Abstract
Rationale
While Bone-marrow endothelial progenitor cell based therapies (BM-EPC) improve the symptoms in patients with ischemic heart disease their limited plasticity and decreased function in patients with existing heart disease limits the full benefit of EPC therapy for cardiac regenerative medicine.
Objective
We hypothesized that reprogramming mouse and/or human EPCs using small molecules targeting key epigenetic repressive marks would lead to a global increase in active gene transcription, induce their cardiomyogenic potential and enhance their inherent angiogenic potential.
Method and Results
Mouse Lin-Sca1+CD31+ EPCs and human CD34+ cells were treated with inhibitors of DNA methyltransferases (5-Azacytidine), histone deacetylases (valproic acid) and G9a histone di-methyltransferase. Forty eight hour treatment led to global increase in active transcriptome including the reactivation of pluripotency associated and CMC specific mRNA expression while EC specific genes were significantly up-regulated. When cultured under appropriate differentiation conditions, reprogrammed EPCs showed efficient differentiation into CMC and vascular smooth muscle cells. Treatment with epigenetic modifying agents show marked increase in histone acetylation on cardiomyocyte and pluripotent cell specific gene promoters. Intra-myocardial transplantation of reprogrammed mouse and human EPCs in an acute myocardial infarction mouse model showed significant improvement in ventricular functions, which was histologically supported by their de novo CMC differentiation and increased capillary density and reduced fibrosis. Importantly, cell transplantation was safe and did not form teratomas.
Conclusions
Taken together, our results suggest that epigenetically reprogrammed EPCs display a safe, more plastic phenotype and improve post-infarct cardiac repair by both neo-cardiomyogenesis and neovascularization.
Keywords: Epigenetic modification, EPC, cardiomyogenesis, myocardial ischemia, cell therapy, histone acetylation, trans-differentiation
Introduction
Despite the pervasive belief that the heart has limited regenerative capacity, progenitor-cell based therapy has been shown to provide substantial clinical benefits for ischemic diseases such as chronic angina, acute myocardial infarction and heart failure1–6. Specifically, murine endothelial progenitor cells (EPC)7, and the human equivalent CD34+ mononuclear cells are capable of homing to infarct and peri-infarct myocardium upon ischemia, inducing angio/vasculogenesis, and augmenting cardiac function and survival through paracrine mediated growth factor secretion 8–10. Although the re-vascularization appears to result in real improvements in quality of life, the ultimate goal of cardiovascular regenerative medicine is to regenerate lost myocytes in addition to neovasculature. There is no convincing evidence that EPCs have cardiomyocyte differentiation potential 11, 12. Also, since the EPCs from aged patients with existing metabolic diseases and cardiovascular risk factors are known to have diminished functional properties, epigenetic modification of EPC may lead to an enhancement in their phenotypic and functional properties thereby further augmenting the clinical relevance of this cellular therapy 13–15. Previous attempts to improve upon EPC therapy involve pretreatment with small molecules or gene therapy, requiring introduction of exogenous DNA 16. In all previous studies, improvements have been limited to incremental enhancements of previously characterized therapeutic effects: survival, homing, proliferation, and paracrine factor release because they only target one gene or one signaling pathway. Importantly, none of these improved strategies have conferred enhanced differentiation capacity.
The epigenetic code, including post-translational modifications in histones, is responsible for adding complexity to gene regulation beyond sequence specificity. It plays a critical role in regulating everything from gene expression, cell cycle, cell fate, differentiation and ultimately the capacity for trans-differentiation in vivo 17, 18. Endothelial cells, vascular smooth muscle cells and cardiomyocytes all differentiate from a common progenitor in the mesoderm, suggesting that reprogramming endothelial cells back to an earlier state in mesodermal development could recapitulate their cardiomyogenic potential. Therefore, in order to remove the transcription-restrictive epigenetic marks and harness all the differentiation potential the endothelial progenitor cell might possess, we treated the EPCs with epigenetic modifying molecules, 5′Azacytidine, valproic acid and BIX-01294. Based on clues from epigenetic silencing mechanisms, we conceptualize that removal of epigenetic repressive marks by the aforementioned inhibitors will remodel chromatin surrounding cardiomyocyte specific genes (as well as global genes), thus providing a window where the cells epigenome is permissive to desired gene transcription and potentially trans-differentiation when cells are cultured under appropriate culture conditions or exposed to the proper microenvironment in vivo.
Our results suggest that small molecule based epigenetic reprogramming of EPCs, both murine and human, results in an open epigenome conducive to enhanced global gene expression including the induction of cardiomyocyte specific gene expression. Further, intramyocardial transplantation of reprogrammed EPCs in the infarcted mouse hearts resulted in enhanced physiological and anatomical repair compared to non-reprogrammed EPCs, suggesting an improved paracrine capacity of reprogrammed cells. Most interestingly, drug treated EPCs, both of murine and human origin, were able to trans-differentiate into cardiomyocytes in the border zone of the infarcted hearts. Collectively, our data suggests that epigenetic reprogramming of EPCs can enhance their therapeutic efficacy both by enhancing their plasticity and functions.
Methods (For extended methods section, please see supplemental information)
FACS sorting
Bone marrow extracted from the femurs, tibiae and hip-bones of 10–12 week old C57BL/6J or eGFP transgenic mice were stained with labeled antibodies against Lineage (CD3e(145-2C11), CD11b(M1/70), B220(RA3-6B2), Ter119(Ly76), Ly6G/C(RB6-8C5)) Sca-1 (D7) and CD31 (MEC13.3) then sorted on a triple-laser Mo-Flo cell sorter (Cytomation).
Real-time PCR
RNA was isolated and reverse transcribed into cDNA from sorted EPCs to assess gene mRNA expression of Oct4, Nanog, Sox2, Nkx2.5, connexin43, cardiac troponin T, eNOS and VE cadherin using the Cells to Ct kit (Invitrogen) according to the suggested protocol. Relative mRNA expression of target genes was normalized to the endogenous 18S control gene.
Myocardial Infarction
Mice underwent surgery to ligate the left anterior descending coronary artery 19 as reported previously 20. 2.0×105 mouse EPCs, 2.5 or 5×104 CD34+ cells re-suspended in 20μL PBS were injected intramyocardially into the LV wall (border zone) at 2 different locations immediately after LAD ligation. Saline group underwent the same surgery but received PBS without cells. Tissue was harvested at d7, d14 or d28 post-AMI for histological analysis.
Echocardiography
Transthoracic 2-dimensional M-mode echocardiography was obtained using the Vevo770 (VisualSonics, Toronto, ON, Canada) equipped with a 30-MHz transducer. Mice were anesthetized for analysis with a mixture of 1.5% isoflurane and oxygen (1L/min) prior to AMI (baseline) and at days 7, 14 and 28 post-AMI. M-mode tracings were used to measure LV wall thickness and LV inner diameter in systole and diastole. The mean value of 3 measurements was determined for each sample. Percentage fractional shortening (%FS) and ejection fraction (%EF) were calculated as described previously 21.
Morphometric studies
Infarcted hearts were perfused with PBS followed by methanol fixation and paraffin embedding. Morphometric analysis including infarct size and percent fibrotic area was performed on Masson’s trichrome-stained tissue sections using ImageJ 1.43u software (US National Institutes of Health;http://rsb.info.nih.gov//ij/).
Chromatin Immunoprecipitation
The ChIP assay was performed as previously described 22, 23.
Methylation analysis by pyrosequencing
Methylation studies were performed as previously described 24.
Statistical analyses
One-tailed, unpaired Student’s t tests (Microsoft Excel) were used to measure statistical differences where P < 0.05 was considered statistically significant.
Results
Staggered valproic acid then 5′Azacytidine treatment results in genome wide enhanced gene expression in EPCs
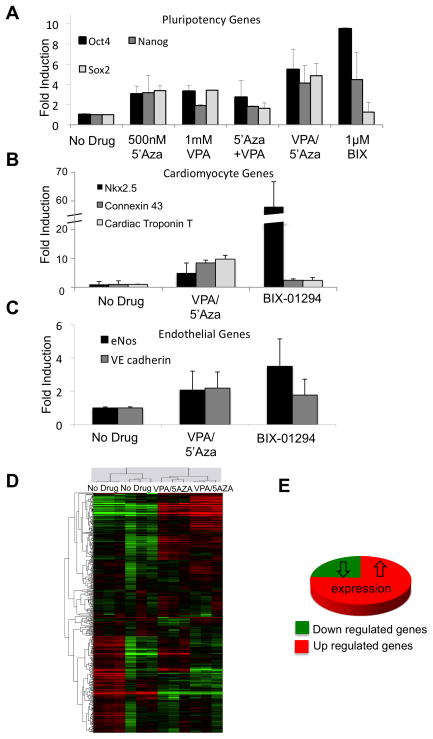
Whole bone marrow was isolated from femurs, tibiae and hip bones of C57BL/6 mice 25. Bone marrow mononuclear cells were FACS sorted to greater than 95% purity for the population of cells characterized as Lineage (Lin: CD11b, Ly6G/C, B220, CD3e, Ter119) negative, Sca-1+ CD31+, which represents approximately 1.4% of total mononuclear cells (Online Figure Ia). This sorting strategy allowed for the isolation of progenitor cell types (Lin-Sca-1+) from the bone marrow with endothelial cell linage (CD31+) 26. Lineage negative Sca-1+CD31+ cells, which will be referred to as EPCs henceforth, showed phenotypic characteristics consistent with their endothelial progenitor identity and incorporated into tubes formed by the mature murine endothelial cell line SVECs on Matrigel (BD Biosciences, Online Figure Ib). This suggests that this sorted population encompasses the functional, effector cells found in the bone marrow-derived cultured EPCs without necessitating in vitro culture or differentiation. In an attempt to increase their plasticity, 2.0×105 sorted EPCs were seeded on fibronectin coated plates then treated for 48 hours with individual or combinations of epigenetic modifying agents; 500nM 5′Azacytidine (5′Aza; DNA methyltransferase inhibitor), 1mM valproic acid (VPA; histone deacetylase inhibitor), 1μM BIX-01294; Histone methyltransferase inhibitor). Drug dosages comply with current literature suggestions 27–29 and were verified as non-toxic by cell viability analysis (data not shown). As determined by real-time PCR, this resulted in a significant induction of pluripotency-associated gene expression (Oct4, Nanog and Sox2) with the highest induction in the cells treated for 48 hours with 1μM BIX-01294 (Oct4 expression: 9.5±2.0 p=0.009), or 24 hours with 1mM VPA followed by an additional 24 hours with 500nM 5′Aza (Oct4 expression: 5.5±1.6 p=0.007) (Fig. 1a). All mRNA expression was normalized to 18S RNA then presented as a fold difference compared to untreated EPCs. Interestingly, one of the most effective conditions included a delayed addition of the DNA methyltransferase inhibitor 5′Azacytidine, indicating that inhibition of HDAC activity prior to DNA de-methylation may be beneficial for enhanced transcriptional activity throughout the genome. Additionally, real-time PCR results show de novo induction of cardiomyocyte-specific transcripts (Fig. 1b) in the two most effective drug treatment conditions immediately after treatment without necessitating cardiomyocyte differentiation conditions. Since the treated cells express cardiomyocyte genes at basal level, they may be more inclined to differentiate into mature CMCs under conditions conducive for CMC differentiation. Similar changes in gene expression were found in human CD34+ cells with the regimen of 2.5mM VPA followed by 500nM 5′Aza (Online Figure II). BIX-01294 treatment, even at reduced doses, was toxic to human CD34+ cells and was therefore not used in human cell studies.
Figure 1.
Drug treatment of sorted EPCs induces gene expression compared to the untreated cells based on Real-time PCR analysis. (a) pluripotency genes; Oct4, Nanog, and Sox2. (b) cardiomyocyte genes; Nkx2.5, Cx43 and Tnnt2. (c) endothelial genes; eNOS and VE cadherin. Values are all fold change compared to the untreated cells. (n=3) (d) Clustering analysis of microarray data comparing human CD34+ cells to those treated with VPA/5′Aza. Up-and down-regulated genes are represented in red and green colors, respectively. CD34+ cells from 2 healthy patient donors were run in triplicate for both conditions. (e) Pie graph depicting pattern of expression changes of statistically significantly affected genes upon VPA/5′Aza treatment of CD34+ cells.
Genome-wide expression profiling of drug-treated human CD34+ cells confirmed that de novo induction of previously silent genes was not limited to any one cell type or cellular function (Online Table I) but was rather reflective of a global transcription permissive chromatin and an open epigenome. Transcriptional profiling of control and treated cells identified 914 genes as significantly up-regulated whereas only 296 genes were significantly down regulated in the staggered VPA/5′Aza treated cells compared to untreated control CD34+ cells (average of two sample sets run in triplicate, Fig. 1d, e). These findings indicate that epigenetic modifying drug treatment results in a global increase in gene expression, which is not limited to induction of pluripotency or cardiomyocyte-specific gene expression. Interestingly, endothelial cell specific gene expression (Fig. 1c and Online Figure II) in both mouse EPCs and human CD34+ cells treated with drugs was either maintained or was marginally increased suggesting that the cells do not acquire pluripotentcy and therefore, a potentially tumorigenic phenotype. This was further supported by the inability of the treated cells to form teratomas when injected into immune-deficient mice and followed up to 4 weeks (Online Figure III).
Chromatin remodeling drugs effectively remove targeted transcription repressive epigenetic marks in endothelial cells
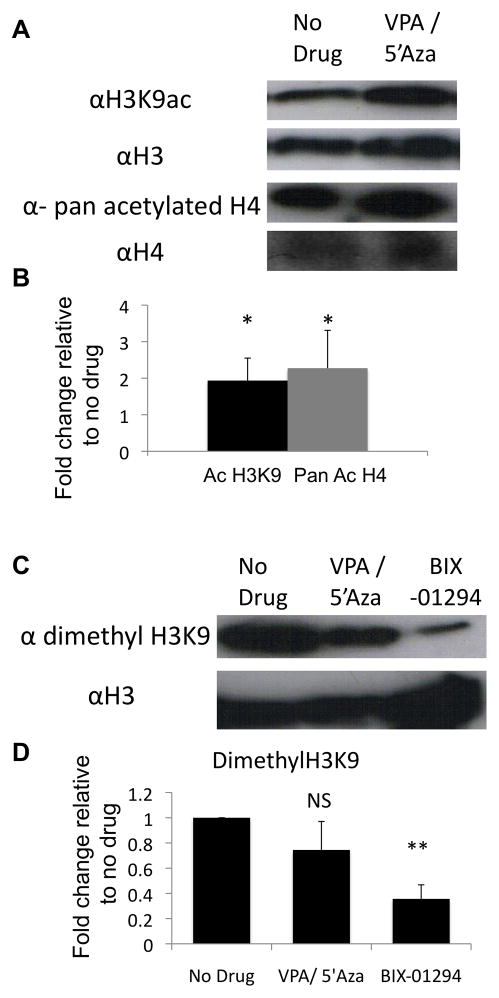
Valproic acid, 5′Azacytidine and BIX-01294 are effector molecules that remodel the chromatin to allow for less restricted gene expression and increased lineage differentiation potential 28, 30–35. VPA and BIX-01294 are direct inhibitors of histone modifying enzymes (histone deacetylase and histone methyltransferase G9a, respectively). Therefore, to confirm activity, murine endothelial cells (SVEC) were treated with either 1μM BIX-01294 or staggered addition of 1mM VPA then 500nM 5′Azacytidine for 48 h and histone acetylation and methylation were assessed by western blot. Acetylation of the lysine 9 position of histone 3 (H3K9, a transcription permissive epigenetic mark) was significantly higher in cells treated with VPA/5′Aza compared to control cells (Fig. 2a, b). Additionally, a pan acetylated histone 4 antibody detected a greater than 2-fold increase in total H4 acetylation in the drug treated cells (Fig. 2a, b). BIX-01294 directly inhibits G9a from forming di-methylated H3K9. H3K9 di-methylation was reduced by 64.3±11% by BIX-01294 compared to untreated control cells (Fig. 2c, d). This suggests both drug conditions, VPA/5′Aza and BIX-01294, actively target the intended epigenetic enzymes.
Figure 2.
VPA/5′Aza or BIX-01294 treatment effects histone modifications in ECs. (a) Western blot analysis of acetylated H3K9, or pan acetylated H4, or (c) di-methyl H3K9 levels from 10 million SVEC cells after 24 hours treatment with 1mM VPA followed by an additional 24 hours with 500nM 5′Azacytidine or 1μM BIX-01294. (b, d) Quantitative assessment of modified histone levels relative to total histone levels. Values are fold change compared to untreated SVEC cells. (n=3)
Drug treatment enhances histone acetylation at pluripotency and cardiac specific gene promoters
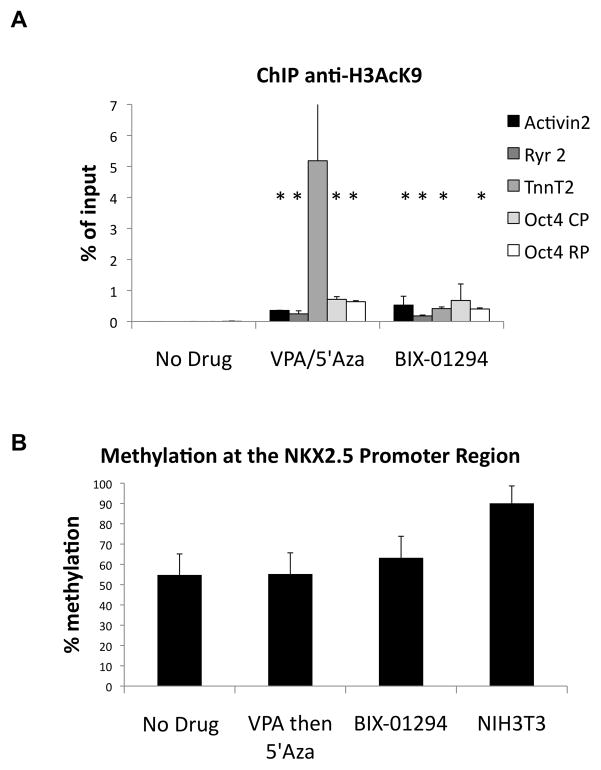
In order to further characterize the epigenetic landscape within the regulatory region of cardiac and pluripotent genes, chromatin immunoprecipitation (ChIP) experiments were carried out to evaluate the level of H3K9-acetylation. Chromatin from control and drug treated SVEC cells was immunoprecipitated with anti-H3K9-acetyl antibodies followed by RT-PCR analysis to quantitate the amount of bound acetylated H3K9 to the Activin 2 (Actn2), Ryanodine receptor (Ryr2), and Troponin T (TnnT2) cardiomyocyte gene promoters or two of the 5′ regulatory regions of Oct4. Cells treated with either BIX-01294 or VPA/5′Aza showed significant increase in the amount of acetylated H3K9 bound to the core (CP) and regulatory promoter (RP) regions of Oct4 compared to the untreated controls (p<0.05; Fig. 3a). Further, all three cardiac-specific gene promoters analyzed in both drug conditions were more heavily bound by acetylated H3K9 than in the non-treated cells (p<0.05; Fig. 3a). This increase in acetylated H3K9 associated with transcription regulatory regions is potentially responsible for the induction of Oct4 and cardiac-specific gene expression in treated EPCs. DNA methylation analysis of Nkx2.5, a cardiomyocyte-specific transcription factor, showed no significant difference in the CpG methylation patterns between treated and control SVEC cells (Fig. 3b). These data suggest that increased H3K9 acetylation rather than DNA methylation, largely accounts for de novo transcription of these previously silent transcripts.
Figure 3.
Epigenetically reprogrammed EPCs have increased acH3K9 associated with cardiac-specific promoters. (a) ChIP assay at CMC-associated gene promoters and 2 regulatory regions of Oct4 CP=core promoter, RP=regulatory promoter. Values represent percent of total input. * p<0.05 (b) Pyro-sequencing of the Nkx2.5 promoter from isolated and bisulfite converted genomic DNA from SVECs with either no drug, VPA/5′Aza or BIX-01294. Converted NIH-3T3 gDNA was used as a positive control.
Transplantation of reprogrammed mouse EPCs improves post-infarct left ventricular function and adverse remodeling to a greater extent than untreated EPCs
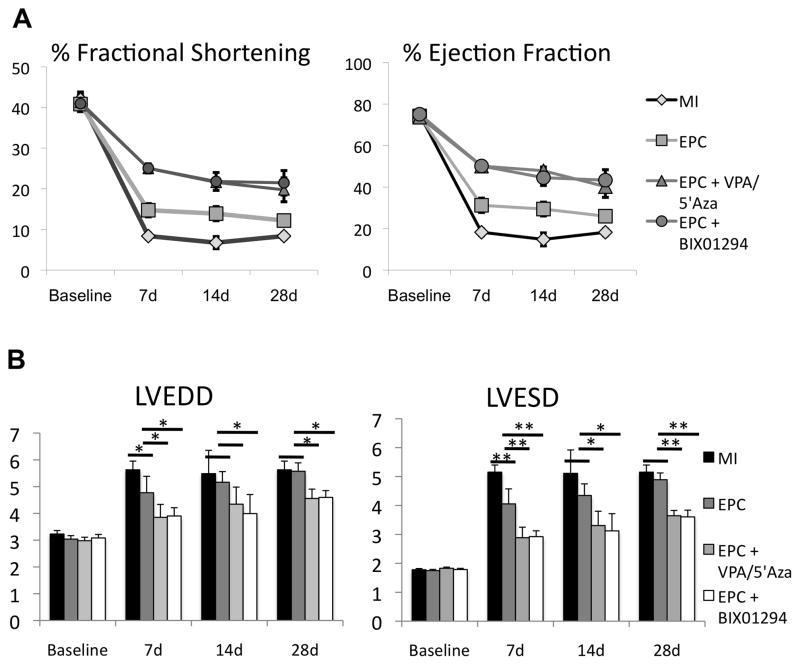
In the next series of experiments, we determined the therapeutic efficacy and differentiation plasticity of drug-treated mouse EPCs in mouse acute myocardial infarction model (AMI). AMI was induced by the permanent ligation of LAD and EPCs, either untreated or treated with BIX-01294 alone or VPA/5′Aza, were intra-myocardially injected at the ischemic border zone. A subset of mice received saline as control. Left ventricular (LV) functions were evaluated by M-mode echocardiography on days 7, 14 and 28, post-AMI. LV function data indicated a significantly improved ejection fraction (%EF), and fractional shortening (%FS) parameters (p<0.01) in mice receiving drug-treated EPCs compared to untreated EPCs (Fig. 4a). Similarly, left ventricle end-diastolic diameter (LVEDD) and left ventricle end-systolic diameter (LVESD) were significantly reduced in mice receiving treated EPCs compared to control EPCs (p<0.01; Fig. 4b).
Figure 4.
Drug treated mouse EPCs improve left ventricular function post myocardial infarction (AMI). (a) Echocardiographic analysis of left ventricular heart function assessed by percent fractional shortening and ejection fraction prior to surgery and at 7, 14 and 28 days post-surgery. MI=saline negative control, EPC=Lin-Sca-1+CD31+ mouse bone marrow cells. (n≥6 at each time point). (b) Left ventricle end-systolic and end-diastolic diameter measured from short-axis m-mode echocardiography. *p≤0.05, **p≤0.01 (n≥6)
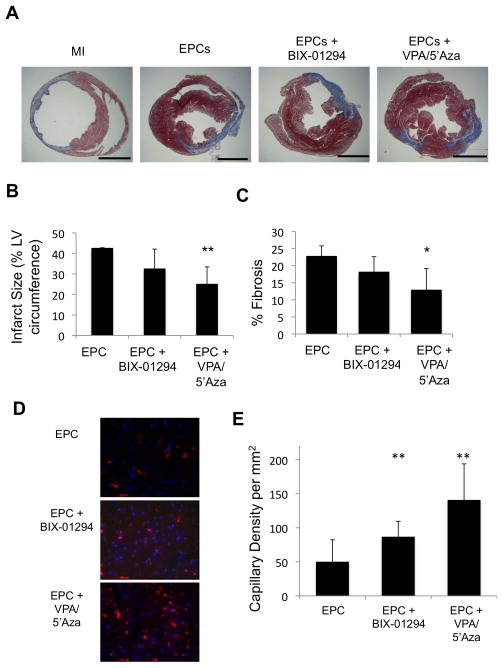
Significant improvement in LV function with drug-treated EPCs was further corroborated by anatomical and histological evidence. An analysis of infarct size and percent LV fibrosis of excised hearts at day 28 post-AMI showed a significant reduction in the infarct size in mice transplanted with drug-treated EPCs, compared to controls (p<0.01; Fig. 5a, b). Similarly mice receiving drug-treated EPCs showed significantly reduced LV fibrosis on day 28, post-AMI. (p<0.01; Fig. 5c).
Figure 5.
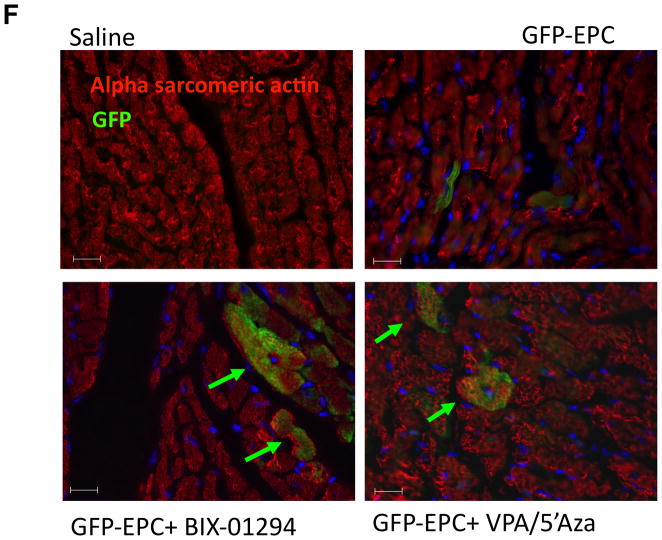
Histological evaluation of infarcted hearts indicates drug treated EPCs confer less severe disease and allow for CMC trans-differentiation in vivo. (a) Masson’s trichrome-stained heart sections (28 d post-AMI). Scale bar is 5mm. (b) Quantitative analysis of infarct size and (c) fibrotic area (%LV area) analysis at 28 d post-AMI. *p≤0.05, **p≤0.01 (n≥3) (d, e) Capillary density per mm2 calculated from 3 high power fields per heart of the border zone of myocardial infarcted mice, minimally 3 mice per condition. (f) IF of heart sections at day 14 post-AMI for GFP and alpha-sarcomeric actin. DAPI was used to stain nuclei. Arrows point to double stained cells. Representative images shown of n=4 animals.
Epigenetically reprogrammed EPCs augment post-AMI vascularity and trans-differentiate into cardiomyocytes
Endothelial progenitor cells have been shown to enhance post-infarct LV functions by augmenting neo-vascularization in the ischemic myocardium, largely by paracrine mechanisms. We assessed capillary density in the border zone of ischemic myocardium at 28 days post-AMI and cell injections. Capillaries were identified as vascular structures staining positive for CD31. EPCs reprogrammed with either VPA/5′Aza or BIX-01294 showed significantly larger number of capillaries, especially in mice receiving EPCs treated with VPA/5′Aza, when compared to untreated EPCs (Fig. 5d, e), suggesting that epigenetic reprogramming of EPCs likely enhances their paracrine abilities leading to increased vascularization and concomitant improved LV function.
More interestingly, epigenetic reprogramming of the EPCs with VPA/5′Aza or BIX-01294 rendered them a more plastic phenotype capable of trans-differentiating to cardiomyocyte lineage, in vivo. eGFP positive Lineage-Sca-1+ CD31+ cells were treated with the combination of drugs before transplantation into the ischemic myocardium following AMI. Although GFP+ cells were located in the EPC control group, none were found to co-stain with the cardiomyocyte specific protein marker, alpha-sarcomeric actin. However, some of the BIX-01294-treated EPCs and an even greater number of VPA/5′Aza treated EPCs co-stained for both GFP and alpha-sarcomeric actin (Fig. 5f). This suggests that, in addition to improving LV function through enhancement of the inherent therapeutic properties of EPCs, treatment of EPCs with VPA/5′Aza or BIX-01294 also enables acquisition of cardiomyocyte differentiation in vivo.
Epigenetically Reprogrammed human CD34+ cells display better therapeutic efficacy, paracrine activity and enhance ischemic myocardial vascularity following AMI in immune-deficient mice
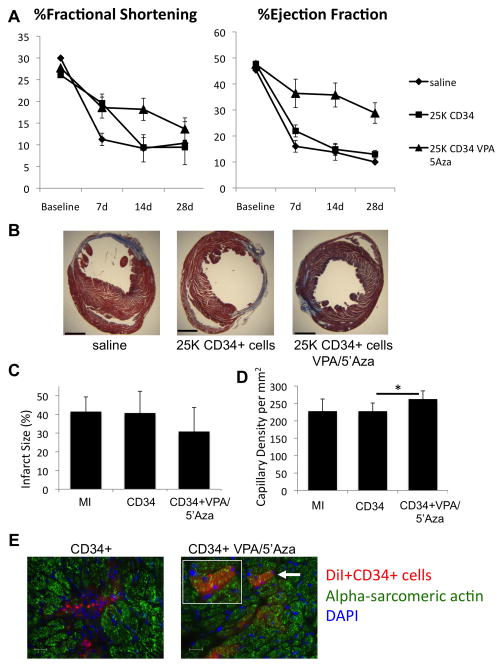
We next determined if the epigenetic reprogramming by these small molecules is limited to mouse EPCs or whether it can also be translated to human EPCs (bone marrow mobilized CD34+ cells), currently used in clinical trials 6. CD34+ cells, obtained from healthy donors and provided by Baxter healthcare, were treated or not with VPA (2.5 mM and 5′Aza (500nM) and mRNA expression of pluripotency and cardiomyocyte-specific transcripts was determined. Despite the promising results seen with BIX-01294 treatment of the mouse EPCs, similar drug doses and even 2-fold reduced levels were toxic to the human CD34+ cells and therefore, omitted. Untreated CD34+ cells did not express significant level of either pluripotent gene or cardiomyocyte gene mRNA, however, VPA/5′Aza treatment significantly induced the expression of these genes (Online figure II). In order to determine enhanced functional capacity of reprogrammed CD34+ cells in the repair of post-infarct myocardium, a previously determined sub-therapeutic dose of 25,000 cells was used for post-AMI transplantation in immune-deficient mouse AMI model. The dose of 25,000 CD34+ cells was chosen deliberately, since our unpublished studies have demonstrated that at this dose, these cells do not confer any therapeutic benefits. The rationale for using a sub-therapeutic dose of cells was to test if the epigenetic reprogramming of cells will enhance the therapeutic efficacy of a cell dose that by itself is not therapeutic. At this dose, mice transplanted with untreated cells did not show any improvement in LV functions, infarct size, or capillary density compared to saline group, whereas transplantation of CD34+ cells treated with VPA/5Aza resulted in a significant enhancement of LV function based on %FS and %EF (%EF at d28 CD34+ 12.96±1.49, VPA/5′Aza 28.82±3.94, p=0.01, Fig. 6a). Additionally, transplantation of reprogrammed CD34+ cells resulted in significantly reduced infarct size and significantly increased capillary density in the border zone of infarcted myocardium (Fig. 6b,-d). Additionally, we observed significantly reduced apoptosis and increased proliferation in the infarcted myocardium in the hearts of mice receiving treated CD34+ cells compared to untreated cells (Online Figure IV).
Figure 6.
Drug treated human CD34+ cells improve LV function based on %FS and %EF. (a) Echocardiographic analysis at baseline and at days 7, 14 and 28 post-AMI for all groups. (b) Masson’s trichrome stained 5μm sections 1mm below suture of infracted hearts 28 days post-AMI. Scale bar is 5mm. (c) Measurements from Masson’s trichrome stained sections for infarct size as a percent of the circumference. (d) Capillary density calculated per mm2 from 3 high-powered fields. (n≥5) for each condition. (e) In vivo cardiomyocyte differentiation identified by immunofluorescence in d7 hearts of mice receiving AMI and DiI labeled CD34+ or VPA/5′Aza treated CD34+ cells in the border zone. DiI labeled donor cells (red), alpha-sarcomeric actin 48 and nuclei (DAPI). White arrow indicates potential donor-derived cardiomyocyte and shown bigger in inset. Scale bar represents 20μm. Representative images from each group are shown.
Because the cardio-protective effects (less apoptosis, increased proliferation, increased V). These data suggests that observed therapeutic effect with treated CD34+ cells in vivo capillary density, improved overall LV function) in mice that receive VPA/5′Aza treated CD34+ cells in the AMI model was considerable, we evaluated whether the paracrine secretory profile is enhanced in these treated cells. The Human Angiogenesis Array (R&D Systems) was used to quantitate changes in angiogenesis promoting proteins in the conditioned medium of control and treated cells. CD34+ cells treated with VPA/5′Aza showed an increase in a majority (15/19) of detected angiogenic proteins (Online Figure was in part due to their enhanced angiogeneic phenotype.
Treatment with VPA/5′Aza confers cardiac differentiation potential to human CD34+ cells in vivo
We also determined if increased repair capacity of treated CD34+ cell in vivo also reflect their increased plasticity towards cardiomyocyte differentiation in vivo. AMI was induced in immune-deficient mice and 4.0×105 DiI-labeled CD34+ cells with or without treatment with VPA/5′Aza were injected into the heart at the time of coronary artery ligation. Heart tissue was harvested 7 days post-AMI and stained for alpha-sarcomeric actin while DiI was used as an indicator of donor cells. Immunofluorescence staining identified donor DiI+ cells that co-stained with alpha-sarcomeric actin in hearts exclusively receiving treated CD34+ cells (Fig. 6e). In hearts receiving untreated cells, no co-staining was observed. This suggests that in addition to enhancing their angiogenic activity, drug treatment also increased their plasticity towards de novo cardiomyocyte differentiation.
Discussion
Despite significant improvement in the prognosis of patients with myocardial ischemia (MI) by available therapies including thrombolysis and urgent revascularization, there remains significant mortality and a significant proportion of survivors are at risk for developing heart failure. Since the main underlying cause of this process represents the loss of cardiomyocytes and microvasculature of the infarcted wall, the development of treatment strategies aimed at preserving or regenerating myocardial tissue is currently acknowledged as a central therapeutic challenge. The available evidence demonstrating improvement in myocardial function following transplantation of autologous bone marrow (BM)-derived stem/progenitor cells including endothelial progenitor cells (EPC), both in pre-clinical as well as in available clinical trials, remains a potent force driving discovery and clinical development simultaneously and has provided new hope for patients with debilitating heart diseases. In the clinical setting, the functional improvement in patients receiving BM-EPCs has been reported and these findings have been replicated in recently completed randomized, double-blind phase II clinical trial 6. In another randomized trial,36 authors reported an increase in ejection fraction and a reduction of end systolic volumes in the group of patients receiving bone marrow cells. Recently these authors published the long-term follow up of these patients, revealing that the early benefit that had been observed was not preserved 37. The reasons for the apparent loss of benefit remain to be resolved however, risk factors for coronary artery disease, including diabetes and age, are reported to be associated with a reduced number and impaired functional activity of EPCs in the peripheral blood of patients 38–41. Moreover, while it is generally accepted that EPCs participate in vascular repair of the ischemic myocardium both via paracrine mechanisms and by physically integrating into neo-vasculature, there exists no convincing evidence that these cells are capable of trans-differentiating into functional cardiomyocytes 11, 12, 38. Since the ultimate goal of cardiac regenerative medicine is to replace both microvasculature as well as lost cardiomyocytes, it is therefore imperative to make efforts towards refining the strategies to improve this autologous source of cardiac cell therapy by augmenting both EPC function and plasticity.
Our studies reported in this manuscript provide evidence that small molecule mediated epigenetic reprogramming of EPCs, both mouse and human, significantly enhances both their angiogenic and functional activity as well as lead to a cellular epigenome that is conducive to a more plastic phenotype capable of trans-differentiation into cardiomyocyte lineage. Several lines of evidence support this conclusion: 1) Treatment of both mouse EPC and human CD34+ cells with small molecule inhibitors of DNA methyltransferase, histone deacetylase and G9a histone methyltransferase leads to globally up-regulated gene transcription indicative of dynamic chromatin remodeling and transcription permissive epigenome; 2) cells treated with these small molecules show de novo induction of both cardiomyocyte specific genes and yet retain their endothelial gene expression; 3) epigenetically reprogrammed EPCs show enhanced angiogenic activity both in terms of paracrine factor secretion and increased ischemic angiogenesis in vivo, including the enhancement of both capillary density and the number of arterioles in the border zone of the infarct, suggesting both angiogenic and vasculogenic activity; 4) epigenetic reprogramming of both mouse and human CD34+ cells significantly enhances their myocardial repair capabilities as evident from improved LV functions and anatomical repair including reduced fibrosis and apoptosis following acute myocardial infarction in mice; and finally 5) reprogrammed cells differentiate into cardiomyocytes, in vivo. We believe that these exciting data have a clear translational bearing on the BM-EPC based cellular therapies for myocardial regenerative medicine.
There is growing recognition that epigenetic mechanisms largely govern the cell fate and function. Much of the information obtained from studies on nuclear cloning and iPS cells on the epigenetic changes in somatic nuclei points towards somatic cell chromatin remodeling mediated via the process of chromatin condensation, DNA methylation/demethylation and post-translational histone modifications (acetylation, phosphorylation and methylation):- two processes that are functionally linked 17, 42. Although complex, the process of epigenetic gene silencing involves 3 critical steps: a) methylation of 5′regulatory regions of target gene, b) deacetylation of histones3/4 by HDACs and c) di-methylation of lysine 9 of histone 3 (H3K9). Chemical inhibitors of DNA methyltransferases (5-Azacytidine) and histone deacetylases (Trichostatin A, Valproic acid), are capable of inducing multipotency which is evidenced by their significant enhancement of reprogramming of somatic cells for somatic cell nuclear transfer (SCNT) or iPS derivation35, 43, 44. Both drugs can change the fate of a given cell by chromatin remodeling leading to a heterochromatin status permissive for silenced gene transcription including reactivation of pluripotency associated genes in somatic cells 30, 44, 45. Specifically in the cardiovascular system, studies have demonstrated that treatment with 5′Azacytidine leads to an increase in cardiomyocyte differentiation of mesenchymal stem cells (MSC) with improved cardiac function after transplantation of 5′Azacytidine–treated MSCs compared to control MSCs 31–34. While these chemical modifiers of key epigenetic repressive marks improve reprogramming efficiency, they are insufficient to induce pluripotency, eliminating the risk of teratoma formation associated with cell therapy 34. Our data corroborates these findings. We observed that treatment of EPCs with VPA/5′Aza or BIX-01294 leads to robust increase in H3K9 acetylation and significantly reduced G9a specific H3K9-di-methyltransferase activity. Importantly, a number of cardiomyocyte specific gene promoters were enriched for H3K9 acetylation indicating inhibition of HDACs as primary epigenetic modification for de novo induction of cardiomyocyte genes. We did not see much change in the Nkx2.5 promoter methylation patterns in control and VPA/5′Aza treated EPCs. There could be several explanations to this unexpected finding. First, we only looked at the promoter region of Nkx2.5 which harbors only few CpG residues; it is possible that an analysis of methylation pattern 5′ to the Nkx2.5 promoter including enhancer region and CpG islands would have yielded different methylation patterns. Secondly, since EPCs are progenitor cell and phylogenetically related to the common mesodermal precursor from which both endothelial cells and cardiomyocytes are derived, the cardiomyocyte specific gene promoters are still hypo-methylated. Indeed, when compared to fibroblasts, we did find significantly less methylated Nkx2.5 promoter region in EPCs.
Although the mouse EPC population used in our studies and characterized as lineage-Sca-1+ CD31+ has only recently been characterized in the literature, our data supports the claim that these cells function similarly to the human CD34+ cell population mobilized from patient bone marrow and is currently used clinically for cardiac cell therapy. The human population is heterogeneous and may contain cell types similar to out-growth populations known as early or late EPCs found in mice 10. Though it is unclear whether the cell type we are using is also present in humans, the surface marker profile of these cells most closely resembles the early EPC population characterized by Asahara 46 that arises after 7–10 days of culture in EBM-2 medium supplemented with IGF-1, VEGF, FGF and EGF. These cells have been shown to acquire both Sca-1 and CD31 surface marker expression during culture 47. Potentially, the same well-characterized early EPC population created in culture already exists endogenously in the bone marrow.
In summary, the results described above clearly suggest the current myocardial cellular therapy using EPC/CD34+ cells can be improved upon both in terms of functional outcome and potential regenerative capacity by pre-treatment with epigenetic modifying small molecules. Indeed, our data indicates that epigenetic reprogramming of CD34+ cells boosted the functional cardiac repair capacity of an otherwise sub-therapeutic dose of cells. Importantly, unlike pluripotent cells, transient reprogramming of EPC is safe since these cells did not show any evidence of teratoma formation in mice. Our approach to use small molecules to create a less condense, more accessible epigenome is a creative way to induce multipotency and cardiomyogenic differentiation potential in EPCs, without the undesired complications involved with induced pluripotent cells, is novel. Additionally, our studies have a direct bearing on translation regenerative medicine and could potentially augment the efficacy of an existing clinical therapy for cardiovascular diseases.
Supplementary Material
Novelty and Significance.
What is known?
Transplantation of Endothelial progenitor cells (EPC) enhances neo-vascularization in the ischemic tissues.
EPC possess little to no cardiomyocyte trans-differentiation ability.
Removal of inhibitory epigenetic marks can improve cellular plasticity.
What new information does this article contribute?
Drugs targeting repressive epigenetic marks induce myogenic plasticity in EPC
Epigenetic reprogramming up-regulates genome wide transcription, including cardiomyocyte-specific gene expression in EPCs.
Reprogrammed EPCs are therapeutically superior to untreated cells resulting in improved left ventricular function in an acute myocardial infarction model.
Secretion of pro-angiogenic factors is enhanced in drug-treated EPCs.
Drug-treated EPCs from both mouse and human show cardiomyocyte differentiation potential in vivo.
Summary of the Novelty and Significance
The therapeutic benefits of BM-EPC therapy in pre-clinical and clinical trials have been attributed to paracrine factor-mediated vascular repair without myogenesis and/or myocardial regeneration. Although the revascularization appears to improve the quality of life, the ultimate goal is regeneration and repair of the afflicted myocardium. Therefore, it is of interest to improve the cardiomyogenic properties of existing autologous cell therapy that has already been approved for clinical use. This study demonstrates that removal of inhibitory epigenetic modifications in both mouse and human EPCs confers enhanced therapeutic potential a mouse model of acute myocardial infarction. Not only is the inherent paracrine activity greater as evidenced by improved capillary density, cell survival and proliferation within the border zone of the infarct but the modified cells also acquire cardiomyogenic potential. The suggested mechanism for the enhanced functionality and differentiation potential is the positive effect epigenetic modifying drugs have on global gene transcription, which primes the cell to respond to environmental stimuli. Clinically, this may be an effective way of modifying an existing cellular therapy with potentially significant improvements not only in revascularization of the ischemic tissue, but also regeneration of the damaged myocardium.
Acknowledgments
Sources of Funding:
Work described in this manuscript was supported in part by National Institute of Health grants HL091983, HL105597, HL095874, HL053354 and HL108795 to R.K. and NIH NRSA F32 postdoctoral award HL107093 to M.A.T.
Non-Standard Abbreviations and Acronyms
- 5′Aza
5′ Azacytidine
- AMI
acute myocardial infarction
- BIX
BIX-01294
- BM
bone marrow
- ChIP
chromatin immunoprecipitation
- CMC
cardiomyocyte
- EF
ejection fraction
- EPC
endothelial progenitor cell
- FACS
fluorescence activated cell sorting
- FS
fractional shortening
- HDAC
histone deacetylase
- LAD
left anterior descending
- Lin
lineage
- LV
left ventricle
- LVEDD
left ventricular end diastolic dimension
- LVESD
left ventricular end systolic dimension
- VPA
valproic acid
Footnotes
Disclosures:
The authors have nothing to disclose.
References
- 1.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (topcare-ami): Mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 2.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: Part ii: Cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 3.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part i: Angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 4.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the repair-ami trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 5.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 6.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasinski K, Spyridopoulos I, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. doi: 10.1161/01.cir.95.7.1768. [DOI] [PubMed] [Google Scholar]
- 8.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18:33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pesce M, Burba I, Gambini E, Prandi F, Pompilio G, Capogrossi MC. Endothelial and cardiac progenitors: Boosting, conditioning and (re)programming for cardiovascular repair. Pharmacol Ther. 2011;129:50–61. doi: 10.1016/j.pharmthera.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 12.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 13.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 14.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 15.Michowitz Y, Goldstein E, Wexler D, Sheps D, Keren G, George J. Circulating endothelial progenitor cells and clinical outcome in patients with congestive heart failure. Heart. 2007;93:1046–1050. doi: 10.1136/hrt.2006.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4 (Suppl 1):S110–113. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 17.Trojer P, Reinberg D. Histone lysine demethylases and their impact on epigenetics. Cell. 2006;125:213–217. doi: 10.1016/j.cell.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 19.Asahara H, Hasunuma T, Kobata T, Inoue H, Muller-Ladner U, Gay S, Sumida T, Nishioka K. In situ expression of protooncogenes and fas/fas ligand in rheumatoid arthritis synovium. J Rheumatol. 1997;24:430–435. [PubMed] [Google Scholar]
- 20.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. Il–10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of stat3 and suppression of hur. Circ Res. 2009;104:e9–18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamurthy P, Lambers E, Verma S, Thorne T, Qin G, Losordo DW, Kishore R. Myocardial knockdown of mrna-stabilizing protein hur attenuates post-mi inflammatory response and left ventricular dysfunction in il-10-null mice. FASEB J. 2010;24:2484–2494. doi: 10.1096/fj.09-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J, Wan M, Zhang Y, Gu P, Xin H, Jung SY, Qin J, Wong J, Cooney AJ, Liu D, Songyang Z. Nanog and oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat Cell Biol. 2008;10:731–739. doi: 10.1038/ncb1736. [DOI] [PubMed] [Google Scholar]
- 23.Rajasingh J, Lambers E, Hamada H, Bord E, Thorne T, Goukassian I, Krishnamurthy P, Rosen KM, Ahluwalia D, Zhu Y, Qin G, Losordo DW, Kishore R. Cell-free embryonic stem cell extract-mediated derivation of multipotent stem cells from nih3t3 fibroblasts for functional and anatomical ischemic tissue repair. Circ Res. 2008;102:e107–117. doi: 10.1161/CIRCRESAHA.108.176115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie H, Wang M, Bonaldo Mde F, Smith C, Rajaram V, Goldman S, Tomita T, Soares MB. High-throughput sequence-based epigenomic analysis of alu repeats in human cerebellum. Nucleic Acids Res. 2009;37:4331–4340. doi: 10.1093/nar/gkp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Celso C, Scadden D. Isolation and transplantation of hematopoietic stem cells (hscs) J Vis Exp. 2007:157. doi: 10.3791/157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived cd31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56:593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T. Reversal of h3k9me2 by a small-molecule inhibitor for the g9a histone methyltransferase. Mol Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only oct4 and sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 29.Lynch CA, Tycko B, Bestor TH, Walsh CP. Reactivation of a silenced h19 gene in human rhabdomyosarcoma by demethylation of DNA but not by histone hyperacetylation. Mol Cancer. 2002;1:2. doi: 10.1186/1476-4598-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milhem M, Mahmud N, Lavelle D, Araki H, DeSimone J, Saunthararajah Y, Hoffman R. Modification of hematopoietic stem cell fate by 5aza 2′deoxycytidine and trichostatin a. Blood. 2004;103:4102–4110. doi: 10.1182/blood-2003-07-2431. [DOI] [PubMed] [Google Scholar]
- 31.Burlacu A, Rosca AM, Maniu H, Titorencu I, Dragan E, Jinga V, Simionescu M. Promoting effect of 5-azacytidine on the myogenic differentiation of bone marrow stromal cells. Eur J Cell Biol. 2008;87:173–184. doi: 10.1016/j.ejcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye NS, Chen J, Luo GA, Zhang RL, Zhao YF, Wang YM. Proteomic profiling of rat bone marrow mesenchymal stem cells induced by 5-azacytidine. Stem Cells Dev. 2006;15:665–676. doi: 10.1089/scd.2006.15.665. [DOI] [PubMed] [Google Scholar]
- 34.Yoon J, Min BG, Kim YH, Shim WJ, Ro YM, Lim DS. Differentiation, engraftment and functional effects of pre-treated mesenchymal stem cells in a rat myocardial infarct model. Acta Cardiol. 2005;60:277–284. doi: 10.2143/AC.60.3.2005005. [DOI] [PubMed] [Google Scholar]
- 35.Ding X, Wang Y, Zhang D, Guo Z, Zhang Y. Increased pre-implantation development of cloned bovine embryos treated with 5-aza-2′-deoxycytidine and trichostatin a. Theriogenology. 2008;70:622–630. doi: 10.1016/j.theriogenology.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 36.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The boost randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 37.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled boost (bone marrow transfer to enhance st-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 39.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of hmg-coa reductase inhibitors. Kidney Int. 2005;67:1672–1676. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 40.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 41.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired cxcr4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 42.Klose RJ, Bird AP. Genomic DNA methylation: The mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maalouf WE, Liu Z, Brochard V, Renard JP, Debey P, Beaujean N, Zink D. Trichostatin a treatment of cloned mouse embryos improves constitutive heterochromatin remodeling as well as developmental potential to term. BMC Dev Biol. 2009;9:11. doi: 10.1186/1471-213X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng HF, Kuo YL, Loo MR, Li CL, Chu TW, Suo H, Liu HS, Lin KH, Chen SL. Valproic acid enhances oct4 promoter activity in myogenic cells. J Cell Biochem. 2010;110:995–1004. doi: 10.1002/jcb.22613. [DOI] [PubMed] [Google Scholar]
- 46.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 47.Sangidorj O, Yang SH, Jang HR, Lee JP, Cha RH, Kim SM, Lim CS, Kim YS. Bone marrow-derived endothelial progenitor cells confer renal protection in a murine chronic renal failure model. Am J Physiol Renal Physiol. 2010;299:F325–335. doi: 10.1152/ajprenal.00019.2010. [DOI] [PubMed] [Google Scholar]
- 48.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D’Agati VD, Friedman AL. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.