P3b”), a large robust fronto-centrally maximal P300 (also called “P3a”) is generated by infrequent distractor, novel or otherwise salient stimuli, with no necessary target value. It has been suggested that P3a is, in fact, a reflection of the orienting response26 perhaps reflecting a shift in attention.
Given that over 100 articles have reported P300 reductions in schizophrenia,27 it is surprising that so few report a relationship between P300 and AVH.28–30 As mentioned above for N1, this could reflect a failure to find a relationship or a failure to try. One study found P3a reductions in hallucinating compared with nonhallucinating patients30 consistent with a deficit in attributing significance to incoming stimuli. Alternatively, though not necessarily contradictorily, the deficits in P300 amplitude observed may be symptomatic of the tonic “tuning” to internal stimuli, over external stimuli, observed in hallucinating patients.31 If schizophrenia patients with auditory hallucinations preferentially attend to voices through internal auditory channels, perhaps there are insufficient cortical resources to switch attention, either automatically or effortfully, to an external stimulus, which would result in diminished P300 amplitude.
Studies of chronic patients are often complicated by comorbidities and medication confounds. A study by van Lutterveld and colleagues32 avoided these problems by using healthy people who hallucinate. Surprisingly, they found that these people had larger P300s than healthy nonhallucinating subjects suggesting P300 reduction typically seen in schizophrenia is not due to the tendency to hallucinate.
Auditory Steady State Response.
Not illustrated in figure 1 is the auditory steady-state response (ASSR). When an auditory stimulus is repeated at a fixed rate, it drives the cortical response at that rate.33 Although both higher and lower frequencies have been tested, the ASSR reaches a maximum at a 40 Hz repetition rate. This likely reflects a resonant response in the auditory system,33 possibly in the primary auditory cortex where the ASSR is generated.
Using dipole source localization, Spencer et al34 found that chronic patients with “greater” intertrial phase coherence of the 40 Hz ASSR in the left primary auditory cortex had more “severe” AVH over their lifetimes. These findings extended their earlier findings of positive correlations between hallucination ratings and oscillation measures in the auditory and visual modalities in first-episode and chronic patients, respectively.34 These findings are also consistent with a case report of abnormally large beta activity in a hallucinating patient.4
Using EEG and MEG to Test Models of AVH
Most studies described above point to auditory cortical involvement in AVH but do not indicate why auditory cortex is busier in hallucinators and during hallucinations and why the resulting percepts are misperceived as coming from external sources. Below we discuss possible mechanisms and the few studies that have used EEG and MEG to assess them.
Spontaneous Neural Activity Model of AVH.
What is the auditory raw material of AVH? Do auditory percepts result from random activity of neural assemblies, from unbidden thoughts during mind wandering, or from thoughts colliding with random noise? Indeed, random noise increases sensitivity to weak signals through stochastic resonance,35 and patients with schizophrenia are known to have “noisier” systems as indexed with EEG methods. This concept is further described below, under “A Neural Network model of AVH.”
Northoff and Qin36 suggested voices may be “traced back to abnormally elevated resting-state activity in auditory cortex itself, abnormal modulation of the auditory cortex by anterior cortical midline regions as part of the default mode network, and neural confusion between auditory cortical resting-state changes and stimulus-induced activity.” The symptom capture studies described above showing greater neural activity in the temporal lobe2–6 and synchrony between frontal and temporal lobes6 are consistent with these ideas.
The Self-Monitoring Model of AVH.
How is this activity in auditory cortex misperceived as voices? It has been suggested that a deficit in self-monitoring of inner speech is responsible. Before describing the neurophysiological studies of this model, we ask, “what is self-monitoring?” and “what is inner speech?”
In its simplest form, the self-monitoring model of AVH suggests that patients misattribute, or misperceive, their thoughts and inner experiences as coming from alien sources. However, it could be argued that “self-monitoring” connotes a higher degree of intention and cognition than is appropriate. Similarly, “inner speech” is a broad term and refers to internal verbal experiences, ranging from the intentional silent rehearsal of an argument to unbidden fleeting thoughts experienced during daydreaming. AVHs are unbidden, but differ from normal daydreaming as the content is often disturbing and disarming, and experienced as coming from external sources.
In spite of these limitations, the self-monitoring of inner speech model has been the one most studied using functional imaging.7 One early version of this model was proposed by Feinberg37 who suggested that self-monitoring failures could result from specific dysfunctions of the efference copy and corollary discharge mechanisms. These mechanisms act across the animal kingdom to suppress sensations resulting from self-initiated motor actions and tag them as coming from self. Feinberg37 linked these concepts to AVH and suggested thinking is our most complex motor act and, as such, it might conserve and utilize the computational and integrative mechanisms evolved for physical movement. Feinberg37 reasoned that in the motor systems of thought, these mechanisms would act to distinguish self-produced thoughts from externally generated events.
Frith38 expanded this concept and prompted a series of behavioral experiments confirming the possibility of corollary discharge dysfunction in schizophrenia. Ford et al39 tested efference copy and corollary discharge dysfunction in schizophrenia by inspecting auditory cortical responsiveness to speech sounds “ah” during the act of talking. Consistent with the action of the corollary discharge system documented in human and nonhuman primates, N1 amplitude was smaller during talking than listening in healthy controls but less so in patients. The amount of suppression of N1 to speech sounds during talking was not related to AVH; however, neural synchrony in the beta band, 100 ms before speech onset, was. Because prespeech neural synchrony was related to subsequent suppression of N1 during talking in controls, EEG synchrony preceding speech may reflect the action of the efference copy of the motor command to speak.
Interregional Communication in the Brain.
Functional connectivity analyses of brain activity are motivated by findings that coordination between brain regions affects whether neural activity is experienced consciously as percepts.40 Indeed, hyperconnectivity between different regions might contribute to false perceptions, and hypoconnectivity might result in failures of mechanisms, such as efference copy, that tag those percepts as coming from self.37 The lack of EEG theta band coherence (hypoconnectivity) between frontal and temporal lobes during talking has been associated with a tendency to hallucinate in patients with schizophrenia,41 and fMRI hyperconnectivity within the corticostriatal loop has been implicated in the hallucination itself.42 Functional connectivity analyses of EEG and fMRI data will provide further tests of these ideas.
Methodological Issues
Assessments of State
One clear advantage of symptom capture work is the ability to observe neural activity preceding, and during, a hallucinatory experience. Although mechanisms cannot be directly inferred from observation of the neural activity associated with the phenomenon, EEG can provide temporal information. In spite of this potential advantage, few studies have used EEG in symptom capture perhaps because symptom capture studies require patience from the research team and cooperation and insight from the patient. Another disadvantage is the unknown contributions of shifting attention away from the voices and toward signaling, and of the motor responses themselves, at the onset of a hallucination. Although symptom capture studies are infeasible in animal models, the neural signature of auditory hallucinations (eg, increased power in temporal lobe and synchrony between frontal and temporal lobes) may provide a target for testing pharmacologic challenges and genetic models.
Assessments of Trait
Comparing patients who do and do not hallucinate is far simpler than comparing periods with and without hallucinations. Successful studies using this method are consistent with findings from the symptom capture literature: Auditory cortex is “busy” in people who tend to hallucinate. However, it is not always easy to find relationships between our biological measures and symptoms for reasons listed under “Impediments to Progress,” below.
Mechanistic Studies
Mechanistic studies offer translation to bench neuroscience and translation to other species, and hence open the door to invasive manipulations that are not possible with in vivo human studies while not requiring the animal to hallucinate. For example, studies of the corollary discharge mechanism can be studied in animals that make social calls, such as songbirds and nonhuman primates. In such experiments, perturbations of the neurotransmitters implicated in schizophrenia might produce a neural signature of the mechanism that resembles the pattern seen in schizophrenia patients who hallucinate. Excessive spontaneous neural noise and both hypo and hyperconnectivity among brain regions could also be studied in animals using similar approaches. In spite of their ability to elucidate mechanisms underlying AVH, these studies would lack the intuitive appeal of symptom capture studies.
EEG/MEG Methodologies
EEG and MEG provide noninvasive measures of brain activity by recording electrical and magnetic activity at the scalp. Furthermore, due to their superior temporal resolution (milliseconds), they have the appropriate temporal resolution for the investigation of rapidly occurring processes that are likely to underlie transitory hallucinations. Another advantage of EEG and MEG methodologies is the relative silence in which the auditory cortex can be investigated compared with the noisy environment of the MR scanner. Compared with fMRI, EEG, and MEG perform poorly when separating activity from brain regions that are not separated by a sufficient spatial distance.
Time-Voltage Analyses.
EEG and MEG derived event-related components are elicited in response to a discrete event (ie, tones, light flashes); their amplitudes and latencies allow for an objective assessment of the strength and timing of perceptual and cognitive processes tightly locked in time to the event. The primary advantage is that information processing can be probed without requiring any active overt response from the subject. This feature provides 2 advantages: First, they are ideal for studies of psychiatric populations, who may be unable to perform behavioral tasks due to cognitive and/or motor deficits; second, they allow assessment of sensation and perception of events people have not been asked to attend to.
Frequency and Time Frequency Analysis of EEG.
The spontaneous or “background” EEG is typically assessed in the frequency domain and yields unique information about the functional state (namely arousal) of brain regions.2–5 Ongoing EEG reflects a mixture of oscillations synchronized within neuronal assemblies that are involved in activities of the mind, including sensing, perceiving, thinking, and responding. Furthermore, a stimulus not only elicits an ERP but also elicits changes in the EEG frequency spectrum that reflect adaptive changes of brain state. Changes in EEG related to a stimulus or response are typically quantified in time-frequency analyses.
EEG data are also analyzed in the spatial domain to test the hypothesis that problems experienced by schizophrenia patients might result from dysfunctional communication between regions. Interest in this hypothesis coupled with novel analytic tools and computation power has triggered a wealth of studies on connectivity and synchronization. Besides confusion with terminology (eg, “coherence” can refer to spatial coherence of signals between areas or to temporal coherence with an area across trials), there is an ever-widening gap between the findings themselves and the ability of the larger schizophrenia research community to understand them.
Microstates.
Some groups have begun to take advantage of algorithms enabling calculation of microstates. Microstates are scalp potential maps that remain quasi-stable for ∼70–125 ms43 and indicate transient states of highly coordinated brain activity. Different microstates represent different modes of information processing; indeed, the content of spontaneous mentation is influenced by microstate class.43 Resting-state data show 4 different classes of microstates that are reliable within and between subjects.44 They are closely related to specific resting-state networks, as measured by the blood oxygen level–dependent response in fMRI.8 They may offer a view into the resting-state activity of the brain preceding AVH and interrupted by them.
Impediments to Progress
Here, we list some impediments to relating neurobiology to AVH. First, our success is limited by our ability to understand and quantify the patients’ symptoms; patients can be guarded, and clinicians may not give them time to “leak psychoticism.” Furthermore, many interview instruments fail to assess important details about the AVH experience. Second, the preponderance of schizophrenia patients are medicated, and medication may decouple the symptoms from the neurobiology by attenuating symptoms but not affecting the sensitivity of the neurobiological measures to the “propensity” to experience those symptoms. Third, other symptoms may combine with hallucinations to affect the neurobiology but not the severity of the hallucinations themselves. Fourth, some drugs of abuse might affect the neurobiology but not the current severity of the symptoms. Fifth, ERP component amplitudes can be both a personal trait of the patient and a reflection of AVH, making cross-sectional comparisons problematic.
Knowledge Gaps
The research described above has primarily addressed AVH in general. With few exceptions, (Smith D.M., Grant B., Fisher D.J., Borracci G., Labelle A., and Knott V.J., unpublished data) the field has not addressed some of the specific features and content of the voices such as the typically negative content, the predominance of male voices even in female patients, the number of voices, and the familiarity of the voices. While these features are intriguing in their own right, it may not be necessary to study them in order to understand the mechanism by which unbidden thoughts are heard as voices. Similarly, music hallucinations should work by the same mechanism as AVH; most people experience unwilled, unbidden musical jingles (music “worms”), but normal people understand the origin of those sounds and do not develop odd beliefs to explain them.
The “nonverbal” auditory hallucinatory experiences reported in clinical ultra high-risk patients have also not been studied. While healthy normal people have unbidden verbal experiences constantly during the day, we do not typically experience “inner” footsteps or bonks.
Future Research
Symptoms vs Syndrome
Most AVH research using EEG and MEG methods has focused on patients with schizophrenia. Although it is unlikely that biology cleaves at current diagnostic joints, with the exception of the study by van Lutterveld et al32 of healthy voice-hearers, we know of no other EEG or MEG study of other groups who hear voices. Indeed, to get traction on the differential contribution of symptoms and syndromes to our neurobiological assays, it may be important to include other groups who hear voices but do not have a diagnosis of schizophrenia, such as psychotic depression, bipolar depression with psychotic features, temporal lobe epilepsy, and hypothyroidism. In addition, our efforts to understand perceptions in the absence of external stimulation might be promoted by studying tinnitus and dreaming.
The symptom-dimensional approach has many obvious advantages over the diagnosis approach. However, we have been more successful at relating biology to enduring features of the disease (the diagnosis itself, or its subtypes) than to symptoms. Perhaps the diagnosis of schizophrenia breeds truer than the symptoms it comprises.45
New Approaches
As a field, we welcome new approaches to understanding the pathophysiology of AVH using neurophysiological methods. Here, we describe 2 such approaches.
Contributions of Baseline States to AVH.
We know various regions of the brain are active during an AVH; however, we do not know what happens seconds before the voices are heard and whether voices can be predicted from fluctuations in the baseline state. Nor do we know how specific baseline states affect the processing of internal and external information that might serve as a trigger for, or interruptions of, AVH. These type questions can be addressed using a combination of methods. For example, global measures of poststimulus N1 amplitude could be used as a regressor for prestimulus EEG spectral power. Also, change point analysis46 of EEG data might reveal whether state changes precede the onset of voices and how soon they occur before voices are signaled.
EEG and fMRI data acquired simultaneously in a symptom capture study could provide information about the fleeting spontaneous EEG activity immediately preceding AVH and the involvement of auditory cortex in the default mode network during the hallucination. In addition, it would allow the identification of the EEG spectral signature of arousal, attention, and impaired function heralding the onset of voices.
A Neural Network Model of AVH.
If a neural network is constantly stimulated with a certain set of stimuli, synapses that lead to the neurons that respond to these stimuli will be strengthened as has been shown in a simulation study using spiking neurons.47 In the absence of external stimuli, the neurons with strong synaptic connections will be the most likely to respond to noise. They are the neurons that represent objects that have been repeatedly perceived—such as one’s own name. The human brain cannot differentiate between the neurons that fire due to noise (hallucination) and those that fire due to external stimulation. Thus, AVH might be the neural response of strongly connected neurons in the absence of external input.48 One example of evidence for such processes are the so-called Ganzfeld-induced hallucinatory experiences.49
Data Sharing
Given the difficulty of gathering sufficient amounts of data, we should consider sharing analysis tools and/or data. The application of different methods to the same data would allow us to compare and contrast findings resulting from different analytic methods. In addition, we should attempt to establish simple but comprehensive standards for clinical and neurobiological data, which can be gathered for meta-analyses.
Conclusions
In the 1960s, improving the accuracy of the diagnosis was a primary goal of neurophysiological studies of schizophrenia. As the field matured and evidence accumulated that P1, N1, MMN, and P300 were all were disrupted in schizophrenia, efforts were made to understand which symptoms were responsible for the group effects. As discussed above, these efforts have been modestly successful: In the “trait” studies discussed above, P1, MMN, and P300 were all reduced in patients who hallucinate compared with those who do not. In the “state” studies, N1 was smaller during periods of hallucinations than during periods free of hallucinations. Also, EEG power across many frequencies was greater over left temporal lobe during the experience of hallucinations, as was phase coupling between frontal and temporal lobes. Together, these data suggest that both the hallucination state and trait tend to render auditory cortex “busy” or otherwise unavailable to process external auditory events. Attempts to use EEG and MEG data to study basic neural mechanisms, which may be responsible for AVH, have also met with some success.
Our efforts to use human EEG and MEG data to understand the pathophysiology of AVH has been hindered by a number of factors, including poor anatomical resolution of the measures, poor assessment of symptoms, poor understanding of the phenomenon, poor models of the phenomenon, and decoupling of the symptoms from the neurophysiology with medications, and various medical and psychiatric comorbidities. Nevertheless, we are optimistic that thoughtful application and combination of new methods will provide critical information about antecedents of AVH, the circuitry supporting them, and the basic neural mechanisms responsible for them.
Funding
National Institutes of Health (MH080187 to K.M.S., MH076989 to D.H.M., and MH058262 to J.M.F.); VA Merit Award (K.M.S. and J.M.F.); National Alliance for Research on Schizophrenia and Depression (to D.H.M); Canadian Institutes of Health Research (to D.J.F.); Dutch Science Organization (Nederlandse Wetenschappelijke Organisatie) (to R.v.L.).
Acknowledgments
The authors wish to thank Flavie Waters, Iris Summer, Paul Allen, Andre Aleman, Frank Laroi, and Todd Woodward for organizing the International Consortium for Hallucination Research, at the Institute of Psychiatry, London, England, September 13–14, 2011, where this review was presented. None of the authors has any conflicts of interest related to this work.
References
- 1.van Lutterveld R, Sommer IE, Ford JM. The neurophysiology of auditory hallucinations—a historical and contemporary review. Front Psychiatry. 2011;2:28. doi: 10.3389/fpsyt.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sritharan A, Line P, Sergejew A, Silberstein R, Egan G, Copolov D. EEG coherence measures during auditory hallucinations in schizophrenia. Psychiatry Res. 2005;136:189–200. doi: 10.1016/j.psychres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Ishii R, Shinosaki K, Ikejiri Y, et al. Theta rhythm increases in left superior temporal cortex during auditory hallucinations in schizophrenia: a case report. Neuroreport. 2000;11:3283–3287. doi: 10.1097/00001756-200009280-00047. [DOI] [PubMed] [Google Scholar]
- 4.Ropohl A, Sperling W, Elstner S, et al. Cortical activity associated with auditory hallucinations. Neuroreport. 2004;15:523–526. doi: 10.1097/00001756-200403010-00028. [DOI] [PubMed] [Google Scholar]
- 5.Reulbach U, Bleich S, Maihofner C, Kornhuber J, Sperling W. Specific and unspecific auditory hallucinations in patients with schizophrenia: a magnetoencephalographic study. Neuropsychobiology. 2007;55:89–95. doi: 10.1159/000103907. [DOI] [PubMed] [Google Scholar]
- 6.Angelopoulos E, Koutsoukos E, Maillis A, Papadimitriou GN, Stefanis C. Cortical interactions during the experience of auditory verbal hallucinations. J Neuropsychiatry Clin Neurosci. 2011;23:287–293. doi: 10.1176/jnp.23.3.jnp287. [DOI] [PubMed] [Google Scholar]
- 7.Allen P, Aleman A, McGuire PK. Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int Rev Psychiatry. 2007;19:407–415. doi: 10.1080/09540260701486498. [DOI] [PubMed] [Google Scholar]
- 8.Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage. 2010;52:1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 9.Kindler J, Hubl D, Strik WK, Dierks T, Koenig T. Resting-state EEG in schizophrenia: auditory verbal hallucinations are related to shortening of specific microstates. Clin Neurophysiol. 2010;122:1179–1182. doi: 10.1016/j.clinph.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Rissling AJ, Makeig S, Braff DL, Light GA. Neurophysiologic markers of abnormal brain activity in schizophrenia. Curr Psychiatry Rep. 2010;12:572–578. doi: 10.1007/s11920-010-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picton TW, Hillyard SA, Krausz HI, Galambos R. Human auditory evoked potentials. I: evaluation of components. Electroencephalogr Clin Neurophysiol. 1974;36:179–190. doi: 10.1016/0013-4694(74)90155-2. [DOI] [PubMed] [Google Scholar]
- 12.Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 13.Hubl D, Koenig T, Strik WK, Garcia LM, Dierks T. Competition for neuronal resources: how hallucinations make themselves heard. Br J Psychiatry. Jan 2007;190:57–62. doi: 10.1192/bjp.bp.106.022954. [DOI] [PubMed] [Google Scholar]
- 14.Tiihonen J, Hari R, Naukkarinen H, Rimon R, Jousmaki V, Kajola M. Modified activity of the human auditory cortex during auditory hallucinations. Am J Psychiatry. 1992;149:255–257. doi: 10.1176/ajp.149.2.255. [DOI] [PubMed] [Google Scholar]
- 15.Näätänen R. Attention and Brain Function. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- 16.Dittmann-Balcar A, Thienel R, Schall U. Attention-dependent allocation of auditory processing resources as measured by mismatch negativity. Neuroreport. 1999;10:3749–3753. doi: 10.1097/00001756-199912160-00005. [DOI] [PubMed] [Google Scholar]
- 17.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Fisher DJ, Labelle A, Knott VJ. The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clin Neurophysiol. 2008;119:909–921. doi: 10.1016/j.clinph.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Fisher DJ, Labelle A, Knott VJ. Auditory hallucinations and the mismatch negativity: processing speech and non-speech sounds in schizophrenia. Int J Psychophysiol. 2008;70:3–15. doi: 10.1016/j.ijpsycho.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Youn T, Park HJ, Kim JJ, Kim MS, Kwon JS. Altered hemispheric asymmetry and positive symptoms in schizophrenia: equivalent current dipole of auditory mismatch negativity. Schizophr Res. 2003;59:253–260. doi: 10.1016/s0920-9964(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 21.Hirayasu Y, Potts GF, O'Donnell BF, et al. Auditory mismatch negativity in schizophrenia: topographic evaluation with a high-density recording montage. Am J Psychiatry. 1998;155:1281–1284. doi: 10.1176/ajp.155.9.1281. [DOI] [PubMed] [Google Scholar]
- 22.Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ. Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified ‘optimal’ multi-feature paradigm. Int J Psychophysiol. 2011;81:245–251. doi: 10.1016/j.ijpsycho.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 24.Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37:131–140. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neckelmann G, Specht K, Lund A, Ersland L, Smievoll AI, Hugdahl K. MR morphometry analaysis of grey matter density reducution in schizophrenia: interactions with hallucinations. Int J Neurosci. 2006;116:9–23. doi: 10.1080/00207450690962244. [DOI] [PubMed] [Google Scholar]
- 26.Roth WT, Kopell BS. P 300—an orienting reaction in the human auditory evoked response. Percept Mot Skills. 1973;36:219–225. doi: 10.2466/pms.1973.36.1.219. [DOI] [PubMed] [Google Scholar]
- 27.Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- 28.Havermans R, Honig A, Vuurman E, et al. A controlled study of temporal lobe structure volumes and P300 responses in schizophrenic patients with persistent auditory hallucinations. Schizophr Res. 1999;38:151–158. doi: 10.1016/s0920-9964(99)00006-7. [DOI] [PubMed] [Google Scholar]
- 29.Turetsky B, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: II. Longitudinal stability and relationship to symptom change. Biol Psychiatry. 1998;43:31–39. doi: 10.1016/s0006-3223(97)00261-8. [DOI] [PubMed] [Google Scholar]
- 30.Fisher DJ, Labelle A, Knott VJ. Auditory hallucinations and the P3a: attention-switching to speech in schizophrenia. Biol Psychol. 2010;85:417–423. doi: 10.1016/j.biopsycho.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Ford JM, Roach BJ, Jorgensen KW, et al. Tuning in to the voices: a multisite FMRI study of auditory hallucinations. Schizophr Bull. 2009;35:58–66. doi: 10.1093/schbul/sbn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Lutterveld R, Oranje B, Kemner C, et al. Increased psychophysiological parameters of attention in non-psychotic individuals with auditory verbal hallucinations. Schizophr Res. 2010;121:153–159. doi: 10.1016/j.schres.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Brenner CA, Krishnan GP, Vohs JL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaramillo F, Wiesenfeld K. Mechanoelectrical transduction assisted by Brownian motion: a role for noise in the auditory system. Nat Neurosci. 1998;1:384–388. doi: 10.1038/1597. [DOI] [PubMed] [Google Scholar]
- 36.Northoff G, Qin P. How can the brain's resting state activity generate hallucinations? A ‘resting state hypothesis' of auditory verbal hallucinations. Schizophr Res. 2010;127:202–214. doi: 10.1016/j.schres.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 38.Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- 39.Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- 40.Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69:407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann D, Strik WK, Henggeler B, Koenig T, Koukkou M. Brain electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. Int J Psychophysiol. 1998;29:1–11. doi: 10.1016/s0167-8760(97)00098-6. [DOI] [PubMed] [Google Scholar]
- 44.Koenig T, Prichep L, Lehmann D, et al. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. Neuroimage. 2002;16:41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- 45.Berenbaum H, Oltmanns TF, Gottesman II. Formal thought disorder in schizophrenics and their twins. J Abnorm Psychol. 1985;94:3–16. doi: 10.1037//0021-843x.94.1.3. [DOI] [PubMed] [Google Scholar]
- 46.Lindquist MA, Waugh C, Wager TD. Modeling state-related fMRI activity using change-point theory. Neuroimage. 2007;35:1125–1141. doi: 10.1016/j.neuroimage.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Fründ I, Ohl FW, Herrmann CS. Spike-timing-dependent plasticity leads to gamma band responses in a neural network. Biol Cybern. 2009;101:227–240. doi: 10.1007/s00422-009-0332-7. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Wackermann J, Pütz P, Allefeld C. Ganzfeld-induced hallucinatory experience, its phenomenology and cerebral electrophysiology. Cortex. 2008;44:1364–1378. doi: 10.1016/j.cortex.2007.05.003. [DOI] [PubMed] [Google Scholar]