Abstract
The objective of this study was to determine the association of seizures and cognitive decline in adults with Down syndrome (DS) and Alzheimer's-type dementia. A retrospective data analysis was carried out following a controlled study of antioxidant supplementation for dementia in DS. Observations were made at baseline and every 6 months for 2 years. Seizure history was obtained from study records. The primary outcome measures comprised the performance-based Severe Impairment Battery (SIB) and Brief Praxis Test (BPT). Secondary outcome measures comprised the informant-based Dementia Questionnaire for Mentally Retarded Persons and Vineland Adaptive Behavior Scales. Because a large proportion of patients with seizures had such severe cognitive decline as to become untestable on the performance measures, time to “first inability to test” was measured. Adjustments were made for the potentially confounding co-variates of age, gender, APOE44 status, baseline cognitive impairment, years since dementia onset at baseline, and treatment assignment. The estimated odds ratio for the time to “first inability to test” on the SIB comparing those with seizures to those without is 11.02 (95% CI: 1.59, 76.27), a ratio that is significantly different from 1 (p = 0.015). Similarly, we estimated an odds ratio of 9.02 (95% CI: 1.90, 42.85) on the BPT, a ratio also significantly different than 1 (p = 0.006). Results from a secondary analysis of the informant measures showed significant decline related to seizures. We conclude that there is a strong association of seizures with cognitive decline in demented individuals with DS. Prospective studies exploring this relationship in DS are indicated.
Keywords: Alzheimer's disease, dementia, Down syndrome, seizures
INTRODUCTION
There is a common platform of seizure-related cognitive decline in both Alzheimer's disease (AD) in the general population as well as in Down syndrome (DS) but the association has been studied more in AD. Cognitive impairment appears to be greater in AD patients in the general population with seizures compared to those without seizures [1]. This seizure-related decline in cognitive performance primarily affects language with a subsequent reduction in autonomy, a greater risk of injury, and a higher mortality rate [2]. In particular, partial complex seizures have been associated with cognitive decline and disruptions in the performance of activities of daily living [3]. In patients with AD and seizures together, with homeostatic responses to this epileptiform activity is considered contributory to the dysfunction of the circuitry that underlies memory formation [4, 5].
Seizures in DS have a bimodal distribution with the first peak occurring in the first two decades of life and a late-onset peak occurring in the 5th to 6th decades. Individuals with DS over 45 years with new-onset seizures are more likely to develop AD and their seizures may herald the onset of dementia [6, 7]. The electro-clinical findings of seizures in adults with DS include myoclonic jerks on awakening and generalized spike and wave discharges on EEG [8]. The incidence of myoclonic epilepsy in adults with DS appears to be under appreciated and may lead to falls associated with dementia [9]. The association of seizures and cognitive decline in demented individuals is not well understood.
Mouse models of both AD and DS exhibit seizures [4, 10]. Seizures in AD and DS may share a common pathogenetic basis in which the toxic accumulation of amyloid-β (Aβ) peptides triggers synaptic degeneration, circuit remodeling, and abnormal synchronization of neuronal networks [11]. Increased metabolite levels of amyloid-β protein precursor (AβPP) occur in the brains of Ts65Dn mice, considered to have homologous chromosome abnormalities to those seen in human trisomy 21 [12]. Experimental data suggest that abnormalities in the cognitive phenotype in DS are reflected in these mice and causally associated with dosage increases of specific chromosome gene orthologs such as App [13]. While the Ts65Dn mouse has limited behavioral deficits at 4 months of age, these deficits increase in severity with aging, a phenotypic worsening that may drive or be driven by the apparent age-dependent dysregulation of various triplicated gene transcripts, including App [14].
Seizures have been linked to both sporadic and early-onset Alzheimer's disease (EOAD). Between 10–22% of patients with sporadic AD in the general population have had at least one unprovoked seizure [15]. There are some parallels between seizures and dementia in EOAD versus DS. In autosomal dominant EOAD, seizures are associated with mutations in AβPP, presenilin-1, and presenilin-2 and by duplications of wild-type AβPP [16–18]. Common to these genetic alterations is an increase in production of Aβ42 or the relative ratio of Aβ42 to Aβ40 peptides [19]. Seizures may develop in 84% of individuals with DS and AD [20]. The increased expression of AβPP in DS has been linked to increased Aβ deposition in brain similar to the patterns seen in EOAD [21]. Autosomal dominant EOAD is associated with an earlier age of onset, generally in the 5th and 6th decades of life. Individuals with DS often have an onset of dementia during these same time epochs [22, 23]. DS shares in common with both sporadic AD and EOAD neuropathological features including neuritic plaques, neurofibrillary tangles, tissue atrophy, and neuronal loss [24, 25].
In this report, we explore the association of seizures and cognitive decline in individuals with DS and AD-type dementia.
MATERIALS AND METHODS
Study population
The data in this report were derived retrospectively from a randomized, double-blind, placebo-controlled study of antioxidant supplementation in DS adults with AD type dementia [26]. No treatment effect was noted over two years of observation. At the completion of the study, ad hoc analyses of the study sample revealed an association between the presence of seizures and severe cognitive decline to the point of subjects becoming “untestable” on the performance-based outcome measures. This report explores the association of seizures and cognitive decline. The study was approved by the UC Irvine Institutional Review Board. Written informed consent was obtained from the participant's legally-authorized representative. Written or verbal assent was obtained from all participants as appropriate.
Sample groups
For the current analysis and irrespective of treatment group, the records of each subject were reviewed for the presence or absence of a seizure disorder. The criteria for inclusion in the seizure group comprised a diagnosis of seizure disorder made by a neurologist before study entry and/or during the study period along with a prescription of antiepileptic medication. Subjects with remote seizures unassociated with dementia were not included. Data on seizure history occurrence was obtained at baseline and at each study visit through caretaker history and a review of the medical record available.
Assessments
The diagnosis of dementia was established according to DSM-IV criteria [27]. A battery of standardized cognitive and neuropsychological tests was administered at baseline and at 6 monthly intervals for the 2-year period of the trial. The battery consisted of performance-based measures that were administered directly to the participant and informant-based interviews with the principle caregiver.
The performance-based measures comprised the Severe Impairment Battery (SIB) [28], and the Brief Praxis Test (BPT) [29]. The SIB assesses domains of attention, language, orientation, memory, praxis, visuospatial ability, construction, social interaction, and orientating to name and has been validated as a protocol specific outcome measure in studies of dementia in DS [30]. Lower scores on the SIB indicate greater cognitive impairment. The BPT is a measure of dyspraxia in DS as expressed by performance of simple, short sequences of voluntary movement. The BPT test consists of 20 items derived from a 62-item cognitive evaluation of praxis. (Dyspraxia Scale for Adults with Down Syndrome).
The informant-based measures were the Dementia Questionnaire for Mentally Retarded Persons (DMR) [31, 32] and the Vineland Adaptive Behavior Scales (VABS) [33]. The DMR was administered as a questionnaire by means of a structured interview. Simple “yes”, “no” or “sometimes” answers are given to 50 items. The test results are stratified into Sum of Cognitive (SC) and Sum of Social (SS) Scores. Since the primary interest of the trial was cognition, only the DMR SC was used in the study. The DMR has been demonstrated to have good reliability and validity over time to measure dementia in DS [34]. The VABS is an informant-based scale covering domains of communication, daily living skills, socialization, motor skills, and maladaptive behavior. It has been widely used and validated in the DS population. Although it is not a primary measure of dementia, the VABS have been useful in defining behavioral phenotypes of many genetic syndromes associated with intellectual disability, including DS [35].
Definition of “untestable”
A subject was deemed untestable if they were unable to score a single point on the first 10 items of the SIB or the BPT. In this circumstance, the individual test was discontinued to refrain from upsetting the subject by multiple failures. In coming to this determination, the items were presented sequentially with verbal instruction followed by repeated verbal prompting with cues and in the case of the BPT followed by item-specific imitation then physical prompting with hand over hand intervention. Only when a subject failed all of these approaches was the term untestable applied for the specific test. For all participants, the informant-based measures were administered and completed throughout the study.
Statistical analysis
Because a large proportion of study subjects were untestable, the scores on the SIB and BPT were unavailable. Therefore, we used the time to first inability to test with adjustment for baseline scores as a surrogate for cognitive dysfunction. Intellectual disability at baseline was reflected in the DMR SC. Kaplan-Meier estimates were plotted to display the probability of becoming untestable using the performance-based outcome measures of SIB and BPT for each group.
Because the observable times were discrete in nature (6 month intervals), the continuation-ratio model was used to analyze the data [36]. This method models the odds ratio of the probability that a subject is untestable at a particular time given that the subject was testable before and had not dropped out of the study for other reasons. Seizure status is treated as a time-varying covariate, reflecting seizure occurrence in the period prior to the current visit. The following factors were a priori identified as potentially confounding factors and adjusted for in the model: age at baseline, gender, presence of the e4 allele of the APOE gene, pre-morbid level of cognitive impairment, antioxidant treatment versus placebo group, years since dementia onset at baseline, and baseline DMR SC. All inference is reported using robust standard errors to account for potential model mis-specification [37]. To investigate the potential for effect modification by treatment assignment in the original trial, a multiplicative interaction between seizure status and treatment arm assignment was considered in post-hoc analysis.
In a secondary analysis, linear mixed-effects models incorporating random intercepts and slopes were used to analyze the informant-based DMR SC, VABS Communication, VABS Daily Living, VABS Socialization, and VABS Motor Skills domains scores longitudinally since the data were complete throughout the study [38]. We consider the retrospectively-defined seizure group indicator instead of the time-varying seizure status to simplify interpretations. Specifically, we hypothesized that the average scores would differ by seizure group. The decision not to contrast the slope of each score by seizure status was determined prior to model fitting because we were aware that the ceiling/floor effects of the scores would force the slopes to be biased toward 0. In other words, the scores were so high (or low) that they could not significantly change over time. Hence, we estimated and tested the average adjusted difference in scores between the seizure group and non-seizure group. For each model, we adjusted for age at baseline, gender, presence of the e4 allele of the APOE gene, pre-morbid level of cognitive impairment, antioxidant treatment versus placebo group, years since dementia onset at baseline and baseline DMR SC as potential confounding factors.
RESULTS
Demographics and seizure characteristics
A total of 53 adults with DS with AD-type dementia were included in this report. The groups had similar demographic, pre-morbid level of cognitive impairment, and APOE genotype characteristics at baseline (Table 1). The seizure group comprised 24 participants (45%). Sixteen of the group of 24 had seizure onset prior to baseline and 8 had seizure onset while on study. Both the seizure and non-seizure group were evenly distributed into the treatment/placebo arms of the parent study. Of the 24 participants in the seizure group, 18 had tonic clonic seizures, five had complex partial seizures, and one had no description available. Co-occurring myoclonic jerks were reported in 13 of the 24 cases. Good seizure control (<1 seizure per year) was noted in 52% of subjects with the remaining having more than this amount. Seizure length was less than one minute in 60% with 20% lasting 1–5 minutes and 20% lasting 5 minutes or longer. The average time period that elapsed between the date of dementia onset and seizure onset was 2.55 years (SD:1.35, range 0.0 to 5.67).
Table 1.
Demographic characteristics of groups at baseline
| Variable | Non-seizure (n = 29) | Seizure (n = 24) |
|---|---|---|
| Baseline age (years) | 50.86±4.68 | 50.38±4.70 |
| Gender | ||
| Female | 17 (59%) | 11 (46%) |
| Male | 12 (41%) | 13 (54%) |
| Race/ethnicity | ||
| White or Caucasian | 25 (86%) | 21 (88%) |
| Hispanic or Latino | 2 (7%) | 3 (12%) |
| American Indian or Alaska Native | 1 (3%) | 0 (0%) |
| Asian | 1 (3%) | 0 (0%) |
| Pre-morbid level of cognitive impairment | ||
| Mild | 9 (31%) | 5 (21%) |
| Moderate | 11 (38%) | 12 (50%) |
| Severe | 7 (24%) | 6 (25%) |
| Profound | 1 (3%) | 1 (4%) |
| Unknown | 1 (3%) | 0 (0%) |
| APOE | ||
| 2/2 | 0 (0%) | 1 (4%) |
| 2/3 | 5 (17%) | 2 (8%) |
| 2/4 | 2 (7%) | 0 (0%) |
| 3/3 | 12 (41%) | 15 (62%) |
| 3/4 | 9 (31%) | 5 (21%) |
| 4/4 | 1 (3%) | 1 (4%) |
| Treatment group | ||
| Placebo | 15 (52%) | 11 (46%) |
| Antioxidant | 14 (48%) | 13 (54%) |
| Years since dementia onset at baseline | 2.10±1.26 | 3.07±1.50 |
Continuous variables are reported as mean±standard deviation, and categorical variables are reported as counts and proportions. Whether a subject is in the seizure group is identified retrospectively based on the diagnosis of seizure disorder and prescription of antiepileptic medication.
The average time from the approximate date of dementia onset to baseline in the seizure group was 3.07 years (SD:1.50, range 0.31 to 6.65) and 2.10 years (SD:1.26, range 0.41 to 5.04) in the non-seizure group. This difference of 0.97 years is significant at the 0.05 level (95% CI: −20.9, −2.32; p = 0.015) based on a two-sample t-test with unequal variance.
EEGs were available in the medical records in 50% of subjects and showed slowing in 75% with paroxysmal spike/wave activity noted in 25%. There was no consistent pattern of antiepileptic drug use. The following medications were noted (# of participants): phenobarbital (2), levetiracetam (8), topiramate (3), lamotrigine (4), phenytoin (12), carbamazepine (6), and valproic acid (9).
Seizures and cognitive test data
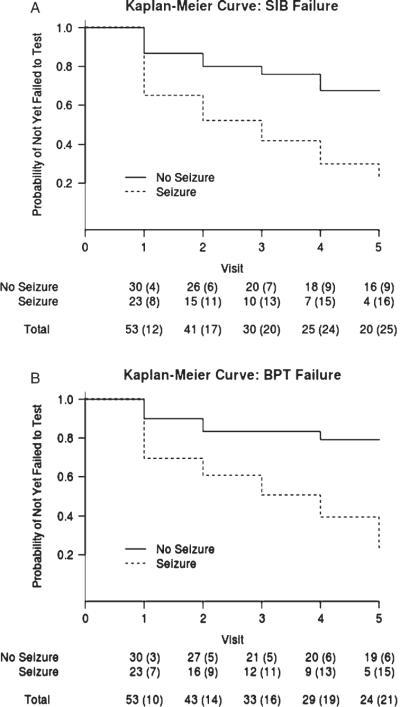
Unadjusted Kaplan-Meier curves in the seizure and non-seizure groups for the SIB and the BPT are presented in Fig. 1A and B. The estimated probability of becoming untestable is greater in the seizure group across all of the time epochs of the study. The differences between seizure and non-seizure groups are summarized more formally in our statistical analyses below.
Fig. 1.
Survival curves showing the probability of being untestable on SIB (A) and BPT (B) using the Kaplan-Meier estimator. The seizure group has higher probability to become untestable in both performance-based measures across all of the time epochs. The corresponding table shows the number of subjects that came in for testing at each visit, with cumulative number of events (failure to test) in parentheses.
Estimated odds ratios along with corresponding 95% confidence intervals, and p-values for the time to first inability to test on the SIB and BPT analysis are reported in Table 2. After adjustment for age at baseline, gender, presence of the e4 allele of the APOE gene, pre-morbid level of cognitive impairment, treatment assignment, years since dementia onset at baseline, and baseline DMR SC, the estimated odds ratio for the time to first inability to test on the SIB was 11.02 (95% CI: 1.59, 76.27) in the seizure group compared to those without seizures, a ratio that is significantly different from 1 (p = 0.015). Similarly, the estimated odds ratio for inability to test on the BPT was 9.02 (95% CI: 1.90, 42.85); it is also significantly different than 1 (p = 0.006). Tests of the multiplicative interaction between seizure status and treatment assignment provided no evidence that the effect of seizures differed by treatment assignment (p = 0.379 for the model of SIB; p = 0.152 for the model of BPT). The main effect of pre-morbid CI is not reported in the table for SIB (p = 0.853) and BPT (p = 0.863) since they cannot be interpreted in a meaningful fashion. Point estimates, 95% confidence intervals, and p-values for all other adjustment covariates are also reported in Table 2 for completeness.
Table 2.
Continuation ratio model for the time to first inability to test fitted to the SIB and BPT scores
| SIB |
BPT |
|||
|---|---|---|---|---|
| Variable | Est. odds ratio (95% CI) | p-value | Est. odds ratio (95% CI) | p-value |
| Baseline age (years) | 1.12 (0.98, 1.29) | 0.104 | 1.10 (0.96, 1.26) | 0.154 |
| Female | 0.52 (0.14, 1.89) | 0.321 | 0.52 (0.15, 1.79) | 0.297 |
| APOE4 | 1.50 (0.36, 6.30) | 0.582 | 0.50 (0.11, 2.29) | 0.373 |
| Pre-morbid cognitive impairment: Severe or profound | 0.56 (0.00, 502.66) | 0.869 | 0.62 (0.00, 373.09) | 0.882 |
| Treatment group (antioxidant vs. placebo) | 4.61 (1.22, 17.35) | 0.024 | 2.70 (0.68, 10.75) | 0.160 |
| Baseline DMR SC score (pre-morbid CI: not severe or profound) | 1.10 (1.00, 1.21) | 0.041 | 1.04 (0.95, 1.14) | 0.358 |
| Baseline DMR SC score (pre-morbid CI: severe or profound) | 1.08 (0.88, 1.32) | 0.477 | 1.05 (0.87, 1.27) | 0.621 |
| Years since dementia onset at baseline | 1.42 (0.94, 2.15) | 0.099 | 1.36 (0.91, 2.03) | 0.138 |
| Seizure status (yes vs. no) | 11.02 (1.59, 76.27) | 0.015 | 9.02 (1.90, 42.85) | 0.006 |
Point estimates are reported on the odds ratio scale of the conditional probability of inability to test at a particular time given the subject is at risk at that particular time. Regression estimates are adjusted for all variable listed in the table. CI-Cognitive impairment.
Point estimates and 95% confidence intervals from the longitudinal analysis of indirect cognitive scores are reported in Table 3. After adjustment for age at baseline, gender, presence of the e4 allele of the APOE gene, pre-morbid level of cognitive impairment, treatment assignment, years since dementia onset at baseline and baseline DMR SC, we estimate that subjects with a history of seizures experienced a mean DMR SC 9.32 points higher (worsening scores) over time when compared to similar subjects without a history of seizures; this difference is statistically significant using a level 0.05 test (95% confidence interval does not contain 0). In addition, we estimate that after adjustment for potential confounding factors subjects with a history of seizures experienced a mean VABS Communication, VABS Daily Living, VABS Socialization, and VABS Motor Skills scores 12.7, 26.7, 17.4, and 12.0 points lower (worsening scores), respectively, when compared to similar subjects without a history of seizures. Each of the above reported associations were statistically significant (p < 0.05). These estimates support our hypothesis, that the seizure group is associated with worse scores (lower cognitive function).
Table 3.
Linear mixed effects models for the indirect measures of cognitive ability as a function of time
| Variable | DMR SC | VABS Communication | VABS Daily Living | VABS Socialization | VABS Motor Skills |
|---|---|---|---|---|---|
| Baseline age | 0.98 (0.51, 1.44) | −2.26 (−3.43, −1.09) | −3.43 (−5.14, −1.72) | −1.78 (−3.01, −0.54) | −1.44 (−2.20, −0.68) |
| Female | −2.07 (−6.52, 2.39) | 7.51 (−3.69, 18.7) | 2.78 (−13.6, 19.1) | 5.06 (−6.73, 16.9) | −1.09 (−8.41, 6.23) |
| APOE4 | 0.70 (−3.97, 5.36) | 4.30 (−7.42, 16.0) | 4.12 (−13.0, 21.2) | 4.80 (−7.51, 17.1) | 4.74 (−2.90, 12.39) |
| Pre-morbid CI level: severe/profound | 6.43 (1.56, 11.3) | −16.1 (−28.3, −3.92) | −20.7 (−38.6, −2.87) | −15.5 (−28.4, −2.58) | −12.1 (−20.0, −4.09) |
| Treatment | 1.81 (−2.51, 6.13) | −0.79 (−11.6, 10.1) | −5.32 (−21.2, 10.5) | −0.65 (−12.1, 10.8) | −3.80 (−10.90, 3.29) |
| Years since dementia onset at baseline | 1.07 (−0.50, 2.65) | −3.72 (−7.70, 0.26) | −4.33 (−10.1, 1.45) | −3.80 (−7.98, 0.38) | −2.84 (−5.44, −0.24) |
| Follow-up | 1.88 (1.05, 2.71) | −4.13 (−5.90, −2.37) | −7.76 (−10.4, −5.16) | −4.84 (−7.20, −2.47) | −3.66 (−4.95, −2.36) |
| Seizure group | 9.32 (4.72, 13.9) | −12.7 (−24.2, −1.19) | −26.7 (−43.5, −9.82) | −17.4 (−29.5, −5.23) | −12.0 (−19.5, −4.47) |
Subjects with a history of seizures experience a mean DMR SC (Sum of Cognitive Scores) 9.32 points higher (worsening scores) over time when compared to similar subjects without a history of seizures; this difference is statistically significant. Regression estimates are adjusted for all variable listed in the table. CI-Cognitive impairment.
DISCUSSION
Our findings show that cognitive decline is more marked in demented individuals with DS who have seizures as opposed to those who do not. Despite the relatively small sample size, this finding was evident in the primary analysis where the time to inability to test on performance-measures were used as primary endpoints and in the secondary analysis where the informant-measures were used longitudinally. At baseline, the seizure group had more severe cognitive impairment and a longer course of dementia, which might lead one to believe that the association of seizures and cognitive decline can be attributed to these factors. Put another way, one might assume that those with seizures and dementia had a higher degree of cognitive disability independent of the association with epilepsy. In order to address this potential confound, we adjusted for DMR SC, as a measure of disease severity, pre-morbid level of cognitive impairment, a measure of the subject's baseline developmental disability, and the years since dementia onset at baseline, a measure of a subject's dementia stage. The DMR SC measures the functional domains of short-term memory, long-term memory, spatial and temporal orientation. This cognitive scale and its published thresholds for the degree of cognitive impairment have been validated in studies of DS and dementia [34, 39, 40]. Even after adjustment for these variables, we observed a strong effect size of seizure upon cognitive decline in all of our analyses.
The time between the approximate onset of dementia to the onset of seizures is better understood in AD in the general population than for individuals with DS. For the former, some studies have found a median of approximately 3.3 years between the onset of dementia and the occurrence of seizures while other investigators have concluded that seizures occur only in the late stages of dementia [41]. Clark et al. found cumulative rates of 1.2 and 15% after 5 and 10 years of dementia, respectively [42].
In our analysis, we chose to use the continuation ratio model since the time values were inherently discrete due to the pre-specified biannual follow-up schedule. Other statistical analyses such as the Cox proportional hazards regression were considered less appropriate for this data. We cannot determine from this retrospective analysis whether seizures were the cause of the steeper cognitive decline compared to the non-seizure group or whether both seizures and dementia were part of a more virulent underlying neurodegenerative process. This apparent weakness, and the possibility that the observed association can be explained by some unobserved confounder, is inherent to all observational studies.
Slowing and disorganization of EEG background was noted in the majority of our seizure cases, a finding that is similar to the loss of band synchronization associated with cognitive impairment within AD in the general population [43]. EEG slowing in DS between 20–60 years has been associated with decline in memory and cognition [44]. In adults with DS over age 45 years, those with seizures are more likely to develop dementia [7]. Decreasing EEG frequency in the occipital lobes in DS has been related to dementia [45]. Paroxysmal activity was noted in a minority of our subjects on sporadic EEGs. This finding may be consistent with the lack of surface epileptic discharges in the temporal lobe despite simultaneous electrical status in the hippocampus of the AD transgenic mouse [4]. The degree of oligomeric Aβ aggregation in AD brain may affect the degree of spiking and epileptic sequelae [46].
There was no consistent pattern of antiepileptic drug usage and it was not possible from this retrospective analysis to determine how long any given anticonvulsant medication was administered for those subjects with seizure onset prior to baseline. Potential cognitive confounds of antiepileptic drugs include sedation, insomnia, distractibility and dizziness, particularly with the older generation of medications such has phenytoin and phenobarbital [2, 47–49]. A more favorable cognitive profile has been associated with levetiracetam and lamotrigine in which the former medication has been shown to ameliorate myoclonic epilepsy in DS [50, 51]. The broad spectrum of medications used in our subjects makes it unlikely that a major component of the cognitive decline observed was drug related.
While the association between seizures and cognitive decline is strongly delineated in our population of demented adults with DS, there may be other co-factors that affect the outcome. In AD these medical complications include hypertension, diabetes mellitus, hyperlipidemia, affective disturbances, and corticosteroid-induced stress [52, 53].
The mechanisms relating seizures to cognitive decline in DS has not been elucidated but high brain levels of Aβ are likely to be a factor. The high levels of Aβ in the brains of individuals with DS may interfere with normal neuronal and synaptic activity. In human AβPP transgenic mice, Aβ-induced epileptic activity was associated with sprouting of inhibitory axons in the molecular layer of the dentate gyrus [4, 54]. Fragments of Aβ (including soluble oligomers) from aberrant expression of alleles from App have been associated with lowered convulsive thresholds in mouse models [55–59]. In these mice and by inferential transfer to patients with DS and AD, the Aβ-based aberrant neuronal activity may be causally related to cognitive decline.
Prospective studies of the relationship between seizures, DS, and AD-type dementia are indicated.
ACKNOWLEDGMENTS
We thank the participants and their families/caregivers for participating in this study. Dr. Jack Lin participated in the preparation of this manuscript. This study was supported by grant R01 AG21912 (ITL, ED & AT), Alzheimer Disease Research Center P50 AG16573 (ITL, ED, VQN & DLG), R01 HD65160 (ITL, ED, AT, NM & DLG) at University of California, Irvine, and “My Brother Joey” Neuroscience Fund. The Institute for Clinical and Translational Science, at University of California, Irvine has provided resources in support of this project (UL1 RR031985).
Footnotes
Authors' disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1073).
REFERENCES
- [1].McAreavey MJ, Ballinger BR, Fenton GW. Epileptic seizures in elderly patients with dementia. Epilepsia. 1992;33:657–660. doi: 10.1111/j.1528-1157.1992.tb02343.x. [DOI] [PubMed] [Google Scholar]
- [2].Hommet C, Mondon K, Camus V, De Toffol B, Constans T. Epilepsy and dementia in the elderly. Dement Geriatr Cogn Disord. 2008;25:293–300. doi: 10.1159/000119103. [DOI] [PubMed] [Google Scholar]
- [3].Armon C, Peterson GW, Liwnicz BH. Alzheimer's disease underlies some cases of complex partial status epilepticus. J Clin Neurophysiol. 2000;17:511–518. doi: 10.1097/00004691-200009000-00011. [DOI] [PubMed] [Google Scholar]
- [4].Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Leonard AS, McNamara JO. Does epileptiform activity contribute to cognitive impairment in Alzheimer's disease? Neuron. 2007;55:677–678. doi: 10.1016/j.neuron.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [6].Menendez M. Down syndrome, Alzheimer's disease and seizures. Brain Dev. 2005;27:246–252. doi: 10.1016/j.braindev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- [7].Puri BK, Ho KW, Singh I. Age of seizure onset in adults with Down's syndrome. Int J Clin Pract. 2001;55:442–444. [PubMed] [Google Scholar]
- [8].Möller JC, Hamer HM, Oertel WH, Rosenow F. Late-onset myoclonic epilepsy in Down's syndrome (LOMEDS) Seizure. 2001;10:303–306. doi: 10.1053/seiz.2000.0500. [DOI] [PubMed] [Google Scholar]
- [9].De Simone R, Puig XS, Gélisse P, Crespel A, Genton P. Senile myoclonic epilepsy: Delineation of a common condition associated with Alzheimer's disease in Down syndrome. Seizure. 2010;19:383–389. doi: 10.1016/j.seizure.2010.04.008. [DOI] [PubMed] [Google Scholar]
- [10].Westmark CJ, Westmark PR, Malter JS. Alzheimer's disease and Down syndrome rodent models exhibit audio-genic seizures. J Alzheimers Dis. 2010;20:1009–1013. doi: 10.3233/JAD-2010-100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer's dementia intersect in the hippocampal formation. Epilepsia. 2011;52:39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Choi JH, Berger JD, Mazzella MJ, Morales-Corraliza J, Cataldo AM, Nixon RA, Ginsberg SD, Levy E, Mathews PM. Age-dependent dysregulation of brain amyloid precursor protein in the Ts65Dn Down syndrome mouse model. J Neurochem. 2009;110:1818–1827. doi: 10.1111/j.1471-4159.2009.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- [14].Hunter CL, Isacson O, Nelson M, Bimonte-Nelson H, Seo H, Lin L, Ford K, Kindy MS, Granholm AC. Regional alterations in amyloid precursor protein and nerve growth factor across age in a mouse model of Down's syndrome. Neurosci Res. 2003;45:437–445. doi: 10.1016/s0168-0102(03)00005-1. [DOI] [PubMed] [Google Scholar]
- [15].Mendez M, Lim G. Seizures in elderly patients with dementia: Epidemiology and management. Drugs Aging. 2003;20:791–803. doi: 10.2165/00002512-200320110-00001. [DOI] [PubMed] [Google Scholar]
- [16].Alberici A, Bonato C, Borroni B, Cotelli M, Mattioli F, Binetti G, Gennarelli M, Luca MD, Simonati A, Perani D, Rossini P, Padovani A. Dementia, delusions and seizures: Storage disease or genetic AD? Eur J Neurol. 2007;14:1057–1059. doi: 10.1111/j.1468-1331.2007.01664.x. [DOI] [PubMed] [Google Scholar]
- [17].Larner AJ. Epileptic seizures in AD patients. Neuro-molecular Med. 2010;12:71–77. doi: 10.1007/s12017-009-8076-z. [DOI] [PubMed] [Google Scholar]
- [18].Doran M, Larner AJ. Prominent behavioural and psychiatric symptoms in early-onset Alzheimer's disease in a sib pair with the presenilin-1 gene R269G mutation. Eur Arch Psychiatry Clin Neurosci. 2004;254:187–189. doi: 10.1007/s00406-004-0467-4. [DOI] [PubMed] [Google Scholar]
- [19].Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- [21].Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N Engl J Med. 1989;320:1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- [22].Schupf N, Sergievsky GH. Genetic and host factors for dementia in Down's syndrome. Br J Psychiatry. 2002;180:405–410. doi: 10.1192/bjp.180.5.405. [DOI] [PubMed] [Google Scholar]
- [23].Prasher VP, Sajith SG, Rees SD, Patel A, Tewari S, Schupf N, Zigman WB. Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer's disease and mortality in persons with Down syndrome. Int J Geriatr Psychiatry. 2008;23:1134–1140. doi: 10.1002/gps.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer's disease and animal models. Annu Rev Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- [25].Selkoe DJ, Physicians ACo, Society AP. Alzheimer disease: Mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- [26].Lott IT, Doran E, Nguyen VQ, Tournay A, Head E, Gillen DL. Down syndrome and dementia: A randomized, controlled trial of antioxidant supplementation. Am J Med Genet A. 2011;155:1939–1948. doi: 10.1002/ajmg.a.34114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Association AP. Diagnostic and statistical manual for mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- [28].Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol. 1994;51:41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- [29].Dalton A, Fedor B. DYSPRAXIA scale for adults with Down syndrome. NYS Institute for Basic Research in Developmental Disabilities; Staten Island, NY, 10314, USA: 1997. [Google Scholar]
- [30].Prasher VP, Huxley A, Haque MS, Down syndrome Ageing Study Group A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Down syndrome and Alzheimer's disease-pilot study. Int J Geriatr Psychiatry. 2002;17:270–278. doi: 10.1002/gps.587. [DOI] [PubMed] [Google Scholar]
- [31].Evenhuis HM. Evaluation of a screening instrument for dementia in ageing mentally retarded persons. J Intellect Disabil Res. 1992;36(Pt 4):337–347. doi: 10.1111/j.1365-2788.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- [32].Evenhuis HM. Further evaluation of the dementia questionnaire for persons with mental retardation (DMR) J Intellect Disabil Res. 1996;40(Pt 4):369–373. doi: 10.1046/j.1365-2788.1996.786786.x. [DOI] [PubMed] [Google Scholar]
- [33].Sparrow S, Cicchetti D. The behavior inventory for rating development (BIRD): Assessments of reliability and factorial validity. Appl Res Ment Retard. 1984;5:219–231. doi: 10.1016/s0270-3092(84)80003-x. [DOI] [PubMed] [Google Scholar]
- [34].Strydom A, Hassiotis A. Diagnostic instruments for dementia in older people with intellectual disability in clinical practice. Aging Ment Health. 2003;7:431–437. doi: 10.1080/13607860310001594682. [DOI] [PubMed] [Google Scholar]
- [35].Di Nuovo S, Buono S. Behavioral phenotypes of genetic syndromes with intellectual disability: Comparison of adaptive profiles. Psychiatry Res. 2011;189:440–445. doi: 10.1016/j.psychres.2011.03.015. [DOI] [PubMed] [Google Scholar]
- [36].Berridge DM, Whitehead J. Analysis of failure time data with ordinal categories of response. Stat Med. 1991;10:1703–1710. doi: 10.1002/sim.4780101108. [DOI] [PubMed] [Google Scholar]
- [37].Huber P. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability.1967. pp. 221–233. [Google Scholar]
- [38].Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- [39].Strydom A, Hassiotis A, King M, Livingston G. The relationship of dementia prevalence in older adults with intellectual disability (ID) to age and severity of ID. Psychol Med. 2009;39:13–21. doi: 10.1017/S0033291708003334. [DOI] [PubMed] [Google Scholar]
- [40].Deb S, Braganza J. Comparison of rating scales for the diagnosis of dementia in adults with Down's syndrome. J Intellect Disabil Res. 1999;43(Pt 5):400–407. doi: 10.1046/j.1365-2788.1999.043005400.x. [DOI] [PubMed] [Google Scholar]
- [41].Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46:727–730. doi: 10.1212/wnl.46.3.727. [DOI] [PubMed] [Google Scholar]
- [42].Clark C, Heyman A, Earl N, Utley C, Haynes C. Myoclonus in Alzheimer's disease. In: Iqbal K, McLachlan D, Winblad B, Wisniewski H, editors. Alzheimer's Disease: Basic Mechanisms, Diagnosis, and Therapeutic Strategies. Wiley; Chichester, NY: 1991. pp. 35–40. [Google Scholar]
- [43].Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer's disease. Acta Neurol Scand. 2003;108:90–96. doi: 10.1034/j.1600-0404.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- [44].Soininen H, Partanen J, Jousmäki V, Helkala EL, Vanhanen M, Majuri S, Kaski M, Hartikainen P, Riekkinen P. Age-related cognitive decline and electroencephalogram slowing in Down's syndrome as a model of Alzheimer's disease. Neuroscience. 1993;53:57–63. doi: 10.1016/0306-4522(93)90284-m. [DOI] [PubMed] [Google Scholar]
- [45].Katada A, Hasegawa S, Ohira D, Kumagai T, Harashima T, Ozaki H, Suzuki H. On chronological changes in the basic EEG rhythm in persons with Down syndrome - with special reference to slowing of alpha waves. Brain Dev. 2000;22:224–229. doi: 10.1016/s0387-7604(00)00107-8. [DOI] [PubMed] [Google Scholar]
- [46].Orbán G, Völgyi K, Juhász G, Penke B, Kékesi KA, Kardos J, Czurkó A. Different electrophysiological actions of 24- and 72-hour aggregated amyloid-beta oligomers on hippocampal field population spike in both anesthetized and awake rats. Brain Res. 2010;1354:227–235. doi: 10.1016/j.brainres.2010.07.061. [DOI] [PubMed] [Google Scholar]
- [47].Tsiouris JA, Patti PJ, Tipu O, Raguthu S. Adverse effects of phenytoin given for late-onset seizures in adults with Down syndrome. Neurology. 2002;59:779–780. doi: 10.1212/wnl.59.5.779. [DOI] [PubMed] [Google Scholar]
- [48].Mula M, Monaco F. Antiepileptic drugs and psychopathology of epilepsy: An update. Epileptic Disord. 2009;11:1–9. doi: 10.1684/epd.2009.0238. [DOI] [PubMed] [Google Scholar]
- [49].Bernardi S, Scaldaferri N, Vanacore N, Trebbastoni A, Francia A, D'Amico A, Prencipe M. Seizures in Alzheimer's disease: A retrospective study of a cohort of outpatients. Epileptic Disord. 2010;12:16–21. doi: 10.1684/epd.2010.0290. [DOI] [PubMed] [Google Scholar]
- [50].Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer's disease. Epilepsy Behav. 2010;17:461–466. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- [51].Sangani M, Shahid A, Amina S, Koubeissi M. Improvement of myoclonic epilepsy in Down syndrome treated with levetiracetam. Epileptic Disord. 2010;12:151–154. doi: 10.1684/epd.2010.0306. [DOI] [PubMed] [Google Scholar]
- [52].Dhikav V, Anand K. Potential predictors of hippocampal atrophy in Alzheimer's disease. Drugs Aging. 2011;28:1–11. doi: 10.2165/11586390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [53].McDonald RJ, Craig LA, Hong NS. The etiology of age-related dementia is more complicated than we think. Behav Brain Res. 2010;214:3–11. doi: 10.1016/j.bbr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- [54].Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc Natl Acad Sci U S A. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G. The Alzheimer's A beta peptide induces neurode-generation and apoptotic cell death in transgenic mice. Nat Genet. 1995;9:21–30. doi: 10.1038/ng0195-21. [DOI] [PubMed] [Google Scholar]
- [56].Moechars D, Lorent K, De Strooper B, Dewachter I, Van Leuven F. Expression in brain of amyloid precursor protein mutated in the alpha-secretase site causes disturbed behavior, neuronal degeneration and premature death in transgenic mice. EMBO J. 1996;15:1265–1274. [PMC free article] [PubMed] [Google Scholar]
- [57].Lalonde R, Dumont M, Staufenbiel M, Strazielle C. Neurobehavioral characterization of APP23 transgenic mice with the SHIRPA primary screen. Behav Brain Res. 2005;157:91–98. doi: 10.1016/j.bbr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- [58].Steinbach JP, Müller U, Leist M, Li ZW, Nicotera P, Aguzzi A. Hypersensitivity to seizures in beta-amyloid precursor protein deficient mice. Cell Death Differ. 1998;5:858–866. doi: 10.1038/sj.cdd.4400391. [DOI] [PubMed] [Google Scholar]
- [59].Del Vecchio RA, Gold LH, Novick SJ, Wong G, Hyde LA. Increased seizure threshold and severity in young transgenic CRND8 mice. Neurosci Lett. 2004;367:164–167. doi: 10.1016/j.neulet.2004.05.107. [DOI] [PubMed] [Google Scholar]