Abstract
Social cognitive impairments are consistently reported in schizophrenia and are associated with functional outcome. We currently know very little about whether these impairments are stable over the course of illness. In the current study, 3 different aspects of social cognition were assessed (emotion processing, Theory of Mind [ToM], and social relationship perception) at 3 distinct developmental phases of illness: prodromal, first episode, and chronic. In this cross-sectional study, participants included 50 individuals with the prodromal risk syndrome for psychosis and 34 demographically comparable controls, 81 first-episode schizophrenia patients and 46 demographically comparable controls, and 53 chronic schizophrenia patients and 47 demographically comparable controls. Outcome measures included total and subtest scores on 3 specialized measures of social cognition: (1) emotion processing assessed with the Mayer-Salovey-Caruso Emotional Intelligence Test, (2) ToM assessed with The Awareness of Social Inference Test, and (3) social relationship perception assessed the Relationships Across Domains Test. Social cognitive performance was impaired across all domains of social cognition and in all clinical samples. Group differences in performance were comparable across phase of illness, with no evidence of progression or improvement. Age had no significant effect on performance for either the clinical or the comparison groups. The findings suggest that social cognition in these 3 domains fits a stable pattern that has outcome and treatment implications. An accompanying article prospectively examines the longitudinal stability of social cognition and prediction of functional outcome in the first-episode sample.
Keywords: social cognition, illness phase, schizophrenia
Introduction
Cognitive factors in schizophrenia can be roughly divided into 2 general types: nonsocial neurocognition and social cognition. Most research on cognition in schizophrenia has focused on neurocognition, including learning and memory, vigilance/attention, speed of processing, reasoning and problem solving, and working memory.1,2 Social cognition generally refers to mental operations that underlie social interactions, including perceiving, interpreting, managing, and generating responses to socially relevant stimuli, such as the intentions and behaviors of others.3–5
Research on social cognition in schizophrenia has increased dramatically over the past decade.6,7 Social cognition may provide insights into the development and persistence of functional disability in schizophrenia.8 Furthermore, several independent data sets that have modeled determinants of outcome found that social cognition acts as a mediator between neurocognition and real world functioning, suggesting that it is more proximal to daily functioning.9–12 Consistent with its role as a mediator, social cognition can explain variance in certain areas of functioning beyond that explained by neurocognition alone (ie, has incremental validity).11–13
Several reviews and meta-analyses have established that patient-control differences on a range of social cognitive measures are large and persistent,14–17 at least in the chronic phase of illness. However, it is unclear whether the impairment is present at the start of illness, whether it exists prior to the onset of illness, whether the degree of impairment progressively increases or decreases, or whether such changes apply across multiple social cognitive domains. Only a few studies have examined social cognition across different phases of illness. One study examined face and voice affect recognition in separate groups of patients with early-stage and chronic schizophrenia compared with a single control group and found that the impairment was greater in the chronic group.18 Three publications from a North American network of early intervention sites compared social cognition performance in prodromal (clinical high risk), first-episode, and chronic patient samples with a single control group.19–21 Findings from these studies are somewhat mixed regarding social cognition in the prodromal sample. One study reported no difference between prodromal subjects and controls in face and voice emotion perception,20 1 reported no differences between these groups on a Theory of Mind (ToM) task,21 and 1 reported differences with controls on emotion discrimination but not emotion identification.19 Two of these studies included a chronic sample and reported comparable levels of social cognitive impairment for both early stage and chronic groups.19,20
The existing studies are mixed but suggest that social cognition measures (eg, facial affect identification and discrimination) start out relatively intact in the prodromal phase and then worsen with onset of illness. The results are equivocal regarding differences between recent- onset and chronic schizophrenia patients. The design of the prior studies with prodromal samples, however, makes it difficult to make strong claims about progression because they used a single control group for all clinical samples, even though the samples differed in key demographic variables. A more direct test of progression of social cognitive impairment would include separate comparison groups that match the clinical groups in terms of age, gender, and parental education. Also, most of the existing studies focused on perception of affect and only 1 considered ToM, so including a range of social cognitive domains is valuable.
For the current study, our selection of the subdomains of social cognition was guided by the 3 “cognitive constituents” of meaningful social interaction identified by social scientists—(1) models or rules for interactions, (2) capacities to understand other minds, and (3) emotional communication.22 We identified areas of social cognition that map directly onto these 3 cognitive constituents, namely social/relationship perception, ToM, and emotion processing. Previously developed measures of ToM and emotion processing were available; however, no such measure of social relationship perception existed so we developed and validated a new measure in this area.23
For each social cognitive measure, we assessed 6 groups of subjects: participants who were considered to be putatively prodromal for psychosis (referred to as prodromal in this article for simplicity), first-episode schizophrenia patients, and chronic schizophrenia patients, as well as demographically comparable control subjects for each of the clinical groups. We previously demonstrated the psychometric properties and validity for these measures in subjects drawn from the same sample of chronic schizophrenia patients,23–25 but social cognitive performance data for prodromal risk syndrome and first-episode patients have not been published. The goal of this cross-sectional study was to assess the stability of any deficits in these 3 domains of social cognition across phases of illness. An accompanying article follows up on these findings to examine the longitudinal stability and prediction of outcome within the first-episode sample.26
Methods
Participants
The participants were recruited through the University of California Los Angeles (UCLA) Center for Neurocognition and Emotion in Schizophrenia. Participants included 50 subjects with the prodromal risk syndrome for psychosis and 34 demographically comparable controls, 81 first-episode schizophrenia patients and 46 demographically comparable controls, and 53 chronic schizophrenia patients and 47 demographically comparable controls (see table 1). The research was approved by the UCLA Institutional Review Board, and all participants provided written informed consent or assent (parental consent was also obtained for minors) after study procedures were fully explained.
Table 1.
Sample Characteristics by Group Across Phase of Illness
| Demographic | Clinical Samples | Comparison Samples | Statistics | |
| Prodromal | Sample size | 50 | 34 | |
| Number of Female (%) | 14 (28) | 15 (44) | X 2 (1 df) = 2.33, P = .13 | |
| Age | 18.25 (3.12) | 18.95 (2.91) | t 82 = 1.03, P = .30 | |
| Parental education (SD) | 15.56 (2.73) | 16.73 (3.03) | t 82 = 1.85, P = .07 | |
| Clinical ratings | SANS total = 8.06 (4.26) | |||
| SAPS total = 3.49 (2.53) | ||||
| First episode | Sample size | 81 | 46 | |
| Number of Female (%) | 20 (25) | 17 (37) | X 2 (1 df) = 2.14, P = .14 | |
| Age (SD) | 22.02 (4.18) | 22.20 (3.51) | t 125 = 0.23, P = .81 | |
| Personal education (SD) | 12.50 (1.96) | 13.86 (1.97) | t 125 = 3.73, P < .01 | |
| Parental education (SD) | 13.88 (3.66) | 13.52 (4.07) | t 121 = 0.51, P = .61 | |
| Clinical ratings | SANS total = 11.65 (4.47) | |||
| SAPS total = 6.87 (4.24) | ||||
| Chronic | Sample size | 53 | 47 | |
| Number of Female (%) | 18 (34) | 13 (28) | X 2 (1 df) = 0.46, P = .50 | |
| Age | 34.77 (7.89) | 33.02 (5.32) | t 98 = 1.29, P = .20 | |
| Personal education (SD) | 13.96 (1.64) | 14.45 (1.69) | t 98 = 1.45, P = .15 | |
| Parental education (SD) | 15.09 (3.01) | 14.44 (2.82) | t 98 = 1.11, P = .27 | |
| Clinical ratings | SANS total = 8.92 (5.14) | |||
| SAPS total = 3.88 (3.13) |
Note: SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms.
Psychiatric diagnosis was established (in the case of chronic participants, they were reconfirmed) with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV) (SCID-P).27 Training of diagnostic interviewers was conducted within the Center and involved viewing videotapes and conducting live interviews to establish adequate inter-rater reliability. A minimum kappa of 0.75 was required of raters on symptom presence. Final diagnosis was determined during case conferences following presentation and review of interview data and collateral information (eg, medical records, informants).
Prodromal Samples.
The prodromal participants were between the ages of 15 and 35 years and did not meet DSM-IV criteria for schizophrenia, schizophreniform, or schizoaffective disorder as assessed by the SCID-P.27 Instead, they met criteria for 1 of 3 prodromal syndrome categories, including: (1) attenuated (subthreshold) psychotic symptoms (n = 37), (2) transient recent-onset psychotic symptoms (n = 13), or (3) a substantial drop in social/role functioning in conjunction with schizotypal personality disorder diagnosis or a first-degree relative with a psychotic disorder (n = 5), as assessed by the Structural Interview for Prodromal Symptoms (SIPS)28 ; 1 participant qualified for both syndromes 1 and 3 and 4 for both syndromes 2 and 3. The control participants for the prodromal sample did not meet DSM-IV criteria for any psychiatric disorder as determined by the SCID-P,27 did not meet criteria for any of the 3 prodromal syndromes, and did not have a first-degree family member with a psychotic disorder. Clinical interviews were conducted both with the patient and with a collateral informant (typically the mother) whenever possible. Additional exclusion criteria for both clinical and control participants included the presence of a neurological disorder, drug or alcohol abuse or dependence within the last 6 months (based on interview), pregnancy (based on self-report), insufficient English fluency, and/or intelligence quotient (IQ) below 70 (based on review of records).
Regarding medication, 10 of the 50 prodromal clinical subjects had no history of psychiatric treatment and were not taking medication at the time of assessment. Seventeen were taking a second-generation antipsychotic mediation at the time of assessment, 12 were taking selective serotonin reuptake inhibitor antidepressants, 3 were taking other antidepressants, 5 were taking a mood stabilizer, 3 were taking benzodiazepines, and 1 was taking a psychostimulant. All participants were recruited via advertising through the Staglin Music Festival Center of the Assessment and Prevention of Prodromal States at UCLA. Detailed information on recruitment procedures, inclusion criteria, inter-rater reliability, and case consensus procedures is described elsewhere.29
First-Episode Samples.
The first-episode clinical sample included outpatients between 18 and 45 years of age (though only 1 subject was >35 years) with a diagnosis of schizophrenia (n = 46), schizoaffective disorder (n = 10), or schizophreniform disorder (n = 25) determined by a SCID-P27 with confirmation of their diagnosis by a senior diagnostician from the Center’s Functional Outcome and Symptom Assessment Core. All first-episode patients were participants in the Aftercare Research Program, which recruits patients from a number of local public and private hospitals and from referrals from community outpatient facilities and providers. Aftercare Research Program patients receive treatment while participating in ongoing research projects. All patients had their first psychotic episode within 2 years prior to participation in this study, with most patients recovering from their first episode of schizophrenia at the time of entry. All participants understood spoken English sufficiently to comprehend testing procedures and exhibited no physical or language impairment that could adversely affect task performance. Patients with IQ < 70 or histories of traumatic brain injury or clinically significant neurological disorder (based on medical records) were excluded. Patients were also excluded if there was evidence of alcohol and or substance abuse in the past 6 months, if psychotic symptoms were drug induced, or if substance use was a dominant factor in the course of illness (all based on interview and records). Female patients were excluded if they were pregnant at time of study entry (based on self-report). At the time of testing, patients were clinically stabilized on oral risperidone. Twelve patients received concomitant anticholinergic medications. When clinically acceptable, anticholinergic medications were discontinued for at least 48 hours before the test session to reduce anticholinergic effects.
Healthy control participants were recruited through local newspapers, websites, and posted advertisements. Exclusion criteria for healthy controls included a diagnosis of any DSM-IV Axis I psychotic disorder, bipolar disorder, recurrent or current major depressive disorder, obsessive-compulsive disorder, post-traumatic stress disorder, current or past alcohol or substance dependence or current abuse assessed with the SCID-P, and/or paranoid, schizoid, or schizotypal personality disorder, assessed using the SCID-II.30 Controls were also excluded if they met criteria for a prodromal state, as assessed by the SIPS. Potential controls who had histories of neurological disorder or traumatic brain injury, had IQ < 70, had limited fluency in English, were currently pregnant, or had a first-degree relative with a psychotic disorder (all based on self-report) were also excluded. The goal was to recruit control participants who would be similar to the patients in gender, age, race, and parental education.
Chronic Samples.
The clinical chronic sample included outpatients with diagnosis of schizophrenia (n = 48) or schizoaffective disorder depressive type (n = 5), determined by the SCID-P,27 and all available collateral information. All patients were required to have been past participants of the Aftercare Research Program (described above). Inclusion criteria required the first psychotic episode to have been at least 5 years prior to study participation. Other than time, since first psychotic episode and maximum age, the selection criteria were the same as described above for the first-episode sample. At the time of testing, patients were clinically stabilized on a variety of first-generation (n = 12) and second-generation (n = 43) antipsychotic medications, with 6 patients receiving both types. Four patients were not receiving antipsychotic medications. Eleven patients received concomitant anticholinergic medications, and as with the first-episode sample, medication was stopped for at least 48 hours when clinically acceptable. The healthy participants were recruited through local newspaper, website, and posted advertisements. Exclusion criteria for healthy controls were the same as for the first-episode sample.
Measures
Mayer-Salovey-Caruso Emotional Intelligence Test 2.0.
The Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) is a self-report instrument that consists of 141 items and 8 ability subscales, which assess 4 components (branches) of emotion processing.31 In this study, the tester administered the MSCEIT test booklet individually to the participant and responses were later entered for computer scoring. The first branch, “Identifying Emotions,” has 2 subscales measuring emotion perception in faces and pictures (eg, identifying the degree to which certain feelings are expressed by a color photograph of a human face). The second branch, “Using Emotions” (to facilitate cognition), contains 2 subscales examining how mood enhances thinking and reasoning and which emotions are associated with which sensations (eg, asking subjects to evaluate the usefulness of different emotions that best assisted a specific cognitive task and behavior). The third branch, “Understanding Emotions,” has 2 subscales that measure the ability to comprehend emotional information, including blends and changes between and among emotions (eg, asking participants to select which 1 of 5 emotions best described a situation). The fourth branch, “Managing Emotions,” has 2 subscales that examine the regulation of emotions in oneself and in one’s relationships with others by presenting vignettes of various situations, along with ways to cope with the emotions depicted in these vignettes. For the current study, we examined the MSCEIT total score, as well as the 4 branch scores, using a general consensus approach (not age corrected). The MSCEIT has shown good reliability and discriminant validity in studies with schizophrenia patients.24,32,33
The Awareness of Social Inference Test (Part III: Social Inference—Enriched).
The Awareness of Social Inference Test (TASIT)34 (Part III) was administered to all participants. Part III of the TASIT consists of 16 videoed scenes, each lasting 15–60 seconds, depicting lies or sarcasm (8 of each presented in a fixed random order). The lie scenes involved either white lies or sympathetic lies. A prologue/epilogue provided information to the viewer about the nature of the conversational exchange. Participants were provided a record form and asked to answer 4 types of forced-choice (yes/no) questions: The first question asks the participant to think about what 1 character in the scene is doing to the other (ie, what he/she is trying to make the other person think or feel). The second question asks what the character is trying to say to the other person (ie, what is the message he/she is trying to get across). The third question asks what the character is thinking (ie, what is his/her underlying belief). The fourth question asks what the character is feeling (ie, what emotion he/she is feeling or how he/she feels toward the other person or the situation). A practice scene is provided at the beginning to familiarize participants with the questions. During administration, the videotape is paused between each scene to allow the participant time to answer the 4 questions respective to that scene. The test is not timed and lasted approximately 15 minutes. The test provides an overall total score (maximum = 64) as well as scores on the type of scene (lie vs sarcasm). Test-retest reliability of Part III was 0.83 in a small sample of traumatic brain injury patients (n = 18).35 In a previous study from this Center, Part III discriminated between chronic schizophrenia patients and controls.25
Relationships Across Domains.
The Relationships Across Domains (RAD) is a 75-item paper and pencil measure of competence in relationship perception. Understanding social relationships is a type of social perception that goes beyond perception of individuals.36 The content and format of the RAD are based on relational models theory.37 The content of the RAD’s vignettes and items reflects the theory’s contention that 4 relational models (communal sharing, authority ranking, equality matching, and market pricing) govern social behavior across many domains of social life (eg, material transactions, contributions, organization of work, social decision-making, moral judgment). The RAD reflects the theory’s assertion that persons use their implicit knowledge of the 4 relational models to understand social relationships and make inferences about the behavior of social partners in future interactions. The RAD contains 25 two to four- sentence vignettes, each involving a differently named male-female dyad whose interpersonal behaviors are consistent with 1 of the 4 relational models. Each vignette is followed by 3 statements that describe that dyad’s interpersonal behavior in domains of social life different from that of the vignette. Each of the 3 statements is consistent with 1 of the relational models.
The order of relational models of the vignettes was varied throughout the RAD. Participants are asked to use what they learned about the dyad from the vignette to indicate whether the behaviors described in the 3 statements are likely or unlikely to occur by answering “yes” or “no.” An example of a vignette would be “Alan and Patty buy gifts for each other whenever they see something they think the other would like, just because they like to make each other happy. They recently had to decide where to locate their restaurant. Alan and Patty thought about how each potential location would affect their relationship with each other. They picked a site that they thought would allow them to spend the most time together.” An example of a question about Alan and Patty would be “Alan keeps track of the time he spends with Patty relative to the time he spends with other people (yes or no?).” (Correct answer is no because this relationship fits communal sharing). Thus, participants use their implicit knowledge of the relational models to correctly answer the items of the RAD. The RAD has been validated for use in schizophrenia: It has good internal consistency in patients and controls, good group separation, and associations to community functioning.23
Clinical Rating Scales.
Psychiatric symptoms were assessed using the Scale for the Assessment of Negative Symptoms (SANS38) and Scale for the Assessment of Positive Symptoms (SAPS39 ). All raters received extensive training from Center staff on these measures to ensure a minimum intraclass coefficient of 0.75. From the SANS, the total of 4 global subscale scores were used in the current study: affective flattening, alogia, anhedonia-asociality, and avolition-apathy. From the SAPS, the total of 4 global scores from the hallucinations, delusions, bizarre behavior, and thought disorder subscales were used.
Data Analysis
The sample for this article includes any participant who received at least 1 social cognition measure. To determine if there were significant differences between the clinical subjects and controls within each phase, demographic variables were compared using chi-square tests for categorical variables and independent samples t-tests for continuous variables. Additionally, we tested whether certain demographic variables (ie, gender and parental education) were different across phases of illness using the same methodology. Demographic variables that were confounded with phase were statistically controlled in all further analyses.
The effects of phase of illness and the group by phase interaction were tested for each social cognitive dependent measure using a 2 × 3 ANOVA with group (clinical subjects or controls) as 1 independent factor and phase of illness (prodromal, first-episode, and chronic) as the second independent variable. We realize that phase of illness only refers to the clinical samples but we will refer to a main effect of phase (across clinical and control samples) for simplicity. All analyses are between-subject analyses, ie, if a subject did not take a particular social cognition measure, they were not included in the analysis of that particular measure. In addition, correlations among the social cognitive measures and the relationships between social cognition and clinical symptoms were evaluated with Pearson’s r.
Results
Table 1 shows the demographic variables for each sample and the tests of significance within each phase. In general, the groups were well matched on key demographics within phase. The first-episode samples differed in personal but not parental education.
We next examined any difference among the phases of illness. There were no significant differences in the gender distribution across phases for either clinical samples or controls (chi-square [2 df] = 2.41, P = .30; chi-square [2 df] = 1.76, P = .41, respectively). For controls, there was a significant difference in parental education between the samples at different phases (F 2,123 = 9.08, P < .01). Post hoc tests using a Tukey correction showed that the parents of the prodromal controls (M = 16.73) were significantly more educated than the parents of the first-episode and chronic control groups (M = 13.52, P < .001 and M = 14.45, P = .009, respectively). A similar difference in parental education was found for the clinical groups across phase of illness (F 2,178 = 4.62, P = .011). Parents of the prodromal risk syndrome patients (M = 15.56) had significantly higher education than the parents of first-episode patients (M = 13.88, P = .014) but did not differ from parents of chronic patients (M = 15.09, P = .747). The first-episode and chronic patients showed a marginal difference in terms of parental education (P = .093). To control for potential bias due to these differences, parental education was included as a covariate in the key analyses. There were significant differences in symptom levels among the 3 clinical groups (SANS: F 2,169 = 10.3, P < .01 and SAPS: F 2,171 = 17.7, P < .01) that were driven mainly by the first-episode sample being more symptomatic than either the prodromal group (SANS: P < .01 and SAPS, P < .01) or the chronic group (SANS: P < .01 and SAPS: P < .01). The prodromal and the chronic samples did not significantly differ from each other (SANS: P = .32 and SAPS: P = .60).
We examined the degree of intercorrelations among the 3 domains, controlling for parental education (see table 2). All the measures, including the newly developed measure of social relationship perception, were moderately intercorrelated within clinical and comparison samples. The same pattern was seen at each phase, with the associations tending to be stronger in the clinical than in the comparison samples. For simplicity, we show the associations for the samples combined across phase: Clinical samples are shown above the diagonal and control samples are below the diagonal.
Table 2.
Partial Correlations Among Social Cognitive Measures
| Measures | MSCEIT Total | TASIT Total | RAD |
| MSCEIT total | — | .545** | .621** |
| TASIT total | .419** | — | .635** |
| RAD | .322** | .520** | — |
Note: MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test; RAD, Relationships across Domains Test; TASIT, The Awareness of Social Inference Test. Partial correlations above the diagonal are the 3 clinical samples combined, controlling for parental education. Partial correlations below the diagonal are the 3 comparison samples combined, controlling for parental education.
P < .001.
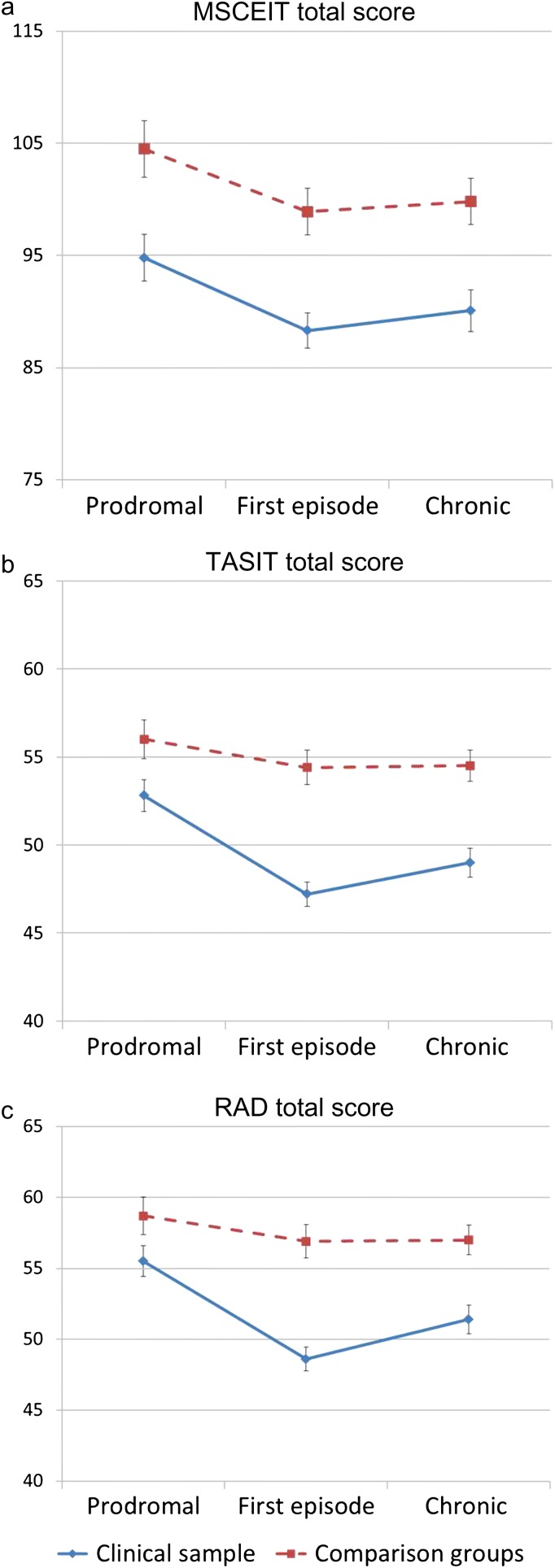
The primary results of the study are shown in table 3 and the figure 1. The table displays the results from the 9 dependent measures: total score and each of the 4 branches of the MSCEIT, the total score and 2 subtest scores of the TASIT, and the total score for the RAD. Each measure showed a strong effect of group, indicating that all tests detected impairment in the clinical groups. One measure showed a significant effect of phase (Branch 1 of the MSCEIT) and 2 others showed trends (TASIT total score and sarcasm subtest). The significant effect in the MSCEIT Branch 1 was due to a slight decline in both groups with later phase; the trends in the TASIT appeared to be due to slightly higher performance in the prodromal samples (both clinical and control) than the first-episode and chronic samples.
Table 3.
Data for Social Cognitive Measures by Group
| Measure | Prodromal | First Episode | Chronic | Statisticsa | |||||
| Clinical | Control | Clinical | Control | Clinical | Control | Group Effect | Phase Effect | Group × Phase Interaction | |
| MSCEIT total | 94.79 (15.63) | 104.48 (8.62) | 88.26 (15.14) | 98.94 (11.85) | 90.05 (14.44) | 99.79 (12.47) | F 1,275 = 33.88 | F 2,275 = 1.62 | F 2,275 = 0.13 |
| P < .001 | P = .200 | P = .879 | |||||||
| Branch 1 | 106.00 (15.41) | 114.98 (11.58) | 103.73 (16.83) | 107.58 (14.41) | 98.49 (14.16) | 105.30 (15.64) | F 1,275 = 10.76 | F 2,275 = 6.68 | F 2,275 = 1.135 |
| P = .001 | P = .001 | P = .323 | |||||||
| Branch 2 | 97.15 (15.85) | 102.95 (10.91) | 93.85 (16.51) | 99.86 (14.47) | 94.07 (16.75) | 100.42 (15.06) | F 1,275 = 9.96 | F 2,275 = 0.15 | F 2,275 = 0.16 |
| P = .002 | P = .861 | P = .852 | |||||||
| Branch 3 | 89.78 (13.98) | 100.16 (10.95) | 82.95 (14.30) | 94.20 (9.18) | 86.13 (12.72) | 96.43 (10.32) | F 1,275 = 49.70 | F 2,275 = 1.53 | F 2,275 = 0.24 |
| P < .001 | P = .218 | P = .788 | |||||||
| Branch 4 | 90.99 (10.95) | 95.21 (8.34) | 86.06 (11.79) | 94.30 (11.24) | 90.57 (9.92) | 96.10 (8.88) | F 1,275 = 21.07 | F 2,275 = 1.41 | F 2,275 = 1.13 |
| P < .001 | P = .245 | P = .325 | |||||||
| TASIT total | 52.79 (6.55) | 56.03 (3.00) | 47.18 (7.48) | 54.41 (5.16) | 48.96 (5.95) | 54.46 (5.46) | F 1,278 = 55.70 | F 2,278 = 2.93 | F 2,278 = 2.344 |
| P < .001 | P = .055 | P = .098 | |||||||
| Sarcasm | 25.14 (4.62) | 27.93 (2.74) | 22.20 (5.10) | 26.77 (3.64) | 23.06 (4.54) | 26.78 (3.42) | F 1,278 = 49.26 | F 2,278 = 2.80 | F 2,278 = 1.144 |
| P < .001 | P = .062 | P = .320 | |||||||
| Lies | 27.14 (3.46) | 28.10 (2.55) | 24.97 (3.81) | 27.64 (2.91) | 25.80 (3.91) | 27.77 (3.11) | F 1,278 = 17.26 | F 2,278 = 0.78 | F 2,278 = 1.697 |
| P < .001 | P = .46 | P = .185 | |||||||
| RAD | 55.49 (7.85) | 58.70 (4.84) | 48.61 (9.11) | 56.88 (5.72) | 51.40 (6.89) | 57.09 (8.00) | F 1,282 = 41.62 | F 2,282 = 2.10 | F 2,282 = 3.364 |
| P < .001 | P = .124 | P = .028 | |||||||
Note: Abbreviations are explained in the first footnote to table 2.
The statistical analyses were conducted controlling for parental education.
Fig. 1.
Social cognitive performance across phase of illness. Panel a (top) shows the data for the MSCEIT total score, panel b (middle) for the TASIT total score, and panel c (bottom) for the RAD for the clinical samples (blue lines) and comparison groups (red lines). MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test; RAD, Relationships across Domains Test; TASIT, The Awareness of Social Inference Test.
The group by phase interaction would be an indication of change or progression of impairment over the course of illness, and this was significant in only 1 of 9 analyses—for the RAD. Although significant, the effect was small (eta square = 0.025), it was not predicted, and it would not have withstood any correction for multiple comparisons. On inspection, this interaction is due to relatively reduced performance in the first-episode clinical sample, as opposed to a general worsening or improvement across phases. These results for the RAD are shown in the bottom panel in figure 1.
Relationships with positive and negative symptoms were examined by correlating summary scores from each social cognitive measure with the sum of the 4 global scores of the SANS and the sum of the 4 global scores of the SAPS. The results (see table 4) reveal correlations between symptoms levels and social cognitive measures primarily for the prodromal and chronic samples. In particular, the TASIT and RAD showed correlations that were slightly stronger with negative symptoms in the prodromal risk syndrome group and slightly stronger for positive symptoms in the chronic group, but these differences in correlation did not reach statistical significance. Correlations with the separate global scores for the SANS and SAPS are available in online supplementary material.
Table 4.
Correlations With Clinical Symptoms
| MSCEIT | n | TASIT | n | RAD | n | |
| Prodromal | ||||||
| SANS | −.393* | 38 | −.502* | 40 | −.409* | 41 |
| SAPS | −.278 | 39 | −.428* | 41 | −.341* | 42 |
| First episode | ||||||
| SANS | −.204 | 77 | −.197 | 76 | −.299* | 78 |
| SAPS | −.120 | 78 | −.052 | 77 | −.124 | 79 |
| Chronic patients | ||||||
| SANS | −.399* | 50 | −.214* | 50 | −.317* | 50 |
| SAPS | −.295* | 50 | −.513* | 50 | −.408* | 50 |
Note: Abbreviations are explained in the first footnote to tables 1 and 2.
P < .05.
To examine the age effects on the dependent measures and any differences in age-related changes between groups, we conducted regression analyses separately for the clinical samples combined and the comparison samples combined, controlling for parental education. The results were similar for each social cognitive measure: Age accounted for a nonsignificant and very small amount variance in performance for both the clinical and the comparison groups (all R square values < .01).
Discussion
Although patterns of neurocognitive performance across phases of illness in schizophrenia have been well established, the course of social cognitive impairment across phases has previously been largely unknown. In this study, we considered the stability of deficits across phases of illness for 3 domains of social cognition that are required for meaningful social interaction: emotion processing, ToM, and social relationship perception. The measures for each of these domains revealed clear impairment in schizophrenia across phase of illness. Importantly, in this cross-sectional cohort study, we did not see any evidence of progression or improvement over the 3 phases of illness. Age had a very small effect on performance for both the clinical and the comparison groups.
This study suggests that social cognitive impairment starts early in the course of illness and remains stable. Only 1 measure, the RAD, showed a significant group by phase interaction and that would not have been significant with a correction for multiple analyses. It was due to relatively lower performance in the first-episode clinical sample (effect sizes are shown in table 5). Figure 1 shows a hint of the same pattern in the TASIT as well. There are a couple of possible explanations for this pattern of increased group differences in the first-episode samples. One factor is that the first-episode clinical sample was close to a psychotic episode and was more symptomatic than the other clinical groups. The assessments typically occurred 2–3 months after a hospitalization, as soon as outpatient maintenance medication level had been stabilized. Thus, the patients were assessed during a period in which they were still adapting medically and socially to the onset of a psychotic illness, including adjusting to the interruption of work or school, and the possible reduction of social support networks. Hence, the performance in this sample may partially reflect the disruption of this period following an acute episode. Alternatively, the prodromal clinical sample may have performed better than the first-episode sample because the prodromal sample is heterogeneous. As mentioned above, this sample is putatively prodromal for psychosis, meaning that some individuals who will go on to develop a psychotic disorder and some will not. At a later time, it will be possible to identify those who do not have true prodromal psychosis and determine if their performance differs from those who do.
Table 5.
Effect Sizes for Between-Group Effects (Cohen's d Between Each Clinical Sample and Their Comparison Group)
| MCSEIT | TASIT | RAD | |
| Prodromal | 0.73 | 0.86 | 0.47 (.13) |
| First episode | 0.76 | 1.06 | 1.02 (<.01) |
| Chronic | 0.72 | 0.96 | 0.76 (<.01) |
Note: Abbreviations are explained in the first footnote to table 2.
The f 2 values for the group by phase interaction are .001, .017, and .025 for the MSCEIT, TASIT, and RAD, respectively. For the 1 significant phase × group interaction with the RAD, the P-values of the post hoc test are included in parentheses.
The results help to resolve findings from previous studies of social cognition across phase of illness. A few studies have examined social cognition across patients with early-stage and chronic schizophrenia18 and/or in prodromal, first-episode, and chronic patient samples,19–21 and the results suggested that some aspects of social cognition were relatively intact in prodromal samples. The demographics of the prodromal risk sample in this study are comparable with those of previous studies,19,20 so that factor does not seem to account for any differences. Interpretation of the previous studies was complicated by the use of a single control group in which the comparison group was not demographically matched to all of the clinical samples. Also, most of the previous studies that examined social cognition over phase of illness focused on affect perception, whereas the current study examined 3 subdomains.
This demonstration that social cognitive impairment is present early and is consistent in magnitude across phases of illness fits the pattern of a vulnerability indicator, as opposed to an indicator of severity or chronicity.40,41 This pattern suggests the possible value of social cognitive measures as endophenotypes in genomic studies in schizophrenia.42,43 This study alone cannot make strong claims in this regard because we did not assess family members or other at-risk groups. There is some evidence of impairment in at risk groups that would be consistent with a vulnerability indicator. For example, studies that examined psychometrically defined schizotypy have reported impairment in emotional processing,44,45 but the findings are inconsistent for ToM with some studies showing impairment46–48 and others not.49,50 In addition, social cognitive impairment has been found in some studies of unaffected relatives of schizophrenia patients.51–54 Hence, the current study demonstrates that the selected measures show 2 characteristics that would be expected from an endophenotype; they reveal impairment early in the course of illness and are relatively stable across phase. In addition, a companion article demonstrates a third feature associated with endophenotypes: good longitudinal stability across a 12-month follow-up period.26
One recurring question is whether nonsocial neurocognition and social cognition in schizophrenia are sufficiently distinct to be considered separately. Clearly, neurocognition and social cognition are correlated and share some cognitive processes in common (eg, basic auditory and visual perception, working memory, etc.). However, several studies using confirmatory factor analyses have shown that models fit better when the 2 domains are separated, as opposed to combined.11,55,56 It appears that these 2 domains are associated but not wholly redundant. This view of partially overlapping and partially distinct constructs is consistent with studies from neuroimaging in nonclinical social neuroscience.57,58 This partial overlap raises the question of whether the group differences and the stability across phase observed in social cognitive tasks stems from neurocognition. The current study cannot address that question directly.
A data analytic consideration was the presence of missing data. Because all analyses are between-subject analyses, we did not expect a bias due to missing data—if a participant did not complete a particular measure, he/she was excluded from the analyses of that variable. The missingness pattern was not significantly associated with any of our variables of interest or demographics. Hence, although the samples used in different analyses are slightly different from each other, there does not seem to be a systematic bias as to why a participant completed 1 part of the study but not the other.
This study had several limitations. First, the first-episode and chronic patient samples were taking antipsychotic medications (nearly all second-generation medications) that might have influenced performance. However, the social cognitive impairment is unlikely to be entirely due to medications because only a small subgroup of the prodromal risk syndrome sample was taking antipsychotic medications and because other studies have reported impairment in unmedicated samples.59,60 Second, this Center did not include a broad battery of nonsocial neurocognitive tasks, so a direct comparison could not be undertaken for possible progression or stability of neurocognitive domains. Similarly, we were unable to control for the effects of selected neurocognitive domains (eg, verbal memory, reasoning, and problem solving) on the results. Third, the social cognitive measures covered 3 domains, but not other aspects of social cognition that are of interest in schizophrenia, including attributional bias and empathy. Fourth, the age range of this sample does not extend into later adulthood. Hence, we do not know if the stability in performance observed across phases of illness extends to older patients or whether the very small age effects on social cognition generalize to later life. Fifth, it would have been informative to consider subgroups of prodromal subjects based on their clinical features, but most of these patients fell into the attenuated symptom group, which precluded meaningful subgroup comparisons. Sixth, this was a cross-sectional study in which we are making inferences about progression of impairment over time. The most direct way to assess this question is with a longitudinal follow-up design (see companion article). Finally, while we measured3 cognitive capacities crucial for social relationships, we did not assess motivation to engage in or sustain relationships.22,61 Social motivation is difficult to measure and rarely studied in schizophrenia, although it is clearly functionally important and related to social cognition.
These findings have implications for outcome and interventions. Training programs for social cognitive impairments in psychosis are currently being developed and validated.62–64 Much of the impetus for these interventions comes from the associations between social cognition and daily functioning of patients,8–11 as well as theoretical links between social cognitive deficits and symptom formation.65 The observation that impairments are present in all phases suggests that the relationships to daily functioning, which are well established for the chronic phase, likely apply to earlier phases as well. This possibility is directly tested in an accompanying article that examined the cross-sectional and longitudinal associations between social cognition and outcome in the first-episode sample at a 12-month follow-up.26
Funding
National Institute of Mental Health Center grant (P50 MH066286 and MH037705 to K.H.N.; MH043292 to M.F.G.).
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We gratefully acknowledge the contributions of the patients and staff of the UCLA Center for Assessment and Prevention of Prodromal States and the UCLA Aftercare Research Program. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Brothers L. The neural basis of primate social communication. Motiv Emotion. 1990;14:81–91. [Google Scholar]
- 4.Kunda Z. Social Cognition: Making Sense of People. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 5.Fiske ST, Taylor SE. Social Cognition. 2nd ed. New York, NY: McGraw-Hill Book Company; 1991. [Google Scholar]
- 6.Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green MF, Leitman DI. Social cognition in schizophrenia. Schizophr Bull. 2008;34:670–672. doi: 10.1093/schbul/sbn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in schizophrenia? Schizophr Res. 2006;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- 11.Vauth R, Rusch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res. 2004;128:155–165. doi: 10.1016/j.psychres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Brekke JS, Kay DD, Kee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. 2005;80:213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Roncone R, Falloon IR, Mazza M, et al. Is theory of mind in schizophrenia more strongly associated with clinical and social functioning than with neurocognitive deficits? Psychopathology. 2002;35:280–288. doi: 10.1159/000067062. [DOI] [PubMed] [Google Scholar]
- 14.Brune M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 15.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96:135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- 19.Addington J, Penn DL, Woods SW, Addington D, Perkins D. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008;192:67–68. doi: 10.1192/bjp.bp.107.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinkham AE, Penn DL, Perkins DO, Graham K, Siegel M. Emotion perception and the course of psychosis: a comparison of individuals at risk, and early and chronic schizophrenia spectrum illness. Cogn Neuropsychiatry. 2007;12:198–212. doi: 10.1080/13546800600985557. [DOI] [PubMed] [Google Scholar]
- 21.Couture SM, Penn DL, Woods SW, Addington J, Perkins DO. Assessment of social judgments and complex mental states in the early phases of psychosis. Schizophr Res. 2008;100:237–241. doi: 10.1016/j.schres.2007.12.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiske AP. Socio-moral emotions motivate action to sustain social relationships. Self Identity. 2002;1:169–175. [Google Scholar]
- 23.Sergi MJ, Fiske AP, Horan WP, et al. Development of a measure of relationship perception in schizophrenia. Psychiatry Res. 2009;166:54–62. doi: 10.1016/j.psychres.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Kee KS, Horan WP, Salovey P, et al. Emotional intelligence in schizophrenia. Schizophr Res. 2009;107:61–68. doi: 10.1016/j.schres.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Kern RS, Green MF, Fiske AP, et al. Theory of mind deficits for processing counterfactual information in persons with chronic schizophrenia. Psychol Med. 2009;39:645–654. doi: 10.1017/S0033291708003966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horan WP, Green MF, de Groot M, et al. Schizophr Bull. Social cognition in schizophrenia, Part 2: 12 month prediction of outcome in first-episode patients. doi:10.1093/schbul/sbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- 28.McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001;27:563–570. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- 29.Meyer SE, Bearden CE, Lux SR, et al. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. J Child Adolesc Psychopharmacol. 2005;15:434–451. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Avis II Personality Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 31.Mayer JD, Salovey P, Caruso DR. Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) User’s Manual. Toronto, Canada: MHS Publishers; 2002. [Google Scholar]
- 32.Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophr Bull. 2010;36:370–380. doi: 10.1093/schbul/sbn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eack SM, Pogue-Geile MF, Greeno CG, Keshavan MS. Evidence of factorial variance of the Mayer-Salovey-Caruso Emotional Intelligence Test across schizophrenia and normative samples. Schizophr Res. 2009;114:105–109. doi: 10.1016/j.schres.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald S, Flanagan S, Rollins J. The Awareness of Social Inference Test. Suffolk, UK: Thames Valley Test Company, Ltd; 2002. [Google Scholar]
- 35.McDonald S, Bornhofen C, Shum D, Long E, Saunders C, Neulinger K. Reliability and validity of The Awareness of Social Inference Test (TASIT): a clinical test of social perception. Disabil Rehabil. 2006;28:1529–1542. doi: 10.1080/09638280600646185. [DOI] [PubMed] [Google Scholar]
- 36.Fiske AP, Haslam N. Social cognition is thinking about relationships. Curr Dir Psychol Sci. 1996;5:143–148. [Google Scholar]
- 37.Fiske AP. The four elementary forms of sociality: framework for a unified theory of social relations. Psychol Rev. 1992;99:689–723. doi: 10.1037/0033-295x.99.4.689. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 39.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 40.Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull. 1984;10:300–312. doi: 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- 41.Zubin J, Spring B. Vulnerability: a new view of schizophrenia. J Abnorm Psychol. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]
- 42.Gur RE, Calkins ME, Gur RC, et al. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 44.Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophr Bull. 2008;34:888–903. doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aguirre F, Sergi MJ, Levy CA. Emotional intelligence and social functioning in persons with schizotypy. Schizophr Res. 2008;104:255–264. doi: 10.1016/j.schres.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Henry JD, Bailey PE, Rendell PG. Empathy, social functioning and schizotypy. Psychiatry Res. 2008;160:15–22. doi: 10.1016/j.psychres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Meyer J, Shean G. Social-cognitive functioning and schizotypal characteristics. J Psychol. 2006;140:199–207. doi: 10.3200/JRLP.140.3.199-207. [DOI] [PubMed] [Google Scholar]
- 48.Langdon R, Coltheart M. Mentalizing, schizotypy, and schizophrenia. Cognition. 2004;71:43–71. doi: 10.1016/s0010-0277(99)00018-9. [DOI] [PubMed] [Google Scholar]
- 49.Fernyhough C, Jones SR, Whittle C, Waterhouse J, Bentall RP. Theory of mind, schizotypy, and persecutory ideation in young adults. Cogn Neuropsychiatry. 2008;13:233–249. doi: 10.1080/13546800801936516. [DOI] [PubMed] [Google Scholar]
- 50.Jahshan CS, Sergi MJ. Theory of mind, neurocognition, and functional status in schizotypy. Schizophr Res. 2007;89:278–286. doi: 10.1016/j.schres.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Kee KS, Horan WP, Mintz J, Green MF. Do the siblings of schizophrenia patients demonstrate affect perception deficits. Schizophr Res. 2004;67:87–94. doi: 10.1016/s0920-9964(03)00217-2. [DOI] [PubMed] [Google Scholar]
- 52.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 53.Toomey R, Seidman LJ, Lyons MJ, Faraone SV, Tsuang MT. Poor perception of nonverbal social-emotional cues in relatives of schizophrenic patients. Schizophr Res. 1999;40:121–130. doi: 10.1016/s0920-9964(99)00036-5. [DOI] [PubMed] [Google Scholar]
- 54.Eack SM, Mermon DE, Montrose DM, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr Bull. 2010;36:1081–1088. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sergi MJ, Rassovsky Y, Widmark C, et al. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr Res. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 56.van Hooren S, Versmissen D, Janssen I, et al. Social cognition and neurocognition as independent domains in psychosis. Schizophr Res. 2008;103:257–265. doi: 10.1016/j.schres.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell JP. Contributions of functional neuroimaging to the study of social cognition. Curr Dir Psychol Sci. 2008;17:142–146. [Google Scholar]
- 58.Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behere RV, Venkatasubramanian G, Arasappa R, Reddy N, Gangadhar BN. Effect of risperidone on emotion recognition deficits in antipsychotic-naive schizophrenia: a short-term follow-up study. Schizophr Res. 2009;113:72–76. doi: 10.1016/j.schres.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 60.Herbener ES, Hill SK, Marvin RW, Sweeney JA. Effects of antipsychotic treatment on emotion perception deficits in first-episode schizophrenia. Am J Psychiatry. 2005;162:1746–1748. doi: 10.1176/appi.ajp.162.9.1746. [DOI] [PubMed] [Google Scholar]
- 61.Fiske AP. Dispassionate heuristic rationality fails to sustain social relationships. In: Mates AW, Mikesell L, Smith MS, editors. Discourse, Sociality, and Frontotemporal Dementia: Reverse Engineering the Social Brain. London, UK: Equinox; 2010. pp. 197–239. [Google Scholar]
- 62.Wolwer W, Frommann N, Haufmann S, Piaszek A, Streit M, Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program. Schizophr Res. 2005;80:295–303. doi: 10.1016/j.schres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Roberts DL, Penn DL. Social cognition and interaction training (SCIT) for outpatients with schizophrenia: a preliminary study. Psychiatry Res. 2009;166:141–147. doi: 10.1016/j.psychres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Horan WP, Kern RS, Sergi MJ, Shokat-Fadai K, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res. 2009;107:47–54. doi: 10.1016/j.schres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bentall RP, Corcoran R, Howard R, Blackwood N, Kinderman P. Persecutory delusions: a review and theoretical integration. Clin Psychol Rev. 2001;21:1143–1192. doi: 10.1016/s0272-7358(01)00106-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.