Abstract
While the majority of cognitive studies on auditory hallucinations (AHs) have been conducted in schizophrenia (SZ), an increasing number of researchers are turning their attention to different clinical and nonclinical populations, often using SZ findings as a model for research. Recent advances derived from SZ studies can therefore be utilized to make substantial progress on AH research in other groups. The objectives of this article were to (1) present an up-to-date review regarding the cognitive mechanisms of AHs in SZ, (2) review findings from cognitive research conducted in other clinical and nonclinical groups, and (3) integrate these recent findings into a cohesive framework. First, SZ studies show that the cognitive underpinnings of AHs include self-source-monitoring deficits and executive and inhibitory control dysfunctions as well as distortions in top-down mechanisms, perceptual and linguistic processes, and emotional factors. Second, consistent with SZ studies, findings in other population groups point to the role of top-down processing, abnormalities in executive inhibition, and negative emotions. Finally, we put forward an integrated model of AHs that incorporates the above findings. We suggest that AHs arise from an interaction between abnormal neural activation patterns that produce salient auditory signals and top-down mechanisms that include signal detection errors, executive and inhibition deficits, a tapestry of expectations and memories, and state characteristics that influence how these experiences are interpreted. Emotional factors play a particular prominent role at all levels of this hierarchy. Our model is distinctively powerful in explaining a range of phenomenological characteristics of AH across a spectrum of disorders.
Keywords: symptoms, psychosis, hallucinosis, research, domain, criteria, auditory network, model
Introduction
Auditory hallucinations (AHs) are auditory experiences that occur in the absence of a corresponding external stimulation and which resemble a veridical perception. Strongly identified with psychotic disorders such as schizophrenia (SZ), AHs have traditionally been investigated in SZ populations. A recent shift in AH research, however, has led to a strategic focus on other clinical and nonclinical groups on the basis of observations that hallucinations and hallucination-like experiences are common in several psychiatric and also nonpsychiatric populations (ie, they are “transdiagnostic”). That such experiences do not map closely onto specific disorders has recently prompted the National Institute of Mental Health–driven Research Domain Criteria to devise new ways of classifying psychopathology based on symptom dimensions.1 This approach lends itself to investigations of symptoms such as AH, which comprise multiple phenomenological features. While acknowledging that the phenomenological features differ somewhat between groups, transdiagnostic studies may shed some light on the mechanisms that are specific to AH independently of other symptoms associated with SZ.
Cognitive methods have been widely used since early studies of AH. The power of cognitive approaches lies in their ability to provide plausible and intuitive explanations for subjective (ie, nonobservable) symptoms and to generate predictions regarding associated neural mechanisms that are testable using neuroscientific methodologies such as magnetic resonance imaging and electroencephalographs. Increasingly, researchers are investigating AH in different population groups, often applying cognitive findings and behavioral paradigms developed for SZ research. An up-to-date review regarding the current knowledge on cognitive mechanisms of AH is therefore needed so that advances can be made on the basis of the existing body of evidence.
The first aim of this article is to present the most recent theoretical developments in cognitive research pertaining to AH. While several high-quality reviews of cognitive mechanisms in AH have been published in the past, this is the first time that different phenomenological features of AH have been so comprehensively linked to their theoretical model in one article. The second aim is to provide an overview of findings from cognitive investigations of AH in non-SZ populations and compare the findings with the SZ literature. The third aim of this article is to amalgamate these findings into a new theoretical model of AH that can provide explanations for a range of phenomenological features in clinical and nonclinical populations.
Cognitive Explanations for the Phenomenological Features of AH in SZ
Approximately 70% of people with a diagnosis of SZ report AH. It is widely accepted that AH in SZ are multidimensional and heterogenous.2 In keeping with this dimensional view of AH, separate explanations have been used to account for the different phenomenological features of AH, each potentially representing a particular circuitry of brain structures and functions. We focus on 4 features that have been the most intensively researched: (1) the failure of self-recognition, whereby the experience is perceived as alien and separate from one’s own mental processes; (2) reduced sense of control over the onset, content, and frequency of AH; (3) the perceptual quality of AH; and (4) the contribution of emotions.
Failure of Self-recognition (alienation)
One core feature of AH in SZ is that they are experienced as somewhat separate from one’s own mental processes: the core experience is that the hallucinated percept is lacking in “self” attributes. In addition, and perhaps secondarily, the origin of these events tends to be incorrectly attributed to an external agent.
Several explanations have been proposed for such failures of self-recognition. One explanation centers around impairments in “self-monitoring” processes, whose role is to predict the sensory consequences of one’s own actions via forward modelling/efference copy mechanisms.3,4 Such difficulties may cause mental events (particularly inner speech) to become isolated from predictive mechanisms and misinterpreted as originating from an external source. Analogous processes are thought to exist for language (including inner speech), actions, and thoughts, so that a failure in this system would lead to broad difficulties in self-recognition. Another model proposes dysfunctions in “source-monitoring,” which refer to judgment processes that are used to make internal/external discriminations. These typically engage memory and other decision-making processes that retrieve and evaluate memory records in order to form a cohesive representation of an experience. It has been proposed that such source-monitoring disturbance results in an incomplete representation of mental events and consequently a failure to identify their origins.5
Theoretical aspects of these models have been criticized on the grounds that they do not provide a comprehensive account of the phenomenological diversity of AH.6 The cognitive tasks used to assess self- or source monitoring have also been criticized on methodological grounds. Typically, these require participants to monitor voluntary movements online or to identify whether items in memory originated from the self or another (“agency” tasks). However, errors may be the result of cognitive processes other than self- or source monitoring, such as response biases or difficulties in appraising ambiguous stimuli that could overshadow patients’ ability to recognize their own actions or mental events.7
Despite these criticisms, evidence supporting the link between AH and self- and source-monitoring deficits is strong, and few studies have failed to replicate these findings. A recent meta-analysis showed that self- and source-monitoring impairments were consistently reported across a range of paradigms, interstimulus intervals, and modalities in patients with SZ and particularly those with AH.8 Such dysfunctions were thought to occur in the earlier, rather than later, stages of information processing, although such early deficits would undoubtedly impact on higher-order processes. One problem with this literature is that monitoring deficits also occur with other symptoms (eg, Frith et al4). While a possibility exists that the cognitive underpinnings of AH may be shared across some symptoms, it also suggests that such impairments are not sufficient for AH to occur.
A related approach to recognition problems has been provided by Signal Detection Theory (SDT) models, which state that all information recognition takes place in the presence of some uncertainty and that processing relies on both pattern recognition (perceptual sensitivity) and biases in responding.9 This approach has been used to test the source-monitoring hypothesis, and the theory suggests that hallucinating individuals may have relatively unimpaired perceptual sensitivity but show lax decision criteria about accepting an signal as real and biases in responding that misattribute events to a nonself source.9 A strength of this model is the differentiation between signal detection and response biases, consistent with the idea that attributions must be differentiated from earlier processing stages. It is also compatible with the above models because self-monitoring problems might contribute to incorrect decisions about the source of information, particularly for predictive mechanisms linked to self-generated actions. Recent versions of this SDT model10 posit that perceptual hypervigilance (perhaps linked to anxiety) enhances biases in responding and thus produces a higher likelihood of errors in cognitive processing and of accepting a signal as real. The desire to reduce uncertainty under threat also leads to increased detection of ambiguous signals, a reduction in auditory threshold, and thus hallucinatory experiences.
As above, one problem with this theory is that SDT impairments are not specific to AH (eg, Harvey11). In addition, the ability to make rapid and overconfident judgments about the nature of perceptions is a processing style that is commonly linked to delusions, pointing to the possibility that such underpinnings of AH may be related to cognitive processes common to many positive symptoms.
While the above models provide explanations for verbal and nonverbal types of AH, inner speech theories have focused on providing accounts of verbal AH. They suggest that information regarding the misattribution of inner speech might be gained by comparing the phenomenology of inner speech in hallucinating individuals with “normal” inner speech.12 One such model builds on the observed distinction between “expanded” (ie, possessing an overt dialogic structure) and “condensed” (ie, abbreviated) forms of inner speech and proposes that AH occur during the transition from condensed to expanded inner dialog, particularly during periods of high cognitive load or stress.12 Although existing data on the phenomenology of inner speech in hallucinating individuals cannot yet fully address this model (see Langdon et al13), such phenomenology-based models are useful in pointing to the evolution and transformation of neural information into increasingly differentiated signals that are subject to modification by factors such as emotions.
In summary, despite different explanations for self-recognition deficits in AH, studies generally converge on the finding that AH are linked to monitoring deficits and misattributions. One key difficulty with this literature is that cognitive deficits in self-/source monitoring and SDT have been linked to other symptoms of SZ. Thus, while these deficits may still play an important role for AH formation and maintenance, it appears that such cognitive mechanisms may underpin a range of other psychotic symptoms.
Sense of Control
From the patients’ perspective, hallucinations are often described as unintentional and intrusive.14,15 This reduced sense of control may be used by individuals to differentiate hallucinated voices from one’s own verbal thoughts,16 although much variability exists in the degree to which AH (and indeed verbal thoughts) are perceived as controllable.17 Cognitive explanations have thus incorporated the idea that AH involve “a failure to control the contents of consciousness,”18 which is generally assumed to reflect a breakdown in one or more of the executive functions which control and regulate thought and action. The differentiation of executive functions into separable (though correlated) components has provided a useful framework for considering reduced sense of control in AH.
Inhibition.
Early studies of AH tended to apply the term “inhibition” fairly loosely and reported negative findings on measures of negative priming and interference (eg, Peters et al19). Since that time, there has been accumulating evidence demonstrating a link between AH and a particular type of suppression, termed “intentional cognitive inhibition” (eg, Waters et al5). These studies draw on extensive evidence from cognitive neuroscience that inhibition involves a family of processes, each with its own characteristic operating mechanisms. For example, different aspects of inhibitory processing may be differentiated, particularly between cognitive vs behavioral inhibition, intentional vs automatic inhibition, and inhibition vs interference control. Studies that have used this model together with tasks demanding the volitional suppression of memory events and irrelevant memories tested the prediction that AH in SZ involve a deficit in intentional cognitive inhibition.5 The degree of inhibitory impairment was significantly correlated with the severity of AH. Moreover, the association was specific to AH because the number of inhibitory failures was not associated with other symptoms. Stated differently, a particular form of prefrontal inhibitory control may allow auditory signals to be relatively functionally autonomous and difficult to control effectively.
Attention and Working Memory Updating.
There has been a long-standing interest in the contribution of attention and working memory processes to AH,14 although early studies concluded that tasks assessing the phonological store and loops were unrelated to positive symptoms (eg, David and Lucas20). Nonetheless, recent functional imaging data show that patients with AH exhibit reduced activity in verbal working memory circuits, albeit in the absence of deficits in working memory performance.21 That verbal working memory neural circuits are associated with AH but not behavioral performance, perhaps reflects a broader pattern of language processing deficits in AH.22 While cognitive evidence for attention and working memory updating remains inconclusive, the role of attention is clearly relevant in early auditory sensory detection mechanisms. Though not directly linked to the sense of control, attentional processes might operate in AH through the determination of resource allocated toward processing and correction of errors during information processing.10
Set-Shifting.
Hugdahl23 has argued that a proper understanding of the neurocognitive basis of AH demands inclusion of “the ability to shift attention away from the voices”. He proposes that AH involve both bottom-up internal activation of left-hemisphere speech perception areas and dysfunctional top-down executive control. On a dichotic listening paradigm, which lateralizes stimulus input, patients with SZ and frequent AH failed to demonstrate the expected right ear advantage, indicative of a functional deficit in the left perisylvian region. Reduced responsiveness to right ear stimulation is thought to arise because left-hemisphere language regions are already engaged in processing; ie, patients are already “tuned in” to the voices. In addition, patients with AH exhibited difficulties in shifting attentional focus to the opposite ear. The implication is that AH involve a difficulty in the modulation of attention and in achieving top-down executive control of voices and that the inability to shift might be a negative consequence of increased attentional focus on hallucinated voices.
In summary, executive and inhibitory control dysfunctions have been linked to AH. Intentional inhibition deficits have also been linked to a reduced sense of control associated with AH. Theoretically, deficits in intentional inhibition can cause mental events to be experienced as unintended and intrusive.5 A lack of anticipatory representation would also contribute to this reduced sense of control. Deficits in attention and set shifting may play an altogether different role, by determining expectations and resources to be allocated to these unintended auditory signals and by limiting the ability to reallocate and transfer attention to other adaptive information.
Perceptual Quality
A cardinal phenomenological feature of hallucinations is their perceptual quality. A person who hallucinates “hears” sounds and voices that can be described in terms of parameters of loudness, pitch, and clarity. These distinctive characteristics of AH have been explained in terms of top-down perceptual processing.
Top-down factors reflect the influence of internal factors and stored representations on perception, which include prior knowledge and memories, perceptual expectations, and mental imagery. Given that all acts of perception are composed of an interaction between bottom-up input and top-down constraints, an imbalance between these factors in perception has been suggested to activate percepts in the absence of external stimulation. Several theories posit that a blend of distorted input from bottom-up sensory information and aberrant top-down factors cause AH.24 Recent models25 suggest that impairments in hierarchical perceptual processing, underpinned by structural and functional neurological abnormalities, are a core abnormality in SZ. The model is also based on the fact that processing of sensory information from the environment is not passive, as it is quickly combined with stored memory representations, other sensory experiences, and top-down expectations. What is experienced is thus a cascade of processes that produces a subjective perception of reality. Over time, this inaccurate processing leads to “hard wiring” of networks which produce a distorted subjective experience of sensory input and reality distortions.
Empirical evidence for increased top-down processing in SZ patients with “verbal” AH is mostly derived from SDT studies with tasks using verbal stimulus detection in noisy circumstances,14,26 suggestibility and expectancies,27,28 and semantic expectations.26 These results support the conclusion that aberrant top-down processing, particularly in the form of strong semantic expectations, may contribute to the experience of AH, at least those of a verbal nature. In addition, the notion of suggestibility provides an interesting explanation for the reporting of a phenomenological experience in the absence of a clear external signal. If so, individuals with AH would be more amenable to conditioning and effects of implicit or explicit suggestion.29 However, it does not explain results in their entirety given that differences are reported in decision-making biases rather than sensitivity to detect biases.9
Consistent with an auditory sensory-conditioning model, one recent study indicated that hallucinating patients acquire auditory conditioned hallucinations more quickly than do nonhallucinating controls, and these AH are more resistant to extinction.30 This effect may determine whether subjective experiences are reported as hallucinations or real percepts.31
Emotional Quality
A range of positive and negative emotions are associated with AH, and studies now view emotions as intrinsically linked to the content, frequency, and beliefs about AH (eg, ref. 32,33). Evidence comes from multiple sources. The affective consequences of AH in psychiatric patients tend to be negative (though not always), and common feelings reported by patients with AH include anxiety and depression.16,32 Furthermore, emotional responses to voices are often related to beliefs and appraisals of the voice rather than to the experience per se.33 For example, beliefs that voices are malevolent tend to be associated with negative emotions (eg, fear and depression), whereas beliefs that voices are benevolent tend to be associated with positive emotions (eg, enthusiasm and respect). Increased distress in voice hearers is also related to a lack of ability to control the AH, perhaps through the influence of rumination processes on the frequency of the experience.34 Finally, a host of evidence indicates the important role of emotions as a trigger of AH, as a maintaining factor,32 and in determining the need for care.35
Further support for the role of emotions is derived from links between dissociation and hearing voices.36 Many studies have also reported a specific association between hallucinations and childhood trauma, prompting recent suggestions of a “2 hit” model of AH in which a combination of cognitive deficit and trauma lead to a high risk of hallucinations in adulthood.37 However, the presence of AH in nonhelp seeking community samples suggests that the experience itself is not always problematic, with evidence for a more positive emotional valence of AH experiences in those not seeking psychiatric help.38
Methodological Issues in Cognitive Studies of AH in SZ
A number of methodological lessons can be gained from a long history of AH research in SZ. First, with some exceptions, cognitive tools can be limited in their ability to engage fully the theoretical constructs of interest, emphasizing the importance of striving for high construct validity when choosing tasks. Cross-disciplinary work might help to identify knowledge gaps and to bridge those gaps through the integration of different methodologies. Increased collaborations between cognitive (and linguistic) sciences, electrophysiological approaches, and neuroimaging will benefit the development of theories. For example, different tools and approaches maybe used to test hypotheses derived from one discipline. This rests upon collaborative efforts and possibly the pooling of data between centers.
Second, the selection and grouping of participants is critical. An adequate design might compare patients with the same diagnosis who differ on the presence/absence of state-related AH, although a group of patients who have never experienced AH would be necessary to tease out state/trait factors. Few longitudinal studies of AH exist, although these are useful for observing the fluctuating nature of symptoms and underlying mechanisms in the same participants. Issues of symptom assessment are also of decisive importance. The presence and severity of different AH features must be assessed using well-validated scales2 and clearly detailed in scientific articles. In addition to AH symptoms, the assessment and reporting of other symptoms are important. Many studies of AH, for instance have shown that cognitive deficits are shared across other symptoms, often delusions, or passivity symptoms. The role of insight has received little attention, although poor insight might play an important role in some aspects of AH phenomenology (see “Cognitive Model of AH” section). Thus, studies must report in a transparent manner on the type and range of clinical experiences seen in their samples so that the specificity of findings to AH may be examined. Similarly, duration of illness and clinical status are important variables that will influence test results. Finally, a number of other confounds can limit the interpretability of cognitive findings. These include the contributing effects of variability in intelligence, attention and working memory, effects of medication, psychiatric comorbidities, and possible social and psychological results of the stigma and negative experiences. While these variables cannot always be controlled, researchers should record these in detail and take into consideration that multiple factors may impact on performance.
Cognitive Investigations of AH in Populations Other Than SZ
When compared with the number of cognitive studies in SZ, fewer studies have been conducted in other population groups. Yet, the study of AH in non-SZ populations is a particularly useful methodological strategy given its potential for understanding the mechanisms of AH independently of other symptoms associated with SZ. Studies with non-SZ populations can therefore elucidate and test hypotheses about the causes of AH without interference from other symptom profiles. In this section, we review available cognitive findings derived from studies in different groups.
The most intensively studied population includes healthy individuals who at times experience AH. The estimated prevalence of AH in this group is approximately 15%. Phenomenologically, AH in these nonclinical populations may be different to those in SZ, mostly in the domain of emotional content of hallucinations and the amount of control over AH.38 By contrast, nonself recognition, perceived location of voices, number of voices, loudness, and personification do not tend to differentiate between psychotic and healthy individuals. Cognitive studies have often focused on individuals who score high on scales such as the revised Launay-Slade Hallucination Scale (LSHS-R). Such individuals are thought to be “prone” to AH, although it is important to note that the LSHS assesses a range of psychological constructs, including different hallucination modalities, intrusive thoughts, and vivid daydreams. Although not strictly assessing AH, such studies can be potentially informative given that potential confounds associated with psychiatric research, such as medication and chronicity, can be avoided. Cognitive investigations show that their pattern of performance tends to be similar to that of SZ groups, albeit at attenuated levels. Consistent with the observation that self-recognition problems and complex perceptual quality are phenomenological features that are present in both clinical and nonclinical samples, studies in healthy people demonstrate spontaneous biases and misattribution39 as well as excessive top-down processing.40 By contrast, findings on self-monitoring tasks have been rather mixed, with some studies showing self-recognition difficulties,41 and others finding no relation between hallucination-proneness (incorporating all modalities) and self-recognition for actions42.
While the reduced sense of control is a less salient feature in nonclinical individuals compared with SZ groups, studies still show broad dysfunctions on tasks of intentional inhibition.43 Other support for executive dysfunctions in this population comes from links between AH proneness and intrusive thoughts, ruminations, and attempts at thought suppression.44 Certain aspects of metacognitive style such as beliefs about the uncontrollability of thoughts have also been reported,44 although these are thought to be largely mediated by the effects of comorbid symptoms.45
Phenomenological studies of AH in nonclinical groups show that the emotional quality of AH is less negative and intrusive than in SZ, yet findings show more negative emotions, and dysfunctional emotional regulation strategies, when compared with healthy individuals without these symptoms.46 Executive dysfunctions and emotion processing difficulties perhaps reflect the potential vulnerability of these individuals to experiencing psychosis. Another approach has been to define an AH analog group in terms of susceptibility to hypnagogic and hypnopompic hallucinations. Studies have showed that these may also be linked to executive and inhibitory dysfunctions.47
Despite the informative nature of transdiagnostic studies, there is a paucity of cognitive investigations conducted in other clinical populations. Approximately 15% of individuals with bipolar disorder report AH, although these have been rarely described phenomenologically. Studies show that AHs in this group are linked to difficulties on a task of self-monitoring, as demonstrated on a voice-distortion paradigm.48
Studies of borderline personality disorder show that AHs have an estimated prevalence of 20%–50%, with phenomenological features that are similar to those in SZ. Cognitive studies in this group have demonstrated links between AH and deficits in executive functions (particularly inhibition).43 Negative emotions might also be important as an etiological factor of AH in borderline personality disorder, with studies reporting increased incidence of childhood trauma and emotional abuse (independent of the presence of paranoid delusions) and a loss of emotional regulation, eg, Kingdon et al.49
It is interesting to note that visual hallucinations in Parkinson’s disease (PD) are linked to both source-monitoring and inhibitory control difficulties.50 As in SZ, poor source-monitoring and inhibitory control thus appear to be a risk factor for the presence of hallucinations in PD. Deficits in executive control have also been reported in individuals with epilepsy presenting with a history of frequent AH and to (visual) hallucinations in eye disease.51 Thus, there may be general, ie, cross-modal mechanisms that underlie hallucinations regardless of the diagnostic category.
Together, the evidence points to shared impairment in inhibitory functions, emotional problems, and top-down mechanisms across different population groups. While nonself recognition is a key clinical feature of AH across all groups, evidence regarding the role of self-/source monitoring is inconsistent, although this might be a reflection of the lack of investigations in this area. Altogether, these findings mirror the pattern of cognitive performance demonstrated in SZ. Conclusions from these observations are limited, however, given publication biases that ensure that only positive findings are reported in the literature. While similarities in deficits are emphasized, much less is known regarding how cognitive profiles differ between groups.
Cognitive Model of AH
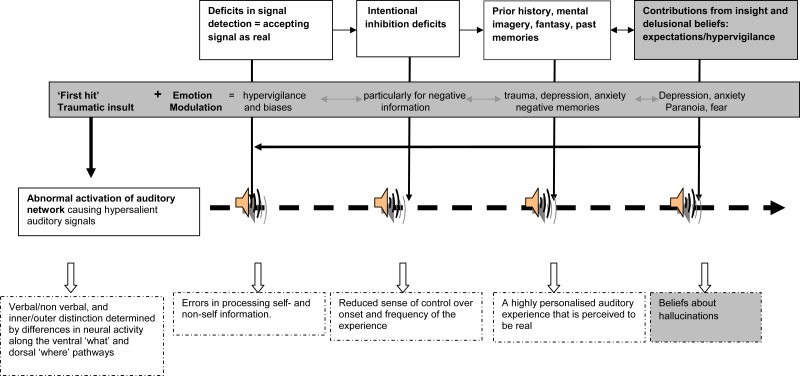
Here, we combine evidence from the above into a cohesive model. We build on a model that was first proposed by Frith and Dolan52 and subsequently elaborated by Aleman et al24 and Hugdahl.23 This model (see figure 1) comprises multidimensional elements, but it focuses on the fact that AHs are essentially perceptions. Like all perceptions, AHs arise through an interaction between information arising from neural activations and top-down activity. Cognitive, imaging, electrophysiological, and phenomenological findings associated with AH can complement and support this explanation.
Fig. 1.
Temporal unfolding of auditory hallucinations (AHs) in clinical and nonclinical populations (the boxes shaded in gray represent processing that maybe more characteristic of schizophrenia and that differentiate clinical and nonclinical AH): In this model, AH arise from an interaction between (a) signals arising from overactivation of auditory brain neural activity and (b) a range of top-down mechanisms that produce a highly complex and multidimensional experience. These top-down mechanisms include: (1) deficits in signal detection that lead to errors in processing; (2) intentional inhibition deficits that contribute to a diminished sense of control over this perceptual experience; (3) a background of expectations, imagery, and memories that provide information that is personally relevant; (4) contributions from lack of insight and delusional beliefs that provide a set of beliefs about AH; and (5) the contribution of emotions that impacts on all aspects of processing and that ensures that emotional material is processed over neutral information. This model can be used to explain variations in phenomenological features (bottom row, dotted lines), so that the severity or location of the cognitive deficits determine individual differences in the extent to which AH features are present.
Model of AH in SZ
There are 2 types of functional brain systems that are needed for this interaction. One (the source) involves salient auditory stimuli that provide the basic signal for AH. This is thought to arise from hyperactivation in functional networks involving the auditory cortex that generate aberrant auditory signals, possibly due to a deviant trigger of activations in language-related areas responsible for AH.22,53 Anomalous activations might be determined by environmental factors and/or internal (eg, emotional) conditions. One consequence of such abnormal neural activation includes auditory signals that exceed perceptual threshold, thus causing unexpectedly intense (hypersalient) sensory information. This may go some way toward explaining source-monitoring difficulties because it would bias such internal material toward being perceived as alien and separate from one’s internal mental processes and as arising from external influences. Specific forms of auditory signals (eg, forms of inner speech, intrusive memories) may be particularly more likely to be converted into AH and conceivably account for some of the verbal phenomenological properties of the AH.
The other processes involve top-down mechanisms that influence the form, content, and meaning of AH. Different modes of attention, cognitive control capacity, prior knowledge/experience, and emotional processes exert influence over form and content. The sequence of processes might be as follows: First, deficits in signal detection produce increased detection of ambiguous or salient signals and increased likelihood of accepting the signal as real and meaningful. Second, such information fails to be suppressed by faulty intentional inhibition mechanisms and becomes functionally autonomous. This would contribute to the failure to contain and control effectively the onset and frequency of these auditory signals. Over time, expectations and hypervigilance would increase the likelihood of such experiences being repeated (creating a sort of “cognitive cue”), leading to increased biases and a reduction in threshold in accepting the signal as being real. The content of AH may be determined by factors such as perceptual expectations, mental imagery, and prior experience/knowledge (eg, memories) that shape a perception of reality that is idiosyncratic and highly personalized. As such, voices of family members and radio personalities, the voice of God, and sounds of dogs barking, can be recognized.
Finally, the meaning of AH is determined by state and trait characteristics, influencing how these experiences are interpreted. In the case of SZ, the presence of reduced insight, delusional beliefs, negative schemas/beliefs about oneself (e.g. low self-esteem), beliefs about the world, and negative affect all combine to produce a complex and elaborate system of beliefs. For example, the perception may be seen as a plot from the Central Intelligence Agency (CIA) or as a message about the need to save the world.
Emotions play a particularly prominent role at all levels of this model (source, form, content, and meaning), also perhaps by providing the first traumatic insult (hit) in this ontogeny.37 Emotional events linked to trauma, dissociations, and other intense negative emotions may influence the source of AH by increasing the rate of firing of neural activation and aberrant auditory signals. They are also likely to shape the form and content of signals, due to an “automatic” prioritizing of emotional processing, which ensures that emotional and personally salient material is preferentially processed over neutral information. This would produce biases toward negative information, hypervigilance, and negative schemas that will further enhance the processing and memory recall of affective material. Traumatic life-events also produce intrusive memories that will impact on the frequency of the experience and perception about uncontrollability. Finally, over time, this would influence more broadly aspects such as beliefs and meaning attributed to AH (omnipotence etc.).
We noted above that space limitations prevent us from reviewing other relevant phenomenological features such as variability in verbal vs nonverbal content and the inner-outer space distinction. While little evidence exists regarding cognitive underpinnings of these AH features, neuroimaging evidence points to different pathways that can provide explanations for variations in such features. Verbal and nonverbal AH may stem from abnormalities at different hierarchical levels along the ventral “what” neural pathway, whose role is to process sound identity in the temporal cortices and inferior frontal cortex. By contrast, inner-outer space localization may be due to differences in activation in the dorsal “where” neural pathway, which projects along the planum temporale, frontal, and parietal cortical areas. In sum, differences in neural activity provide the functional basis for verbal and nonverbal AH and for inner-outer distinctions, which therefore may provide the basic auditory material (the “source”) for AH. Top-down processes, as explained above, would further “shape” this signal into a perceived reality that is personally relevant. As an example, neural activity in the planum temporale with projection to the inferior parietal cortex may contribute to external sound localization, while increased activation in premotor areas might engage “voices” rather than nonverbal sounds. A person showing hypervigilance for a stimulus that is emotional and self-relevant would be likely to perceive this signal as a critical verbal remark, ie, located outside the head.
In summary, we propose that AH arise through an interaction between hypersalient auditory signals (the source) and top-down mechanisms comprising different modes of error-processing, cognitive control, prior knowledge/experience, that govern the form and content of AH, together with an influence of state characteristics (insight, belief systems, etc.) that determine the meaning. Emotional processing plays a prominent role, with an initial traumatic insult creating a vulnerability for experiencing psychosis, and impacting at all levels of processing in this hierarchy. Finally, phenomenological variations may be explained by individual differences in severity of deficits and localization of neural activity. Clearly, there are many subtypes of AH2 that require different combinations of processes.54
Cognitive Model of AH in Non-SZ Populations
The model proposed above can be used to explain variations in AH features and cognitive findings in populations other than SZ (figure 1). Essentially, an auditory signal is an obligatory element for hallucinations to occur. Though this has not been yet pursued in non-SZ populations, it should be possible to examine for increased spontaneous auditory neural signals and reduced neural suppression during covert speech in these groups. Here, we propose that, similarly to processes involved in SZ and consistent with evidence to date, top-down mechanisms comprising a mix of deficits in error processing, and faulty intentional inhibition mechanisms would lead to increased attention of these aberrant perceptual signals and a failure to suppress such signals.
Other top-down processes, however, may be the key factors differentiating clinical from nonclinical hallucinations. Emotional factors may be one such differentiating factor. The timing, or severity, of trauma and negative prior experiences may occur outside a critical window, leading to a different AH experience with more positive emotional valence, and reduced hypervigilance and help-seeking behavior in nonpsychotic individuals. Personal traits (eg, presence of insight) in healthy populations would also influence how these experiences are perceived, such that these experiences are interpreted in the context of a benign rationale, with an absence of an “active” search for a meaning as is the case in psychotic individuals.
Clearly, a number of issues require clarification. For example, we must determine the origin of auditory signals, and the processes by which these are activated, in particular, which cognitive or environmental cues contribute to spontaneous activation, and under what conditions. Further differentiation is also needed in the processes underpinning AH that distinguish psychotic, nonpsychotic clinical, and nonclinical individuals. Unfortunately, insufficient evidence currently exists regarding the phenomenological characteristics and cognitive and biological underpinnings of AH in different conditions. Emerging evidence will lead to greater understanding and clearer predictions regarding the processes underlying AH in different groups.
Concluding Comments
Altogether, our knowledge of AH is slowly accumulating, and we are now in a position to provide increasingly comprehensive models of AH that can incorporate the broad phenomenological variations. The above is part of a process of building a research foundation that may, in the short term, inform novel practical and theoretical approaches toward investigations of AH. It is hoped that this review on the current state of knowledge will help researchers in selecting and incorporating the most appropriate research focus for their transdiagnostic research on AH. In the longer term, such approaches may facilitate the classification of patients in research and guide the best approaches for clinical diagnosis and treatment. In the meantime, investigations using novel task design, careful phenomenological assessment, and cross-disciplinary research protocols are urgently needed to improve the power of cognitive approaches to assess mechanisms underlying AH. It is clear that an important avenue for research is to cross borders between cognition, phenomenology, imaging, and neurobiological tools. Such a framework that interfaces directly with different branches of science will facilitate progress in understanding underlying etiology and maintenance of AH and in developing new therapeutic interventions.
Acknowledgments
We would like to thank Richard Bentall and Daniel Freeman for their input in an earlier version of this manuscript. We also wish to sincerely apologize to the authors whose important work could not be included due to a journal cap in number of references. Conflicts of interest: The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
FW is funded by a National Health and Medical Research Council grant.
References
- 1.Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephane M. Standardized assessment of hallucinations. In: Jardri R, Pins D, Cachia A, Thomas P, editors. The Neuroscience of Hallucinations. New York, NY: Springer; In press. [Google Scholar]
- 3.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 4.Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 5.Waters FA, Badcock JC, Michie PT, Maybery MT. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cogn Neuropsychiatry. 2006;11:65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher S. Neurocognitive models of schizophrenia: a neurophenomenological critique. Psychopathology. 2004;37:8–19. doi: 10.1159/000077014. [DOI] [PubMed] [Google Scholar]
- 7.Allen PP, Johns LC, Fu CH, Broome MR, Vythelingum GN, McGuire PK. Misattribution of external speech in patients with hallucinations and delusions. Schizophr Res. 2004;69:277–287. doi: 10.1016/j.schres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Waters F, Woodward T, Allen P, Aleman A, Sommer I. Self-recognition deficits in schizophrenia patients with auditory hallucinations: a meta-analysis of the literature [published online ahead of print December 08, 2010] Schizophr Bull. doi: 10.1093/schbul/sbq144. doi: 10.1093/schbul/sbq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentall RP, Slade PD. Reality testing and auditory hallucinations: a signal detection analysis. Br J Clin Psychol. 1985;24(pt 3):159–169. doi: 10.1111/j.2044-8260.1985.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 10.Dodgson G, Gordon S. Avoiding false negatives: are some auditory hallucinations an evolved design flaw? Behav Cogn Psychother. 2009;37:325–334. doi: 10.1017/S1352465809005244. [DOI] [PubMed] [Google Scholar]
- 11.Harvey P. Reality Monitoring in Mania and Schizophrenia. J Nerv Ment Dis. 1985;173:67–73. doi: 10.1097/00005053-198502000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Fernyhough C. Alien voices and inner dialogue: towards a developmental account of auditory verbal hallucinations. New Ideas Psychol. 2004;22:49–68. [Google Scholar]
- 13.Langdon R, Jones SR, Connaughton E, Fernyhough C. The phenomenology of inner speech: comparison of schizophrenia patients with auditory verbal hallucinations and healthy controls. Psychol Med. 2009;39:655–663. doi: 10.1017/S0033291708003978. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman RE, Rapaport J, Mazure CM, Quinlan DM. Selective speech perception alterations in schizophrenic patients reporting hallucinated “voices”. Am J Psychiatry. 1999;156:393–399. doi: 10.1176/ajp.156.3.393. [DOI] [PubMed] [Google Scholar]
- 15.Morrison AP, Baker CA. Intrusive thoughts and auditory hallucinations: a comparative study of intrusions in psychosis. Behav Res Ther. 2000;38:1097–1106. doi: 10.1016/s0005-7967(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RE, Varanko M, Gilmore J, Mishara AL. Experiential features used by patients with schizophrenia to differentiate 'voices' from ordinary verbal thought. Psychol Med. 2008;38:1167–1176. doi: 10.1017/S0033291707002395. [DOI] [PubMed] [Google Scholar]
- 17.Moritz S, Laroi F. Differences and similarities in the sensory and cognitive signatures of voice-hearing, intrusions and thoughts. Schizophr Res. 2008;102:96–107. doi: 10.1016/j.schres.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Bullen JG, Hemsley DR. Schizophrenia: a failure to control the contents of consciousness. Br J Clin Psychol. 1987;26:25–33. doi: 10.1111/j.2044-8260.1987.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 19.Peters ER, Pickering AD, Kent A, et al. The relationship between cognitive inhibition and psychotic symptoms. J Abnorm Psychol. 2000;109:386–395. doi: 10.1037/0021-843X.109.3.386. [DOI] [PubMed] [Google Scholar]
- 20.David AS, Lucas PA. Auditory-verbal hallucinations and the phonological loop: a cognitive neuropsychological study. Br J Clin Psychol. 1993;32(pt 4):431–441. doi: 10.1111/j.2044-8260.1993.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 21.Wible CG, Lee K, Molina I, et al. fMRI activity correlated with auditory hallucinations during performance of a working memory task: data from the FBIRN consortium study. Schizophr Bull. 2009;35:47–57. doi: 10.1093/schbul/sbn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 23.Hugdahl K. “Hearing voices”: auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand J Psychol. 2009;50:553–560. doi: 10.1111/j.1467-9450.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 24.Aleman A, Bocker KB, Hijman R, de Haan EH, Kahn RS. Cognitive basis of hallucinations in schizophrenia: role of top-down information processing. Schizophr Res. 2003;64:175–185. doi: 10.1016/s0920-9964(03)00060-4. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan RR, Keefe R, Kraus M. Schizophrenia is a disorder of higher order hierarchical processing. Med Hypotheses. 2009;72:740–744. doi: 10.1016/j.mehy.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 26.Vercammen A, de Haan EH, Aleman A. Hearing a voice in the noise: auditory hallucinations and speech perception. Psychol Med. 2008;38:1177–1184. doi: 10.1017/S0033291707002437. [DOI] [PubMed] [Google Scholar]
- 27.Haddock G, Slade P, Bentall RP. Auditory hallucinations and the verbal transformation effect: the role of suggestions. Pers Individ Dif. 1995;19:301–305. [Google Scholar]
- 28.Ilankovic LM, Allen PP, Engel R, et al. Attentional modulation of external speech attribution in patients with hallucinations and delusions. Neuropsychologia. 2011;49:805–812. doi: 10.1016/j.neuropsychologia.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Bentall RP. The illusion of reality: a review and integration of psychological research on hallucinations. Psychol Bull. 1990;107:82–95. doi: 10.1037/0033-2909.107.1.82. [DOI] [PubMed] [Google Scholar]
- 30.Kot T, Serper M. Increased susceptibility to auditory conditioning in hallucinating schizophrenic patients: a preliminary investigation. J Nerv Ment Dis. 2002;190:282–288. doi: 10.1097/00005053-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Kirsch I, Wickless C, Moffitt KH. Expectancy and suggestibility: are the effects of environmental enhancement due to detection? Int J Clin Exp Hypn. 1999;47:40–45. doi: 10.1080/00207149908410021. [DOI] [PubMed] [Google Scholar]
- 32.Freeman D, Garety PA. Connecting neurosis and psychosis: the direct influence of emotion on delusions and hallucinations. Behav Res Ther. 2003;41:923–947. doi: 10.1016/s0005-7967(02)00104-3. [DOI] [PubMed] [Google Scholar]
- 33.Birchwood M, Meaden A, Trower P, Gilbert P, Plaistow J. The power and omnipotence of voices: subordination and entrapment by voices and significant others. Psychol Med. 2000;30:337–344. doi: 10.1017/s0033291799001828. [DOI] [PubMed] [Google Scholar]
- 34.Morrison R, O'Connor RC. The role of rumination, attentional biases and stress in psychological distress. Br J Psychol. 2008;99(pt 2):191–209. doi: 10.1348/000712607X216080. [DOI] [PubMed] [Google Scholar]
- 35.Romme MAJ, Escher A. Hearing voices. Schizophr Bull. 1989;15:209–216. doi: 10.1093/schbul/15.2.209. [DOI] [PubMed] [Google Scholar]
- 36.Offen L, Thomas G, Waller G. Dissociation as a mediator of the relationship between recalled parenting and the clinical correlates of auditory hallucinations. Br J Clin Psychol. 2003;42(pt 3):231–241. doi: 10.1348/01446650360703357. [DOI] [PubMed] [Google Scholar]
- 37.Varese F, Barkus E, Bentall RP. Dissociation mediates the relationship between childhood trauma and hallucination-proneness. Psychol Med. 2011;6:1–12. doi: 10.1017/S0033291711001826. [DOI] [PubMed] [Google Scholar]
- 38.Daalman K, Boks MP, Diederen KM, et al. The same or different? A phenomenological comparison of auditory verbal hallucinations in healthy and psychotic individuals. J Clin Psychiatry. 2011;72:320–325. doi: 10.4088/JCP.09m05797yel. [DOI] [PubMed] [Google Scholar]
- 39.Barkus E, Stirling J, Hopkins R, McKie S, Lewis S. Cognitive and neural processes in non-clinical auditory hallucinations. Br J Psychiatry Suppl. 2007;51:s76–s81. doi: 10.1192/bjp.191.51.s76. [DOI] [PubMed] [Google Scholar]
- 40.Vercammen A, Aleman A. Semantic expectations can induce false perceptions in hallucination-prone individuals. Schizophr Bull. 2010;36:151–156. doi: 10.1093/schbul/sbn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johns LC, Allen P, Valli I, et al. Impaired verbal self-monitoring in individuals at high risk of psychosis. Psychol Med. 2010;40:1433–1442. doi: 10.1017/S0033291709991991. [DOI] [PubMed] [Google Scholar]
- 42.Jones SR, de-Wit L, Fernyhough C, Meins E. A new spin on the Wheel of Fortune: priming of action-authorship judgements and relation to psychosis-like experiences. Conscious Cogn. 2008;17:576–586. doi: 10.1016/j.concog.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Paulik G, Badcock JC, Maybery MT. Dissociating the components of inhibitory control involved in predisposition to hallucinations. Cogn Neuropsychiatry. 2008;13:33–46. doi: 10.1080/13546800701775683. [DOI] [PubMed] [Google Scholar]
- 44.Jones SR, Fernyhough C. Rumination, reflection, intrusive thoughts, and hallucination-proneness: towards a new model. Behav Res Ther. 2009;47:54–59. doi: 10.1016/j.brat.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Varese F, Bentall RP. The metacognitive beliefs account of hallucinatory experiences: a literature review and meta-analysis. Clin Psychol Rev. 2011;31:850–864. doi: 10.1016/j.cpr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 46.van 't Wout M, Aleman A, Kessels RP, Laroi F, Kahn RS. Emotional processing in a non-clinical psychosis-prone sample. Schizophr Res. 2004;68:271–281. doi: 10.1016/j.schres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Jones S, Fernyhough C, Meads D. In a dark time: development, validation, and correlates of the Durham Hypnagogic and Hypnopompic Hallucinations Questionnaire. Pers Individ Dif. 2009;46:30–34. [Google Scholar]
- 48.Johns L, Gregg L, Allen P, McGuire P. Impaired verbal self-monitoring in psychosis: effects of state, trait and diagnosis. Psychol Med. 2006;36:465–474. doi: 10.1017/S0033291705006628. [DOI] [PubMed] [Google Scholar]
- 49.Kingdon DG, Ashcroft K, Bhandari B, et al. Schizophrenia and borderline personality disorder: similarities and differences in the experience of auditory hallucinations, paranoia, and childhood trauma. J Nerv Ment Dis. 2010;198:399–403. doi: 10.1097/NMD.0b013e3181e08c27. [DOI] [PubMed] [Google Scholar]
- 50.Barnes J, Boubert L. Executive functions are impaired in patients with Parkinson's disease with visual hallucinations. J Neurol Neurosurg Psychiatry. 2008;79:190–192. doi: 10.1136/jnnp.2007.116202. [DOI] [PubMed] [Google Scholar]
- 51.Graham G, Dean J, Mosimann UP, et al. Specific attentional impairments and complex visual hallucinations in eye disease. Int J Geriatr Psychiatry. 2011;26:263–267. doi: 10.1002/gps.2522. [DOI] [PubMed] [Google Scholar]
- 52.Frith C, Dolan RJ. Brain mechanisms associated with top-down processes in perception. Philos Trans R Soc Lond B Biol Sci. 1997;352:1221–1230. doi: 10.1098/rstb.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diederen KM, Neggers SF, Daalman K, et al. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry. 2010;167:427–435. doi: 10.1176/appi.ajp.2009.09040456. [DOI] [PubMed] [Google Scholar]
- 54.Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr Bull. 2010;36:566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]