Abstract
Background
Chronic obstructive pulmonary disease (COPD) is an under-diagnosed condition. General practitioners meet and examine the patients in early stages of the disease, and symptoms represent the starting point of the diagnostic process.
Aim
To evaluate the diagnostic value of respiratory symptoms in the diagnosis of airflow limitation.
Methods
Spirometry was performed in a cross-sectional population-based study of 3954 subjects 60 years and older (54.5% women), who also filled in a questionnaire on symptoms.
Results
The prevalence of any airflow limitation was 15.5% and 20.8%, in women and men, respectively, whereas the corresponding prevalence of severe airflow limitation (FEV1<50% predicted) was 3.4% and 4.9%. The positive predictive value of chronic cough with phlegm for any airflow limitation was 37.0% in women and 40.4% in men, and 17.3% and 14.2%, respectively, for severe airflow limitation. Wheezing was a symptom which persisted despite smoking cessation, whereas coughing was considerably less common in ex-smokers than in current smokers. Wheezing, dyspnoea on unhurried walking, dyspnoea on quick walking, and coughing with phlegm were independent predictors of any airflow limitation, OR 1.5, 1.8, 1.4, and 1.6 respectively. (The ORs for severe airflow limitation were 2.4, 2.4, 2.4, and 1.6 respectively.) To be an ex-smoker (OR 2.4) or a current smoker (OR 5.8) was of greater importance. In never- and ex-smokers the chance of having airflow limitation was almost doubled when having two or more, compared with one, of the three symptoms: wheezing, dyspnoea, and coughing with phlegm. Ex-smokers reporting two symptoms had a similar risk of airflow limitation to current smokers not reporting any symptoms.
Conclusion
Respiratory symptoms are valuable predictors of airflow limitation and should be emphasized when selecting patients for spirometry.
Keywords: Airflow limitation, COPD, elderly, family practice, spirometry, symptoms
Respiratory symptoms are important clues in the diagnosis of airflow limitation in the elderly, and should be emphasized when selecting patients for spirometry.
In general practice there should be a low threshold for doing spirometry in current smokers.
This study has demonstrated that lung function testing also should be kept in mind in ex-smokers with respiratory symptoms.
Chronic obstructive pulmonary disease (COPD) is well known as a leading cause of morbidity and mortality in the developed countries worldwide [1–4]. The criteria for diagnosing COPD are, by definition, based on spirometry in the Global initiative for chronic Obstructive Lung Disease (GOLD) criteria [1], and in other guidelines [5–7]. FEV1/FVC < 70% post-bronchodilator is the criterion for diagnosing COPD [1], regardless of age and symptoms. The one-dimensioned grading, based on spirometry only, is an item for discussion [8–10].
It is well known that smokers are at risk of getting COPD [1], and the prevalence of the disease increases with increasing age [9], [10]. Dyspnoea is known as the hallmark symptom of COPD and is often the reason for seeking a doctor [1], [11], whereas chronic cough may be the first symptom in the development of COPD [1]. Assessments of symptoms are, however, subjective and can be evaluated in different ways. Symptoms are often underreported by patients and not recognized by physicians, especially in the early stages of COPD [12]. A weak correlation between reduced FEV1 and patients’ symptoms has been demonstrated in evaluation of pulmonary rehabilitation [12], [13]. Improvements in dyspnoea after such rehabilitation could not be detected by spirometric tests [12].
We wanted to evaluate the diagnostic value of respiratory symptoms in the elderly, and the potential role a combination of symptoms in the diagnosis of airflow limitation might have. Our aim was increased insight regarding symptoms as guide for general practitioners in the diagnosis of COPD. We have argued for new criteria of COPD after the age of 70 years [10], and in this paper we have defined airflow limitation to occur only when FEV1/FVC < 65% for subjects 70 years and older.
Material and methods
Subjects
The survey took place in Tromsø, a city in the northern part of Norway, with about 61 000 inhabitants, and 7842 inhabitants aged 60 years or more (as at January 2001). There is little occupational or environmental pollution in Tromsø. The survey started in March 2001 and ended in February 2002 [10].
Among subjects aged 60 years or more, a total of 5328 subjects were eligible, and 4519 (85%) took part [10]. Spirometry was performed in 4102 subjects, 90% of the attendees and 77% of the eligible. Absence of staff and technical problems were reasons for spirometry not being performed in 10% of the attendees.
Questionnaire
A questionnaire including smoking habits and different symptoms and diseases was sent by mail together with the invitation to participate. The questions concerning respiratory symptoms were the following: Wheezing: Have you experienced episodes with wheezing in your chest? Dyspnoea: Do you get dyspnoeic when (1) You walk quickly on level ground or slightly upwards? (2) You walk unhurriedly on level ground? Coughing: Do you cough daily in periods of the year? If yes: (1) Do you usually bring up phlegm with the cough? (2) Did you have such a cough for more than three months during the last two years? Participants answering positively to all three questions concerning cough were defined as having chronic cough with phlegm. The answers to all the questions were yes, or no. The questions are based on the MRC questionnaire on respiratory symptoms [14].
Examinations
Spirometry was carried out with the use of one spirometer only, a “Sensormedics Vmax 20”. The American Thoracic Society criteria for spirometry testing [15] were followed. Calibration of the instrument was performed every morning and at the machine's demand. Three trained technicians shared the conducting of the spirometry. The subjects were sitting, using a nose clip, and were instructed to blow for as long as possible, and for at least six seconds. At least three exhalations were required. The difference between best and next best FEV1 and FVC should not exceed 5% or 200 ml, whichever was the greater. A reversibility test was not performed. Current drug therapy was not interrupted before the test. Height was measured barefoot.
In week 41 and 42 in 2001 an inter- and intra-observer agreement test was done with two of our three technicians (A and B) and 80 participants. It implied repeated testing in both weeks. The two technicians were blinded to each other's results. Kappa values ranged from 0.77 to 0.92 (mean 85.5) [10].
Statistical analysis
Spirometric results were analysed according to sex, age, smoking habits, and reported symptoms. Any airflow limitation was defined as: FEV1/FVC < 70% for subjects under 70 years of age, and FEV1/FVC < 65% for subjects 70 years and older [10]. Severe airflow limitation was defined as: When in addition FEV1<50% was predicted, by using a Norwegian reference value [16]. Differences in prevalence were evaluated by chi-squared statistics. Binary logistic regression was used in calculating odds ratios (OR). A three-symptom score was made based on the independent predictors determined by logistic regression. Each symptom gave 1+ when present, and zero when absent. Positive predictive values (PPV) for airflow limitation of separate symptoms, and the three-symptom score were calculated. ROC curves were used to illustrate the value of the three symptoms in the diagnosis of airflow limitation. The SPSS 14.0 for Windows (SPSS Inc., Chicago, Illinois, USA) was used in the statistical analyses.
The Regional Committee for Medical Research Ethics approved the study. All the participants gave informed written consent.
Results
Spirometry was performed in 4102 persons aged 60 years or more, 2269 women and 1833 men. At the completion of the data collection, 25 women and 12 men had withdrawn their consent to participate. Ninety women and 21 men (2.7% of the total) were excluded due to inadequate performance (of these 84 women and 20 men blew for less than three seconds) [10], and a total of 2154 women aged 60–89 and 1800 men aged 60–85 years were included in the analysis (Table I). The material has been described in more details in a previous paper [10]. Significantly more women (47%) than men (18%) were never smokers (p < 0.001). The prevalence of any airflow limitation was 15.5% in women and 20.8% in men. The corresponding prevalence of severe airflow limitation was 3.4% and 4.9%, respectively (Table I). Wheezing and two levels of dyspnoea were reported with about the same frequencies in women and men, but men did more often report cough, with and without phlegm, than women (Table I). Wheezing was reported significantly more frequently in ex- and current smokers than in never-smokers (Table II). In men, dyspnoea on quick walking was reported significantly less frequently in never smokers compared with ex-smokers. This symptom was more common in never-smoking women than in never-smoking men. Among those with BMI ≥ 30, never-smoking women reported both forms of dyspnoea more frequently than did never-smoking men (on unhurried walking 8.6% versus 1.8%, on quick walking 65.0% versus 40.4%). Reporting cough, with or without phlegm, was significantly more common in current smokers than in ex-smokers, and more common in smoking men than in smoking women.
Table I.
Characteristics of the 3954 participants in the study.
| Women |
Men |
|||
| n | % | n | % | |
| Total | 2154 | 54.5 | 1800 | 45.5 |
| Age (years) | ||||
| 60–69 | 1152 | 53.5 | 957 | 53.2 |
| 70–79 | 889 | 41.3 | 748 | 41.6 |
| 80 + | 113 | 5.2 | 95 | 5.3 |
| Smoking habit1 | ||||
| Never smoker | 999 | 46.9 | 327 | 18.3 |
| Ex-smoker | 633 | 29.7 | 1043 | 58.3 |
| Current smoker | 498 | 23.4 | 419 | 23.4 |
| Body mass index (BMI) | ||||
| < 30 | 1636 | 76.0 | 1492 | 82.9 |
| ≥ 30 | 518 | 24.0 | 308 | 17.1 |
| Airflow limitation | ||||
| Any airflow limitation2 | 333 | 15.5 | 375 | 20.8 |
| Severe airflow limitation3 | 73 | 3.4 | 88 | 4.9 |
| Reported symptoms | ||||
| Wheezing | 483 | 22.4 | 437 | 24.3 |
| Dyspnoea on unhurried walking | 88 | 4.1 | 55 | 3.1 |
| Dyspnoea on quick walking | 1044 | 48.5 | 834 | 46.3 |
| Daily cough in periods | 346 | 16.1 | 407 | 22.6 |
| Daily cough with phlegm | 227 | 10.5 | 295 | 16.4 |
| Chronic cough with phlegm | 127 | 5.9 | 183 | 10.2 |
Notes: 1Missing data in 24 women and 11 men. 2Defined as FEV1/FVC < 70% for persons 69 years and younger and FEV1/FVC < 65% for persons 70 years and older. 3In addition FEV1 < 50% predicted according to the equation of Langhammer.
Table II.
Frequency of reported symptoms (%) by smoking status in 2130 women and 1789 men aged 60 years or more.
| Women |
Men |
|||||
| Reported symptoms | Never smoker | Ex-smoker1 | Smoker2 | Never smoker | Ex-smoker1 | Smoker2 |
| Wheezing | 16.8 | 25.8 p = 0.000 | 29.3 p = 0.202 | 11.9 | 22.7 p = 0.000 | 37.7 p = 0.000 |
| Dyspnoea on unhurried walking | 4.1 | 3.9 p = 1.000 | 4.2 p = 0.880 | 1.5 | 3.0 p = 0.232 | 4.3 p = 0.202 |
| Dyspnoea on quick walking | 45.7 | 50.7 p = 0.053 | 51.4 p = 0.857 | 33.3 | 47.3 p = 0.000 | 53.7 p = 0.028 |
| Daily cough in periods | 12.3 | 13.6 p = 0.449 | 26.9 p = 0.000 | 15.0 | 17.8 p = 0.274 | 40.3 p = 0.000 |
| Daily cough with phlegm | 6.4 | 9.6 p = 0.022 | 20.1 p = 0.000 | 10.1 | 13.2 p = 0.150 | 28.9 p = 0.000 |
| Chronic cough with phlegm | 2.9 | 6.0 p = 0.003 | 11.8 p = 0.001 | 6.7 | 7.9 p = 0.551 | 18.1 p = 0.000 |
Notes: 1p-values: Ex-smokers are compared with never smokers. 2p-values: Smokers are compared with ex-smokers.
The PPVs of coughing, in any form, for any airflow limitation, were higher in men than in women (Table III). Cough with phlegm had higher PPV for severe airflow limitation in women than in men, although women had lower prevalence of this condition (Tables I and III). The independent role of sex, age, smoking status, and the different symptoms for airflow limitation is shown in Table IV. Four significant symptoms had ORs between 1.4 and 1.8 for any airflow limitation, and between 1.6 and 2.4 for severe airflow limitation. Current smoking had the highest OR for both airflow limitation categories (5.8 and 3.3, respectively). A three-symptom-score based on the significant symptoms in Table IV, wheezing, dyspnoea (either on unhurried or quick walking), and daily cough with phlegm was calculated, and positive predictive values of the scores according to smoking status are given in Table V. Among subjects with no symptoms 5.1% never smokers and 31.2% current smokers had airflow limitation. In never smokers and ex-smokers the risk of having any airflow limitation increased by 2–3 times at between one and two symptoms. Ex-smokers with two or three symptoms had a similar risk of any airflow limitation to all current smokers, 31.2% and 35.3%, respectively, whereas never smokers did not reach this risk even with a full score of symptoms. In all smoking groups the risk of severe airflow limitation increased by any increase in symptom score.
Table III.
Positive predictive value (PPV) of reported symptoms for any airflow limitation1 and severe airflow limitation1 for 2154 women and 1800 men 60 years and older.
| Any airflow limitation |
Severe airflow limitation |
|||
| Women | Men | Women | Men | |
| Reported symptoms | PPV (%) | PPV (%) | PPV (%) | PPV (%) |
| Wheezing | 25.5 | 32.7 | 9.7 | 10.5 |
| Dyspnoea on unhurried walking | 30.7 | 36.4 | 15.9 | 16.4 |
| Dyspnoea on quick walking | 18.7 | 27.2 | 5.6 | 8.2 |
| Daily cough in periods | 26.6 | 35.4 | 10.1 | 10.8 |
| Daily cough with phlegm | 31.7 | 40.0 | 14.5 | 11.9 |
| Chr. cough with phlegm | 37.0 | 40.4 | 17.3 | 14.2 |
Notes: See notes 2 and 3 to Table I, respectively.
Table IV.
Odds ratios (OR), with 95% confidence intervals (CI), of gender, age, smoking status, and symptoms, in the development of any airflow limitation and severe airflow limitation, in 3919 subjects aged 60 years or more.
| Any airflow limitation |
Severe airflow limitation |
|||
| OR | 95% CI | OR | 95% CI | |
| Male gender | 1.2 | 1.0–1.4 | 1.1 | 0.8–1.6 |
| Age over 70 | 1.0 | 1.0–1.0 | 1.1 | 1.0–1.1 |
| Ex-smoker | 2.4 | 1.9–3.1 | 2.4 | 1.4–4.0 |
| Current smoker | 5.8 | 4.5–7.5 | 3.3 | 1.9–5.6 |
| Wheezing | 1.5 | 1.2–1.8 | 2.4 | 1.7–3.5 |
| Dyspnoea on unhurried walking | 1.8 | 1.2–2.6 | 2.4 | 1.4–4.1 |
| Dyspnoea on quick walking | 1.4 | 1.1–1.6 | 2.4 | 1.6–3.6 |
| Daily cough | 1.1 | 0.8–1.5 | 1.3 | 0.8–2.2 |
| Daily cough with phlegm | 1.6 | 1.1–2.4 | 1.6 | 0.9–3.0 |
| Chronic cough with phlegm | 1.2 | 0.8–1.7 | 1.4 | 0.8–2.5 |
Notes: See notes 2 and 3 to Table I, respectively.
Table V.
The risk of having any airflow limitation1 or severe airflow limitation1 according to number of symptoms (wheezing, both forms of dyspnoea, and cough with phlegm), according to smoking status, in 3919 subjects aged 60 years or more (women and men are analysed together).
| Never smokers |
Ex smokers |
Current smokers |
|||||||
| Number of symptoms | n | Moderate to severe airflow limitation (%) | Severe airflow limitation (%) | n | Moderate to severe airflow limitation (%) | Severe airflow limitation (%) | n | Moderate to severe airflow limitation (%) | Severe airflow limitation (%) |
| 0 | 688 | 5.1 | 0.6 | 735 | 10.6 | 1.1 | 317 | 31.2 | 2.8 |
| 1 | 443 | 6.3 | 1.6 | 556 | 15.6 | 2.9 | 293 | 32.4 | 4.4 |
| 2 | 155 | 15.5 | 4.5 | 295 | 27.1 | 10.5 | 201 | 36.8 | 9.5 |
| 3 | 40 | 15.0 | 5.0 | 90 | 44.4 | 20.0 | 106 | 52.8 | 22.6 |
Note: See notes 2 and 3 to Table I, respectively.
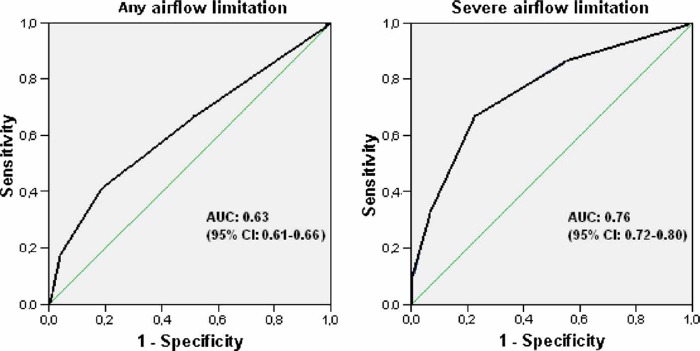
The ROC curves of the symptoms score for airflow limitation and severe airflow limitation gave areas under curve of 0.63 and 0.76, respectively (Figure 1).
Figure 1. .
Diagnostic values of a three-symptom score for any airflow limitation and severe airflow limitation for women and men together. Note: AUC = area under curve.
Discussion
Symptoms play an important role and should be emphasized when searching for airflow limitation in the elderly. The risk of any airflow limitation is considerable in ex-smokers with two or more respiratory symptoms, as it is in all current smokers. The risk of severe airflow limitation increases exponentially by increasing amount of symptoms in both ex-smokers and current smokers. The study confirms what is known from previous epidemiological studies [1], [12], [17–20]: that smoking is a strong contributor to both respiratory symptoms and airflow limitation.
Wheezing was a common symptom and was reported in 22–24% of all our participants, and significantly less frequently in those who were never smokers compared with ex- and current smokers (see Tables I and II). It was found to be an independent predictor of any airflow limitation (see Table IV). Vandevoorde et al. [20] also found wheezing to be strongly linked to COPD. It was the only sign to be significantly more frequent in heavily smoking men with COPD (50%) than in heavily smoking men without COPD (26.9%). Van Schayck et al. [17] found in their review paper cough, phlegm, dyspnoea, and wheezing as discriminating symptoms in COPD (OR 2.4–3.6). Freeman et al. [21] found the best questions discriminating for COPD were age, dyspnoea on exertion, and wheezing.
Reporting dyspnoea on unhurried walking was rarely reported (3–4%), and there were no significant differences in never smokers compared with ex-smokers or current smokers (see Table II). However, this symptom turned out to be an important predictor of any airflow limitation, with the highest OR of the four significant symptoms.
Never-smoking women with BMI ≥ 30 reported being breathless more often than never-smoking men, on both unhurried and quick walking. Obese subjects have an enhanced perception of dyspnoea [22]. Smokers usually have lower BMI than the general population [23]. Overweight may be an explanation for over-reporting breathlessness in never-smoking women. When a normal weight patient reports dyspnoea on unhurried walking, the doctor has a particular reason to consider airflow limitation.
Reporting dyspnoea when walking quickly on level ground or slightly upwards was very common (see Tables I and II). Almost half of all our participants report this symptom, regardless of sex or smoking status, except for never-smoking men. Vrijhoef et al. [18] regarded breathlessness in moderate and heavy smokers as an early sign of COPD. The patients in their study were few and very selected, and hard to compare with our survey. All our participants were 60 years and older. Dyspnoea is usually more prominent in the elderly [11]. GOLD [1] considers dyspnoea as a hallmark symptom, and Ries [12], who deals with dyspnoea in a review article, says: “Yet in the primary care setting, even a few simple questions related to the presence and extent of dyspnoea may yield substantial diagnostic and prognostic information”. Among the elderly, however, dyspnoea on quick walking as the only symptom seems to be of limited discriminating value when searching for airflow limitation.
In our study coughing in any form had PPVs for any airflow limitation of 26.6–37.0% in women, and 35.4–40.4% in men, and was more common in men than in women, irrespective of smoking status (see Table II). All types of coughing were significantly more common in smokers than in ex-smokers and never smokers (see Table II). Calverley et al. [24] found similar frequencies of coughing to ours: chronic cough in 5.3% of non-COPD and 13.6% of COPD patients. They considered wheeze and phlegm to be the two most important symptoms in the diagnosis of COPD. To report cough, especially with phlegm, may be viewed to be not very feminine and therefore reported less by females. It was interesting that coughing with phlegm in our study was a stronger predictor of severe airflow limitation in women than in men (see Table III), despite a lower reporting rate of the condition. Phlegm was also an important symptom according to Holleman et al. [19]. In their review paper, wheezing, coughing, and sputum production were the most important symptoms of airflow limitation. Geijer et al. also found coughing to be independently associated with the presence of mild COPD [25].
Lindberg et al. [26] followed a Swedish population for 10 years, to determine the cumulative incidence of COPD. They evaluated respiratory symptoms prior to the development of COPD, and found that those who developed COPD during the 10-year period reported significantly more symptoms at the start of the survey than those who did not develop COPD. The GOLD COPD stage 0, defined as “at risk”: “Chronic cough and sputum production; lung function is still normal” [1], has been removed in the latest version [4]. Although there are limited data supporting the relation between development of COPD and people at risk [27], [28], the exclusion of stage 0 can be questioned. Excluding symptoms may reduce the GP's attention towards the clinical presentation when considering COPD.
The omission of reversibility testing is a weakness of our study, which we have discussed previously [10]. Not all subjects with moderate airflow limitation have COPD. That is why we have chosen to use the term airflow limitation in this study. Further examinations are needed before the diagnosis of COPD can be established.
In conclusion, respiratory symptoms are important clues in the diagnosis of airflow limitation in the elderly. In general practice there should be a low threshold for undertaking spirometry in current smokers. This study has demonstrated that lung function testing should also be kept in mind in ex-smokers with respiratory symptoms.
Acknowledgements
The survey was funded by the University of Tromsø, the National Health Screening Service, and the Lung Fund, Department of Pulmonology, University Hospital of North Norway. Thanks are offered to all the inhabitants of Tromsø City who participated in this survey and to the three spirometry technicians Anne Britt Larssen, Liv Kirsti Jørgensen, and Eva Solstad.
References
- 1.Lenfant Claude, Khaltaev Nikolai., editors. Global initiative for Chronic Obstructive Lung Disease, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease NHLBI/WHO workshop report. 2701; National Institutes of Health, National Heart, Lung, and Blood Institute. 2001; 2005. [Google Scholar]
- 2.Sullivan SD, Buist AS, Weiss K. Health outcomes assessment and economic evaluation in COPD: Challenges and opportunities. Eur Respir J. 2003;21:1S–3S. doi: 10.1183/09031936.03.00077603. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 5.Pierson DJ. Clinical practice guidelines for chronic obstructive pulmonary disease: A review and comparison of current resources. Respir Care. 2006;51:277–88. [PubMed] [Google Scholar]
- 6.BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax. 1997;52((Suppl 5)):S1–28. [PMC free article] [PubMed] [Google Scholar]
- 7.Celli BR, Macnee W. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 8.Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:822–32. doi: 10.1183/09031936.06.00145104. [DOI] [PubMed] [Google Scholar]
- 9.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20:1117–22. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 10.Medbo A, Melbye H. Lung function testing in the elderly: Can we still use FEV(1)/FVC < 70% as a criterion of COPD? Respir Med. 2007;101:1097–1105. doi: 10.1016/j.rmed.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen F, Mehlsen J, Raymond I, Atar D, Skjoldborg US, Hildebrandt PR. Evaluation of dyspnoea in a sample of elderly subjects recruited from general practice. Int J Clin Pract. 2007;61:1481–91. doi: 10.1111/j.1742-1241.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 12.Ries AL. Impact of chronic obstructive pulmonary disease on quality of life: The role of dyspnea. Am J Med. 2006;119:12–20. doi: 10.1016/j.amjmed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Chapman KR. The needs of patients with COPD can be met by more aggressive treatment of symptoms. Prim Care Resp J. 2002;11:15–19. [Google Scholar]
- 14.Questionnaire on Respiratory Symptoms. (1986). Medical Research Council; [Google Scholar]
- 15.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 16.Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Forced spirometry reference values for Norwegian adults: The Bronchial Obstruction in Nord-Trondelag Study. Eur Respir J. 2001;18:770–9. doi: 10.1183/09031936.01.00255301. [DOI] [PubMed] [Google Scholar]
- 17.Van Schayck CP, Halbert RJ, Nordyke RJ, Isonaka S, Maroni J, Nonikov D. Comparison of existing symptom-based questionnaires for identifying COPD in the general practice setting. Respirology. 2005;10:323–33. doi: 10.1111/j.1440-1843.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 18.Vrijhoef HJ, Diederiks JP, Wesseling GJ, van Schayck CP, Spreeuwenberg C. Undiagnosed patients and patients at risk for COPD in primary health care: Early detection with the support of non-physicians. J Clin Nurs. 2003;12:366–73. doi: 10.1046/j.1365-2702.2003.00736.x. [DOI] [PubMed] [Google Scholar]
- 19.Holleman DR, Jr, Simel DL. Does the clinical examination predict airflow limitation? JAMA. 1995;273:313–9. [PubMed] [Google Scholar]
- 20.Vandevoorde J, Verbanck S, Gijssels L, et al. Early detection of COPD: A case finding study in general practice. Respir Med. 2007;101:525–30. doi: 10.1016/j.rmed.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Freeman D, Nordyke RJ, Isonaka S, et al. Questions for COPD diagnostic screening in a primary care setting. Respir Med. 2005;99:1311–18. doi: 10.1016/j.rmed.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG. Int J Obes. 2007. Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Baum CL. Health Econ. 2008. The effects of cigarette costs on BMI and obesity. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Calverley PM, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D. Development of a population-based screening questionnaire for COPD. COPD. 2005;2:225–32. [PubMed] [Google Scholar]
- 25.Geijer RM, Sachs AP, Verheij TJ, Lammers JW, Salome PL, Hoes AW. Are patient characteristics helpful in recognizing mild COPD (GOLD I) in daily practice? Scand J Prim Health Care. 2006;24:237–42. doi: 10.1080/02813430601016894. [DOI] [PubMed] [Google Scholar]
- 26.Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, Lundback B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127:1544–52. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 27.Ekberg-Aronsson M, Pehrsson K, Nilsson JA, Nilsson PM, Lofdahl CG. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res. 2005;6:98. doi: 10.1186/1465-9921-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166:329–32. doi: 10.1164/rccm.2112048. [DOI] [PubMed] [Google Scholar]