Abstract
BACKGROUND
A recent meta-analysis of genome-wide linkage scans of blood pressure (BP) in the large (N=13,044) Family Blood Pressure Program (FBPP) identified 5 quantitative trait loci (QTLs) on chromosomes 6, 8, 20, and 21. We conducted follow-up fine mapping studies in 1,251 African (AA) and 1,254 European American (EA) participants of the Hypertension Genetic Epidemiology Network (HyperGEN).
METHODS
Ethnic-specific linear mixed effects models were used to test associations of BP with genotyped and imputed single nucleotide polymorphisms (SNPs) contained in the LOD score ≥2 interval under each of the QTL peaks. We used multipoint variance components models to perform linkage analysis conditional on each significant SNP in the QTL region to see if the SNP explained the QTL.
RESULTS
Three intergenic SNPs (rs898164, rs2876587, rs6935795) on chromosome 6p22.3 were significantly associated with pulse pressure (using appropriate Bonferroni correction). Conditioning on the significant SNPs reduced the chromosome 6 QTL linkage evidence by 14%–30%. Both AAs and EAs exhibited suggestive associations between BP and intronic SNPs on chromosomes 8q24.12 (genes: OPG in AAs, SAMD12 in EAs), 20q13.12 (genes: SLC13A3 in AAs, SLC12A5 in EAs), and 21q21.1 (genes: C21orf34 in AAs, BC039377 in EAs).
CONCLUSIONS
Significant associations with common SNPs explained less than 1/3 of the QTL evidence. Our results cannot refute the hypothesis that rare variants account for most of the statistical evidence for the FBPP linkage peaks. Whole genome resequencing can identify the variants driving the linkage peaks and GWAS hits thereby spurring investigations to deepen our understanding of hypertension pathophysiology.
Keywords: hypertension, blood pressure, GWAS, genetics, SNP, fine mapping
INTRODUCTION
Hypertension afflicts approximately 26.4% (or 972 million people) of the global population1. Determining causal genes and pathological biochemical pathways could spur new pharmacogenomic interventions to substantially reduce the global health burden. Blood pressure (BP) heritability remains elusive, with less than 2% of the interindividual BP variation explained by common variants using massive sample sizes2–3. In a recent genome-wide linkage meta-analysis of the National Heart, Lung, and Blood Institute Family Blood Pressure Program (FBPP), Simino et al. identified 5 BP quantitative trait loci (QTLs) on chromosomes 6, 8, 20, and 214. The QTL shoulders [with logarithm of odds (LOD) score ≥ 2 under the linkage peaks] were broad (spanning 11.5 Mbps to 38.4 Mbps) and encompassed a multitude of genes (79 to 541 in each QTL). We fine mapped the QTLs using dense single nucleotide polymorphisms (SNPs) from the genome-wide association study (GWAS) of the Hypertension Genetic Epidemiology Network (HyperGEN), one of four FBPP networks included in the previous BP linkage meta-analysis (and in which GWAS data are available).
HyperGEN provided support (LOD≥1) for the linkage of BP to the QTLs on chromosome 6 for African Americans (AAs) and chromosome 21 for AAs and European Americans (EAs) but lacked linkage evidence for the QTLs on chromosomes 8 and 204. Simino et al.4 argued that low-frequency variants drove the FBPP linkage peaks. Mounting evidence from lipid studies suggests that rare and low-frequency variants discovered through linkage reside in the same genes as common variants identified by GWAS5–6. We capitalized on the much better resolution of SNPs than linkage markers (average intermarker distance of ≈1 kbps versus ≈7 Mbps) and the less extended linkage disequilibrium in the participants of recent African ancestry5 to narrow the list of candidate genes and prioritize genomic regions for future sequencing analysis. We tested all QTL regions (even those without linkage evidence in HyperGEN) because genes harboring at least 1 trait-affecting variant in any population may be more likely to harbor additional variants altering the gene's expression and/or function6. We investigated whether common variants identified in this process explained the linkage peaks by performing linkage conditional on the significant SNPs found.
METHODS
Subjects
HyperGEN is one of four FBPP networks created to identify and characterize genes affecting hypertension. Genotyped participants were recruited by 4 field centers: Minneapolis, Minnesota; Salt Lake City, Utah; Forsyth County, North Carolina; and Birmingham, Alabama7. Two types of AA and EA participants were recruited: hypertensive sibships (with ≥2 sibs having SBP≥140 mmHg, DBP≥90 mmHg, or taking hypertensive medication(s)), and unmedicated adult offspring of one or more hypertensive siblings7. Sibships with at least one severely hypertensive (SBP≥160 mmHg, DBP≥100 mmHg, or taking ≥2 classes hypertensive medications) member were preferentially ascertained7. Individuals with hypertension onset past age 60 or hypertension secondary to primary kidney disease were excluded.7 The institutional review board at each field center approved the study protocol and informed consent collection procedure.
GWAS DATA
All 1,264 EAs and 175 AAs were genotyped on the Affymetrix Genome-wide Human SNP Array 5.0 (BRLMM calling algorithm). The remaining 1,083 AAs were genotyped using the Affymetrix Genome-wide Human SNP Array 6.0 (Birdseed calling algorithm); 67 AA families had members genotyped on both arrays. Graphical Representation of Relationships (GRR) was run on approximately 20,000 autosomal SNPs to determine pedigree errors. Individuals with chip wide call rates <97% in EAs and <99% in AAs were excluded. Mendelian errors detected by PLINK (using an integrated Affymetrix 5.0 and 6.0 dataset in AAs) were zeroed out by setting the offspring's genotype to missing. By race and genotyping platform, SNPs with missing rate >5%, minor allele frequency (MAF) <1%, or Hardy-Weinberg p-value <10−6 were removed. SNPs with Hardy-Weinberg p-values <10−6 in the AA 5.0 and 6.0 integrated dataset were also removed.
Within each race, MACH8 was used to derive hidden Markov imputation models on the 200 unrelated individuals with the lowest missing rate of quality-controlled SNPs. To form a "revised union" for the AA imputation, SNPs in the intersection of the CEU and YRI HapMap phased data were joined with SNPs in the phased data of CEU or YRI only with a near-zero MAF in the unphased data of the other. The AA (HapMap release 22, phased data, "revised union" of CEU and YRI populations) and EA (HapMap release 22, phased data, CEU population) imputation models yielded 3.01 million and 2.54 million SNPs, respectively. We analyzed the allele dosages (the expected number of copies of the coded allele) for each SNP. Microsatellite marker data for the conditional linkage analysis was previously described in Simino et al.4
PHENOTYPE
Centrally trained and certified personnel measured systolic BP (SBP) and diastolic BP (DBP) using automated Dinamap devices; the average of two measurements of SBP and DBP were calculated for each participant. Mean arterial pressure (MAP) was estimated by the sum of two-thirds the average DBP and one-third the average SBP. Pulse pressure (PP) was computed as the difference between the average SBP and DBP.
For each phenotype (SBP, DBP, MAP, and PP), we used 3 methods (called "raw", "+10/5", and "+15/10") to adjust for antihypertensive medication status. The "raw" method used the observed BP values without any adjustment. The "+10/5" medication adjusted values9 were derived by adding 10mmHg to SBP and 5mmHg to DBP of those known to be taking antihypertensive or diuretic medications. Similarly, the “+15/10” medication adjusted values10 were obtained by adding 15mmHg to SBP and 10 mmHg to DBP of those known to be taking antihypertensives or diuretics. The observed BP phenotypes were always used for those untreated or with unknown medication status. Medication adjusted PP and MAP were calculated from the medication adjusted SBP and DBP values. Both the “+10/5” and “+15/10” medication adjustments result in the same values of PP (PP+5 for medication users).
We excluded the BP phenotypes of 29 individuals with BMI, SBP, DBP, MAP, or PP values 4 or more standard deviations from their race and sex specific mean values. Phenotypes were adjusted for age, age-squared, age-cubed, BMI, and field center within each race and sex subgroup, retaining covariates that were significant at the 5% level during a stepwise linear regression. The residual phenotypes were standardized to a mean of 0 and standard deviation of 1. We excluded 7 covariate adjusted phenotype values that were in excess of 4 standard deviations from the mean of that race and sex subgroup. Table 1 displays the summary statistics for the analysis sample.
Table 1.
Descriptive Statistics of Sample
| Characteristic | African American (Nfam=466) | European American (Nfam=297) | ||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | |||||
| Number of participants | 845 | 406 | 630 | 624 | ||||
| Age | 45.7 | (13.1) | 44.2 | (13.7) | 50.9 | (13.6) | 48.7 | (14.3) |
| % Hypertensives | 70.5 | 62.8 | 56.0 | 52.9 | ||||
| % Taking Antihypertensives or Diuretics | 65.0 | 53.4 | 53.9 | 47.6 | ||||
| Body Mass Index (BMI) | 33.8 | (8.2) | 29.8 | (6.4) | 29.5 | (6.7) | 29.1 | (4.8) |
| SBP | 128.6 | (22.5) | 130.0 | (20.6) | 121.5 | (19.7) | 124.9 | (17.4) |
| DBP | 72.5 | (10.9) | 76.8 | (12.3) | 67.5 | (9.2) | 73.9 | (9.6) |
| MAP | 91.2 | (13.5) | 94.5 | (14.1) | 85.5 | (11.1) | 90.9 | (11.2) |
| PP | 56.1 | (17.0) | 53.3 | (13.9) | 54.0 | (17.0) | 51.0 | (13.0) |
Nfam is the number of families in that ethnic group. Continuous variables are represented as: mean (standard deviation). SBP=systolic blood pressure; DBP=diastolic blood pressure; MAP=mean arterial pressure; PP=pulse pressure.
STATISTICAL ANALYSIS
We analyzed HyperGEN genotyped and imputed SNPs contained in the QTL shoulders (defined by LOD score≥2 interval under each linkage peak). We used the NCBI 36 (March 2006) assembly on the UCSC Genome Browser to deduce physical position(http://genome.ucsc.edu/)11. The first microsatellite marker on chromosome 21 had a LOD score≥2, thus we extended the interval to the beginning of the chromosome. Genotyped SNPs were analyzed without any imputation of missing values. Genotyped SNPs exclusive to the Affy 5.0 platform in AAs (called less than 175 individuals) were excluded from analysis. Table 2 quantifies the number of SNPs analyzed within each QTL region by ethnicity.
Table 2.
Number of SNPs analyzed in each QTL region
| Chr | Start Position (in bp) |
End Position (in bp) |
African American | European American | ||||
|---|---|---|---|---|---|---|---|---|
| Genotyped | Imputed | Total | Genotyped | Imputed | Total | |||
| 6 | 20,579,309 | 39,703,884 | 6,429 | 20,672 | 27,101 | 3,032 | 20,944 | 23,976 |
| 8 | 102,586,989 | 120,290,804 | 4,974 | 15,780 | 20,754 | 2,271 | 15,043 | 17,314 |
| 20 | 37,179,214 | 48,644,168 | 4,143 | 9,576 | 13,719 | 1,714 | 9,059 | 10,773 |
| 21 | 0 | 38,378,064 | 8,780 | 19,884 | 28,664 | 3,769 | 19,119 | 22,888 |
The R package GWAF12 was employed to fit linear mixed models of the BP residuals onto the fixed additive effect SNP and the random polygenic effect. To control for the testing of multiple traits with SNPs across 4 QTL regions, we applied a significance threshold of 0.05 divided by twice the total number of genotyped SNPs in all QTL regions. This is analogous to the conventional Bonferroni correction (for the total number of tested genotyped SNPs) applied during GWAS. This multiple testing correction also accounted for the two measured (SBP and DBP) but not derived (PP and MAP) phenotypes. The significance threshold for all association tests in AAs and EAs is 0.05/(2*24,326)= 1.03*10−6 and 0.05/(2*10,786)= 2.32*10−6, respectively. Similarly, a "suggestive" association threshold, defined as 1 divided by twice the total number of genotyped SNPs in all QTL regions, took values 2.06*10−5 and 4.64*10−5 in AAs and EAs, respectively.
Inflation factors using all genotyped SNPs for each BP trait ranged from 0.91 to 1.03 in AAs and 0.96 to 1.08 in EAs (for imputed SNPs the maximum inflation factors are also 1.03 and 1.08 in AAs and EAs, respectively). Q-Q plots of the −log10(p-values) revealed no evidence of population stratification. Merlin13 multipoint variance components models were used to perform linkage conditional on each individual significant SNP. We compared the genome-wide scans that included and omitted the SNP, restricting both analyses to BP phenotypes of participants with non-missing values of the included SNP.
RESULTS
Three intergenic SNPs (rs898164, rs2876587, and rs6935795) on chromosome 6p22.3 were significantly associated with PP phenotypes in AAs (after applying Bonferroni-corrected thresholds). Genotyped SNP rs2876587 was significantly (p-value=9.85*10−7) associated with medication adjusted PP while imputed SNPs rs898164 and rs6935795 were significantly associated with raw PP (respective p-values 4.45*10−7 and 4.54*10−7) and medication adjusted PP (respective p-values 1.27*10−7 and 1.26*10−7). SNPs rs898164, rs2876587, and rs6935795 have strong pairwise linkage disequilibriums; using the SNP Annotation and Proxy Search database14 with either Youribans or CEU 1000 Genomes Pilot 1 data yielded pairwise D' values of 1 and R2 values ranging from 0.925 to 1. These SNPs have MAFs of 0.32, individually explain over 2% of the medication adjusted PP heritability (for heritability formula see12), and are within 3.05 Mbps of the marker (D6S2439) significantly linked to PP (LOD=3.76) and medication adjusted PP (LOD=3.23) in FBPP AAs. Figure 1 overlays the FBPP AA linkage evidence on the HyperGEN AA association results for the chromosome 6 QTL region and medication adjusted PP. SNPs rs898164, rs2876587, and rs6935795 were not significantly associated with medication adjusted PP in EAs (respective p-values of 0.06,0.27, and 0.06) but the MAFs were much smaller (respective values 0.13, 0.12, and 0.13) than in AAs.
Figure 1.
Association of the chromosome 6 QTL region with medication adjusted pulse pressure in African Americans. The red plus (+) and blue circle indicate association results (−log10(P-values) for genotyped and imputed SNPs, respectively. The green dashed line indicates the Bonferroni-corrected threshold for significance. The black connected line represents the linkage evidence (logarithm of odds (LOD) scores) from the meta-analysis of medication adjusted pulse pressure in FBPP African Americans.
None of the tested SNPs achieved statistical significance in EAs. Table 3 displays the most-associated SNP within each chromosomal region that yielded significant or suggestive evidence for that race. Seventy-one SNPs exhibited suggestive associations (14 in AAs, 57 in EAs) that implicated 6 additional chromosomal bands. Both AAs and EAs exhibited suggestive associations for intronic SNPs on chromosomes 8q24.12 (genes: OPG in AAs, SAMD12 in EAs), 20q13.12 (genes: SLC13A3 in AAs, SLC12A5 in EAs), and 21q21.1 (genes: C21orf34 in AAs, BC039377 in EAs).
Table 3.
Most-associated SNP within each chromosomal region that yielded significant or suggestive evidence for blood pressure
| Chr | Position (in bp) |
Ethnic Group |
Most associated SNP ID (NCBI 36) |
SNP Type | Genomic Location |
Allele Coded |
Allele Freq |
N | Trait | Beta (s.e.) | h2 | P-value | # BP- associated SNPs in Chr Band† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6p22.3 | 21,367,307 | AA | rs6935795 | imputed | Intergenic | T | 0.68 | 1246 | Med. Adj. PP | 0.25 (0.05) | 2.11 | 1.26E-07 | 6 |
| 6p21.31 | 33,899,908 | AA | rs1408501 | imputed | Intergenic | A | 0.93 | 1246 | PP | 0.45 (0.09) | 1.95 | 1.40E-06 | 1 |
| 8q24.12 | 119,326,037 | EA | rs17455085 | imputed | SAMD12 intron | G | 0.92 | 1254 | SBP | −0.38 (0.08) | 1.78 | 8.21E-06 | 1 |
| 8q24.12 | 120,015,322 | AA | rs3134053 | imputed | OPG intron | C | 0.87 | 1246 | PP | 0.30 (0.06) | 1.78 | 3.28E-06 | 1 |
| 20q12 | 39,965,971 | EA | rs16986255 | genotyped | Intergenic | G | 0.02 | 1232 | +15/10 MAP | −0.60 (0.14) | 1.46 | 2.80E-05 | 1 |
| 20q13.12 | 44,106,652 | EA | rs4812987 | imputed | SLC12A5 intron | T | 0.98 | 1254 | MAP | −0.69 (0.16) | 1.63 | 2.41E-05 | 2 |
| 20q13.12 | 44,650,935 | AA | rs6066029 | imputed | SLC13A3 intron | C | 0.92 | 1248 | +15 SBP | −0.42 (0.09) | 1.62 | 1.70E-06 | 5 |
| 20q13.13 | 46,469,909 | EA | rs6012418 | imputed | Intergenic | G | 0.92 | 1254 | +15/10 MAP | −0.36 (0.08) | 1.91 | 4.60E-06 | 23 |
| 21q21.1 | 16,790,159 | AA | rs9977640 | imputed | C21orf34 intron | G | 0.96 | 1246 | PP | 0.48 (0.11) | 1.78 | 7.80E-06 | 4 |
| 21q21.1 | 22,305,480 | EA | rs9982805 | genotyped | BC039377 intron | A | 0.03 | 1254 | PP | 0.54 (0.12) | 1.60 | 1.30E-05 | 30 |
Indicates the number of SNPs that were significantly or suggestively associated with blood pressure traits within that chromosomal band NOTE: AA=African American; EA=European American; N=number of individuals included in the association analysis of the SNP with the trait; Beta=coefficient of the SNP in the mixed model; s.e.=standard error of beta; h2=SNP-specific heritability as calculated by R GWAF; SBP=systolic blood pressure; MAP=mean arterial pressure; PP=pulse pressure; Med. Adj. PP is PP+5 for antihypertensive/diuretic users and raw PP for non-users; +15 SBP indicates 15 mmHg was added to the raw SBP for individuals taking antihypertensive/diuretic medications (the raw SBP was used for non-medication takers); +15/10 MAP was computed from +15 SBP and +10 DBP for those taking antihypertensive/diuretic medications (raw MAP used for non-medication takers). The statistically significant result is bolded.
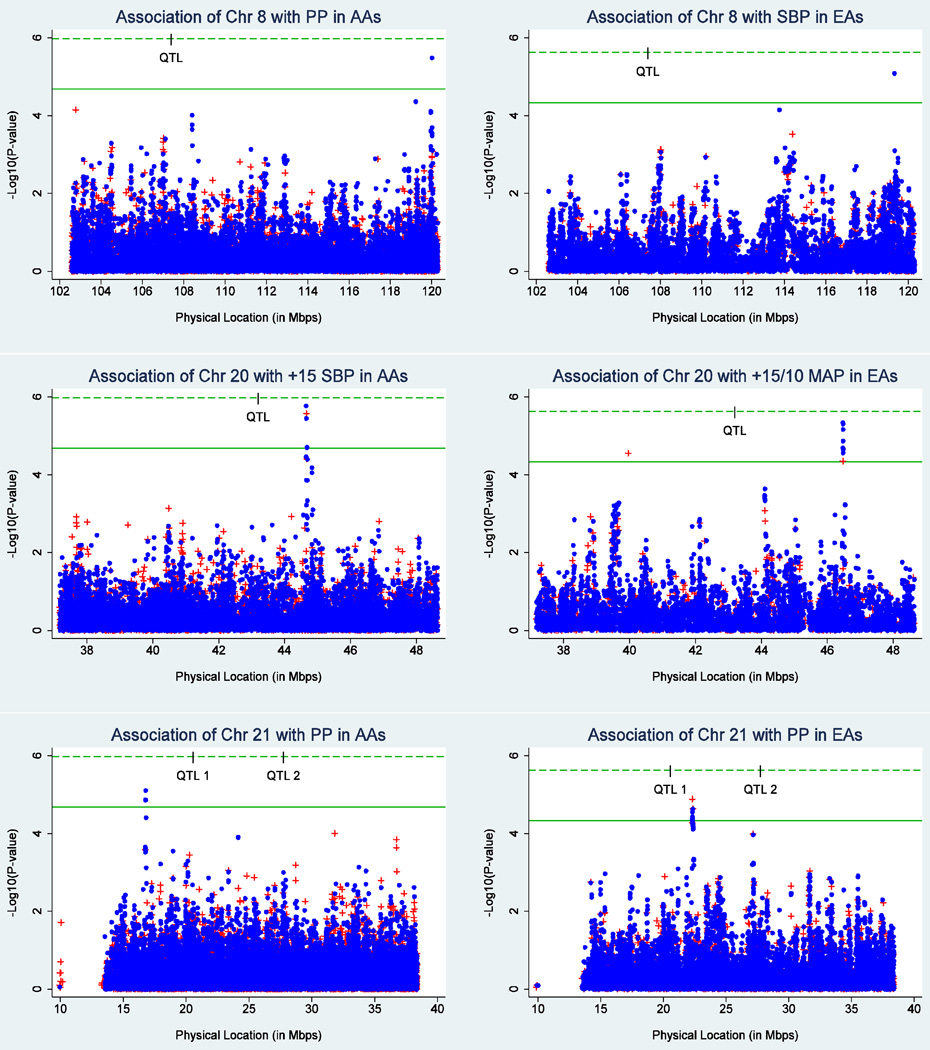
The phenotype significantly linked to a QTL in FBPP was not always the same as that suggestively associated in HyperGEN. Chromosome 8 was linked to DBP in FBPP but was suggestively associated with PP and SBP in HyperGEN AAs and EAs, respectively. Chromosome 20 was linked to PP in FBPP but was suggestively associated with +15/10 MAP in HyperGEN EAs. Figure 2 displays the ethnic-specific association results for the BP phenotype that yielded the smallest p-value within each chromosome 8, 20, and 21 QTL region (for all graphs see the online supplement). A hash mark on the Bonferroni-corrected significance threshold line indicates the physical location of the FBPP QTL.
Figure 2.
Ethnic-specific association results for the blood pressure trait most-associated with each of the chromosome 8, 20, and 21 QTL regions. The red plus (+) indicates a genotyped SNP while the blue circle represents an imputed SNP. The green dashed and solid lines indicate the Bonferroni-corrected thresholds for significant and suggestive association, respectively; the hash mark on the significance threshold indicates the physical location of the LOD score peak in the FBPP QTLs (Simino et al., 2011). AAs=African Americans; EAs=European Americans
Conditioning on any of the three significant SNPs decreased the linkage evidence for the chromosome 6 QTL region by up to 30%. Peak QTL LOD scores decreased by 14% to 30% depending on the SNP and PP phenotype (raw or medication adjusted). However, the maximum LOD for PP phenotypes was only 0.79 at the chromosome 6 FBPP peak QTL marker using individuals with GWAS data. Common SNPs failed to explain much of the FBPP linkage evidence at the 5 QTLs.
DISCUSSION
This investigation reports three PP-associated variants harbored in the chromosome 6 QTL region previously identified by the FBPP genome-wide linkage meta-analysis of AAs4. Given our limited knowledge of the genome, the associations between PP and the three intergenic SNPs currently lack functional justification. Forty-three percent of trait-associated SNPs identified via GWAS are intergenic15; these SNPs could inhabit unidentified genes16 or important BP regulatory elements such as enhancers, insulators, transcription factor binding sites, or microRNA coding sequences17. Alternatively, the significant SNPs may be correlated with the BP-influencing variant, contained in the haplotype tagging the BP-influencing variant, or linked to a BP-influencing copy number variant15. In this case, the effect size of the BP-influencing variant may be vastly underestimated17.
We cannot refute the hypothesis that low-frequency variants in a small number of families were the driving force behind the FBPP linkage peaks. The significant associations with common SNPs explained some of the chromosome 6 QTL as evidenced by the 14–30% decrease in QTL LOD scores during the conditional linkage analysis. However, we failed to identify significant SNPs in the chromosome 8, 20, and 21 QTL regions, even though both HyperGEN ethnic groups provided linkage evidence for the latter. The lack of significant SNPs in the chromosome 21 QTL region might be attributable to insufficient power based on the sample size, LD between the causal variant and the SNP, the frequency of the causal variant, and/or the effect of the causal variant18. A rare variant driving a linkage peak may not be detected in GWAS even if it has a large effect18. Therefore we cannot dismiss the suggestive association results for the linkage phenotype (Medication adjusted PP) in both ethnicities on chromosome 21q21.1 (see supplementary figure).
Both AAs and EAs failed to provide any linkage evidence in the chromosome 8 QTL region but both exhibited suggestive association between BP and intronic SNPs on chromosome 8q24.12. These common causal variants may contribute too small an effect on BP to surpass the stringent significance thresholds applied to our limited sample size18. The phenotypes producing suggestive associations (PP in AAs and SBP in EAs) on 8q24.12 differed from those linked in FBPP (DBP), perhaps implicating different causal genes for the two types of evidence. However, SNP rs11573901 (Illumina Golden Gate Assay), 419 bp away from the suggestively associated SNP in AAs (rs3134053) and also intronic to OPG, was associated with DBP (the FBPP linkage phenotype) in 1,070 elderly participants of the Health in Men Study (Perth, Western Australia)19.OPG codes osteoprotegerin which functions as a negative regulator of bone resorption20. Several studies have shown an association between circulating levels of osteoprotegerin and BP21–23. A biologically plausible link between BP and OPG is vascular calcification and blood vessel stiffness. Vascular calcification induced by vitamin D3 and nicotine in rats was accompanied by an increase in SBP and up-regulation of osteoprotegerin mRNA expression24. Osteoprotegerin was associated with aortic pulse wave velocity (a measure of arterial stiffness) in Estonian men25.
The suggestive association between BP and SNP rs17455085 intronic to SAMD12 (8q24.12) in EAs also has external evidence. SNP rs4514016 intronic to SAMD12 yielded a p-value of 4.52*10−5 in a family-based association test of DBP (the FBPP linkage phenotype) in 1,233 Framingham Heart Study participants26. In Yoruban (YRI) HapMap samples, SNP rs17455085 has been tentatively associated with expression of chromosome 18 open reading frame 1 (C18orf1) (p-value=9*10−5)27. SNP rs8096897 intronic to C18orf1 was associated (p-value=3.2*10−8) with SBP (the HyperGEN EA suggestively associated phenotype) in the CHARGE meta-analysis2.
Similarly, both ethnic groups lacked linkage evidence but provided suggestive association for the chromosome 20 QTL region. Follow up investigations searching for variants within the QTLs are amply justified. Ten of 17 BP-associated novel SNPs from a large unpublished consortium of 200,000 individuals of European descent (Ehret et al., 2011) are contained in the LOD score ≥ 0.5 interval of primary and secondary (LOD score≥2 in any meta- or cohort-specific analysis) QTLs4. Sequencing holds promise to detect novel variants from the whole allele frequency spectrum. Whole genome resequencing is declining in cost and offers an unbiased interrogation of the entire genome rather than a narrow region or exons that may miss the causal variant. Furthermore, whole genome resequencing of diverse populations and multiple BP traits can identify new structural and regulatory variants28 that may be vital to parse the complex genetic architecture of hypertension.
Supplementary Material
Acknowledgments
We thank all HyperGEN participants. The following investigators are associated with the HyperGEN Network: Steven C. Hunt (Network Director), Janet Hood, Donna Arnett, James S. Pankow, John H. Eckfeldt, R. Curtis Ellison, Richard H. Myers, C. Charles Gu, Gerardo Heiss, Kari North, Paul Hopkins, Aldi T. Kraja, Jean-Marc Lalouel, Mark Leppert, Albert Oberman, Cora E. Lewis, Michael A. Province, D.C. Rao, Treva Rice, and Robert Weiss. This research was partly supported by Grants T32 HL091823, U01 HL54473, R21 HL095054, and R01 HL55673 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement: None
DISCLOSURE: None
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simino J, Shi G, Kume R, Schwander K, Province MA, Gu CC, Kardia S, Chakravarti A, Ehret G, Olshen RA, Turner ST, Ho LT, Zhu X, Jaquish C, Paltoo D, Cooper RS, Weder A, Curb JD, Boerwinkle E, Hunt SC, Rao DC. Five blood pressure loci identified by an updated genome-wide linkage scan: Meta-analysis of the Family Blood Pressure Program. Am J Hypertens. 2011;24:347–354. doi: 10.1038/ajh.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gloyn AL, McCarthy MI. Variation across the allele frequency spectrum. Nat Genet. 2010;42:648–650. doi: 10.1038/ng0810-648. [DOI] [PubMed] [Google Scholar]
- 7.Williams RR, Rao DC, Ellison RC, Arnett DK, Heiss G, Oberman A, Eckfeldt JH, Leppert MF, Province MA, Mokrin SC Hunt SC for the HyperGEN Investigators. NHLBI Family Blood Pressure Program: Methodology and recruitment in the HyperGEN network. Ann Epidemiol. 2000;10:389–400. doi: 10.1016/s1047-2797(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- 10.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 11.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen MH, Yang Q. GWAF: An R package for genome-wide association analyses with family data. Bioinformatics. 2010;26:580–581. doi: 10.1093/bioinformatics/btp710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI. SNAP: A web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein RJ, Xu X, Mukherjee S, Willis J, Hayes J. Successes of genome-wide association studies. Cell. 2010;142:350–351. doi: 10.1016/j.cell.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Ku CS, Loy EY, Pawitan Y, Chia KS. The pursuit of genome-wide association studies: Where are we now? J Hum Genet. 2010;55:195–206. doi: 10.1038/jhg.2010.19. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, Visscher PM. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golledge J, Biros E, Clancy P, Cooper M, Palmer LJ, Norman PE. A single-nucleotide polymorphism in the gene encoding osteoprotegerin is associated with diastolic blood pressure in older men. Am J Hypertens. 2009;22:1167–1170. doi: 10.1038/ajh.2009.177. [DOI] [PubMed] [Google Scholar]
- 20.NCBI Entrez gene: http://www.ncbi.nlm.nih.gov/gene/.
- 21.Uemura H, Yasui T, Miyatani Y, Yamada M, Hiyoshi M, Arisawa K, Irahara M. Circulating osteoprotegerin is associated with age and systolic blood pressure, but not with lipid profile or fasting glucose, in postmenopausal women. Menopause. 2008;15:180–184. doi: 10.1097/gme.0b013e318046369b. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen LM, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol. 2006;154:75–81. doi: 10.1530/eje.1.02049. [DOI] [PubMed] [Google Scholar]
- 23.Lieb W, Gona P, Larson MG, Massaro JM, Lipinska I, Keaney JF, Jr, Rong J, Corey D, Hoffmann U, Fox CS, Vasan RS, Benjamin EJ, O'Donnell CJ, Kathiresan S. Biomarkers of the osteoprotegerin pathway: Clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler Thromb Vasc Biol. 2010;30:1849–1854. doi: 10.1161/ATVBAHA.109.199661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Zhou YB, Cai Y, Teng X, Song JQ, Duan XH, Zhang WZ, Qi YF. [effect of age on vascular calcification induced by vitamin D3 and nicotine] Beijing Da Xue Xue Bao. 2010;42:131–136. [PubMed] [Google Scholar]
- 25.Zagura M, Serg M, Kampus P, Zilmer M, Zilmer K, Eha J, Unt E, Lieberg J, Kals J. Association of osteoprotegerin with aortic stiffness in patients with symptomatic peripheral artery disease and in healthy subjects. Am J Hypertens. 2010;23:586–591. doi: 10.1038/ajh.2010.38. [DOI] [PubMed] [Google Scholar]
- 26.Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang SJ, Vasan RS, Mitchell GF. Framingham Heart Study 100k Project: Genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet. 2007;8(Suppl 1):S3. doi: 10.1186/1471-2350-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox N, Nicolae D, Dolan ME, Gamazon E. SCAN SNP and CNV Annotation Database. doi: 10.1093/bioinformatics/btp644. http://www.scandb.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobreira NLM, Cirulli ET, Avramopoulos D, Wohler E, Oswald GL, Stevens EL, Ge DL, Shianna KV, Smith JP, Maia JM, Gumbs CE, Pevsner J, Thomas G, Valle D, Hoover-Fong JE, Goldstein DB. Whole-genome sequencing of a single proband together with linkage analysis identifies a mendelian disease gene. Plos Genet. 2010;6 doi: 10.1371/journal.pgen.1000991. e1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.