Abstract
HSP40 family member MRJ (DNAJB6) has been in the spot light for its relevance to Huntington’s, Parkinson’s diseases, limb-girdle muscular dystrophy, placental development, neural stem cells, cell cycle and malignancies such as breast cancer and melanoma. This gene has two spliced variants coding for 2 distinct proteins with significant homology. However, MRJ(L) (large variant) is predominantly localized to the nucleus whereas MRJ(S) (small variant) is predominantly cytoplasmic. Interestingly MRJ(S) translocates to the nucleus in response to heat shock. The classical heat shock proteins respond to crises (stress) by increasing the number of molecules, usually by transcriptional up-regulation. Our studies imply that a quick increase in the molar concentration of MRJ in the nuclear compartment is a novel method by which MRJ responds to stress. We found that MRJ(S) shows NLS (nuclear localization signal) independent nuclear localization in response to heat shock and hypoxia. The specificity of this response is realized due to lack of such response by MRJ(S) when challenged by other stressors, such as some cytokines or UV light. Deletion analysis has allowed us to narrow down on a 20 amino acid stretch at the C-terminal region of MRJ(S) as a potential stress sensing region. Functional studies indicated that constitutive nuclear localization of MRJ(S) promoted attributes of malignancy such as proliferation and invasiveness overall indicating distinct phenotypic characteristics of nuclear MRJ(S).
Keywords: MRJ, DnaJB6, NLS, Heat Shock
INTRODUCTION
Mammalian relative of DNAJ (MRJ) is a class II J-domain protein [1]. MRJ has been known for its roles in murine placental development, neural stem cell renewal, reducing the formation and toxicity of misfolded protein aggregates, cell cycle and multiple pathologies such as Huntington’s, Parkinson’s diseases, limb-girdle muscular dystrophy and malignancies such as breast cancer and melanoma [2–14]. This member of the HSP40 family of chaperones is expressed in humans in two separate spliced variants: 326-aa large form viz. MRJ(L), and 242-aa small viz. MRJ(S) [6, 15]. However, MRJ(L) (large variant) is predominantly localized to the nucleus whereas MRJ(S) (small variant) is predominantly cytoplasmic.
Expression of MRJ(L) is lost in advanced breast cancers and restoration of its expression resulted in reduced tumor xenograft growth and metastasis in athymic mice [6]. Wnt/β-catenin signaling is negatively modulated by MRJ(L) [16]. Additionally, MRJ(L) has been implicated in limiting epithelial-mesenchymal-transition, regulating murine placental development and modulating neural stem cell renewal [7, 9, 11, 16]. It is important to note that unlike most heat shock proteins, transcription of MRJ is not significantly up-regulated in response to heat shock (Supplemental Figure 1), this raises the question, and how MRJ may respond to heat shock?
We have previously demonstrated that MRJ(L) exhibits predominantly nuclear localization due to the presence of a C-terminal nuclear localization sequence (NLS) [6]. Here, we report that despite the lack of an NLS, MRJ(S) is capable of translocating to the nucleus in response to heat shock and hypoxia. In fact, our observations indicate that once in the nucleus, MRJ(S) is capable of localizing to the nucleolus in response to heat stress. Interestingly, nuclear MRJ(S) exhibits distinct additional functionalities such as promotion of invasive phenotype and proliferation that are seemingly contrasting to the functions exhibited by MRJ(L).
Experimental Procedures
Plasmids and Constructs
pcDNA3.1-Flag-MRJ(S) was generated by PCR amplification of chemically synthesized cDNA of MRJ(S) (Accession: NM_005494.2, GI:24234719) (GenScript Corp., Piscataway, NJ, USA) with primers that allowed incorporation of an in-frame N-terminal FLAG tag. The product was cloned into pcDNA3.1(−) (Invitrogen, Carlsbad, CA). pcDNA-Flag-MRJ(S)-NLS was cloned in pcDNA3.1(−) following PCR using primers that allowed in-frame incorporation of the NLS of SV40T. pcDNA-HA-MRJ(L) was generated by cloning a PCR product that was generated by amplifying cDNA of MRJ(L) with a primer that allowed incorporation of an in-frame HA tag. pEGFP-MRJ(S) and pEGFP-MRJ(L) were generated by cloning cDNA of MRJ(S) and MRJ(L) into the MCS of pEGFP-N1. Truncations were generated by amplifying MRJ(S) cDNA with reverse primers introducing stop codons at aa119, aa179, and aa220, respectively.
Cell Culture, Cell Lines and Transfection
COS7 and MDA-MB-435 cells were cultured in DMEM/F12 medium supplemented with 5% fetal bovine serum, 1.0 mg/mL sodium pyruvate and 1% non-essential amino acids. Cells were maintained in 5% CO2 at 37°C. Transfections were performed with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), or with Fugene 6 (Promega, Madison, WI) following manufacturers' recommendations. Stable cell lines were generated by maintaining transfected cells in media containing the selection agent G418 at a concentration of 500 μg/mL.
Heat shock and Cytokine Treatment
Cells were plated on cover slips or in glass-bottomed dishes (World Precision Instruments, Sarasota, FL), and were transfected with pEGFP-MRJ(S) after twenty-four hours. Heat shock was induced by replacing growth media with media pre-warmed to 42°C and placing cells in an incubator set and pre-warmed to 42°C. Cells expressing GFP fusions were imaged before and after two hours of heat shock treatment. Cytokine treatment was performed by adding serum-free media containing IFN-α (1000 units/ml) or IFN-γ (1000 units/ml) or IL-2 (1000 units/ml) or IL-1 alpha (500 IU/ml). Images were captured before and after two hours of cytokine treatment.
Hypoxia
Cells were seeded into dishes and transfected with pEGFP-MRJ(S). After 24 hours incubation at 37°C, 5% CO2 and normoxic conditions, dishes were placed in a a Billups-Rothenberg Modular Incubator Chamber 101 (Billups-Rothenberg, Inc., Del Mar, CA) that was flushed with a gas mixture of 3% O2, 5% CO2 and 92% N2 and incubated at 37°C. Images were captured after 5–24 hours of exposure to hypoxia.
UV Irradiation
Cells were plated in glass-bottomed dishes and after twenty-four hours, cells were gently washed and kept in PBS. Dishes were placed with the lid off in a Spectrolinker XL-1000 UV Crosslinker (Spectronics, Westbury, NY), and cells were irradiated with either 5 mJ/cm2 or 20 mJ/cm2 of 254 nm UV. Following irradiation, PBS was replaced with growth media and cells were placed in the incubator. Images were recorded immediately before and at 30 minute intervals following irradiation.
3D Cell Culture
Cells were seeded into Cultrex 3-D Culture Matrix BME (Trevigen Inc,, Gaithersburg, MD) in chambered slides at a density of 2000 cells per well and incubated at 5% CO2, 37°C. Images were recorded using a Nikon TE-2000E over a time course of eight days. For 3-D hypoxia experiments, the same cells were placed in the hypoxic chamber as above, with 3% O2, 5% CO2, 92% N2 and incubated at 37°C. Images of hypoxic cells were captured after six days.
Foci Formation
Single cell suspension was seeded at a density of either 5×103 cells per plate into 10 cm2 culture plates and incubated at 5% CO2 at 37°C. After 7 days of incubation, cells were washed with PBS and stained with crystal violet. Images were captured using a NikonTE-2000E microscope and analyzed using a custom macro written in Nikon NIS-Elements software.
Proliferation
Cells were seeded into 6-well culture plates at a density of 10×103 cells/well and incubated at 5% CO2, 37°C. Cells were removed from wells with trypsin and counted using a hemocytometer at 24 hour intervals, and the calculated total cell number was plotted and analyzed using Graphpad Prizm software (Graphpad Software, Inc., La Jolla, CA).
Immunocytochemistry
Cells were grown on glass-bottomed dishes and treated experimentally. Cells were then fixed in 4% paraformaldehyde in PBS, pH 7.4 for ten minutes and solubilized in 0.1 % Triton X-100 in PBS, pH 7.4 for ten minutes. Following 40 a minute blocking step in 10% normal goat serum in PBS, cells were incubated with rabbit monoclonal anti-fibrillarin antibody (Cell Signaling, Beverly, MA ), and in some cases with mouse monoclonal anti-Flag antibody (Sigma Aldrich, St. Louis, MO). After washing, cells were incubated with goat anti-rabbit antibody conjugated to Alexafluor 594 and in some cases goat anti-mouse antibody conjugated to Alexafluor 488 (Invitrogen, Carlsbad, CA). After a final wash, coverslips were mounted using Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA).
Quantitative Real time PCR
Total RNA was isolated from cultured cells using the RNEasy kit (Qiagen, Valencia, CA) per manufacturer's instructions. cDNA synthesis was carried out using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) with 2 μg of total RNA as the template and random primers. Real-time quantitative (RTQ-PCR) analysis was performed using an iQ5 iCycler instrument (BioRad, Hercules, CA) with primers from Applied Biosystems. Gene expression in each sample was expressed as a relative Ct value that was normalized against the expression of GAPDH.
Image Acquisition and Analysis
Images were captured using either a Nikon TE2000-E inverted fluorescent microscope equipped with Roper Coolsnap HQ2 camera, Live Cell cell-culture chamber, automated stage, and Perfect Focus system (Nikon, Melville, NY), or an Axiovert inverted fluorescent microscope equipped with Axiosnap camera (Zeiss). Image processing and measurement was performed with either NIS-Elements 3.2 (Nikon) or Axiovision 4.8.2 (Zeiss, Thornwood, NY).
Note: All experiments were repeated at least ones and all quantitations were performed from N=3 in each experiment and represented with error bars corresponding to SEM.
RESULTS
MRJ(S) localizes to nucleus in response to heat shock and hypoxia
The amino acid sequence of MRJ(S) is 96% identical to MRJ(L) (Figure 1A) [6] but MRJ(S) does not show a distinct NLS. However, some previous reports have alluded to a possibility of nuclear presence for MRJ(S) [17, 18]. Hence we wanted to test the possibility if MRJ(S) will display NLS-independent nuclear transport. To test this contention, we evaluated the effect of heat shock on EGFP-tagged MRJ(S). Concurrent with our hypothesis, we found that the predominantly cytoplasmic MRJ(S) translocates to the nucleus (in ~45% cells) in response to heat shock (Figure 1B and Table 1). This translocation is clearly evident from the video attached (Supplemental Figure 2). Cells show this translocation as early as 20 min. but a predominant response is seen within 120 minutes.
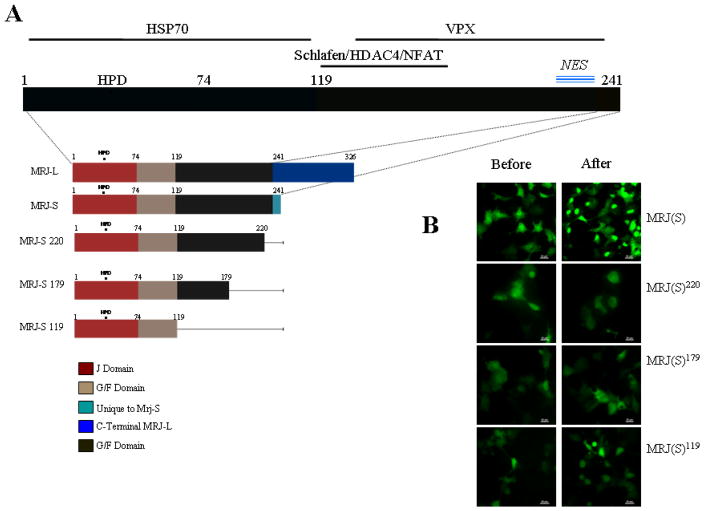
Figure 1. Evaluation of response of MRJ(S) and its deletions to cellular stress.
(A) Diagrammatic representation of domains of MRJ. The regions important for interaction with known interactors (HSP70, VPX, Schlafen, HDAC4 and NFAT) that could possibly carry MRJ to the nucleus are shown with solid line parallel to the domain structure. This figure also shows the comparison of linear structure of all the deletion mutants.
(B) COS7 cells at 80% confluence were transfected with MRJ(S) or deletions of MRJ(S) (MRJ-S 119, 179, 220). Thirty hours after the transfection, cells were subjected to a 42°C heat shock for 120 minutes. Representative photomicrographs recorded before and after the heat shock are presented. (Scale Bar = 20μm)
Table 1.
This table summarizes the quantitation of extent of nuclear localization of MRJ(S) and its deletions in response to cellular stresses. Photomicrographs were recorded before and after the heat shock treatment and percent increase in cells showing nuclear localization of MRJ(S) was calculated by manual counts from 6 random, independent fields. The numerical value is the average number obtained from those counts. (NS= no specific difference)
| Isoform or Deletion | Treatment | % Nuclear Localization |
|---|---|---|
| MRJ-S | Heat Shock | 45 |
| MRJ-S | Hypoxia | 45 |
| MRJ-S | Cytokines | NS |
| MRJ-S | UV | NS |
| MRJ-S 119 | Heat Shock | NS |
| MRJ-S 179 | Heat Shock | NS |
| MRJ-S 220 | Heat Shock | NS |
Heat shock is a form of cellular stress. Cells face a variety of stresses under varying physiologic conditions. We evaluated the cellular localization of MRJ(S) in response to hypoxia, UV irradiation, and treatment with cytokines (IFNα, IFNγ, IL1α and IL2). As seen in Table 1, at resting conditions, MRJ(S) is distributed throughout the cell with predominance in the cytoplasm. We found that MRJ(S) nuclear localization was noticeably increased and was comparable to heat shock stress in response to hypoxia (Supplemental Figure 3). However MRJ(S) failed to show any significant nuclear localization in response to cytokine treatment or UV irradiation.
The C-terminal sequence of MRJ(S) is critical for nuclear translocation
To identify the protein domain(s) of MRJ(S) that play a deciding role in its nuclear transport in response to stress, we created a series of mutations (or truncations) in the EGFP tagged MRJ(S) (Figure 1A). These fusion proteins were assessed for their cellular localization in response to heat shock. As seen in Figure 1B and Table 1, we found that deletion of the last 20 amino acids was capable of abrogating the nuclear translocation ability of MRJ(S) in response to heat shock.
MRJ(S) localizes to the nucleolus after heat shock
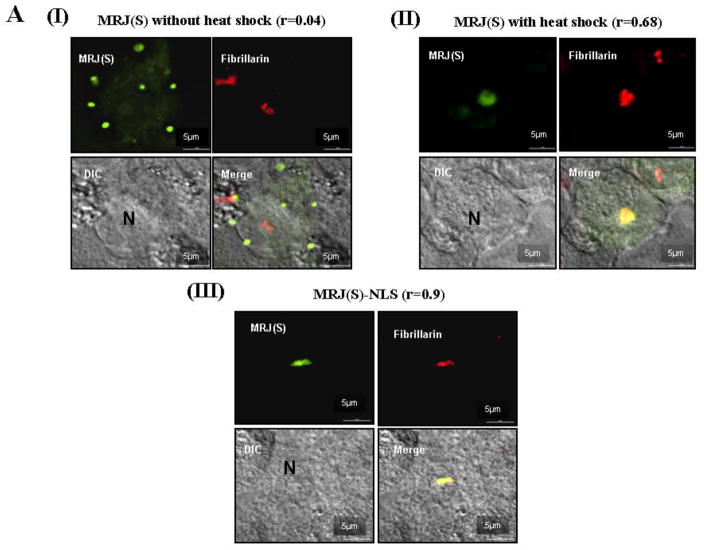
We used high resolution fluorescent imaging to identify if MRJ(S) localized to a specific sub-part of the nucleus upon heat shock. We found that after heat shock, MRJ(S) was localized to the nucleoplasm, but was more specifically focused to the nucleolus. The nucleolar localization is also evident in the time-lapse-video (Supplemental Figure 2). Co-staining with fibrillarin (a nucleolar marker) [19] and overlays of images confirmed this nucleolar localization (Figure 2AI, II). It is interesting to point out that for most of the control (without heat shock) MRJ(S) cells, we observe a punctate localization with a background diffused staining (Figure 2AI). At this point we are unable to comment on the significance of this punctate staining but we speculate these to be multiprotein complex(es).
Figure 2. MRJ(S) localizes to nucleolus upon heat shock.
(A) Sub-nuclear location of MRJ(S) after heat shock was compared to the sub-nuclear location of MRJ(S)-NLS under normal conditions. MDA-MB-435 cells were grown on glass-bottomed dishes, grown to 80% confluency and transfected with appropriate plasmids. 30 hours after transfection, cells were heat shock treated. The treated and control cells were fixed using paraformaldehyde and stained to visualize fibrillarin in red, MRJ(S) in green, and DAPI was used as a nuclear stain, shown in blue. The scale bar represents 5μm length. Values for Pearson's coefficient of correlation (r) given in this figure were calculated using NIS-Elements software following background subtraction to reduce noise in the images.
Nuclear MRJ(S) promotes in vitro attributes of tumor growth and invasiveness
Heat shock or other stresses that stimulate MRJ(S) translocation can activate and mobilize a large number of MRJ independent signaling networks. We wished to isolate the unique effects of nuclear localization of MRJ(S). Hence we added an exogenous, strong nuclear localization signal, derived from the SV40 large T antigen, to constitutively localize MRJ(S) to the nucleus. As was the case with heat shock, addition of the SV40-NLS drove MRJ(S) to the nucleus and predominantly to nucleolus as confirmed by co-localization with fibrillarin co-staining (Figure 2AIII).
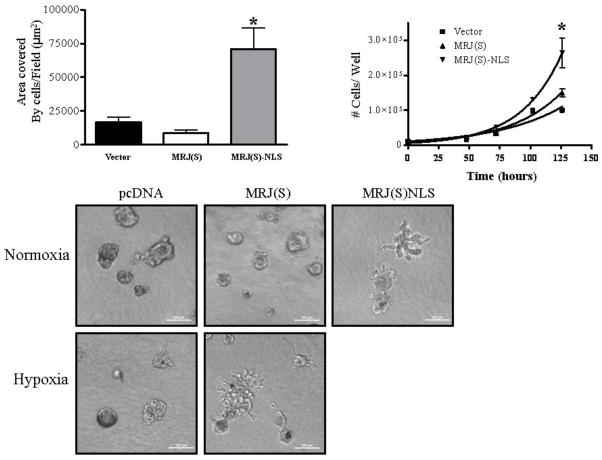
Next, we investigated the functional significance of MRJ(S) localizing to the nucleus by performing in vitro assays to assess some of the attributes of malignancy using MDA-MB-435 cells stably expressing either MRJ(S), MRJ(S)NLS or vector. Cells expressing MRJ(S)NLS showed more than 60% increases in contact-independent growth relative to vector or MRJ(S) cells (Figures 3A). These cells also exhibited significantly accelerated proliferation rate with 60% reduction in the mean doubling time increase (Figure 3B). We tested these cells for capacity to show invasive growth in 3-D matrix. The MRJ(S)NLS cells exhibited a highly branched, invasive morphology relative to vector, whereas MRJ(S) cells had a rounded, less invasive morphology relative to vector cells (Figure 3C). To test whether this effect was unique to MRJ(S)NLS, we also grew MRJ(S) and vector cells in 3-D matrix under hypoxic conditions. As seen in Figure 3C, MRJ(S) cells grown under hypoxia have a morphology strikingly similar to that of MRJ(S)NLS cells grown in normoxia, suggesting that the phenotype is potentially due to nuclear localization of MRJ(S).
Figure 3. Nuclear MRJ(S) promotes in vitro measures of tumor growth and invasiveness.
(A) MDA-MB-435 cells stably expressing MRJ(S), MRJ(S)NLS, or vector were seeded into 10 cm2 tissue culture coated plates at a density of 10,000 cells/plate, and incubated at 37°C, 5% CO2 for seven days. Dishes were then fixed and stained with crystal violet. The area covered by cells in 10 randomly selected fields was measured with a custom macro in NIS-Elements. ( * = p<0.05)
(B) MDA-MB-435 cells stably expressing MRJ(S), MRJ(S)NLS, or vector were seeded into 6-well culture plates at a density of 10×103 cells/well and incubated at 5% CO2, 37°C. Cells were detached using trypsin and counted using a hemocytometer at 24 hour intervals. The mean doubling time(calculated using nonlinear regression) was 38hr for Vector, 30 hr for MRJ(S) and 23 hr for MRJ(S)-NLS. ( * = p<0.05)
(C) MDA-MB-435 cells stably expressing MRJ(S), MRJ(S)NLS, or vector were seeded into a 3-D cell culture matrix in chambered slides at a density of 2,000 cells per well and incubated at 5% CO2, 37°C. To measure the effect of hypoxia, MRJ(S) and vector cells were also incubated at 3% O2, 5% CO2, 92% N2 at 37°C. Images were collected with a Nikon TE-2000E over a time course of eight days. Images shown are from day 8 for normoxia and day 6 for hypoxia. (Scale Bar = 100μm)
Discussion
Our studies show that MRJ(S) is capable of translocating to the nucleus upon exposure to at least two specific cellular stresses, heat shock and hypoxia. These results emphasize the participation of this protein in a physiologically relevant stress-response pathway(s) beyond the namesake heat shock. The name heat shock protein derives from the identification of families of proteins that are up-regulated in response to heat shock in E. coli and yeast, and has been applied to other proteins that share the structural features of these proteins. The HSP40 family of protein chaperones is best known as part of the HSP40-HSP70 chaperone machinery, in which HSP40 presents client proteins to HSP70 and induces the ATPase activity of HSP70, driving cycles of protein denaturation and re-folding [20]. There are over 40 HSP40 family members coded by the human genome [20]. They all exhibit some degree of homology, at least within the conserved J domain [1]. Multiple regulatory mechanisms such as tissue specific expression, stress, and cell physiology specific (temporal) expression controls may regulate their cellular availability. However, not all heat shock proteins change their cellular expression levels in response to heat shock; some show relocation from one cellular compartment to another (e.g heat shock cognate protein, HSC70) [21, 22]. Compartmentalization (spatial distribution) within the cell is another important mechanism that proteins can use to respond to environmental stimuli [23–26]. In fact, nuclear import and stress-regulated nucleo-cytoplasmic shuttling of mammalian heat-shock factor 1 has also been reported [27]. Thus, in response to a specific need of a cell, proteins can be recruited in an organelle to execute a specific task [25, 28, 29].
MRJ(L) has been shown to be responsible for regulation of transcription of a variety of genes [4, 6, 14, 16, 17]. This is apparent from its possible participation in multiple transcription regulatory complexes [4, 17, 30]. However, there are two spliced variants of MRJ and they tend to have distinct locations. One could speculate that MRJ(S) acts as a carrier protein to transport client proteins to cogent HSP70 [4, 15] whereas MRJ(L) would be a part of housekeeping transcription complexes [30]. Most of the downstream signaling regulated by MRJ(L) is critical for the normal functioning of a cell, such as regulated Wnt signaling, maintaining epithelial state or ensuring appropriate function of neurons [4, 16]. However, if the cell encounters a stress situation such as hypoxia or micro-environmental alterations such as the presence of certain cytokines, MRJ may need to ensure (or step-up) expression of certain genes. Since the transcription levels of MRJ do not significantly change in response to heat shock stress (Supplemental Figure 1), we speculates that the cell responds to a crisis situation by recruiting MRJ(S) to the nucleus. Once in the nuclear compartment, MRJ(S) would provide a desired stoichiometric boost to MRJ(L)
Several proteins such as HSP70, Schlafen, HDAC4, NFATC3 and VPX, which have been reported to bind to MRJ, may play a putative role in it’s nuclear translocation [4, 15, 17, 31]. However, our observations show that this activity depends on the C-terminal end region of MRJ(S). Thus far this region has not been shown to be relevant to any protein interactions reported for MRJ. Interestingly, a search for any putative characteristics that are embedded in this stretch of amino acids revealed a nuclear export signal at amino acids 224–230 (NetNES server prediction, Tech. Univ. Denmark). Notably, this amino acid stretch is common to small and large spliced variants of MRJ. However the relevance of this to the nuclear export signal remains to be explored.
We have shown that MRJ(S) also translocates to the sub-nuclear body known as the nucleolus upon heat shock. Various stress stimuli have also been shown to induce changes in the localization of proteins in and out of nucleolar compartment. Tumor suppressor proteins like VHL and p53 are stabilized by sequestration in the nucleolus [32, 33]. Independently, nucleolin and B23 are translocated from the nucleolus into the nucleoplasm, where they play a regulatory role in inducing cell cycle arrest [34, 35]. Cells respond to a variety of extracellular and intracellular forms of stress by down-regulating rRNA synthesis which is believed to be one of the core functions of the nucleolus. Thus the nucleolus takes control of protein trafficking under cellular stress [36]. The nucleolus can be seen as a master stress sensor that orchestrates the chain of events the cell uses to properly respond to stress signals [37, 38]. Perturbation of nucleolar function can initiate neurodegeneration as activation of an endogenous suicide response after perturbation of rRNA synthesis leads to neurodegeneration in mice [39]. These observations are particularly interesting considering the proposed role of MRJ in neurodegenerative diseases, muscular dystrophy, cell cycle and cancer. Trafficking involving nuclear import and export is quite common in cellular functions, however trafficking of one isoform to boost the function of another is not frequently reported, and hence is a novel aspect of MRJ biology.
Given the nearly identical sequences of MRJ(S) and MRJ(L), it may be expected that the functional effects of driving MRJ(S) to the nucleus, where MRJ(L) is constitutively located, would be similar to the anti-tumor effects of overexpressing MRJ(L). Unexpectedly, constitutive nuclear localization of MRJ(S) actually increased in vitro measures of tumor progression. How do we then account for the pro-malignant function of nuclear MRJ(S)?
Signaling events are compartmentalized in time as well as space; after the removal of an inducing stress, clearance of MRJ(S) from the nucleus is normally rapid, occurring within four hours (unpublished data). The normal function of MRJ(S) may be to give a short-term boost to the housekeeping functions of MRJ(L), however, chronic localization of MRJ(S) to the nucleus may result in downstream effects distinct from those of MRJ(L). This suggestion becomes even more intriguing in the context of hypoxia, which is known to promote tumor growth, chemoresistance, invasiveness, and metastasis [40–43]. We have shown that hypoxia drives MRJ(S) to the nucleus. With tumor growth, the intra-tumoral microenvironment of a solid tumor becomes hypoxic and tumor cells show more aggressive properties [44]. We therefore suggest that the following mechanism: as the tumor grows, more and more cells become exposed to chronic levels of hypoxia, which drive MRJ(S) to the nucleus and maintain it there, promoting proliferation, invasiveness and the further growth of the tumor. Thus our observations have revealed a yin-yang type of functional relationship when MRJ(L) and nuclear MRJ(S) functions are compared. Such an antagonistic relationship of spliced variants of DNAJ proteins has been previously shown eg. spliced variants eg. DNAJ family member TID1 [26].
Supplementary Material
MCF10A cells (immortalized human breast epithelial line) were subjected to heat shock treatment for 30 min. and 120 min. Total RNA was isolated and levels of MRJ transcript were measured using quantitative RT-PCR.
COS7 cells were seeded in 6 well culture plates and grown to 80% confluency in 5% CO2 at 37°C. Cells were then transfected with eGFP-MRJ(S) and after twenty-four hours incubated for an additional 24 hours in normoxia or hypoxia (3%O2, 5% CO2, 92%N2) at 37°C. Normoxic cells were then heat shocked at 42°C for 120 minutes, and images were captured. Heat shock and hypoxia resulted in ~50% increase in cells showing nuclear localization of MRJ(S). Asterisk (*) indicates a significant difference, p<0.05.
COS7 cells were seeded in 6 well culture plates and grown to 80% confluency in 5% CO2 at 37°C. Cells were then transfected with eGFP-MRJ(S) and after twenty-four hours incubated for an additional 24 hours in normoxia or hypoxia (3%O2, 5% CO2, 92%N2) at 37°C. As a positive control for nuclear and nucleolar localization, cells were heat shocked at 42°C for 120 minutes. Under normal conditions (A), MRJ(S) shows a mostly cytoplasmic distribution. Heat shock (B) induced nuclear and nucleolar localization, whereas hypoxia (C) resulted in nuclear, but not nucleolar localization. White arrows indicate location of nucleoli in each panel based on morphology visible in phase contrast.
Time-lapse video was captured using a Nikon TE2000-E inverted fluorescent microscope equipped with a 40x objective, Roper Coolsnap HQ2 camera, automated stage, Perfect Focus system and a Live Cell incubation chamber (Pathology Devices, Westminster, MD). MDA-MB-435 cells were grown on glass-bottomed dishes (World Precision Instruments, Sarasota FL) and transfected with MRJ(S)GFP. Images were captured twenty-four hours after transfection at intervals of two minutes for duration of two hours following heat shock. Heat shock was induced by addition of growth medium pre-warmed to 42°C, and the incubation chamber was maintained at 42°C for the duration of the video. Image processing was performed using Nikon Elements 3.2 software.
Acknowledgments
Grant support: USPHS grants CA140472 (R. S. Samant) and CA138850 (L. A. Shevde).
L. J. Sykora received support from UCUR Summer Research Program and T. Barik received support from National Science Foundation Research Experiences for Undergraduates (NSF-REU).
R. S. Samant is the recipient of the Mayer Mitchell Award for Excellence in Cancer Research and acknowledges the support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitra A, Shevde LA, Samant RS. Multi-faceted role of HSP40 in cancer. Clin Exp Metastasis. 2009;26:559–567. doi: 10.1007/s10585-009-9255-x. [DOI] [PubMed] [Google Scholar]
- 2.Durrenberger PF, Filiou MD, Moran LB, Michael GJ, Novoselov S, Cheetham ME, Clark P, Pearce RK, Graeber MB. DnaJB6 is present in the core of Lewy bodies and is highly up-regulated in parkinsonian astrocytes. J Neurosci Res. 2009;87:238–245. doi: 10.1002/jnr.21819. [DOI] [PubMed] [Google Scholar]
- 3.Rose JM, Novoselov SS, Robinson PA, Cheetham ME. Molecular chaperone-mediated rescue of mitophagy by a Parkin RING1 domain mutant. Hum Mol Genet. doi: 10.1093/hmg/ddq428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang JZ, Zhou H, Zhu M, Li SH, Li XJ, Sung CH. Characterization of a brain-enriched chaperone, MRJ, that inhibits Huntingtin aggregation and toxicity independently. J Biol Chem. 2002;277:19831–19838. doi: 10.1074/jbc.M109613200. [DOI] [PubMed] [Google Scholar]
- 5.Li ZF, Wu X, Jiang Y, Liu J, Wu C, Inagaki M, Izawa I, Mizisin AP, Engvall E, Shelton GD. Non-pathogenic protein aggregates in skeletal muscle in MLF1 transgenic mice. J Neurol Sci. 2008;264:77–86. doi: 10.1016/j.jns.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Mitra A, Fillmore RA, Metge BJ, Rajesh M, Xi Y, King J, Ju J, Pannell L, Shevde LA, Samant RS. Large isoform of MRJ (DNAJB6) reduces malignant activity of breast cancer. Breast Cancer Res. 2008;10:R22. doi: 10.1186/bcr1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson ED, Mattar P, Schuurmans C, Cross JC. Neural stem cell self-renewal requires the Mrj co-chaperone. Dev Dyn. 2009;238:2564–2574. doi: 10.1002/dvdy.22088. [DOI] [PubMed] [Google Scholar]
- 8.Dey S, Banerjee P, Saha P. Cell cycle specific expression and nucleolar localization of human J-domain containing co-chaperone Mrj. Mol Cell Biochem. 2009;322:137–142. doi: 10.1007/s11010-008-9950-y. [DOI] [PubMed] [Google Scholar]
- 9.Hunter PJ, Swanson BJ, Haendel MA, Lyons GE, Cross JC. Mrj encodes a DnaJ-related co-chaperone that is essential for murine placental development. Development. 1999;126:1247–1258. doi: 10.1242/dev.126.6.1247. [DOI] [PubMed] [Google Scholar]
- 10.Tateossian H, Powles N, Dickinson R, Ficker M, Maconochie M. Determination of downstream targets of FGF signalling using gene trap and cDNA subtractive approaches. Exp Cell Res. 2004;292:101–114. doi: 10.1016/j.yexcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Watson ED, Geary-Joo C, Hughes M, Cross JC. The Mrj co-chaperone mediates keratin turnover and prevents the formation of toxic inclusion bodies in trophoblast cells of the placenta. Development. 2007;134:1809–1817. doi: 10.1242/dev.02843. [DOI] [PubMed] [Google Scholar]
- 12.Sarparanta J, Jonson PH, Golzio C, Sandell S, Luque H, Screen M, McDonald K, Stajich JM, Mahjneh I, Vihola A, Raheem O, Penttila S, Lehtinen S, Huovinen S, Palmio J, Tasca G, Ricci E, Hackman P, Hauser M, Katsanis N, Udd B. Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat Genet. doi: 10.1038/ng.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms MB, Sommerville RB, Allred P, Bell S, Ma D, Cooper P, Lopate G, Pestronk A, Weihl CC, Baloh RH. Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann Neurol. doi: 10.1002/ana.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra A, Menezes ME, Pannell LK, Mulekar MS, Honkanen RE, Shevde LA, Samant RS. DNAJB6 chaperones PP2A mediated dephosphorylation of GSK3beta to downregulate beta-catenin transcription target, osteopontin. Oncogene. doi: 10.1038/onc.2011.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X, Belshan M, Ratner L. Hsp40 facilitates nuclear import of the human immunodeficiency virus type 2 Vpx-mediated preintegration complex. J Virol. 2008;82:1229–1237. doi: 10.1128/JVI.00540-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra A, Menezes ME, Shevde LA, Samant RS. DNAJB6 induces degradation of beta-catenin and causes partial reversal of mesenchymal phenotype. J Biol Chem. 285:24686–24694. doi: 10.1074/jbc.M109.094847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai YS, Xu J, Molkentin JD. The DnaJ-related factor Mrj interacts with nuclear factor of activated T cells c3 and mediates transcriptional repression through class II histone deacetylase recruitment. Mol Cell Biol. 2005;25:9936–9948. doi: 10.1128/MCB.25.22.9936-9948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Z, Sikandar S, Witherspoon M, Dizon D, Nguyen T, Benirschke K, Wiley C, Vrana P, Lipkin SM. Impaired placental trophoblast lineage differentiation in Alkbh1(−/−) mice. Dev Dyn. 2008;237:316–327. doi: 10.1002/dvdy.21418. [DOI] [PubMed] [Google Scholar]
- 19.Endo A, Matsumoto M, Inada T, Yamamoto A, Nakayama KI, Kitamura N, Komada M. Nucleolar structure and function are regulated by the deubiquitylating enzyme USP36. J Cell Sci. 2009;122:678–686. doi: 10.1242/jcs.044461. [DOI] [PubMed] [Google Scholar]
- 20.Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 22.Shim JK, Jung DO, Park JW, Kim DW, Ha DM, Lee KY. Molecular cloning of the heat-shock cognate 70 (Hsc70) gene from the two-spotted spider mite, Tetranychus urticae, and its expression in response to heat shock and starvation. Comp Biochem Physiol B Biochem Mol Biol. 2006;145:288–295. doi: 10.1016/j.cbpb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Tkach JM, Glover JR. Nucleocytoplasmic trafficking of the molecular chaperone Hsp104 in unstressed and heat-shocked cells. Traffic. 2008;9:39–56. doi: 10.1111/j.1600-0854.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- 24.Peng ZG, Zhou MY, Huang Y, Qiu JH, Wang LS, Liao SH, Dong S, Chen GQ. Physical and functional interaction of Runt-related protein 1 with hypoxia-inducible factor-1alpha. Oncogene. 2008;27:839–847. doi: 10.1038/sj.onc.1210676. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi M, Imanaka-Yoshida K, Yoshida T, Wood M, Fearns C, Tatake RJ, Lee JD. A crucial role of mitochondrial Hsp40 in preventing dilated cardiomyopathy. Nat Med. 2006;12:128–132. doi: 10.1038/nm1327. [DOI] [PubMed] [Google Scholar]
- 26.Syken J, De-Medina T, Munger K. TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions. Proc Natl Acad Sci U S A. 1999;96:8499–8504. doi: 10.1073/pnas.96.15.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vujanac M, Fenaroli A, Zimarino V. Constitutive nuclear import and stress-regulated nucleocytoplasmic shuttling of mammalian heat-shock factor 1. Traffic. 2005;6:214–229. doi: 10.1111/j.1600-0854.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 28.Dastoor Z, Dreyer J. Nuclear translocation and aggregate formation of heat shock cognate protein 70 (Hsc70) in oxidative stress and apoptosis. J Cell Sci. 2000;113 (Pt 16):2845–2854. doi: 10.1242/jcs.113.16.2845. [DOI] [PubMed] [Google Scholar]
- 29.Ducret C, Maira SM, Dierich A, Wasylyk B. The net repressor is regulated by nuclear export in response to anisomycin, UV, and heat shock. Mol Cell Biol. 1999;19:7076–7087. doi: 10.1128/mcb.19.10.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst DR, Mehta A, Moore BP, Phadke PA, Meehan WJ, Accavitti MA, Shevde LA, Hopper JE, Xie Y, Welch DR, Samant RS. Breast cancer metastasis suppressor 1 (BRMS1) is stabilized by the Hsp90 chaperone. Biochem Biophys Res Commun. 2006;348:1429–1435. doi: 10.1016/j.bbrc.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Yang Z, Cao Y, Zhang S, Li H, Huang Y, Ding YQ, Liu X. The Hsp40 family chaperone protein DnaJB6 enhances Schlafen1 nuclear localization which is critical for promotion of cell-cycle arrest in T-cells. Biochem J. 2008;413:239–250. doi: 10.1042/BJ20071510. [DOI] [PubMed] [Google Scholar]
- 32.Mekhail K, Gunaratnam L, Bonicalzi ME, Lee S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nat Cell Biol. 2004;6:642–647. doi: 10.1038/ncb1144. [DOI] [PubMed] [Google Scholar]
- 33.Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurki S, Peltonen K, Latonen L, Kiviharju TM, Ojala PM, Meek D, Laiho M. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell. 2004;5:465–475. doi: 10.1016/s1535-6108(04)00110-2. [DOI] [PubMed] [Google Scholar]
- 35.Daniely Y, Dimitrova DD, Borowiec JA. Stress-dependent nucleolin mobilization mediated by p53-nucleolin complex formation. Mol Cell Biol. 2002;22:6014–6022. doi: 10.1128/MCB.22.16.6014-6022.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nalabothula N, Indig FE, Carrier F. The Nucleolus Takes Control of Protein Trafficking Under Cellular Stress. Mol Cell Pharmacol. 2:203–212. [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer C, Bierhoff H, Grummt I. The nucleolus as a stress sensor: JNK2 inactivates the transcription factor TIF-IA and down-regulates rRNA synthesis. Genes Dev. 2005;19:933–941. doi: 10.1101/gad.333205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer C, Grummt I. Cellular stress and nucleolar function. Cell Cycle. 2005;4:1036–1038. doi: 10.4161/cc.4.8.1925. [DOI] [PubMed] [Google Scholar]
- 39.Parlato R, Kreiner G, Erdmann G, Rieker C, Stotz S, Savenkova E, Berger S, Grummt I, Schutz G. Activation of an endogenous suicide response after perturbation of rRNA synthesis leads to neurodegeneration in mice. J Neurosci. 2008;28:12759–12764. doi: 10.1523/JNEUROSCI.2439-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 41.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 42.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 43.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 44.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MCF10A cells (immortalized human breast epithelial line) were subjected to heat shock treatment for 30 min. and 120 min. Total RNA was isolated and levels of MRJ transcript were measured using quantitative RT-PCR.
COS7 cells were seeded in 6 well culture plates and grown to 80% confluency in 5% CO2 at 37°C. Cells were then transfected with eGFP-MRJ(S) and after twenty-four hours incubated for an additional 24 hours in normoxia or hypoxia (3%O2, 5% CO2, 92%N2) at 37°C. Normoxic cells were then heat shocked at 42°C for 120 minutes, and images were captured. Heat shock and hypoxia resulted in ~50% increase in cells showing nuclear localization of MRJ(S). Asterisk (*) indicates a significant difference, p<0.05.
COS7 cells were seeded in 6 well culture plates and grown to 80% confluency in 5% CO2 at 37°C. Cells were then transfected with eGFP-MRJ(S) and after twenty-four hours incubated for an additional 24 hours in normoxia or hypoxia (3%O2, 5% CO2, 92%N2) at 37°C. As a positive control for nuclear and nucleolar localization, cells were heat shocked at 42°C for 120 minutes. Under normal conditions (A), MRJ(S) shows a mostly cytoplasmic distribution. Heat shock (B) induced nuclear and nucleolar localization, whereas hypoxia (C) resulted in nuclear, but not nucleolar localization. White arrows indicate location of nucleoli in each panel based on morphology visible in phase contrast.
Time-lapse video was captured using a Nikon TE2000-E inverted fluorescent microscope equipped with a 40x objective, Roper Coolsnap HQ2 camera, automated stage, Perfect Focus system and a Live Cell incubation chamber (Pathology Devices, Westminster, MD). MDA-MB-435 cells were grown on glass-bottomed dishes (World Precision Instruments, Sarasota FL) and transfected with MRJ(S)GFP. Images were captured twenty-four hours after transfection at intervals of two minutes for duration of two hours following heat shock. Heat shock was induced by addition of growth medium pre-warmed to 42°C, and the incubation chamber was maintained at 42°C for the duration of the video. Image processing was performed using Nikon Elements 3.2 software.