Abstract
HIV-1 infection of the brain commonly leads to cognitive impairments (CI). In its most severe form, HIV-associated dementia is associated with advanced immune suppression and debilitating loss of memory, behavioral, and motor functions. Despite significant research activities, diagnosis remains one of exclusion. Bioimaging, neuropsychological testing, and viral and immune biomarkers serve to support but not define a diagnosis of HIV-1 associated CI. This is timely and required as brain injury triggered by HIV-1 can be controlled, in part, by antiretroviral medicines. The recent development of proteomics has opened new ways to study viral-host interactions which may provide new insight into treatment and disease monitoring. To this end we developed a proteomics platform for HIV-1 associated CI biomarker discovery was used it to perform a pilot study for sera-associated HIV-1 associated dementia (HAD) proteins. A 2-dimensional electrophoresis (2-DE) map of a serum proteome was focused on differentially expressed proteins. Differential expression of 2 proteins was validated by Western blot tests identifying afamin and ceruloplasmin as potential biomarkers for CI associated with advanced HIV-1 infection.
Keywords: 2D DIGE, biomarker, cognitive impairment, HIV-1, proteomics
1 Introduction
HIV-1 enters the brain at an early stage of infection and remains persistent through disease. Cognitive, motor, and behavioral impairments develop in 20 to 50% of infected individuals [1, 2]. In its most severe form HIV-associated dementia (HAD) [3–5] occurs at the terminal stages of disease. Introduction of highly active antiretoviral therapy (HAART) had a profound effect on slowing disease progression, increasing survival, and overall decreasing the number of new incidences of HAD [2]. Nevertheless, the rate of HIV-1 infected patients with CI ranging from mild to the most severe remains the same [2] and the prevalence of HAD has increased due to increased survival of these individuals [1, 6–9]. HAD also remains as a significant cause of morbidity in HIV-1 infected individuals [10, 11]. This suggests that HAART provides only partial protection against neurological damage in HIV-infected people [12]. Current diagnosis and identification of HAD is based on neuropsychological tests and exclusion of other potential causes such as opportunistic infections, tumor etc. Laboratory measures of CI progression, although valuable, are not diagnostic. Thus there is a need for more accurate and reliable markers to monitor HAD progression [13].
A number of recent works have uncovered a series of biomarkers linked to CI in HIV-1 infected people and have been linked, in large measure, to viral infection, immune function, and neuroinflammation [14–18]. The most recent approach in search of biomarkers for HAD is proteomics. In our own proteomic analysis of cerebrospinal fluid (CSF) from HAD patients [19, 20] we demonstrated several differentially expressed proteins which can be potential biomarkers. Although CSF surrounding the brain and spinal cord can be reflective of ongoing pathological processes [21, 22] and provide valuable information about the central nervous system (CNS) in disease [23–27], evaluations of the CSF proteome pose a challenge for a number of reasons. First, CSF protein concentration range from 0.2 to 0.8 mg/ml and is approximately 100 times less than what is present in sera [28]. Second, albumin, transferrin and immunoglobulins constitute more than 70% of the protein CSF content which can and often does confound detection [29, 30]. Third, biomarkers are commonly present in low abundance and because of the high turnover and dynamic nature of CSF detection may be lost [31]. CSF is potentially a good source of biomarkers for HAD, as it is an ultrafiltrate of plasma and is a drainage route for waste products of brain metabolism. Therefore, because of CSF direct excretion into the capillary blood vessels across the blood-brain barrier, we posit that sera could contain proteins that will be useful for biomarker discovery in HAD and for characterizing ongoing neuropathological processes [32–36]. Therefore, proteins reflecting changes outside the brain may be also relevant for prediction and/or disease prognosis of neurological disorders. [37]. These, taken together, suggest that alternative body fluids need be considered for biomarker detection paradigms, one of which is sera.
2-dimensional electrophoresis (2 DE) is a technique that has become a fundamental approach for studies of complex protein mixtures, mostly in connection with mass spectrometry for identification of differentially expressed proteins. 2D Difference Gel Electrophoresis (2D DIGE) with minimal labeling enables running on a single gel two samples labeled with different fluorescent dye along with internal standard (a mixture of all samples used in the experiment, labeled with a third dye). The same internal standard is applied across multiple gels and used for spot matching and normalization of relative abundance of proteins between all gels included in the experiment. Another method used for labeling proteins before 2D DIGE is saturated labeling, which uses two dyes, one for labeling of analyzed samples, the second for labeling of internal standard. One sample and internal standard are run on the same gel and the internal standard is used for signal normalization between samples. In our study we used saturated labeling to detect differences in protein profile between sera from HIV-1 infected patients with and without CI. We propose that levels of afamin and ceruloplasmin in conjunction with other measures can be indicative of developing cognitive impairment due to HIV-1 infection of the brain.
Overall, our works suggest that multiple protein fingerprints would be required to assess disease onset and progression [38, 39]. These combination biomarker levels can be measured relative to baseline for any individual with disease or suspected disease and the measurements combined with psychological and brain imaging tests [40]. Discovery of biomarkers, which could be used to predict dementia and monitor disease progression, is important for the development of early and effective treatments designed to maintain normal cognition and quality of life [14, 41].
2 Materials and Methods
2.1 Sera samples used in the study
Sera samples were provided from HIV-1 infected individuals with (HAD) or without (ND) HIV-1-associated dementia by the National NeuroAIDS Tissue Consortium (NNTC, https://web.emmes.com/study/hbb/) under Request# R101. Sample classification was based on clinical diagnosis performed by NNTC participating centers according to diagnostic criteria recommended at the time. We acknowledge the evolving criteria for HIV-associated neurocognitive disorders (HAND) [42], however in this manuscript we refer to patient classification (HAD and ND) originally provided to us by NNTC. No other criteria (age, race, gender, T-cell count, viral load, etc.) were applied for sample selection. Samples were shipped frozen to the University of Nebraska Medical Center (UNMC) on dry ice. Use of sera samples in this study has been approved by UNMC Institutional Review Board (#196-05-EX). We used 14 samples in this study, seven from each group.
2.2 Immunodepletion (partitioning)
Twelve most abundant serum proteins: serum albumin, IgG, fibrinogen, transferrin, IgA, IgM, haptoglobin, apo A-I, apo A-II, α1-antitrypsin, α1-acid glycoprotein, α2 – macroglobulin were removed by immunodepletion. We used the ProteomeLab IgY-12 High Capacity Proteome Partitioning Kit (Beckman Coulter, Fullerton, CA), which included a HPLC affinity column LC10 (12.7 × 79.0 mm) with a capacity of 0.25 ml of human serum per cycle and optimized buffers for sample loading, washing, eluting and regenerating. To pre-clear and delipidate samples before chromatography, serum samples were centrifuged at 18000 × g at 4°C for 15 minutes. The middle layer of pre-cleared serum was collected, diluted (0.25 ml of serum and 0.375 ml of dilution buffer, 10 mM Tris-HCL pH 7.4, 0.15 M NaCl), and filtered through 0.45 μm spin filters. Diluted samples (0.625 ml) were loaded at flow rate 0.5 ml/minute. Flow-through fractions were collected and then concentrated using Amicon Ultra-15 centrifugal filters (Millipore, Billerica, MA). Eluted fractions were then obtained with 0.1 M glycine-HCL pH 2.5, neutralized with 0.1 M Tris-HCl pH 8.0 and equilibrated with 10 mM Tris-HCL pH 7.4, 0.15 M NaCl at a flow rate 2 ml/min. Eluted fractions were saved for further studies.
2.3 Sample preparation and protein labeling
After sample partitioning, proteins were purified by precipitation using 2D Clean-Up Kit (GE Healthcare, Piscataway, NJ) according to the manufacturer’s instructions. Precipitated protein pellets were re-suspended in 0.1% Triton X-100 and protein concentration was determined (Bio-RadDc Protein Assay, Bio-Rad Laboratories, Hercules, CA). Protein samples were dried in a speedvac and re-suspended in 30 mM Tris-HCl pH 8.0, 7 M urea, 2 M thiourea, 4% CHAPS. For analytical runs, 5 μg of protein was reduced with 2 nM TCEP [tris-(2-carboxyethyl) phosphine hydrochloride] for 1 hr at 37°C and labeled with 4 nM of Cy5 saturation dye for 30 min at 37°C. Then one volume of sample buffer (7 M urea, 2 M thiourea, 4% CHAPS, 2% Pharmalyte, 130 mM DTT) was added to stop the reaction. To prepare internal standards, 5 μg of each sample was mixed together, speed vacuum dried, re-suspended in 30 mM Tris-HCl pH 8.0, 7 M urea, 2 M thiourea, 4% CHAPS, reduced with TCEP for 1 hr at 37°C (2 nM of TCEP per 5 μg of protein) and labeled with Cy3 saturation dye for 30 min at 37°C (4 nM of Cy3 per 5 μg protein). Next, sample buffer (7 M urea, 2 M thiourea, 4% CHAPS, 2% Pharmalyte, 130 mM DTT) was added to the final volume 0.45 ml.
Another labeling reaction was performed to prepare material for preparative gel. Aliquots taken from all samples were pooled and labeled with Cy3 saturation dye for preparative labeling. The sample pool containing 220 μg of protein was speed vacuum dried and pellet was re-suspended in 0.25 ml of 30 mM Tris-HCl pH 8.0, 7 M urea, 2 M thiourea, 4% CHAPS. Proteins were reduced with TCEP (2 nM per 5 μg proteins) at 37°C for 30 min and labeled with Cy3 (4 nM per 5 μg proteins) for 30 min at 37°C. Sample buffer (7 M urea, 2 M thiourea, 4% CHAPS) was added to the final volume 445.5 μl. Then 4.5 μl of Pharmalytes pH 3–10 (1% final concentration) and 4.5 mg of DTT was added (final concentration of 130 mM).
2.4 2D electrophoresis and gel analysis
The first dimension was performed in 24 cm long Immobiline DryStrips™ gels with linear immobilized gradient pH 3–10 (GE Healthcare, Piscataway, NJ). For one analytical gel, 5 μg of sample labeled with Cy5 was mixed with 5 μg of internal standard labeled with Cy3 and rehydration buffer (7 M urea, 2 M thiourea, 2% CHAPS, 60 mM DTT, 1% Pharmalyte pH 3–10) was added to final volume of 450 μl. Samples were loaded on gels by overnight rehydration. Isoelectrofocusing (IEF) step was carried out with an IPGphor II™ apparatus (GE Healthcare, Piscataway, NJ), at a constant temperature of 20°C, with a total 45 kVh (500 V for 0.5 kVh, gradient to 1000 V for 0.8 kVh, gradient to 8000 V for 13.5 kVh, 8000 V for 30.2 kVh). For the preparative run, 220 μg of protein labeled with Cy3 was loaded on one immobiline strip by overnight rehydration. IEF step was carried out at 65 kVh (500 V for 0.5 kVh, gradient to 1000 V for 0.8 kVh, gradient to 8000 V for 13.5 kVh, 8000 V for 50.2 kVh). Before the second dimension separation, strips were incubated with an equilibration solution (50 mM Tris-HCL pH 8.8, 6 M urea, 30% glycerol, 2% sodium dodecyl sulfate, 0.01% bromophenol blue) containing 100 mM DTT for 15 min. Then the strips were loaded on the top of 10 – 20% gradient polyacrylamide gels and fixed with 0.5% agarose. Second dimension was carried out with an Ettan Daltsix Electrophoresis System™ (GE Healthcare, Piscataway, NJ) at 20°C. Power was held constant to 5W per gel for the initial 30 min and 17W per gel until bromophenol blue reached the gel bottom. Typhoon 9410 Variable Mode Imager (GE Healthcare Piscataway, NJ) was used for gel scanning. Scans were taken at resolution of 100 μm with emission wavelengths of 520 and 620 nm, optimal for Cy3 and Cy5 dyes respectively. Image analysis was performed using DeCyder 2D 6.5™ software (GE Healthcare, Piscataway, NJ). All 14 analytical gels were processed with DIA module and matched in BVA module of DeCyder™ software. The BVA module t-test was used to determine differences between ND and HAD. Differentially expressed spots with p<0.05 were selected for protein identification and matched with the preparative gel. Selected spots were picked from preparative gel by automatic Ettan™ Spot Picker (GE Healthcare, Piscataway, NJ) with a 2 mm diameter tip.
2.5 Protein identification
Gel pieces excised from 2-DE gels were washed for 1 hr at room temperature with 200 μl 50% acetonitrile (ACN)/50 mM NH4HCO3, dried, then incubated at 37°C overnight in 0.1 μg trypsin (Promega, Madison, WI) plus 50 mM NH4HCO3. After incubation, resulting peptides were extracted with 60% ACN/0.1% trifluoroacetic acid (TFA), dried, and re-suspended in 0.5% TFA. Next, samples were purified using C18 Zip Tips® (Millipore, Billerica, MA) according to manufacturer’s procedure and re-suspended in 0.1% formic acid in water prior to mass spectrometric analysis. Protein identification was performed as described previously [19]. Peptides were analyzed using ESI-LC-MS/MS system (ProteomeX System with LCQDecaPlus, ThermoElectron, Inc., San Jose, CA) in a nano-spray configuration. For fractionation, a microcapillary RP-C18 column (New Objectives, Woburn, MA) was used. The spectra were searched using Sequest™ search engine in BioWorks 3.2 software (ThermoElectron Inc., San Jose, CA). Threshold for Dta generation parameters was 10000; precursor ion mass tolerance was set at 1.4, peptide tolerance at 2.00 and fragment ions tolerance at 1.00. Database NCBI.fasta from ftp.ncbi.nih.gov was used with two missed cleavage sites allowed and at least two peptides were required for protein identification.
2.6 One-dimensional Electrophoresis (1-DE) and Western Blot Analysis
1-DE SDS PAGE electrophoresis was performed using NuPAGE gel system (Invitrogen Corp., Carlsbad, CA) in 4–12% gradient gels under reducing conditions. For Western blot analyses, 3 μg of serum protein immunodepleted on an IgY column were loaded per lane. Electrophoresis followed by transfer and immunodetection was performed as previously described [43]. The following primary antibodies were used: rabbit anti-afamin (GenWay Biotech Inc., San Diego, CA), chicken anti-ceruloplasmin (ICL Inc., Newberg, OR), and chicken anti-complement C3 (GenWay Biotech Inc., San Diego, CA). Secondary antibodies included HRP-conjugated goat anti-rabbit and donkey anti-chicken IgY (Jackson Immunoresearch, West Grove, PA). All antibodies were used at concentrations recommended by the respective manufacturers. The SuperSignal West Pico™ Chemiluminescent Substrate (Pierce, Rockford, IL) for HRP was used and signal was recorded on X-ray films. Images were scanned and signal was evaluated using Image Quant™ software (GE Healthcare, Piscataway, NJ).
3 Results and discussion
Because of CSF direct excretion into the capillary blood vessels across the blood-brain barrier, we posited that sera would contain proteins useful for biomarker discovery in HAD and for characterizing ongoing neuropathological processes [32–36]. Therefore, proteins reflecting changes outside the brain may be also relevant for prediction and/or disease prognosis of neurological disorders. [37].
Clinically classified sera samples were obtained from the NNTC repository. All samples analyzed by 2D DIGE and western blots were from HIV-1 infected individuals with (7 samples) or without HAD (7 samples). All samples were subjected to partitioning using IgY immunoaffinity HPLC column and resulting flow-through fractions were immunodepleted from 12 most abundant proteins: serum albumin, IgG, IgA, IgM, fibrinogen, transferring, haptoglobin, apolipoprotein A-I, apolipoprotein A-II, α1-antitrypsin, α1-acid glycoprotein, α2-macroglobulin. In the next step, immunodepleted samples were concentrated and prepared for isoelectric focusing step using 2D Clean-up Kit. Protein yield from an initial 1 ml of serum ranged between 0.78 and 1.9 mg.
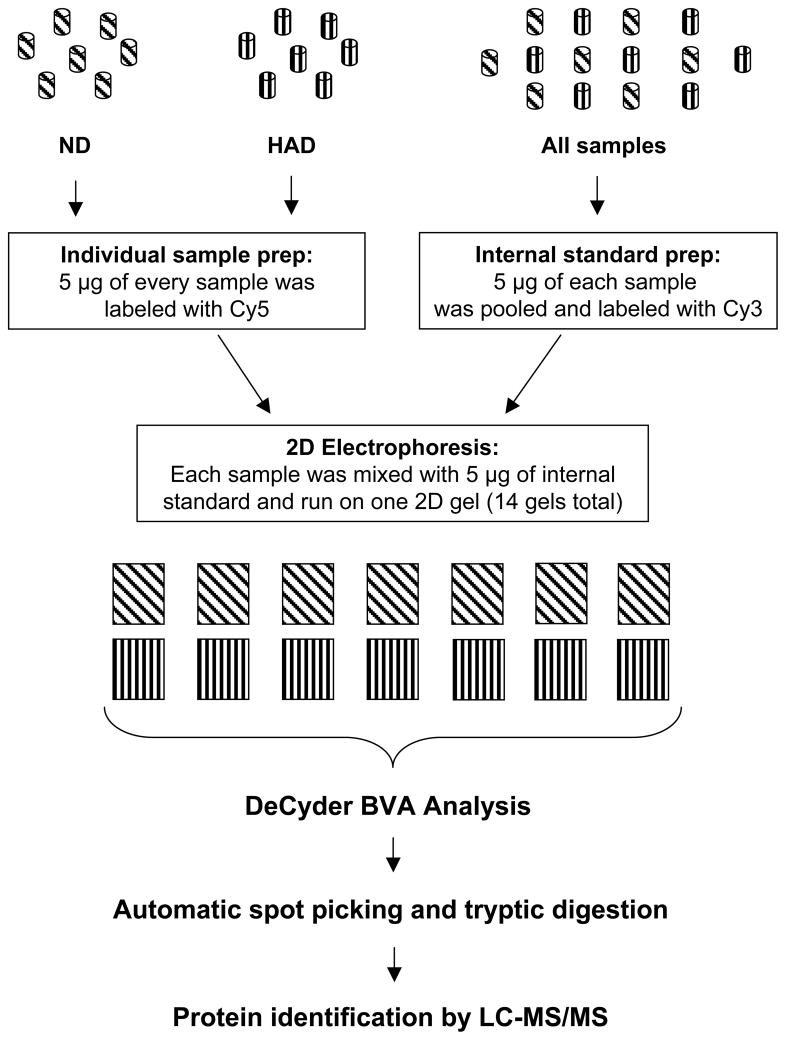
We used 2D DIGE with saturated labeling as a method of protein profiling. Figure 1 shows our experimental design. The internal control, which enabled statistical analysis across all gels, consisted of a mixture of equal amounts of protein derived from all 14 samples, pooled, labeled with Cy3, divided into 12 equal portions, and added to all tested samples. Although 1 ml of serum can provide enough protein for 2D DIGE with minimal labeling, we decided to use saturated labeling approach to avoid complications associated with spot alignment. In minimal labeling 2D DIGE, only 5% of each protein is labeled with Cy dye and it is shifted vertically due to 500 Da mass difference between labeled and unlabeled fractions of the same protein(s). As this might not be a problem in analysis of relatively low complexity samples, serum contains approximately 3000 proteins. We have experienced this difficulty in analyzing proteomes of cerebrospinal fluids [19].
Figure 1.
Experimental design of 2D DIGE analysis of serum samples using saturation labeling method. Seven sera samples from HIV patients without dementia (ND) and 7 from demented patients (HAD) were used. Before labeling, 12 most abundant proteins were removed by immunoaffinity chromatography.
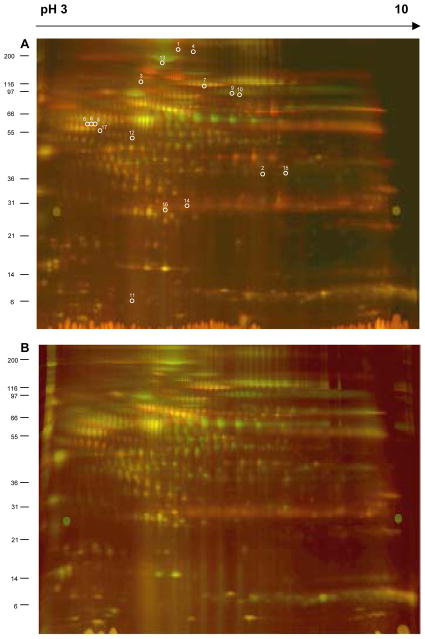
After electrophoresis gels were scanned and images analyzed using DeCyder™ software. The DIA module was used for spot detection and normalization. In spot detection, we limited the maximum number of spots to 2500 per gel and detected between 2180 and 2500 spots per gel in the 14 analytical gels. Representative gels for non-demented and demented patients are shown in Fig. 2. Our level of spot detection was comparable to the levels reported in the literature. For example, Nagalla et. al reported 1816 and 1842 spots detected in analyzed sera [44] and Corzett and co-authors reported 2511 in plasma samples [45]. Increasing limit of spot detection did not result in detection of more protein spots in our experiments. Because some proteins were represented by more than one spot and in some spots more than one protein was identified, we conclude that the detection limit for 2D DIGE analysis of serum/plasma samples will be around this level. The Human Plasma Protein Project reported identification of 3015 proteins, thus other proteomic approaches may be necessary for more complete profiling. Certainly, additional steps of pre-2DE fractionation may be one such approach. It has to be noted that we did not analyze the albuminome fraction, thus we are not able to assess at this time how many proteins were removed from our samples during immunodepletion of 12 most abundant proteins.
Figure 2.
Representative gels from 2D DIGE experiment, which included seven sera samples from patients without dementia (ND) and seven with dementia (HAD). Samples labeled with fluorescent dyes were resolved in pH gradient from 3 to 10 (first dimension IEF in 24 cm immobiline gels) and in gradient polyacrylamide gels 10–20% (second dimension SDS PAGE). After electrophoresis gels scanned at resolution of 100 μm and wavelength of 520 and 620 nm, optimal for Cy3 and Cy5 dyes respectively. A=ND; B=HAD. White circles and numbers shown in gel A correspond to Spot No in Table 1.
The next step of data analysis was performed using BVA module of DeCyder™ software which performed spot matching across all gels. The gel with the highest number of spots detected (2500), was selected to be a Master Gel and remaining 13 gels were aligned with it. Among these gels, 1379 to 1564 spots were matched. Fourteen gels were included in one workspace for proper spot matching between gels and statistical significance of changes in relative abundance observed between groups (ND and HAD) was evaluated by the BVA module t-test. We found 81 spots with statistically significant differences (p<0.05) in relative abundance. These spots were individually evaluated for their quality based on shape and volume, resulted in selection of 30 spots for protein identification by LC-MS/MS.
In 2D DIGE with saturated labeling, analytical gels do not provide enough material for protein identification using LC-MS/MS. Therefore, we prepared 220 mg of pooled proteins from all samples and ran a preparative gel. Proteins were labeled with Cy3 under saturated conditions. This approach removed a difficulty of matching labeled labeled proteins, thus being larger by 500 Da with those not labeled. Spots of interest were excised from the preparative gel by automated spot picking and used for protein identification.
Our primary method of protein identification was nano-LC-MS/MS using LCQDecaPlus mass spectrometer interfaced with Surveyor HPLC system. Elution gradient was 1.44% of acetonitrile per min. Resulting data were submitted to NCBI database search using the Sequest algorithm. Details of search parameters are described in materials and methods. Search using reversed database was also performed and yield no significant hits (data not shown). Results are presented in Table 1. Negative values of DIGE Index indicates fold decrease in spot volume comparing to control (Cy3 labeled) in samples from individuals with HAD. Consequently, positive DIGE index indicated up-regulation in samples from ND individuals. Positive, high confidence identification of proteins in 17 spots out of 30 analyzed was obtained. 11 spots with negative DIGE Index (Table 1) corresponded to proteins down-regulated in HAD. In one spot, two proteins were identified and in six instances some proteins were identified in more than one spot.
Table 1.
Identification of differentially expressed proteins in sera from HIV1 infected patients with and without dementia
| Spot No | Protein name | MW | Swiss-Prot | NCBI | T test | DIGE Index |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | complement C3 precursor | 187162.3 | P01024 | 116594 | 0.016 | −5.24 |
|
| ||||||
| 2 | L Chain L, Crystal Structure Of Tissue Factor | 23499.0 | 1JPS_L | 18655500 | 0.014 | −2.4 |
| complement C3 precursor | 187162.3 | P01024 | 116594 | |||
|
| ||||||
| 3 | afamin precursor | 69068.8 | NP_001124 | 4501987 | 0.023 | −2.25 |
|
| ||||||
| 4 | complement C4-A | 51033.7 | CAA30403 | 758073 | 0.0044 | −2.14 |
| complement factor I | 37661.4 | Q03591 | 543981 | |||
|
| ||||||
| 5 | serpin peptidase inhibitor, clade A, member 3 precursor | 47650.6 | NP_001076 | 50659080 | 0.006 | −2.04 |
| alpha-2-HS-glycoprotein | 39324.4 | NP_001613 | 4502005 | |||
|
| ||||||
| 6 | serpin peptidase inhibitor, clade A, member 3 precursor | 47650.6 | NP_001076 | 50659080 | 0.011 | −2.03 |
| alpha-2-HS-glycoprotein | 39324.4 | NP_001613 | 4502005 | |||
|
| ||||||
| 7 | complement component 4 binding protein | 67032.9 | NP_000706 | 4502503 | 0.0075 | −2.01 |
|
| ||||||
| 8 | serpin peptidase inhibitor, clade A, member 3 precursor | 47650.6 | NP_001076 | 50659080 | 0.022 | −1.97 |
| alpha-2-HS-glycoprotein | 39324.4 | NP_001613 | 4502005 | |||
|
| ||||||
| 9 | complement C3 precursor | 187162.3 | P01024 | 116594 | 0.0016 | −1.92 |
|
| ||||||
| 10 | complement C3 precursor | 187162.3 | P01024 | 116594 | 0.047 | −1.62 |
|
| ||||||
| 11 | kininogen | 47883.0 | NP000884 | 4504893 | 0.049 | −1.32 |
|
| ||||||
| 12 | CO4A_HUMAN Complement C4-A precursor(Acidic complement C4) | 192769.5 | P0C0L4 | 81175238 | 0.04 | 1.2 |
| CFAI_HUMAN Complement factor I precursor (C3B/C4B inactivator) | 65720.0 | P05156 | 116133 | |||
|
| ||||||
| 13 | ceruloplasmin precursor | 122204.3 | NP000087 | 4557485 | 0.028 | 1.56 |
|
| ||||||
| 14 | complement C3 precursor | 187162.3 | P01024 | 116594 | 0.041 | 1.67 |
|
| ||||||
| 15 | complement factor D precursor | 27004.0 | P00746 | 3915626 | 0.049 | 1.71 |
|
| ||||||
| 16 | ceruloplasmin precursor | 122204.3 | NP000087 | 4557485 | 0.013 | 2.09 |
|
| ||||||
| 17 | NBHUA2 leucine-rich alpha-2-glycoprotein | 34346.1 | NBHUA2 | 72059 | 0.042 | 2.72 |
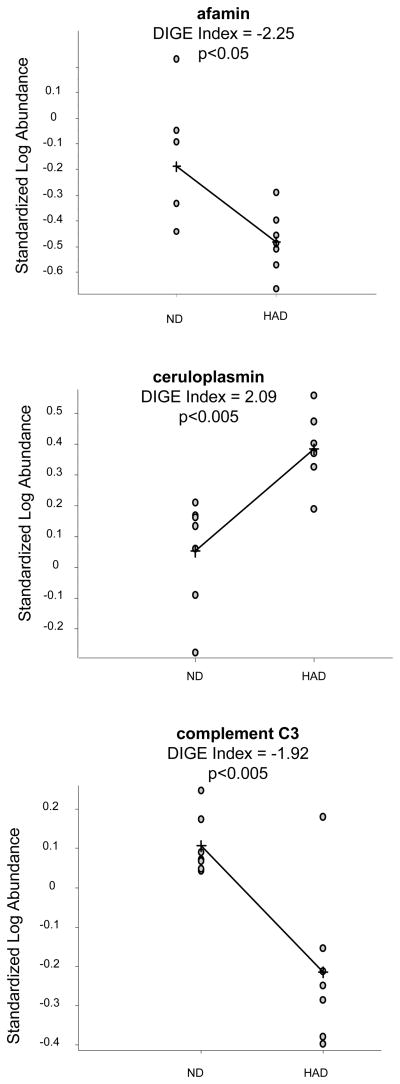
Figure 3 shows comparison of results obtained for those proteins from DIGE analysis using the BVA module of DeCyder software. Quantification using the BVA module is based on standardized abundances derived from normalized spot volumes and gives an average ratio of spots volumes between groups (DIGE Index).
Figure 3.
Results of analysis of spots in which afamin, ceruloplasmin and complement C3 were identified. DIGE Index (average ratio of spot volumes) was calculated in BVA module of DeCyder™ software. Negative values indicate down-regulation in HAD thus positive values indicate up-regulation in HAD. Statistical analysis was performed using T-test in BVA module. p<0.05 was considered statistically significant.
We selected three differentially expressed proteins: afamin, ceruloplasmin and complement C3 to be further validated using western blot analysis. For this experiment we used the same 14 immunodepleted sera samples. Resulting bands were scanned and relative intensities of chemiluminescence signals were calculated using Image Quant 5.2 (GE Healthcare, Inc.) software. Figure 4 shows a boxplot analysis to assess the distribution of results. The differences between HAD and ND groups were statistically significant (p<0.05) for afamin and ceruloplasmin, but not for complement C3 (p>0.05). This analysis confirmed down-regulation of afamin (DIGE Index −2.25 and p<0.05) and ceruloplasmin (DIGE Index 2.9 and p<0.013).
Figure 4.
Box plots of statistical analysis for values obtained from Western blot images quantified in Image Quant™ software (GE Healthcare). Box plots indicate normal distribution of proteins. p values indicate statistical significance of protein level differences between ND and HAD for respective proteins; p<0.05 is considered statistically significant. ND – non-demented; HAD – demented.
Afamin is a vitamin E binding protein which together with alpha-fetoprotein, serum albumin, and vitamin D binding protein belongs to albumin gene family [46] and is predominantly expressed in liver [47]. It has been proposed that the neuroprotective effect of afamin involves two mechanisms. One is the synergy with vitamin E which enhances the survival of cortical neurons exposed to either oxidative stress (H2O2) or beta-amyloid peptide [48]. The other, yet unknown mechanism, is independent from vitamin E [48]. Although afamin has been found to be quite abundant in CSF (0.28+/−0.16 μg/mL) [49] we did not find this protein to be differentially expressed in CSF of cognitively impaired HIV-1-infected individuals [19]. It is also not clear whether afamin present in CSF entirely originates from serum or is also expressed by any cell type within central nervous system.
2D DIGE showed that ceruloplasmin was up-regulated in sera samples from individuals with HAD (Table 1, Fig. 3B) and this differential expression was confirmed by Western blot analysis (Fig. 4). Ceruloplasmin is a 132 kDa copper containing protein mostly synthesized in the liver however it is also synthesized in the brain [50]. It has ferroxidase activity oxidizing Fe+2 to the less toxic Fe+3 form, thus contributing to iron homeostasis. Other functions include copper transport, coagulation, angiogenesis, defense against oxidant stress by scavenging superoxide radicals and oxidation of low-density lipoproteins [51]. Ceruloplasmin also contains 95% of the copper circulating in plasma [52]. Low levels of ceruloplasmin and its ferroxidase activity leads to pathological iron overload in the brain and other organs and has been linked to neurodegenerative disorders such as Alzheimer’s Disease [53]. The elevated iron concentration is associated with increased lipid peroxidation in the brains of aceruloplasminemia patients. Increased levels of ceruloplasmin in sera of patients with HAD could be linked to ongoing inflammation. It has been also proposed that ceruloplasmin plays a pathological role in chronic inflammation and its serum level is increased in patients with rheumatoid arthritis [54] and arteriosclerosis [55]. The exact role of elevated levels of ceruloplasmin in sera of patients with HAD is not clear at this moment and will require further studies.
Sera complement C3 was found in multiple spots resembling its distribution in CSF samples (Table 1). The difference between sera and CSF samples was that in this study the DIGE Index for protein spots containing complement C3 ranged from −5.24 (down-regulation in HAD) to 1.67 (up-regulation in ND) while in CSF samples all spots containing complement C3 showed down-regulation. Observed difference, regardless of up- or down-regulation were statistically significant. Therefore, it was not unexpected when Western blot analysis did not confirm significant down-regulation in sera samples. In addition, these data show that composition of forms derived from complement C3 precursor due to processing and/or degradation as well as accumulation might be different in sera than in CSF.
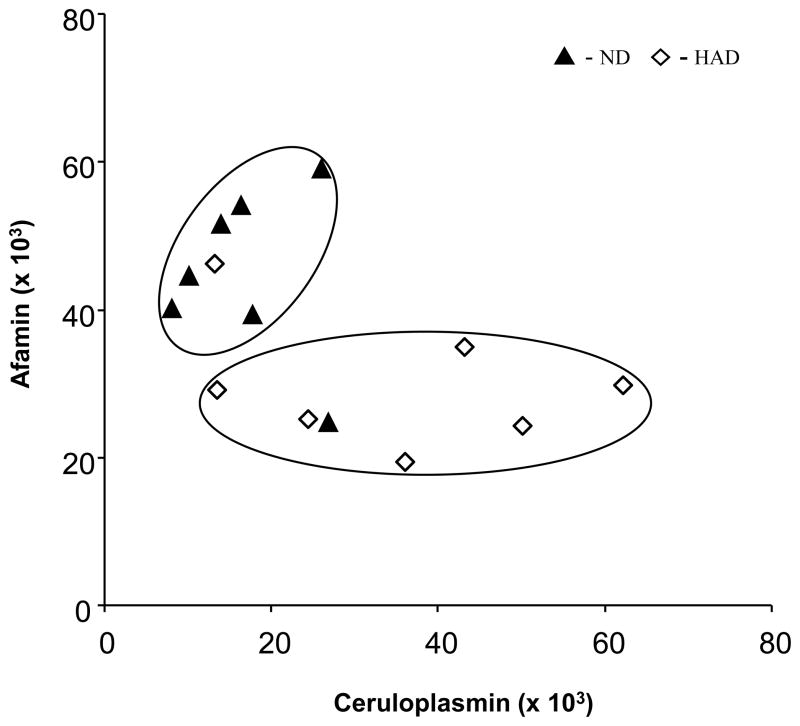
The two dimensional plot of relative intensities of chemiluminescence signals for afamin and ceruloplasmin presented in Fig. 5 shows that 12 of 14 samples used in this study can be correctly classified as either ND or HAD. Two separate groups encircled on a graph contain one outlier per 7 samples (~15%) from ND and HAD groups. These results also indicate afamin as the potential primary predictor of HAD. Note that using a value below 38,000 as “test positive” for HAD, one has sensitivity: Prob(T+ |D+) = 6/7 and specificity: Prob(T− | D−) = 6/7. Addition of ceruloplasmin did not add to the prediction model. It was confirmed using a logistic regression model of the probability of HAD as a function of protein levels. It is important to note that presented sensitivity and specificity values are highly variable due to small sample size and therefore final conclusions about utility of afamin and ceruloplasmin as diagnostic biomarkers cannot be made at this time. Besides larger number of samples, future studies should also include additional parameters for sample selection which are not only directly related to HIV-1 infection, but also those related to concurrent infections, e.g. chronic hepatitis C (HCV) and other diseases. Nevertheless, we show for the first time differences in levels of afamin and ceruloplasmin in sera of HAD and ND individuals. Whether observed changes in proteomes are linked to HAD directly or are reflective of other conditions associated with HAD such as brain inflammation remains an open question.
Figure 5.
Classification of samples based on afamin and ceruloplasmin levels determined by densitometry measurements of Western blots films. ND – non-demented; HAD – demented. s
4 Concluding remarks
Proteomic profiling of sera presented in this study is a continuation of our previously published profiling of CSF from HIV-1 infected individuals with or without cognitive impairments [16]. This current study is a first attempt to find biomarkers for HIV-1-associated cognitive impairment in sera. Our profiling approach using 2D DIGE (saturated labeling) to analyze 14 samples (7 in each group) after immunodepletion of 12 most abundant proteins yielded 80 protein spots which were matched in a BVA module of DeCyder 6.1 and showed statistical significance in relative abundance. Only 17 protein spots yielded protein identification with high confidence. Eventually, differential expression of only 2 proteins: afamin, ceruloplasmin was validated by Western blot analysis. These two proteins may be used as potential biomarkers, however, in any event their utility will certainly require a larger sample cohort. The small number of differentially expressed proteins also indicates that further proteomic analysis is necessary and should involve at least two avenues. First, would be probe further into the proteome. Second, would be an investigation of differences in post-translational modifications. This work provides a foundation for the role of differentially expressed proteins in HIV-1-associated cognitive impairment biomarker discovery. Nevertheless, future diagnosis of neurodegenerative disorders will combine laboratory tests using CSF and sera samples with other methods such as neuroimaging and psychiatric/psychological evaluations.
Supplementary Material
Acknowledgments
The authors thank Ms. Robin Taylor for outstanding administrative and computer support and for her effort in helping us put this manuscript together in a timely manner. This work was funded by NIH grants 1R21 MH075662-01, 20 RR15635 from the COBRE Program of the National Center for Research Resources.
Abbreviations
- 1-DE
one-dimensional electrophoresis
- 2D DIGE
2D Difference Gel Electrophoresis
- 2-DE
2-dimensional electrophoresis
- CI
cognitive impairment
- CSF
cerebrospinal fluid
- HAART
highly active antiretoviral therapy
- HAD
HIV-1 associated dementia
- HAND
HIV-associated neurocognitive disorders
- ND
without HIV-1 associated dementia
- NNTC
National NeuroAIDS Tissue Consortium
- UNMC
University of Nebraska Medical Center
Literature
- 1.Bottiggi KA, Chang JJ, Schmitt FA, Avison MJ, et al. The HIV Dementia Scale: predictive power in mild dementia and HAART. J Neurol Sci. 2007;260:11–15. doi: 10.1016/j.jns.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 3.Navia BAaRWP. In: The Neurology of AIDS. Gendelman HE, SAL, Epstein L, Swindells S, editors. Chapman and Hall; New York: 1998. pp. 229–240. [Google Scholar]
- 4.Wilkie FL, Goodkin K, Khamis I, van Zuilen MH, et al. Cognitive functioning in younger and older HIV-1-infected adults. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S93–S105. doi: 10.1097/00126334-200306012-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 6.Nath A, Sacktor N. Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Curr Opin Neurol. 2006;19:358–361. doi: 10.1097/01.wco.0000236614.51592.ca. [DOI] [PubMed] [Google Scholar]
- 7.Nath A, Schiess N, Venkatesan A, Rumbaugh J, et al. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- 8.Wojna V, Skolasky RL, Hechavarria R, Mayo R, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12:356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- 9.McArthur JC, Haughey N, Gartner S, Conant K, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 10.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 11.McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–150. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- 12.Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price RW, Epstein LG, Becker JT, Cinque P, et al. Biomarkers of HIV-1 CNS infection and injury. Neurology. 2007;69:1781–1788. doi: 10.1212/01.wnl.0000278457.55877.eb. [DOI] [PubMed] [Google Scholar]
- 14.Bandaru VV, McArthur JC, Sacktor N, Cutler RG, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gisslen M, Hagberg L, Brew BJ, Cinque P, et al. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 16.Sui Z, Sniderhan LF, Schifitto G, Phipps RP, et al. Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J Immunol. 2007;178:3226–3236. doi: 10.4049/jimmunol.178.5.3226. [DOI] [PubMed] [Google Scholar]
- 17.Dunfee RL, Thomas ER, Gorry PR, Wang J, et al. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc Natl Acad Sci U S A. 2006;103:15160–15165. doi: 10.1073/pnas.0605513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurado A, Rahimi-Moghaddam P, Bar-Jurado S, Richardson JS, et al. Genetic markers on HIV-1 gp120 C2-V3 region associated with the expression or absence of cognitive motor complex in HIV/AIDS. J NeuroAIDS. 1999;2:15–28. doi: 10.1300/J128v02n02_02. [DOI] [PubMed] [Google Scholar]
- 19.Rozek W, Ricardo-Dukelow M, Holloway S, Wojna V, et al. Cerebrospinal fluid proteomics profiling of HIV-1-infected patients with or at risk for cognitive impairments. J Proteome Res. 2007 doi: 10.1021/pr070220c. in press. [DOI] [PubMed] [Google Scholar]
- 20.Laspiur JP, Anderson ER, Ciborowski P, Wojna V, et al. CSF proteomic fingerprints for HIV-associated cognitive impairment. J Neuroimmunol. 2007 doi: 10.1016/j.jneuroim.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonsen AH, McGuire J, Hansson O, Zetterberg H, et al. Novel panel of cerebrospinal fluid biomarkers for the prediction of progression to Alzheimer dementia in patients with mild cognitive impairment. Arch Neurol. 2007;64:366–370. doi: 10.1001/archneur.64.3.366. [DOI] [PubMed] [Google Scholar]
- 22.Simonsen AH, McGuire J, Podust VN, Davies H, et al. Identification of a novel panel of cerebrospinal fluid biomarkers for Alzheimer’s disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Huang JT, Leweke FM, Oxley D, Wang L, et al. Disease biomarkers in cerebrospinal fluid of patients with first-onset psychosis. PLoS Med. 2006;3:e428. doi: 10.1371/journal.pmed.0030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonteh AN, Harrington RJ, Huhmer AF, Biringer RG, et al. Identification of disease markers in human cerebrospinal fluid using lipidomic and proteomic methods. Dis Markers. 2006;22:39–64. doi: 10.1155/2006/202938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan X, Desiderio DM. Proteomics analysis of human cerebrospinal fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815:179–189. doi: 10.1016/j.jchromb.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Yuan X, Desiderio DM. Proteomics analysis of phosphotyrosyl-proteins in human lumbar cerebrospinal fluid. J Proteome Res. 2003;2:476–487. doi: 10.1021/pr025589a. [DOI] [PubMed] [Google Scholar]
- 27.Khwaja FW, Nolen JD, Mendrinos SE, Lewis MM, et al. Proteomic analysis of cerebrospinal fluid discriminates malignant and nonmalignant disease of the central nervous system and identifies specific protein markers. Proteomics. 2006;6:6277–6287. doi: 10.1002/pmic.200600135. [DOI] [PubMed] [Google Scholar]
- 28.Thompson EJ. Proteins of the Cerebrospinal Fluid: Analysis & Interpretation in the Diagnosis and Treatment of Neurological Disease. Academic Press; 2005. [Google Scholar]
- 29.Ogata Y, Charlesworth MC, Muddiman DC. Evaluation of protein depletion methods for the analysis of total-, phospho- and glycoproteins in lumbar cerebrospinal fluid. J Proteome Res. 2005;4:837–845. doi: 10.1021/pr049750o. [DOI] [PubMed] [Google Scholar]
- 30.Wittke S, Mischak H, Walden M, Kolch W, et al. Discovery of biomarkers in human urine and cerebrospinal fluid by capillary electrophoresis coupled to mass spectrometry: towards new diagnostic and therapeutic approaches. Electrophoresis. 2005;26:1476–1487. doi: 10.1002/elps.200410140. [DOI] [PubMed] [Google Scholar]
- 31.Terry DE, Desiderio DM. Between-gel reproducibility of the human cerebrospinal fluid proteome. Proteomics. 2003;3:1962–1979. doi: 10.1002/pmic.200300463. [DOI] [PubMed] [Google Scholar]
- 32.Carrette O, Demalte I, Scherl A, Yalkinoglu O, et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics. 2003;3:1486–1494. doi: 10.1002/pmic.200300470. [DOI] [PubMed] [Google Scholar]
- 33.Puchades M, Hansson SF, Nilsson CL, Andreasen N, et al. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer’s disease. Brain Res Mol Brain Res. 2003;118:140–146. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Ruetschi U, Zetterberg H, Podust VN, Gottfries J, et al. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol. 2005;196:273–281. doi: 10.1016/j.expneurol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Jiang L, Lindpaintner K, Li HF, Gu NF, et al. Proteomic analysis of the cerebrospinal fluid of patients with schizophrenia. Amino Acids. 2003;25:49–57. doi: 10.1007/s00726-003-0356-6. [DOI] [PubMed] [Google Scholar]
- 36.Hammack BN, Fung KY, Hunsucker SW, Duncan MW, et al. Proteomic analysis of multiple sclerosis cerebrospinal fluid. Mult Scler. 2004;10:245–260. doi: 10.1191/1352458504ms1023oa. [DOI] [PubMed] [Google Scholar]
- 37.Teunissen CE, Dijkstra C, Polman C. Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol. 2005;4:32–41. doi: 10.1016/S1474-4422(04)00964-0. [DOI] [PubMed] [Google Scholar]
- 38.Hsu HS, Chen TP, Hung CH, Wen CK, et al. Characterization of a multiple epigenetic marker panel for lung cancer detection and risk assessment in plasma. Cancer. 2007 doi: 10.1002/cncr.23001. [DOI] [PubMed] [Google Scholar]
- 39.Brouard S, Mansfield E, Braud C, Li L, et al. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinerman JR, Honig LS. Laboratory biomarkers in Alzheimer’s disease. Curr Neurol Neurosci Rep. 2007;7:381–387. doi: 10.1007/s11910-007-0059-6. [DOI] [PubMed] [Google Scholar]
- 41.Vanacore N, Galeotti F, Maggini M, Raschetti R. Biomarkers in dementia. Arch Neurol. 2007;64:1356. doi: 10.1001/archneur.64.9.1356. [DOI] [PubMed] [Google Scholar]
- 42.Antinori A, Arendt G, Becker JT, Brew BJ, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciborowski P, Enose Y, Mack A, Fladseth M, Gendelman HE. Diminished matrix metalloproteinase 9 secretion in human immunodeficiency virus-infected mononuclear phagocytes: modulation of innate immunity and implications for neurological disease. J Neuroimmunol. 2004;157:11–16. doi: 10.1016/j.jneuroim.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Nagalla SR, Canick JA, Jacob T, Schneider KA, et al. Proteomic analysis of maternal serum in down syndrome: identification of novel protein biomarkers. J Proteome Res. 2007;6:1245–1257. doi: 10.1021/pr060539h. [DOI] [PubMed] [Google Scholar]
- 45.Corzett TH, Fodor IK, Choi MW, Walsworth VL, et al. Statistical analysis of the experimental variation in the proteomic characterization of human plasma by two-dimensional difference gel electrophoresis. J Proteome Res. 2006;5:2611–2619. doi: 10.1021/pr060100p. [DOI] [PubMed] [Google Scholar]
- 46.Lichenstein HS, Lyons DE, Wurfel MM, Johnson DA, et al. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J Biol Chem. 1994;269:18149–18154. [PubMed] [Google Scholar]
- 47.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Heiser M, Hutter-Paier B, Jerkovic L, Pfragner R, et al. Vitamin E binding protein afamin protects neuronal cells in vitro. J Neural Transm Suppl. 2002:337–345. doi: 10.1007/978-3-7091-6139-5_32. [DOI] [PubMed] [Google Scholar]
- 49.Jerkovic L, Voegele AF, Chwatal S, Kronenberg F, et al. Afamin is a novel human vitamin E-binding glycoprotein characterization and in vitro expression. J Proteome Res. 2005;4:889–899. doi: 10.1021/pr0500105. [DOI] [PubMed] [Google Scholar]
- 50.Loeffler DA, LeWitt PA, Juneau PL, Sima AA, et al. Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Res. 1996;738:265–274. doi: 10.1016/s0006-8993(96)00782-2. [DOI] [PubMed] [Google Scholar]
- 51.Das D, Tapryal N, Goswami SK, Fox PL, Mukhopadhyay CK. Regulation of ceruloplasmin in human hepatic cells by redox active copper: identification of a novel AP-1 site in the ceruloplasmin gene. Biochem J. 2007;402:135–141. doi: 10.1042/BJ20060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- 53.Klevay LM. Alzheimer’s disease as copper deficiency. Med Hypotheses. 2007 doi: 10.1016/j.mehy.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 54.Louro MO, Cocho JA, Mera A, Tutor JC. Immunochemical and enzymatic study of ceruloplasmin in rheumatoid arthritis. J Trace Elem Med Biol. 2000;14:174–178. doi: 10.1016/S0946-672X(00)80007-3. [DOI] [PubMed] [Google Scholar]
- 55.Fox PL, Mukhopadhyay C, Ehrenwald E. Structure, oxidant activity, and cardiovascular mechanisms of human ceruloplasmin. Life Sci. 1995;56:1749–1758. doi: 10.1016/0024-3205(95)00146-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.