ABSTRACT
Meiosis in mammalian females is marked by two arrest points, at prophase I and metaphase II, which must be tightly regulated in order to produce a haploid gamete at the time of fertilization. The transition metal zinc has emerged as a necessary and dynamic regulator of the establishment, maintenance, and exit from metaphase II arrest, but the roles of zinc during prophase I arrest are largely unknown. In this study, we investigate the mechanisms of zinc regulation during the first meiotic arrest. Disrupting zinc availability in the prophase I arrested oocyte by treatment with the heavy metal chelator N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) causes meiotic resumption even in the presence of pharmacological inhibitors of meiosis. We further show that the MOS-MAPK pathway mediates zinc-dependent prophase I arrest, as the pathway prematurely activates during TPEN-induced meiotic resumption. Conversely, inhibition of the MOS-MAPK pathway maintains prophase I arrest. While prolonged zinc insufficiency ultimately results in telophase I arrest, early and transient exposure of oocytes to TPEN is sufficient to induce meiotic resumption and bypass the telophase I block, allowing the formation of developmentally competent eggs upon parthenogenetic activation. These results establish zinc as a crucial regulator of meiosis throughout the entirety of oocyte maturation, including the maintenance of and release from the first and second meiotic arrest points.
Keywords: egg, MAPK, meiosis, MOS, oocyte, zinc
Zinc homeostasis maintains prophase I arrest in mouse oocytes by inhibiting premature activation of the MOS-MAPK pathway, expanding the role of zinc signaling to encompass both physiological meiotic arrest points in the oocyte.
INTRODUCTION
During embryonic development, mammalian oocytes initiate meiosis and subsequently arrest at the first prophase (prophase I [PI]). This arrest can last from months to years, depending on the species, and is maintained by high intraoocyte cAMP levels. The preovulatory luteinizing hormone (LH) surge causes degradation of cAMP and signals the oocyte to resume meiosis. The reinitiation of meiosis, also known as meiotic maturation, can also occur spontaneously in an in vitro setting (in vitro maturation [IVM]) with release of fully grown oocytes from the follicle environment [1]. Whether it occurs in vivo or in vitro, the first clear sign of meiotic maturation is germinal vesicle breakdown (GVBD), with disassembly of the nuclear membrane and formation of the first meiotic spindle at metaphase I (MI). The oocyte then asymmetrically divides to give off the first polar body (PB), progresses into meiosis II, and arrests a second time at metaphase of meiosis II (MII). The egg remains arrested at this stage until fertilization, which, if successful, results in the completion of meiosis with extrusion of the second PB. The two arrest points at PI and MII are unique hallmarks of female meiosis, and require intricate temporal and spatial regulation.
Several factors are known to regulate meiotic maturation in the oocyte. Maturation-promoting factor (MPF), a heterodimer consisting of cyclin-dependent kinase 1 and cyclin B1, regulates meiotic resumption through phosphorylation events that initiate GVBD, chromatin condensation, and spindle formation [2]. MPF activation is inhibited during the PI arrest by elevated cAMP levels due to high protein kinase A (PKA) activity [3]. In addition, MPF remains an important regulatory factor in the establishment and maintenance of the second meiotic arrest at MII.
Another important regulator of meiotic maturation is the MOS-mitogen-activated protein kinase (MAPK) pathway. Transcripts of the c-Mos proto-oncogene (Mos) are highly and almost exclusively expressed in the oocyte during M phase, suggesting its role in female meiosis [4]. MOS is a MAPK kinase kinase (MAP3K), and its accumulation triggers the phosphorylation of MAPK kinase 1 and 2 (MAP2K1/2; previously known as MEK1/2), which, in turn, phosphorylate and activate MAPK 3 and 1 (MAPK3/1; previously known as ERK1/2) [5, 6]. In Xenopus laevis oocytes, MOS is required for meiotic resumption, activating both MAPK and MPF [7–9]. In rodent species, MOS protein is translated after GVBD [4], and is not necessary for meiotic resumption [10, 11]. MOS overexpression, however, is sufficient to induce GVBD, likely via indirect activation of MPF [12, 13]. Furthermore, MOS plays an evolutionarily conserved role in preventing parthenogenesis by maintaining arrest of the mature egg (MII in mammals) and allowing it to await fertilization [10, 11, 14, 15]. Altogether, the process of meiotic maturation comprises a complex array of events that rely on the coordinated activities of multiple factors and parallel pathways.
Recently, the transition metal zinc has emerged as an essential and novel regulator of the events surrounding MII arrest (Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org). From PI to MII arrest, a transition that occurs within 12–14 h in the mouse oocyte, the total intracellular concentration of zinc increases by more than 50% [16]. Such dramatic influxes in elemental zinc have been shown to induce cell death in other cell types [17–19], and attests to the unique status of the oocyte in adopting zinc-dependent mechanisms for the regulation of meiosis. Furthermore, this accrual is necessary for the establishment of MII arrest, as limiting zinc uptake during IVM using the heavy metal chelator N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) results in premature meiotic arrest at telophase I (TI; Supplemental Fig. S1; [16]).
The spindle and meiotic division abnormalities induced by zinc insufficiency during IVM are reminiscent of those observed in MOS null mice [20–22]. However, detailed studies by our group determined that MOS does not mediate the effects of zinc in the establishment of MII arrest [23]. Instead, we found that zinc-insufficient oocytes fail to increase MPF activity after MI, explaining the failure to reach MII [23]. The MPF regulator early meiosis inhibitor 2 (EMI2; official gene symbol, Fbxo43) is a zinc-binding protein that is first detectable at MI and increases to maintain high MPF activity during MII arrest [24]. EMI2 appears to act in concert with zinc levels during the MI-MII transition [25, 26]. In the proposed model, the observed increase in total cellular zinc during IVM directly modulates EMI2 activity to initiate MII entry and arrest [25]. Zinc insufficiency consequently disrupts EMI2 function and prevents the establishment of MII (Supplemental Fig. S1).
Additionally, zinc also participates in the exit from MII arrest upon fertilization. Egg activation by either fertilization or parthenogenesis initiates the exocytosis of zinc through a series of coordinated, calcium-dependent events called zinc sparks [27]. Activation can also be induced artificially by treating eggs with TPEN, thereby bypassing a need for the calcium oscillations that normally precede egg activation [26, 27]. Finally, increasing zinc content in the egg using zinc pyrithione, a zinc ionophore, prevents egg activation by strontium chloride (Supplemental Fig. S1; [25]). These results establish the reduction in intracellular zinc availability at the time of egg activation as both necessary and sufficient to allow meiotic progression beyond MII. Furthermore, because EMI2 protein is normally rapidly degraded upon egg activation to allow MII exit, the results observed with inactivation of EMI2 by zinc insufficiency are also consistent with a role for EMI2 in mediating the effects of zinc insufficiency (Supplemental Fig. S1).
Several lines of evidence support a critical role for zinc throughout the establishment of, maintenance of, and release from the second meiotic arrest, which represents a critical window of zinc activity largely mediated via the cell cycle regulator EMI2, and not the MOS-MAPK pathway (Supplemental Fig. S1). The role of zinc in regulating the first meiotic arrest, however, is only beginning to be defined. Recently, it was shown that oocytes exposed to TPEN in vitro or collected from mice fed a zinc-deficient diet spontaneously undergo GVBD in the absence of an LH surge [28]. The mechanism by which this occurs, however, is unknown. In the present study, we provide a detailed analysis of the kinetics and dynamics of TPEN-induced meiotic maturation. In contrast to the later effects of zinc during MII arrest, this early effect of zinc in maintaining PI arrest is, intriguingly, mediated through the MOS-MAPK pathway.
MATERIALS AND METHODS
Reagents and Antibodies
All media, fetal bovine serum (FBS), Alexa Fluor 488-conjugated goat anti-mouse IgG, rhodamine-phalloidin, 10% Bis-Tris NuPAGE gels, penicillin-streptomycin, MOPS-SDS running buffer, and horseradish peroxidase (HRP)-conjugated anti-mouse IgG were purchased from Invitrogen (Carlsbad, CA). HRP-conjugated anti-rabbit IgG and ECL Advance Western Blotting Detection Kit were from GE Healthcare (Piscataway, NJ). Immobilon-P polyvinylidene fluoride (PVDF) membranes and potassium simplex optimized medium (KSOM) were purchased from Millipore (Billerica, MA). Anti-phospho-MAP2K1/2 and anti-phospho-MAPK3/1 antibodies, and the MAP2K1/2 inhibitor U0126 were purchased from Cell Signaling Technology (Beverly, MA). Anti-MOS antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-α-tubulin (T9026 for immunofluorescence, T6199 for Western blot) and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Animals
CD1 mice were housed and bred in a controlled barrier facility and provided food and water ad libitum. Mice were exposed to a 14L/10D cycle with constant humidity and temperature. To obtain cumulus-oocyte complexes (COCs) for most experiments, immature CD1 female mice, 19–25 days old, were given intraperitoneal injections of 5 IU equine chorionic gonadotropin (eCG; Calbiochem, La Jolla, CA) 44–48 h prior to euthanization. For parthenogenetic activation experiments, COCs were obtained from 7- to 10-wk-old CD1 female mice, which were given intraperitoneal injections of 10 IU eCG 44–48 h prior to euthanization. To collect MII-stage eggs from in vivo ovulation (IVO), we primed 7- to 10-wk-old females with 10 IU eCG and then with 10 IU human chorionic gonadotropin (hCG) 46–48 h later. Eggs were isolated from the oviducts 14 h after hCG treatment. All animal use was approved by Northwestern University's Institutional Animal Care and Use Committee and maintained according to the National Institutes of Health's guidelines.
Oocyte Collection and In Vitro Culture
Ovaries were collected in Leibovitz's L-15 medium (L-15) supplemented with 1% (v/v) FBS and 10 μM milrinone. Large antral follicles were punctured using insulin-gauge needles to release COCs, which were denuded by gentle aspiration through a narrow-bore pipette. Denuded oocytes (DOs) were cultured in groups of 40–60 in four-well dishes (Nalge Nunc International, Rochester, NY). The base medium used was minimum essential medium (MEM)-alpha with GlutaMAX L-15 supplemented with 10% FBS and pre-equilibrated at 37°C in a humid atmosphere of 5% CO2. TPEN was prepared as a 1-mM stock solution in Milli-Q water and used at a final concentration of 10 μM. TPEN was included in the culture medium for the duration of each experiment, unless stated otherwise. The moment at which DOs were transferred into culture medium was designated as t = 0 h. For exogenous metal supplementation experiments, sulfate derivatives of Mg2+, Fe2+, Cu2+, and Zn2+ were prepared as 10-mM stock solutions in Milli-Q water and used at a final concentration of 10 μM. Cycloheximide was prepared as a 100 mg/ml stock solution in dimethyl sulfoxide (DMSO) and used at a final concentration between 1 μg/ml and 100 μg/ml. U0126 was prepared as a 10-mM stock solution in DMSO and used at a final concentration between 1 μM and 10 μM.
To obtain MII eggs, oocytes were cultured in milrinone-TPEN medium for the indicated times and transferred to milrinone medium for a total culture period of 24 h. To obtain MII eggs after TPEN treatment and for activation, oocytes were cultured in milrinone-TPEN medium for 5 h, transferred to milrinone medium for 1 h, then cultured in IVM medium for 14 h. Because a percentage of oocytes undergo GVBD even after transfer out of TPEN-containing medium, we utilized the 1-h incubation in milrinone to maximize collection of GVBD oocytes. IVM eggs were cultured in parallel to TPEN treatment, and were thus maintained in milrinone medium for 6 h prior to transfer to IVM medium. Ovulated eggs were denuded using 0.3% (w/v) hyaluronidase. All reagents were added to the base culture medium and pre-equilibrated prior to the beginning of culture. Brightfield images were captured at the beginning and end of each culture period using a DM IRB inverted microscope (Leica Microsystems, Heidelberg, Germany).
Immunofluorescence and Confocal Microscopy
Oocytes were prepared for immunofluorescence as previously described [16]. Briefly, oocytes were fixed and extracted with 2% (v/v) formaldehyde and 1% (v/v) Triton X-100 for 0.5 h, then blocked for 1 h in blocking solution containing 0.2% (w/v) sodium azide, 0.2% (w/v) milk, 2% (v/v) normal goat serum, 1% (w/v) BSA, 100 mM glycine, and 0.1% (v/v) Triton X-100. Samples were incubated with monoclonal α-tubulin antibody (1:100) for 1 h, washed three times in the blocking solution, and incubated with a mixture of Alexa Fluor 488-conjugated goat anti-mouse IgG (1:250) and rhodamine-phalloidin (1:100) for 1 h. All fixation, staining, and wash steps were done at 37°C. Samples were washed in blocking solution and mounted in VectaShield solution containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were acquired on a Leica TCS SP5 inverted laser-scanning confocal microscope with 63× oil immersion objective and processed using LAS AF software (Leica Microsystems).
SDS-PAGE and Immunoblotting
Oocyte samples were collected throughout culture as indicated and washed in L-15 medium supplemented with 0.01% PVA to remove serum. Samples were lysed in 8 μl of 1× SDS-PAGE sample buffer [29] and stored at −80°C until use. Samples were heated at 95°C for 5 min, electrophoresed on 10% Bis-Tris gels, and transferred to PVDF membranes. Blocking and antibody incubations were done in 2% w/v ECL Advance Blocking Reagent prepared according to manufacturer's instructions. Blots were blocked for 1 h at room temperature and then incubated with primary antibodies diluted 1:1000 (anti-MOS was used at 1:500 dilution) overnight at 4°C. After washing, blots were incubated with HRP-conjugated secondary antibodies at 1:10 000 dilution for 1 h at room temperature and detected using ECL Advance Detection Reagent. Blots were incubated in Restore Western Blot Stripping Buffer (Thermo Fisher Scientific, Rockford, IL) for 30 min at room temperature and reprobed. Analysis of band intensity was performed using ImageJ version 1.44o. All protein levels were normalized to α-tubulin.
Oocyte Microinjection
Mos siRNA was purchased from Invitrogen based on the published sequence UAG GGG GCA CGU ACA CGC A [30] and used at a final concentration of 100 μM. The Mos inverted repeated pCRII plasmid was kindly provided by Dr. Paula Stein and Dr. Richard Schultz (University of Pennsylvania, Philadelphia, PA) [31]. Mos dsRNA was made by in vitro transcription of linearized plasmid using the mMESSAGE mMACHINE T7 kit (Life Technologies, Grand Island, NY). RNA was purified on Qiagen RNeasy columns (Qiagen, Valencia, CA), and eluted and diluted in nuclease-free water to obtain a concentration of 106 molecules/5 pl for microinjection. Mos siRNA or Mos dsRNA (5–10 pl) was injected into the oocyte cytoplasm using an Eppendorf FemtoJet pressure microinjector with Femtotip injection capillaries (Eppendorf, Hauppauge, NY). Injected oocytes were held in αMEM with 10% FBS and 10 μM milrinone for 10–14 h and then either maintained in this medium or transferred to medium containing 10 μM TPEN. Following an additional 14 h of culture, oocytes were imaged to score meiotic progression and processed for immunoblotting.
Parthenogenetic Activation and Development
Ionomycin was prepared as a 5 mg/ml stock solution in DMSO and diluted to a working concentration of 2.5 μg/ml in calcium-free KSOM. Eggs were incubated in ionomycin for 5 min, washed through three drops of KSOM with 10% FBS, and transferred to KSOM with 10 μg/ml cycloheximide for 6 h. Eggs were diploidized by culturing in 5 μg/ml cytochalasin B for 4 h following ionomycin treatment. At 6 h post-activation (hpa), activated eggs were washed and transferred to fresh KSOM for extended culture. Embryos of the correct developmental stage were transferred to fresh KSOM every other day. Images were acquired at 8, 24, and 120 hpa to score for pronuclei, two-cell, and blastocyst formation rates, respectively.
Statistical Analysis
Densitometry analysis of immunoblots, rates of GVBD, and rates of embryo development were analyzed either by two-tailed Student t-tests in Excel (Microsoft) or by one-way ANOVA followed by Bonferroni post hoc test if statistically significant (Prism4; Graph Pad Software). The level of statistical significance is indicated in each figure legend.
RESULTS
Specific Disruption of Intracellular Zinc Causes Meiotic Resumption
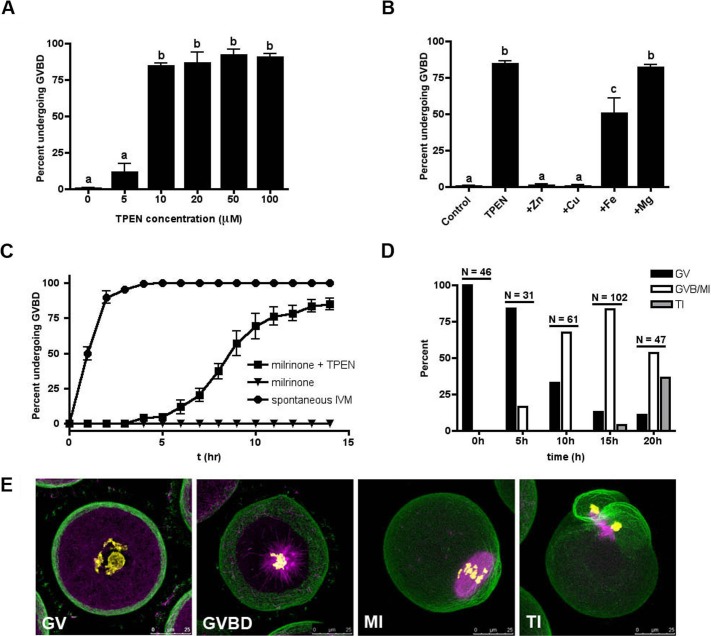
We first sought to investigate the functional significance of zinc regulation during the first meiotic arrest at PI. Because oocytes released from their follicular environment spontaneously undergo meiotic maturation, the phosphodiesterase (PDE) 3 inhibitor milrinone was added to all culture conditions to prevent spontaneous maturation by inhibiting the degradation of cAMP. To determine whether zinc insufficiency could override the meiotic inhibitory effects of milrinone in maintaining PI arrest, fully grown DOs were cultured for 14 h in the presence of increasing concentrations of TPEN, a heavy metal chelator, and 10 μM of milrinone. As expected, oocytes cultured in milrinone maintained PI arrest as scored by the presence of an intact germinal vesicle (GV) (Fig. 1A; Supplemental Fig. S2A). In contrast, addition of 10 μM TPEN overrode the milrinone-maintained arrest, and approximately 85% of oocytes cultured in these conditions resumed meiosis, as evidenced by GVBD (Fig. 1A; Supplemental Fig. S2C). Immunofluorescence of the meiotic spindle confirmed that these oocytes progressed to form MI spindles (Supplemental Fig. S2C, inset). Quantification of GVBD rates revealed that concentrations of TPEN less than 10 μM were ineffective in causing premature meiotic resumption, while all concentrations higher than 10 μM resulted in approximately 90% GVBD (Fig. 1A). High TPEN concentrations, however, caused increasing cytoplasmic granularity and spindle disorganization in the oocyte (Supplemental Fig. S2, A–F); thus, the concentration of TPEN in subsequent experiments was limited to 10 μM. TPEN (10 μM) was also able to induce meiotic resumption in the presence of other well-characterized meiotic inhibitors, including the pan-PDE inhibitor IBMX, the PDE3-specific inhibitor cilostamide, and the cAMP analog dibutyryl-cAMP (Supplemental Fig. S3). This suggests that premature meiotic resumption as induced by zinc insufficiency bypasses the oocyte's physiological maturation mechanism, which requires a reduction in cAMP levels. In addition, we utilized DOs in these experiments to clearly visualize the nuclear status of the oocytes throughout culture, but also confirmed that TPEN could induce meiotic resumption in milrinone-held COCs (Supplemental Fig. S4; [28]).
FIG. 1. .
Zinc insufficiency induces meiotic resumption with prolonged meiosis I kinetics. A) The incidence of GVBD was scored following 14-h culture of DOs in the presence of milrinone and increasing concentrations of TPEN. B) To determine the zinc specificity of TPEN-mediated GVBD, medium containing milrinone and 10 μM TPEN was supplemented with equimolar concentrations of zinc, iron, copper, or magnesium. Oocytes were scored for incidence of GVBD at 14 h. C) The kinetics of GVBD were determined in DOs cultured for 14 h in medium without milrinone (i.e., in vitro matured; circles), medium containing milrinone (triangles), or medium containing milrinone and TPEN (squares). D) The specific meiotic stage of oocytes cultured in the presence of milrinone and TPEN was quantified by analysis of α-tubulin (magenta), F-actin (green), and chromatin (yellow) immunostaining. Oocytes were scored as GV, GVBD/MI, or TI according to the status of the cytoskeleton and the chromosome configuration. “N” represents the total number of oocytes examined at each time point. E) Representative confocal images of each category of oocytes are shown as Z stack projections of sections containing the spindle and/or chromatin. Bars = 25 μm. Experiments in A–C were conducted at least three times and the results represent the mean ± SEM. Between 30 and 60 oocytes were examined per group per experimental repeat. In A and B, statistical differences in the incidence of GVBD were calculated according to one-way ANOVA with Bonferroni post hoc test and labeled as indicated (P < 0.001).
TPEN binds multiple transition metals, with the highest binding affinities for copper, zinc, and iron. To determine whether the effect of TPEN is specifically due to zinc chelation or its effect on other metals, oocytes were cultured in milrinone-containing medium with 10 μM TPEN and equimolar amounts of zinc, copper, iron, or magnesium. Magnesium was included as a negative control due to its low affinity for TPEN [32]. Zinc and copper fully rescued PI arrest, while iron only partially rescued the phenotype (Fig. 1B). Supplementation with magnesium, as expected, did not rescue meiotic arrest. To test whether TPEN-induced meiotic resumption was a direct result of altered intracellular copper homeostasis, milrinone-treated oocytes were exposed to two independent membrane-permeable copper chelators. In the presence of milrinone, neither neocuproine nor tetrathiomolybdate caused GVBD at concentrations equivalent to TPEN (10 μM; Supplemental Fig. S1, C and D) or ten-fold higher (100 μM; data not shown). These results suggest that copper rescues the effects of TPEN by sequestering the chelator and preventing it from perturbing zinc, for which it has a lower affinity. Similarly, iron can also sequester the chelator, but due to TPEN's lower affinity for iron than for zinc, the rescue of meiotic arrest, as expected, was only partial. Taken together, the data indicate that zinc-dependent pathways play a crucial role in the maintenance of the first meiotic arrest at PI.
Oocytes that Undergo GVBD by TPEN Induction Have a Prolonged Meiosis I and Arrest at Telophase I
When oocytes are removed from milrinone-containing medium, spontaneous meiotic maturation occurs on a rapid timescale [33]. To determine the kinetics of TPEN-induced meiotic resumption, oocytes in culture were observed at 1-h intervals for up to 14 h. In contrast to spontaneously maturing oocytes, where GVBD occurs in over 90% of oocytes within 2 h of milrinone washout (Fig. 1C), those rendered zinc-insufficient progressed at a slower rate: only 50% of oocytes had undergone GVBD after 9 h of culture in milrinone and TPEN (Fig. 1C). As expected, oocytes cultured in the presence of milrinone alone remained arrested at PI (Fig. 1C).
To track TPEN-induced meiotic progression more precisely, milrinone-treated oocytes were fixed at 0, 5, 10, 15, and 20 h after continuous exposure to 10 μM TPEN and examined for chromatin, α-tubulin, and F-actin. Oocytes were categorized as GV, GVBD/MI, or TI based on spindle morphology and chromatin configuration (Fig. 1D; representative images shown in Fig. 1E). The majority of oocytes at 0 and 5 h after TPEN treatment remained arrested at PI (Fig. 1E, GV). At 10 h after TPEN treatment, 67% of oocytes had undergone GVBD: some had formed MI spindles (Fig. 1E, MI), while others were captured in the process of spindle formation, as visualized by the presence of condensed chromatin and nucleating microtubules (Fig. 1E, GVBD). By 15 h, most oocytes had reached the MI stage. No oocytes cultured in the presence of TPEN and milrinone reached the MII stage. In fact, 36% were found to have a TI-like microtubule conformation by 20 h. This is consistent with and reminiscent of results when oocytes were allowed to undergo IVM in the presence of TPEN without milrinone [16]. These results suggest at least two independent roles for zinc during meiotic maturation: first in maintaining PI arrest and later in establishing MII arrest.
The MOS-MAPK Pathway Is Prematurely Activated prior to GVBD in TPEN-Induced Meiotic Maturation
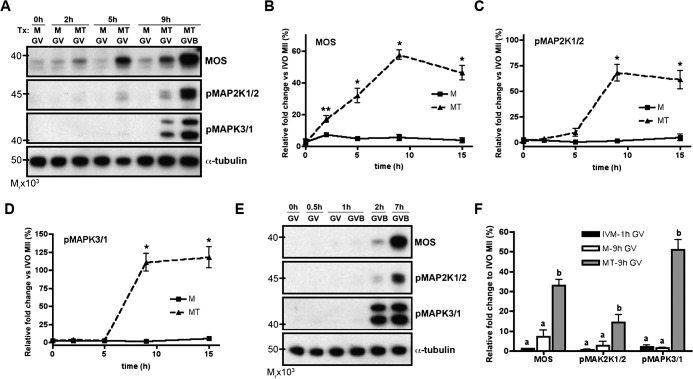
Given the known roles of the MOS-MAPK pathway during meiotic maturation, we investigated whether TPEN treatment affected the activation and function of this pathway at PI. While previous work excluded this pathway in mediating the effects of TPEN-induced TI arrest [23], its role during TPEN-induced meiotic resumption is largely unknown. Lysates obtained from oocytes treated with milrinone only (M, control) or milrinone and TPEN (MT) were subjected to immunoblotting for levels of MOS protein and the phosphorylated, activated forms of the downstream components MAP2K1/2 and MAPK3/1 (Fig. 2A). Compared to control oocytes in milrinone alone, which maintained low levels of MOS throughout culture, the levels of MOS in MT oocytes increased significantly within 2 h, continued to rise up to 9 h in culture, and remained elevated thereafter (Fig. 2B). All oocytes collected prior to 9 h for immunoblot analysis were GV intact. At 9 h, GV- and GVBD-stage oocytes were separated into individual samples, since approximately 50% had undergone GVBD by this time (Fig. 2C). Levels of the downstream components pMAP2K and pMAPK remained low until 9 h, at which time both the GV and GVBD samples showed increased phosphorylation of MAP2K1/2 and MAPK3/1 (Fig. 2, A, C, and D). Thus, all components of the MOS-MAPK pathway were activated during the timeframe of GVBD as induced by zinc insufficiency.
FIG. 2. .
TPEN-treated oocytes prematurely accumulate MOS and activate the MOS-MAPK pathway. A) Lysates from oocytes cultured in milrinone alone (M) or milrinone and TPEN (MT) were collected at the indicated time points and immunoblotted for MOS, phospho-MAP2K1/2, and phospho-MAPK3/1. B–D) Protein levels of MOS (B), phospho-MAP2K1/2 (C), and phospho-MAPK3/1 (D) ± SEM were quantified by densitometric analysis of at least three experiments normalized to α-tubulin. *P < 0.05, **P < 0.005 according to Student t-test, comparing TPEN-treated (MT) to untreated (M) oocytes at each time point. E) Lysates from oocytes were collected at the indicated time points during IVM for immunoblotting. Note that, as compared to TPEN-induced GVBD, protein levels of MOS, phospho-MAP2K1/2, and phospho-MAPK3/1 in in vitro matured oocytes do not increase until after GVBD has occurred. F) Blots similar to those represented in A and E were analyzed to compare the protein levels of GV intact oocytes collected after 1-h culture in IVM medium (black bars), 9-h culture in milrinone medium (white bars), and 9-h culture in milrinone-TPEN medium (grey bars). Analysis was performed by densitometry of at least three experiments and normalized to α-tubulin. For each protein, groups with uncommon letters indicate statistical differences in the protein level (P < 0.05). All immunoblots contain 20 oocytes per lane.
To determine whether the observed changes in MOS-MAPK activation were unique to TPEN-induced meiotic resumption or simply a consequence of the physiological sequence of events an oocyte undergoes during meiotic maturation, we next compared the time course of MOS-MAPK activation with respect to the onset of GVBD in control and TPEN-treated oocytes. Control oocytes were washed out of milrinone and matured in vitro. Since GVBD occurs rapidly during IVM (Fig. 1C), these samples were collected over a shorter time course relative to samples collected over the course of TPEN treatment. Notably, levels of MOS, pMAP2K1/2, and pMAPK3/1 were undetectable until after GVBD had occurred in oocytes undergoing IVM (Fig. 2E). This is consistent with previously published reports, which show little to no MOS-MAPK activation until after GVBD [4, 34]. In contrast, levels of these proteins rose before GVBD in TPEN-treated oocytes, as discussed above (Fig. 2A). To better compare the activation of the MOS-MAPK pathway between IVM and TPEN-treated oocytes, we plotted the levels of MOS, pMAP2K1/2, and pMAPK3/1 in GV-stage oocytes obtained after 1 h of culture in IVM medium or 9 h of culture in MT medium (Fig. 2F). These time points were selected based on the kinetics of GVBD: approximately 50% of oocytes remained GV intact (PI arrested) in their respective culture conditions at those times (Fig. 1C). For all three proteins evaluated, expression or phosphorylation levels were significantly higher in GV oocytes after 9 h culture in milrinone-TPEN medium than in GV oocytes after 1 h culture in IVM medium or 9 h culture in milrinone medium (Fig. 2F). In summary, components of the MOS-MAPK pathway are prematurely activated with zinc insufficiency, and may account for the phenotype observed, whereby TPEN overrides the first meiotic arrest to induce GVBD.
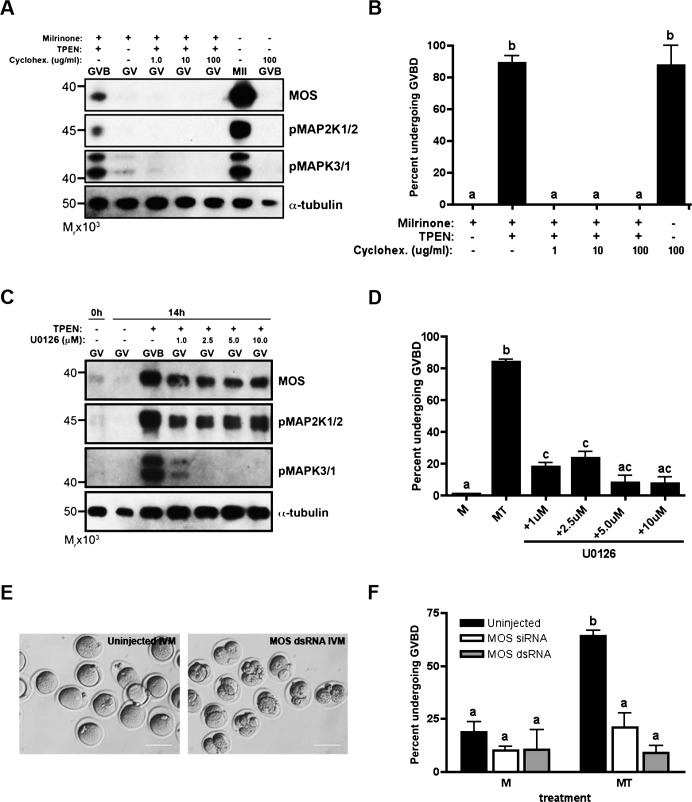
Inhibition of Protein Synthesis or Components of the MOS-MAPK Pathway Prevent TPEN-Induced Meiotic Resumption
The previous results suggest a possible role of the MOS-MAPK pathway in mediating the meiotic resumption effects of TPEN. Therefore, we tested whether inhibition of the pathway could prevent meiotic resumption and maintain PI arrest. Because translation is required for a rise in MOS protein levels, we first tested whether inhibition of protein synthesis could prevent meiotic resumption in the presence of TPEN. Oocytes were cultured in the presence of 10 μM milrinone and 10 μM TPEN with increasing concentrations of the global protein synthesis inhibitor cycloheximide for 14 h. Cycloheximide prevented activation of the MOS-MAPK pathway, as evidenced by a lack of expression of MOS, pMAP2K1/2, and pMAPK3/1 on immunoblot (Fig. 3A). At all concentrations tested, cycloheximide completely inhibited TPEN-induced meiotic resumption in the presence of milrinone (Fig. 3B). In comparison, cycloheximide did not prevent meiotic resumption in oocytes cultured under control IVM conditions, consistent with previous reports showing that the initiation of meiotic maturation, namely GVBD, is protein synthesis independent in the mouse oocyte [35–37].
FIG. 3. .
TPEN-induced meiotic resumption is dependent on activation of the MOS-MAPK pathway. Global protein synthesis was inhibited by addition of cycloheximide to culture (A and B). The MOS-MAPK was specifically inhibited by treatment with U0126 (C and D), or microinjection of Mos hairpin dsRNA or siRNA (E and F). A and C) Lysates obtained from TPEN-treated and in vitro-matured oocytes cultured for 14 h in the presence of cycloheximide (A) or U0126 (C) were immunoblotted for MOS, phospho-MAP2K1/2, and phospho-MAPK3/1 expression. Each lane contains 20 oocytes. B and D) The incidence of GVBD after cycloheximide (B) or U0126 (D) treatment was scored by light microscopy. E) Oocytes were injected with MOS hairpin dsRNA, held for 10–14 h, and in vitro matured. Functionality of dsRNA injection was determined by visualization of parthenogenetic activation after 36-h culture. Bars = 80 μm. F) Oocytes injected with MOS hairpin dsRNA or MOS siRNA were held for 10–14 h and either maintained in milrinone or transferred to medium containing milrinone and TPEN for an additional 14 h. The incidence of GVBD was scored. In B, D, and F, experiments were performed at least three times and the results represent the mean ± SEM. Between 30 and 60 oocytes were analyzed per group per experimental repeat. Groups with uncommon letters indicate statistical differences in the percentage of oocytes undergoing GVBD (P < 0.001). M, milrinone only; MT, milrinone and TPEN.
To specifically inhibit the MOS-MAPK pathway, we used two approaches: treatment with the MAP2K1/2 inhibitor U0126, and knockdown by microinjection of Mos siRNA or dsRNA. Oocytes cultured in the presence of U0126, milrinone, and TPEN for 14 h showed decreased phosphorylation of the downstream target MAPK3/1 on immunoblot (Fig. 3C), confirming the function of the pharmacological inhibitor. At all concentrations tested, U0126 treatment significantly reduced the percentage of oocytes undergoing GVBD in the presence of milrinone and TPEN to less than 25% (Fig. 3D).
Finally, as a nonpharmacological approach to the inhibition of MOS-MAPK activation, we attempted to knockdown the Mos transcript in oocytes prior to TPEN exposure. To test knockdown efficiency, oocytes were injected with in vitro-transcribed Mos hairpin dsRNA [31] and held for 10–14 h to allow transcript degradation, followed by IVM. Uninjected oocytes progressed to and arrested at the MII stage even after 36 h in IVM culture, while Mos dsRNA-injected oocytes parthenogenetically activated, as evidenced by pronuclear (PN) formation and cleavage (Fig. 3E). This result confirmed functional knockdown of Mos [38]. To test the effect of Mos knockdown on TPEN-induced meiotic resumption, oocytes injected with Mos hairpin dsRNA were cultured as described above, then either maintained in milrinone medium or transferred to milrinone-TPEN medium for an additional 14 h of culture. Compared to uninjected oocytes cultured in milrinone and TPEN, in which 64% underwent GVBD, the percentage of oocytes that underwent GVBD was reduced by seven-fold after Mos dsRNA injection. Similar results were obtained with injection of Mos siRNA, which showed a three-fold reduction in GVBD frequency with milrinone-TPEN treatment. The percentage of oocytes undergoing GVBD in milrinone medium was not significantly different between uninjected and injected oocytes. Taken together, these results show that the maintenance of meiotic arrest at PI requires the zinc-dependent inhibition of the MOS-MAPK pathway.
Transient Zinc Insufficiency Can Trigger GVBD and Allow Meiotic Maturation to Progress to MII
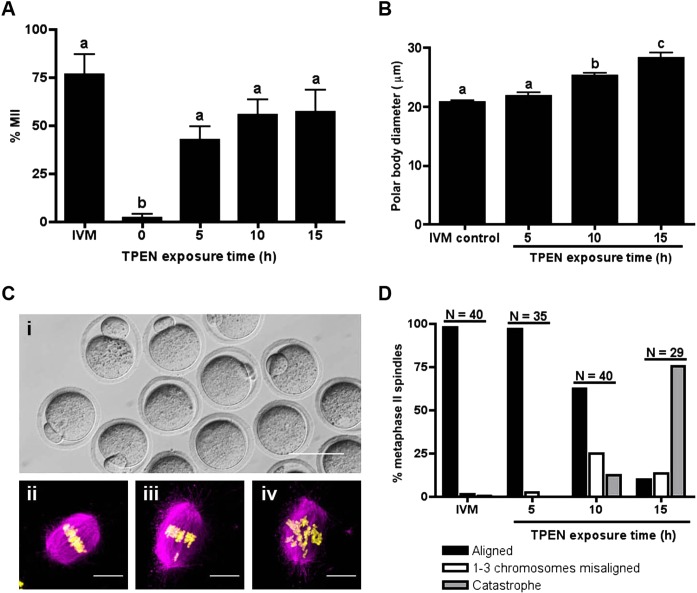
Given that long-term exposure to TPEN in the presence of meiotic inhibitors initiated meiotic resumption in oocytes, but not to completion, we investigated whether a transient exposure to TPEN would suffice to overcome PI arrest and allow normal progression to MII. To test this hypothesis, oocytes held in the presence of milrinone were exposed to 10 μM TPEN for 5, 10, or 15 h, then washed and cultured in TPEN-free medium with milrinone for a total of 24 h. Of oocytes cultured in milrinone and TPEN medium for 5 h, 44% progressed to the MII stage (Fig. 4A), as scored by extrusion of the first PB. Oocytes cultured in TPEN for 10 and 15 h showed PB formation in 55% and 57% of oocytes, respectively (Fig. 4A), though significantly larger PBs were noted as compared to IVM eggs (Fig. 4B). To further analyze egg quality, oocytes that had a visible PB by light microscopy (Fig. 4C, i) were fixed and stained to visualize spindle morphology. Chromosomes on the metaphase plate were categorized as aligned (Fig. 4C, ii), one to three misaligned (Fig. 4C, iii), or catastrophe (more than three chromosomes misaligned; Fig. 4C, iv). We found an increasing incidence and severity of chromosome misalignment with longer TPEN exposure time (Fig. 4D). Thus, by transiently inducing zinc insufficiency during the first meiotic arrest at PI, we show that these oocytes have the capacity to successfully complete the maturation program and arrest at MII.
FIG. 4. .
Transient TPEN exposure permits meiotic progression to MII. A) Oocytes were cultured in milrinone-TPEN medium for the indicated times and transferred to medium without TPEN for a total of 24 h. Progression to MII was assessed by scoring for the presence of a PB. The experiment was repeated three times and the results represent the mean ± SEM. Approximately 30 oocytes were used per group per experiment. B) PB diameter was measured by taking the average of two perpendicular lengths and compared among treatment groups. Results represent the mean ± SEM. C, i) A representative image of MII eggs obtained from TPEN exposure followed by culture in milrinone. C, ii–iv) To determine the quality of the MII spindle, eggs that had a visible PB by light microscopy were fixed and stained for α-tubulin (magenta) and chromatin (yellow). Chromosomes on the metaphase plate were categorized as aligned (i), one to three misaligned (ii), or catastrophe (iii). Representative confocal images of each category are shown as Z stack projections. Bar in i = 80 μm; bars in ii–iv = 10 μm. D) Chromosome alignment was scored according to the categories described in C. “N” represents the total number of eggs examined for each culture condition. Only spindles oriented approximately parallel to the plane of the Z stacks were scored. In A and B, groups with uncommon letters indicate statistical differences (P < 0.01).
MII Eggs Collected after Transient TPEN Exposure Are Developmentally Competent
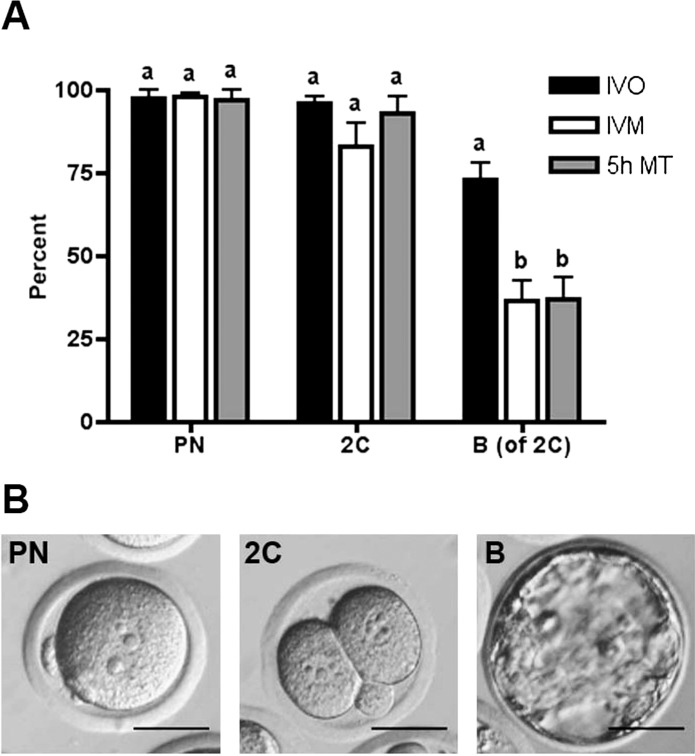
Subsequently, we tested whether MII eggs obtained after TPEN exposure were capable of supporting early embryonic development. MII eggs matured after a 5-h exposure to 10 μM TPEN were chosen for these experiments, because they had PB sizes comparable to control IVM eggs (Fig. 4B) and proper alignment of chromosomes on the meiotic spindle (Fig. 4C). After a 5-h culture in milrinone and TPEN medium followed by a 1-h culture in milrinone-only medium, oocytes that had undergone GVBD were selected and matured in vitro for an additional 14 h. Eggs reaching the MII stage as visualized by the presence of PBs were parthenogenetically activated and scored for progression to the PN, two-cell (2C), and blastocyst stages (Fig. 5). Eggs matured after transient TPEN and milrinone exposure progressed to the PN and 2C stages with 97% and 95% efficiency, respectively (Fig. 5A), similar to the developmental competency of eggs obtained by IVM or IVO. Of 2Cs obtained after TPEN exposure, 37% developed to blastocysts. While lower than the 73% blastocyst formation rate of IVO eggs, this percentage is comparable to the 37% blastocyst rate of IVM eggs, suggesting that MII eggs obtained after early zinc modulation acquire the necessary machinery to undergo preimplantation embryo development.
FIG. 5. .
MII eggs derived after TPEN exposure are developmentally competent. MII eggs obtained after transient TPEN exposure followed by a 14-h culture in IVM medium were parthenogenetically activated. A) Following parthenogenetic activation, the preimplantation developmental progression of eggs derived from TPEN-treatment (5h MT; gray bars), in vivo ovulation (black bars), and IVM (white bars) was scored. Specifically, the progression to pronuclear (PN), two-cell (2C), and blastocyst stage (B) were analyzed by light microscopy, and representative images are shown in B. The percentages of PN and 2C were calculated based on total number of MII eggs. The percentage of blastocysts was calculated based on number of 2C embryos. The experiment was performed three times and results represent the mean ± SEM. Between 20 and 40 eggs were used per group per experiment. For each embryo stage, groups with uncommon letters indicate statistical differences (P < 0.05). Bars = 40 μm.
DISCUSSION
We report here the necessity of zinc availability in maintaining the first meiotic arrest at PI. Oocytes cultured under zinc-insufficient conditions, as induced by addition of the heavy metal chelator TPEN, resume meiosis even in the presence of potent meiotic inhibitors. This phenotype is mediated by activation of the MOS-MAPK pathway, and is regulated by a previously unappreciated zinc-dependent mechanism. Finally, developmentally competent MII eggs with blastocyst formation rates comparable to IVM eggs are obtained after early and transient zinc modulation, indicating the physiological relevance of the oocyte's meiotic zinc signal, and, as discussed below, its potential clinical utility.
In a physiological setting, the oocyte accrues 20 billion atoms of zinc during meiotic maturation, and subsequently releases a fraction of its total zinc content during intense exocytosis events termed “zinc sparks” [16, 27]. Such a large, nonpathological change in transition metal content has not been described in any other cell type. Thus, it is of particular interest that the oocyte should harness the power of the transition metal zinc as a signaling ligand to drive meiotic maturation. Because the oocyte maintains a transcriptionally quiescent state throughout the entirety of maturation [39], an inorganic regulator like zinc provides the missing link in the carefully orchestrated meiotic progression pathway.
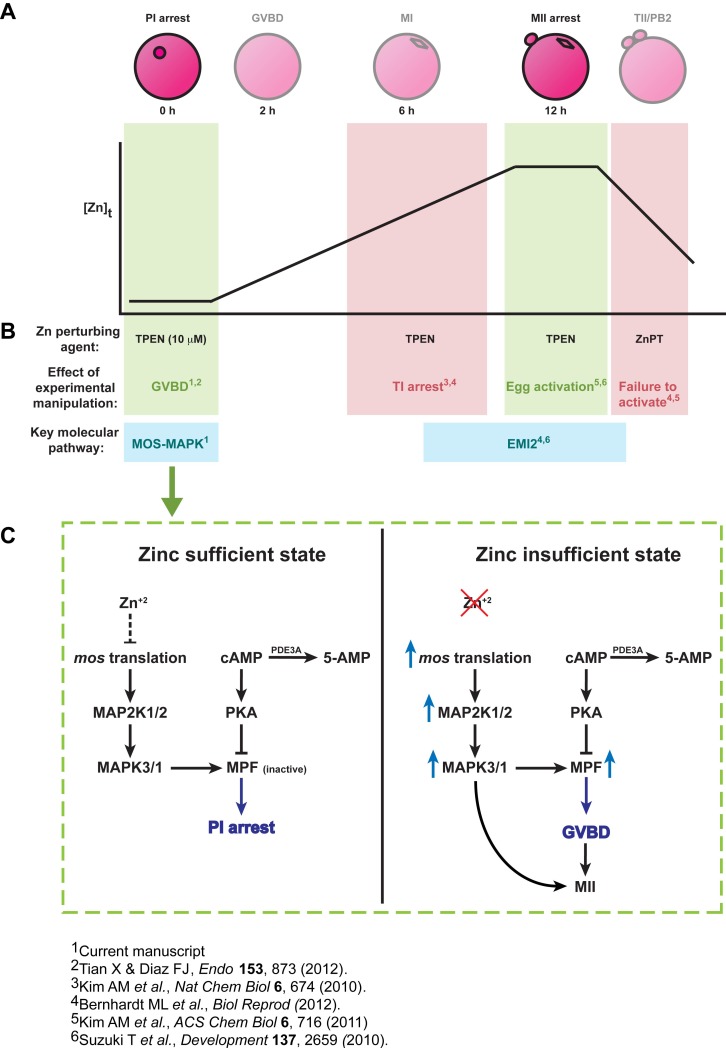
Indeed, several reports over the past 2 yr have elucidated crucial regulatory functions of zinc during both PI and MII arrest in mammalian oocytes. Results described in this article show that zinc-insufficient oocytes escape the first meiotic arrest at PI and resume meiosis prematurely, albeit on a slower time course than oocytes undergoing IVM. These results are consistent with previously reported data, which show zinc-dependent regulation of the periovulatory period [28]. Several lines of evidence have also established zinc-dependent mechanisms during the later events of meiosis surrounding the establishment, maintenance, and exit from MII. Preventing zinc uptake during IVM, for example, even if initiated as late as MI, leads to premature meiotic arrest and spindle defects [16, 25]. Preventing the decline in intracellular zinc after MII exit causes the reformation of a metaphase spindle [27]. Finally, MII eggs cultured under zinc-insufficient conditions fail to maintain MII arrest, and undergo parthenogenetic egg activation robust enough to support live births in mice [26]. These data suggest that zinc dynamics sensitively regulate the progression of meiotic maturation, particularly at the transitions surrounding each physiological meiotic arrest point (Fig. 6A). Alteration of zinc content during any of these transitions results in profound disturbances to the oocyte's maturation program (Fig. 6A). All together, this suggests very precise mechanisms by which zinc temporally exerts its effects, likely correlated to the “on” or “off” status of various pathways necessary to achieve proper meiotic progression.
FIG. 6. .
A) Total cellular zinc ([Zn]t) increases by 50% during meiotic maturation from the PI arrested oocyte to the MII arrested egg, and then drops upon fertilization in events described as zinc sparks. B) Perturbation of zinc availability using the heavy metal chelator TPEN or the zinc ionophore ZnPT at critical windows of meiotic maturation and fertilization interferes with specific zinc-dependent pathways, resulting in premature arrest or progression through the maturation pathway. Decreasing zinc availability using TPEN at the two crucial arrest points, namely PI and MII, results in premature meiotic progression (green shading). Oocytes that undergo GVBD due to zinc insufficiency have the capacity to develop to MII eggs upon return to zinc-replete medium and blastocysts upon egg activation. This effect of zinc insufficiency in causing meiotic resumption from PI arrest is mediated via the MOS-MAPK pathway. As described in Supplemental Figure S1, experimental manipulation of zinc levels before or after MII arrest, when zinc is actively being acquired or released, respectively, results in meiotic arrest (red shading). C) Model showing proposed action of a zinc-mediated pathway during the first meiotic arrest at PI. In a zinc-sufficient state, the MOS-MAPK pathway is maintained in a quiescent state, MPF activity remains low, and the oocyte remains arrested at the PI stage. Zinc insufficiency, as induced by chelation, relieves the inhibition on the MOS-MAPK pathway, allowing meiotic resumption, GVBD, and progression toward MII.
Intriguingly, lowering zinc availability at either meiotic arrest point causes the meiotic program to progress forward. As mentioned in the Introduction, this premature progression from MII arrest appears to involve EMI2. While EMI2 is not present during PI arrest, early meiosis inhibitor 1 (EMI1), the mitotic homolog of EMI2, is active. However, a parallel mechanism of zinc insufficiency causing inactivation of EMI1 would not explain the premature progression from PI arrest observed with TPEN treatment. In fact, EMI1 depletion in oocytes delays GVBD and prevents MI spindle formation [40]. Thus, the meiotic resumption observed with zinc insufficiency likely act through separate pathways at the two arrest points.
In investigating a mechanism for TPEN-induced meiotic resumption from PI arrest, we observed that the MOS-MAPK pathway was prematurely activated. MOS overexpression in GV-stage oocytes allows meiotic maturation to progress in the presence of pharmacological meiotic inhibitors, albeit with slower maturation kinetics than that of IVM [12]. Similarly, the kinetics of meiotic resumption observed with TPEN treatment in the presence of milrinone are also slower than in control IVM. Thus, we hypothesized that the premature activation of the MOS-MAPK pathway in TPEN-treated oocytes caused the GVBD phenotype observed.
This hypothesis is supported by experiments described here in which inhibition of the MOS-MAPK pathway significantly decreases the ability of TPEN to induce meiotic resumption. The addition of the global protein synthesis inhibitor cycloheximide completely abrogates the ability of TPEN to cause GVBD, consistent with a requirement for MOS protein synthesis in mediating the TPEN-induced meiotic resumption. Cycloheximide does not, however, prevent GVBD in oocytes undergoing IVM. This difference suggests that cycloheximide prevents TPEN-induced meiotic resumption as a direct result of inhibiting protein synthesis, and not due to inhibition of the physiological machinery that normally allows GVBD to occur. Similarly, targeted inhibition of the MOS-MAPK pathway using U0126 or Mos siRNA significantly decreased the ability of TPEN to induce meiotic resumption. Interestingly, while immunoblot of U0126-treated oocytes confirmed decreased phosphorylation of the downstream target MAPK3/1, levels of the upstream components MOS and pMAP2K1/2 were high compared to freshly isolated or milrinone-held oocytes. In all conditions, the oocytes are arrested at PI and remain GV intact. This indicates that TPEN-induced GVBD proceeds via full activation of the MOS-MAPK pathway, since high expression of the upstream components are insufficient to induce GVBD in the absence of MAPK phosphorylation. While other substrates besides MAP2K have been proposed to be direct targets for MOS phosphorylation [21, 41, 42], these are unlikely to mediate TPEN-induced meiotic resumption given the above results. Taken together, these experiments clearly indicate that the primary mechanism through which zinc insufficiency induces meiotic resumption from PI arrest is through premature activation of the MOS-MAPK pathway (Fig. 6B).
This is a newly discovered mechanism by which zinc homeostasis regulates the first meiotic arrest via MOS expression, although how zinc triggers this pathway is not yet known. Because translation of Mos mRNA is controlled, in part, through polyadenylation, one explanation may be that a certain threshold level of zinc is required for the maintenance of translational quiescence. While the biochemical events that govern polyadenylation in mouse oocytes are not well characterized, the possibility exists that one or more of these regulators may be zinc-binding proteins that normally repress translation. Zinc insufficiency would therefore prevent the function of these repressors and cause activation of otherwise dormant translational pathways. Further studies are required to dissect the underlying relationship between zinc availability and translational regulation in the mouse oocyte.
While our data definitively support a link between zinc availability and MOS expression in mediating PI arrest, we previously reported that an MOS-independent pathway is responsible for the premature TI arrest induced by TPEN during IVM [23]. This further suggests that two separate zinc-dependent mechanisms regulate the two physiological meiotic arrest points at PI and MII. During IVM, the oocyte rapidly undergoes GVBD, and thus any effect of TPEN in abrogating PI arrest would be masked by the rapid kinetics of the oocyte's endogenous maturation mechanisms. Thus, the only effect of TPEN noticeable during IVM is its effect toward the end of meiotic maturation, when the oocytes fail to progress to MII. In the presence of milrinone or other meiotic inhibitors, we unmasked the effects of TPEN during the first meiotic arrest, since the endogenous maturation pathways are inhibited. In this situation, oocytes undergo GVBD, but also fail to progress to MII, stalling at TI. Thus, while zinc levels control both two meiotic arrest points, different cell cycle regulatory mechanisms mediate the effects of zinc.
Indeed, by transiently exposing oocytes to TPEN in the presence of milrinone, we were able to isolate the effect of zinc insufficiency during PI arrest from that during MII arrest. Eggs collected from a 5-h TPEN exposure followed by IVM had rates of parthenogenetic activation to the PN, two-cell, and blastocyst stages that were nearly identical to those of IVM eggs. While the blastocyst rate was less than that from eggs obtained by IVO, it is consistent with previous reports that the developmental competency of IVM eggs is less than that of IVO eggs following egg activation [43]. These results suggest that isolating the effect of TPEN in inducing GVBD initiates a robust meiotic maturation program, and that the zinc-dependent pathways required for establishing MII arrest are correctly accessed. Furthermore, the capacity to obtain developmentally competent MII eggs by modulating zinc levels at PI could be utilized in a clinical setting to improve IVM, an increasingly utilized form of assisted reproduction technology worldwide [44]. While IVM does not require the high levels of ovarian hyperstimulation that are often used in IVF cycles, which can be costly and cause major side effects, the rates of embryo formation and live births after IVM remain significantly lower in comparison to IVF [45, 46]. Thus, the modulation of zinc during the first meiotic arrest may be a particularly useful approach to assist the initiation of the meiotic maturation program and increase the total number of developmentally competent oocytes obtained.
In a physiological setting, nutritional zinc deficiency in humans is a rare but recognized disease, with associated symptoms affecting multiple organ systems, including the reproductive organs (reviewed in [47]). A recent study found that acute exposure to a zinc-deficient diet can cause infertility due to premature GVBD and decreased ovulation in mice [28]. How the oocyte regulates its zinc content in a physiological context, however, is still largely unknown. One possibility is the presence and activity of particular zinc transporters, which are sensitive regulatory mechanisms by which zinc homeostasis is tightly maintained within the body. In mammalian species, 24 zinc transporters are known to modulate zinc concentration in the cell, (reviewed in [48]), suggesting their importance and functional redundancy. It is currently unknown which of these transporters are active and regulated in the oocyte, particularly during the period of meiotic maturation and egg activation.
In summary, we propose a novel mechanism by which zinc availability maintains meiotic arrest at PI by inhibiting the premature activation of the MOS-MAPK pathway. Transient zinc modulation limited to overcoming the first meiotic arrest not only allowed successful completion of maturation and formation of MII eggs, but also produced early embryos upon parthenogenetic activation. Coupled with our previous results, our study positions zinc in the center of an essential and unifying regulatory mechanism controlling the precise timing of meiosis.
ACKNOWLEDGMENT
The authors thank Paula Stein and Richard Schultz for the generous gift of the Mos hairpin inverted repeat expression vector. They also thank Rafael Fissore for insightful discussions on egg activation, and Francesca Duncan, Thomas McGarry, and Emily Que for advice and critical reading of the manuscript. They gratefully acknowledge Dragan Mackovic for providing animal care and Katy Ebbert for providing technical assistance.
Footnotes
Supported by National Institutes of Health grant P01 HD021921 and a Medical Research Award from the W. M. Keck Foundation.
REFERENCES
- Tsafriri A. Mammalian oocyte maturation: model systems and their physiological relevance. Adv Exp Med Biol 1979; 112: 269 281 [DOI] [PubMed] [Google Scholar]
- Josefsberg LB, Galiani D, Lazar S, Kaufman O, Seger R, Dekel N. Maturation-promoting factor governs mitogen-activated protein kinase activation and interphase suppression during meiosis of rat oocytes. Biol Reprod 2003; 68: 1282 1290 [DOI] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol 2002; 187: 153 159 [DOI] [PubMed] [Google Scholar]
- Paules RS, Buccione R, Moschel RC, Vande Woude GF, Eppig JJ. Mouse Mos protooncogene product is present and functions during oogenesis. Proc Natl Acad Sci U S A 1989; 86: 5395 5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell 1993; 4: 781 790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn NG, Vande Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol 1993; 13: 2546 2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N, Daar I, Oskarsson M, Showalter SD, Vande Woude GF. The product of the mos proto-oncogene as a candidate “initiator” for oocyte maturation. Science 1989; 245: 643 646 [DOI] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J 1994; 13: 2131 2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol 1995; 168: 677 682 [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, Aizawai S. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 1994; 370: 68 71 [DOI] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 1994; 370: 65 68 [DOI] [PubMed] [Google Scholar]
- Choi T, Rulong S, Resau J, Fukasawa K, Matten W, Kuriyama R, Mansour S, Ahn N, Vande Woude GF. Mos/mitogen-activated protein kinase can induce early meiotic phenotypes in the absence of maturation-promoting factor: a novel system for analyzing spindle formation during meiosis I. Proc Natl Acad Sci USA 1996; 93: 4730 4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Kubiak JZ, Umbhauer M, Rassinier P, Colledge W, Maro B. Mos activates MAP kinase in mouse oocytes through two opposite pathways. The EMBO Journal 2000; 19: 6065 6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 1989; 342: 512 518 [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science 1993; 262: 1262 1265 [DOI] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 2010; 6: 674 681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 1996; 272: 1013 1016 [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci 1998; 21: 347 375 [DOI] [PubMed] [Google Scholar]
- Watjen W, Haase H, Biagioli M, Beyersmann D. Induction of apoptosis in mammalian cells by cadmium and zinc. Environ Health Perspect 2002; 110 (Suppl 5): 865 867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod 1996; 55: 1315 1324 [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Géraud G, Colledge WH, Evans MJ, Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 1996; 122: 815 822 [DOI] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci U S A 1996; 93: 7032 7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc requirement during meiosis I-meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol Reprod 2011; 84: 526 536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol 2006; 174: 791 801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TKA. Zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 2012; [DOI] [PMC free article] [PubMed]
- Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry ACF. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development 2010; 137: 2659 2669 [DOI] [PubMed] [Google Scholar]
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O'Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol 2011; 6: 716 723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 2011; 153: 873 886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680 685 [DOI] [PubMed] [Google Scholar]
- Kim MH, Yuan X, Okumura S, Ishikawa F. Successful inactivation of endogenous Oct-3/4 and c-mos genes in mouse preimplantation embryos and oocytes using short interfering RNAs. Biochem Biophys Res Commun 2002; 296: 1372 1377 [DOI] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Schultz RM. RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem Biophys Res Commun 2001; 287: 1099 1104 [DOI] [PubMed] [Google Scholar]
- Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem 1985; 260: 2719 2727 [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 1965; 208: 349 351 [DOI] [PubMed] [Google Scholar]
- Verlhac MH, de Pennart H, Maro B, Cobb MH, Clarke HJ. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol 1993; 158: 330 340 [DOI] [PubMed] [Google Scholar]
- Schultz RM, Wassarman PM. Specific changes in the pattern of protein synthesis during meiotic maturation of mammalian oocytes in vitro. Proc Natl Acad Sci U S A 1977; 74: 538 541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM, Wassarman PM. Biochemical studies of mammalian oogenesis: protein synthesis during oocyte growth and meiotic maturation in the mouse. J Cell Sci 1977; 24: 167 194 [DOI] [PubMed] [Google Scholar]
- Moor RM, Crosby IM. Protein requirements for germinal vesicle breakdown in ovine oocytes. J Embryol Exp Morphol 1986; 94: 207 220 [PubMed] [Google Scholar]
- Stein P, Svoboda P, Schultz RM. Transgenic RNAi in mouse oocytes: a simple and fast approach to study gene function. Dev Biol 2003; 256: 187 193 [DOI] [PubMed] [Google Scholar]
- Moore GP, Lintern-Moore S. Transcription of the mouse oocyte genome. Biol Reprod 1978; 18: 865 870 [DOI] [PubMed] [Google Scholar]
- Marangos P, Verschuren EW, Chen R, Jackson PK, Carroll J. Prophase I arrest and progression to metaphase I in mouse oocytes are controlled by Emi1-dependent regulation of APC(Cdh1). J Cell Biol 2007; 176: 65 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy LM, Singh B, Gautier J, Arlinghaus RB, Nordeen SK, Maller JL. The cyclin B2 component of MPF is a substrate for the c-mos(xe) proto-oncogene product. Cell 1990; 61: 825 831 [DOI] [PubMed] [Google Scholar]
- Zhou RP, Shen RL. Pinto da Silva P, Vande Woude GF. In vitro and in vivo characterization of pp39mos association with tubulin. Cell Growth Differ 1991; 2: 257 265 [PubMed] [Google Scholar]
- Nogueira D, Cortvrindt R, De Matos DG, Vanhoutte L, Smitz J. Effect of phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitro. Biol Reprod 2003; 69: 2045 2052 [DOI] [PubMed] [Google Scholar]
- Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril 2006; 86: 1277 1291 [DOI] [PubMed] [Google Scholar]
- Child TJ, Phillips SJ, Abdul-Jalil AK, Gulekli B, Tan SL. A comparison of in vitro maturation and in vitro fertilization for women with polycystic ovaries. Obstet Gynecol 2002; 100: 665 670 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ, Wigglesworth K, Nicholson A, Zhang W, King BA. Effect of in vitro maturation of mouse oocytes on the health and lifespan of adult offspring. Hum Reprod 2009; 24: 922 928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS. Zinc: an overview. Nutrition 1995; 11: 93 99 [PubMed] [Google Scholar]
- Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 2009; 29: 153 176 [DOI] [PubMed] [Google Scholar]