ABSTRACT
Fetal growth restriction (FGR) greatly increases the risk of perinatal morbidity and mortality and is associated with increased uterine artery resistance and levels of oxidative stress. There are currently no available treatments for this condition. The hypothesis that the antioxidant 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (Tempol) would improve uterine artery function and rescue fetal growth was tested in a mouse model of FGR, using the endothelial nitric oxide synthase knockout mouse (Nos3−/−). Pregnant Nos3−/− and control C57BL/6J mice were treated with the superoxide dismutase-mimetic Tempol (1 mmol/L) or vehicle from Gestational Day 12.5 to 18.5. Tempol treatment significantly increased pup weight (P < 0.05) and crown–rump length (P < 0.01) in C57BL/6J and Nos3−/− mice. Uterine artery resistance was increased in Nos3−/− mice (P < 0.05); Tempol significantly increased end diastolic velocity in Nos3−/− mice (P < 0.05). Superoxide production in uterine arteries did not differ between C57BL/6J and Nos3−/− mice but was significantly increased in placentas from Nos3−/− mice (P < 0.05). This was not reduced by Tempol treatment. Placental System A activity was reduced in Nos3−/− mice (P < 0.01); this was not improved by treatment with Tempol. Treatment of Nos3−/− mice with Tempol, however, was associated with reduced vascular density in the placental bed (P < 0.05). This study demonstrated that treatment with the antioxidant Tempol is able to improve fetal growth in a mouse model of FGR. This was associated with an increase in uterine artery blood flow velocity but not an improvement in uterine artery function or placental System A activity.
Keywords: intrauterine growth restriction, nitric oxide, oxidative stress, placental transport, pregnancy
Treatment with the antioxidant Tempol increases uterine artery blood velocity and improves fetal growth in a mouse model of fetal growth restriction.
INTRODUCTION
Fetal growth restriction (FGR), defined as a fetus that fails to achieve its genetic growth potential, complicates up to 10% of pregnancies worldwide [1]. The perinatal mortality rate is 4–10 times higher in FGR fetuses than in normally grown infants, and 5%–10% of pregnancies complicated by FGR will result in either stillbirth or neonatal death [2]. There are also long-term consequences for FGR infants: neurological and developmental delays may occur [3], and there is also the well-documented relationship between size at birth and an increased risk of developing cardiovascular disease or diabetes in later life [3, 4]. Hence, understanding the underlying mechanisms of FGR, as well as identifying potential therapeutic interventions, could have a significant impact.
The cause of FGR is complex and likely multifactorial. It is, however, still poorly understood because there are a number of factors that are known to negatively impact fetal growth, including maternal factors such as undernutrition [5], chronic hypertension [6], and pre-eclampsia [7]. Furthermore, abnormal placentation and fetal factors such as chromosomal anomalies and congenital malformations may also affect birth weight [8].
Abnormal placentation results in FGR through placental insufficiency, that is, the compromised ability of the placenta to exchange nutrients and waste products to and from the fetus. One aspect of placental insufficiency is related to a reduction in blood flow in the uterine circulation. Increased uterine artery resistance is a feature common to human FGR and leads to irregular flow and reduced uteroplacental perfusion [9, 10]. Such reduced perfusion will lead to a reduced efficiency of gaseous and other nutrient exchange across the placenta with direct effects on fetal growth. Abnormal flow in the uterine circulation could also cause hypoxia in the placenta and ischemia/reperfusion events leading to oxidative stress and the production of superoxide anions and other free radicals [11], which might damage structure and function of the placenta.
One putative therapy is the use of an antioxidant. Increased uterine artery resistance causes irregular blood flow and hypoperfusion of the placenta, which in turn leads to an increase in oxidative stress, namely an increased production of superoxide anions. These can react with nitric oxide (NO) in the vasculature, leading to the production of peroxynitrite, which reduces vasodilation, both through the scavenging of NO and through modification of proteins. Reduced vasodilation as a result of increased oxidative stress will therefore further reduce uteroplacental perfusion. Treatment during pregnancy with a specific antioxidant may improve uterine artery vasodilation, increase fetoplacental perfusion, and rescue fetal growth.
4-Hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (Tempol) is an antioxidant drug, specifically, a superoxide dismutase mimetic agent, which is able to metabolize superoxide anions due to the presence of a reducible nitroxide group [12]. This drug has previously been used in a mouse model of pre-eclampsia, the BPH/5 mouse. This is a model which spontaneously develops some of the hallmark features of pre-eclampsia, namely, onset of hypertension and proteinuria [13]. Administration of Tempol to BPH/5 mice was able to ameliorate placental oxidative stress and rescue fetal growth [14]. An advantage of using Tempol, as opposed to other antioxidant therapies, is that it is able to permeate the membrane and specifically targets superoxide anions that are produced intracellularly. Here we have investigated the effectiveness of Tempol in another mouse model that also shows FGR, the endothelial nitric oxide synthase knockout (Nos3−/−) mouse.
The enzyme endothelial nitric oxide synthase (eNOS) catalyzes the cellular conversion of arginine to the potent vasodilator NO, which plays a crucial role in the cardiovascular adaptations of pregnancy that ensure adequate uteroplacental perfusion [15–17]. It has been previously observed that the activity of eNOS in umbilical artery endothelial cells is reduced in pregnancies complicated by FGR [18]. The female Nos3−/− mouse demonstrates an increase in systolic blood pressure both before [19] and during pregnancy [20]. Although the uterine artery of nonpregnant Nos3−/− mice does not demonstrate any structural differences compared with wild-type (WT) controls, the increase in radius which occurs during normal pregnancy is impaired in Nos3−/− mice [21], suggesting abnormal uterine artery remodeling which may impact uterine artery blood flow and hence placental perfusion. There are no differences between litter sizes of Nos3−/− mice and those of WT mice [20], but there is a reported 10% decrease in fetal growth in the knockout mouse [20]. From the previous considerations this FGR in the Nos3−/− mouse could be due to the effect of oxidative stress on the remodeling of the uterine artery, with consequent reduced uteroplacental perfusion and/or an effect on placental exchange function.
This study first tested the hypothesis that Tempol would normalize fetal growth in the Nos3−/− mouse through amelioration of the effects of oxidative stress on uterine artery blood flow and function. We tested this hypothesis by investigating fetal and placental growth and uterine artery Doppler flow in vivo and function in vitro, as well as levels of oxidative stress in vessels from control C57BL/6J and Nos3−/− mice. We next investigated whether the effect of Tempol in increasing pup weight in both C57BL/6J and Nos3−/− mice, without improving uterine artery function, was mediated though amelioration of free radical levels in the placenta and improved transport (System A amino acid transporter activity) function.
MATERIALS AND METHODS
Animals and Treatments
All protocols were approved by the University of Alberta Health Sciences Animal Policy and Welfare committee in accordance with the Canadian Council on Animal Care and conformed to the Guide for the Care and Use of Laboratory Animals (1996, National Academy of Science). Female Nos3−/− and C57Bl/6J mice were purchased from Jackson Laboratories (8–12 weeks); C57BL/6J mice were the background strain used to produce Nos3−/− mice and were used here as the control group. Both groups of mice were mated nightly with males of the corresponding genotype. The day a copulation plug was detected was designated Gestational Day (GD) 0.5. A Tempol solution (1 mmol/L in drinking water) [14] or normal drinking water was administered from GD 12.5, which is when placental development was equivalent to the end of the first trimester in the human, to GD 18.5 (term is GD 19.5). Food and water intake were monitored in all animals and were not altered by Tempol treatment.
Ultrasound Biomicroscopy
Uterine artery blood flow velocity was assessed on one occasion at Day 17.5 of gestation. Before the procedure, mice were anesthetized with isoflurane (3%) in air. Maternal heart rate, respiratory rate, and rectal temperature were continuously monitored, and anesthetic concentration was adjusted (∼0.5%–1.5%) to maintain a constant maternal heart rate of 550 ± 50 bpm and a respiratory rate of 120 ± 20 cpm. Heating was adjusted to maintain rectal temperature between 36° and 38°C. Hair was removed from the abdomen by shaving, followed by a chemical hair remover. Prewarmed gel was used as an ultrasound-coupling medium. Mice were then imaged transcutaneously using an ultrasound biomicroscope (model Vevo 770; VisualSonics, Toronto, ON, Canada) and a 30-MHz transducer operating at 100 frames/sec, as previously described [22]. In Doppler mode, the high-pass filter was set at 6 Hz, and the pulse repetition frequency was set between 4 and 48 kHz. A 0.2- to 0.5-mm pulsed Doppler gate was used, and the angle between the Doppler beam and the vessel was <30°. Images were recorded for offline analysis. Doppler waveforms were obtained in both uterine arteries near the lateral-inferior margin of the uterocervical junction close to the iliac artery on each side. Peak systolic velocity (PSV) and end diastolic velocity (EDV) were measured from at least 3 consecutive cardiac cycles that were not affected by motion caused by maternal breathing, and the results were averaged. Left-to-right uterine artery PSV Pearson correlation was 0.96 (95% confidence interval [CI], 0.91%–0.99%, P < 0.001). The resistance index, calculated using the equation (PSV − EDV)/PSV, was obtained when EDV was >0 to quantify the pulsatility of arterial blood velocity waveforms.
Fetal and Placental Measurements
Mice were culled on GD 18.5, and pups and placentas were dissected. Pups from the right uterine horn were blotted dry and weighed, and fetal crown-to-rump length and abdominal circumference were determined. The gross anatomy of the pups was also examined. Placentas were blotted dry and weighed and then dried at 40°C, and dry weight was recorded. Placentas and the main uterine artery from the left horn were collected and snap frozen in OCT and stored at −80°C until processed for detection of reactive oxygen species (ROS). Uterine arteries from the right horn were dissected clean all of the fat and connective tissue in cold physiological salt solution and mounted in a wire myograph for assessment of vascular function.
Ex Vivo Vascular Function
Four uterine artery segments were mounted in a wire myograph (catalog no. 610M; Danish Myo Technology, Aarhus, Denmark) using 25-μm tungsten wire and warmed to 37°C for 20 min before they were normalized according to standard procedures. Vessels were then activated with phenylephrine (PE; μmol/L) twice, with a 15-min wash between activation periods. Methacholine (MCh; 10 μmol/L) was added at the end of the second PE-induced contraction to determine the integrity of the endothelium.
A PE dose-response curve (0.1 nmol/L to 10 μmol/L in nine steps) was performed, followed by careful washing. The vessels were then preconstricted with PE (at the effective concentration 80 (EC80) calculated after the PE dose-response curve), and the endothelium-dependent relaxation response was tested by a cumulative dose-response curve to MCh (0.1 nmol/L to 10 μmol/L in nine steps). Finally, a dose curve was determined in response to sodium nitroprusside (SNP; 0.1 nmol/L to 10 μmol/L in eight steps) to validate endothelium-independent NO-mediated smooth muscle relaxation. All chemicals were obtained from Sigma (St. Louis, MO).
Detection of Superoxide and Nitrotyrosine
Cryosections of placentas and uterine arteries were taken (20 μm), and dihydroethidium (DHE) was used to assess for the presence of superoxide as described previously [23]. Immunohistochemistry assay was used to detect nitrotyrosine, the footprint of peroxynitrite, in 8-μm cryosections of placenta and uterine arteries. Sections were incubated at room temperature for 1 h with an anti-nitrotyrosine antibody (1:125 dilution; Millipore). This was followed by a 1-h incubation with a goat anti-rabbit secondary antibody (1:250 dilution; AlexaFluor). Sections were then counterstained with .4′,6-diamidino-2-phenylindole (DAPI).
Images were then obtained with fluorescence microscopy (Olympus model IX 81) using the CY3 filter. Images were analyzed using Adobe Photoshop to determine mean fluorescence intensity/pixel. Four sections of each uterine artery and four sections of one associated placenta per animal were used, and mean values were determined.
Placental System A Amino Acid Transport
Placental System A amino acid transport was measured in vivo, using the method described by Constancia et al. [24], by determining unidirectional maternofetal transfer of 14C-labeled α-methylaminoisobutyric acid ([14C]MeAIB). Mice were anesthetized with fentanyl/fluanisone and midazolam solutions in water (1:1:2 water; i.p.). A cannula was inserted into the tail vein (using a 25-G needle attached to 0.58-mm inside diameter tubing), and a bolus dose of [14C]MeAIB, a nonmetabolizable amino acid, was delivered (100 μl, activity of 3.5 μCi). A maternal blood sample was taken by terminal cardiac puncture at 2 min postdose. Fetuses and their corresponding placentas were dissected and then placed in liquid scintillation medium (Biosol; National Diagnostics, Humberside, U.K.) overnight at 55°C until dissolved. Scintillation fluid was then added, and samples were counted on a β scintillation counter. Counts from each fetus were then compared to counts within maternal plasma, and maternofetal clearance per gram of placenta (Kmf) was calculated as described previously [25].
Vascular Casting of the Maternal Bed of the Placenta
Mice were injected with heparinized saline solution (50 U/kg, i.v.) 10 min prior to the procedure. They were then anesthetized with isoflurane, and a terminal blood sample was taken by cardiac puncture. The abdomen was opened, and both uterine horns were exteriorized; the descending aorta was identified and cannulated infrarenally. The uterine circulation was then perfused with 10 ml of heparinized saline (50 U/ml) at a rate of 1 ml/min. This was followed by perfusion with 6.3 ml of contrast agent, a freshly mixed radiopaque silicone polymer containing lead chromate (Microfil MV 122; Flow Tech Inc., Carver, MA), at a rate of 1 ml/min. The polymer agent was allowed to cure for 1 h; the uterus was removed, wrapped in plastic film, and kept at 4°C overnight. Individual placentas were then dissected and stored in 4% neutral-buffered formalin until analysis. Placentas were scanned at 0.3° increments, using a microtomography scanner (SkyScan 1076 system; Micro Phonetics Inc.), and x-ray transmission images were acquired in each angle of view at a resolution of 9 μm and digitized to a 16-byte gray scale. Three-dimensional volume images were reconstructed using a filtered back-projection algorithm and displayed on a computer workstation by volume rendering for display and analysis of renal MV, using Analyze software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN), and the spatial density of microvessels (diameters of 1–500 μm) were calculated as previously described [26, 27].
Statistical Analysis
All normally distributed data are expressed as means ± SEM and were compared using the Student t-test and one-way ANOVA or two-way ANOVA that included genotype and treatment as factors of variation, followed by Bonferroni post hoc test. A repeated measures two-way ANOVA was used to compare endothelium-dependent relaxation. A P value of <0.05 was considered statistically significant. Data that were not normally distributed are expressed as median values and were compared using the Kruskal-Wallis test, followed by the Dunn multiple comparison test. Sigmoidal curve fitting was performed on wire myography concentration-response curve data, using GraphPad Prism version 5.0 software; curves were then used to determine either EC50 or EC80 values.
RESULTS
Fetal and Placental Growth
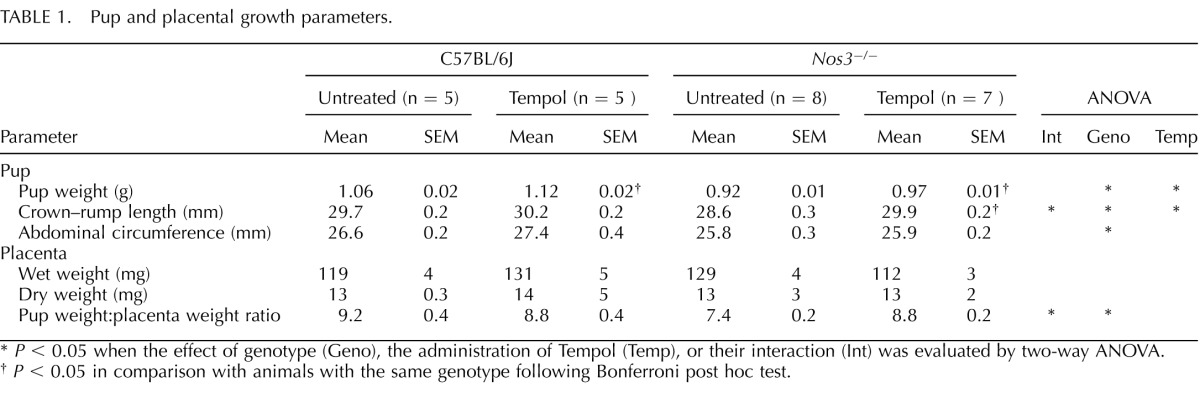
Litter sizes did not differ between groups on GD 18.5, and no gross fetal anomalies were noted in any of the groups. Pup growth, as evaluated on GD 18.5, showed that Nos3−/− mice had reduced body weight, crown–rump length, and abdominal circumference compared with those from C57BL/6J mice (P < 0.01) (Table 1). Tempol significantly increased pup weight in both C57BL/6J and Nos3−/− mice (P < 0.05) (Table 1). Tempol also significantly increased pup crown–rump length in Nos3−/− mice (P < 0.01), and there was a significant interaction between genotype and treatment (P < 0.05) (Table 1). There was no effect of Tempol on abdominal circumference. There were no differences in placental wet or dry weight between Nos3−/− and C57BL/6J mice; furthermore, Tempol treatment had no effect on placental wet or dry weight (Table 1). The body weight:placental weight ratio was lower in Nos3−/− mice than in C57BL/6J mice; this was normalized in Tempol-treated Nos3−/− mice, and there was a significant interaction between genotype and treatment (P = 0.004) (Table 1).
TABLE 1. .
Pup and placental growth parameters.
P < 0.05 when the effect of genotype (Geno), the administration of Tempol (Temp), or their interaction (Int) was evaluated by two-way ANOVA.
P < 0.05 in comparison with animals with the same genotype following Bonferroni post hoc test.
Uterine Artery Blood Flow Velocity
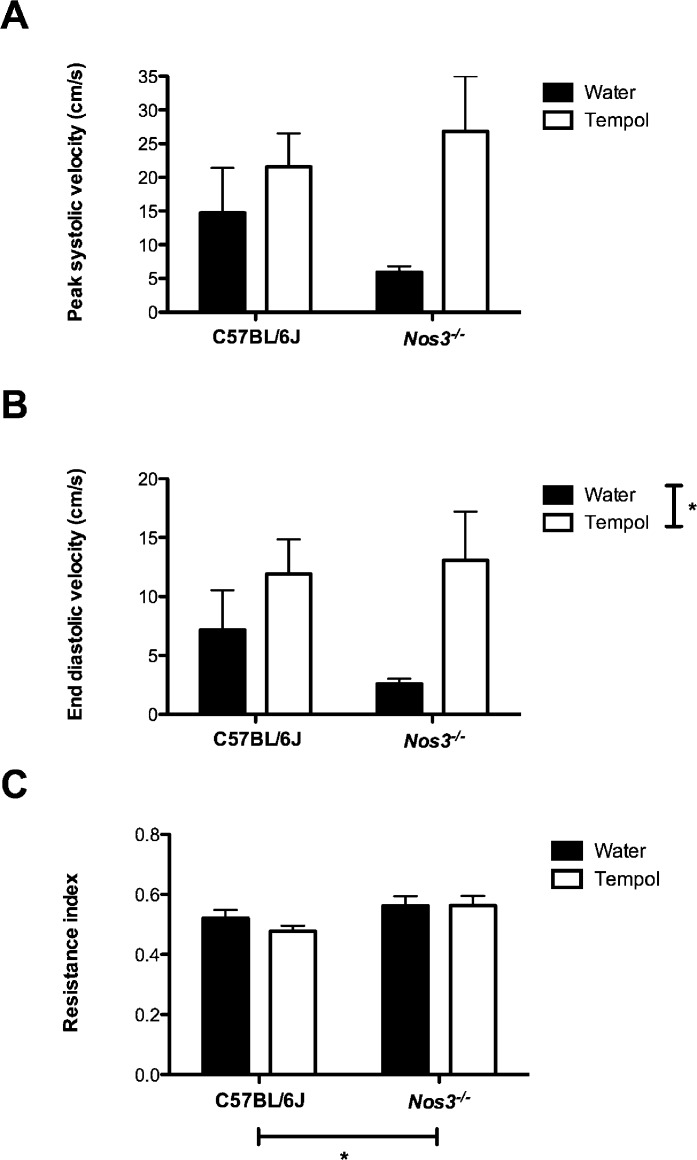
No differences in uterine artery blood flow velocity were found between Nos3−/− and C57BL/6J mice (C57BL/6J PSV was 14.8 ± 6.6 cm/sec, and EDV was 7.2 ± 3.4 cm/sec; Nos3−/− PSV was 6.0 ± 0.9 cm/sec, and EDV was 2.6 ± 0.4 cm/sec) (Fig. 1, A and B). There was, however, a significant increase in the resistance index in the uterine artery of Nos3−/− mice compared with that in C57BL/6J mice (0.6 ± 0.03 vs. 0.5 ± 0.03; P < 0.05) (Fig. 1C). Although Tempol increased both PSV and EDV in both groups of mice compared with their genotype control, this change was statistically significant only when EDV was considered (i.e., C57BL/6J PSV of 21.6 ± 5.0 cm/sec, and EDV of 11.9 ± 2.9 cm/sec; Nos3−/− PSV of 26.8 ± 8.2 cm/sec, EDV of 13.1 ± 4.2 cm/sec) (Fig. 1, A and B). There was no effect of Tempol on the resistance index in either C57BL/6J or Nos3−/− mice (0.5 ± 0.02 vs. 0.6 ± 0.03, respectively) (Fig. 1C).
FIG. 1. .
Uterine artery blood flow velocity waveforms were unaffected by genotype or Tempol treatment. There were no differences in uterine artery PSV (A), EDV (B), or resistance index (C) between C57BL/6J and Nos3−/− mice. There was no effect of treatment with Tempol. Means ± SEM are shown for n = 5–8 mice; two-way ANOVA; *P < 0.05.
Uterine Artery Function
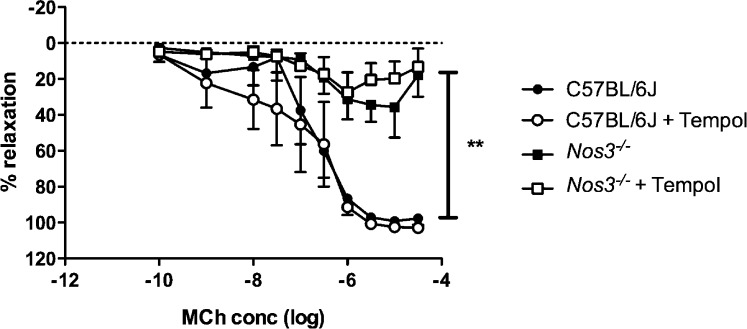
Uterine artery function was assessed using either the endothelium-dependent vasodilator MCh or the endothelium-independent NO donor SNP. Uterine arteries from Nos3−/− mice showed reduced endothelium-dependent relaxation compared to arteries from C57BL/6J mice (P < 0.01) (Fig. 2); there was no effect of Tempol treatment on endothelium-dependent relaxation in either group (Fig. 2). There were no differences in the relaxation response in response to SNP between arteries from Nos3−/− and those from C57BL/6J mice (data not shown).
FIG. 2. .
Endothelium-dependent relaxation was significantly impaired in the uterine artery of Nos3−/− mice. Endothelium-dependent relaxation was significantly reduced in uterine artery sections from Nos3−/− mice compared with that from C57BL/6J mice. There was no effect of Tempol treatment in either group. Data are means ± SEM for n = 6 mice; two-way ANOVA followed by Bonferroni post hoc test; **P < 0.01.
Placental and Uterine Artery ROS
When uterine arteries from C57BL/6J and Nos3−/− mice were examined, there were no differences in intensity of DHE staining (superoxide production; Nos3−/− mice showed 100% ± 26% compared to that of C57BL/6J) or nitrotyrosine staining (103 ± 12 vs. 110 ± 23 pixels, respectively) between the two groups.
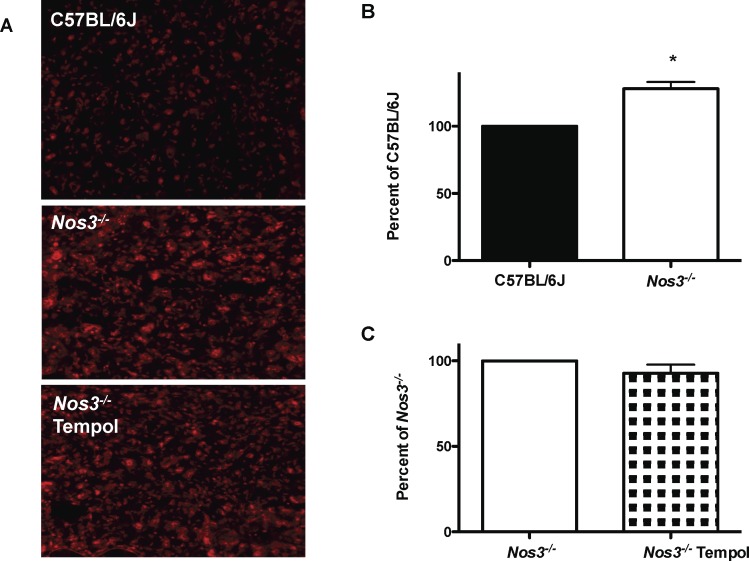
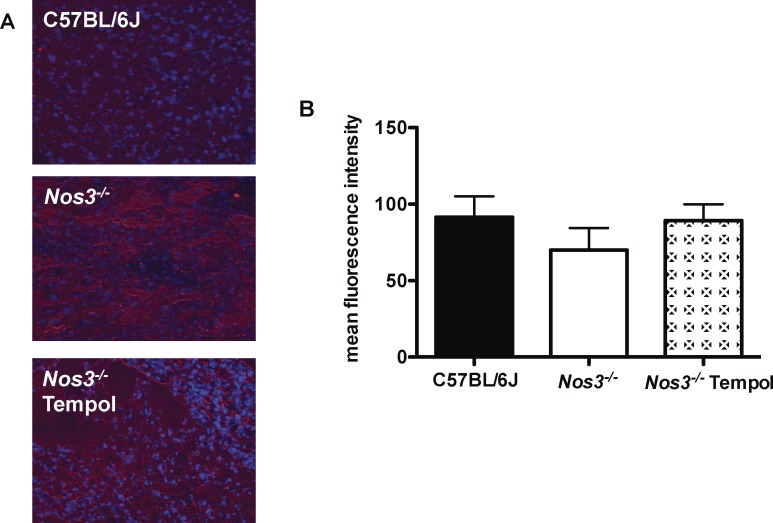
Superoxide production in placentas from Nos3−/− mice was significantly increased compared with that in placentas from C57BL/6J mice (128% ± 5%; P < 0.001) (Fig. 3). Because superoxide production was increased in Nos3−/− mice, we then tested the ability of Tempol to reduce this. There was, however, no effect of Tempol on superoxide production in placentas from Nos3−/− mice (93% ± 5%) (Fig. 3). The mean intensity of nitrotyrosine staining in the placentas did not differ among Nos3−/− (70 ± 14 pixels), C57BL/6J (92 ± 14 pixels), and Nos3−/− mice treated with Tempol (89 ± 11 pixels) (Fig. 4).
FIG. 3. .
Tempol treatment did not normalize superoxide production in placentas from Nos3−/− mice. A) Representative images of DHE staining in placental sections from C57BL/6J, Nos3−/−, and Tempol-treated Nos3−/− mice. Original magnification ×100. B) Superoxide production was significantly increased in placentas from Nos3−/− mice compared with that in C57BL/6J mice. This was not attenuated by treatment of Nos3−/− mice with Tempol. Data are means ± SEM from n = 9–10 mice; Student t-test; *P < 0.05.
FIG. 4. .
Placental nitrotyrosine formation was not affected by genotype or treatment. A) Representative images are shown of nitrotyrosine staining in placental sections from C57BL/6J, Nos3−/−, and Tempol-treated Nos3−/− mice. Original magnification ×100. B) There were no difference between nitrotyrosine formation in placentas from C57BL/6J and that in Nos3−/− mice. Furthermore, treatment of Nos3−/− mice with Tempol had no affect on placental nitrotyrosine formation. Data show means ±SEM from n = 6–7 mice; one-way ANOVA; DAPI (blue) staining.
Placental System A Amino Acid Transport
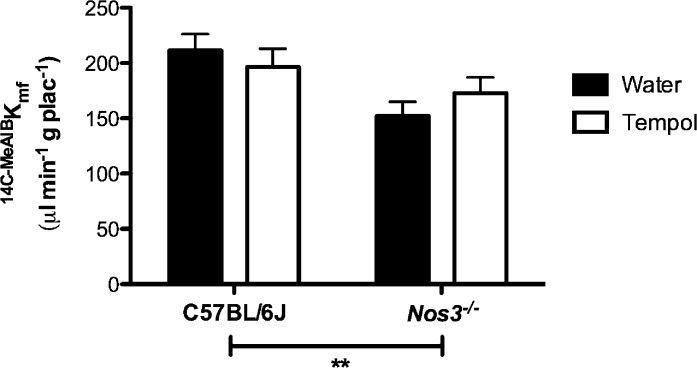
Unidirectional maternofetal transfer of [14C]MeAIB (Kmf) was significantly reduced in Nos3−/− mice compared with that in C57BL/6J mice (P < 0.05) (Fig. 5); this was not altered by Tempol treatment in either group.
FIG. 5. .
Reduced System A amino acid transport in Nos3−/− mice was not rescued by treatment with Tempol. Placental System A amino acid transport was significantly reduced in Nos3−/− compared with that in C57BL/6J mice. There was no effect of Tempol in either group. Data show means ± SEM in n = 7–12 mice; two-way ANOVA followed by Bonferroni post hoc test; **P < 0.01.
Vascular Casting of the Maternal Bed of the Placenta
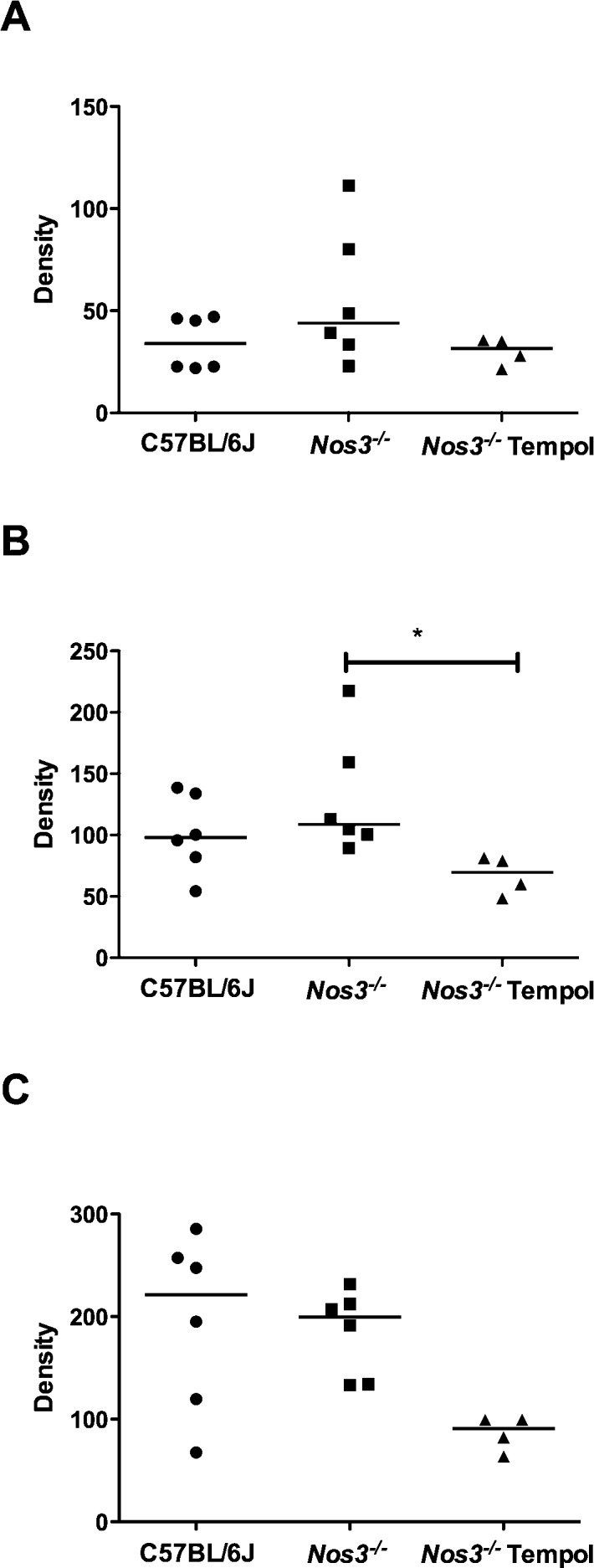
Following microtomography reconstruction of the maternal bed microvasculature, we calculated the density of vessels in the following ranges: 0–80 μm, 0–200 μm, and 0–500 μm. There were no differences in vascular density between C57BL/6J and Nos3−/− mice in any of the ranges (Fig. 6, A–C). Microvascular density was significantly decreased in Nos3−/− mice treated with Tempol compared with that in untreated mice in the 0–200 μm range (P < 0.05) (Fig. 6B).
FIG. 6. .
Treatment with Tempol reduced vascular density in placentas from Nos3−/− mice. Vascular density, in ranges of 0–80 μm (A), 0–200 μm (B), and 0–500 μm (C), did not differ between placentas from C57BL/6J and those from Nos3−/− mice. Treatment of Nos3−/− mice with Tempol resulted in a significant decrease in vascular density in the 0–200 μm range (B). Line denotes median, n = 4–6 mice; Kruskal-Wallis test followed by Dunn multiple comparison test; *P < 0.05.
DISCUSSION
The current study shows that treatment with the antioxidant Tempol is able to improve fetal growth in a mouse model of FGR. This may be due, in part, to an increase in uterine artery blood flow velocity. It should be noted that Tempol was also found to increase pup weight in WT control mice.
Increased oxidative stress has been implicated as a key pathophysiological mediator in a number of diseases, including FGR [28]. Consequently, antioxidants have been proposed as a therapeutic strategy for FGR. It is important, however, to specifically identify and target the oxidant molecule(s) that mediates a particular pathological process. Additionally, the ideal antioxidant therapy would act at the appropriate location. Disruption of the pro-/antioxidant balance may have both beneficial and negative effects. This was highlighted by a large-scale, randomized placebo-controlled trial [29] which studied the ability of the antioxidant vitamins C and E to prevent pre-eclampsia in high-risk pregnant women. Although vitamin supplementation had no effect on the incidence of pre-eclampsia, it did increase the rate of babies born with low birth weight. There may be a number of reasons why this particular antioxidant therapy failed to have beneficial effects, including the inability to administer sufficient concentrations at which antioxidant effects are observed or the inability of vitamins C and E to localize to the site of production. The use of Tempol as an antioxidant therapy, therefore, has a number of advantages. It is a superoxide dismutase-mimetic agent and, as such, specifically targets superoxide anions. Superoxide anions that are produced intracellularly after, for instance, uncoupling of the mitochondrial respiratory chain, remain trapped inside the cell where they can mediate cell injury. Tempol is able to cross biological membranes, and it has previously been observed that treatment of rats with Tempol resulted in a significant decrease in both cytosolic and mitochondrial ROS production [30]. Specific targeting of increased production of intracellular superoxide anions by Tempol may therefore be less likely to mediate unwanted side effects than other proposed antioxidant therapies.
Previous studies observed that Nos3−/− mice deliver growth-restricted pups [20]; this observation was confirmed here. Following treatment with Tempol, body weight was significantly increased in fetuses from both C57BL/6J control and Nos3−/− mice; Tempol also caused an increase in crown–rump length in the Nos3−/− mice. Furthermore, the fetal weight:placental weight ratio was significantly decreased in Nos3−/− mice, that is, there was a lower gram-weight fetus produced per gram-weight of placenta, suggesting that the efficiency of Nos3−/− placentas was reduced. This ratio was normalized following treatment with Tempol, suggesting that an increase in placental efficiency may be one mechanism by which Tempol was able to increase pup growth; this is considered further below.
It is appropriate to consider uterine artery hemodynamic changes observed in mouse pregnancy, as a previous study observed that the characteristics of the uterine artery waveform in the mouse were similar to those of humans [22]; this includes the loss of a diastolic notch in the last third of gestation and an increase in EDV and subsequent reduction in the resistance index such that it approaches 0.5 at term. Hemodynamic changes subsequent to therapy may be one underlying mechanism by which Tempol improves fetal growth. Data presented here demonstrate that the resistance of the uterine artery was increased at GD 17.5 in Nos3−/− mice, suggesting that in line with previous reports of aberrant uterine artery remodeling in pregnant Nos3−/− mice [20, 21], there may be reduced uteroplacental perfusion in this model. The significant increase in EDV in Tempol-treated Nos3−/− mice suggests that Tempol may cause a small increase in uterine artery blood flow and hence increase uteroplacental perfusion in mice and may be one mechanism by which pup growth was increased.
In order to further investigate possible changes to uterine artery function, endothelium-dependent relaxation was assessed at GD 18.5 and found to be significantly impaired in Nos3−/− mice, as indicated by reduced response to MCh but not SNP. The dysfunction observed was not ameliorated by treatment with Tempol. Production of superoxide and the presence of nitrotyrosine, the permanent footprint of peroxynitrite, was similar in uterine arteries from pregnant Nos3−/− and C57BL/6J mice, suggesting that the endothelial dysfunction observed in these arteries was therefore not mediated by ROS. Thus, the inability of Tempol to ameliorate the dysfunction observed is unsurprising given this observation.
Following the small effect of Tempol treatment on uterine artery blood flow velocity and function and the suggested positive effect of Tempol on placental efficiency, we explored the effect of Tempol on placental function. It has become increasingly apparent that placental insufficiency may be associated with other abnormalities besides reduced uteroplacental blood flow, including changes in structure as well as alterations at the molecular level [31]. One such molecular change is the altered activity of plasma membrane nutrient transporters, such as the System A amino acid transporter. A reduction in placental System A activity has been demonstrated to be associated with FGR in humans [32]. Furthermore, there are data from both normal mice [33] and those with a deletion of the placental Igf2 transcript [34] that suggest that when the fetal:placental weight ratios are increased (as they were here with our Tempol-treated Nos3−/− mice), this is mediated through upregulation of System A transporter activity per gram-weight of placenta. Additionally, it is known that expression of System A transporters is downregulated as a result of reduced oxygen concentrations [35]. Given the positive effect of the antioxidant Tempol on fetal growth in Nos3−/− mice and on the fetal:placental weight ratio, we hypothesized that increased placental oxidative stress was associated with reduced amino acid transport via System A and that this was normalized by Tempol treatment. First, we examined placental sections which demonstrated an increase in superoxide but not peroxynitrite production in Nos3−/− mice; this was likely due to a decrease in NO production in Nos3−/− mice. A decrease in System A activity in vivo was also observed in Nos3−/− mice, suggesting that an increase in ROS may well mediate a reduction in activity of System A transporters. Treatment with Tempol, however, did not reduce superoxide production. A previous study demonstrated the ability of Tempol to normalize placental oxidative stress [14]; that study, however, was carried out in a different mouse model. Although the concentration of drug and route of administration were identical to those used here, treatment was commenced 2 days before mating and continued throughout gestation, and that may explain the differing effects on placental oxidative stress. Furthermore, Tempol treatment did not significantly increase placental System A activity; the increases in pup growth and fetal:placental weight ratio observed following treatment with Tempol were therefore not mediated by increased System A activity. Although other changes in placental changes, namely lipid deposition, were investigated, no changes were observed between genotype and the potential effect of Tempol was not pursued.
Finally, vascular casts of the placental bed were constructed in order to assess the effect of antioxidant treatment on vascular density. Treatment with Tempol actually reduced vascular density in the maternal bed, further suggesting that the increase in pup growth was not mediated by an increase in delivery of oxygen or nutrients to the placenta. Antioxidant treatment has previously been associated with a decrease in oxidative stress and a concomitant reduction in microvascular density in a swine model of hypercholesterolemia [26], indicating potential oxidative stress-mediated pathological neovascularization. Although we used a different animal and model in our study, our findings show a similar effect by reducing placental vascular density after Tempol treatment. Hence, while this finding does not explain the observed increase in pup growth, it does highlight a potentially negative effect of this antioxidant therapy during pregnancy.
The ability of Tempol to improve pup growth in a mouse model of FGR is an exciting finding that could have considerable significance for human pregnancies complicated by this condition. Although this study has highlighted one potential mechanism for increased growth (increased uterine artery EDV, which may increase placental perfusion), further studies will be necessary to fully elucidate the mechanisms by which Tempol increases pup growth. These studies will include assessment of trophoblast invasion of the spiral arteries, as this may be one mechanism of increasing uterine artery blood flow velocity and hence increasing uteroplacental perfusion. Furthermore, microarray analysis coupled with gene expression, protein quantification, and estimation of transporter activity may indicate an effect of Tempol on other placental transporters, which may affect fetal growth.
In summary, treatment of a mouse model of FGR with the antioxidant Tempol resulted in an increase in pup growth. This may be mediated, in part, by increases in uterine artery blood flow velocity, although endothelial function was not improved. Furthermore, there were no increases in placental System A activity or placental bed vascular density. Although this study provides further evidence that antioxidant therapy during pregnancy may be able to at least partially rescue fetal growth, the mechanisms by which this is mediated remain to be fully elucidated. Furthermore, it also provides evidence that such therapy may have a negative effect on placental bed vascular density, and as such, further studies would be required before moving to a clinical trial.
ACKNOWLEDGMENT
We gratefully acknowledge the technical assistance of B. Baker and L. Renshall in carrying out placental System A activity studies. We are also grateful for helpful discussions with Drs. Susan Greenwood and Mark Wareing.
Footnotes
Supported by a Medical Research Council Programme grant (U.K.) to P.N.B. and C.P.S. and National Institutes of Health grant HL095638 to A.R.C.
REFERENCES
- Lewis G for the Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers' Lives: Reviewing Maternal Deaths To Make Motherhood Safer (2003–2005). London, UK: Royal College of Obstetricians and Gynaecologists; 2007. [Google Scholar]
- McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 1999; 340: 1234 1238 [DOI] [PubMed] [Google Scholar]
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004; 364: 513 520 [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol 1992; 99: 275 276 [DOI] [PubMed] [Google Scholar]
- Naeye RL, Blanc W, Paul C. Effects of maternal nutrition on the human fetus. Pediatrics 1973; 52: 494 503 [PubMed] [Google Scholar]
- Gilbert WM, Young AL, Danielsen B. Pregnancy outcomes in women with chronic hypertension: a population-based study. J Reprod Med 2007; 52: 1046 1051 [PubMed] [Google Scholar]
- Ferrazzani S, Caruso A, De Carolis S, Martino IV, Mancuso S. Proteinuria and outcome of 444 pregnancies complicated by hypertension. Am J Obstet Gynecol 1990; 162: 366 371 [DOI] [PubMed] [Google Scholar]
- Baschat AA. The fetal circulation and essential organs-a new twist to an old tale. Ultrasound Obstet Gynecol 2006; 27: 349 354 [DOI] [PubMed] [Google Scholar]
- Moore RJ, Ong SS, Tyler DJ, Duckett R, Baker PN, Dunn WR, Johnson IR, Gowland PA. Spiral artery blood volume in normal pregnancies and those compromised by pre-eclampsia. NMR Biomed 2008; 21: 376 380 [DOI] [PubMed] [Google Scholar]
- Moore RJ, Strachan BK, Tyler DJ, Duncan KR, Baker PN, Worthington BS, Johnson IR, Gowland PA. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 2000; 21: 726 732 [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol 2001; 159: 1031 1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 2008; 60: 418 469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension 2002; 39: 337 342 [DOI] [PubMed] [Google Scholar]
- Hoffmann DS, Weydert CJ, Lazartigues E, Kutschke WJ, Kienzle MF, Leach JE, Sharma JA, Sharma RV, Davisson RL. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension 2008; 51: 1058 1065 [DOI] [PubMed] [Google Scholar]
- Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol 1997; 272: H1730 1740 [DOI] [PubMed] [Google Scholar]
- Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res 2000; 87: 406 411 [DOI] [PubMed] [Google Scholar]
- Weiner C, Martinez E, Zhu LK, Ghodsi A, Chestnut D. In vitro release of endothelium-derived relaxing factor by acetylcholine is increased during the guinea pig pregnancy. Am J Obstet Gynecol 1989; 161: 1599 1605 [DOI] [PubMed] [Google Scholar]
- Casanello P, Sobrevia L. Intrauterine growth retardation is associated with reduced activity and expression of the cationic amino acid transport systems y+/hCAT-1 and y+/hCAT-2B and lower activity of nitric oxide synthase in human umbilical vein endothelial cells. Circ Res 2002; 91: 127 134 [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995; 377: 239 242 [DOI] [PubMed] [Google Scholar]
- Hefler LA, Reyes CA, O'Brien WE, Gregg AR. Perinatal development of endothelial nitric oxide synthase-deficient mice. Biol Reprod 2001; 64: 666 673 [DOI] [PubMed] [Google Scholar]
- van der Heijden OW, Essers YP, Fazzi G, Peeters LL, De Mey JG, van Eys GJ. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod 2005; 72: 1161 1168 [DOI] [PubMed] [Google Scholar]
- Mu J, Adamson SL. Developmental changes in hemodynamics of uterine artery, utero- and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am J Physiol Heart Circ Physiol 2006; 291: H1421 1428 [DOI] [PubMed] [Google Scholar]
- Andersson IJ, Sankaralingam S, Davidge ST. Restraint stress up-regulates lectin-like oxidized low-density lipoprotein receptor-1 in aorta of apolipoprotein E-deficient mice. Stress 2010; 13: 454 460 [DOI] [PubMed] [Google Scholar]
- Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A 2005; 102: 19219 19224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth MR, Kusinski LC, Cowley E, Ward BS, Husain SM, Constancia M, Sibley CP, Glazier JD. Placental-specific Igf2 knockout mice exhibit hypocalcemia and adaptive changes in placental calcium transport. Proc Natl Acad Sci U S A 2010; 107: 3894 3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chade AR, Bentley MD, Zhu X, Rodriguez-Porcel M, Niemeyer S, Amores-Arriaga B, Napoli C, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol 2004; 15: 1816 1825 [DOI] [PubMed] [Google Scholar]
- Iliescu R, Chade AR. Progressive renal vascular proliferation and injury in obese Zucker rats. Microcirculation 2010; 17: 250 258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karowicz-Bilinska A, Kedziora-Kornatowska K, Bartosz G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic Res 2007; 41: 870 873 [DOI] [PubMed] [Google Scholar]
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 2006; 367: 1145 1154 [DOI] [PubMed] [Google Scholar]
- Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol 2007; 293: H2726 2737 [DOI] [PubMed] [Google Scholar]
- Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res 2005; 58: 827 832 [DOI] [PubMed] [Google Scholar]
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 1997; 42: 514 519 [DOI] [PubMed] [Google Scholar]
- Coan PM, Angiolini E, Sandovici I, Burton GJ, Constancia M, Fowden AL. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol 2008; 586: 4567 4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 2002; 417: 945 948 [DOI] [PubMed] [Google Scholar]
- Nelson DM, Smith SD, Furesz TC, Sadovsky Y, Ganapathy V, Parvin CA, Smith CH. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. Am J Physiol Cell Physiol 2003; 284: C310 315 [DOI] [PubMed] [Google Scholar]