Abstract
Previous studies showed that solute carrier family 22 member 18 (SLC22A18) is involved in tumorigenesis. The aim of this study was to examine the role of SLC22A18 in glioma cells. Glioma U251 cells were transfected with the human SLC22A18 gene. Transfection of the empty vector pcDNA3.1 was used as a negative control. Sensitivity to BCNU was measured by Annexin V staining. The expression of caspase-3 and bcl-2 was determined by immunohistochemistry. The transfection was confirmed by PCR, RT-PCR and Western blotting. Augmented apoptotic cell death was observed in the SLC22A18-transfected cells, compared to the non-transfected cells or cells with the empty vector. Caspase-3 expression increased in U251-SLC22A18 cells, whereas the bcl-2 expression decreased. These results indicated that SLC22A18 has a pro-apoptotic function in glioma cells.
Keywords: solute carrier family 22 member 18, apoptosis, glioma, BCNU
Introduction
Solute carrier family 22 (organic cation transporter) member 18 (SLC22A18) is located within the 11p15.5 cluster, and has been assigned a number of different nomenclatures (1,2). Blast homology analysis suggests that SLC22A18 is a member of a family of polyspecific transporters and multidrug resistance genes (2). SLC22A18 has been shown to be a tumor suppressor candidate, as well as a substrate for RING105 (3). Structural mutations in SLC22A18 are rare, with isolated reports of point mutations in a breast cancer cell line, a rhabdomyosarcoma cell line (2), and Wilms’ and lung tumors (1). Exonic deletions in Wilms’ tumors and loss of heterozygosity in hepatoblastomas were also reported (1,4), indicating that SLC22A18 may play a role in tumorigenesis.
In this study, a eukaryotic expression vector of human SLC22A18 (pcDNA3.1-SLC22A18) was constructed and the vector was stably transfected into the U251 glioma cell line, which did not express SLC22A18 under normal growth conditions, in order to investigate the role of SLC22A18 in glioma cells. The elevated expression of SLC22A18 was found to increase the sensitivity of U251 cells to the anticancer drug BCNU in vitro.
Materials and methods
Construction of the expression vector
The 890-bp SLC22A18 cDNA was amplified using reverse transcription-polymerase chain reaction (RT-PCR) (5). The PCR reaction (10 μl) contained 1 μl cDNA, l μl 10X buffer (MgCl2), 0.4 mM dNTPs, 1 μmol primer and 1 unit TaqDNA polymerase. After denaturation at 95°C for 5 min, PCR was performed for 35 cycles (30 sec at 95°C, 30 sec at 50°C and 30 sec at 72°C) and extended at 72°C for 5 min. The linear NHeI-EcoRI fragment containing the SLC22A18 cDNA was subcloned into pcDNA3.1 (Invitrogen Corp., Carlsbad, CA, USA), which yielded pcDNA3.1-SLC22A18 by T4 ligase (Takara Bio Inc., Otsu, Shiga, Japan ). The insertion of SLC22A18 in pcDNA3.1 was confirmed by PCR, restriction enzyme digestion analysis (NHeI and EcoRI) and DNA sequencing.
Cell culture and determination of the optimal concentration of G418
The U251 human glioma cells, obtained from the Wuhan University of China, Wuhan, China, were incubated at 37°C in RPMI-1640 supplemented with 10% calf serum, 100 μg/ml penicillin and 100 μg/ml streptomycin, in an atmosphere of 5% CO2 at a saturation humidity. The culture medium was changed every 48 h. G418 is an aminoglycoside and is commonly used as a selective agent for the bacterial neo r/kan r genes. To determine the optimal concentration of G418 for the selection of resistance, U251 cells were plated at the same concentration of 5×104/well, in 24-well plates containing 2 ml culture medium per well. G418 (Sigma, St. Louis, MO, USA) was added to the wells at 10 different concentrations (50, 100, 150, 200, 300, 400, 500, 600, 700 and 800 μg/ml). The culture media were changed once every 48 h. The lowest G418 concentration, in which all cells died after 12–14 days of culture, was selected as the optimal concentration for resistance selection.
Transfection of U251 cells with pcDNA3.1-SLC22A18 and PCR confirmation of SLC22A18 in U251 cells
For the stable transfection of the SLC22A18 gene, U251 cells (1×106) were plated in 6-well plates 24 h prior to transfection. Lipofectamine 2000 (Invitrogen) was used to mediate transfection using 5.0 μg of pcDNA3.1-SLC22A18 vector or 5.0 μg of the empty pcDNA3.1 vector as a control according to the manufacturer’s instructions. After 48 h of transfection, the cells were suspended in media supplemented with G418 (150 μg/ml). The medium was changed every 48 h. Non-transfected U251 cells died within 2 weeks. G418-resistant cells were selected and denoted as U251-SLC22A18. Cells with empty vector pcDNA3.1 were named as U251-EV. DNA from cells of normal U251, U251-EV and U251-SLC22A18 was isolated using a DNA extraction kit (Puregene® DNA isolation kit, Gentra Systems, Inc., Minneapolis, MN, USA). A section of the SLC22A18 gene was used to design the primers (6). The upstream primer sequence was 5′-AGCTGAGCAGCCACTTCTC-3′ and the downstream was 5′-AAAGCTGCGGTACAGGAGG-3′. The PCR reaction system (50 μl) was: 3 μl cDNA, 5 μl 10X buffer, 4 μl MgC12, 1 μl dNTP, 1 μl primer and 0.3 μl TaqDNA polymerase. The conditions for the PCR reaction comprised an initial denaturation step of 94°C for 7 min, followed by 30 cycles of a three-step program of 94°C for 30 sec, 56°C for 30 sec, 72°C for 45 sec and a final extension step of 72°C for 7 min. All products were electrophoresed on agarose gel.
RT-PCR of SLC22A18 mRNA
The analysis of SLC22A18 mRNA expression in the three cell groups (normal U251, U251-EV and U251-SLC22A18) was performed by RT-PCR. Total RNA was extracted from glioma tissues, their adjacent brain tissues and the glioma cell line U251 with TRIzol-reagent (Invitrogen) following the manufacturer’s instructions. The RT reaction was performed on 2 μg of total RNA with the SuperScript II first-strand synthesis using an oligo(dT) primer system (Life Technologies, Corp., Carlsbad, CA, USA). Primer sequences and conditions for the RT-PCR product were previously described (forward, 5′-GCTTCGGCGTCGGAGT CAT-3′ and reverse, 5′-AGCCTGGGCGTCAGTTTT-3′) (7). The housekeeping gene GAPDH was used as an internal control of the RT reaction (forward primer, 5′-GGGAGC CAAAAGGGTCATCATCTC-3′ and reverse primer, 5′-CCA TGCCAGTGAGCTTCCCGTTC-3′) (7). PCR consisted of 35 cycles at 94°C for 1 min, at 62°C for 1 min and at 72°C for 1 min followed by a final extension at 72°C for 5 min. PCR products were analyzed on 2% agarose gels.
Western blot analysis
The three cell groups (normal U251, U251-EV and U251-SLC22A18) were washed in ice-cold phosphate-buffered saline (PBS) and lysed in a buffer using standard methods (8). After centrifugation at 10,000 × g for 10 min, the supernatants were stored at −70°C. Lysate equalized for protein content was separated in 150 g/l sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidine fluoride membrane. The membranes were blocked for 1 h at room temperature in 10 g/l bovine serum albumin and incubated overnight at 4°C with SLC22A18 antibody, followed by incubation with sheep antirabbit second antibody conjugated to HRP (Dako, Glostrup, Denmark) for 120 min. Finally, the membranes were developed with DAB and incubated until color developed sufficiently.
Apoptosis analysis
Normal U251, U251-EV and U251-SLC22A18 cells were incubated in 6-well plates at 1×106 cells/well in medium with or without 50 μM BCNU (Medical Isotopes, Inc., Pelham, NH, USA ) for 24 h. Apoptosis was detected using the Annexin V-FITC apoptosis detection kit (Jingmei Biotech Co., Ltd., Shenzhen, Guangdong, China) (9). Briefly, cells were harvested and then resuspended in 1 ml buffer prior to the addition of 5 μl Annexin V and 10 μl propidium iodite (PI). Cells were incubated in the dark at room temperature for 15 min. Cell death was determined using a flow cytometer (Backman Co.). Data were obtained and analyzed using CellQuest software (Largo Co.).
Immunohistochemical analysis for caspase-3 and bcl-2
Normal U251, U251-EV and U251-SLC22A18 cells were incubated in 6-well plates at 1×106 cells/well in medium with or without 50 μM BCNU (Medical Isotopes, Inc.). AC for 24 h. Cells were washed in 0.05 M (PBS) (pH 7.4) for 15 min, then fixed in 4% paraformaldehyde for 20 min. The streptavidin/biotin-peroxidase method was used for immunohistochemical staining. The primary antibodies, i.e., antibodies against caspase-3 and bcl-2 (Wuhan Boster Biological Technology, China), were diluted at a concentration of 1:100. PBS was used as a control. Labeled cells were photographed and the integrated optical density (IOD) was measured using Image-Pro Plus 5.02 (Media Cybernetics, Inc., Bethesda, MD, USA).
Statistical analysis
The differences between the controls and treated groups were analyzed using the χ2-test. SPSS 10.0 statistical software was used to analyze the results. P<0.05 was considered to be statistically significant.
Results
SLC22A18 cDNA amplification and identification of the expression vector
First, the primers were designed and then the 890-bp SLC22A18 gene was amplified. The PCR product was collected and purified. The purified SLC22A18 fragment and the pcDNA3.1 vector were digested by NHeI and EcoRI, followed by ligation with T4 ligase at 16°C overnight. The ligated plasmid was transformed into the DH5α strain of E. coli. Single colonies were selected and PCR amplification confirmed a single band of 890 bp. The plasmids were isolated from DH5α and digested by NHeI and EcoRI. DNA gel showed two bands, one corresponding to the 890-bp fragment and the second corresponding to the vector pcDNA3.1. DNA sequencing showed that the 890-bp fragment corresponded with the DNA sequence of the SLC22A18 gene.
Cell morphology and U251-SLC22A18 screening
Normal U251 cells were usually elongated and elliptical (Fig. 1A). The cells died within 2 weeks when cultured in the presence of 150 μg/ml G418 (Fig. 1B). Cells transfected with pcDNA3.1-SLC22A18 were resistant to G418 and were screened under G418 for ~3 weeks and denoted as U251-SLC22A18. The U251-SLC22A18 cells had a similar appearance to the non-transfected cells and occasionally formed clusters (Fig. 1C and D).
Figure 1.
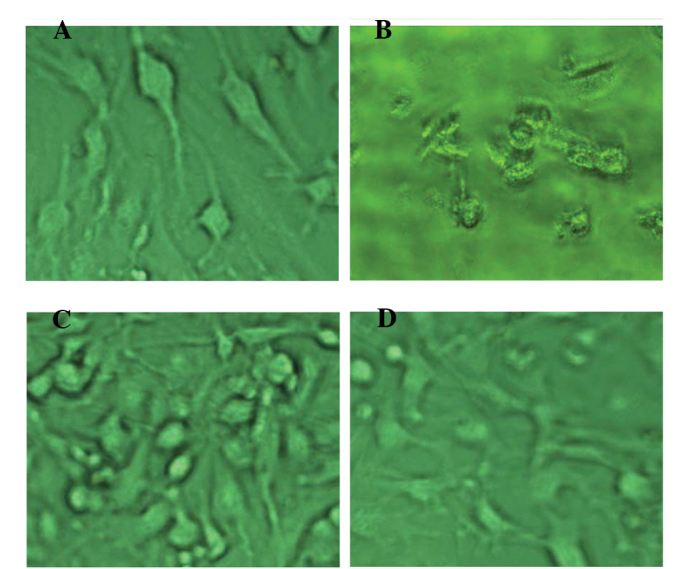
Microscopic images of different groups cells in the selection. (A) Normal U251; (B) Normal U251 cells cultured in the presence of G418 for 2 weeks; (C and D) U251-SLC22A18 cells cultured in the presence of G418 for 3 weeks.
PCR analysis of SLC22A18 in U251 cells
DNA was extracted from the cells of normal U251, U251-EV and U251-SLC22A18. The extracted DNA was amplified by PCR using the primer pair described above. As expected, a 170-bp fragment was observed in U251-SLC22A18 cells, but not in normal U251 or U251-EV cells (Fig. 2). This result further confirmed the specific transfection of the SLC22A18 gene into U251 cells.
Figure 2.
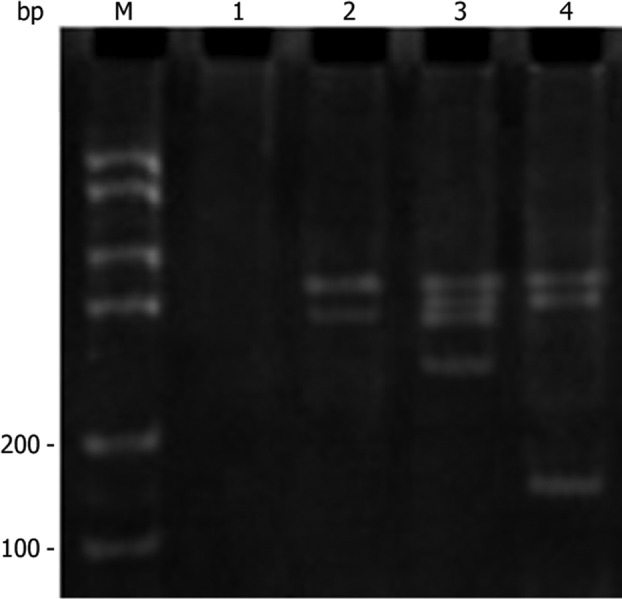
PCR amplification of SLC22A18. Lane 1, control (culture medium only); lane 2, U251-EV cells; lane 3, normal U251 cells; lane 4, U251-SLC22A18 cells; M, the marker for standard DNA molecular mass.
SLC22A18 mRNA expression in U251 cells
RNA extracted from normal U251, U251-EV and U251-SLC22A18 cells was amplified by RT-PCR and subsequently analyzed using DNA gel. A prominent 170-bp band was detected in U251-SLC22A18 cells, but was non-detectable in U251-EV cells or normal U251 cells (Fig. 3).
Figure 3.
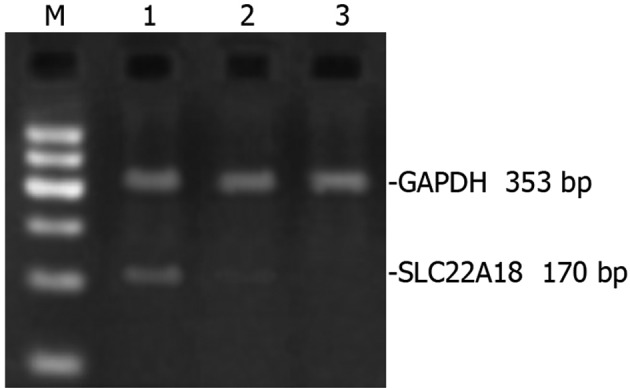
RT-PCR analysis of SLC22A18 mRNA expression. Lane 1, U251-SLC22A18 cells; lane 2, U251-EV cells; lane 3, normal U251 cells; M, the marker of standard DNA molecular mass.
SLC22A18 protein expression in U251 cells
Total protein extracted from normal U251, U251-EV and U251-SLC22A18 cells was separated using 12% SDS-PAGE and was subsequently analyzed by Western blot analysis. A 50-kDa band corresponding to the size of the SLC22A18 protein was observed in U251-SLC22A18 cells, but not in U251-EV or normal U251 cells (Fig. 4).
Figure 4.
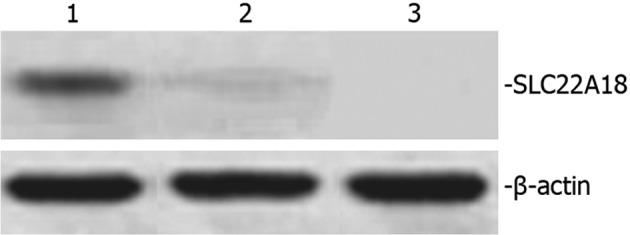
Western blot analysis of SLC22A18 protein. Lane 1, U251- SLC22A18 cells; lane 2, U251-EV cells; lane 3, normal U251 cells. β-actin was used as a loading control.
BCNU-induced apoptosis
BCNU is an anticancer drug and an inducer of apoptotic cell death. BCNU was used to further assess the role of SLC22A18 in U251 cells. Apoptosis was observed in the three cell groups (normal U251, U251-EV and U251-SLC22A18). The average apoptosis ratio of normal U251, U251-EV and U251-SLC22A18 cells was 2.4±0.2, 2.2±0.3 and 9.2±0.3%, respectively. The apoptosis ratio of U251-SLC22A18 cells was significantly (P<0.05) higher than that of normal U251 or U251-EV cells. Minimal apoptosis was observed in all three cell groups in the absence of BCNU.
Immunohistochemistry
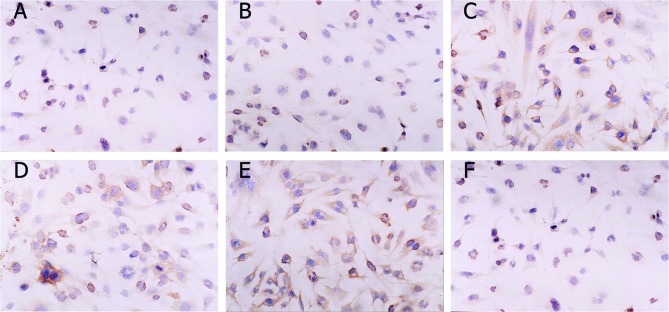
Images of caspase-3 and bcl-2 staining are shown in Fig. 5. The positive reaction located in cytosol was stained brown. The color of the stain is positively correlated to the protein expression. The IOD of each group revealed that in U251-SLC22A18 cells the expression of caspase-3 increased, whereas the expression of bcl-2 decreased (Fig. 6).
Figure 5.
Caspase-3 and bcl-2 protein expression in the three cell groups (original magnification, ×200). (A) Caspase-3 normal U251; (B) caspase-3 U251-EV; (C) caspase-3 U251-SLC22A18; (D) bcl-2 normal U251; (E) bcl-2 U251-EV; (F) bcl-2 U251-SLC22A18.
Figure 6.
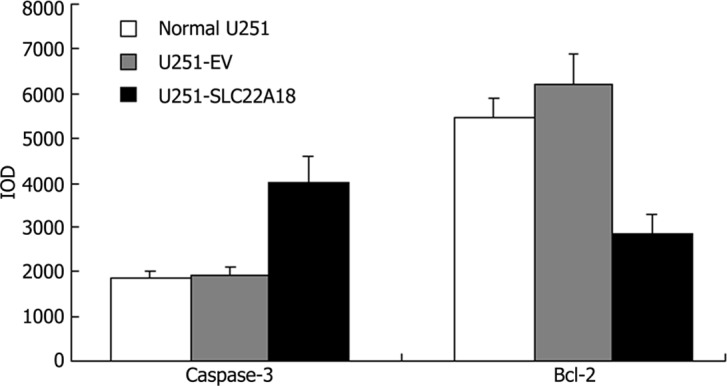
Caspase-3 and bcl-2 expression (in IOD) in normal U251, U251-EV and U251-SLC22A18 cells (n=6).
Discussion
SLC22A18 is located within the 11p15.5 cluster and has been assigned a number of different nomenclatures (1,2). Blast homology analysis suggests that SLC22A18 is a member of a family of polyspecific transporters and multidrug resistance genes (2). SLC22A18 has also been shown to be a tumor suppressor candidate and a substrate for RING105 (3). Structural mutations in SLC22A18 are infrequent, with isolated reports of point mutations in a breast cancer cell line, a rhabdomyosarcoma cell line (2), and Wilms’ and lung tumors (1). Exonic deletions in Wilms’ tumors and loss of heterozygosity in hepatoblastomas have also been reported (1,4). Thus, SLC22A18 may be involved in tumorigenesis.
The role SLC22A18 plays in tumorigenesis is functionally unclear. In this study, a eukaryotic expression vector of human SLC22A18 (pcDNA3.1-SLC22A18) was constructed and the vector was stably transfected into the U251 glioma cell line, which did not express SLC22A18 under normal growth conditions. Data from the present study revealed that apoptotic cell death observed in the SLC22A18-transfected cells was more abundant than in the non-transfected cells or in cells with the empty vector. Moreover, the expression of caspase-3 increased, whereas the expression of bcl-2 decreased in the U251-SLC22A18 cells, indicating that the expression of SLC22A18 increased the sensitivity of U251 cells to the anticancer drug BCNU in vitro, and that SLC22A18 has a pro-apoptotic function in glioma. We speculate that the function of SLC22A18 may be tissue- or cell type-specific. Further studies, in particular a direct comparison of SLC22A18 in different cell types under the same conditions, are required in order to better understand the complex functions of SLC22A18 in relation to tumor development.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of China (30901535), the Research Fund of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (10XHJT01), and the New One Hundred Person Project of Shanghai Jiao Tong University School of Medicine (10XBR01).
References
- 1.Lee MP, Reeves C, Schmitt A, Su K, Connors TD, Hu RJ, Brandenburg S, Lee MJ, Miller G, Feinberg AP. Somatic mutation of TSSC5, a novel imprinted gene from human chromosome 11p15.5. Cancer Res. 1998;58:4155–4159. [PubMed] [Google Scholar]
- 2.Schwienbacher C, Sabbioni S, Campi M, Veronese A, Bernardi G, Menegatti A, Hatada I, Mukai T, Ohashi H, Barbanti-Brodano G, Croce CM, Negrini M. Transcriptional map of 170-kb region at chromosome 11p15.5: identification and mutational analysis of the BWR1A gene reveals the presence of mutations in tumor samples. Proc Natl Acad Sci USA. 1998;95:3873–3878. doi: 10.1073/pnas.95.7.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada HY, Gorbsky GJ. Tumor suppressor candidate TSSC5 is regulated by UbcH6 and a novel ubiquitin ligase RING105. Oncogene. 2006;25:1330–1339. doi: 10.1038/sj.onc.1209167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht S, Hartmann W, Houshdaran F, Koch A, Gärtner B, Prawitt D, Zabel BU, Russo P, von Schweinitz D, Pietsch T. Allelic loss but absence of mutations in the polyspecific transporter gene BWR1A on 11p15.5 in hepatoblastoma. Int J Cancer. 2004;111:627–632. doi: 10.1002/ijc.20280. [DOI] [PubMed] [Google Scholar]
- 5.Mohammad F, Mondal T, Guseva N, Pandey GK, Kanduri C. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development. 2010;137:2493–2499. doi: 10.1242/dev.048181. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher E, McGoldrick A, Chung WY, McCormack O, Harrison M, Kerin M, Dervan PA, McCann A. Gain of imprinting of SLC22A18 sense and antisense transcripts in human breast cancer. Genomics. 2006;88:12–17. doi: 10.1016/j.ygeno.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Xu HM, Zhang HW, He HY, Hou YY, Zhao ZQ. Methylation and mRNA expression of imprinted gene SLC22A18/TSSC5/BWR1A in breast cancer. Chin J Pathophysiol. 2008;24:1007–1012. [Google Scholar]
- 8.Chu SH, Yuan XH, Li ZQ, Jiang PC, Zhang J. C-Met antisense oligodeoxynucleotide inhibits growth of glioma cells. Surg Neurol. 2006;65:533–538. doi: 10.1016/j.surneu.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Li W, Xu Q, Huang Y. Elevated expression of Dickkopf-1 increases the sensitivity of human glioma cell line SHG44 to BCNU. J Exp Clin Cancer Res. 2010;29:131. doi: 10.1186/1756-9966-29-131. [DOI] [PMC free article] [PubMed] [Google Scholar]