Abstract
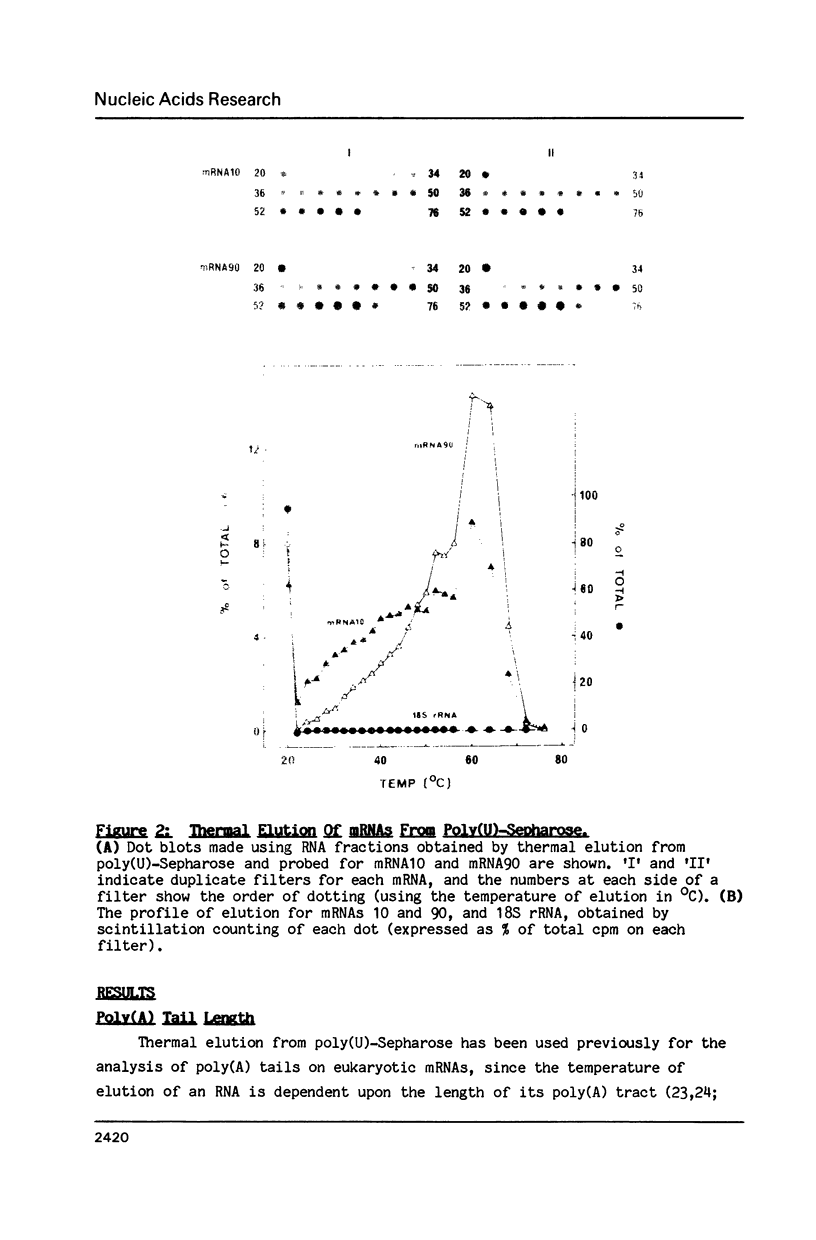
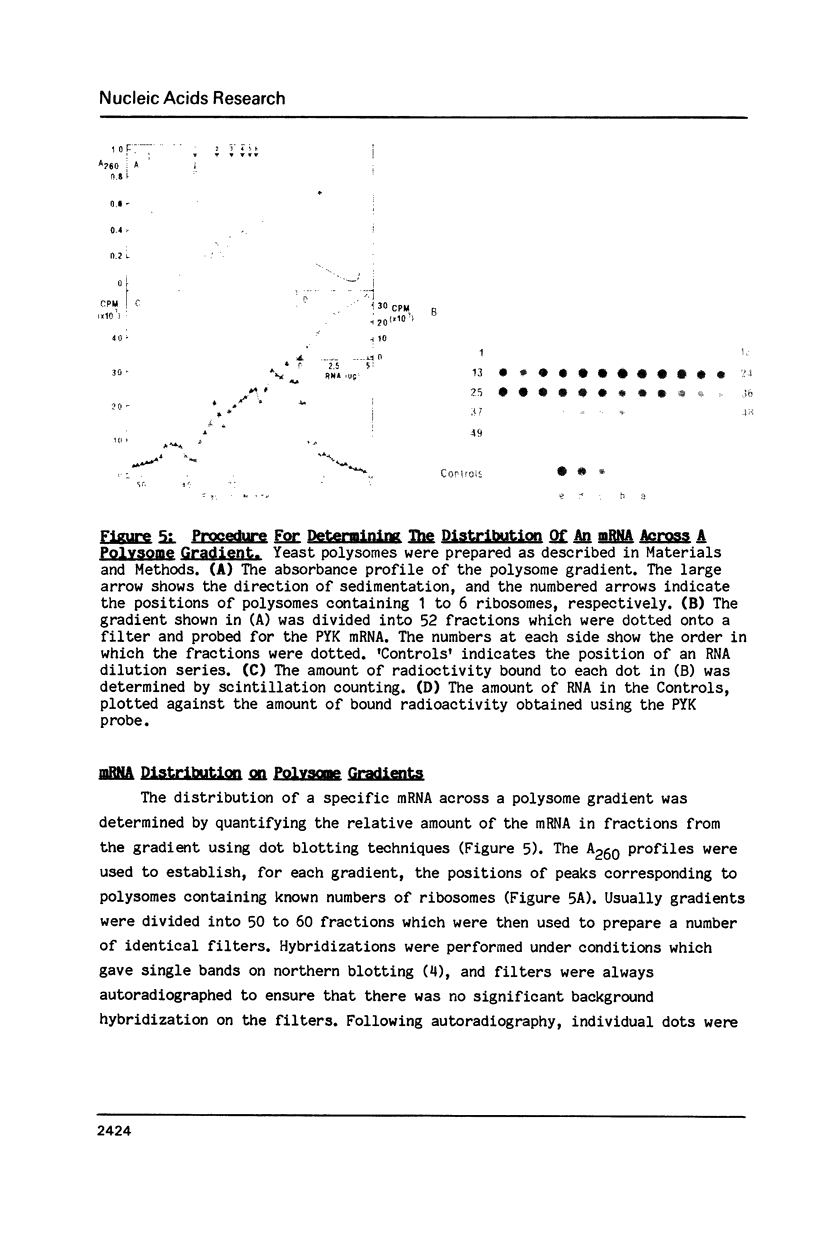
A comparison between the half-lives of 10 specific yeast mRNAs and their distribution within polysomes (fractionated on sucrose density gradients) was used to test the relationship between mRNA translation and degradation in the eukaryote Saccharomyces cerevisiae. Although the mRNAs vary in their distribution across the same polysome gradients, there is no obvious correlation between the stability of an mRNA and the number of ribosomes it carries in vivo. This suggests that ribosomal protection against nucleolytic attack is not a major factor in determining the stability of an mRNA in yeast. The relative lengths of the poly(A) tails of 9 yeast mRNAs were analysed using thermal elution from poly(U)-Sepharose. No dramatic differences in poly(A) tail length were observed amongst the mRNAs which could account for their wide ranging half-lives. Minor differences were consistent with shortening of the poly(A) tail as an mRNA ages.
Full text
PDF





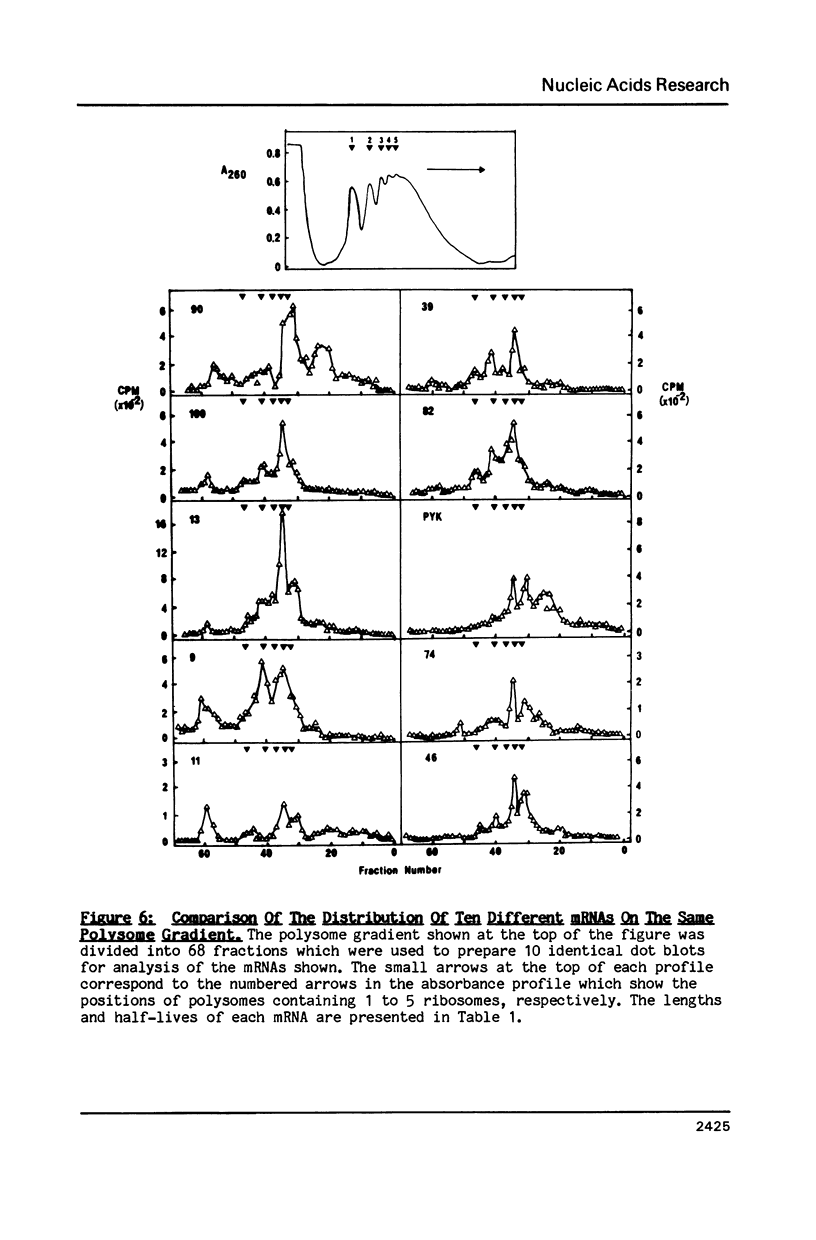




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baim S. B., Pietras D. F., Eustice D. C., Sherman F. A mutation allowing an mRNA secondary structure diminishes translation of Saccharomyces cerevisiae iso-1-cytochrome c. Mol Cell Biol. 1985 Aug;5(8):1839–1846. doi: 10.1128/mcb.5.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Belasco J. G., Nilsson G., von Gabain A., Cohen S. N. The stability of E. coli gene transcripts is dependent on determinants localized to specific mRNA segments. Cell. 1986 Jul 18;46(2):245–251. doi: 10.1016/0092-8674(86)90741-5. [DOI] [PubMed] [Google Scholar]
- Brown A. J., Hardman N. The effect of age on the properties of poly(A)-containing messenger RNA in Physarum polycephalum. J Gen Microbiol. 1981 Jan;122(1):143–150. doi: 10.1099/00221287-122-1-143. [DOI] [PubMed] [Google Scholar]
- Brown A. J., Hardman N. Utilization of polyadenylate mRNA during growth and starvation in Physarum polycephalum. Eur J Biochem. 1980 Sep;110(2):413–420. doi: 10.1111/j.1432-1033.1980.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Tekamp-Olson P., Najarian R. The isolation, characterization, and sequence of the pyruvate kinase gene of Saccharomyces cerevisiae. J Biol Chem. 1983 Feb 25;258(4):2193–2201. [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. beta 0 thalassemia, a nonsense mutation in man. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2886–2889. doi: 10.1073/pnas.76.6.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia L. L., McLaughlin C. The half-life of mRNA in Saccharomyces cerevisiae. Mol Gen Genet. 1979 Feb 26;170(2):137–144. doi: 10.1007/BF00337788. [DOI] [PubMed] [Google Scholar]
- De Kloet S. R., Andrean B. A. Methylated nucleosides in polyadenylate-containing yeast messenger ribonucleic acid. Biochim Biophys Acta. 1976 Apr 2;425(4):401–408. doi: 10.1016/0005-2787(76)90004-6. [DOI] [PubMed] [Google Scholar]
- Fahrner K., Yarger J., Hereford L. Yeast histone mRNA is polyadenylated. Nucleic Acids Res. 1980 Dec 11;8(23):5725–5737. doi: 10.1093/nar/8.23.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Fiori M., Mach B. A new nonsense mutation as the molecular basis for beta thalassaemia. J Mol Biol. 1982 Jan 25;154(3):537–540. doi: 10.1016/s0022-2836(82)80012-0. [DOI] [PubMed] [Google Scholar]
- Graham M. Y., Tal M., Schlessinger D. lac Transcription in Escherichia coli cells treated with chloramphenicol. J Bacteriol. 1982 Jul;151(1):251–261. doi: 10.1128/jb.151.1.251-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Schlessinger D. Coupling of rates of transcription, translation, and messenger ribonucleic acid degradation in streptomycin-dependent mutants of Escherichia coli. J Bacteriol. 1976 Jan;125(1):84–93. doi: 10.1128/jb.125.1.84-93.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. G., Ruth M. E. The dissociation of rat liver ribosomes by ethylenediaminetetraacetic acid; molecular weights, chemical composition, and buoyant densities of the subunits. Biochemistry. 1969 Mar;8(3):851–856. doi: 10.1021/bi00831a013. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Yanofsky C. Hyper-labile messenger RNA in polar mutants of the tryptophan operon of Escherichia coli. J Mol Biol. 1972 Dec 14;72(1):103–110. doi: 10.1016/0022-2836(72)90072-1. [DOI] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Koch H., Friesen J. D. Individual messenger RNA half lives in Saccharomyces cerevisiae. Mol Gen Genet. 1979 Feb 26;170(2):129–135. doi: 10.1007/BF00337787. [DOI] [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Soreq H. The regulatory function of poly(A) and adjacent 3' sequences in translated RNA. Prog Nucleic Acid Res Mol Biol. 1982;27:53–83. doi: 10.1016/s0079-6603(08)60597-8. [DOI] [PubMed] [Google Scholar]
- Losson R., Fuchs R. P., Lacroute F. In vivo transcription of a eukaryotic regulatory gene. EMBO J. 1983;2(12):2179–2184. doi: 10.1002/j.1460-2075.1983.tb01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Morse D. E. Polarity induced by chloramphenicol and relief by suA. J Mol Biol. 1971 Jan 14;55(1):113–118. doi: 10.1016/0022-2836(71)90285-3. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. doi: 10.1038/224329a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Jacobson A. Fractionation and functional analysis of newly synthesized and decaying messenger RNAs from vegetative cells of Dictyostelium discoideum. J Mol Biol. 1979 Mar 5;128(3):371–395. doi: 10.1016/0022-2836(79)90093-7. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago T. C., Purvis I. J., Bettany A. J., Brown A. J. The relationship between mRNA stability and length in Saccharomyces cerevisiae. Nucleic Acids Res. 1986 Nov 11;14(21):8347–8360. doi: 10.1093/nar/14.21.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Blundell M., Kennell D. Translation and mRNA decay. Mol Gen Genet. 1978 Apr 6;160(2):121–129. doi: 10.1007/BF00267473. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Puckett L., Darnell J. E. Possible relationship of poly(A) shortening to mRNA turnover. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1077–1081. doi: 10.1073/pnas.72.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripati C. E., Groner Y., Warner J. R. Methylated, blocked 5' termini of yeast mRNA. J Biol Chem. 1976 May 25;251(10):2898–2904. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Sawicki S. G., White P. A., Darnell J. E., Jr A correlation between the rate of poly(A) shortening and half-life of messenger RNA in adenovirus transformed cells. J Mol Biol. 1978 Nov 25;126(1):23–36. doi: 10.1016/0022-2836(78)90277-2. [DOI] [PubMed] [Google Scholar]
- Wong H. C., Chang S. Identification of a positive retroregulator that stabilizes mRNAs in bacteria. Proc Natl Acad Sci U S A. 1986 May;83(10):3233–3237. doi: 10.1073/pnas.83.10.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. Mutationally altered 3' ends of yeast CYC1 mRNA affect transcript stability and translational efficiency. J Mol Biol. 1984 Jul 25;177(1):107–135. doi: 10.1016/0022-2836(84)90060-3. [DOI] [PubMed] [Google Scholar]