ABSTRACT
Sphingosine is a structural component of sphingolipids. The metabolism of phosphoethanolamine ceramide (sphingomyelin) by sphingomyelinase (SMase), followed by the breakdown of ceramide by ceramidase (CDase) yields sphingosine. Female tsetse fly is viviparous and generates a single progeny within her uterus during each gonotrophic cycle. The mother provides her offspring with nutrients required for development solely via intrauterine lactation. Quantitative PCR showed that acid smase1 (asmase1) increases in mother's milk gland during lactation. aSMase1 was detected in the milk gland and larval gut, indicating this protein is generated during lactation and consumed by the larva. The higher levels of SMase activity in larval gut contents indicate that this enzyme is activated by the low gut pH. In addition, cdase is expressed at high levels in the larval gut. Breakdown of the resulting ceramide is likely accomplished by the larval gut-secreted CDase, which allows absorption of sphingosine. We used the tsetse system to understand the critical role(s) of SMase and CDase during pregnancy and lactation and their downstream effects on adult progeny fitness. Reduction of asmase1 by short interfering RNA negatively impacted pregnancy and progeny performance, resulting in a 4–5-day extension in pregnancy, 10%–15% reduction in pupal mass, lower pupal hatch rates, impaired heat tolerance, reduced symbiont levels, and reduced fecundity of adult progeny. This study suggests that the SMase activity associated with tsetse lactation and larval digestion is similar in function to that of mammalian lactation and represents a critical process for juvenile development, with important effects on the health of progeny during their adulthood.
Keywords: ceramide, Glossina, lactation, milk, sphingomyelin
Sphingomyelinase (SMase) is generated during tsetse fly lactation, but is only activated by acidic conditions within the larval gut contents; reduced SMase levels in tsetse milk leads to impaired progeny development and health.
INTRODUCTION
Sphingolipids are critical components of cell membranes of eukaryotes and some bacteria [1–5]. These lipids not only are necessary for cell membrane integrity, but are critical for cell signaling [1–5]. Sphingomyelinases (SMases) catalyze the hydrolysis of phosphocholine (PC)-ceramide (mammalian sphingomyelin) to phosphocholine and ceramide in mammals [1–3]. Following the breakdown of sphingomyelin, ceramide can be metabolized to sphingosine and free fatty acid by ceramidase (CDase) [6, 7]. The presence of SMase and CDase during digestion is critical for the provision of sphingomyelin-based lipids from food sources for cell membrane development and sphingosine-based cell signaling processes [3, 8, 9].
In humans, defects in SMase encoding genes causes Niemann-Pick disease, a neurodegenerative disorder resulting in death before 3 years of age in severe cases [2, 10, 11]. Insects differ from mammals in that SMase hydrolyzes phosphoethanolamine (PE)-ceramide (insect sphingomyelin) to yield PE and ceramide. Although the substrate differs between the mammalian and insect SMases in the chemical moiety attached to the ceramide, the basic structure and function of the SMase enzymes and their metabolic products are similar [3]. Thus, insects could serve as model systems for studying metabolic diseases related to sphingomyelinase deficiency [2, 3].
SMases are classified according to the pH at which they are activated; acid SMases (aSMases) function at low pH, neutral SMases (nSMases) near pH 7, and alkaline SMase near pH 9 [2, 3]. Few studies have addressed the role of SMases in relation to insect physiology [3]. In Drosophila, asmases and nsmase are widely expressed during embryonic development [1]. Microarray studies revealed that an asmase (CG15533) and neutral cdase are downregulated in Drosophila after sugar feeding and may play a role in suppressing fatty acid synthesis [12].
In tsetse (Glossina morsitans morsitans), lipid metabolism plays a major role in the reproductive cycle [13–16]. Tsetse reproduction is viviparous. The female tsetse carries a single intrauterine larva and the only source of essential nutrients for immature development is female milk products generated from the accessory gland. This is drastically different than most insects, which are oviparous, and deposit many eggs during each gonotrophic cycle. About 50% of the nutrients in the tsetse milk are lipids [13–16]. The evolution of viviparity has driven dramatic adaptations in the tsetse's reproductive physiology [17]. These modifications include reduced ovary capacity and production (a single oocyte maturing during each gonotrophic cycle), increased uterine capacity (capable of accommodating a developed third instar larva equal in mass to the mother), and expansion of the female accessory gland into a lactating organ (milk gland) to provision nourishment to the intrauterine progeny via its secretions [17–20]. The first progeny is deposited 20–22 days post-adult eclosion, with each subsequent larva being deposited every 10–11 days thereafter [17]. This slow reproductive rate limits tsetse mothers to 8–12 births of single progeny in their lifetime [17]. Thus, interference with tsetse pregnancy could drastically reduce tsetse populations.
The only source of nutrients for adult tsetse is vertebrate blood. Blood is extremely rich in some nutrients such as proteins and lipids, but nearly devoid of essential vitamins and other micronutrients. These lacking nutrients are provided to tsetse flies by their obligate symbiont, Wigglesworthia glossinidia [21]. Specifically, this bacterium is thought to produce B vitamins that are critical for many biochemical processes [21]. It is important to note that presence of this symbiont is critical to tsetse reproduction because elimination of the bacteria via antibiotic treatment leads to a significant reduction in fecundity [22].
Lactation in tsetse and mammals has evolved independently, but essential requirements inherent to the lactation process appear to be conserved between the two systems. These include the development of specialized secretory cells in the lactating tissue, high lipid content and composition of the milk, functional conservation of milk proteins (i.e., lactoferrin and lipocalins in mammals and transferrin and milk gland protein in tsetse, respectively), and transfer of beneficial symbiotic bacteria from mother to progeny via milk secretions [19, 23–31]. Thus, tsetse could represent a novel model system for the study of the mechanisms underlying lactation.
Previous work showed that mammalian milk secretions contain an activated SMase function, which is stimulated during digestion in newborns [7, 32, 33]. Here, we first analyze SMase function(s) during tsetse fly pregnancy and lactation to determine if this enzyme is fulfilling an orthologous function. We report on the spatial and temporal expression of smases and cdases throughout tsetse pregnancy. We next use an RNA interference-based gene silencing approach to evaluate the functional roles of these enzymes in pregnancy and the downstream impact of their modification on the health of the progeny. Our findings suggest that the presence of SMase in tsetse milk is critical for the provision of essential sphingosine products to intrauterine larva during development. Modification in SMase activity within tsetse milk negatively impacts female fecundity and the health of progeny. We discuss the role of tsetse as an important invertebrate model with which to study the role of sphingolipids and SMases during lactation.
MATERIALS AND METHODS
Flies
Glossina morsitans morsitans, utilized at Yale University and the Slovak Academy of Sciences, originated from a population collected in Zimbabwe and had been in culture for at least 20 years. Flies were maintained at 25°C and 50%–60% RH using an artificial membrane feeding system [34]. Females were mated 3–5 days after emergence. Flies were collected according to established developmental markers based on oocyte, embryo, and larva presence [29, 34].
Phylogenetic Analysis of Tsetse smase and cdase
BLASTX analysis of the tsetse genome using Drosophila sequences was utilized to identify the tsetse genomic scaffold containing the smase and cdase sequences. Full length sequences were obtained by mapping Illumina high-throughput reads against identified genomic scaffolds using the CLC Genomics software package (CLC Bio). Predicted protein sequences were aligned using ClustalX [35] and formatted with BioEdit [36]. Pairwise phylogenic tree construction and bootstrap analysis (10 000 replicates) were performed using the MEGA5 sequence analysis suite [37].
RNA and Protein Extraction
RNA and protein were extracted from whole flies and tissues using Trizol reagent according to the manufacturer's protocol (Invitrogen). Complementary DNA was synthesized from 1 μg of total RNA from two combined flies using Superscript III reverse transcriptase kit (Invitrogen) based on the manufacturer's protocol, with the exception that cDNA synthesis was extended to 60 min. RNA and cDNA were stored at −70°C until use. Protein was stored in protein pellet solubilization buffer (8 M urea, 3 M thiourea, 1% dithiothreitol, and 4% CHAPS) at −20°C.
Analysis of smase and cdase Expression
Levels of asmase1–4 (JQ308535, JQ308537–JQ308539), nsmase (JQ308535), cdase (JQ308540), brain washing (bwa, a protein with potential CDase-like activity, JQ308541), milk gland protein 1 (mgp1, a lipocalin that is expressed highly during tsetse pregnancy [13], DQ294227.1) and lipophorin receptor (lpr, a gene utilized as a control because it varies little throughout tsetse pregnancy, DQ294226.1) were determined by quantitative PCR (qPCR) utilizing the iCycler iQ real-time PCR detection system (Bio-Rad) using gene-specific primers (Supplemental Table S1; all Supplemental Data are available online at www.biolreprod.org). The data were obtained in triplicate samples and were normalized to tsetse tubulin (tub, DQ377071.1) expression levels and analyzed with CFX Manager software version 3.1 (Bio-Rad).
Knockdown of SMase
Short interfering RNA (siRNA) consisting of two Duplex sequences (CAGCAAUAUUUCAACUUGGUGAUACUC and AAAUAAUUUCAAAGCUUUGUUCACCAA) was obtained commercially (IDT). Control siRNA were designed against green fluorescent protein (GFP; GAUGCCAUUCUUUGGUUUGUCUCCCAU and CUUGACUUCAGCACGUGUCUUGUAGUU). Previous studies on insects, and specifically tsetse, have demonstrated gene knockdown by injection of siRNA [16]. The siRNA concentration was determined by spectrophotometer and adjusted to 700–750 ng/μl. Each fly was injected with 1.5 μl siRNA 8–10 days after adult emergence. Previous studies show that injection of siRNA into the mother has no discernable effect on larval transcript levels [13]. Expression levels were determined by qPCR (as described) 5 days after siRNA injection and normalized to tubulin. Mass of the pupae was determined utilizing an electrobalance (Cahn 25) and wing length was measured by the Comstock-Needham method [38]. For pupal hatch assessment, pupae were held in a Petri dish for 60 days and the number of adults that emerged was measured. Fecundity of progeny from mothers with reduced aSMase1 was compared to that from mothers injected with siGFP and mothers injected with siMilk gland protein (siMGP) for comparative analysis to previous studies [29]. Pupae where no adults emerged were dissected to determine if development had ceased and mortality occurred.
Heat Tolerance
Within 24 hours of emergence teneral female offspring of aSMase1 knockdown flies were subjected to 40, 42.5, and 45°C in groups of 10 for 2 h. Subsequently, the flies were returned to normal colony rearing conditions at 25°C. One day postexposure to heat challenge, flies were analyzed and survival was indicated when an individual responded to mechanical disturbance. Each experiment was replicated three times.
Symbiont Levels Within Teneral Flies and Expression of Wigglesworthia BioF
Symbiont genome numbers were quantified by qPCR using the groEL (AF321516.1) primer set specific for obligate W. glossinidia, with data normalized to host tubulin [39]. BioF is an enzyme involved in biotin synthesis [21], but is structurally similar to serine palmitoyl transferase (SPT), which is an enzyme involved in the sphingolipid biosynthetic pathway [4]. Transcript levels for BioF were determined in the bacteriome (the organ that harbors the symbiont in the midgut), in the adult carcass (which has milk gland tissue), and in larval gut contents (where Wigglesworthia transferred in the milk is exposed to sphingosine products). Levels of bioF were normalized according to groEL, which was shown to be consistently expressed in the bacteriome, mother, and larvae [39]. Total RNA was extracted from five teneral female flies within 48 h of adult emergence and cDNA for qPCR analysis was synthesized as described previously. qPCR primers utilized in this section are provided in Supplemental Table S1.
pH Determination of Milk Gland, Uterine Fluid, and Larval Gut Contents
Milk gland, uterine fluid, and larval gut contents were collected from females pregnant with late second/early third instar larvae. Females were immobilized on ice and the reproductive tract was exposed by dissection. Uterine fluid was collected using a 20-μl pulled glass capillary needle from within the uterus using negative pressure according to Benoit et al. [13]. Milk glands were removed at the attachment site to the uterus, separated from the fat body, and stored in groups of 10 at −20°C until treatment. Individual larvae were obtained from the uterus by removing the upper portion of the reproductive tract and extracting the progeny with soft-tip forceps. Larval guts were removed by dissection. Milk gland tissue and larval guts were macerated without buffer and centrifuged at 2000 rpm. The supernatant was removed and pH was tested with a microprobe (Sigma-Aldrich). Each tissue type was replicated five times and represents combined tissue from five flies.
SMase Activity
SMase activity was determined by a standard fluorescent assay (SMase Enzymatic Assay; Caymen Chemical). Milk gland, uterine fluid, and larval gut contents were collected as described. Samples were analyzed without buffer (to maintain the physiological pH within the organs) or buffered to pH 6.0 with citrate buffer or 7.8 with PBS according to manufacturer's protocol. Samples were stored at −20°C until use. Each sample was measured using a fluorescent plate reader (Biotek) with an excitation wavelength of 535 nm and absorbance at 590 nm. Activity was determined by comparison to a phosphocholine standard. Each tissue type was replicated three times and each replicate represents combined tissue from five flies. Protein concentrations were standardized according to total protein using a Bradford assay.
Western Blot Analysis
Proteins from each tissue represent combined samples from at least 10 flies. Rabbit antisera against tubulin (GmmTub) were previously described [29] and rabbit polyclonal human-SMase antisera were commercially obtained (Abcam). Blots were blocked overnight in PBS, 3% bovine serum albumin, and 0.5% Tween 20 (blocking buffer). The aSMase antibodies were used at 1:1000 concentration. The equivalent of 1/400 of a fly was loaded into each well. Blots were visualized with Supersignal West Pico Substrate (Pierce) on an Image Station 2000R (Kodak).
Immunohistochemistry of SMase
Immunohistochemical analysis was performed with aSMase antisera in blocking buffer (1:200). Goat anti-rabbit IgG conjugated with DyLight 488 (Thermo Scientific) was used as the secondary antibody at 1:500 concentration. Tissues from pregnant flies were dissected, fixed, and stained as described [29, 40].
RESULTS
Tsetse SMase and CDase Genes Are Orthologous to Those Found in Drosophila
Sequences for five smases (asmase1, asmase2, asmase3, asmase4, and nsmase) and two cdases (cdase and bwa) were identified from tsetse fly genome data via BLAST using Drosophila SMase protein sequences (Supplemental Fig. S1). Structural analysis of all five predicted tsetse SMases highlighted the presence of a conserved SMase metallophosphatase domain in each of the sequences. Phylogenic analysis of the predicted tsetse SMases (four aSMases and one nSMase) with Drosophila SMases revealed that each tsetse sequence is orthologous to individual Drosophila sequences (Supplemental Fig. S1). The percentage identity and similarity between the tsetse and Drosophila SMases range from 50% to 73% and 69% to 84% respectively. A search for CDase genes using a similar approach as described above yielded two genes. Although there are no defined domain structures associated with CDases, the tsetse genes show high homology to characterized CDases in Drosophila. Each of the two tsetse CDases has representative orthologs in Drosophila (CDase and Bwa; Supplemental Fig. S2). These data suggest that the SMase and CDase enzyme families are conserved between Drosophila and tsetse.
Tsetse aSMase1 Is Regulated and Expressed in a Pregnancy- and Milk Gland-Specific Manner
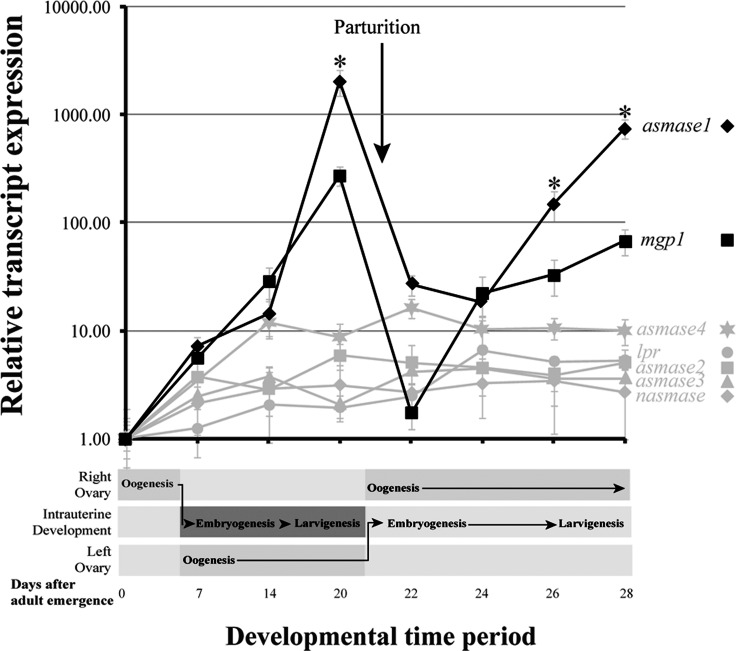
The expression profile of the tsetse smase and cdase genes was followed in fertile females over the course of the first two gonotrophic cycles by qPCR analysis. Within the first 24–48 hours of adult emergence, transcripts for asmase1–4 and nsmase1 increased to a baseline level. Levels of asmase2–4 and nsmase remained constitutive for the remainder of the time course (Fig. 1). In contrast, levels of asmase1 increased by over 1000-fold during pregnancy/lactation (13–20 days), but declined rapidly to baseline levels after birth (parturition at 20 days). This expression pattern for asmase1 was repeated again during the second gonotrophic cycle (Fig. 1). The asmase1 expression profile matched that of the major milk protein (gmmmgp1), which is a lactation-associated lipocalin. The expression of gmmmgp1 was found to increase over 100-fold during pregnancy/lactation, but was down dramatically within 48 h after parturition (Fig. 1). A recently completed high-throughput sequencing project, comparing the transcriptome of pregnant versus postpregnancy samples, confirmed that asmase1 is the only SMase gene that increased in expression in pregnant flies compared to those that had undergone parturition (Supplemental Fig. S3). Transcripts for cdase or brain washing (bwa), a protein with potential CDase activity [40], increased within 24–48 h of adult emergence, but did not vary throughout pregnancy. This is similar to what is observed with the lipophorin receptor (lpr), which was previously shown to vary little throughout pregnancy [13] (Supplemental Fig. S4).
FIG. 1. .
Time course of asmase1–4 and nsmase expression during the first two tsetse gonotrophic cycles. Milk gland proteins 1 (mgp1) and lipophorin receptor (lpr) transcript levels are shown for comparative purposes. Transcript levels were determined by qPCR utilizing the iCycler iQ real-time PCR detection system (Bio-Rad). The data were analyzed with software version 3.1 (Bio-Rad). Data represent the mean ± SEM of three replicates and were normalized to tubulin. Significant differences (*P < 0.01) were determined by ANOVA followed by Tukey test in comparison to samples that do not increase in relation to pregnancy.
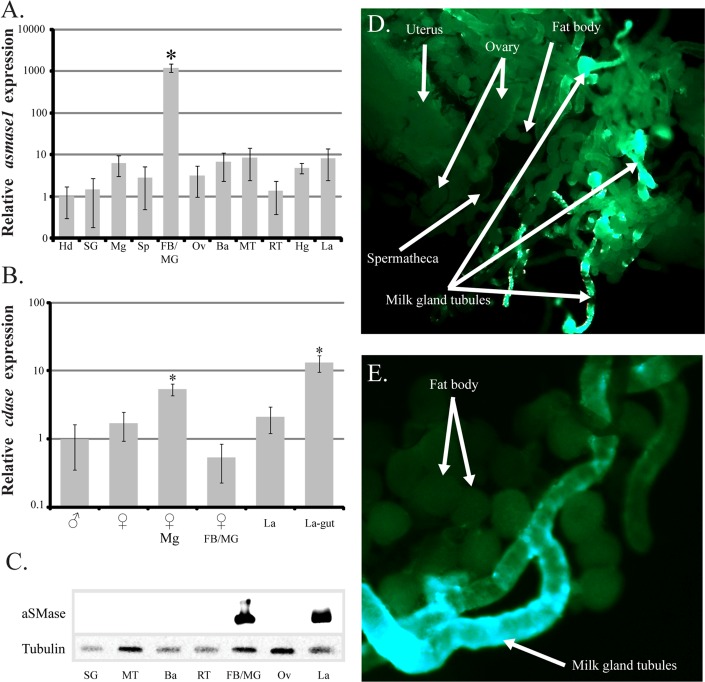
Spatial analysis of asmase1 expression revealed that this gene is preferentially expressed in the milk gland/fat body of pregnant mothers, although some transcripts could be detected in the bacteriome and malpighian tubules as well. In contrast, cdase is expressed in the guts of the developing intrauterine larvae and mother (Fig. 2, A and B). Western analysis of SMase proteins indicated the presence of high levels of these proteins primarily in the milk gland/fat body and larvae (Fig. 2C). Whole mount immunohistochemical staining further confirmed that SMase proteins are localized in the milk gland tubules of lactating mothers (Fig. 2, D and E). This localization profile is consistent with proteins generated in the mother and transferred in the milk to the nursing larva [29, 30].
FIG. 2. .
Spatial analysis of transcript expression and immunolocalization of SMase. A and B) Acid SMase 1 (asmase1; A) and CDase (cdase; B). Transcript levels were determined by qPCR using the iCycler iQ real-time PCR detection system (Bio-Rad). The data were analyzed with software version 3.1 (Bio-Rad). Data represent the mean ± SEM for three samples and was normalized to tubulin. *Significant difference determined by ANOVA followed by Tukey test (P < 0.01). C) Western blot analysis of SMase tissue distribution. Tsetse female midgut contents were not tested because of cross-reaction of the human SMase antibody and SMase present within the cow blood utilized to feed the flies. Hd, hindgut; SG, salivary gland; Mg, midgut; Sp, spermatheca; FB/MG, fat body/milk gland; Ov, ovaries; Ba, bacteriome; MT, malpighian tubules; RT, reproductive tract; Hg, hindgut; La, larvae. D) Immunolocalization of SMase in reproductive tract during pregnancy. E) Immunolocalization of SMase in fat body and milk gland. Images are representative of three separate tissue stainings.
SMase Enzymatic Activity in Milk Secretions Is Regulated by Environmental pH
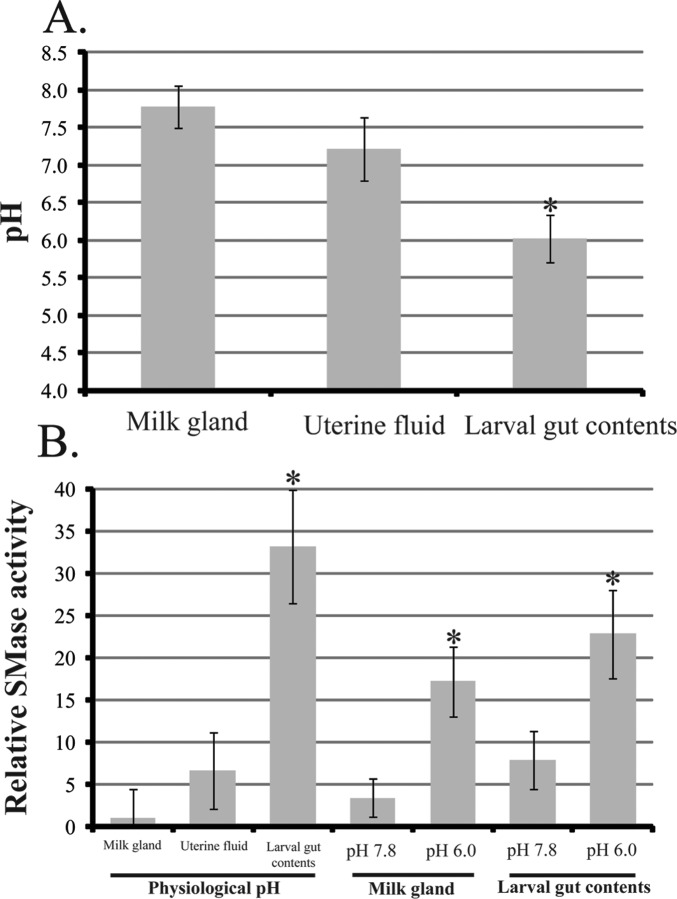
The pH of the milk gland, uterine fluid, and larval gut contents was determined to be 7.8, 7.2, and 6.0, respectively (Fig. 3A). SMase activity in the larval gut was over 30 times higher than that measured from the milk gland and uterine fluid (Fig. 3B). However, when the pH of the milk gland contents was decreased to that of the larval gut contents (pH 6.0), SMase enzymatic activity increased by 5–6-fold (Fig. 3B). In addition, when the larval gut contents were buffered to milk gland pH (pH 7.8), SMase activity in the larval contents was reduced by 3–4-fold (Fig. 3B). These results indicate that milk SMase is activated by the low pH environment of the larval gut contents. This system likely prevents the premature digestion of sphingomyelins during synthesis/storage in the milk gland and facilitates digestion and uptake of these nutrients within the digestive tract of developing larva.
FIG. 3. .
The pH and SMase activity in milk gland tissue, uterine fluid, and larval gut content of pregnant tsetse flies and their larval offspring. A and B) pH, mean ± SEM of five measurements (A), and activity of SMase, mean ± SEM of three measurements (B). The mean physiological pH for milk gland is 7.8, uterine fluid is 7.2, and larval gut contents is 6.0. Milk gland and larval gut content were buffered to pH 7.4 with PBS and to pH 6.0 with sodium citrate buffer. *Significance following ANOVA with Tukey test at P < 0.01.
RNAi Knockdown of aSMase1 Extends Larval Development and Negatively Affects Offspring Health
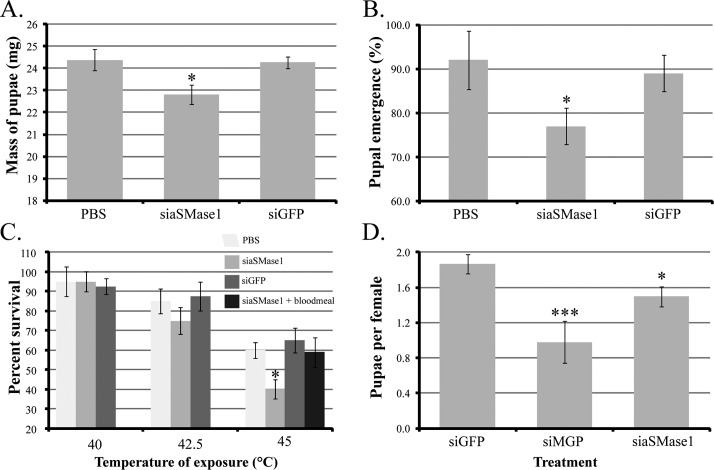
To determine the role of aSMase1 during pregnancy and larval development, pregnant females were treated with siRNA targeting aSMase1 and subsequently observed for phenotypes associated with pregnancy and offspring performance. Quantitative PCR of asmase1 after siaSMase1 treatment showed an approximately 60% decrease relative to transcript levels in control siGFP-treated flies. Accordingly, SMase activity in the larval midgut was reduced by 50%–60% in progeny from siaSMase1-treated mothers (Supplemental Fig. S5). The total number of pupae produced from control and asmase1 knockdown flies did not differ over the two gonotrophic cycles analyzed (Fig. 4, A and B). However, the duration of pregnancy was extended by 4–5 days in the asmase1 knockdown group (Fig. 4C). These results suggest that aSMase1 activity is an important component of the milk for larval development as its reduction causes a significant delay in larvigenesis.
FIG. 4. .
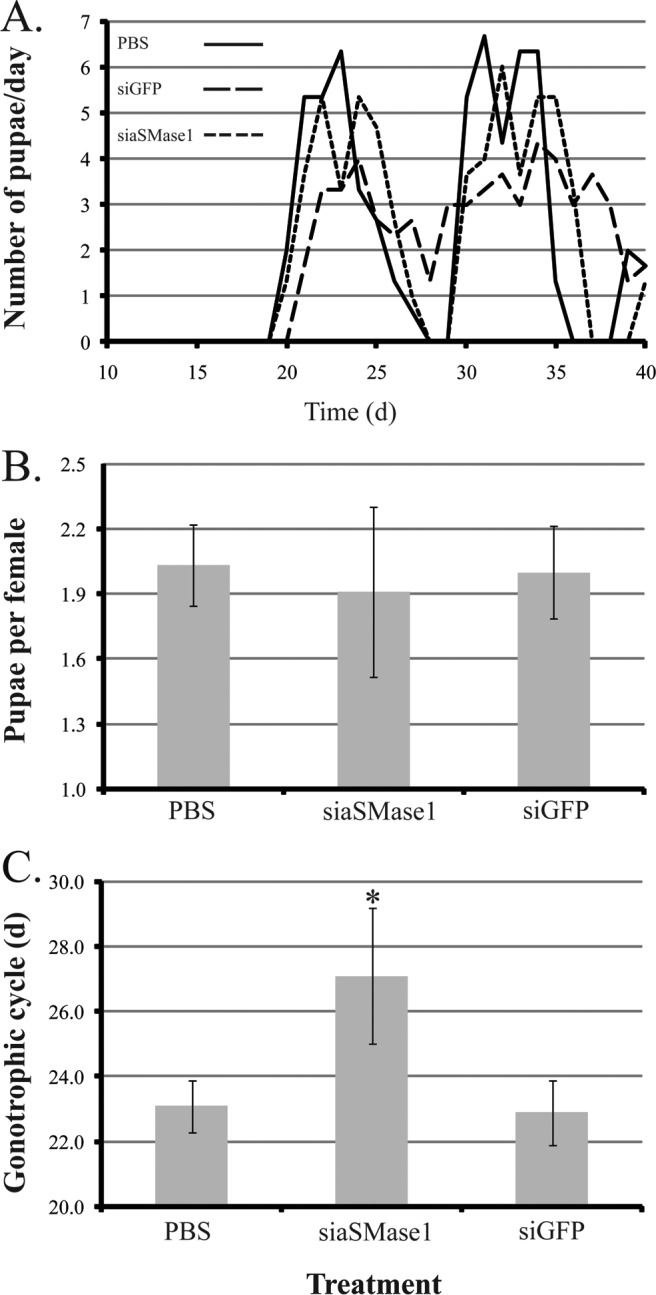
Phenotype of pregnant female tsetse following injection of aSMase1-specific siRNA. A) Daily deposition rates for a representative group of 30 females over 40 days. B) Number of pupae per female over 40 days, mean ± SEM of three groups of 30 flies. C) Duration of pregnancy, mean ± SEM of three groups of 15 flies. *Significant difference from siGFP-injected control following ANOVA with Tukey test at P < 0.01.
We evaluated the impact of asmase1 knockdown in the mother on her emerging progeny. The mass of the pupae from siaSMase1-treated mothers measured 5 days after deposition was lower by 1–2 mg (5%–8% reduction compared to the controls; Fig. 5A) and hatch rate of the pupae from asmase1 knockdown flies was suppressed by 15%–20% (Fig. 5B). The siaSMase1 knockdown in the mothers caused no significant differences in the wing length of emerging adult progeny compared to the controls (0.77 ± 0.04 after siaSMase1 treatment vs. 0.75 ± 0.05 after siGFP treatment). However, heat tolerance of emerging adult progeny from aSMase1 knockdown mothers was negatively impacted at 45°C, although not at 40 or 42.5°C (Fig. 5C). The impaired heat tolerance of adult progeny from siaSMase1 mothers returned to normal levels when analyzed after a blood meal (Fig. 5C). We also evaluated the long-term effects of acquiring reduced SMase1 levels during juvenile development by measuring the fecundity of the emerging adult progeny. Progeny from siaSMase1 mothers produced fewer pupae compared to siGFP controls, although this reduction was not as drastic as suppression of MGP in pregnant mothers (Fig. 5D). Additionally, pregnancy duration was extended in flies from siaSMase-deficient mothers by 2–3 days (21.9 ± 0.7 days after siGFP treatment vs. 23.9 ± 0.8 days after siaSMase1 treatment). These results indicate that reduction of aSMase1 in milk has significant negative effects upon pregnancy duration along with reduced progeny emergence, progeny thermal stress tolerance, and progeny reproduction output.
FIG. 5. .
Phenotypes of tsetse progeny after aSMase1 knockdown in their mother. A) Dry mass of pupae 5 days after deposition, mean ± SEM of three groups of 10 pupae. B) Pupal hatch rate of progeny from treated mothers, mean ± SEM of three groups of 15 pupae. C) Heat tolerance of teneral female flies, mean ± SEM of three groups of 10 flies. Control flies were injected with siGFP (1 μg/μl) and PBS (1 μl). D) Pupal production per female over two gonotrophic cycles, mean ± SEM of three groups of 15 individuals. A group of adult females was injected with siMGP for comparison. In the case of heat tolerance, an additional control group was sham injected. *Significant difference from siGFP-injected control following ANOVA with Tukey test (P < 0.01). ***Statistically lower than siGFP and siaSMase1 treatments (P < 0.01).
SMase Levels Impact Density of the Obligate Symbiont, Wigglesworthia, in Emerging Progeny
We assessed if aSMase1 reduction in mother's milk impacted the levels of the obligate Wigglesworthia transmitted to the intrauterine larva. This symbiont is required for females to maintain fecundity and is transferred to the developing larva in the milk [19, 22]. Suppression of asmase1 in the mother resulted in reduced density levels of Wigglesworthia (40%–50% reduction compared to controls) in emerging progeny (Fig. 6A). Given the negative effect maternal SMase knockdown has on Wigglesworthia transmission to offspring, we measured the expression of a Wigglesworthia gene, biof. We reasoned that bacterial dependence upon host sphingolipids could explain the decrease in Wigglesworthia numbers we observed in progeny of siaSMase1 knockdown mothers. Levels of Wigglesworthia biof were measured in the female milk gland and bacteriome organs as well as in the developing larva. Expression levels of biof were nearly 8-fold higher in the adult bacteriome than in the milk gland and larva (Fig. 6B). These results suggest that BioF likely plays a role in Wigglesworthia biotin synthesis in the adult bacteriome rather than in utilization of sphingolipids present in the milk gland or larvae. Our study shows that symbiont transfer effectiveness was reduced by aSMase1 knockdown in the mother, which in turn could compromise the health of the progeny leading to the negative indicators we observed.
FIG. 6. .
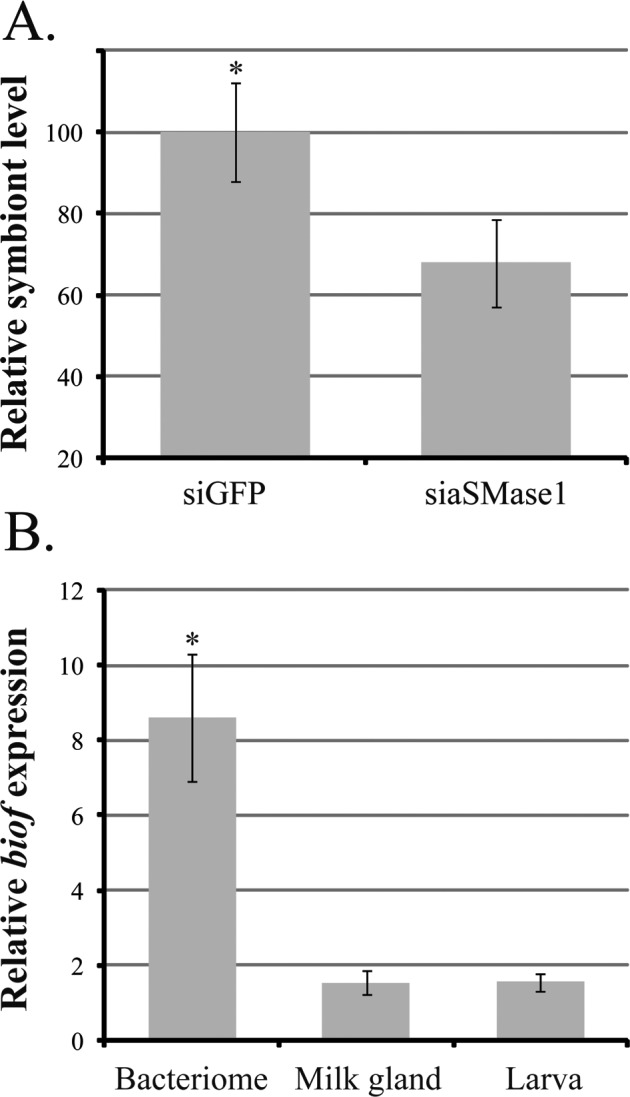
Levels of obligate symbiont, Wigglesworthia glossinidia, and W. glossinidia biof in pregnant flies and specific tissues. A) Wigglesworthia glossinidia density based on groEL in teneral female flies in relation to tsetse tubulin, mean ± SEM of six flies. B) Levels of biof expression, a protein with potential SPT activity, in W. glossinidia analyzed from tsetse fly bacteriome, milk gland, and larvae. Expression was normalized in relation to Wigglesworthia groEL. Data are presented as mean ± SEM of four measurements. *Significant difference determined by ANOVA followed by Tukey test (P < 0.01).
DISCUSSION
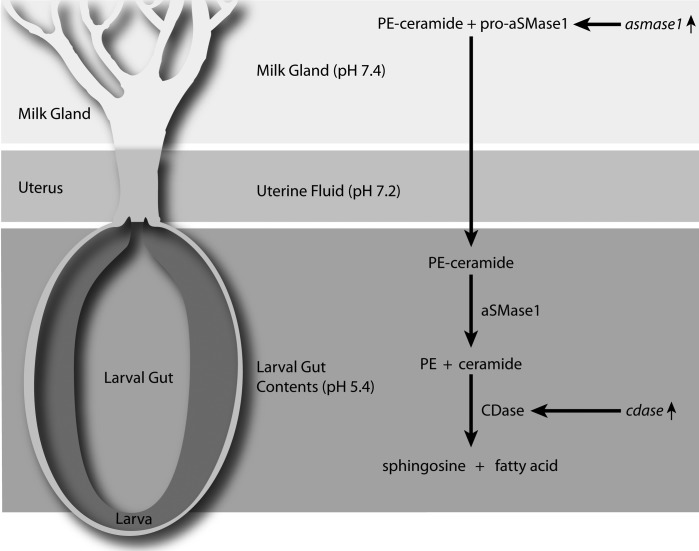
This study demonstrates that SMase is an important component of the tsetse milk secretions and is critical for success of pregnancy and subsequent health of her progeny. Our results show that asmase1 transcripts increase nearly 1000-fold during pregnancy and that asmase1 is the only SMase upregulated during pregnancy. Acid SMase1 is secreted, is transported to the intrauterine larva as a component of the milk, and is activated by the low pH within the larval gut to utilize milk-associated sphingomyelins. In addition, cdase expression in the larval gut indicates that ceramide generated by aSMase1 activity is metabolized to sphingosine and free fatty acid for absorption. Based on these results, aSMase1 activity is required for larval utilization of essential sphingolipids and their byproducts in tsetse. Furthermore, aSMase1 activity is also important for the tsetse's microbiome, which is transmitted in the milk to juvenile progeny. We have developed a model for aSMase1 synthesis, secretion, and function during pregnancy (Fig. 7). This system likely provides the majority of sphingosine derivatives required for progeny development because milk secretions are the sole nutrient source during intrauterine larval growth and pupal development.
FIG. 7. .
Model for the role of aSMase1 during tsetse lactation and larval feeding.
Insect sphingomyelin metabolism is an understudied field of research. The only other studies on insect sphingomyelin metabolism are in Drosophila and consist primarily of transcript localization studies [3]. Expression of SMase transcripts varies significantly between tissues and throughout development in Drosophila based on data provided by FlyBase [41]. Similar to Drosophila, tsetse has four aSMases and one nSMase, indicating these genes are conserved between these two flies. Comparison of Drosophila cg3376 (asmase1 ortholog) to the pregnancy-specific tsetse asmase1 indicates that both have relatively low levels in all tissues with the exception of asmase1 in tsetse accessory gland (milk gland) and cg3376 in the larval fat body of Drosophila. Currently it is not known if cg3376 or any other smase gene is expressed in Drosophila female accessory gland (paraovaria), because of the small size of these glands in Drosophila and the difficulty of their removal from the surrounding fat body. Future studies will be useful to determine if cg3376 is expressed similarly to tsetse asmase1 in Drosophila paraovaria during egg production and oviposition or if the evolutionary changes associated with tsetse viviparous reproductive physiology are responsible for the pregnancy-specific nature of tsetse asmase1.
Based on our knockdown studies, aSMase1 deficiency in the milk resulted in the extension of intrauterine larval development and reduced performance of emerging progeny. The aSMase1 knockdown females gave birth to slightly smaller progeny with a lower rate of pupal eclosion. In addition, emerging adult offspring displayed reduced heat tolerance. The products of sphingomyelin, sphingosine and ceramide, are associated with apoptosis, cell growth/proliferation, and metabolic processes [2, 3]. Thus, reduction of aSMase1 could impair development and contribute to the reduced pupal hatch rates. Additionally, changes in sphingolipid availability may alter cell membrane composition and signaling properties and could explain the reduced heat tolerance observed in emerging progeny from aSMase1 knockdown mothers [42, 43]. The reduced heat tolerance phenotype of the progeny was rescued following a blood meal when nutrients are made available to adult flies, given that blood is an excellent source of sphingomyelin [44]. Additionally, female progeny from SMase knockdown mothers had lower fecundity and extended pregnancy when compared to control flies. This indicates that there are prolonged negative consequences for the progeny when juveniles do not receive adequate levels of SMase in the milk.
We also observed a reduction in the levels of Wigglesworthia in progeny produced by mothers with suppressed levels of asmase1. Reduction in Wigglesworthia levels is detrimental to tsetse fly physiology, as this bacterium is critical for vitamin production, reproductive fitness, and tsetse immune priming [22, 45]. It appears that proper sphingomyelin metabolism during pregnancy is important for the maintenance and/or establishment of Wigglesworthia populations in the larval progeny. The exact cause for reduced levels of Wigglesworthia following aSMase1 knockdown remains to be studied, but could result from inadequate sphingosine levels in the larval gut, which can inhibit bacterial proliferation or colonization. In Bacteroides, which is part of the human intestinal microflora, external sources of sphingolipids are incorporated into the bacterial cell membrane [4]. Regardless of the exact mechanism, reduced transfer of Wigglesworthia through the milk could account (at least partially) for the reduced performance of progeny produced by aSMase1 knockdown mothers. We found that the potential SPT ortholog, biof, has been retained in the small genome of Wigglesworthia, but orthologs for the two other genes involved in sphingolipid metabolism, 3-ketosphinganine reductase and ceramide synthase, are absent [21]. However, several bacteria known to utilize sphingolipids have been shown to lack functional orthologs of 3-ketosphinganine reductase and ceramide synthase, indicating that their absence may not be indicative of Wigglesworthia's inability to process sphingolipids. Specific bacteria have been documented to utilize SMase, such as Staphylococcus aureus [46], but similar to Bacteroides and most other bacteria [4], Wigglesworthia lacks a distinct SMase ortholog [4]. It is possible that bacteria may use pathways different than those that occur in eukaryotes to process sphingolipids [4]. We investigated whether BioF could function in sphingolipid metabolism. Given the high levels of sphingolipids and their derivatives in the milk gland and larval gut contents, we predicted that expression of Wigglesworthia biof would be higher in the milk gland and larva. It was, however, significantly higher in the midgut bacteriome organ, which houses the intracellular form of Wigglesworthia. The function of BioF within the bacteriome is likely for biotin synthesis [21]. Even though these findings suggest that Wigglesworthia may not metabolize sphingolipids, the abundance of sphingolipids and sphingolipid products in the milk may lead to their incorporation into cell membrane. In Bacteroides, as much as 10% of the total lipid contents were documented to be ceramide even though the proper metabolic pathways for sphingolipid processing this species have yet to be identified [4, 5]. Thus, direct analysis of Wigglesworthia cell membranes will be necessary to determine if sphingolipids are incorporated into the cell membrane by this symbiont.
Sphingomyelins are one of the most abundant phospholipids present in mammalian milk [47, 48]. In human milk, aSMase activity is responsible for the hydrolysis of half of the milk's sphingomyelin [31]. After the initial breakdown of sphingomyelin by the SMase present within mammalian milk, the ceramide product can be metabolized by a bile salt-stimulated lipase (also present in the milk) [31]. Sphingomyelin digestion occurs in the mammalian intestinal tract by endogenous alkaline SMase and CDase activities as shown in humans and mice [7, 32, 49, 50]. Regardless, sphingomyelin in tsetse or mammalian milk requires the presence of SMase activity that is specific to and active at the proper physiological pH of the gut/intestine for the initial breakdown of sphingomyelin to PE/PC and ceramide. The co-occurrence of a CDase or CDase-like activity to metabolize ceramide to sphingosine and free fatty acid for proper digestive absorption is also a functional orthology between mammals and tsetse flies [this study, 7, 31, 32]. The similarities between the mammalian and tsetse systems suggest that the presence of SMase and CDase either in the milk or within the digestive tract of the nursing juvenile is a factor critical to species that rely on the mother's milk as the sole source of nutrients during early development to obtain the sphingosine-containing molecules.
The analogies between tsetse and mammal milk production, including development of specialized lactation-associated cells, functionally conserved milk proteins, comparable lipid content of the milk, and transfer of microbial symbionts in the milk, suggests that convergent mechanisms may operate in organisms that utilize lactation as the sole source of nutrients for their developing offspring [19, 23–26]. The critical nature of SMase activity during lactation and milk consumption is an additional similarity between tsetse and mammal lactation. Functional studies with SMase in tsetse could provide information on the role of sphingomyelin digestion, metabolism, and utilization during early progeny development in lactating animals. Lastly, reduced fecundity in progeny after inadequate SMase transfer from mother to progeny indicates long-term negative consequences. Given the ease of insect cultivation, shorter generation times, and the availability of gene silencing methodologies, the tsetse system can be readily used to understand the mechanisms underlying transgenerational effects that are more complicated to tease out in the mammalian systems.
ACKNOWLEDGMENT
We thank Oleg Kruglov and Yineng Wu for their technical expertise.
Footnotes
Supported by the National Institutes of Health (AI081774 and F32AI093023) and Ambrose Monell Foundation Awards.
REFERENCES
- Renault AD, Starz-Gaiano M, Lehmann R. Metabolism of sphingosine 1-phosphate and lysophosphatidic acid: a genome wide analysis of gene expression in Drosophila. Mech Dev 2002; 119: S293 S301 [DOI] [PubMed] [Google Scholar]
- Sabourdy F, Kedjouar B, Sorli SC, Colie S, Milhas D, Salma Y, Levade T. Functions of sphingolipid metabolism in mammals—lessons from genetic defects. Biochim Biophys Acta 2008; 1781: 145 183 [DOI] [PubMed] [Google Scholar]
- Kraut R. Roles of sphingolipids in Drosophila development and disease. J Neurochem 2011; 116: 764 778 [DOI] [PubMed] [Google Scholar]
- An D, Na C, Bielawski J, Hannun YA, Kasper DL. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc Natl Acad Sci USA 2011; 108: 4666 4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa E, Azuma R, Suto T, Yano I. Occurrence of free ceramides in Bacteroides fragilis Nctc-9343. J Biochem 1979; 86: 311 320 [DOI] [PubMed] [Google Scholar]
- Acharya U, Acharya JK. Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol Life Sci 2005; 82: 128 142 [DOI] [PubMed] [Google Scholar]
- Duan RD. Sphingomyelinase and ceramidase in the intestinal tract. Euro J Lipid Sci Tech 2007; 109: 987 993 [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Bba-Biomembranes 2006; 1758: 1864 1884 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 2002; 277: 25847 25850 [DOI] [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, Mock MB, Fredrick DS. Metabolism of sphingomyelin. 2. Evidence of an enzymatic deficiency in Niemann-Pick disease. Proc Natl Acad Sci USA 1966; 55: 366 370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH. Acid sphingomyelinase deficient mice—a model of Type-A and Type-B Niemann-Pick disease. Nat Genet 1995; 10: 288 293 [DOI] [PubMed] [Google Scholar]
- Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J 2002; 21: 6162 6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JB, Yang G, Krause TB, Patrick KR, Aksoy S, Attardo GM. Lipophorin acts as a shuttle of lipids to the milk gland during tsetse fly pregnancy. J Insect Physiol 2011; 57: 1553 1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimley RW, Langley PA. Hormonal control of lipid synthesis in the fat body of the adult female tsetse fly, Glossina morsitans. J Insect Physiol 1981; 27: 839 847 [Google Scholar]
- Attardo GM, Strickler-Dinglasan P, Perkin SAH, Caler E, Bonaldo MF, Soares MB, El-Sayeed N, Aksoy S. Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): regulation of yolk and milk gland protein synthesis. Insect Mol Biol 2006; 15: 411 424 [DOI] [PubMed] [Google Scholar]
- Attardo GM, Benoit JB, Michalkova V, Yang G, Roller L, Bohova J, Takac P, Aksoy S. Analysis of lipolysis underlying lactation in the tsetse fly, Glossina morsitans. Insect Biochem Mol Biol 2012; 45: 360 370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe SS, Langley PA. Reproductive physiology of Glossina. Annu Rev Entomol 1978; 23: 283 307 [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Ma WC. Dynamics of the pregnancy cycle in the tsetse Glossina morsitans. J Insect Physiol 1974; 20: 1015 1026 [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Ma WC. Maternal nutritive secretions as possible channels for vertical transmission of microorganisms in insects: the tsetse fly example. Ann N Y Acad Sci 1975; 266: 162 165 [DOI] [PubMed] [Google Scholar]
- Ma WC, Denlinger DL, Jarlfors U, Smith DS. Structural modulations in the tsetse fly milk gland during a pregnancy cycle. Tissue Cell 1975; 7: 319 330 [DOI] [PubMed] [Google Scholar]
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet 2002; 32: 402 407 [DOI] [PubMed] [Google Scholar]
- Aksoy S, Pais R, Lohs C, Wu YN, Wang JW. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol 2012; 74: 5965 5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton P. The Natural History of Tsetse Flies. London: H.K. Lewis and Co. Ltd; 1955. [Google Scholar]
- Moloo SK. Oocyte differentiation and vitellogenesis in Glossina morsitans Westw. Acta Trop 1971; 28: 334 340 [PubMed] [Google Scholar]
- Lara-Villoslada F, Olivares M, Sierra S, Rodriguez JM, Boza J, Xaus J. Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr 2007; 98: S96 S100 [DOI] [PubMed] [Google Scholar]
- Martin R, Langa S, Reviriego C, Jiminez E, Marin ML, Xaus J, Fernandez L, Rodriguez JM. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 2003; 143: 754 758 [DOI] [PubMed] [Google Scholar]
- McManaman JL, Neville MC. Mammary physiology and milk secretion. Adv Drug Deliv Rev 2003; 55: 629 641 [DOI] [PubMed] [Google Scholar]
- Cmelik SHW, Bursell E, Slack E. Composition of the gut contents of third-instar tsetse larvae (Glossina morsitans Westwood). Comp Biochem Physiol 1969; 29: 447 453 [Google Scholar]
- Attardo GM, Guz N, Strickler-Dinglasan P, Aksoy S. Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): regulation of yolk and milk gland protein synthesis. J Insect Physiol 2006; 52: 1128 1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz N, Attardo GM, Wu Y, Aksoy S. Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans). J Insect Physiol 2007; 53: 715 723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Farooqi A, Blackberg L, Duan RD, Nilsson A, Hernell O. Digestion of ceramide by human milk bile salt-stimulated lipase. J Pediatr Gastroenterol Nutr 1998; 27: 560 567 [DOI] [PubMed] [Google Scholar]
- Duan RD. Physiological functions and clinical implications of sphingolipids in the gut. J Digest Dis 2011; 12: 60 70 [DOI] [PubMed] [Google Scholar]
- Moloo SK. An artificial feeding technique for Glossina. Parasitology 1971; 63: 507 512 [DOI] [PubMed] [Google Scholar]
- Yang G, Attardo GM, Lohs C, Aksoy S. Molecular characterization of two novel milk proteins in the tsetse fly (Glossina morsitans morsitans). Insect Mol Biol 2010; 19: 253 262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997; 25: 4876 4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999; 41: 95 98 [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA. 3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 2004; 5: 150 163 [DOI] [PubMed] [Google Scholar]
- Hu CY, Rio RVM, Medlock J, Haines LR, Nayduch D, Savage AF, Guz N, Attardo GM, Pearson TW, Galvani AP, Aksoy S. Infections with immunogenic trypanosomes reduce tsetse reproductive fitness: potential impact of different parasite strains on vector population structure. PLoS Negl Trop Dis 2008; 12: e192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio RVM, Symula RE, Wang J, Lohs C, Wu YN, Snyder AK, Bjornson RD, Oshima K, Biehl BS, Perna NT, Hattori M, Aksoy S. Insight into transmission biology and species-specific functional capabilities of tsetse's obligate symbiont Wigglesworthia. mBio 2012; 3: e00240 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Rao RP, Jesmin N, Bamba T, Nagashima K, Pascual A, Preat T, Fukusaki E, Acharya U, Acharya JK. CDase is a pan-ceramidase in Drosophila. Mol Biol Cell 2011; 22: 33 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart WM, Emmert DB. FlyBase high throughput expression pattern data, beta version FlyBase Analysis. 2011. http://www.flybase.org.
- Overgaard J, Tomcala A, Sorensen JG, Holmstrup M, Krogh PH, Simek P, Kostal V. Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J Insect Physiol 2008; 54: 619 629 [DOI] [PubMed] [Google Scholar]
- Steels EL, Learmonth RP, Watson K. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 1994; 140: 569 576 [DOI] [PubMed] [Google Scholar]
- Hooghwinkel GJ, van Gelderen HH, Staal A. Sphingomyelin of red blood cells in lipidosis and in dementia of unknown origin in children. Arch Dis Child 1969; 44: 197 202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss BL, Wang JW, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol 2011; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walev I, Weller U, Strauch S, Foster T, Bhakdi S. Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus. Infect Immun 1996; 64: 2974 2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris AM, Jensen RG. Lipids in human milk: a review. 1: Sampling, determination, and content. J Pediatr Gastroenterol Nutr 1994; 3: 108 122 [DOI] [PubMed] [Google Scholar]
- Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr 1997; 17: 159 183 [DOI] [PubMed] [Google Scholar]
- Lillienau J, Cheng Y, Nilsson A, Duan RD. Development of intestinal alkaline sphingomyelinase in rat fetus and newborn rat. Lipids 2003; 38: 545 549 [DOI] [PubMed] [Google Scholar]
- Duan RD, Cheng Y, Jonsson BA, Ohlsson L, Herbst A, Hellstrom-Westas L, Nilsson A. Human meconium contains significant amounts of alkaline sphingomyelinase, neutral ceramidase, and sphingolipid metabolites. Pediatr Res 2007; 61: 61 66 [DOI] [PubMed] [Google Scholar]