ABSTRACT
Prenatal testosterone (T) excess leads to reproductive dysfunctions in sheep, which include increased ovarian follicular recruitment and persistence. To test the hypothesis that follicular disruptions in T sheep stem from changes in the developmental ontogeny of ovarian proliferation and apoptotic factors, pregnant Suffolk sheep were injected twice weekly with T propionate or dihydrotestosterone propionate (DHT; a nonaromatizable androgen) from Days 30 to 90 of gestation. Changes in developmental expression of proliferating cell nuclear antigen (PCNA), BCL2, BAX, activated CASP3, and FAS/FASLG were determined at Fetal Days 90 and 140, 22 wk, 10 mo, and 21 mo of age by immunocytochemisty. Prenatal T treatment induced changes in expression of proliferative and apoptotic markers in a follicle-, age-, and steroid-specific manner. Changes in BAX were evident only during fetal life and PCNA, BCL2, and CASP3 only postnatally. Prenatal T and not DHT increased PCNA and decreased BCL2 in granulosa/theca cells of antral follicles at 10 and 21 mo but decreased CASP3 in granulosa/theca cells of antral follicles at 22 wk (prepubertal) and 10 and 21 mo. Both treatments decreased BAX immunostaining in granulosa cells of Fetal Day 90 primordial/primary follicles. Neither treatment affected FAS expression at any developmental time point in any follicular compartment. Effects on BAX appear to be programmed by androgenic actions and PCNA, BCL2, and CASP3 by estrogenic actions of T. Overall, the findings demonstrate that fetal exposure to excess T disrupts the ovarian proliferation/apoptosis balance, thus providing a basis for the follicular disruptions evidenced in these females.
Keywords: BAX, BCL2, CASP3, PCNA, PCOS
Fetal exposure to excess testosterone disrupts the ovarian cell proliferation/apoptosis balance in sheep, providing a basis for the follicular persistence seen in these animals.
INTRODUCTION
Folliculogenesis is a complex process that involves constant remodeling involving several regulatory molecules that are subject to influence from external signals [1]. Disruption of follicular growth is also part of reproductive defects seen in women with polycystic ovary syndrome (PCOS) [2, 3]. Animal models of ovarian disruptions provide a unique resource to help unravel the underlying mechanisms by which ovarian follicular differentiation progresses. For instance, prenatal testosterone (T) treatment leads to multifollicular ovarian morphology [4], enhanced follicular recruitment and depletion during early reproductive life [5], and follicular persistence [6, 7] in sheep, contributing to the disrupted reproductive phenotype of these animals [8–11].
The underlying mechanisms mediating enhanced follicular recruitment and persistence in prenatal T-treated animals remain to be elucidated. Several paracrine factors act locally in concert with FSH to promote or inhibit follicular growth and differentiation [12, 13]. These paracrine factors regulate the balance between survival and apoptotic factors modulating whether a follicle continues to develop or undergo atresia [1, 14–16]. Proliferating cell nuclear antigen (PCNA) is a proliferation-associated protein required for DNA synthesis and appears to be involved in follicular growth. It is a common marker of proliferation and the level of its expression increases during the gonadotropin-dependent stages of preovulatory follicular development [17–19]. On the other hand, regulation of apoptosis in the ovary is mediated by members of BCL2 family and the FAS system [15, 20, 21]. Members of the BCL2 family include those that have antiapoptotic (e.g., BCL2, BCL2L2, and BCL2L1) and those that have proapoptotic (e.g., BAX, BAD, BCL2L11, and BOK) functions. The antiapoptotic factors block the activation of effector caspases that transduce the apoptotic signals [22, 23].
To determine the contributing role of apoptotic and proliferative factors in the enhanced follicular recruitment and persistence seen across the reproductive life span of the prenatal T-treated sheep, we compared ovarian expression levels of PCNA, BCL2 family members (BCL2 and BAX), activated caspase-3 (CASP3), and FAS/FASLG in prenatal T-treated females with controls at several critical developmental time points. Specifically, we tested the hypothesis that prenatal T excess disrupts the balance of key regulatory and effector proteins involved in the control of intraovarian proliferation/apoptosis in a development-specific manner. In addition, since T can be aromatized to estrogen, we compared findings from prenatal T-treated animals with that of prenatal dihydrotestosterone (DHT)-treated animals to determine if prenatal T-induced perturbations are facilitated by androgenic or estrogenic programming.
MATERIALS AND METHODS
Breeding and Prenatal Treatment
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of the University of Michigan and consistent with the National Research Council's Guide for the Care and Use of Laboratory Animals. Prenatal T and DHT treatment and the diet of breeder sheep and lambs have been previously described [7, 24]. Generation of prenatal T- and DHT-treated sheep involved injecting pregnant ewes twice weekly with 100 mg of T propionate (1.2 mg/kg; Sigma-Aldrich Corp.) or 100 mg DHT propionate (Steraloids, Inc.) suspended in cottonseed oil (Sigma-Aldrich) from Days 30 to 90 of gestation. Ovaries from control, prenatal T-treated, and DHT-treated females at Fetal Day 90, 22 wk of age (prepubertal), 10 mo of age (end of first breeding season), and 21 mo of age (end of second breeding season) were utilized in this study. Ovaries from 10- and 21-mo-old sheep were collected during the presumptive follicular phase after administration of prostaglandin F2α, twice 11 days apart. Sample size ranged between five and eight for each treatment group at each developmental time point. There were insufficient DHT-treated females born to include a 21-mo-old prenatal DHT-treated group. Details of euthanasia, ovarian collection, and processing have been previously published [5]. Paraffin-embedded sections from one ovary from each animal were used in this study. Developmental changes in ovarian morphometry [5], steroid receptors [25], and members of insulin-signaling cascade [26] from the same set of animals have been previously published, enabling integration of findings across mediators.
Immunohistochemistry
Antigen/antibody specificity.
The homology between the target peptide of each antibody and the correspondent ovine protein was tested using Basic Local Alignment Search Tool (BLAST software; http://www.ncbi.nlm.nih.gov/BLAST) to determine the peptide locations and to confirm antigen specificity. To test specificity of the primary antibodies used in this study (Table 1), sheep ovarian tissue extracts were separated in SDS-PAGE (15% resolving gel), as previously described [25, 26]. Proteins were transferred onto nitrocellulose membranes (Amersham), blocked for 1 h in 2% nonfat milk in TBS containing 0.05% Tween-20 (Sigma-Aldrich Corp.) and then incubated overnight at 4°C with specific primary antibodies (Table 1). Following washing, membranes were treated for 1 h with corresponding secondary peroxidase-conjugated antibody (Table 1). Immunopositive bands were visualized with a chemiluminescent detection kit (ECL-Plus; GE-Amersham).
TABLE 1. .
Used antibodies, suppliers, and dilutions.
For immunohistochemistry, the streptavidin-biotin immunoperoxidase method was employed as described previously [25, 26]. Briefly, the sections were deparaffinized, and antigen retrieval was performed by heating in a microwave. Endogenous peroxidase was blocked with 3% H2O2 in methanol, and nonspecific binding was blocked with 10% (v/v) normal goat serum. Sections were incubated with primary antibodies (Table 1) for 18 h at 4°C and then with the respective biotinylated secondary antibody for 30 min at room temperature. Detection was by a streptavidin-peroxidase solution (BioGenex) with 3.3-diaminobenzidine (DAB; DAKO) as chromogen. The sections were then counterstained with Mayer hematoxylin, dehydrated, and mounted. Blocking of the endogenous peroxidase activity was confirmed by incubating some sections with DAB alone, and the specificity of the secondary antibodies was tested replacing the primary antibodies with nonimmune serum. As multiple series of histological processing were involved, serial sections of a nonexperimental set of sheep ovaries were included with each series to allow normalization across series. Each immunohistochemical series included randomly selected slides with ovarian sections from different ages and treatments. Follicle classes were distinguished using criteria previously established [27] and include primordial, primary, small preantral, large preantral, and antral follicles.
Image Analysis
Details of image analysis have been described earlier [25, 26]. For each marker, to avoid duplicate counting of follicles, two sections (one-third and two-thirds into the ovary) were used for immunohistochemical quantification. All growing follicles in both sections were analyzed (ranged between 8 and 15 for each follicular class). For primordial follicles, the slides were scanned left to right from the top, and the first 20 primordial follicles that were distinct and showed no overlap with neighboring follicles were utilized. Only healthy follicles without pycnotic nucleus (indicative of atresia) were evaluated. To avoid subjectivity and differences in location of proteins, all the analyzed images covered granulosa from antrum to theca.
For each ovary, 10 images of cortical stromal tissue were analyzed. Stromal areas (31 884 μm2) devoid of follicular structures were selected by scanning the ovarian section left to right from the top.
The image analysis was performed using the Image Pro-Plus 3.0.1 system (Media Cybernetics), as detailed earlier [25, 26]. Images were digitized with an Olympus C5060 digital camera mounted on a conventional light microscope (Olympus BH-2; Olympus Co.). The average density (% of immunopositive area) was calculated as a percentage of total area evaluated through color segmentation analysis, which extracts objects by locating all objects of a specific color (brown stain). These values were verified and normalized with the controls carried across various runs using the same region (verified by image comparison) for calibration. The percentage of immunopositive area was calculated separately for each follicular compartment (granulosa, theca interna, and theca externa) and stroma. Sections were analyzed with the observer blinded to treatment. Quantitative comparisons across proteins are not possible, as immunostaining and image analyses were optimized for each protein.
Statistical Analyses
The average density (% of immunopositive area) of each follicular compartment (granulosa and theca) within a follicle class was first averaged, and then a group mean across follicles was derived for each follicle type within an animal. A statistical software package (SPSS 11.0 for Windows; SPSS Inc.) was used for performing the statistical tests. Data were compared by analyses of variance, followed by Duncan multiple range tests. A P < 0.05 value was considered significant. Results are expressed as mean ± SEM.
RESULTS
Antibody Specificity
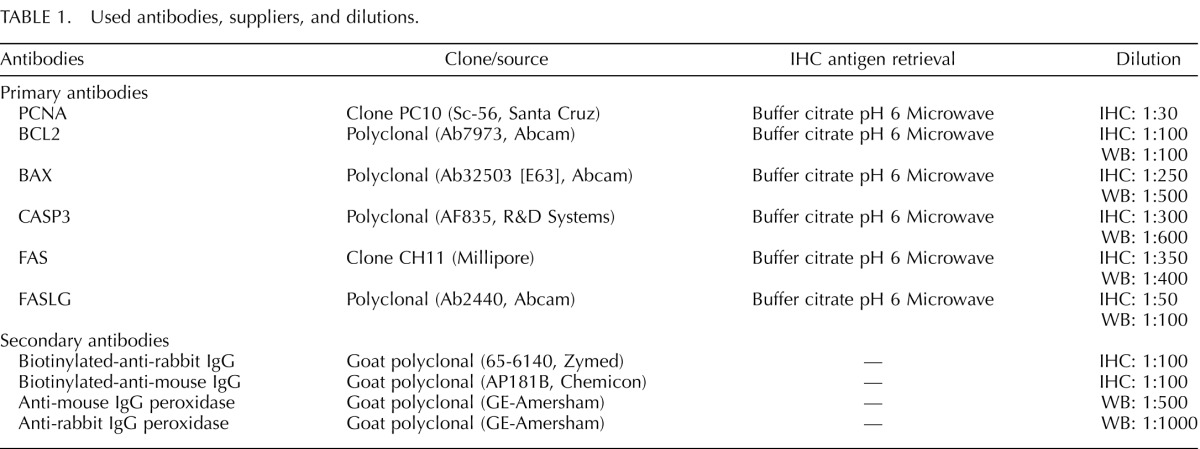
Western blot recognition of proteins in ovarian homogenate and immunohistochemical localization of the six proteins in ovarian sections is summarized in Figure 1. Western blot analysis revealed positive bands of appropriate sizes for each of the protein studied (Fig. 1, left). The PCNA, BCL2, BAX, FAS, and FASLG antibodies detected a single band at 35, 26, 22, 45, and 40 kDa, respectively, while two bands at 20 and 32 kDa were observed for CASP3. In the absence of the primary antibodies, no specific staining were observed (Fig.1, negative control). Immunostaining of key markers in antral follicles of control females are shown in Figure 1 (Antral 10/21 mo). All proteins except PCNA, which showed nuclear staining, had cytoplasmic localization (see results from segmentation analyses in Fig. 1, right panel). With the exception of FASLG, which was evident only in atretic follicles (the proportion of healthy to atretic follicles did not differ between treatment groups [28]), all follicles expressed PCNA, BCL2, BAX, CASP3, and FAS.
FIG. 1. .
Representative images of PCNA, BCL2, BAX, CASP3, FAS, and FASL immunostaining in antral follicles are shown in the third column. Verification of antibody specificity by Western blot analyses of ovarian homogenate and negative controls for immunostaining demonstrating the specificity of the antibody are shown in the left two columns, respectively. The fourth column shows segmentation analyses results of immunostaining. G, granulosa cells; TE, theca externa; TI, theca interna. Bar = 25 μm.
Developmental Changes in Expression Pattern of PCNA, BCL2, BAX, CASP3, FAS, and FASLG
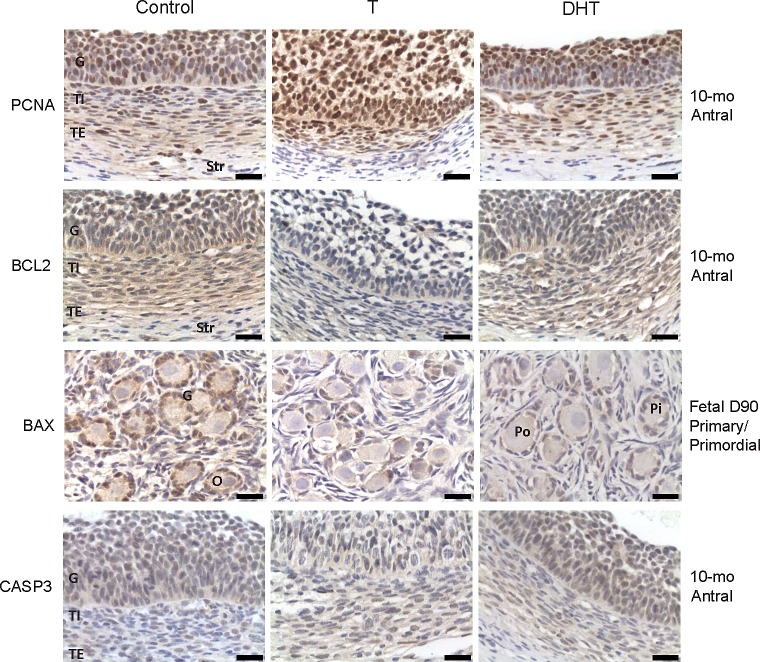
PCNA was localized in the nucleus of granulosa and theca interna cells (antral follicles) (Fig. 1). Expression of PCNA was lowest in primordial and primary follicles of Fetal Day 90 ovaries. PCNA expression in the granulosa cells increased with follicular differentiation with highest level seen in preantral/antral follicles (P < 0.05; Fig. 2). BCL2 was highly expressed in the cytoplasm of granulosa cells and theca interna cells (antral follicles) with a lower immunostaining in theca externa. Levels were also lower in theca cells of preantral follicles and stromal cells (Fig. 1). No differences in level of expression were evident across follicular stages (Fig. 3). BAX was expressed mainly in the cytoplasm of granulosa cells with a weak immunostaining in thecal and stromal cells. A significantly higher (P< 0.05) expression in the granulosa cells of antral follicles was observed relative to other follicular stages (Fig. 4).
FIG. 2. .
Relative expression (measured as % of immunopositive area) of PCNA in ovaries of control, prenatal T-, and prenatal DHT-treated Day 90 and Day 140 fetuses, 22-wk-old, 10-mo-old, and 21-mo-old sheep. Significant differences across follicle classes for each given age are shown by differing letters. *Significant treatment effect within follicular compartment for each follicular class at each age. Prim., primordial; Pry., primary; Small PreA., small preantral; Large PreA., large preantral; Gr, granulosa.
FIG. 3. .
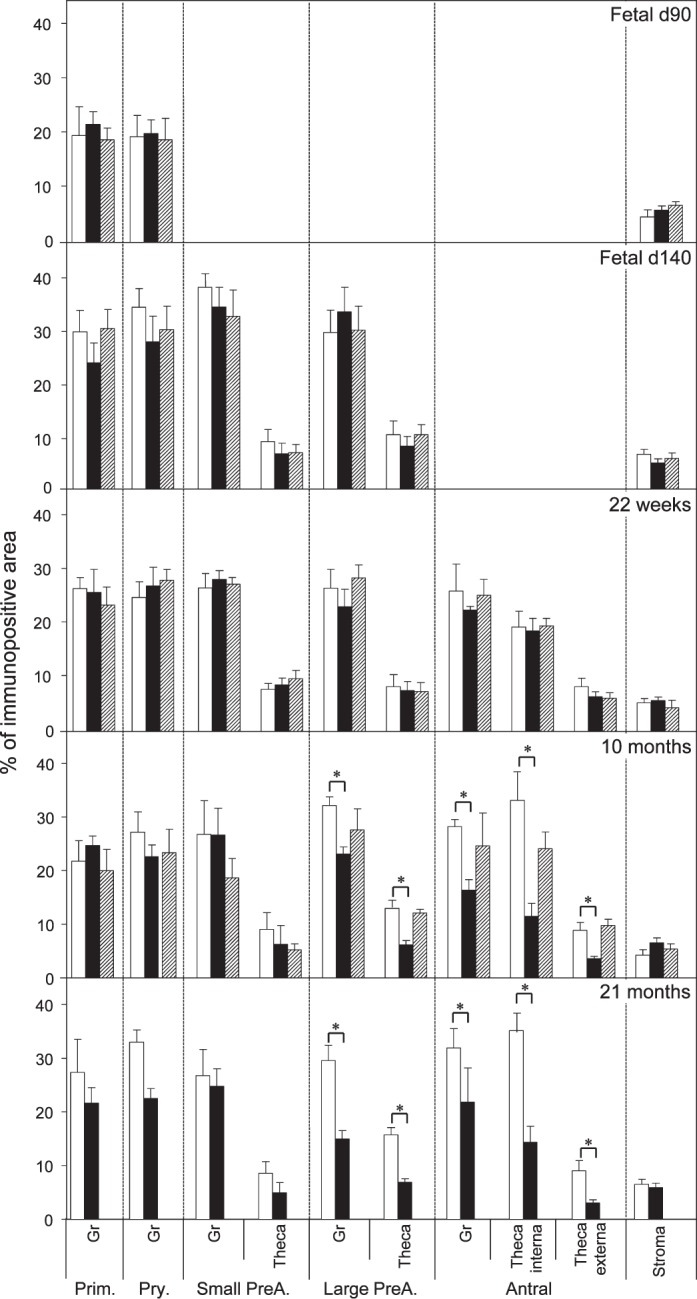
Relative expression (measured as % of immunopositive area) of BCL2 in ovaries of control, prenatal T-, and prenatal DHT-treated Day 90 and Day 140 fetuses, 22-wk-old, 10-mo-old, and 21-mo-old sheep. *Significant treatment effect within follicular compartment for each follicular class at each age. Prim., primordial; Pry., primary; Small PreA., small preantral; Large PreA., large preantral; Gr, granulosa.
FIG. 4. .
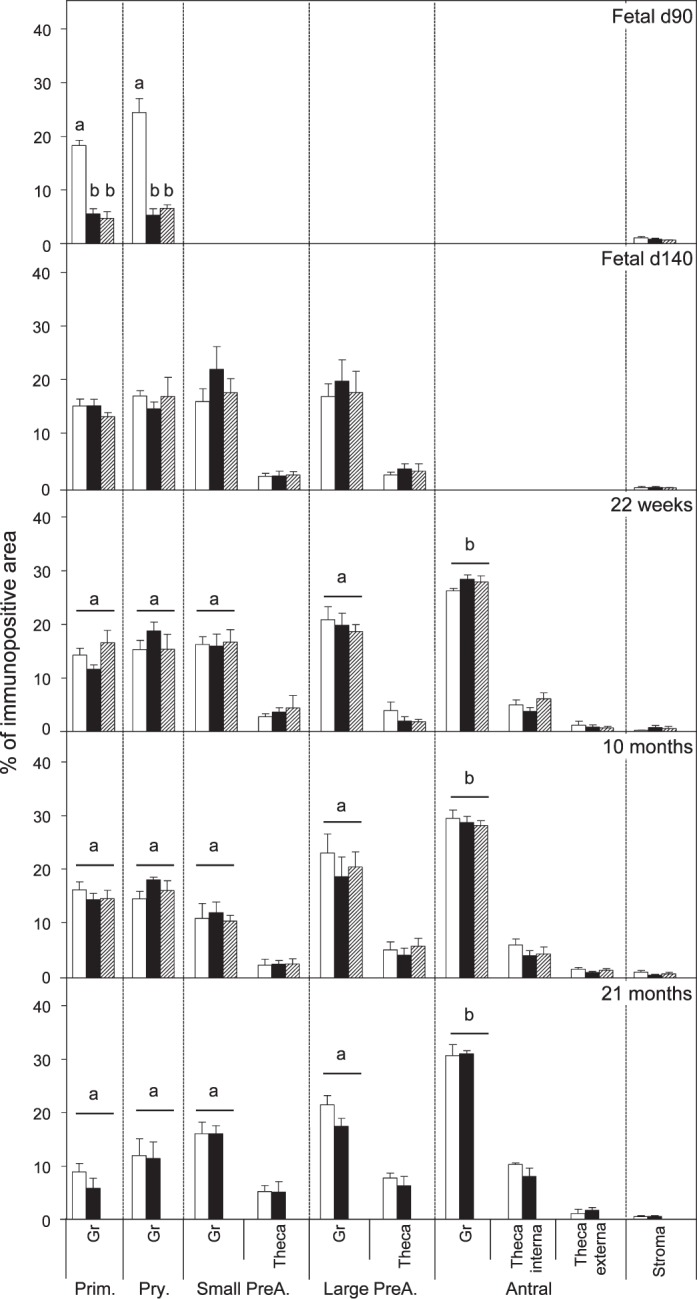
Relative expression (measured as % of immunopositive area) of BAX in ovaries of control, prenatal T-, and prenatal DHT-treated Day 90 and Day 140 fetuses, 22-wk-old, 10-mo-old, and 21-mo-old sheep. Significant differences across follicle classes for each given age are shown by differing letters. *Significant treatment effect within follicular compartment for each follicular class at each age. Prim., primordial; Pry., primary; Small PreA., small preantral; Large PreA., large preantral; Gr, granulosa.
CASP3 was localized in the cytoplasm and weakly in the nucleus of granulosa cells, while thecal cells and stromal cells showed low level of immunostaining. Expression level in the granulosa cells increased with follicular differentiation with the highest level seen in preantral but decreasing in antral follicles (P < 0.05; Fig. 5). Expression level of BAX in antral follicles was similar to that seen in primary follicles. FAS was moderately expressed in the cytoplasm of granulosa, theca, and stromal cells without differences in level of expression across follicular stages (Figs. 1 and 6). FASLG expression was localized only in atretic follicles with no immunostaining evident in healthy follicles or stroma (Fig. 1).
FIG. 5. .
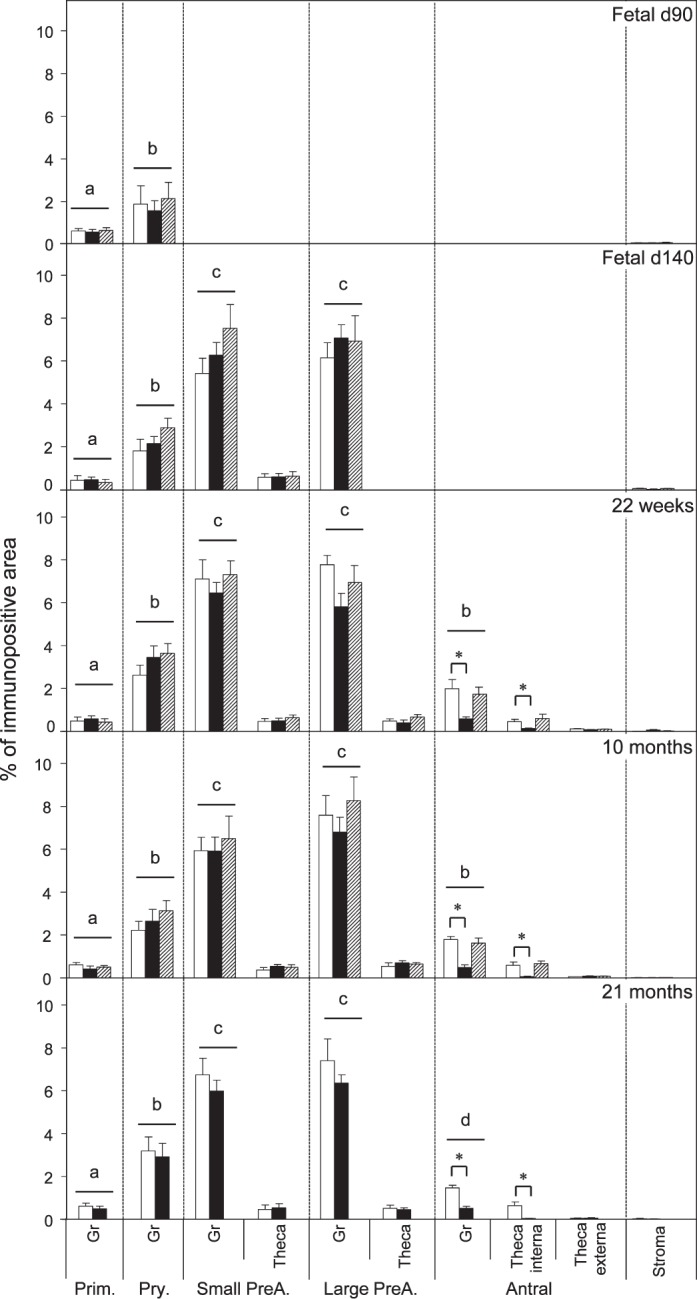
Relative expression (measured as % of immunopositive area) of CASP3 in ovaries of control, prenatal T-, and prenatal DHT-treated Day 90 and Day 140 fetuses, 22-wk-old, 10-mo-old, and 21-mo-old sheep. Significant differences across follicle classes for each given age are shown by differing letters. *Significant treatment effect within follicular compartment for each follicular class at each age. Prim., primordial; Pry., primary; Small PreA., small preantral; Large PreA., large preantral; Gr, granulosa.
FIG. 6. .
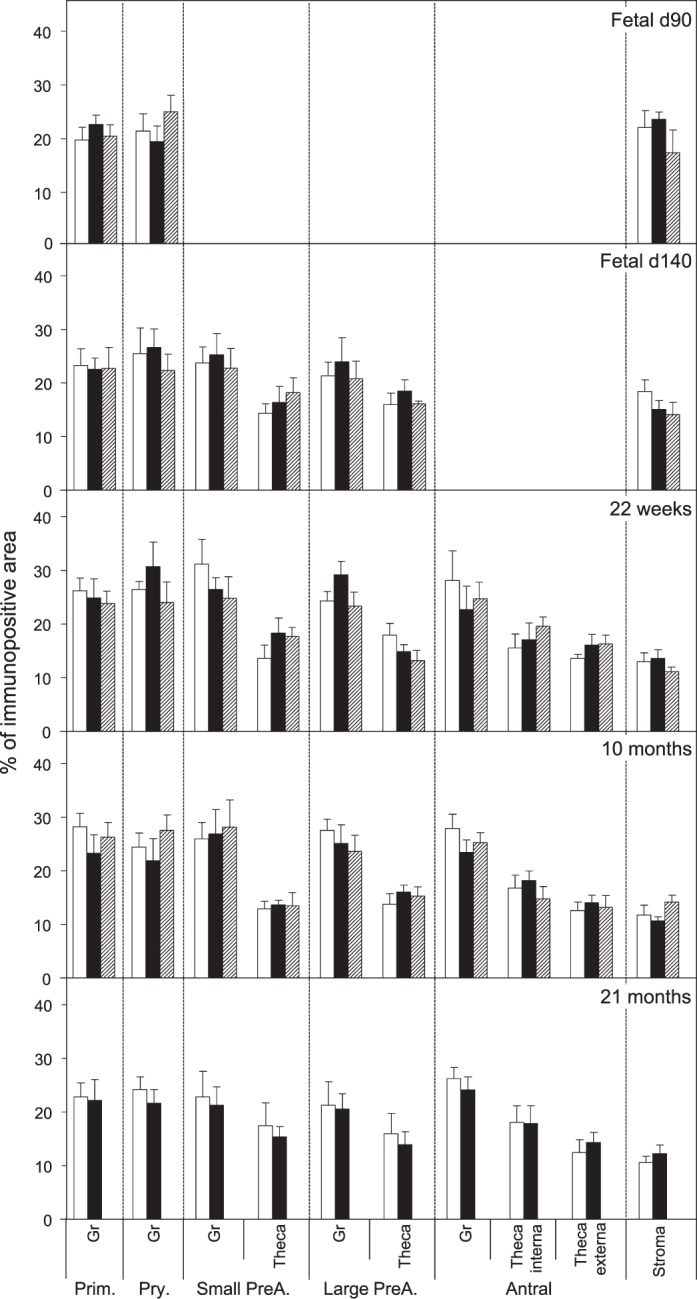
Relative expression (measured as % of immunopositive area) of FAS in ovaries of control, prenatal T-, and prenatal DHT-treated Day 90 and Day 140 fetuses, 22-wk-old, 10-mo-old, and 21-mo-old sheep. Prim., primordial; Pry., primary; Small PreA., small preantral; Large PreA., large preantral; Gr, granulosa.
Effects of Prenatal T and DHT Treatment
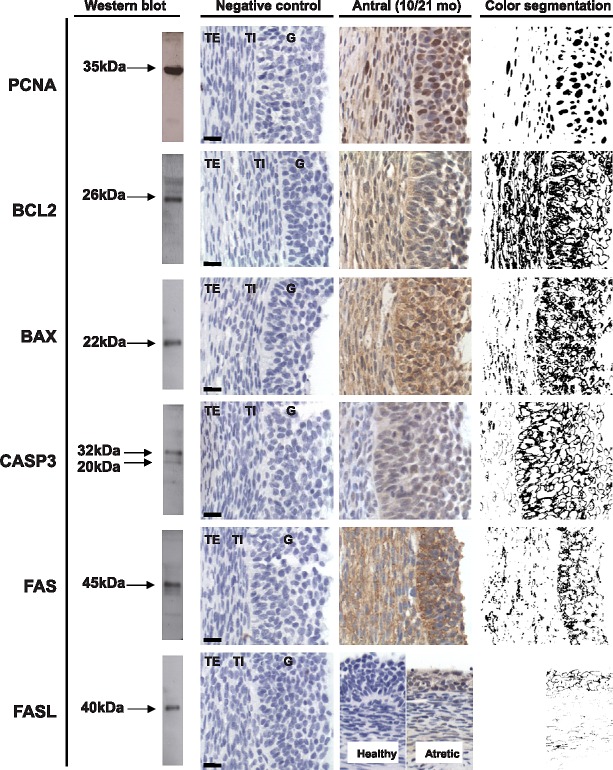
Both prenatal T and DHT treatment failed to have an effect on the expression of PCNA at Fetal Days 90 and 140 or 22 wk. Prenatal T but not DHT treatment (studied at 10 mo only) increased (P < 0.05) PCNA expression in granulosa and theca cells of antral follicles of 10- and 21-mo-old animals (Figs. 2 and 7). Prenatal T but not DHT treatment decreased (P < 0.05) BCL2 expression in granulosa and theca cells of antral follicles of 10- and 21-mo-old animals (Figs 2, 3, and 7) but not in other follicular classes at any age studied. Prenatal T as well as DHT treatments decreased BAX immunostaining (P < 0.05) in granulosa cells of Fetal Day 90 primordial and primary follicles (Figs. 4 and 7) but not any follicular classes at any other age. Prenatal T treatment but not DHT treatment decreased (P < 0.05) CASP3 expression in granulosa and theca cells of antral follicles of 22-wk and 10- and 21-mo-old animals (Figs. 5 and 7) but not earlier ages. Prenatal T or DHT treatment had no effect on FAS protein expression in any of the cellular compartments at any fetal or postnatal ages studied (Fig. 6). None of these markers were altered by prenatal T or DHT treatment in the stromal cells at any age studied.
FIG. 7. .
Representative images of PCNA and BCL2 in antral (10 mo old), BAX in primordial (primary Fetal Day 90) and CASP3 in antral follicles (10 mo old). G, granulosa cells; TE, theca externa; TI, theca interna; O, oocyte; Pi, primary follicle; Po, primordial follicle. Bar = 25 μm.
DISCUSSION
Findings from this study provide evidence that exposure to excess T/DHT prenatally alters the proliferation/apoptosis indices in a follicle-, age-, and steroid-specific manner. Prenatal T excess induced changes in PCNA, BCL2, and CASP3 expression in antral follicles during adult but not fetal life, while prenatal T and DHT treatment reduced BAX expression in primordial and primary follicles during fetal but not adult life. Furthermore, comparative assessment of developmental changes in prenatal T- and DHT-treated females found that the effects on BAX are programmed by androgenic actions of T, while the effects on PCNA, BCL2, and CASP3 likely by its estrogenic action.
Prenatal Changes in Apoptosis and Proliferation Factors
From a developmental perspective, the increase in expression of PCNA and proapoptotic proteins BAX and CASP3 with advanced follicular differentiation supports the existence of a delicate balance between these factors in controlling cellular events. The finding that the effects of T and DHT treatment on BAX expression is evident only in Fetal Day 90 but not Fetal Day 140 ovaries indicates that this is the direct effect of increased androgen signaling, stemming from the gestational T/DHT treatment per se. In support of this, our recent studies have found that levels of T are elevated in gestational T-treated females fetuses at Fetal Day 90 but not Fetal Day 140 [29], although ovarian AR expression is increased at both fetal ages [25]. The reduction in the proapoptotic factor, BAX, is likely a contributing factor in the increased recruitment evidenced at Fetal Day 140 [5]. This is consistent with the observation that during fetal life, a significant proportion of oocytes and follicles undergo apoptosis and that this is associated with increased BAX expression [30]. Further evidence in support for this premise comes from the finding that BAX knockout mice have increased primordial follicles [31].
While a treatment effect was observed with BAX at Fetal Day 90 following prenatal T/DHT treatment, no changes in expression of BCL2 were evident. The finding of similar level of expression of anti apoptotic BCL2 protein in the granulosa cells of all follicle classes during fetal life is consistent with the concept that BCL2 is expressed in a constitutive manner [32, 33]. Considering that apoptosis is regulated by the balance of proapoptotic and antiapoptotic factors, the lack of change in BCL2 in the face of reduced BAX is supportive of reduced follicular apoptosis during fetal life.
The finding that CASP3 is localized in granulosa cells increasing throughout follicular development is also consistent with its predicted role in granulosa cell apoptosis [34]. Involvement of CASP3 in follicular atresia comes from studies of CASP3 in rat granulosa cells [35, 36] and granulosa-lutein cells obtained from patients undergoing assisted reproductive technology [37]. The finding that neither T nor DHT had an impact on CASP3 during fetal life (this study), in the face of increased recruitment evidenced at Fetal Day 140 [5], indicates that it has limited role in follicular recruitment. This premise is supported by the lack of impact of CASP3 deficiency in altering the ovarian reserve of CASP3 KO mice [34]. Reduction of BAX expression at Fetal Day 90 but not CASP3 in prenatal T/DHT fetuses suggests a block in induction of cytochrome c release from the mitochondria, which activates CASP3. Other studies have shown that active CASP3 has a role in controlling cell cycle and cell differentiation in the developing ovary [38].
Our findings of lack of changes in FAS expression across follicle stages differ from earlier studies in rodents that showed FAS expression to vary during follicular development [39]. This discrepancy may relate to species differences. Lack of impact of prenatal T/DHT excess on FAS and FASL ligand across follicular development is supportive of a lack of a role for these ligands in establishing follicular reserve. This is also supported by the fact that FAS-knockout mice show increased number of germ cells [40]. The finding from this study that FASL expression was evident only in atretic follicles is also consistent with earlier studies that found higher concentrations of FASLG in granulosa cells from atretic follicles [19, 41]. Conceivably, granulosa cells express FAS antigen during various stages of development as a means to facilitate rapid atresia on receipt of cytotoxic signals.
From a proliferation stand point, the finding that PCNA expression changes with advancing follicular differentiation is in agreement with findings in mouse and rat ovaries [42, 43] and its role in granulosa cell proliferation. Increased expression of PCNA has been found to accompany activation of primordial follicles and growth of follicular cells [44, 45]. Lack of effects of prenatal T and DHT treatment on PCNA expression during fetal life, however, does not support a role for PCNA in enhancing follicular recruitment evidenced at Fetal Day 140. Conceivably, enhanced follicular recruitment/persistence evident in prenatal T-treated sheep [5–7, 28] may be mediated by other mediators, such as the cyclin-dependent kinase inhibitor 1B, commonly known as p27. Previous studies with p27-deficient (p27−/−) mice have demonstrated that p27 controls ovarian development by suppressing follicle endowment and activation and by promoting follicle death [46]. Others studies have found that loss of p27 leads to premature activation of primordial follicles and rescue from the massive follicular death that occurs before sexual maturity [47]. Interestingly, DHT downregulates p27 by causing its degradation through the ubiquitin-proteasome proteolytic pathway [48]. Whether p27 underlies the changes in recruitment/depletion/persistence evident across the reproductive life span of prenatal T-treated animals [28] remains to be determined.
Postnatal Changes in Apoptosis and Proliferation Factors
The effects of prenatal T/DHT on apoptotic and proliferation indices at 10 (postpubertal) and 22 mo (adult) of age were opposite to that of found during fetal life. In terms of apoptotic signals, while a decline in BAX was evident in primordial and primary follicles of prenatal T- or DHT-treated females at Fetal Day 90, no change in expression of BAX was evident in any follicular compartment at any stage of follicular development at any age studied. In contrast, while no change in BCL2 was evident during fetal life (Days 90 and 140), effects on BCL2 were evident at 10 mo (postpubertal) and 22 mo (adult) in both the granulosa and the theca cells of large preantral and antral follicles of prenatal T-treated females. An impact of prenatal T was also evident in CASP3 in antral but not preantral follicles. Such aberrations restricted only to postpubertal life suggest contribution from pubertal events and cyclic hormonal changes (or their lack thereof) in the manifestation of defects. Changes in CASP3 without evident changes in BCL2 or BAX during prepubertal life suggest that the executive phase of the apoptotic machinery may be mediated via alternate (e.g., APAF1) pathways [49].
While one would expect that a reduction in the antiapoptotic signal BCL2 may increase effector caspases, the decrease in BCL2 in preantral/antral follicles of 10- and 21-mo-old prenatal T-treated females was accompanied not by an increase but rather by a decrease in active CASP3. The reduction in CASP3 is likely responsible for the antral follicle arrest and persistence [6, 7] and multifollicular ovary morphology [4, 5] evidenced in prenatal T-treated females. The finding that the changes in BCL2 and CASP3 are evident only in prenatal T- but not DHT-treated animals is in concert with follicular persistence observed in prenatal T- but not DHT-treated females. These findings are also consistent with estrogenic reprogramming of these disruptions. In support, an increase in estradiol release is clearly evident in the female fetuses of prenatal T-treated females [29].
Considering that estrogens enhance the responsiveness of ovarian follicles to gonadotropin stimulation and increase granulosa cell proliferation, the overexpression of PCNA may be related to altered ESR1:ESR2 balance in postpubertal and adult females [25]. The differences in apoptosis and cellular proliferation observed in our study may also be a reflection of disrupted androgen signaling. However, while BCL2 and CASP3 were downregulated in the granulosa as well as theca cells of the prenatal T-treated females, AR expression underwent opposing changes in granulosa and theca cells; AR expression was increased in granulosa but decreased in theca cells [25]. The opposing changes in AR in these two cellular compartments in the face of similar changes in apoptotic signals suggest the involvement of cell-specific pathways.
The finding that BCL2 is downregulated in preantral and antral follicles while downregulation of CASP3 was restricted to the antral follicular stage of adult animals implies that changes in BCL2 are the likely trigger for induction of effector CASP3. Similarly, occurrence of BCL2 changes at a preantral stage, when AR changes are first evident [25], supports their involvement in follicular persistence [6]. Studies in cancer cell lines have suggested a link between AR overexpression and resistance to apoptosis [50].
Paradoxically, the increase in expression of the proliferative marker PCNA in antral follicles of prenatal T-treated females was found to go hand in hand with decreased BCL2, an antiapoptotic signal. These opposing signals likely disrupt the follicular environment and prevent the follicles from either undergoing atresia or proceeding to a preovulatory state, thus leading to follicular arrest. As such, the increase in PCNA may be responsible for overcoming the effects of decreased expression of BCL2, in preventing the increase in effector CASP3, and in arresting the follicles from undergoing atresia.
Translational Relevance
The findings from this study are likely to be of translational relevance to the ovarian phenotype of women with PCOS, whose characteristics the prenatal T-treated females recapitulate [8–11]. Similar to the prenatal T-treated sheep [5, 28] ovaries from PCOS women have increased number of growing follicles [51] suggestive of an intrinsic ovarian abnormality and disordered follicular development [3, 52]. Therefore, an imbalance in proliferative and apoptotic signals seen in the prenatal T females may underlie the follicular arrest and accumulation of multiple antral follicles in ovaries of women with PCOS. Higher proliferation rates [53] and lower apoptotic rates have been found in granulosa cells of women with PCOS compared to women with regular ovulatory cycles [3]. Studies of Almahbobi et al. [54] that document that the majority of the granulosa cells of polycystic ovaries are not apoptotic are in agreement with reduced CASP3 expression seen in prenatal T-treated females. These findings are also in agreement with modulators of cell death playing a role in determining the fate of follicles in the ovary [36, 55] and in the cyclical growth and regression of follicles in the ovary [22].
In summary, findings from this study demonstrate that the exposure to excess T during fetal life disrupts the proliferation:apoptosis balance in follicular cells, thus providing an explanation for the follicular arrest and persistence [5–7, 28] evidenced in these females, and are likely to be of translational relevance to women with PCOS.
ACKNOWLEDGMENT
We are grateful to Mr. Douglas Doop for his expert animal care, facility management, and help with generation of the experimental lambs; Drs. Mohan Manikkam, Teresa Steckler, Ms. Olga Astapova, Ms. Carol Herkimer, and Mr. James S. Lee for assistance with prenatal steroid treatment and/or collection, processing, and sectioning of ovaries; and staff members of the Laboratory of Molecular and Cellular Biology (FCV-UNL) for their technical support during processing of the slides.
Footnotes
Supported by National Institutes of Health grant P01-HD-44232 (to V.P.).
REFERENCES
- Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt MA, Dupont J, Fortune JE, Gilchrist RB, Martin GB, McNatty KP, McNeilly AS, Monget P, Monniaux D. et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev 2011; 23: 444 467 [DOI] [PubMed] [Google Scholar]
- Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update 2008; 14: 367 378 [DOI] [PubMed] [Google Scholar]
- Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocr Metab 2008; 93: 881 887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol 2001; 185: 51 59 [DOI] [PubMed] [Google Scholar]
- Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod 2009; 80: 726 736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology 2006; 147: 1997 2007 [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology 2007; 148: 3532 3540 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 2006; 246: 165 174 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V. Veiga-Lopez, Abbott DH, Dumesic DA. Developmental programming of ovarian disruption. : Gonzalez-Bulnes A. (ed.), Novel Concepts in Ovarian Endocrinology. Trivandrum, Kerala, India: Research Signpost; 2007: 329 352 [Google Scholar]
- Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int J Androl 2009; 33: 394 404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: androgenic vs. estrogenic reprogramming. Semin Reprod Med 2011; 29: 173 186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatty KP, Fidler AE, Juengel JL, Quirke LD, Smith PR, Heath DA, Lundy T, O'Connell A, Tisdall DJ. Growth and paracrine factors regulating follicular formation and cellular function. Mol Cell Endocrinol 2000; 163: 11 20 [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132: 191 206 [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev 1994; 15: 707 724 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Tissue-specific Bcl-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev 2000; 80: 593 614 [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Sazón R, Keren-Tal I, Aharoni D, Dantes A, Rimon E, Land A, Cohen T, Dor Y, Hirsh L. Alternative pathways of ovarian apoptosis: death for life. Biochem Pharmacol 2003; 66: 1355 1362 [DOI] [PubMed] [Google Scholar]
- Maruo T, Laoag-Fernandez JB, Takekida S, Peng X, Deguchi J, Samoto T, Kondo H, Matsuo H. Regulation of granulosa cell proliferation and apoptosis during follicular development. Gynecol Endocrinol 1999; 13: 410 419 [DOI] [PubMed] [Google Scholar]
- Salvetti NR, Panzani CG, Gimeno EJ, Neme LG, Alfaro NS, Ortega HH. An imbalance between apoptosis and proliferation contributes to follicular persistence in polycystic ovaries in rats. Reprod Biol Endocrinol 2009; 7: 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti NR, Stangaferro ML, Palomar MM, Alfaro NA, Rey F, Gimeno EJ, Ortega HH. Cell proliferation and survival mechanisms underlying the abnormal persistence of follicular cysts in bovines with cystic ovarian disease induced by ACTH. Anim Reprod Sci 2010; 122: 98 110 [DOI] [PubMed] [Google Scholar]
- Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update 2005; 11: 162 177 [DOI] [PubMed] [Google Scholar]
- Slot KA, Voorendt M, De Boer-Brouwer M, Van Vugt HH, Teerds KJ. Estrous cycle dependent changes in expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and pro- and active CASP3 in the rat ovary. J Endocrinol 2006; 188: 179 192 [DOI] [PubMed] [Google Scholar]
- Tilly JL. Apoptosis and ovarian function. Rev Reprod 1996; 1: 162 172 [DOI] [PubMed] [Google Scholar]
- Johnson AL. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim Reprod Sci 2003; 78: 185 201 [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 2004; 145: 790 798 [DOI] [PubMed] [Google Scholar]
- Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction 2009; 137: 865 877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HH, Rey F, Velazquez MML, Padmanabhan V. Effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins. Biol Reprod 2010; 82: 1065 1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH, Hudson NL, Moeller CL, Cranfield M, Reader KL, Laitinen MP, Groome NP, Sawyer HR. et al. Oocyte-derived growth factors and ovulation rate in sheep. Reprod Suppl 2003; 61: 339 351 [PubMed] [Google Scholar]
- Padmanabhan V, Smith P, Veiga-Lopez A. Developmental programming: Impact of prenatal testosterone treatment and postnatal obesity on ovarian follicular dynamics. J Dev Origins Health Dis 2012; (in press) Published online ahead of print 7 March 2012; DOI 10.1017/S2040174412000128 [DOI] [PMC free article] [PubMed]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod 2011; 84: 87 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, te Velde ER, Stenbäck F, Heikinheimo M, Tapanainen JS. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab 2001; 86: 3421 3429 [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995; 270: 96 99 [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI, Kenton ML, Johnson AL. Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger ribonucleic acid levels. Endocrinology 1995; 136: 232 241 [DOI] [PubMed] [Google Scholar]
- Kim MR, Tilly JL. Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochim Biophys Acta 2004; 1644: 205 210 [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, Tilly JL. CASP3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology 2001; 142: 2468 2480 [DOI] [PubMed] [Google Scholar]
- Flaws JA, Kugu K, Trbovich AM, DeSanti A, Tilly KI, Hirshfield AN, Tilly JL. Interleukin-1 beta-converting enzyme-related proteases (IRPs) and mammalian cell death: dissociation of IRP-induced oligonucleosomal endonuclease activity from morphological apoptosis in granulosa cells of the ovarian follicle. Endocrinology 1995; 136: 5042 5053 [DOI] [PubMed] [Google Scholar]
- Boone DL, Tsang BK. CASP3 in the rat ovary: localization and possible role in follicular atresia and luteal regression. Biol Reprod 1998; 58: 1533 1539 [DOI] [PubMed] [Google Scholar]
- Kugu K, Ratts VS, Piquette GN, Tilly KI, Tao XJ, Martimbeau S, Aberdeen GW, Krajewski S, Reed JC, Pepe GJ, Albrecht ED, Tilly JL. Analysis of apoptosis and expression of bcl-2 gene family members in the human and baboon ovary. Cell Death Differ 1998; 5: 67 76 [DOI] [PubMed] [Google Scholar]
- Los M, Stroh C, Janicke RU, Engels IH, Schulze-Osthoff K. Caspases: more than just killers? Trends Immunol 2001; 22: 31 34 [DOI] [PubMed] [Google Scholar]
- Porter DA, Vickers SL, Cowan RG, Huber SC, Quirk SM. Expression and function of Fas antigen vary in bovine granulosa and theca cells during ovarian follicular development and atresia. Biol Reprod 2000; 62: 62 66 [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M, Sakamaki K, Akazawa Y, Miyano T. Oocyte growth and follicular development in KIT-deficient Fas-knockout mice. Reproduction 2007; 133: 117 125 [DOI] [PubMed] [Google Scholar]
- Porter DA, Harman RM, Cowan RG, Quirk SM. Susceptibility of ovarian granulosa cells to apoptosis differs in cells isolated before or after the preovulatory LH surge. Mol Cell Endocrinol 2001; 176: 13 20 [DOI] [PubMed] [Google Scholar]
- Muskhelishvili L, Wingard SK, Latendresse JR. Proliferating cell nuclear antigen—a marker for ovarian follicle counts. Toxicol Pathol 2005; 33: 365 368 [DOI] [PubMed] [Google Scholar]
- Balla M, Angelopoulou R, Lavranos G, Kittas C. Follicular cells versus oocytes: cell population dynamics in the developing ovary. Tissue Cell 2008; 40: 373 381 [DOI] [PubMed] [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol 2000; 163: 53 60 [DOI] [PubMed] [Google Scholar]
- Xu B, Hua J, Zhang Y, Jiang X, Zhang H, Ma T, Zheng W, Sun R, Shen W, Sha J, Cooke HJ, Shi Q. Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS ONE 2011; 6: e16046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldis P. Another piece of the p27Kip1 puzzle. Cell 2007; 128: 241 244 [DOI] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P, Liu K. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol 2007; 21: 2189 2202 [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang Y, Tong X, Yang Y, Shao Z. Dihydrotestosterone induces p27 degradation via direct binding with SKP2 in ovarian and breast cancer. Int J Mol Med 2011; 28: 109 114 [DOI] [PubMed] [Google Scholar]
- Liu X, Zou H, Slauter C, Wang X. DFF. a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 1997; 89: 175 184 [DOI] [PubMed] [Google Scholar]
- Wang LG, Ossowski L, Ferrari AC. Overexpressed androgen receptor linked to p21WAF1 silencing may be responsible for androgen independence and resistance to apoptosis of a prostate cancer cell line. Cancer Res 2001; 61: 7544 7551 [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis.” Obstet Gynecol Surv 1982; 37: 59 77 [DOI] [PubMed] [Google Scholar]
- Franks S, McCarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl 2006; 29: 278 285 [DOI] [PubMed] [Google Scholar]
- Stubbs SA, Stark J, Dilworth SM, Franks S, Hardy K. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab 2007; 92: 4418 4426 [DOI] [PubMed] [Google Scholar]
- Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO. Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol 1996; 44: 571 580 [DOI] [PubMed] [Google Scholar]
- Jääskeläinen M, Nieminen A, Pökkylä RM, Kauppinen M, Liakka A, Heikinheimo M, Vaskivuo TE, Klefström J, Tapanainen JS. Regulation of cell death in human fetal and adult ovaries—role of Bok and Bcl-X(L). Mol Cell Endocrinol 2010; 330: 17 24 [DOI] [PubMed] [Google Scholar]