Abstract
Human milk oligosaccharides (HMOs) are a family of structurally diverse unconjugated glycans that are highly abundant in and unique to human milk. Originally, HMOs were discovered as a prebiotic “bifidus factor” that serves as a metabolic substrate for desired bacteria and shapes an intestinal microbiota composition with health benefits for the breast-fed neonate. Today, HMOs are known to be more than just “food for bugs”. An accumulating body of evidence suggests that HMOs are antiadhesive antimicrobials that serve as soluble decoy receptors, prevent pathogen attachment to infant mucosal surfaces and lower the risk for viral, bacterial and protozoan parasite infections. In addition, HMOs may modulate epithelial and immune cell responses, reduce excessive mucosal leukocyte infiltration and activation, lower the risk for necrotizing enterocolitis and provide the infant with sialic acid as a potentially essential nutrient for brain development and cognition. Most data, however, stem from in vitro, ex vivo or animal studies and occasionally from association studies in mother–infant cohorts. Powered, randomized and controlled intervention studies will be needed to confirm relevance for human neonates. The first part of this review introduces the pioneers in HMO research, outlines HMO structural diversity and describes what is known about HMO biosynthesis in the mother's mammary gland and their metabolism in the breast-fed infant. The second part highlights the postulated beneficial effects of HMO for the breast-fed neonate, compares HMOs with oligosaccharides in the milk of other mammals and in infant formula and summarizes the current roadblocks and future opportunities for HMO research.
Keywords: dietary glycans, infections, inflammation, nutrition, prebiotics
Origins of HMO research
The discovery of human milk oligosaccharides (HMOs) was driven by scientists and physicians with two very different perspectives and interests. Pediatricians and microbiologists were trying to understand the observed health benefits associated with human milk feeding. Chemists were trying to characterize the highly abundant carbohydrates uniquely found in human milk.
Already at the end of the 19th century, when the overall infant first-year mortality rates were as high as 30%, it was observed that breast-fed infants had a much higher chance of survival and had lower incidences of infectious diarrhea and many other diseases than “bottle-fed” infants. At that time, Escherich (1886), an Austrian pediatrician and microbiologist, had just discovered a relationship between intestinal bacteria and the physiology of digestion in infants. Guided by observations that infant health is linked to both breast-feeding and intestinal bacteria, Moro (1900), one of Escherich's former students, and Tissier (1900), a graduate student in Paris, independently found differences in the bacterial composition in the feces of breast-fed compared with bottle-fed infants. Which components in human milk determined the bacterial composition in the infant's intestine remained unknown until more than half a century later.
In parallel to the observations by pediatricians and microbiologists, the chemists' interest in HMO evolved when Eschbach noted in 1888 that human milk contained “a different type of lactose” than bovine milk (reviewed in Montreuil 1992). Shortly after, Deniges found that lactose in human and bovine milk is the same but that human milk contains an additional unknown carbohydrate fraction (reviewed in Montreuil 1992). In the early 1930, more than 40 years later, Polonowski and Lespagnol (1929, 1930, 1931, 1933) were able to characterize this carbohydrate fraction and called it “gynolactose”. It was only weakly soluble in methanol, contained nitrogen and hexosamines and consisted of various components. A few years later, Polonowski and Montreuil (1954) introduced two-dimensional paper chromatography to separate “gynolactose” into individual oligosaccharides. The exact structures and potential functions of these oligosaccharides were, however, unknown.
HMO research experienced a breakthrough when the chemist Richard Kuhn and the pediatrician Paul György, one of Moro's former students, started to collaborate. Earlier, in 1926, Schönfeld (1926) had reported that the whey fraction of human milk contains a growth-promoting factor for Lactobacillus bifidus (later reclassified as Bifidobacterium bifidus). The chemical nature of the “bifidus factor” in human milk was unknown, but Kuhn and György hypothesized a connection between Moro's and Tissier's work on bacteria and Polonowski's and Lespagnol's work on “gynolactose”. In the end, they were able to confirm that the “bifidus factor” indeed consists of oligosaccharides (Gauhe et al. 1954; György, Hoover, et al. 1954; György et al. 1954a, 1954b; Rose et al. 1954).
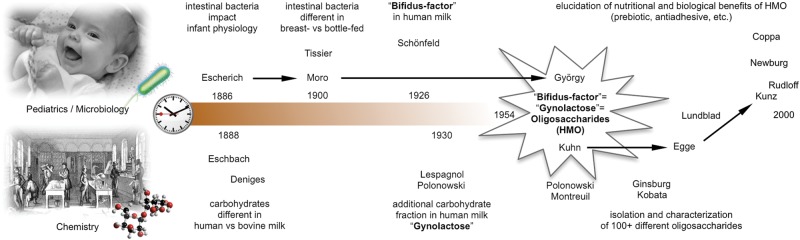
In the following years, Montreuil's group in France and Richard Kuhn's group in Germany discovered and characterized more than a dozen individual HMO (Kuhn and Baer 1956; Kuhn et al. 1956, 1958, 1960; Kuhn and Gauhe 1958, 1962; Montreuil 1960). Some of the oligosaccharides showed activities of blood group determinants, which contributed to the structural characterization of H and Lewis (Le) blood group determinants (Grollman and Ginsburg 1967; Grollman et al. 1969, 1970; Kobata 2010). In return, the interest in blood group glycans led to the development of new methods and tools that supported the discovery and characterization of many additional HMOs with Victor Ginsburg and Akira Kobata as some of the most influential investigators (Kobata and Ginsburg 1969, 1972a, 1972b; Kobata et al. 1969; Kobata 2010). Heinz Egge, one of Kuhn's former students in Germany, was one of the first to use fast atom bombardment mass spectrometry to characterize additional HMOs (Egge et al. 1983), and his former student, Clemens Kunz, a nutritional scientist, continues to study the nutritional and biological properties of HMO (Kunz et al. 1996; Rudloff et al. 1996, 2002, 2006, 2011; Gnoth et al. 2000, 2001; Bode, Kunz, et al. 2004; Bode, Rudloff, et al. 2004; Kuntz et al. 2008, 2009). Figure 1 shows a timeline with key events in HMO discovery until the end of the 20th century.
Fig. 1.
Pioneers in HMO research in the 20th century. HMO research originates at the end of the 19th century with parallel work by pediatricians and microbiologists that studied the health benefits of human milk (top half of the time-scale) and chemists that characterized the carbohydrates abundant in human milk (bottom half of the time-scale). Toward the middle of the 20th century scientists from both disciplines closely collaborated, which led to the discovery that human milk “gynolactose” is the “bifidus factor” and consists of oligosaccharides. Since then, more than a hundred different HMOs have been isolated and characterized, and accumulating data suggest that HMOs benefit the breast-fed neonate in multiple ways (black arrows indicate direct mentor–mentee relationships over the course of the century).
Concentration, composition and variation
Oligosaccharide amount and composition vary between women and over the course of lactation (reviewed in Kunz et al. 2000). Colostrum, the thick, yellowish fluid secreted by the mammary gland a few days before and after parturition, contains as much as 20–25 g/L of HMO (Coppa et al. 1999; Gabrielli et al. 2011). As milk production matures, HMO concentrations decline to 5–20 g/L (Coppa et al. 1999; Kunz et al. 1999; Newburg et al. 2000; Chaturvedi, Warren, Altaye, et al. 2001; Davidson et al. 2004; Bao et al. 2007; Gabrielli et al. 2011), which still exceeds the concentration of total milk protein. Milk of mothers delivering preterm infants has higher HMO concentrations than term milk (Gabrielli et al. 2011). The wide range in HMO concentrations reflects interpersonal variations as well as differences in the non-standardized analytical methods used in various laboratories. Table I lists macromolecule concentrations in mature human milk and compares it with bovine milk, the basis of most infant formula.
Table I.
Macronutrients and HMOs in mature human and bovine milk (approximate values)
| Human | Bovine | |
|---|---|---|
| Protein (g/L) | 8a | 32a |
| Fat (g/L) | 41a | 37a |
| Lactose (g/L) | 70a | 48a |
| Oligosaccharides (g/L) | 5–15b | 0.05c |
| Number of identified oligosaccharides | 100+b,d | ∼40e |
| %fucosylated | 50–80%b,d,f | ∼1%e |
| %sialylated | 10–20%b,d | ∼70%e |
aData from Hale and Hartmann (2007).
bData complied from the following references: Coppa et al. (1999), Kunz et al. (1999), Newburg et al. (2000), Chaturvedi, Warren, Altaye, et al. (2001), Davidson et al. (2004), Bao et al. (2007) and Gabrielli et al. (2011).
cData from Gopal and Gill (2000).
dData complied from the following references Ninonuevo et al. (2006) and Wu et al. (2010; 2011) and reviewed in Kobata (2010).
eData from Tao et al. (2008) and Tao et al. (2009).
fDepending on the woman's Se/Le blood group status.
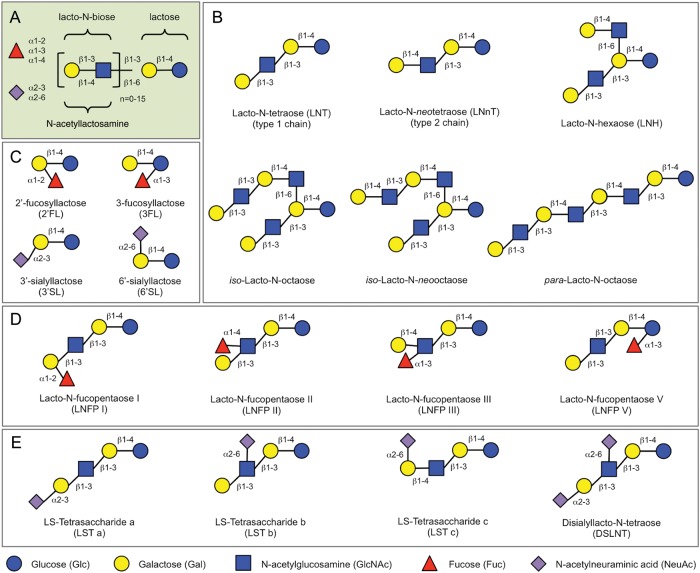
HMOs are composed of the five monosaccharides glucose (Glc), galactose (Gal), N-acetylglucosamine (GlcNAc), fucose (Fuc) and sialic acid (Sia), with N-acetylneuraminic acid (Neu5Ac) as the predominant if not only form of Sia. HMO biosynthesis appears to follow a basic blueprint (Figure 2A): all HMOs contain lactose (Galβ1-4Glc) at their reducing end, which can be elongated by the addition of β1-3- or β1-6-linked lacto-N-biose (Galβ1-3GlcNAc-, type 1 chain) or N-acetyllactosamine (Galβ1-4GlcNAc-, type 2 chain). Elongation with lacto-N-biose appears to terminate the chain, whereas N-acetyllactosamine can be further extended by the addition of one of the two disaccharides. A β1-6 linkage between two disaccharide units introduces chain branching. Branched structures are designated as iso-HMO; linear structures without branches as para-HMO (Figure 2B). Lactose or the elongated oligosaccharide chain can be fucosylated in α1-2, α1-3 or α1-4 linkage and/or sialylated in α2-3 or α2-6 linkage (Figure 2C–E). Some HMOs occur in several isomeric forms, e.g. lacto-N-fucopentaose (LNFP, Figure 2D) or sialyllacto-N-tetraose (LST, Figure 2E).
Fig. 2.
HMO blueprint and selected HMO structures. (A) HMOs follow a basic structural blueprint. (Monosaccharide key is shown at the bottom of the figure.) (B) Lactose can be fucosylated or sialylated in different linkages to generate trisaccharides. (C) Lactose can be elongated by addition of either lacto-N-biose (type I) or N-acetyllactosamine (type II) disaccharides. Addition of disaccharides to each other in the β1-3 linkage leads to linear chain elongation (para-HMO); a β1-6 linkage between two disaccharides introduces chain branching (iso-HMO). (D) Elongated type I or II chains can be fucosylated in different linkages to form a variety of structural isomers, some of which have Le blood group specificity (Figure 3). (E) The elongated chains can also be sialylated in different linkages to form structural isomers. Disialylated lacto-N-tetraose (bottom right) prevents NEC in neonatal rats.
More than a hundred different HMOs have been identified so far, but not every woman synthesizes the same set of oligosaccharides (reviewed in Kobata 2010). The HMO composition mirrors blood group characteristics, which depend on the expression of certain glycosyltransferases. Four milk groups can be assigned based on the Secretor (Se) and Le blood group system, which is determined by the activity of two gene loci encoding for the α1-2-fucoslyltransferase FUT2 (encoded by the Se gene) and the α1-3/4-fucosyltransferase FUT3 (encoded by the Le gene; Kumazaki and Yoshida 1984; Viverge et al. 1990; Johnson and Watkins 1992; Thurl et al. 1997; Chaturvedi, Warren, Altaye, et al. 2001; Stahl et al. 2001; Thurl et al. 2010). Individuals with an active Se locus are classified as Secretors. Milk of Secretor women is abundant in 2′-fucosyllactose (2′FL), LNFP I and other α1-2-fucosylated HMOs. In contrast, non-Secretor lack a functional FUT2 enzyme and their milk does not contain α1-2-fucosylated HMO. Individuals with an active Le locus are classified as Le positive. They express FUT3, which transfers Fuc in the α1-4 linkage to subterminal GlcNAc on type 1 chains (Xu et al. 1996). In contrast, the milk of Le negative women lacks these specific α1-4-fucosylated HMOs, e.g. LNFP II. Figure 3 shows that breast milk can be assigned to one of the four groups based on the expression of FUT2 and FUT3: Le-positive Secretors (Se+Le+), Le-negative Secretors (Se+Le−), Le-positive non-Secretors (Se−Le+) and Le-negative non-Secretors (Se−Le−). The classification of individual milk samples into these four groups is, however, an oversimplification of HMO complexity. For example, FUT2 and FUT3 compete for some of the same substrates (Kumazaki and Yoshida 1984; Johnson and Watkins 1992; Xu et al. 1996) and the levels of enzyme expressions and activities create a continuum of HMO profiles throughout the population. Even the milk of Le-negative non-Secretors women that express neither FUT2 nor FUT3 contains fucosylated HMO like 3FL or LNFP III, suggesting that other Se- and Le-independent FUTs (FUT4, 5, 6, 7 or 9) may be involved (Newburg et al. 2005). In addition, α1-2-fucosylated HMOs have been found in the milk of non-Secretor women toward the end of lactation, and Newburg et al. suggested that FUT1 may also participate in HMO fucosylation (Newburg et al. 2005).
Fig. 3.
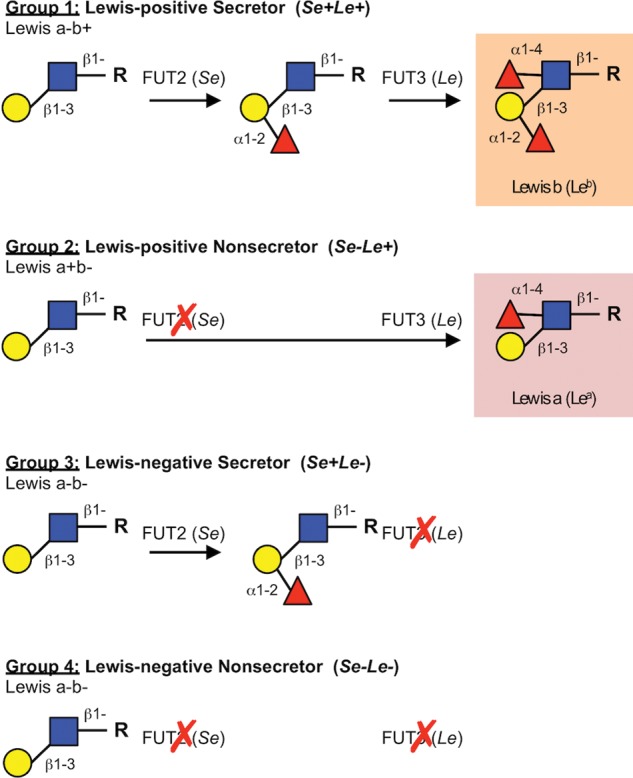
Se- and Le-dependent HMO fucosylation. HMO fucosylation highly depends on a woman's Se and Le blood group status and allows for the distinction of four milk groups. FUT2 is encoded by the Se gene (Se) and facilitates the addition of Fuc to terminal Gal in an α1-2 linkage. FUT3 is encoded by the Le gene (Le) and catalyzes the addition of Fuc to subterminal GlcNAc on type I chains in an α1-4 linkage. If both FUT2 and FUT3 are expressed, milk contains HMO with Le b antigens (highlighted). If only FUT3 is expressed, milk contains HMO with Le a antigens (highlighted). If FUT3 is not expressed, HMOs contain neither Le a nor Le b antigens.
In addition to these genetic variations, other factors such as nutritional and environmental aspects may also affect oligosaccharide amount and composition, but there is currently no data to support these speculations.
Biosynthesis
Since all HMOs carry lactose at their reducing end, it is likely that HMO biosynthesis is an extension of lactose biosynthesis, which occurs in the Golgi and starts with Glc (Figure 4). Some of the cytosolic Glc is activated to UDP-Glc and converted to UDP-Gal. Studies with 13C-labeled Gal suggest that exogenous dietary Gal might be directly incorporated into lactose and HMO without prior conversion to Glc in the liver and reconversion to Gal in the mammary gland (Rudloff et al. 2006). Eventually, both UDP-Gal and Glc are transported into the Golgi and are linked by the lactose synthase complex that consists of two components: the “A” protein is a constitutively expressed β1-4 galactosyltransferase (β4GalT1) that by itself transfers UDP-Gal to terminal GlcNAc during glycoconjugate biosynthesis. In combination with the “B” protein α-lactalbumin, which is expressed under the control of lactation hormones, β4GalT1 shifts its acceptor specificity from GlcNAc to Glc to yield lactose (Ramakrishnan et al. 2002).
Fig. 4.
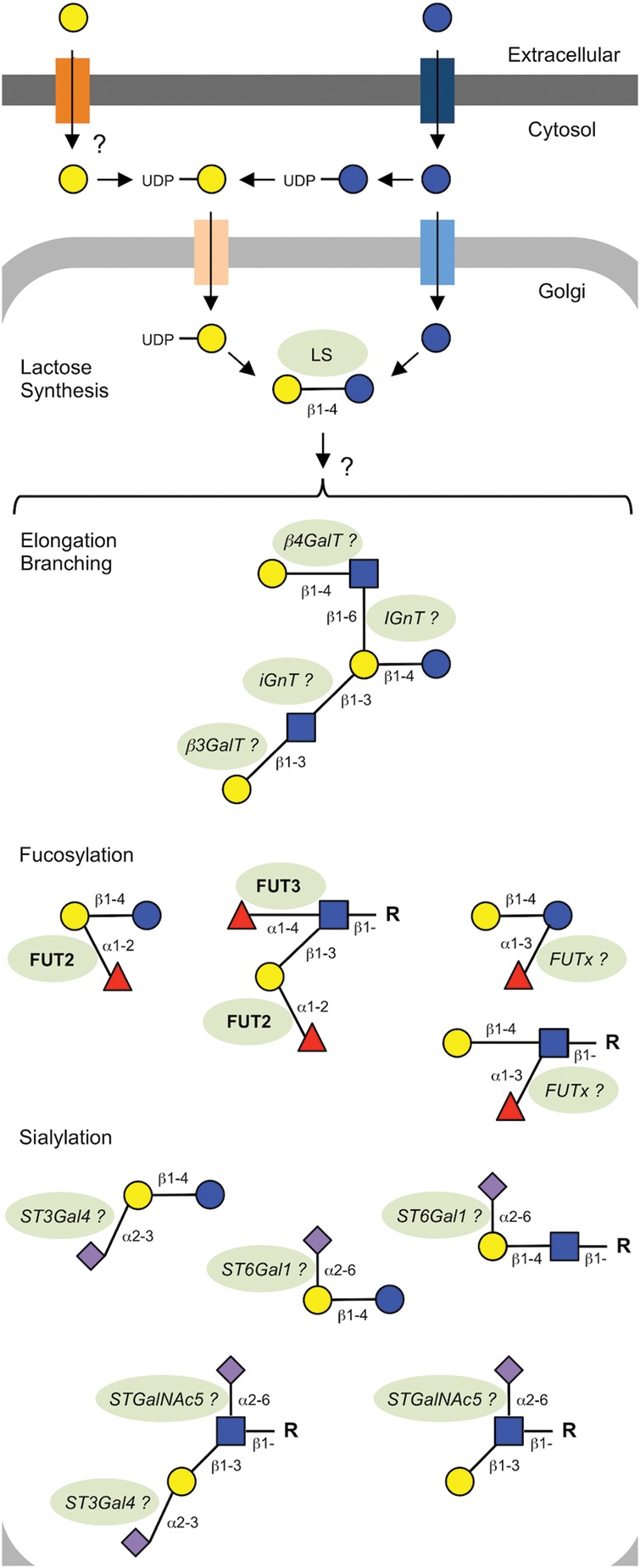
Postulated HMO biosynthesis. Although lactose synthesis in the Golgi of mammary gland epithelial cells has been well described and is catalyzed by lactose synthase (LS), subsequent HMO biosynthesis remains poorly understood. Speculations on enzymes (shaded background) involved in HMO biosynthesis are based on other known glycan synthesis pathways, but data to prove their involvement in HMO biosynthesis are often missing, which raises many questions that are pointed out in the figure with question marks and are further explained in the text. Enzymes known to be involved are highlighted in bold. Enzymes that are speculated to be involved are italicized.
How lactose is extended to form the different HMOs remains poorly understood. As described in a later section of this review, milk oligosaccharide amount and composition highly vary between species, which makes it difficult to study HMO biosynthesis in animal models. A battery of glycosylation knockout mice would be available to investigate which parts of the glycosylation machinery are involved in milk oligosaccharide biosynthesis. Unfortunately, mouse milk contains only 3′- and 6′-sialyllactose, but none of the elongated, branched or fucosylated oligosaccharides found in human milk (Fuhrer et al. 2010). Others and we have used immortalized or transformed human mammary gland epithelial cells to study HMO biosynthesis, with limited success. None of the cell lines synthesized HMO, not even lactose. Most of them did not even express α-lactalbumin, essential for lactose synthesis. This lack of suitable models limits our current understanding of which enzymes and transporters participate in HMO biosynthesis and how it is regulated.
Kobata suggested that HMO elongation and branching follows the example of poly-N-acetyllactosamine synthesis on glycoconjugates and that the lactose core is extended by alternating actions of N-acetylglucosaminyltransferases (GlcNAcT) and GalT (Kobata 2003). A β3GlcNAcT (iGnT) would initiate linear chain elongation, corresponding to the “i” blood group antigen. A β6GlcNAcT (IGnT) would initiate chain branching, corresponding to the “I” antigen. A β3GalT would produce a type 1 disaccharide unit (Galβ1-3GlcNAc-), whereas a β4GalT would generate a type 2 disaccharide unit (Galβ1-4GlcNAc-). Based on our current knowledge in glycan synthesis, it seems likely that these enzymes are involved in HMO elongation and branching. However, direct evidence to support this hypothesis is currently not available.
HMO fucosylation is fairly well understood and has been elucidated based on the involvement of blood group-synthesizing FUTs as outlined in the section on HMO variation. FUT2 adds Fuc in an α1-2 linkage to terminal Gal, and FUT3 adds Fuc in an α1-4 linkage to internal GlcNAc, preferentially on type 1 chains. The expression of FUT2 and FUT3 strictly depends on the activity of the Se and Le gene loci (Kumazaki and Yoshida 1984; Johnson and Watkins 1992; Chaturvedi, Warren, Altaye, et al. 2001; Stahl et al. 2001). As mentioned in the previous section on HMO composition, an additional unknown FUT (FUTx in Figure 4) is Se- and Le-independent and adds Fuc in an α1-3 linkage to the reducing end Glc or internal GlcNAc of type 2 chains. Thus, even the milk of Le-negative non-Secretor (Se−Le−) women contains some fucosylated HMOs, e.g. 3FL or LNFP III (Thurl et al. 1997, 2010; Chaturvedi, Warren, Altaye, et al. 2001; Stahl et al. 2001; Gabrielli et al. 2011). Although mouse milk contains only 3′- and 6′-sialyllactose and no fucosylated oligosaccharides, 2′FL appears in the milk of transgenic mice that express FUT1 under the control of a lactogenic promoter (Prieto et al. 1995).
Several questions remain unanswered with respect to HMO sialylation. Analyzing the milk of β-galactoside sialyltransferase knockout mice revealed that ST3Gal4 and ST6Gal1 are involved in the biosynthesis of 3′- and 6′-sialyllactose, respectively (Fuhrer et al. 2010). Whether these two transferases also sialylate the terminal Gal in complex milk oligosaccharides found in human milk remains unknown. In addition, the subterminal GlcNAc of HMO can be sialylated in an α2-6 linkage, which is a rather unusual structural feature and rarely found on human tissues with the exception of the central nervous system and certain tumors (Tsuchida et al. 2003). Six different ST6GalNAc have been identified in humans, but not a single ST6GlcNAc. In certain colon cancer lines, ST6GalNAc5 facilitates the α2-6-sialylation of subterminal GlcNAc (Tsuchida et al. 2003). However, the terminal Gal needs to be α2-3-sialylated before ST6GalNAc5 adds Sia to the subterminal GlcNAc. Asialo-lacto-N-tetraose is not a substrate for ST6GalNAc5. Human milk contains disialyl-LNT (DSLNT), which is α2-3-sialylated at the terminal Gal and would qualify as an ST6GalNAc5 substrate. However, human milk also contains LST b, which contains α2-6-sialylated subterminal GlcNAc, but the terminal Gal is not sialylated, which would exclude the involvement of ST6GalNAc5 based on previous reports on substrate specificity (Tsuchida et al. 2003). Which transferases contribute to the sialylation of DSLNT and LST b remains to be identified. In general, our knowledge on HMO biosynthesis remains fairly limited. Novel, more suitable models are needed to help close this gap.
Metabolism
Once ingested, HMOs resist the low pH in the infant's stomach as well as digestion by pancreatic and brush border enzymes based on data from in vitro degradation studies (Engfer et al. 2000; Gnoth et al. 2000). Data obtained in the 1980s and 1990s show that HMOs reach the distal small intestine and colon in an intact form and are excreted with the infant's feces (Sabharwal et al. 1984; Sabharwal, Nilsson, Chester, et al. 1988; Sabharwal, Nilsson, Gronberg, et al. 1988; Sabharwal et al. 1991; Chaturvedi, Warren, Buescher et al. 2001; Coppa et al. 2001). More recent studies with capillary electrophoresis and laser-induced fluorescence detection and mass spectrometry confirm and refine these original observations and suggest multistage HMO processing and degradation that depend on infant age, blood group and feeding regime (Albrecht et al. 2010; Albrecht, Schols, van den Heuvel, et al. 2011; Albrecht, Schols, van Zoeren, et al. 2011). In the first stage between birth and ∼2 months of life, feces of breast-fed infants contains sialylated and non-sialylated HMOs that are similar, but not identical to the corresponding milk samples. In the subsequent second stage, the feces contains mainly HMO degradation and processing products that are fairly different from the HMOs in the corresponding milk samples. In the third stage, starting from when feedings other than human milk are introduced, HMOs entirely disappear from the infant's feces (Albrecht, Schols, van den Heuvel, et al. 2011).
Rudloff et al. (1996) were the first to show that intact HMOs appear in the urine of preterm breast-fed infants, but not in formula-fed infants. These results suggest that HMOs are absorbed in the infant's intestine and reach the systemic circulation. In the following years, the same group used a set of elegant and elaborate in vivo 13C-labeling studies to further investigate HMO metabolism (Obermeier et al. 1999; Rudloff et al. 2006, 2011). Lactating women received an oral bolus of 13C-labeled Gal and their mammary glands incorporated the label during HMO synthesis. The breast-fed infant then ingested the in vivo-labeled HMO with the mother's milk and ∼1% appeared in the infant's urine. In fact, the label was still at the same position as in the 13C-label of the orally administered Gal, indicating direct incorporation and minimal rearrangement.
While non-sialylated HMO cross-monolayers of cultured intestinal epithelial cells by receptor-mediated transcytosis and paracytosis, sialylated HMOs use paracytosis only (Gnoth et al. 2001). It remains unknown what receptors facilitate absorption and how rapid HMOs are absorbed, appear in the circulation and are cleared from the system.
Postulated beneficial effects
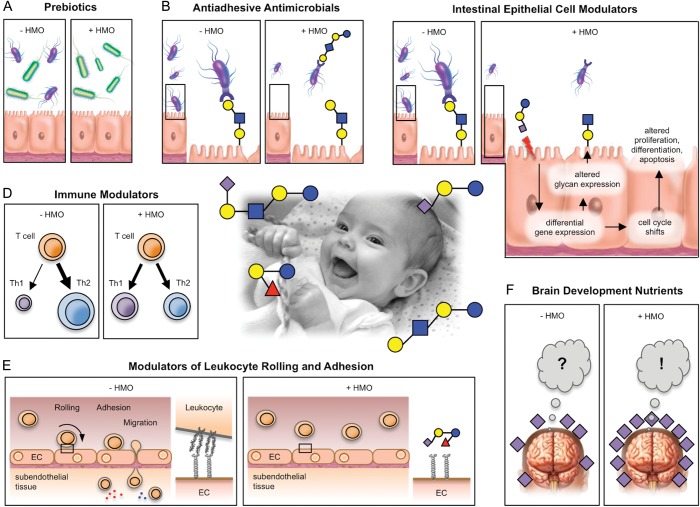
Originally identified as the “bifidus factor” in human milk, HMOs had mostly been recognized for their “bifidogenic” or prebiotic effects. However, since the early 1990s, accumulating evidence suggests that HMOs are more than just a substrate to promote the growth of desired bacteria in the infant's intestine. The following sections outline the postulated effects of HMOs on breast-fed infants and potentially also on breast-feeding mothers. Figure 5 illustrates some of the discussed effects.
Fig. 5.
Postulated HMO effects. HMOs may benefit the breast-fed infant in multiple different ways. (A) HMOs are prebiotics that serve as metabolic substrates for beneficial bacteria (green) and provides them with a growth advantage over potential pathogens (purple). (B) HMOs are antiadhesive antimicrobials that serve as soluble glycan receptor decoys and prevent pathogen attachment. (C) HMOs directly affect intestinal epithelial cells and modulate their gene expression, which leads to changes in cell surface glycans and other cell responses. (D) HMOs modulate lymphocyte cytokine production, potentially leading to a more balanced Th1/Th2 response. (E) HMOs reduce selectin-mediated cell–cell interactions in the immune system and decrease leukocyte rolling on activated endothelial cells, potentially leading to reduced mucosal leukocyte infiltration and activation. (F) HMOs provide Sia as potentially essential nutrients for brain development and cognition. (Center photo taken from author's personal collection).
Prebiotics
Prebiotics are defined as “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora, that confers benefits upon host well-being and health” (Gibson et al. 2004; Roberfroid 2007a). This definition requires that prebiotics are resistant to gastric acidity, hydrolysis by host enzymes and gastrointestinal absorption. HMOs meet all three criteria given that an absorption rate of ∼1% can be neglected in this specific context and that the great majority of HMOs reach the distal small intestine and colon intact and at high concentrations. Bifidobacterium longum subsp. infantis (B. infantis, JCM1222) grows well when HMOs are offered as the only carbohydrate source (LoCascio et al. 2007; Marcobal et al. 2010; Asakuma et al. 2011). Over time, B. infantis consumes HMO entirely, including mono- and disaccharide degradation products (Asakuma et al. 2011). Sequencing of the B. infantis genome revealed entire gene clusters that control the expression of specific glycosidases, sugar transporters and glycan-binding proteins dedicated to HMO utilization (Sela et al. 2008). Compared with B. infantis, B. bifidum (JCM1255) grows slightly slower on HMOs and leaves behind at least some of the monosaccharide degradation products (Asakuma et al. 2011). In contrast, B. longum subsp. longum (JCM1217) and B. breve (JCM1192) hardly grow on HMOs at all and metabolize only lacto-N-tetraose (LNT; Galβ1-3GlcNAC-Lac, type I), but not lacto-N-neotetraose (LNnT; Galβ1-4GlcNAc-Lac, type 2). Hence, it is incorrect to refer to HMO as “bifidogenic” since they promote the growth of certain, but certainly not all Bifidobacteria.
Although most data on prebiotic effects of HMO stem from isolated in vitro fermentation studies, a most recent report in germ-free mice showed that LNnT provides a significant growth advantage for B. infantis over co-inoculated Bacteroides thetaiotaomicron (Marcobal et al. 2011). In vitro, B. infantis grows well on LNnT as the only carbohydrate source; B. thetaiotaomicron does not. In germ-free mice that are co-inoculated with B. infantis and B. thetaiotaomicron, the relative abundance of B. infantis is only around 2% when LNnT is absent from the diet, but increases to over 40% when LNnT is introduced with the drinking water. It remains to be investigated how these results translate to an environment with more complex bacterial communities and the presence of other bioactive milk compounds in human neonates.
The HMO-mediated dominance of B. infantis may keep potentially harmful bacteria in check as they compete for limited nutrient supply. In addition, B. infantis and several other infant-associated bacteria produce short-chain fatty acids and other metabolites (post-biotics) that create an environment favoring the growth of commensals over potential pathogens (Gibson and Wang 1994; Figure 5A).
Antiadhesive antimicrobials
In addition to indirectly keeping pathogens in check by providing a competitive advantage to non-pathogenic commensals, HMOs also directly reduce microbial infections by serving as antiadhesive antimicrobials (Kunz et al. 2000; Newburg et al. 2005). Many viral, bacterial or protozoan pathogens need to adhere to mucosal surfaces to colonize or invade the host and cause disease. Pathogen adhesion is often initiated by lectin–glycan interactions. For example, Escherichia coli with type 1 fimbriae bind to mannose-containing glycans, whereas E. coli with S fimbriae as well as Helicobacter pylori bind to sialylated glycans (Firon et al. 1983; Parkkinen et al. 1983). Glycan-mediated attachment mechanisms have also been described for many viruses like noroviruses or rotaviruses (Hu et al. 2012), which are among the most common causes of severe diarrhea in infants and young children and responsible for almost half a million deaths annually (Tate et al. 2012). Some HMOs resemble mucosal cell surface glycans, serve as soluble decoy receptors to prevent pathogen binding and reduce the risk of infections (Simon et al. 1997; Gustafsson et al. 2006).
The most comprehensive data on HMO as antiadhesive antimicrobials has been reported for Campylobacter jejuni infections, which are one of the most common causes of bacterial diarrhea and infant mortality (Ruiz-Palacios et al. 2003; Morrow et al. 2004). Campylobacter jejuni binds to cultured epithelial cells via type 2 H-antigens, which are α1-2-fucosylated glycans (Fucα1-2Galβ1-4GlcNAc-R; Ruiz-Palacios et al. 2003). Addition of soluble α1-2-fucosylated HMO blocks C. jejuni binding to cultured cells and human intestinal mucosa explants and reduces C. jejuni colonization in mice. The beneficial effect of α1-2-fucosylated HMO on reducing episodes of C. jejuni-associated diarrhea has been confirmed in a prospective study on almost 100 mother–infant pairs from a transitional neighborhood of Mexico City (Morrow et al. 2004). Campylobacter jejuni diarrhea occurred significantly less often in infants whose mother's milk contained high concentrations of 2′FL. In addition, calicivirus diarrhea occurred less often in infants whose mother's milk contained high concentrations of lacto-N-difucohexaose I, another α1-2-fucosylated HMO.
Instead of using lectins to bind to host glycans, some microorganisms express glycans that bind to lectins on host cells. For example, the envelope glycoprotein gp120 facilitates binding of human immunodeficiency virus (HIV) to DC-SIGN (dendritic cell-specific ICAM3-grabbing non-integrin) on human dendritic cells that peek through mucosal surfaces and screen the environment for potential pathogens (Su et al. 2003; van Kooyk and Geijtenbeek 2003; Wu and KewalRamani 2006). The initial gp120/DC-SIGN interaction is important for HIV entry through mucosal barriers during mother-to-child HIV transmission via breast-feeding. While DC-SIGN binds to high-mannose type glycans on gp120 (Zhu et al. 2000; Doores et al. 2010), it has even higher affinities to Le blood group antigens (Naarding et al. 2005; van Liempt et al. 2006). HMOs contain Le blood group antigens and compete with gp120 for binding to DC-SIGN in vitro (Hong et al. 2009). In the breast-fed infant, mucosal surfaces are covered with high concentrations of HMO, which may block HIV entry via DC-SIGN. This may explain why mother-to-child HIV transmission through breast-feeding is rather inefficient with 80–90% of infants not acquiring infections, despite continuous exposure to the virus in milk over many months (Coutsoudis et al. 2004). This hypothesis has been further strengthened by HMO analysis from milk samples collected as part of a larger study of HIV-infected women and their infants followed from birth to 24 months in Lusaka, Zambia (Kuhn et al. 2008). Higher HMO concentrations in the mother's milk were indeed associated with protection against post-natal HIV transmission independent of other known risk factors (Bode et al. manuscript in preparation).
Antiadhesive antimicrobial effects may not be restricted to bacteria and viruses; they might also apply to certain protozoan parasites like Entamoeba histolytica, which causes amoebic dysentery or amoebic liver abscess (Pritt and Clark 2008). Worldwide, ∼50 million people are infected with E. histolytica, resulting in nearly 100,000 deaths annually and making it the third leading cause of death by parasitic diseases, surpassed only by malaria and schistosomiasis (Pritt and Clark 2008). Entamoeba histolytica colonization and invasion requires the attachment to the host's colonic mucosa. Parasites that cannot attach are carried downstream and are excreted with the feces without causing disease. One of E. histolytica's major virulence factors is the Gal/GalNAc lectin, which facilitates parasite attachment as well as the killing and phagocytosis of intestinal epithelial cells (Cano-Mancera and Lopez-Revilla 1987; Saffer and Petri 1991a, 1991b). Some HMOs significantly reduce E. histolytica attachment and cytotoxicity in co-cultures with human intestinal epithelial cell lines (Jantscher-Krenn et al. 2012). In sharp contrast to C. jejuni infections, α1-2-fucosylated HMOs have no effect on E. histolytica attachment and cytotoxicity, suggesting that a non-fucosylated terminal Gal on HMOs is important to bind to the Gal/GalNAc lectin. Unlike other glycans with high Gal/GalNAc lectin affinity such as lactose or Gal (Ravdin and Guerrant 1981; Cano-Mancera and Lopez-Revilla 1987), HMOs are only minimally digested and absorbed in the small intestine and reach the colon as the actual site of E. histolytica infection. If confirmed in vivo, the antiadhesive antimicrobial effects of HMO may provide one explanation for why breast-fed infants are at lower risk to acquire E. histolytica infections than formula-fed infants (Islam et al. 1988).
Antiadhesive antimicrobial effects may not only be relevant to enteric infections. Human milk often covers the mucosal surfaces in the infant's nasopharyngeal regions and occasionally reaches the upper respiratory tract during episodes of aspiration. Breast-fed infants are less likely to develop otitis media caused by Streptococcus pneumoniae, Pseudomonas aeruginosa or Haemophilus influenzae and are also at lower risk to develop respiratory syncytial virus (RSV; Downham et al. 1976; Abrahams and Labbok 2011). These microbial pathogens employ lectin–glycan interactions to initiate infection, and HMOs have been shown to block their attachment, at least in vitro (Andersson et al. 1986; Devaraj et al. 1994; Lesman-Movshovich et al. 2003; Malhotra et al. 2003). Similarly, HMOs are absorbed and excreted with the urine, and they reduce uropathogenic E. coli-induced hemagglutination (Martin-Sosa et al. 2002), suggesting that HMOs also reduce urinary tract infections. In conclusion, the antiadhesive antimicrobial effects of HMO may contribute to the lower incidence of intestinal, upper respiratory and urinary tract infections in breast-fed compared with formula-fed infants (Figure 5B).
Modulators of intestinal epithelial cell responses
HMOs interfere with host–microbial interactions by serving as prebiotics or antiadhesive antimicrobials, but may also directly modulate host intestinal epithelial cell responses. Incubating cultured human intestinal epithelial cell lines with the HMO 3′-sialyllactose lowers the gene expression of sialyltransferases ST3Gal1, ST3Gal2 and ST3Gal4 and diminishes α2-3- and α2-6-sialylation on cell surface glycans (Angeloni et al. 2005). As a consequence, binding of enteropathogenic E. coli is significantly reduced as it uses sialylated cell surface glycans to attach to the host's intestinal epithelial cell. It needs to be further investigated how these results translate to in vivo and whether they are relevant to changing the course of infectious diseases in the neonate. This was, however, the first study to show that HMOs are able to directly affect intestinal epithelial cells, induce differential gene expression and modulate a cell response.
In the meantime, other studies have confirmed that HMOs directly modulate intestinal epithelial cell responses. HMOs reduce cell growth and induce differentiation and apoptosis in cultured human intestinal epithelial cells by altering growth-related cell cycle genes (Kuntz et al. 2008, 2009).
The combined observations from these in vitro studies strongly suggest that HMOs can directly interact with the infant's intestinal epithelial cells, affect gene expression and reprogram the cell cycle as well as cell surface glycosylation (Figure 5C). It requires further investigation to determine which receptors and signaling pathways HMO employ to trigger differential gene expression. Epidermal growth factor receptor (EGFR) and Ras/Raf/ERK signaling may be involved (Kuntz et al. 2009), but whether HMOs directly interact with EGFR or indirectly modulate its signal remains to be determined. Also, whether these in vitro observations are viable in animal models and translate to the human neonate remains to be investigated.
Immune modulators
Although HMO-mediated changes in the infant's microbiota composition or intestinal epithelial cell response may indirectly affect the infant's immune system, results from in vitro studies suggest that HMOs also directly modulate immune responses. HMOs may either act locally on cells of the mucosa-associated lymphoid tissues or on a systemic level since ∼1% of the HMOs are absorbed and reach the systemic circulation (Rudloff et al. 1996, 2011; Gnoth et al. 2001). The number of interferon-γ-producing CD3+CD4+ and CD3+CD8+ lymphocytes as well as interleukin-13 (IL-13)-producing CD3+CD8+ lymphocytes increases when cord blood T-cells are exposed to sialylated HMOs (Eiwegger et al. 2004). This observation led the study authors to speculate that sialylated HMOs influence lymphocyte maturation and promote a shift in T-cell response toward a more balanced Th1/Th2-cytokine production and low-level immunity (Figure 5D). Sialylated HMOs also reduce IL-4 production in a subset of lymphocytes from adult patients with peanut allergy, which led to the conclusion that certain sialylated HMOs may contribute to allergy prevention (Eiwegger et al. 2010).
LNFP III (Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4Glc) and LNnT (Galβ1-4GlcNAcβ1-3Galβ1-4Glc), two HMOs whose structures are also found on helminth parasite glycans, expand peritoneal macrophage populations capable of suppressing naïve CD4+ T-cell responses (Atochina et al. 2001; Terrazas et al. 2001). In addition, LNFP III stimulates macrophage activity in vitro and increases the secretion of prostaglandin E2, IL-10 and TNFα (Atochina and Harn 2005). The physiological relevance of these in vitro observations remains to be elucidated.
It is currently unknown which receptors and signaling pathways transduce HMO-mediated effects on lymphocyte cytokine production or macrophage stimulation. Several different lectins are involved in the immune system, and their glycan-binding specificity suggests that HMOs may interfere with some of these processes. Siglecs (Sia-binding Ig-like lectins), for example, are cell surface receptors and members of the immunoglobulin superfamily that recognize Sia. Siglecs are involved in the immune system in multiple ways (Crocker et al. 2007) and have been shown to bind sialylated HMO (Koliwer-Brandl et al. 2011). Galectins are β-galactoside-binding lectins that modulate immune responses (Rabinovich et al. 2002). HMOs are β-galactosides that often contain β1-3- or β1-4-linked Gal at their non-reducing end and could potentially target galectin-mediated interactions. Whether HMOs modulate siglec- or galectin-mediated immune responses and impact infant physiology needs further investigation. Some in vitro and ex vivo data, however, suggest that HMOs interfere with another family of lectins, the selectins.
Selectins are C-type lectins and cell adhesion molecules that mediate the earliest stages of leukocyte trafficking and platelet–neutrophil complex (PNC) formation. At sites of inflammation, leukocytes need to migrate from the blood stream through the endothelium into subendothelial regions of inflammation (Osborn 1990; Springer 1994). Induced by proinflammatory cytokines, endothelial cells express P- and E-selectin, which bind to glycoconjugates on leukocytes passing by with the blood stream. This initial contact decelerates the leukocytes and makes them roll over the endothelial cell layer. Subsequently, additional adhesion molecules bring leukocytes to a complete stop and facilitate their transmigration into subendothelial regions. Initial selectin-mediated rolling is essential for leukocyte extravasation and mucosal infiltration.
Selectins also initiate PNC formation (Cerletti et al. 1999; Evangelista et al. 1999; Peters et al. 1999). Activated platelets express P-selectin that bind to glycoconjugate ligands on neutrophil surfaces and cause the neutrophils to increase their expression of adhesion molecules, capacity for phagocytosis and production of reactive oxygen species. Again, selectin-mediated PNC formation is essential to activate this highly reactive neutrophil subpopulation.
Selectins bind to glycans that carry sialylated Le blood group epitopes (Varki 1997), which are sialylated and fucosylated lacto-N-bioses (Galβ1-3GlcNAc) or N-acetyllactosamines (Galβ1-4GlcNAc)—very similar to HMO. In fact, HMOs contain Le blood group antigens (Rudloff et al. 2002) and are able to reduce selectin-mediated cell–cell interactions (Bode, Kunz, et al. 2004; Bode, Rudloff, et al. 2004; Figure 5E). Sialylated HMOs reduce leukocyte rolling and adhesion in an in vitro flow model with TNFα-activated human endothelial cells (Bode, Kunz, et al. 2004). Similarly, sialylated HMOs reduce PNC formation and subsequent neutrophil activation in an ex vivo model with whole human blood (Bode, Rudloff, et al. 2004). In both cases, non-sialylated HMOs are ineffective and pooled HMOs are more effective than monovalent sialyl-Le X, indicating the importance of Sia and suggesting potential multivalent interactions with higher molecular HMOs that carry more than one sialylated blood group epitope. Whether these in vitro and ex vivo observations translate to health benefits for the breast-fed neonate remains to be elucidated. Increased mucosal neutrophil infiltration and activation occurs in early stages of diseases like necrotizing enterocolitis (NEC; Stefanutti et al. 2005), but whether HMOs can reduce these potentially detrimental immune responses and protect neonates from disease is currently unknown.
Natural protection against NEC
NEC is one of the most common and often fatal disorders in preterm infants (Uauy et al. 1991; Holman et al. 1997; Neu and Walker 2011). Between 5 and 10% of very low birth weight infants (<1500 g) develop NEC (Holman et al. 2006). More than a quarter of them die, and the survivors are often faced with severe neurological complications (Holman et al. 1997; Rees et al. 2007). NEC etiology and pathogenesis are poorly understood. There are currently no biomarkers to identify neonates at risk to develop NEC. Treatment is limited and the surgical resection of the necrotic intestine often the last remaining option that comes with long-term complications associated with short bowel syndrome. Most intriguingly, breast-fed infants are at a 6–10-fold lower risk to develop NEC than formula-fed infants (Lucas and Cole 1990; Schanler et al. 2005; Sisk et al. 2007). The significant differences in HMO amount and composition between human milk and infant formula led to the hypothesis that HMOs contribute to the protective effects of breast-feeding against NEC. Human intervention studies to test this hypothesis are unfeasible due to the lack of available HMOs required for a well-powered and well-controlled design. Alternatively, studies in an established rat model of the disease show that HMOs indeed protect from NEC (Jantscher-Krenn et al. 2011). Survival rates and pathology scores significantly improve when HMOs are added to the orally gavaged formula. A single HMO, DSLNT (Figure 2E), seems responsible for the protection, which suggests a highly structure-specific and potentially host receptor-mediated effect. It remains to be investigated how DSLNT protects from NEC and whether the results translate from the animal model to human neonates.
Nutrients for brain development
Breast-fed preterm infants have superior developmental scores at 18 months of age and higher intelligence quotients at the age of 7 (Lucas et al. 1990, 1992). A body of evidence suggests that brain development and cognition in part depend on Sia-containing gangliosides and poly-Sia containing glycoproteins (reviewed in Wang 2009). Sia concentrations in the brain more than double between a few months prior to birth and a couple of years after birth (Svennerholm et al. 1989). Animal studies suggest that dietary Sia is an essential nutrient to serve the high Sia demand during pre- and post-natal stages of brain development (Carlson and House 1986; Wang et al. 2007). For example, learning and memory increases in piglets when the sow milk replacer is supplemented with sialylated casein glycomacropeptide (Wang et al. 2007). Human milk is a rich source of Sia (Wang et al. 2001), and post-mortem analysis on human neonates showed that ganglioside- and protein-bound Sia concentrations are significantly higher in the brains of breast-fed infants compared with infants fed with formula that contained lower amounts of Sia than human milk (Wang et al. 2003). Sialylated HMOs contribute to the majority of Sia in human milk. The amount of Sia from HMO is 2–3-fold higher than that from glycoproteins, and Sia in glycolipids accounts for only ∼1% of the total Sia in human milk (Wang et al. 2001). It remains to be investigated whether sialylated HMOs are the primary Sia carrier that provides the developing brain with this seemingly essential nutrient and contribute to superior developmental scores and intelligence quotients in breast-fed infants (Figure 5F).
Effects on the mother
So far, all of the potential benefits of HMOs relate to the breast-fed infant. It is, however, possible that HMOs also affect the breast-feeding mother and the underlying mechanisms may be similar. Human milk per se is not sterile and contains complex bacterial communities that are often highly personalized to the lactating woman (Martin et al. 2007; Hunt et al. 2011). These bacteria can be regarded as natural probiotics to inoculate the infant's intestinal microbiota, but they could also be regarded as potential commensals that modulate the mother's milk composition or pathogens that cause diseases like mastitis. HMOs may influence the bacterial communities in milk in the mammary gland by serving as prebiotics or antiadhesive antimicrobials or by directly modulating mammary gland epithelial cell responses or local immune responses, very similar to what has been described in the context of the neonate. Staphylococcus, for example, is a major cause of mastitis, defined as the inflammation of the breast that is associated with redness, swelling, painful lactation, and sometimes fever or flu-like symptoms (Barbosa-Cesnik et al. 2003; Delgado et al. 2009). Some Staphylococcus strains bind to 2′FL in a biosensor-based assay (Lane et al. 2011), but whether 2′FL or other HMOs interact with Staphylococcus strains to reduce or increase the risk of mastitis is unknown. In addition, HMOs appear in pregnant women's urine shortly prior to parturition (Date 1964; Hallgren et al. 1977; Hallgren and Lundblad 1977a, 1977b). These observations indicate the retrograde “leakage” into the circulation and suggest potential systemic effects not only in the breast-fed infant, but also in the breast-feeding mother.
Oligosaccharides in the milk of other mammals
Oligosaccharides in the milk of many other mammals have been studied over the years, but no other animal matches the high amount and high structural diversity of HMOs (reviewed in (Urashima et al. 2001). Two different groups recently analyzed oligosaccharides in the milk of New and Old World monkeys and apes (Goto et al. 2010; Tao et al. 2011). In general, the oligosaccharides in primate milk, including humans, are more complex and exhibit greater diversity compared to those in non-primate milk. In humans 50–80% of the oligosaccharides are fucosylated depending on the Se/Le group, which is followed by chimpanzees at around 50% and gorillas with only 15%. Most other species show very low levels of fucosylation (<1%). In humans, 10–30% of the oligosaccharides are sialylated and similar values are found in chimpanzees, rhesus and gorillas. Interestingly, primate milk oligosaccharide cluster analysis does not follow primate phylogeny, suggesting an independent emergence of milk oligosaccharides, potentially driven by distinct pathogen exposures (Tao et al. 2011).
Oligosaccharide concentrations in milk of most farm animals including cows, goats, sheep and pigs are 100–1000-fold lower than that in human milk, with a lower number of different oligosaccharides, a higher abundance of sialylated and a lower abundance of fucosylated oligosaccharides (Saito et al. 1981, 1984; Urashima et al. 1991; Martinez-Ferez et al. 2006; Nakajima et al. 2006; Tao et al. 2010). Table I highlights significant differences in oligosaccharide concentration and composition between human milk and bovine milk, which forms the basis for most infant formula. In addition, bovine milk oligosaccharides contain not only Neu5Ac, but also some of the non-human Sia derivative N-glycolylneuraminic acid (Neu5Gc). It remains to be investigated whether incorporation of infant formula-derived exogenous Neu5Gc into rapidly growing neonatal tissues affect infant health (Irie and Suzuki 1998; Tangvoranuntakul et al. 2003; Taylor et al. 2010). In addition, bovine milk also contains α3′-galactosyllactose (Galα1-3Galβ1-4Glc) that is not found in human milk (Urashima et al. 1991). It is unknown whether the concentrations of non-human αGal-containing oligosaccharides in infant formula are sufficient to trigger adverse IgE-mediated responses (Chung et al. 2008; Commins et al. 2009).
Oligosaccharides in infant formula
Oligosaccharides in the milk of farm animals are much less abundant and structurally less complex than oligosaccharides in human milk, and no other natural resources are available to provide access to large amounts of HMO. Hence, infant formula does not provide the human neonate with HMO. As an alternative and in an attempt to mimic the multiple benefits of HMOs, other, non-HMOs are currently added to infant formula, including galactooligosaccharides (GOS) and fructooligosaccharides (FOS).
GOS are Gal oligomers with a degree of polymerization (DP) between 3 and 10 (mostly 3, 4 and 5) that are synthesized from lactose by enzymatic transgalactosylation using β-galactosidases from yeast or bacteria and lactose as the substrate (Fransen et al. 1998). Depending on enzyme source, GOS contain β1-4 and β1-6, but also β1-2 or β1-3 linkages, leading to a variety of different structural isomers (Coulier et al. 2009).
FOS are mostly β2-1-linked fructose oligomers of the inulin-type often extracted from Compositae family plants like chicory (Roberfroid 2005, 2007b). The DP of chicory inulin varies between 2 and more than 60, and the polymers often carry Glc at the reducing end. FOS is produced from inulin using an endoinulinase that cleaves the polymers into smaller oligomers with or without Glc at the reducing end (Cho et al. 2001; Park and Yun 2001). FOS can also be synthesized by transfructosylation using β-fructosidases from yeast or bacteria and sucrose as the substrate (Lafraya et al. 2011; Tian et al. 2011). These synthetic FOS usually carry Glc at the reducing end and their DP is often less than 5.
It is important to note that Gal and fructose oligomers do not naturally occur in human milk. In fact, the fructose monomer itself is not found in human milk. On the other hand, GOS and FOS are neither fucosylated nor sialylated. Despite their structural differences compared to HMO, ingestion of GOS and FOS influences the microbiota composition in the infant's feces and provides other benefits that are extensively reviewed elsewhere (Seifert and Watzl 2007; Boehm and Moro 2008; Macfarlane et al. 2008; Rijnierse et al. 2011). A defined mixture of GOS and FOS reduces the incidence of atopic dermatitis during the first six months of life (Moro et al. 2006) and subsequent allergic manifestations and infections during the first two years of life (Arslanoglu et al. 2008). Long-term health benefits and risks of providing infants with significant amounts of these non-human milk glycans need to be further investigated.
GOS and FOS are non-sialylated, but the carboxyl-group of Sia introduces a negative charge critical to some HMO effects. In an attempt to more closely resemble the composition of oligosaccharides naturally occurring in human milk, a pectin hydrolysate consistent of galacturonic acid oligomers has recently been studied as an additional infant formula oligosaccharide (Westerbeek, Hensgens et al. 2011; Westerbeek, Morch et al. 2011; Westerbeek, van den Berg et al. 2011). While galacturonic acid provides a negatively charged carboxyl-group, its overall structure is very different from Sia. Hence, it is not surprising that pectin-derived acidic oligosaccharides so far failed to affect infant stool viscosity, frequency, pH or feeding tolerance (Westerbeek Hensgens et al. 2011). Additional studies are required to assess short- and long term benefits or adverse effects of introducing non-human milk galacturonic acid oligomers in early infant feeding.
Current roadblocks and future opportunities
Future research on HMOs will likely continue to elucidate and verify the beneficial effects of HMOs for the breast-fed infant, but it will be intriguing to observe whether and how HMOs impact the health of the breast-feeding mother and the composition of other milk components. One of the biggest roadblocks in HMO research remains to be the limited availability of HMO resources needed to better understand the underlying mechanisms of action and to confirm that the observed effects translate to measurable health benefits for the neonate. Due to advances in chemical and enzymatic carbohydrate synthesis, HMO tri- and tetrasaccharides have recently become available in kg quantities, which is going to boost preclinical research and enable first clinical intervention studies. Still, HMOs remain expensive, and government agencies as well as formula companies are faced with making decisions on which of the available HMOs to test and what primary outcome to study. Is there sufficient preclinical data to warrant a multi million-dollar intervention study to assesses whether 2′FL or other HMOs reduce episodes of infectious diarrhea? Is there enough data to support potentially even more expensive studies to determine whether 3′- or 6′-sialyllactose improve infant learning and cognition?
At this point, only a handful of HMO tri- and tetrasaccharides are available in sufficient quantities to move forward, but what if a mix of structurally more complex HMOs is needed to confer significant health benefits? In the end, the human mammary gland produces not only one or two HMOs, but more than one hundred different oligosaccharides. Insights into how HMOs are synthesized in the human mammary gland may help develop novel strategies and technologies to generate complex mixtures of HMOs.
HMO research has come a long way since 1900 when Moro and Tissier first observed that human milk feeding affects infant intestinal microbiota composition (Moro 1900; Tissier 1900). Many questions about HMO biosynthesis, metabolism and health benefits however remain unanswered and create exciting opportunities for future generations of dedicated scientists. History shows that it was the collaboration between György and Kuhn, two scientists with entirely different backgrounds, that eventually led to the discovery of HMO (Gauhe et al. 1954; György, Hoover, et al. 1954; György et al. 1954a, 1954b; Rose et al. 1954). It will likely be these multi-discipline collaborations that involve pediatricians, nutritional scientists, microbiologists, chemists, glycobiologists and many others, that will continue to create the biggest impact on HMO research.
Funding
The author is supported by a Pathway to Independence Award of the National Institutes of Health (DK78668).
Conflict of Interest
None declared.
Abbreviations
DC, dendritic cell-specific ICAM3-grabbing non-integrin; DP, degree of polymerization; DSLNT, disialyllacto-N-tetraose; EGFR, epidermal growth factor receptor; Fuc, fucose; 2′FL, 2′-fucosyllactose; FUT, fucosyltransferase; Gal, galactose; GalT, galactosyltransferase; Glc, glucose; GlcNAc, N-acetylglucosamine; HIV, human immunodeficiency virus; HMO, human milk oligosaccharide; IL, interleukin; Le, Lewis; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; LST, sialyllacto-N-tetraose; NEC, necrotizing enterocolitis; Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; PNC, platelet–neutrophil complex; RSV, respiratory syncytial virus; Se, Secretor; Sia, sialic acid.
Acknowledgements
I expresses my deepest gratitude to my PhD advisors Clemens Kunz and Silvia Rudloff at the Justus-Liebig-University in Giessen, Germany, who initiated my excitement for HMO research, to my postdoctoral advisor Hudson Freeze at the Burnham Institute in La Jolla, CA, who taught me the essentials of glycobiology and to Ajit Varki and Jeff Esko at the University of California, San Diego, for their continuous inspiration and support. I thank the dedicated and hard working members of my lab at the University of California, San Diego, better known as the “Milk Gang”.
References
- Abrahams SW, Labbok MH. Breastfeeding and otitis media: A review of recent evidence. Curr Allergy Asthma Rep. 2011;11:508–512. doi: 10.1007/s11882-011-0218-3. doi:10.1007/s11882-011-0218-3. [DOI] [PubMed] [Google Scholar]
- Albrecht S, Schols HA, van den Heuvel EG, Voragen AG, Gruppen H. CE-LIF-MS n profiling of oligosaccharides in human milk and feces of breast-fed babies. Electrophoresis. 2010;31:1264–1273. doi: 10.1002/elps.200900646. doi:10.1002/elps.200900646. [DOI] [PubMed] [Google Scholar]
- Albrecht S, Schols HA, van den Heuvel EG, Voragen AG, Gruppen H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res. 2011;346:2540–2550. doi: 10.1016/j.carres.2011.08.009. doi:10.1016/j.carres.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Albrecht S, Schols HA, van Zoeren D, van Lingen RA, Groot Jebbink LJ, van den Heuvel EG, Voragen AG, Gruppen H. Oligosaccharides in feces of breast- and formula-fed babies. Carbohydr Res. 2011;346:2173–2181. doi: 10.1016/j.carres.2011.06.034. doi:10.1016/j.carres.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Andersson B, Porras O, Hanson LA, Lagergard T, Svanborg-Eden C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986;153:232–237. doi: 10.1093/infdis/153.2.232. doi:10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, Kochhar S, Sigrist H, Sprenger N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2005;15:31–41. doi: 10.1093/glycob/cwh143. doi:10.1093/glycob/cwh143. [DOI] [PubMed] [Google Scholar]
- Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–1095. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, Yamamoto K, Kumagai H, Ashida H, Hirose J, Kitaoka M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem. 2011;286:34583–34592. doi: 10.1074/jbc.M111.248138. doi:10.1074/jbc.M111.248138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-γ and nitric oxide-dependent mechanism. J Immunol. 2001;167:4293–4302. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- Atochina O, Harn D. LNFPIII/LeX-stimulated macrophages activate natural killer cells via CD40-CD40L interaction. Clin Diagn Lab Immunol. 2005;12:1041–1049. doi: 10.1128/CDLI.12.9.1041-1049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Zhu L, Newburg DS. Simultaneous quantification of sialyloligosaccharides from human milk by capillary electrophoresis. Anal Biochem. 2007;370:206–214. doi: 10.1016/j.ab.2007.07.004. doi:10.1016/j.ab.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Cesnik C, Schwartz K, Foxman B. Lactation mastitis. JAMA. 2003;289:1609–1612. doi: 10.1001/jama.289.13.1609. doi:10.1001/jama.289.13.1609. [DOI] [PubMed] [Google Scholar]
- Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost. 2004;92:1402–1410. doi: 10.1160/TH04-01-0055. [DOI] [PubMed] [Google Scholar]
- Bode L, Rudloff S, Kunz C, Strobel S, Klein N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil β2 integrin expression. J Leukoc Biol. 2004;76:820–826. doi: 10.1189/jlb.0304198. doi:10.1189/jlb.0304198. [DOI] [PubMed] [Google Scholar]
- Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr. 2008;138:1818S–1828S. doi: 10.1093/jn/138.9.1818S. [DOI] [PubMed] [Google Scholar]
- Cano-Mancera R, Lopez-Revilla R. Inhibition of the adhesion of Entamoeba histolytica trophozoites to human erythrocytes by carbohydrates. Parasitol Res. 1987;74:18–22. doi: 10.1007/BF00534926. doi:10.1007/BF00534926. [DOI] [PubMed] [Google Scholar]
- Carlson SE, House SG. Oral and intraperitoneal administration of N-acetylneuraminic acid: Effect on rat cerebral and cerebellar N-acetylneuraminic acid. J Nutr. 1986;116:881–886. doi: 10.1093/jn/116.5.881. [DOI] [PubMed] [Google Scholar]
- Cerletti C, Evangelista V, de Gaetano G. P-selectin-β2-integrin cross-talk: A molecular mechanism for polymorphonuclear leukocyte recruitment at the site of vascular damage. Thromb Haemost. 1999;82:787–793. [PubMed] [Google Scholar]
- Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering LK, Newburg DS. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11:365–372. doi: 10.1093/glycob/11.5.365. doi:10.1093/glycob/11.5.365. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS. Survival of human milk oligosaccharides in the intestine of infants. Adv Exp Med Biol. 2001;501:315–323. doi: 10.1007/978-1-4615-1371-1_39. doi:10.1007/978-1-4615-1371-1_39. [DOI] [PubMed] [Google Scholar]
- Cho YJ, Sinha J, Park JP, Yun JW. Production of inulooligosaccharides from chicory extract by endoinulinase from Xanthomonas oryzae No. 5. Enzyme Microb Technol. 2001;28:439–445. doi: 10.1016/s0141-0229(00)00341-0. doi:10.1016/S0141-0229(00)00341-0. [DOI] [PubMed] [Google Scholar]
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. doi:10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, Woodfolk JA, Platts-Mills TA. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433. doi: 10.1016/j.jaci.2008.10.052. doi:10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Bruni S, Carloni I, Gabrielli O. Characterization of oligosaccharides in milk and feces of breast-fed infants by high-performance anion-exchange chromatography. Adv Exp Med Biol. 2001;501:307–314. doi: 10.1007/978-1-4615-1371-1_38. doi:10.1007/978-1-4615-1371-1_38. [DOI] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. 1999;88:89–94. doi: 10.1111/j.1651-2227.1999.tb01307.x. doi:10.1111/j.1651-2227.1999.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Coulier L, Timmermans J, Bas R, Van Den Dool R, Haaksman I, Klarenbeek B, Slaghek T, Van Dongen W. In-depth characterization of prebiotic galacto-oligosaccharides by a combination of analytical techniques. J Agric Food Chem. 2009;57:8488–8495. doi: 10.1021/jf902549e. doi:10.1021/jf902549e. [DOI] [PubMed] [Google Scholar]
- Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Msellati P, et al. Late postnatal transmission of HIV-1 in breast-fed children: An individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. doi:10.1086/420834. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. doi:10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Date JW. The isolation of some oligosaccharides from the urine of pregnant and lactating women. Scand J Clin Lab Invest. 1964;16:597–603. doi: 10.3109/00365516409055222. doi:10.3109/00365516409055222. [DOI] [PubMed] [Google Scholar]
- Davidson B, Meinzen-Derr JK, Wagner CL, Newburg DS, Morrow AL. Fucosylated oligosaccharides in human milk in relation to gestational age and stage of lactation. Adv Exp Med Biol. 2004;554:427–430. doi: 10.1007/978-1-4757-4242-8_56. [DOI] [PubMed] [Google Scholar]
- Delgado S, Arroyo R, Jimenez E, Marin ML, del Campo R, Fernandez L, Rodriguez JM. Staphylococcus epidermidis strains isolated from breast milk of women suffering infectious mastitis: Potential virulence traits and resistance to antibiotics. BMC Microbiol. 2009;9:82. doi: 10.1186/1471-2180-9-82. doi:10.1186/1471-2180-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj N, Sheykhnazari M, Warren WS, Bhavanandan VP. Differential binding of Pseudomonas aeruginosa to normal and cystic fibrosis tracheobronchial mucins. Glycobiology. 1994;4:307–316. doi: 10.1093/glycob/4.3.307. doi:10.1093/glycob/4.3.307. [DOI] [PubMed] [Google Scholar]
- Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, Burton DR, Crispin M, Scanlan CN. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci USA. 2010;107:13800–13805. doi: 10.1073/pnas.1006498107. doi:10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downham MA, Scott R, Sims DG, Webb JK, Gardner PS. Breast-feeding protects against respiratory syncytial virus infections. Br Med J. 1976;2:274–276. doi: 10.1136/bmj.2.6030.274. doi:10.1136/bmj.2.6030.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egge H, Dell A, Von Nicolai H. Fucose containing oligosaccharides from human milk. I. Separation and identification of new constituents. Arch Biochem Biophys. 1983;224:235–253. doi: 10.1016/0003-9861(83)90207-2. [DOI] [PubMed] [Google Scholar]
- Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R, Szepfalusi Z. Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol. 2010;21:1179–1188. doi: 10.1111/j.1399-3038.2010.01062.x. doi:10.1111/j.1399-3038.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, Dehlink E, Loibichler C, Urbanek R, Szepfalusi Z. Human milk—derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res. 2004;56:536–540. doi: 10.1203/01.PDR.0000139411.35619.B4. doi:10.1203/01.PDR.0000139411.35619.B4. [DOI] [PubMed] [Google Scholar]
- Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71:1589–1596. doi: 10.1093/ajcn/71.6.1589. [DOI] [PubMed] [Google Scholar]
- Escherich T. Die Darmbakterien des Säuglings und ihre Beziehungen zur Physiologie der Verdauung. Enke Verlag: Stuttgart; 1886. [Google Scholar]
- Evangelista V, Manarini S, Sideri R, Rotondo S, Martelli N, Piccoli A, Totani L, Piccardoni P, Vestweber D, de Gaetano G, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: Role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- Firon N, Ofek I, Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983;120:235–249. doi: 10.1016/0008-6215(83)88019-7. doi:10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Fransen CT, Van Laere KM, van Wijk AA, Brull LP, Dignum M, Thomas-Oates JE, Haverkamp J, Schols HA, Voragen AG, Kamerling JP, et al. α-d-Glcp-(1↔1)-β-d-Galp-containing oligosaccharides, novel products from lactose by the action of β-galactosidase. Carbohydr Res. 1998;314:101–114. doi: 10.1016/s0008-6215(98)00285-7. doi:10.1016/S0008-6215(98)00285-7. [DOI] [PubMed] [Google Scholar]
- Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207:2843–2854. doi: 10.1084/jem.20101098. doi:10.1084/jem.20101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C, Coppa GV. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011;128:e1520–1531. doi: 10.1542/peds.2011-1206. doi:10.1542/peds.2011-1206. [DOI] [PubMed] [Google Scholar]
- Gauhe A, György P, Hoover JR, Kuhn R, Rose CS, Ruelius HW, Zilliken F. Bifidus factor. IV. Preparations obtained from human milk. Arch Biochem Biophys. 1954;48:214–224. doi: 10.1016/0003-9861(54)90326-4. doi:10.1016/0003-9861(54)90326-4. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. doi:10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77:412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. doi:10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–3020. doi: 10.1093/jn/130.12.3014. [DOI] [PubMed] [Google Scholar]
- Gnoth MJ, Rudloff S, Kunz C, Kinne RK. Investigations of the in vitro transport of human milk oligosaccharides by a Caco-2 monolayer using a novel high performance liquid chromatography-mass spectrometry technique. J Biol Chem. 2001;276:34363–34370. doi: 10.1074/jbc.M104805200. doi:10.1074/jbc.M104805200. [DOI] [PubMed] [Google Scholar]
- Gopal PK, Gill HS. Oligosaccharides and glycoconjugates in bovine milk and colostrum. Br J Nutr. 2000;84(Suppl. 1):S69–S74. doi: 10.1017/s0007114500002270. [DOI] [PubMed] [Google Scholar]
- Goto K, Fukuda K, Senda A, Saito T, Kimura K, Glander KE, Hinde K, Dittus W, Milligan LA, Power ML, et al. Chemical characterization of oligosaccharides in the milk of six species of New and Old World monkeys. Glycoconj J. 2010;27:703–715. doi: 10.1007/s10719-010-9315-0. doi:10.1007/s10719-010-9315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman EF, Ginsburg V. Correlation between secretor status and the occurrence of 2′-fucosyllactose in human milk. Biochem Biophys Res Commun. 1967;28:50–53. doi: 10.1016/0006-291x(67)90404-4. doi:10.1016/0006-291X(67)90404-4. [DOI] [PubMed] [Google Scholar]
- Grollman EF, Kobata A, Ginsburg V. An enzymatic basis for Lewis blood types in man. J Clin Invest. 1969;48:1489–1494. doi: 10.1172/JCI106115. doi:10.1172/JCI106115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman EF, Kobata A, Ginsburg V. Enzymatic basis of blood types in man. Ann N Y Acad Sci. 1970;169:153–160. doi: 10.1111/j.1749-6632.1970.tb55980.x. doi:10.1111/j.1749-6632.1970.tb55980.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson A, Hultberg A, Sjostrom R, Kacskovics I, Breimer ME, Boren T, Hammarstrom L, Holgersson J. Carbohydrate-dependent inhibition of Helicobacter pylori colonization using porcine milk. Glycobiology. 2006;16:1–10. doi: 10.1093/glycob/cwj031. doi:10.1093/glycob/cwj031. [DOI] [PubMed] [Google Scholar]
- György P, Hoover JR, Kuhn R, Rose CS. Bifidus factor. III. The rate of dialysis. Arch Biochem Biophys. 1954;48:209–213. doi: 10.1016/0003-9861(54)90325-2. doi:10.1016/0003-9861(54)90325-2. [DOI] [PubMed] [Google Scholar]
- György P, Kuhn R, Rose CS, Zilliken F. Bifidus factor. II. Its occurrence in milk from different species and in other natural products. Arch Biochem Biophys. 1954b;48:202–208. doi: 10.1016/0003-9861(54)90324-0. [DOI] [PubMed] [Google Scholar]
- György P, Kuhn R, Rose CS, Zilliken F. Bifidus factor I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954a;48:193–201. doi: 10.1016/0003-9861(54)90323-9. [DOI] [PubMed] [Google Scholar]
- Hale TW, Hartmann PE. Textbook of Human Lactation. 1st ed. Amarillo (TX): Hale Publishing, L.P; 2007. [Google Scholar]
- Hallgren P, Lindberg BS, Lundblad A. Quantitation of some urinary oligosaccharides during pregnancy and lactation. J Biol Chem. 1977;252:1034–1040. [PubMed] [Google Scholar]
- Hallgren P, Lundblad A. Structural analysis of nine oligosaccharides isolated from the urine of a blood group O, nonsecretor, woman during pregnancy and lactation. J Biol Chem. 1977a;252:1014–1022. [PubMed] [Google Scholar]
- Hallgren P, Lundblad A. Structural analysis of oligosaccharides isolated from the urine of a blood group A, secretor, woman during pregnancy and lactation. J Biol Chem. 1977b;252:1023–1033. [PubMed] [Google Scholar]
- Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–2031. doi: 10.2105/ajph.87.12.2026. doi:10.2105/AJPH.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506. doi: 10.1111/j.1365-3016.2006.00756.x. doi:10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) J Nutr. 2009;101:482–486. doi: 10.1017/s0007114508025804. [DOI] [PubMed] [Google Scholar]
- Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK, Prasad BV. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012 doi: 10.1038/nature10996. doi:10.1038/nature10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One. 2011;6:e21313. doi: 10.1371/journal.pone.0021313. doi:10.1371/journal.pone.0021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie A, Suzuki A. CMP-N-acetylneuraminic acid hydroxylase is exclusively inactive in humans. Biochem Biophys Res Commun. 1998;248:330–333. doi: 10.1006/bbrc.1998.8946. doi:10.1006/bbrc.1998.8946. [DOI] [PubMed] [Google Scholar]
- Islam A, Stoll BJ, Ljungstrom I, Biswas J, Nazrul H, Huldt G. The prevalence of Entamoeba histolytica in lactating women and in their infants in Bangladesh. Trans R Soc Trop Med Hyg. 1988;82:99–103. doi: 10.1016/0035-9203(88)90276-3. doi:10.1016/0035-9203(88)90276-3. [DOI] [PubMed] [Google Scholar]
- Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr. 2012 doi: 10.1017/S0007114511007392. doi:http://dx.doi.org/10.1017/S0007114511007392. [DOI] [PubMed] [Google Scholar]
- Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2011 doi: 10.1136/gutjnl-2011-301404. doi:10.1136/gutjnl-2011-301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PH, Watkins WM. Purification of the Lewis blood-group gene associated α-3/4-fucosyltransferase from human milk: An enzyme transferring fucose primarily to Type 1 and lactose-based oligosaccharide chains. Glycoconj J. 1992;9:241–249. doi: 10.1007/BF00731136. doi:10.1007/BF00731136. [DOI] [PubMed] [Google Scholar]
- Kobata A. Possible application of milk oligosaccharides for drug development. Chang Gung Med J. 2003;26:621–636. [PubMed] [Google Scholar]
- Kobata A. Structures and application of oligosaccharides in human milk. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:731–747. doi: 10.2183/pjab.86.731. doi:10.2183/pjab.86.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata A, Ginsburg V. Oligosaccharides of human milk. II. Isolation and characterization of a new pentasaccharide, lacto-N-fucopentaose 3. J Biol Chem. 1969;244:5496–5502. [PubMed] [Google Scholar]
- Kobata A, Ginsburg V. Oligosaccharides of human milk. 3. Isolation and characterization of a new hexasaccharide, lacto-N-hexaose. J Biol Chem. 1972a;247:1525–1529. [PubMed] [Google Scholar]
- Kobata A, Ginsburg V. Oligosaccharides of human milk. IV. Isolation and characterization of a new hexasaccharide, lacto-N-neohexaose. Arch Biochem Biophys. 1972b;150:273–281. doi: 10.1016/0003-9861(72)90036-7. doi:10.1016/0003-9861(72)90036-7. [DOI] [PubMed] [Google Scholar]
- Kobata A, Ginsburg V, Tsuda M. Oligosaccharides of human milk. I. Isolation and characterization. Arch Biochem Biophys. 1969;130:509–513. doi: 10.1016/0003-9861(69)90063-0. [DOI] [PubMed] [Google Scholar]
- Koliwer-Brandl H, Siegert N, Umnus K, Kelm A, Tolkach A, Kulozik U, Kuballa J, Cartellieri S, Kelm S. Lectin inhibition assay for the analysis of bioactive milk sialoglycoconjugates. Int Dairy J. 2011;21:413–420. doi:10.1016/j.idairyj.2011.01.005. [Google Scholar]
- Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, Kasonde P, Scott N, Vwalika C, Walter J, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. doi:10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Baer HH. Die Konstitution der Lacto-N-tetraose. Chem Ber. 1956;89:504–511. doi:10.1002/cber.19560890246. [Google Scholar]
- Kuhn R, Baer HH, Gauhe A. Kristallisation und Konstitutionsermittlung der Lacto-N-fucopentaose I. Chem Ber. 1956;89:2514–2523. doi:10.1002/cber.19560891106. [Google Scholar]
- Kuhn R, Baer HH, Gauhe A. Die Konstitution der Lacto-N-fucopentaose II. Chem Ber. 1958;91:364. doi:10.1002/cber.19580910221. [Google Scholar]
- Kuhn R, Baer HH, Gauhe A. Über ein kristallisiertes, Le a-aktives Hexasaccharid aus Frauenmilch. Chem Ber. 1960;93:647–651. doi:10.1002/cber.19600930317. [Google Scholar]
- Kuhn R, Gauhe A. Über die lacto-difuco-hexaose der Frauenmilch. Justus Liebigs Ann Chem. 1958;611:249–252. doi:10.1002/jlac.19586110123. [Google Scholar]
- Kuhn R, Gauhe A. Über drei saure Pentasaccharide aus Frauenmilch. Chem Ber. 1962;95:513–517. doi:10.1002/cber.19620950230. [Google Scholar]
- Kumazaki T, Yoshida A. Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci USA. 1984;81:4193–4197. doi: 10.1073/pnas.81.13.4193. doi:10.1073/pnas.81.13.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr. 2009;101:1306–1315. doi: 10.1017/S0007114508079622. doi:10.1017/S0007114508079622. [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. doi:10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S, Hintelmann A, Pohlentz G, Egge H. High-pH anion-exchange chromatography with pulsed amperometric detection and molar response factors of human milk oligosaccharides. J Chromatogr B Biomed Appl. 1996;685:211–221. doi: 10.1016/s0378-4347(96)00181-8. doi:10.1016/S0378-4347(96)00181-8. [DOI] [PubMed] [Google Scholar]
- Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr. 2008;99:462–471. doi: 10.1017/S0007114507824068. [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S, Schad W, Braun D. Lactose-derived oligosaccharides in the milk of elephants: Comparison with human milk. Br J Nutr. 1999;82:391–399. doi: 10.1017/s0007114599001798. doi:10.1017/S0007114599001798. [DOI] [PubMed] [Google Scholar]
- Lafraya A, Sanz-Aparicio J, Polaina J, Marin-Navarro J. Fructo-oligosaccharide synthesis by mutant versions of Saccharomyces cerevisiae invertase. Appl Environ Microbiol. 2011;77:6148–6157. doi: 10.1128/AEM.05032-11. doi:10.1128/AEM.05032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JA, Mehra RK, Carrington SD, Hickey RM. Development of biosensor-based assays to identify anti-infective oligosaccharides. Anal Biochem. 2011;410:200–205. doi: 10.1016/j.ab.2010.11.032. doi:10.1016/j.ab.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Lesman-Movshovich E, Lerrer B, Gilboa-Garber N. Blocking of Pseudomonas aeruginosa lectins by human milk glycans. Can J Microbiol. 2003;49:230–235. doi: 10.1139/w03-027. doi:10.1139/w03-027. [DOI] [PubMed] [Google Scholar]
- LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, Mills DA, German JB. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. 2007;55:8914–8919. doi: 10.1021/jf0710480. doi:10.1021/jf0710480. [DOI] [PubMed] [Google Scholar]
- Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. doi:10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- Lucas A, Morley R, Cole TJ, Gore SM, Lucas PJ, Crowle P, Pearse R, Boon AJ, Powell R. Early diet in preterm babies and developmental status at 18 months. Lancet. 1990;335:1477–1481. doi: 10.1016/0140-6736(90)93026-l. doi:10.1016/0140-6736(90)93026-L. [DOI] [PubMed] [Google Scholar]
- Lucas A, Morley R, Cole TJ, Lister G, Leeson-Payne C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet. 1992;339:261–264. doi: 10.1016/0140-6736(92)91329-7. doi:10.1016/0140-6736(92)91329-7. [DOI] [PubMed] [Google Scholar]
- Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008;104:305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Ward M, Bright H, Priest R, Foster MR, Hurle M, Blair E, Bird M. Isolation and characterisation of potential respiratory syncytial virus receptor(s) on epithelial cells. Microbes Infect. 2003;5:123–133. doi: 10.1016/s1286-4579(02)00079-5. doi:10.1016/S1286-4579(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010;58:5334–5340. doi: 10.1021/jf9044205. doi:10.1021/jf9044205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10:507–514. doi: 10.1016/j.chom.2011.10.007. doi:10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Heilig HG, Zoetendal EG, Jimenez E, Fernandez L, Smidt H, Rodriguez JM. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res Microbiol. 2007;158:31–37. doi: 10.1016/j.resmic.2006.11.004. doi:10.1016/j.resmic.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Martin-Sosa S, Martin MJ, Hueso P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J Nutr. 2002;132:3067–3072. doi: 10.1093/jn/131.10.3067. [DOI] [PubMed] [Google Scholar]
- Martinez-Ferez A, Rudloff S, Guadix A, Henkel CA, Pohlenz G, Boza JJ, Guadix EM, Kunz C. Goat's milk oligosaccharides as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int Dairy J. 2006;16:173–181. doi:10.1016/j.idairyj.2005.02.003. [Google Scholar]
- Montreuil J. Les glucides du lait. Bull Soc Chim Biol. 1960;42:1399–1427. [PubMed] [Google Scholar]
- Montreuil J. The saga of human milk gynolactose. In: Renner B, Sawatzki GG, editors. New Perspectives in Infant Nutrition. Stuttgart NY: Georg Thieme Verlag; 1992. pp. 3–11. [Google Scholar]
- Moro E. Morphologie und bakteriologische Untersuchungen über die Darmbakterien des Säuglings: Die Bakterien-flora des normalen Frauenmilchstuhls. Jahrbuch Kinderh. 1900;61:686–734. [Google Scholar]
- Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814–819. doi: 10.1136/adc.2006.098251. doi:10.1136/adc.2006.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145:297–303. doi: 10.1016/j.jpeds.2004.04.054. doi:10.1016/j.jpeds.2004.04.054. [DOI] [PubMed] [Google Scholar]
- Naarding MA, Ludwig IS, Groot F, Berkhout B, Geijtenbeek TB, Pollakis G, Paxton WA. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest. 2005;115:3256–3264. doi: 10.1172/JCI25105. doi:10.1172/JCI25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Kinoshita M, Matsushita N, Urashima T, Suzuki M, Suzuki A, Kakehi K. Capillary affinity electrophoresis using lectins for the analysis of milk oligosaccharide structure and its application to bovine colostrum oligosaccharides. Anal Biochem. 2006;348:105–114. doi: 10.1016/j.ab.2005.10.010. doi:10.1016/j.ab.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. doi:10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. doi:10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- Newburg DS, Shen Z, Warren CD. Quantitative analysis of human milk oligosaccharides by capillary electrophoresis. Adv Exp Med Biol. 2000;478:381–382. doi: 10.1007/0-306-46830-1_37. doi:10.1007/0-306-46830-1_37. [DOI] [PubMed] [Google Scholar]
- Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–7480. doi: 10.1021/jf0615810. doi:10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- Obermeier S, Rudloff S, Pohlentz G, Lentze MJ, Kunz C. Secretion of 13C-labelled oligosaccharides into human milk and infant's urine after an oral [13C]galactose load. Isotopes Environ Health Stud. 1999;35:119–125. doi: 10.1080/10256019908234084. doi:10.1080/10256019908234084. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990;62:3–6. doi: 10.1016/0092-8674(90)90230-c. doi:10.1016/0092-8674(90)90230-C. [DOI] [PubMed] [Google Scholar]
- Park JP, Yun JW. Utilization of chicory roots for microbial endoinulinase production. Lett Appl Microbiol. 2001;33:183–187. doi: 10.1046/j.1472-765x.2001.00977.x. doi:10.1046/j.1472-765x.2001.00977.x. [DOI] [PubMed] [Google Scholar]
- Parkkinen J, Finne J, Achtman M, Vaisanen V, Korhonen TK. Escherichia coli strains binding neuraminyl α2-3 galactosides. Biochem Biophys Res Commun. 1983;111:456–461. doi: 10.1016/0006-291x(83)90328-5. doi:10.1016/0006-291X(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Peters MJ, Dixon G, Kotowicz KT, Hatch DJ, Heyderman RS, Klein NJ. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol. 1999;106:391–399. doi: 10.1046/j.1365-2141.1999.01553.x. doi:10.1046/j.1365-2141.1999.01553.x. [DOI] [PubMed] [Google Scholar]
- Polonowski M, Lespagnol A. Sur la nature glucidique de la substance lévogyre du lait de femme. Bull Soc Biol. 1929;101:61–63. [Google Scholar]
- Polonowski M, Lespagnol A. Sur l'existence de plusieurs glucides dans le lactoserum de femme. C R Soc Biol (Paris) 1930;104:555–557. [Google Scholar]
- Polonowski M, Lespagnol A. Sur deux nouveaux sucres du lait de femme, le gynolactose et l'allolactose. C R Acad Sci. 1931;192:1319. [Google Scholar]
- Polonowski M, Lespagnol A. Nouvelles acquisitions sur les composés glucidiques du lai de femme. Bull Soc Chim Biol. 1933;15:320–349. [Google Scholar]
- Polonowski M, Montreuil J. Etude chromatographique des polyosides du lait de femme. C R Acad Sci Paris. 1954;238:2263–2264. [PubMed] [Google Scholar]
- Prieto PA, Mukerji P, Kelder B, Erney R, Gonzalez D, Yun JS, Smith DF, Moremen KW, Nardelli C, Pierce M, et al. Remodeling of mouse milk glycoconjugates by transgenic expression of a human glycosyltransferase. J Biol Chem. 1995;270:29515–29519. doi: 10.1074/jbc.270.49.29515. doi:10.1074/jbc.270.49.29515. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Clark CG. Amebiasis. Mayo Clin Proc. 2008;83:1154–1159. doi: 10.4065/83.10.1154. quiz 1159–1160 doi:10.4065/83.10.1154. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: Amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. doi:10.1016/S1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan B, Boeggeman E, Qasba PK. β1,4-galactosyltransferase and lactose synthase: Molecular mechanical devices. Biochem Biophys Res Commun. 2002;291:1113–1118. doi: 10.1006/bbrc.2002.6506. doi:10.1006/bbrc.2002.6506. [DOI] [PubMed] [Google Scholar]
- Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981;68:1305–1313. doi: 10.1172/JCI110377. doi:10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F193–F198. doi: 10.1136/adc.2006.099929. doi:10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnierse A, Jeurink PV, van Esch BC, Garssen J, Knippels LM. Food-derived oligosaccharides exhibit pharmaceutical properties. Eur J Pharmacol. 2011;668(Suppl. 1):S117–S123. doi: 10.1016/j.ejphar.2011.07.009. doi:10.1016/j.ejphar.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. Introducing inulin-type fructans. Br J Nutr. 2005;93(Suppl. 1):S13–S25. doi: 10.1079/bjn20041350. doi:10.1079/BJN20041350. [DOI] [PubMed] [Google Scholar]
- Roberfroid M. Prebiotics: The concept revisited. J Nutr. 2007a;137:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. Inulin-type fructans: Functional food ingredients. J Nutr. 2007b;137:2493S–2502S. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- Rose CS, Kuhn R, Zilliken F, György P. Bifidus factor. V. The activity of alpha- and-beta-methyl-N-acetyl-D-glucosaminides. Arch Biochem Biophys. 1954;49:123–129. doi: 10.1016/0003-9861(54)90173-3. [DOI] [PubMed] [Google Scholar]
- Rudloff S, Obermeier S, Borsch C, Pohlentz G, Hartmann R, Brosicke H, Lentze MJ, Kunz C. Incorporation of orally applied (13)C-galactose into milk lactose and oligosaccharides. Glycobiology. 2006;16:477–487. doi: 10.1093/glycob/cwj092. doi:10.1093/glycob/cwj092. [DOI] [PubMed] [Google Scholar]
- Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 2011;107:957–963. doi: 10.1017/S0007114511004016. [DOI] [PubMed] [Google Scholar]
- Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatr. 1996;85:598–603. doi: 10.1111/j.1651-2227.1996.tb14095.x. doi:10.1111/j.1651-2227.1996.tb14095.x. [DOI] [PubMed] [Google Scholar]
- Rudloff S, Stefan C, Pohlentz G, Kunz C. Detection of ligands for selectins in the oligosaccharide fraction of human milk. Eur J Nutr. 2002;41:85–92. doi: 10.1007/s003940200012. doi:10.1007/s003940200012. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H (O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–14120. doi: 10.1074/jbc.M207744200. doi:10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- Sabharwal H, Nilsson B, Chester MA, Lindh F, Gronberg G, Sjoblad S, Lundblad A. Oligosaccharides from faeces of a blood-group B, breast-fed infant. Carbohydr Res. 1988;178:145–154. doi: 10.1016/0008-6215(88)80107-1. doi:10.1016/0008-6215(88)80107-1. [DOI] [PubMed] [Google Scholar]
- Sabharwal H, Nilsson B, Chester MA, Sjoblad S, Lundblad A. Blood group specific oligosaccharides from faeces of a blood group A breast-fed infant. Mol Immunol. 1984;21:1105–1112. doi: 10.1016/0161-5890(84)90121-4. doi:10.1016/0161-5890(84)90121-4. [DOI] [PubMed] [Google Scholar]
- Sabharwal H, Nilsson B, Gronberg G, Chester MA, Dakour J, Sjoblad S, Lundblad A. Oligosaccharides from feces of preterm infants fed on breast milk. Arch Biochem Biophys. 1988;265:390–406. doi: 10.1016/0003-9861(88)90142-7. doi:10.1016/0003-9861(88)90142-7. [DOI] [PubMed] [Google Scholar]
- Sabharwal H, Sjoblad S, Lundblad A. Sialylated oligosaccharides in human milk and feces of preterm, full-term, and weaning infants. J Pediatr Gastroenterol Nutr. 1991;12:480–484. doi: 10.1097/00005176-199105000-00012. doi:10.1097/00005176-199105000-00012. [DOI] [PubMed] [Google Scholar]
- Saffer LD, Petri WA., Jr Entamoeba histolytica: Recognition of α- and β-galactose by the 260-kDa adherence lectin. Exp Parasitol. 1991a;72:106–108. doi: 10.1016/0014-4894(91)90128-j. doi:10.1016/0014-4894(91)90128-J. [DOI] [PubMed] [Google Scholar]
- Saffer LD, Petri WA., Jr Role of the galactose lectin of Entamoeba histolytica in adherence-dependent killing of mammalian cells. Infect Immun. 1991b;59:4681–4683. doi: 10.1128/iai.59.12.4681-4683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Itoh T, Adachi S. Presence of two neutral disaccharides containing N-acetylhexosamine in bovine colostrum as free forms. Biochim Biophys Acta. 1984;801:147–150. doi: 10.1016/0304-4165(84)90223-x. doi:10.1016/0304-4165(84)90223-X. [DOI] [PubMed] [Google Scholar]
- Saito T, Itoh T, Adachi S, Suzuki T, Usui T. The chemical structure of neutral and acidic sugar chains obtained from bovine colostrum kappa-casein. Biochim Biophys Acta. 1981;678:257–267. doi: 10.1016/0304-4165(81)90215-4. doi:10.1016/0304-4165(81)90215-4. [DOI] [PubMed] [Google Scholar]
- Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116:400–406. doi: 10.1542/peds.2004-1974. doi:10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- Schönfeld H. Über die Beziehung der einzelnen Bestandteile der Frauenmilch zur Bifidusflora. Jahrbuch Kinderh. 1926;113:19–60. [Google Scholar]
- Seifert S, Watzl B. Inulin and oligofructose: Review of experimental data on immune modulation. J Nutr. 2007;137:2563S–2567S. doi: 10.1093/jn/137.11.2563S. [DOI] [PubMed] [Google Scholar]
- Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105:18964–18969. doi: 10.1073/pnas.0809584105. doi:10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon PM, Goode PL, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun. 1997;65:750–757. doi: 10.1128/iai.65.2.750-757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007;27:428–433. doi: 10.1038/sj.jp.7211758. doi:10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. doi:10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stahl B, Thurl S, Henker J, Siegel M, Finke B, Sawatzki G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv Exp Med Biol. 2001;501:299–306. doi: 10.1007/978-1-4615-1371-1_37. doi:10.1007/978-1-4615-1371-1_37. [DOI] [PubMed] [Google Scholar]
- Stefanutti G, Lister P, Smith VV, Peters MJ, Klein NJ, Pierro A, Eaton S. P-selectin expression, neutrophil infiltration, and histologic injury in neonates with necrotizing enterocolitis. J Pediatr Surg. 2005;40:942–947. doi: 10.1016/j.jpedsurg.2005.03.027. discussion 947–948 doi:10.1016/j.jpedsurg.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Su SV, Gurney KB, Lee B. Sugar and spice: Viral envelope-DC-SIGN interactions in HIV pathogenesis. Curr HIV Res. 2003;1:87–99. doi: 10.2174/1570162033352129. doi:10.2174/1570162033352129. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Bostrom K, Fredman P, Mansson JE, Rosengren B, Rynmark BM. Human brain gangliosides: Developmental changes from early fetal stage to advanced age. Biochim Biophys Acta. 1989;1005:109–117. doi: 10.1016/0005-2760(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. doi:10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao N, DePeters EJ, Freeman S, German JB, Grimm R, Lebrilla CB. Bovine milk glycome. J Dairy Sci. 2008;91:3768–3778. doi: 10.3168/jds.2008-1305. doi:10.3168/jds.2008-1305. [DOI] [PubMed] [Google Scholar]
- Tao N, DePeters EJ, German JB, Grimm R, Lebrilla CB. Variations in bovine milk oligosaccharides during early and middle lactation stages analyzed by high-performance liquid chromatography-chip/mass spectrometry. J Dairy Sci. 2009;92:2991–3001. doi: 10.3168/jds.2008-1642. doi:10.3168/jds.2008-1642. [DOI] [PubMed] [Google Scholar]
- Tao N, Ochonicky KL, German JB, Donovan SM, Lebrilla CB. Structural determination and daily variations of porcine milk oligosaccharides. J Agric Food Chem. 2010;58:4653–4659. doi: 10.1021/jf100398u. doi:10.1021/jf100398u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao N, Wu S, Kim J, An HJ, Hinde K, Power ML, Gagneux P, German JB, Lebrilla CB. Evolutionary glycomics: Characterization of milk oligosaccharides in primates. J Proteome Res. 2011;10:1548–1557. doi: 10.1021/pr1009367. doi:10.1021/pr1009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. doi:10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010;207:1637–1646. doi: 10.1084/jem.20100575. doi:10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA., Jr The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: A potential mechanism for immune polarization in helminth infections. J Immunol. 2001;167:5294–5303. doi: 10.4049/jimmunol.167.9.5294. [DOI] [PubMed] [Google Scholar]
- Thurl S, Henker J, Siegel M, Tovar K, Sawatzki G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J. 1997;14:795–799. doi: 10.1023/a:1018529703106. doi:10.1023/A:1018529703106. [DOI] [PubMed] [Google Scholar]
- Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104:1261–1271. doi: 10.1017/S0007114510002072. doi:10.1017/S0007114510002072. [DOI] [PubMed] [Google Scholar]
- Tian F, Inthanavong L, Karboune S. Purification and characterization of levansucrases from Bacillus amyloliquefaciens in intra- and extracellular forms useful for the synthesis of levan and fructooligosaccharides. Biosci Biotechnol Biochem. 2011;75:1929–1938. doi: 10.1271/bbb.110315. doi:10.1271/bbb.110315. [DOI] [PubMed] [Google Scholar]
- Tissier H. Recherches sur la flora intestinale de nourissons (état normal et pathologique) Paris, France: 1900. [Google Scholar]
- Tsuchida A, Okajima T, Furukawa K, Ando T, Ishida H, Yoshida A, Nakamura Y, Kannagi R, Kiso M. Synthesis of disialyl Lewis a (Le(a)) structure in colon cancer cell lines by a sialyltransferase, ST6GalNAc VI, responsible for the synthesis of α-series gangliosides. J Biol Chem. 2003;278:22787–22794. doi: 10.1074/jbc.M211034200. doi:10.1074/jbc.M211034200. [DOI] [PubMed] [Google Scholar]
- Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. Necrotizing enterocolitis in very low birth weight infants: Biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1991;119:630–638. doi: 10.1016/s0022-3476(05)82418-7. doi:10.1016/S0022-3476(05)82418-7. [DOI] [PubMed] [Google Scholar]
- Urashima T, Saito T, Nakamura T, Messer M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj J. 2001;18:357–371. doi: 10.1023/a:1014881913541. doi:10.1023/A:1014881913541. [DOI] [PubMed] [Google Scholar]
- Urashima T, Saito T, Ohmisya K, Shimazaki K. Structural determination of three neutral oligosaccharides in bovine (Holstein-Friesian) colostrum, including the novel trisaccharide; GalNAcα1-3Galβ1-4Glc. Biochim Biophys Acta. 1991;1073:225–229. doi: 10.1016/0304-4165(91)90207-w. doi:10.1016/0304-4165(91)90207-W. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Geijtenbeek TB. DC-SIGN: Escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. doi:10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- van Liempt E, Bank CM, Mehta P, Garcia-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. doi:10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands: Will the real ones please stand up? J Clin Invest. 1997;100:S31–S35. [PubMed] [Google Scholar]
- Viverge D, Grimmonprez L, Cassanas G, Bardet L, Solere M. Discriminant carbohydrate components of human milk according to donor secretor types. J Pediatr Gastroenterol Nutr. 1990;11:365–370. doi: 10.1097/00005176-199010000-00014. doi:10.1097/00005176-199010000-00014. [DOI] [PubMed] [Google Scholar]
- Wang B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. 2009;29:177–222. doi: 10.1146/annurev.nutr.28.061807.155515. doi:10.1146/annurev.nutr.28.061807.155515. [DOI] [PubMed] [Google Scholar]
- Wang B, Brand-Miller J, McVeagh P, Petocz P. Concentration and distribution of sialic acid in human milk and infant formulas. Am J Clin Nutr. 2001;74:510–515. doi: 10.1093/ajcn/74.4.510. [DOI] [PubMed] [Google Scholar]
- Wang B, McVeagh P, Petocz P, Brand-Miller J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am J Clin Nutr. 2003;78:1024–1029. doi: 10.1093/ajcn/78.5.1024. [DOI] [PubMed] [Google Scholar]
- Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S, Brand-Miller J. Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr. 2007;85:561–569. doi: 10.1093/ajcn/85.2.561. [DOI] [PubMed] [Google Scholar]
- Westerbeek E, Hensgens R, Mihatsch W, Boehm G, Lafeber H, van Elburg R. The effect of neutral and acidic oligosaccharides on stool viscosity, stool frequency and stool pH in preterm infants. Acta Paediatr. 2011;100:1426–1431. doi: 10.1111/j.1651-2227.2011.02295.x. doi:10.1111/j.1651-2227.2011.02295.x. [DOI] [PubMed] [Google Scholar]
- Westerbeek EA, Morch E, Lafeber HN, Fetter WP, Twisk JW, Van Elburg RM. Effect of neutral and acidic oligosaccharides on fecal IL-8 and fecal calprotectin in preterm infants. Pediatr Res. 2011;69:255–258. doi: 10.1203/PDR.0b013e318206fd25. doi:10.1203/PDR.0b013e318206fd25. [DOI] [PubMed] [Google Scholar]
- Westerbeek EA, van den Berg A, Lafeber HN, Fetter WP, van Elburg RM. The effect of enteral supplementation of a prebiotic mixture of non-human milk galacto-, fructo- and acidic oligosaccharides on intestinal permeability in preterm infants. Br J Nutr. 2011;105:268–274. doi: 10.1017/S0007114510003405. doi:10.1017/S0007114510003405. [DOI] [PubMed] [Google Scholar]
- Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10:856–868. doi: 10.1021/pr101006u. doi:10.1021/pr101006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: Infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. doi:10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9:4138–4151. doi: 10.1021/pr100362f. doi:10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Vo L, Macher BA. Structure-function analysis of human α1,3-fucosyltransferase. Amino acids involved in acceptor substrate specificity. J Biol Chem. 1996;271:8818–8823. doi: 10.1074/jbc.271.15.8818. [DOI] [PubMed] [Google Scholar]
- Zhu X, Borchers C, Bienstock RJ, Tomer KB. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry. 2000;39:11194–11204. doi: 10.1021/bi000432m. doi:10.1021/bi000432m. [DOI] [PubMed] [Google Scholar]