Abstract
Lys49-PLA2 myotoxins, an important component of various viperid snake venoms, are a class of PLA2-homolog proteins deprived of catalytic activity. Similar to enzymatically active PLA2 (Asp49) and to other classes of myotoxins, they cause severe myonecrosis. Moreover, these toxins are used as tools to study skeletal muscle repair and regeneration, a process that can be very limited after snakebites. In this work, the cytotoxic effect of different myotoxins, Bothrops asper Lys49 and Asp49-PLA2, Notechis scutatus notexin and Naja mossambica cardiotoxin, was evaluated on macrophages, cells that have a key role in muscle regeneration. Only the Lys49-myotoxin was found to trigger a rapid asynchronous death of mouse peritoneal macrophages and macrophagic cell lines through a process that involves ATP release, ATP-induced ATP release and that is inhibited by various purinergic receptor antagonists. ATP leakage is induced also at sublytical doses of the Lys49-myotoxin, it involves Ca2+ release from intracellular stores, and is reduced by inhibitors of VSOR and the maxi-anion channel. The toxin-induced cell death is different from that caused by high concentration of ATP and appears to be linked to localized purinergic signaling. Based on present findings, a mechanism of cell death is proposed that can be extended to other cytolytic proteins and peptides.
Keywords: Lys49-PLA2, phospholipase A2, myotoxin, notexin, cardiotoxin, purinergic signaling
Snakebite envenoming is a highly relevant and neglected health problem on a worldwide basis, and most cases of envenoming in Latin America are caused by snakes of the genus Bothrops.1 Bothrops venoms, like those of many other viperid snakes, cause complex pathophysiological alterations with prominent local (necrosis, hemorrhage, blistering and edema) and systemic effects (bleeding, coagulopathy, cardiovascular shock and renal failure).2, 3 Local tissue damage leading to dermonecrosis and myonecrosis is particularly relevant, as it is frequently followed by poor tissue regeneration and permanent sequelae.4
Myotoxins are the main snake venom components causing tissue necrosis and, upon injection into higher animals, they cause irreversible damage on skeletal muscle fibers. They are basic proteins that can be classified into three main groups belonging to structurally distinct protein families: the ‘small' myotoxins, the cardiotoxins and the PLA2 myotoxins.5 The pathology caused by cardiotoxins and PLA2 myotoxins develops rapidly and it is associated with marked damage to the sarcolemma, whereas pathology associated with ‘small' myotoxins has a more delayed onset and sarcolemma damage is not apparent.6 Among fast acting myotoxins, cobra cardiotoxins are basic three-finger toxins deprived of catalytic activity, they cause severe tissue necrosis and systolic heart arrest in snakebite victims through ill-known mechanisms that involve formation of membrane pores.7 The PLA2 myotoxins form the largest group and are divided into ‘Asp49', which catalyze the hydrolysis of the ester bond in the sn-2 position of glycerol-phospholipids, and their enzymatically inactive homologs ‘Lys49'. The mechanisms of myotoxicity of these toxins are largely unknown and, catalytically dependent and independent events are involved; in the case of the enzymatically inactive Lys49-PLA2 homologs, an evident membrane disruption takes place without phospholipid hydrolysis. A common structural characteristic of locally acting myotoxins is a cationic face, which is likely to interact with negatively charged phospholipid bilayers. This initial electrostatic interaction, in the case of B. asper Mt-II, is probably followed by the penetration and disorganization of the membrane by the C-terminal region of the toxin.2, 5, 8
In B. asper venom, various myotoxins have been identified. One enzymatically active PLA2 myotoxin, Mt-I (alternative name Mt-III) (P20474), and three Lys49-PLA2 myotoxins, Mt-II (P24605), Mt-IV (P0C616/Q9PRT7) and M1-3-3 (Q9PVE3), are presently reported in UNIPROT database, and together PLA2s myotoxins account for 15–35% of B. asper venom proteins.9 Cardiotoxins and PLA2 myotoxins are used as tools to study the regeneration and maturation of mammalian skeletal muscle,10 a very complex process that involves multiple types of cells, among which macrophages have a major role.11 These cells are recruited already in the first phase of muscle regeneration, after acute muscle damage, and have the dual role of scavengers that phagocytose necrotic debris and of promoters of myogenic differentiation.11 For these reasons, it is relevant to evaluate whether myotoxins are cytotoxic also for macrophages, as this may have implications in the model of muscle injury by myotoxins and in the reparative and regenerative processes after snakebites.
Notexin, an Asp49-PLA2 of Notechis scutatus (Ntx), and cardiotoxin of Naja mossambica (Ctx) are among the most frequently used myotoxins in the study of the muscle regeneration process.10 In this work, the activity of these toxins was compared with that of Mt-I and Mt-II, on mouse peritoneal macrophages and on three macrophagic cell lines (RAW264.7, J774.A1 and N13). Only Mt-II was found to induce a rapid death of these cells. Moreover, as in C2C12 myotubes B. asper myotoxins induce a massive ATP release,12 the extracellular concentration and the role of this molecule in macrophage death were analyzed. Mt-II was found to induce an initial ATP release, followed by an ATP-induced ATP release, that participates in the onset of a rapid and asynchronous cell burst. This is a novel type of cell death, quite different from that induced by a massive extracellular addition of ATP in J774.A1 and N13 mouse macrophagic cell lines.13, 14
Based on these data, a two-step model of Mt-II-induced cytotoxicity is proposed, with an initial alteration of the plasma membrane associated with purinergic signaling followed by cytolysis due to the insertion of the toxin into the lipid bilayer.
Results
Mt-II, but not other myotoxins, is cytolytic for macrophages
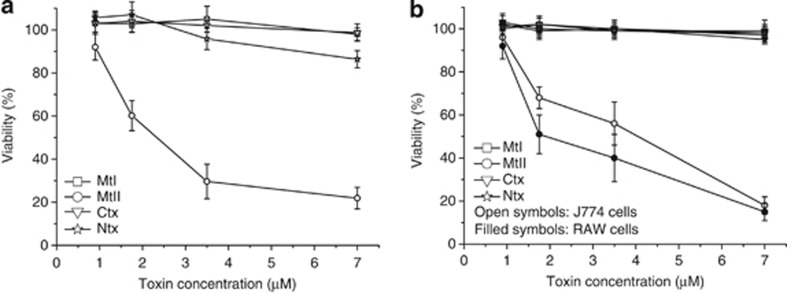
Mt-I, Mt-II, Ntx and Ctx activities were evaluated on isolated peritoneal mouse macrophages. Figure 1a shows that, among the four myotoxins studied and compared here, only Mt-II, the Lys49 myotoxin devoid of PLA2 activity, displays a significant toxicity on these cells. Similar results were obtained with the mouse macrophages cell lines RAW264.7 and J774.A1 (Figure 1b).
Figure 1.
Cell death induced by Mt-I, Mt-II, Ctx and Ntx. Cytotoxicity was measured with the MTS assay on mouse peritoneal macrophages (a) and on macrophagic cell lines RAW264.7 and J774.A1 (b) as a function of the toxin concentration in the medium. Macrophages were incubated with the different toxins in the mKRB medium (see Materials and Methods section) for 1 h, and cell viability was determined. Values are mean±S.D.; n=4–5
Mt-II induces ATP release from macrophages
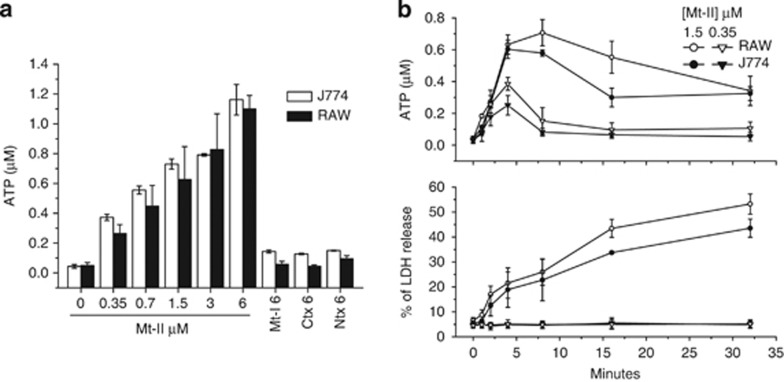
Mt-II induces a dose-dependent release of ATP from RAW264.7 and J774.A1 cells (Figure 2a), whereas Mt-I, Ntx and Ctx, even at concentrations of 6 μM, are without effect. ATP release was detected also at a sublytical concentration of Mt-II (0.35 μM) with no significant LDH release, while at a higher toxin concentration extracellular ATP reaches its maximum level very rapidly, but this occurs always before the peak of LDH release (Figure 2b) and subsequently it decreases, presumably because of the action of ecto-ATP hydrolases (ecto-ATPases). In fact, in the presence of the ectoATPase inhibitor ARL67156 (100 μM), and by sequestering calcium, a cofactor of ecto-ATPases, with EGTA, the extracellular ATP concentration keeps on increasing, together with that the extracellular LDH (Supplementary Figure S1). Altogether these experiments indicate that ATP release is not only due to a general plasma membrane damage but also because of a specific mechanism of ATP release induced by the toxin occurs in the initial 3–5 min after the intoxication.
Figure 2.
ATP release induced by Mt-I, Mt-II, Ctx and Ntx. (a) Mt-II induces a dose-dependent release of ATP from RAW264.7 and J774.A1 cells. Mt-I, Ctx and Ntx (6 μM) do not induce a significant release of ATP. ATP was measured 5 min after intoxication with a luciferin/luciferase-based assay. (b) Kinetics of ATP (top panel) and LDH (bottom panel) release from J774.A1 and RAW264.7 cells induced by 0.35 and 1.5 μM Mt-II. Notice that ATP release temporally precedes that of LDH. Values are mean±S.D.; n =4
The Mt-II-induced ATP release from macrophages is inhibited by anionic channel antagonists and involves intracellular Ca2+ signaling
Different modes for ATP release have been proposed: via vesicle exocytosis, by the opening of channels, such as pannexins and connexins, or through specific anionic channels.15, 16 To investigate the possible pathways for ATP release, RAW264.7 and J774.A1 cells were exposed to Mt-II in the presence of bafilomicyn A1, which prevents ATP exocytosis by inhibiting the v-ATPase proton pump, or in the presence of inhibitors of various potential ATP channels. ATP release was measured after 5 min, at the time of maximum concentration of released ATP and before important percentage of cell lysis, as indicated by the kinetics of LDH release (Figure 2).
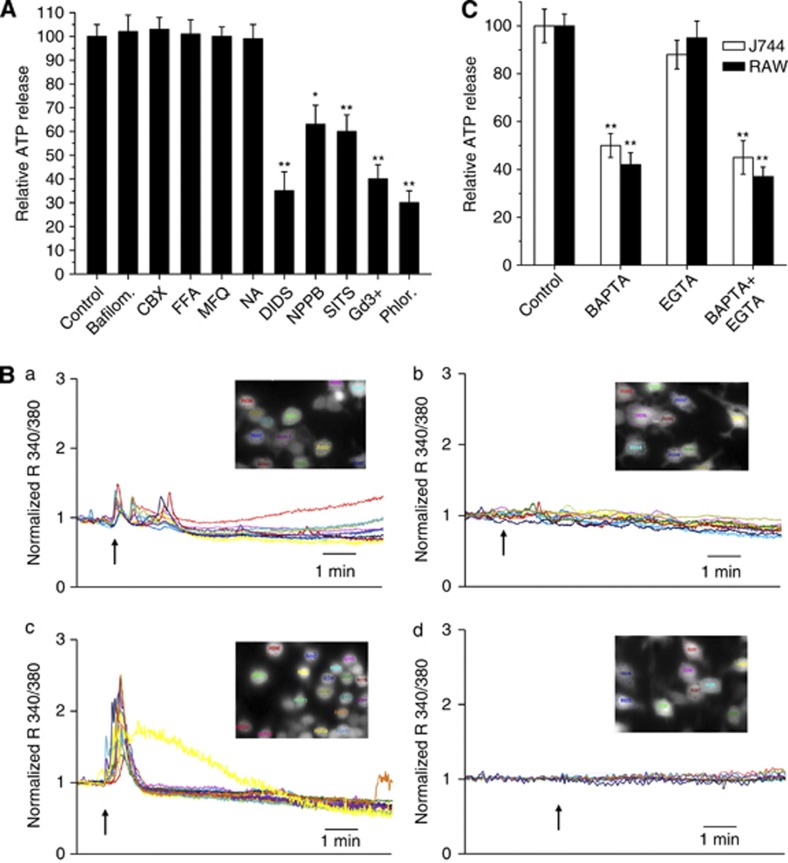
Figure 3A shows that neither bafilomycin A1 nor connexin and pannexin inhibitors (carbenoxolone, flufenamic acid, mefloquine and niflumic acid) reduced the ATP release. In contrast, antagonists of the maxi-anion channel and of VSOR inhibited the process, indicating that these channels are involved. SITS and DIDS inhibit both the maxi-anion channel and VSOR, whereas phloretin is specific for VSOR and gadolinium ion is specific for the maxi-anion channel.17, 18
Figure 3.
Characterization of the ATP release induced by Mt-II (1.5 μM) on macrophages. (A) Effect of bafilomycin and inhibitors of ATP channels. ATP present in the extracellular medium was determined 5 min after the intoxication. Values are mean±S.D. of four independent experiments on J774.A1. RAW264.7 cells give identical results (data not shown). Statistically significant differences were determined via an one-tailed Student's t-test (*P<0.01; **P<0.001). Inhibitors were used at the same concentrations indicated in Table 1. Bafilomycin A1 was used at 0.2 μM. (B) Calcium imaging of RAW264.7 cells loaded with fura-2 AM and treated with Mt-II (1.5 μM) in mKRB (top-left panel) or in Ca2+-free EGTA (0.2 mM)-containing medium (bottom-left panel). Top- right panel shows an addition of the same volume of mKRB without the toxin to fura-2 loaded cells. Bottom-right panel: fura-2 loaded cells were pretreated, before the intoxication with Mt-II in mKRB, with cyclopiazonic acid (20 μM, CPA) and EGTA (0.6 mM) to deplete intracellular calcium stores. The arrows indicate the time at which the toxin was added. The same results were obtained in at least three independent experiments for each condition. The inserts correspond to pictures of the evaluated cells before the addition of toxin or buffer, to correlate each colored trace to a specific cell. Representative ratios from each picture are shown. Similar results were obtained with J 774 cells (data not shown). (C) Release of ATP induced by Mt-II, 5 min after the intoxication, in cells preloaded with BAPTA-AM (2 μM) or in the presence of EGTA (1.5 mM) in the extracellular medium. Values are mean±S.D. of four independent experiments. Statistically significant differences were determined via an one-tailed Student's t-test (*P<0.01; **P<0.001)
As ATP release is elicited by stimulation of receptors that induce cytosolic calcium increase,15 the role of this ion in the mechanism of Mt-II-induced ATP release was investigated. Mt-II was found to cause an increase in cytosolic [Ca2+], as observed by calcium imaging of cells loaded with fura-2, both in the presence or absence of external Ca2+, indicating that this toxin triggers a release of Ca2+ from intracellular stores (Figure 3B, left panels). This was confirmed by depleting the intracellular Ca2+ stores by pre-treating cells with cyclopiazonic acid and EGTA, and observing that in this case the toxin does not induce an increase in cytosolic [Ca2+] (Figure 3B, bottom-right panel).
A link between calcium signaling and ATP release was established by preloading cells with BAPTA-AM, a chelator of cytosolic Ca2+, or by exposing cells to EGTA-containing medium, before intoxication with Mt-II. BAPTA-AM, but not EGTA addition, reduced the released ATP, 5 min after intoxication, to about 50%, confirming that the calcium signal mediating this process is predominantly derived from intracellular stores (Figure 3C).
Mt-II-induced cell death is inhibited by ATP anion channel and purinergic receptor antagonists
The same compounds that inhibit the Mt-II-induced ATP release, protect cells from death due to the intoxication (Table 1, lines 1–9). Also BAPTA confers some protection from Mt-II intoxication, whereas EGTA is not protective (Table 1, lines 10–11). The role of purinergic receptors was then investigated by evaluating the protective action of P2X and P2Y antagonists on Mt-II-induced cell death. P2Xs are ligand-gated cation channels activated by extracellular ATP, whereas P2Ys are G-protein coupled receptors activated by ATP, ADP, UTP, UDP and UDP-glucose. To date, seven members of the P2X family (P2 × 1–7) and eight P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) have been characterized.19 Upon activation, these receptors, which are widely distributed throughout the body, modulate an array of cellular functions, such as plasma membrane permeabilization, Ca2+ influx and cell death.19 Expression profiles of all these receptors, except P2Y11, have been verified in RAW264.7 cells20 (Supplementary Figure S2).
Table 1. Protection percentage (Prot %) of macrophagic cells, from Mt-II-induced cytotoxicity, by inhibitors of ATP channels (first white area), calcium chelators (first shaded area), inhibitors of the purinergic receptors (second white area) and of intracellular signaling activated by GPCRs and/or purinergic receptors (second shaded area).
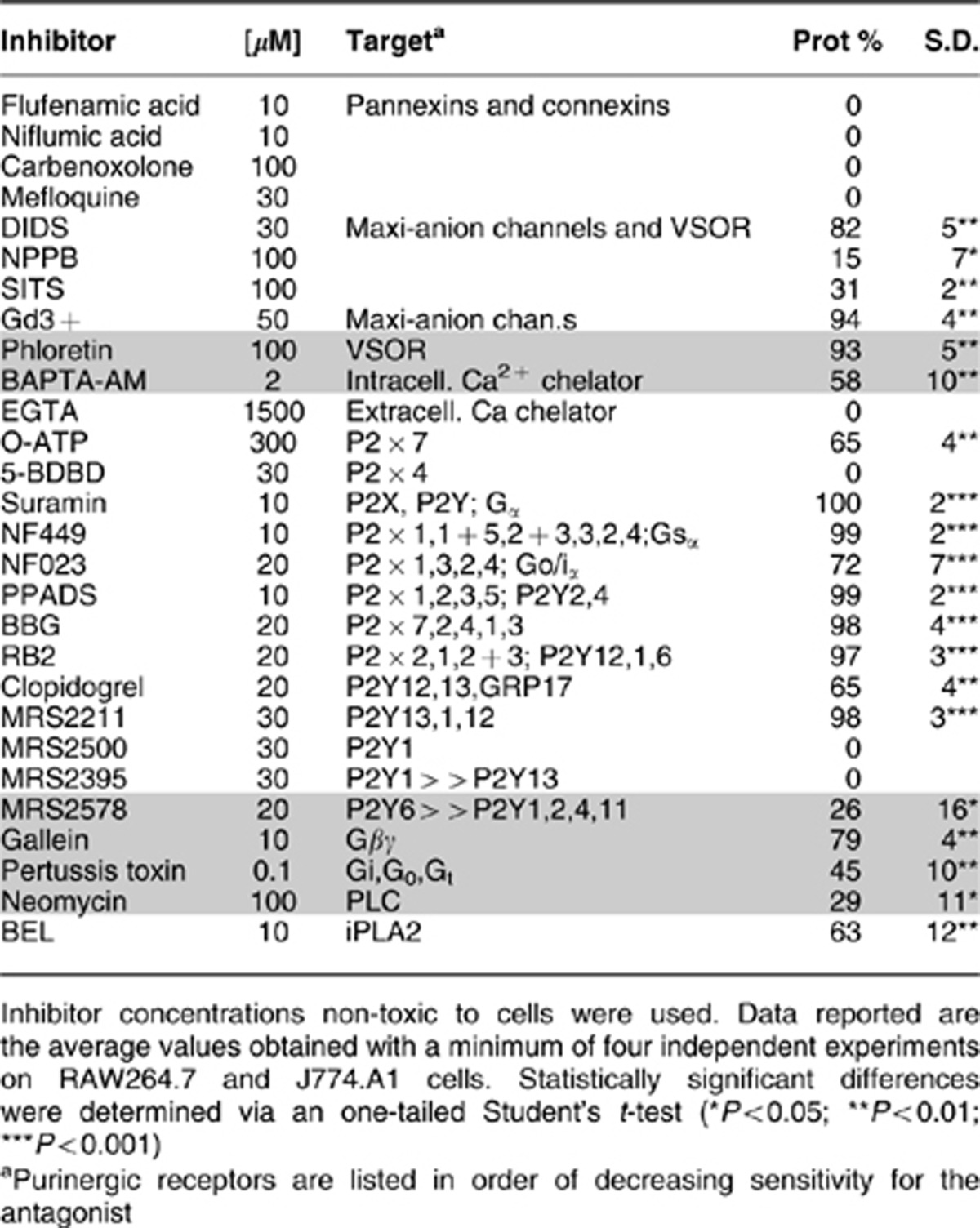
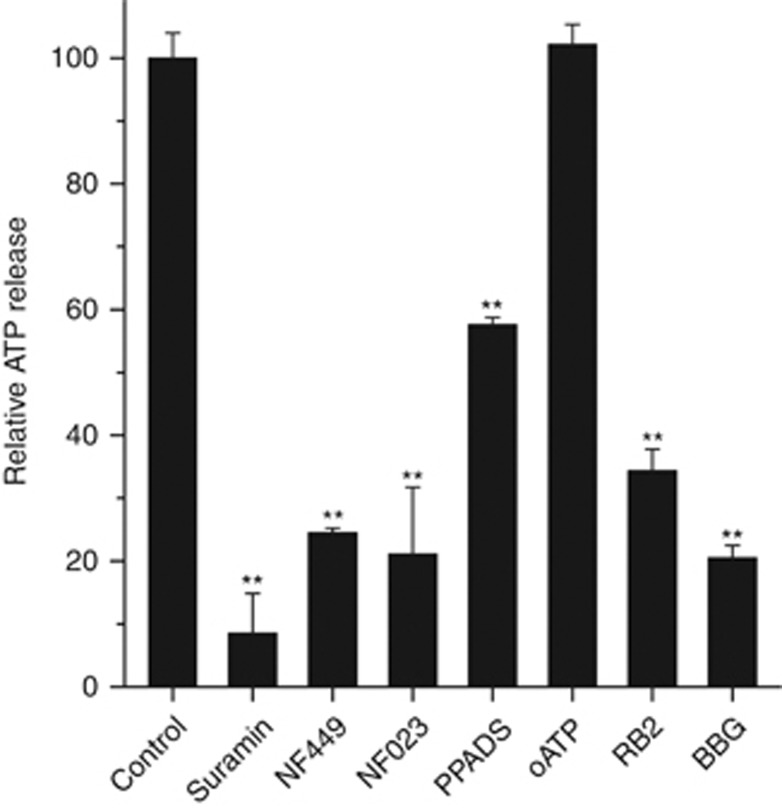
Table 1 (lanes 12–24) reports the protection, of macrophage cell death induced by Mt-II, obtained with various inhibitors of purinergic receptors, as well as the concentration and the role of the inhibitors. From the pattern of inhibitors showing a protective role, it appears that more than one type of membrane purinergic receptor/channel is involved in this cell death process. A prominent role appears to be played by P2 × 1, 2 or 3 (inhibited by NF449, NF023, PPADS, BBG, RB2) and by P2Y12 and 13 (selectively inhibited by MRS2211 and clopidogrel). However, the inhibition by oATP also involves P2 × 7. P2Y1 and P2 × 4 may not have an important role as their specific inhibitors, MRS2500 and 5-BDBD, respectively, are not effective. P2Y6, the purinergic receptor expressed at the highest level in RAW264.7 cells20 (Supplementary Figure S2), is inhibited by MRS2578 that is not fully protective against Mt-II cytotoxicity. Inhibitors of the signal transduction pathways, activated by P2Ys and by GPCRs, are also protective (bottom lines of Table 1), including BEL, an inhibitor of the cytosolic Ca2+-independent phospholipases. Importantly, some of these purinergic antagonists inhibit the ATP release induced by the toxin (Figure 4) indicating that the first released ATP may induce a further release of the same molecule, triggering a positive feedback.
Figure 4.
Effect of some purinergic inhibitors on the ATP release induced by Mt-II in J774.A1 cells. Experiments were performed as reported in Figure 3A. All inhibitors were pre-incubated for 30 min. Statistically significant differences were determined via a one-tailed Student's t test (**P<0.001)
Mt-II-induced cell death differs from that induced by high ATP concentration
High extracellular ATP concentration is toxic for several cells including the J774.A1 macrophage cell line.14 So the death process induced by ATP in J774.A1 macrophages and in N13 microglial cells, another cell line sensitive to ATP,13 was compared with the death induced by Mt-II intoxication. N13 are as sensitive to Mt-II intoxication as macrophages (Supplementary Figure S3), with a similar, though not identical, profile of activity of the array of inhibitors used in the previous section (Supplementary Table S1). When RAW264.7, J774.A1 or N13 cells are intoxicated with Mt-II, lysis occurs in a sudden but not synchronized way: cells undergo volume variations, swelling and shrinkage, some of them form large plasma membrane blebs with exposure of phosphatidylserine, and eventually cells burst with a random distribution (Figure 5, Supplementaty Videos S1 and S2). In contrast, J774.A1 and N13 cells treated with high ATP concentration form little and diffuse membrane blebs and die in a slower synchronized way (Supplementary Video S3). A time to death of 6 h was reported in cells exposed to very high concentration of ATP (> 3 mM),13, 14 whereas at the dose of the toxin (1.5 μM) used in the present work 50% of the cells dies in less than 1 h. Moreover, also the changes of cytosolic [Ca2+] in N13 and J774.A1 cells, induced by high concentration of Mt-II and ATP, were found to be different (see Figure S4 and Figure S5 in Supplementary Materials, Video S4 and Video S5).
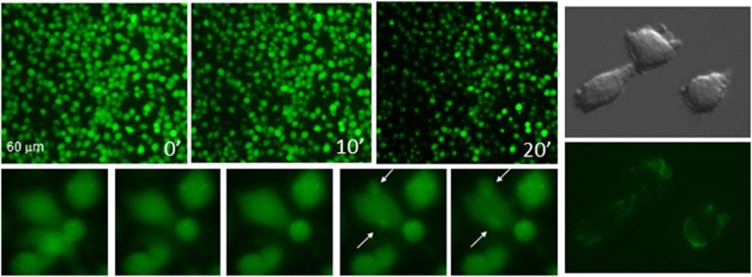
Figure 5.
Observation by fluorescence microscopy of macrophage death induced by Mt-II (1.5 μM). Left: J744.A1 cells were loaded with the fluorescent dye calcein and intoxicated with Mt-II. Top panels: cell death appears as a rapid burst of cells in an asynchronous, ‘spotted-like' way (see also Supplementaty Video S1). Bottom panels: some cells swell and form characteristic membrane blebs (arrows). Images have been recorded with 10 '' time intervals (see also Supplementaty Video S2). Right panels: RAW264.7 cells intoxicated with Mt-II in the presence of annexin-V-fluorescein. Membrane blebs induced by Mt-II intoxication show surface exposure of phosphatidylserine (see also Supplementary Figures S6 A and B). Cell integrity was checked by staining with propidium iodide. All experiments were repeated at least three times
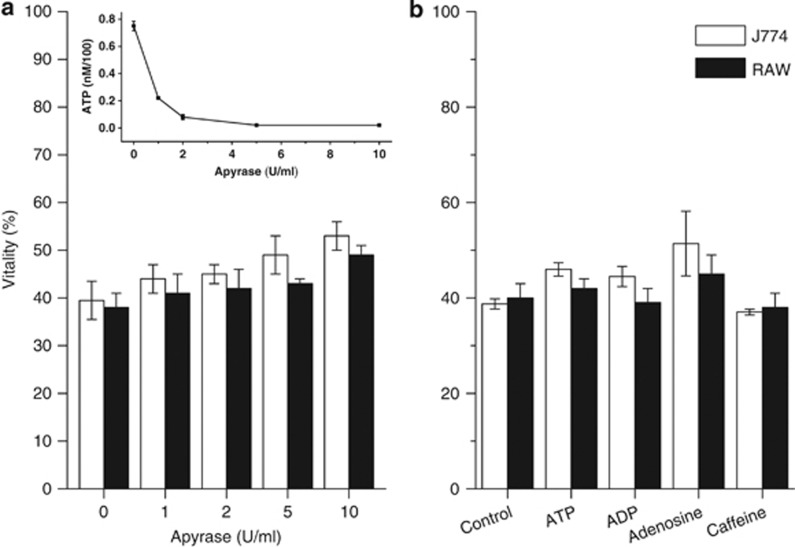
Apyrase from potato, an enzyme mixture that hydrolyzes nucleoside triphosphates and diphosphates, completely protect cells from death induced by ATP (data not shown), whereas it displays only a partial protection of cells exposed to Mt-II (Figure 6a). Moreover, the addition of ATP and ADP does not amplify the death induced by the toxin (Figure 6b). The lack of protection by apyrase can be due to the transformation of ATP into other toxic products as the final product of ATP degradation by apyrase is AMP, which is subsequently transformed into adenosine by extracellular 5′-nucleotidases. Adenosine is present in many snake venoms and it has been proposed to have a role in envenoming effects.21 To test whether adenosine also participates in the cell death process, the effects of caffeine, a generic adenosine receptor inhibitor, and of adenosine itself, were evaluated. The results shown in Figure 6b indicate that adenosine receptors are not involved in the Mt-II-induced cell death mechanism.
Figure 6.
Effect of apyrase (a), of various purine derivatives (100 μM each) and of caffeine (10 μM) (b) on Mt-II-induced cytotoxicity. Apyrase activity was checked by determining ATP in the extracellular medium of J744.A1 cells incubated with Mt-II (1.5 μM, inset). Values are mean±S.D. of four independent experiments on J774.A1 and RAW264.7 cells
Discussion
The results reported here show that Mt-II, a catalytically inactive PLA2-homolog myotoxin, induces an ATP release from mouse macrophages that is involved in a death process through an ATP positive feedback mechanism. This toxin has been reported to cause activation of some functions, that is, phagocytosis and respiratory burst, in mouse peritoneal macrophages at sublytic concentrations.22 The physiological consequences of all these effects, and of the cytotoxicity, in vivo remain to be elucidated. In skeletal muscle, there is a population of resident macrophages,23 which are likely to be affected by these toxins following envenomation. In addition, infiltration of the tissue by macrophages follows the process of myonecrosis. Initially, a population of ‘inflammatory' macrophages predominates, with a role in removal of necrotic debris and, subsequently, a population of ‘non-inflammatory' macrophages appears, releasing factors required for skeletal muscle regeneration.11 It is suggested that, upon venom injection, myotoxins interact with resident macrophages, inducing activation and cytotoxicity. As the inflammatory population of macrophages appears many hours after the onset of myonecrosis, it is possible that the toxin concentration in the tissue, by the time these macrophages reach the affected muscle is low. However, owing to the leading role that macrophages have in the process of muscle regeneration, the possible effect of myotoxins in these cells in vivo warrants further investigations.
In contrast to Mt-II, Mt-I, Ntx and Ctx are not cytolytic for macrophages and do not induce a significant release of ATP from these cells. These results suggest that Mt-II is likely to have an acceptor in macrophages, which mediates the observed effects, and given the wide cytotoxic profile of Mt-II,24 this receptor is likely to be ubiquitous. Mt-I, Ntx and Ctx either do not have an acceptor molecule in macrophages or their binding does not result in ATP release and cytotoxicity. Mt-I, despite the lack of cytotoxic effect on macrophages, induces signaling activation and lipid body formation in these cells25 and ATP release and a rapid cell lysis in C2C12 myotubes.12 Therefore, it is possible that the Mt-I receptor(s) is present in both macrophages and C2C12 but induces different signaling in the two cell types, or the Mt-I receptor is present only in C2C12 and the Mt-I effects in macrophages are due to the PLA2 enzymatic activity.
Present findings on ATP release and cytotoxicity data suggest that, among the possible ways of ATP release, Mt-II induces the opening of maxi-anion channel and VSOR, two anion channels still not identified at molecular levels, that are ubiquitous and involved in many pathological processes.17, 18 These channels are known to be activated by cell swelling, ischemia and hypoxia and by various stimuli acting on GPCRs. Observations on Ca2+ release from intracellular stores induced by Mt-II, indicate a possible involvement of GPCRs in toxin-induced ATP release, via activation of PLC and IP3 production; moreover, various animal toxins, and among them also some PLA2s, have been found to bind GPCRs.26
Another possible mechanism for ATP release is the opening of P2 × 7 channels,16 but the fact that the specific inhibitor, oATP, does not inhibit the ATP release argues against this possibility.
Mt-II-induced cell death process appears to involve many purinergic receptors and also ectonucleotidases that transform ATP into ADP, agonist of the P2Y1, 2, 12 and 13 receptors. Precise information on the receptors involved is difficult to obtain using only a pharmacological approach, as some drugs may affect more than one target and some of them such as suramin but probably also its analog NF449, also directly interact with Mt-II.27 Nonetheless, from the present pharmacological inhibition profile, P2 × 1-3 and P2Y12-13 appear to be main players in the Mt-II-induced macrophage death process. In addition, other receptors that could be involved are P2 × 7, P2Y2 and P2Y6. Purinergic receptors seem to be involved also in a Mt-II-induced, positive feedback mechanism of ATP release, as purinergic inhibitors reduce the amount of released ATP. The alternative possibility that all these inhibitors block the supposed myotoxin receptor is very unlikely, as it would imply that molecules of different structure bind to the same target. Moreover, from previous data, it clearly appears that purinergic receptors, in different cell types, are often involved in positive feedback mechanisms: for example, the P2 × 2 receptor seems to be necessary for an autocrine positive feedback signal amplifying taste-evoked ATP secretion,28 whereas P2 × 3 constitutes a positive autocrine signal for insulin release in human pancreatic beta cells.29 Moreover, P2Y12 together with P2Y2 and adenosine receptors are involved in autocrine purinergic feedback loops induced by the chemoattractant C5a, in macrophages, through the release of ATP.30
Besides Mt-I12 and Mt-II, other membrane-perturbing polypeptides have been reported to stimulate ATP efflux. Some antimicrobial peptides were found to stimulate ATP release from Candida albicans and the released ATP was suggested to participate in the lytic process by an unknown mechanism.31 Alpha-hemolysin, a pore-forming toxin, was shown to involve purinergic signaling via P2X receptors and pannexins,32 and to stimulate surface exposure of phosphatidylserine.33
The current model for the Mt-II cytolytic mechanism posits that this toxin inserts in the plasma membrane and perturbs its permeability properties.5 Moreover, it is known that the presence of anionic lipids34, 35 and the membrane fluidity36 affect the cytolytic activity of Mt-II and other myotoxins, that is, human red blood cells, insensitive to Mt-II, become susceptible to the toxin after enrichment with phosphatidylserine,34 whereas cell membrane cholesterol depletion, by methyl-β-cyclodextrin treatment, enhanced the Mt-II cytolytic action on C2C12.36
Taking together previous and present results, it is suggested that purinergic signaling activation by Mt-II alters the bilayer composition and the asymmetry of the plasma membrane and makes it more susceptible to the lytic action of the toxin. This is consistent with the finding that purinergic signaling activation is involved in membrane ruffling and pseudopod protrusion during immune cell chemotaxis,30, 37 a process that is mediated also by intracellular phospholipases,38 whereas phosphatidylserine distribution and membrane blebbing are known to involve P2 × 7 and iPLA2 activation.39 Moreover, the P2Y2 and other purinergic receptors are involved in ATP-mediated autocrine and paracrine mechanisms stimulated by HIV-1, facilitating fusion between viral and cell membranes.40
The random distribution of the Mt-II-induced cell lysis indicates that the product(s) released by a dying cell does not facilitate the death of neighboring cells. This observation, together with the lack of a significant protective effect of apyrase and of a synergism between ATP or ADP addition and the Mt-II-induced cell death, suggests that the process activated by the myotoxin is probably localized in cell membrane microdomains where ATP is released, by the opening of specific channels, and rapidly intercepted by local ectonucleotidases and purinergic receptors. Purinergic receptor compartmentalization has been observed in different cells.19 Moreover, the maxi-anion channels in adult cardiomyocytes are localized at the opening of T-tubules and along Z-lines17 and this distribution may be relevant to the activity of Mt-II on myotubes.12
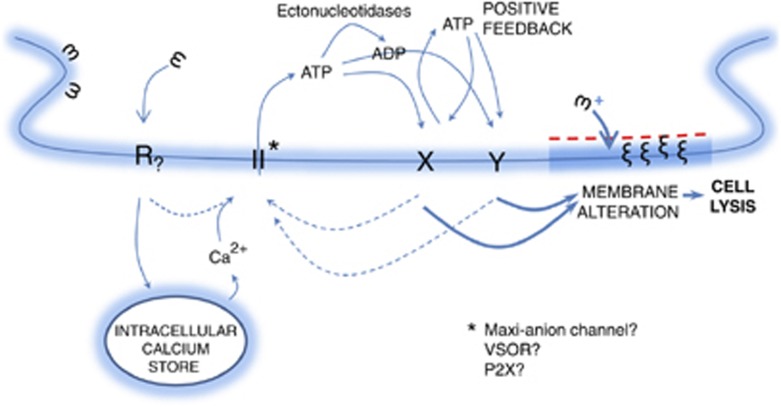
In conclusion, the protection from Mt-II, achieved in macrophagic cells by various inhibitors of purinergic signaling, suggests a novel two-step mechanism for the toxin action. In the first step, cytotoxins interact with and activate, already at low concentrations, a specific receptor, in a membrane microdomain, that triggers a signaling process, which alters the membrane composition and properties. This membrane alteration renders the bilayer more susceptible to the action of Mt-II. Then, in the second step, the toxin molecules insert into the altered membrane and, if present at high concentration, cause a rapid cell lysis (Figure 7). Considering that other cytolysins have been found to induce ATP release, this hypothetic mechanism of action may be extended to other toxins, which act by changing the plasma membrane permeability and suggests that the possible involvement of purinergic mechanisms should be tested. The involvement of a receptor that induces the ATP release can explain the cell specificity of these membrane perturbing toxins although an interaction with specific cell membrane lipids cannot be excluded.
Figure 7.
Model of action of Mt-II. The toxin interacts with a yet unidentified receptor (R), embedded in a membrane microdomain. R activates ATP channels by inducing the release of calcium from intracellular stores and/or by other mechanisms. The released ATP stimulates purinergic receptors within the same plasma membrane microdomain inducing a further release of ATP. The extracellular ATP activates a signaling cascade leading to changes in the composition of the plasma membrane, which in turn promotes the insertion of Mt-II into the lipid bilayer with consequent osmotic cell lysis. It is proposed that this hypothesis may be valid also for other membrane-active toxins and peptides
Finally, the purinergic receptor inhibitors and other compounds, which were found here to be protective in the Mt-II cytotoxicity assay should be evaluated as possible therapeutics for ameliorating the tissue damage induced by snake venoms.
Materials and Methods
Materials
Myotoxins I and II were isolated from the crude venom of Bothrops asper, a pool obtained from at least 30 specimens kept at the serpentarium of Instituto Clodomiro Picado, University of Costa Rica, as described before.12 Notexin from Notechis scutatus was purchased from Latoxan (Valence, France) and Naja mossambica cardiotoxin from Sigma. All inhibitors were purchased from Tocris or Sigma (Milan, Italy). Pertussis toxin was obtained from Tocris (Abingdon, UK) and potato apyrase from Sigma (A7646). BAPTA-AM and calcein were obtained from Life Technologies (Monza, Italy).
Cell cultures
The murine macrophage cell lines J774.A1 (ATCC TIB 67) and Raw264.7 (ATCC TIB 71) were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium supplemented with heat-inactivated fetal bovine serum (EuroClone, Milan, Italy) 10%, 10 μg/ml streptomycin, 10 U/ml penicillin. The microglial N13 cell line13 was cultured in RPMI 1640 medium supplemented with 2 mM glutamine, 10% heat-inactivated FBS, 10 μg/ml streptomycin, 10 U/ml penicillin.
Primary macrophages were isolated from the peritoneum of CD-1 mice. All experimental procedures were carried out in accordance to the European Community Council Directive no. 86/609/EEC and were approved by the local authority veterinary service, project number 24088/ 2011. Mice were intraperitoneally injected with thioglycollate broth. After 3–5 days, animals were killed under CO2 atmosphere, peritoneal cavities were washed with 3 ml of phosphate buffer solution and the fluid was collected, centrifuged at 1200 × g, and the cell sediment was resuspended in a complete medium (RPMI added with 10% fetal calf serum). Cells were seeded in 96-well plates and after adhesion, cultures were washed three times with phosphate buffer solution to remove non-adherent cells. More than 95% of the cell population consisted of macrophages, as determined by conventional morphological criteria.
Cytotoxicity assay
Cells seeded the day before in 96-well plates (40 000/well) were washed once in modified Krebs–Ringer buffer (mKRB) (140 mM NaCl, 2.8 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM Hepes and 11 mM glucose, pH 7.4) and incubated with Mt-II, diluted in mKRB (final volume 100 μl), for the indicated time or, in pharmacological tests, for the time necessary to obtain a 50% of death in the control samples (in presence of toxin and without inhibitors). Purinergic inhibitors, channel inhibitors and apyrase were added at the same time as Mt-II. Pertussis toxin was pre-incubated for 2 h in full cell medium. Gallein, BEL, forskolin and neomycin were pre-incubated for 30 min in mKRB. All inhibitors were checked for their effect on cell viability and used at non-toxic concentrations. Cell viability was measured with the MTS (3-(4,5-dimethylthiazol-2-yl-5-(3-carboxymethoxyphenyl-2-(4-sulfonphenyl)-2H-tetrazolium, inner salt) assay. The CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Milan, Italy) was used and instructions from the manufacturer were followed. The release of LDH was measured as an index of cellular necrosis using the commercial kit TOX7 (Sigma), which is based on the LDH-catalyzed reduction of NAD+, which then converts a tetrazolium dye to a soluble colored formazan derivative.
Protection of macrophage cells from Mt-II-induced cytotoxicity by pharmacological inhibitors was evaluated with both kinds of tests (cell viability and LDH release) with similar results. Results reported in Table 1 have been obtained with LDH release test.
ATP measurement
Cells were seeded, the day before, in 24-well plates, 150 000 for each well. The growth medium was replaced with 225 μl of mKRB, or with mKRB-containing diluted inhibitors, 30 min before the ATP release test, except for 300 μM O-ATP (in mKRB) that was preincubated for 120 min. Inhibitors did not affect per se the total ATP cell content and the luciferase activity. The toxins were diluted in 25 μl of mKRB and added to the wells at the zero time. The release of ATP due to mechanical effect was checked by adding 25 μl of mKRB in control wells. During the pre-incubation and incubation time, cell plates were placed in incubator at 37 °C, 5% CO2. At different time points, the contents of at least three wells were collected and conserved on ice. Released ATP was determined with the ATPlite luciferase assay (Perkin-Elmer, Monza, Italy). Briefly, the supernatant of control and intoxicated samples were collected in a white 96-well plate, and the mammalian lysis solution, included in the assay kit, was added. The plate was shaken for 5 min in an orbital shaker at 700 r.p.m., RT. ATP substrate solution was added and a 5-min shake was performed, protected from light. After 10 min, the luminescence was measured using the Fluoroskan Ascent FL (Thermo Electron Corporation, Milan, Italy). The ATP concentration was calculated from a calibration curve obtained using ATP standard solutions.
Calcium Imaging
Cells plated on coverslips (24-mm diameter in six-well plates, 200 000 cells/well), were loaded with 9 μM Fura-2/AM at 37°C for 20 min, in 1 ml of mKRB containing 0.04% pluronic F-127 (Molecular Probes, Inc., Eugene, OR, USA). To prevent fura-2 leakage and sequestration, sulfinpyrazone (200 μM) was present throughout the loading procedure. Then, 1 ml of mKRB was added to the mixture and cells were incubated for another 10 min at 37°C. Subsequently, coverslips were washed with mKRB, mounted on a thermostatted chamber (Medical System Corp., New York, NY, USA) at 37°C, and placed on an inverted microscope (Zeiss, Milan, Italy, Axiovert 100 TV) equipped for single cell fluorescence measurements and imaging analysis (TILL Photonics, Martinsried, Germany). Where indicated, a Ca2+-free EGTA (200 μM)-containing medium was used. The sample was alternatively illuminated (t=200 ms) by monochromatic light (at 340 and 380 nm wavelengths), every second for 10–20 min after toxin exposure, through a × 40 oil immersion objective (NA=1.30; Zeiss). The emitted fluorescence was passed through a dichroic beam splitter (455DRPL), filtered at 505–530 nm (Omega Optical and Chroma Technologies, Brattleboro, VT, USA) and captured by a cooled CCD camera (Imago, TILL Photonics). For presentation, the ratios (F340/F380) of cells were off-line normalized to the resting value measured within the first minute of the experiment.
Fluorescence microscopy
Cells were plated on coverslips, and treated for 2 h with collagen (BD Biosciences, Milan, Italy). Before experiments, cells were incubated for 15 min, at 37°C, with 1 μM calcein-AM (Life Technologies) diluted in mKRB and then washed three times with mKRB. Alternatively, cells were incubated with annexin-V-fluorescein and propidium iodide (Annexin-V-FLUOS Staining Kit, Roche, Milan, Italy) in the incubation buffer provided with the kit. Effect of intoxication with Mt-II was observed with a wide-field inverted microscope (Leica Microsystems, Milan, Italy, DMI6000B) equipped with a DFC300FXR2 digital camera. Images were acquired sequentially with a FITC and Texas Red filter set (Chroma Technology Corp., Olking, Germany). The acquisition software was LAS AF6000 (Leica Microsystems).
Acknowledgments
We thank P Caccin, I Zornetta and ML Massimino for helpful comments and suggestions. This work was supported by University of Padova Progetto Strategico ‘An in vivo approach to the physiopathology of signal transduction' to CM, a fellowship of the Fondazione CARIPARO to JF and the ICGEB-CRP Program (grant COS-08-03) to BL and JG.
Glossary
- BBG
Coomassie brilliant blue G
- CBX
carbenoxolone
- Ctx
Naja mossambica cardiotoxin
- DIDS
4,4'-Diisothiocyano-2,2'-stilbenedisulfonic acid
- EGTA
ethylene glycol tetraacetic acid
- FFA
flufenamic acid
- GPCR
G-protein coupled receptor
- iPLA2
calcium independent PLA2
- LDH
lactic acid dehydrogenase
- MFQ
mefloquine
- Mt-I
B. asper myotoxin I
- Mt-II
B. asper myotoxin II
- NA
niflumic acid
- Ntx
Notechis scutatus notexin
- PLA2
phospholipase A2
- PPADS
Pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid
- RB2
reactive blue 2
- SITS
4-Acetamido-40-isothiocyanostil-bene-2,20-disulfonic acid
- VSOR
volume-sensitive outwardly rectifying anion channel
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Verkhratsky
Supplementary Material
References
- Gutiérrez JM, Theakston RD, Warrell DA. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med. 2006;3:e150. doi: 10.1371/journal.pmed.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez JM, Rucavado A, Chaves F, Díaz C, Escalante T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon. 2009;54:958–975. doi: 10.1016/j.toxicon.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Escalante T, Rucavado A. Experimental pathophysiology of systemic alterations induced by Bothrops asper snake venom. Toxicon. 2009;54:976–987. doi: 10.1016/j.toxicon.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Hernández R, Cabalceta C, Saravia-Otten P, Chaves A, Gutiérrez JM, Rucavado A. Poor regenerative outcome after skeletal muscle necrosis induced by Bothrops asper venom: alterations in microvasculature and nerves. PLoS One. 2011;6:e19834. doi: 10.1371/journal.pone.0019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte B, Angulo Y, Calderón L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42:885–901. doi: 10.1016/j.toxicon.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Fletcher JE, Hubert M, Wieland SJ, Gong QH, Jiang MS. Similarities and differences in mechanisms of cardiotoxins, melittin and other myotoxins. Toxicon. 1996;34:1301–1311. doi: 10.1016/s0041-0101(96)00105-5. [DOI] [PubMed] [Google Scholar]
- Wu WG, Tjong SC, Wu PL, Kuo JH, Wu K. Role of heparan sulfates and glycosphingolipids in the pore formation of basic polypeptides of cobra cardiotoxin. Adv Exp Med Biol. 2010;677:143–149. doi: 10.1007/978-1-4419-6327-7_12. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Gutiérrez JM, Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell Mol Life Sci. 2008;65:2897–2912. doi: 10.1007/s00018-008-8113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo Y, Lomonte B. Biochemistry and toxicology of toxins purified from the venom of the snake Bothrops asper. Toxicon. 2009;54:949–957. doi: 10.1016/j.toxicon.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Harris JB. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon. 2003;42:933–945. doi: 10.1016/j.toxicon.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des. 2010;16:906–914. doi: 10.2174/138161210790883453. [DOI] [PubMed] [Google Scholar]
- Cintra-Francischinelli M, Caccin P, Chiavegato A, Pizzo P, Carmignoto G, Angulo Y, et al. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc Natl Acad Sci USA. 2010;107:14140–14145. doi: 10.1073/pnas.1009128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, et al. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36:1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Murgia M, Pizzo P, Steinberg TH, Di Virgilio F.Characterization of the cytotoxic effect of extracellular ATP in J774 mouse macrophages Biochem J 1992288(Pt 3):897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriden R, Insel PA.Basal release of ATP: an autocrine-paracrine mechanism for cell regulation Sci Signal 20103re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Okada Y. The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity. J Physiol Sci. 2009;59:3–21. doi: 10.1007/s12576-008-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Sato K, Numata T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol. 2009;587 (Pt 10:2141–2149. doi: 10.1113/jphysiol.2008.165076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonté C, D'Ambrosi N. Membrane compartments and purinergic signaling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 2009;276:318–329. doi: 10.1111/j.1742-4658.2008.06793.x. [DOI] [PubMed] [Google Scholar]
- Bok K, Prikhodko VG, Green KY, Sosnovtsev SV. Apoptosis in murine norovirus-infected RAW264.7 cells is associated with downregulation of survivin. J Virol. 2009;83:3647–3656. doi: 10.1128/JVI.02028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird SD. Ophidian envenomation strategies and the role of purines. Toxicon. 2002;40:335–393. doi: 10.1016/s0041-0101(01)00232-x. [DOI] [PubMed] [Google Scholar]
- Zuliani JP, Gutiérrez JM, Casais e Silva LL, Coccuzzo Sampaio S, Lomonte B, Pereira Teixeira CeF. Activation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 phospholipases A2. Toxicon. 2005;46:523–532. doi: 10.1016/j.toxicon.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578 (Pt 1:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte B, Tarkowski A, Hanson LA. Broad cytolytic specificity of myotoxin II, a lysine-49 phospholipase A2 of Bothrops asper snake venom. Toxicon. 1994;32:1359–1369. doi: 10.1016/0041-0101(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Leiguez E, Zuliani JP, Cianciarullo AM, Fernandes CM, Gutiérrez JM, Teixeira C. A group IIA-secreted phospholipase A2 from snake venom induces lipid body formation in macrophages: the roles of intracellular phospholipases A2 and distinct signaling pathways. J Leukoc Biol. 2011;90:155–166. doi: 10.1189/jlb.0510263. [DOI] [PubMed] [Google Scholar]
- Näreoja K, Näsman J. Selective targeting of G-protein-coupled receptor subtypes with venom peptides. Acta Physiol. 2011. [DOI] [PubMed]
- Murakami MT, Arruda EZ, Melo PA, Martinez AB, Calil-Eliás S, Tomaz MA, et al. Inhibition of myotoxic activity of Bothrops asper myotoxin II by the anti-trypanosomal drug suramin. J Mol Biol. 2005;350:416–426. doi: 10.1016/j.jmb.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, et al. Knocking Out P2X Receptors reduces transmitter secretion in taste buds. J Neurosci. 2011;31:13654–13661. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Silva MC, Correa-Medina M, Cabrera O, Rodriguez-Diaz R, Makeeva N, Fachado A, et al. ATP-gated P2 × 3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci USA. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis Sci Signal 20103ra55. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Sun JN, Edgerton M. The role of released ATP in killing Candida albicans and other extracellular microbial pathogens by cationic peptides. Purinergic Signal. 2007;3:91–97. doi: 10.1007/s11302-006-9040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skals M, Jorgensen NR, Leipziger J, Praetorius HA. Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci USA. 2009;106:4030–4035. doi: 10.1073/pnas.0807044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skals M, Jensen UB, Ousingsawat J, Kunzelmann K, Leipziger J, Praetorius HA. Escherichia coli alpha-hemolysin triggers shrinkage of erythrocytes via K(Ca)3.1 and TMEM16A channels with subsequent phosphatidylserine exposure. J Biol Chem. 2010;285:15557–15565. doi: 10.1074/jbc.M109.082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz C, León G, Rucavado A, Rojas N, Schroit AJ, Gutiérrez JM. Modulation of the susceptibility of human erythrocytes to snake venom myotoxic phospholipases A2: role of negatively charged phospholipids as potential membrane binding sites. Arch Biochem Biophys. 2001;391:56–64. doi: 10.1006/abbi.2001.2386. [DOI] [PubMed] [Google Scholar]
- Konshina AG, Boldyrev IA, Utkin YN, Omel'kov AV, Efremov RG. Snake cytotoxins bind to membranes via interactions with phosphatidylserine head groups of lipids. PLoS One. 2011;6:e19064. doi: 10.1371/journal.pone.0019064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel J, Quesada O, Gutiérrez JM, Angulo Y, Lomonte B. Membrane cholesterol modulates the cytolytic mechanism of myotoxin II, a Lys49 phospholipase A2 homologue from the venom of Bothrops asper. Cell Biochem Funct. 2011;29:365–370. doi: 10.1002/cbf.1758. [DOI] [PubMed] [Google Scholar]
- Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart MK.Signal-activated phospholipase regulation of leukocyte chemotaxis J Lipid Res 200950SupplS231–S236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Dubyak GR. P2 × 7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 2009;5:163–173. doi: 10.1007/s11302-009-9132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séror C, Melki MT, Subra F, Raza SQ, Bras M, Saïdi H, et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med. 2011;208:1823–1834. doi: 10.1084/jem.20101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.