Background: Sirt-1 has been linked to transcriptional silencing and appears to play a key role in inflammation.
Results: Transfection of tenocytes with antisense oligonucleotides against Sirt-1-induced inflammation and apoptosis.
Conclusion: Sirt-1 can regulate p53 and NF-κB activity via deacetylation.
Significance: Down-regulation of Sirt-1 by mRNA interference abrogated the effect of resveratrol on p53 and NF-κB suppression.
Keywords: Antisense RNA, Inflammation, NF-κB Transcription Factor, p53, Resveratrol, Sirt1, Tenocytes
Abstract
Tendon overuse injuries and tendinitis are accompanied by catabolic processes and apoptosis of tenocytes. However, the precise molecular mechanisms of the destructive processes in tendon are not fully understood. Sirt-1, a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, has been linked to transcriptional silencing and appears to play a key role in inflammation. The purpose of this study was to examine whether down-regulation of Sirt-1 using antisense oligonucleotides (ASO) affects inflammatory and apoptotic signaling in tenocytes. Transient transfection of tenocytes with ASO against Sirt-1 induced expression of Bax and other proteins involved in apoptosis (cleaved caspase-3 and poly(ADP-ribose)polymerase), acetylation of tumor suppressor p53, and mitochondrial degradation. Interestingly, Sirt-1 was found to interact directly with p53. In contrast, Sirt-1 activator resveratrol inhibited interleukin-1β (IL-1β)- and nicotinamide-induced NF-κB activation and p65 acetylation and suppressed the activation of IκB-α kinase. Resveratrol also reversed the IL-1β- or nicotinamide-induced up-regulation of various gene products that mediate inflammation (cyclooxygenase-2) and matrix degradation (matrix metalloproteinase-9) that are known to be regulated by NF-κB. Knockdown of Sirt-1 by using ASO abolished the inhibitory effects of resveratrol on inflammatory and apoptotic signaling including Akt activation and SCAX suppression. Down-regulation of histone deacetylase Sirt-1 by mRNA interference abrogated the effect of resveratrol on NF-κB suppression, thus highlighting the crucial homeostatic role of this enzyme. Overall, these results suggest for the first time that Sirt-1 can regulate p53 and NF-κB signaling via deacetylation, demonstrating a novel role for resveratrol in the treatment of tendinitis/tendinopathy.
Introduction
Tenocytes are specialized, fibroblast-like cells of mesenchymal origin that constitute the cellular component of periarticular tendons. They play an important role in producing extracellular matrix and in initiating regenerative responses following injury or degeneration. The poor vascularization of tendon seems to be a major reason for its limited healing capacity (1). Overuse tendon injury and tendinopathies are a growing problem in sports medicine and orthopedic practice that affects a large proportion of the aging Western population (2, 3). They have a major influence on quality of life for the general population and competitive athletes (4).
It has been reported that tenocytes undergo apoptosis in response to hypoxia, oxidative stress, or excessive tensile load (5). Moreover, it has been reported and shown that the genes for the majority of the proinflammatory proteins are regulated by the nuclear transcription factor NF-κB (6, 7). The overexpression of catabolic enzymes by proinflammatory mediators is initiated by a set of proinflammatory signaling pathways. Among them, the activation of NF-κB is critical in the pathophysiology of tendinitis (8). Thus, agents that suppress the expression of inflammatory mediators and the activation of NF-κB have potential for the treatment of tendinopathy.
Treatment of tendon lesions, either primary traumatic or degenerative tendinopathies, is often hampered by contradictory descriptions of the underlying pathologic changes, with a limited repertoire of successful and evidence-based treatments (9). Most treatment strategies, such as the use of non-steroidal anti-inflammatory agents (NSAID) are only able to improve the symptoms. In contrast, other anti-inflammatory agents based on naturally occurring substances, such as resveratrol or curcumin, might actually improve healing. Numerous agents derived from plants can suppress inflammatory cell signaling intermediates (10–12). Furthermore, Aggarwal et al. (10) have shown the inhibition of the NF-κB pathway by naturally occurring anti-inflammatory agents, such as resveratrol or curcumin. Interestingly, these two compounds proved to be the most potent anti-inflammatory and anti-proliferative agents of all agents tested in this study (10).
Resveratrol is a naturally occurring polyphenolic compound that is highly enriched in grapes, red wine, peanuts, and a wide variety of other food sources (10). It is known to have anti-inflammatory, anti-oxidant, anti-viral, and neuroprotective properties (13) and acts as a cancer chemopreventive and chemotherapeutic agent (14). Studies have shown that resveratrol is implicated in the regulation of a variety of cellular responses such as cell cycle arrest, differentiation, and apoptosis in various cancer cell lines (11, 15). Furthermore, resveratrol was identified as a potent activator of Sirtuin activity (16) and additionally as an inhibitor of NF-κB transcription (14, 17, 18).
Antisense oligonucleotides (ASO)2 can be used to selectively down-regulate the translation of target genes (19). Several ASO-based drugs have been developed as gene-silencing therapeutic agents for use in clinical trials and the treatment of diseases such as cancer (20, 21). Thus far there have been no in vitro studies on the effects of ASO against Sirt-1 in the context of tendon biology.
We hypothesize that transcriptional and pharmacological modulation of Sirt-1 regulates inflammation in tendon. To test this hypothesis, we exploited an in vitro model of tendinitis to study the effects of targeting Sirt-1 with ASO on p53 and NF-κB signaling pathways in human tenocytes.
EXPERIMENTAL PROCEDURES
Antibodies
Acetylated lysine (Ac-K-103) antibody was purchased from Cell Signaling Technology (Danvers, MA). Antibodies to MMP-9 and to anti-active caspase-3 were obtained from R&D Systems, Inc. (Heidelberg, Germany). Monoclonal anti-PARP (poly(ADP-ribose)polymerase) antibodies were purchased from BD Biosciences. Cyclo-oxygenase-2 antibody was obtained from Cayman Chemical (Ann Arbor, MI). Antibodies to phospho-Akt were obtained from Biocarta (Hamburg, Germany). Antibodies to β-actin were from Sigma. Antibodies to p53 and Bax were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-scleraxis (SCXA) and polyclonal anti-Sirt-1 were obtained from Abcam PLC (Cambridge, UK). Antibodies against phospho-specific IκBα (Ser-32/36), p65, and phospho-specific p65 (Ser-536) were obtained from Cell Technology (Beverly, MA). Anti-IκB kinase (anti-IKK)-α and (anti-IKK)-β antibodies were obtained from Imgenex (Hamburg, Germany). Secondary antibodies for immunofluorescence were purchased from Dianova (Hamburg, Germany). Alkaline phosphatase-linked sheep anti-mouse and sheep anti-rabbit secondary antibodies for immunoblotting were purchased from Millipore (Schwalbach, Germany). All antibodies were used at concentrations and dilutions recommended by the manufacturer (dilutions ranged from 1:100 for immunomorphological experiments to 1:10,000 for Western blot analysis).
Growth Media, Chemicals, and Cytokines
Growth medium (Ham's F-12/Dulbecco's modified Eagle's medium (50:50) supplemented with 10% fetal calf serum, 25 μg/ml ascorbic acid, 50 IU/ml streptomycin, 50 IU/ml penicillin, 2.5 μg/ml amphotericin B, essential amino acids, and l-glutamine) was obtained from Seromed (Munich, Germany). Trypsin/EDTA (EC 3.4.21.4) was purchased from Sigma. Epon was obtained from Plano (Marburg, Germany). Nicotinamide, protein A/G-Sepharose beads, and resveratrol with purity greater than 98% was purchased from Sigma. A 100 mm stock solution of resveratrol (Mr 228.2) was prepared in ethanol and further diluted in cell culture medium for working concentrations. The maximum final concentration of ethanol in culture was less than 0.1% and had no cytotoxic effects. IL-1β was obtained from Acris Antibodies GmbH (Herold, Germany).
Antisense and Lipofectin-mediated Transfection
The Sirt-1 antisense sequences used in these experiments were designed using a computational neural network mode (55). Tenocytes were plated in 3-cm2 tissue culture dishes or in a 4-well glass plate at a concentration of 2 × 105 cells/dish or 1 × 104 cells/well and were grown to confluence. All transfection experiments were carried out on 50% confluent monolayer cultures. Phosphorothioate-specific oligonucleotides (21-mer) in antisense (ASO) (sequence 5′-GTATTCCACATGAAACAGACA-3′) corresponding to Sirt-1 mRNA and control 21-mer sense oligonucleotides (SO) (sequence 5′-TGTCTGTTTCATGTGGAATAC-3′) were synthesized by eurofins (mwg/operon, Ebersberg, Germany). All ASO and SO were phosphorothioate-modified to protect them from the cell nucleases. Subconfluent tenocytes were transfected with ASO or SO by use of Lipofectin reagent (Invitrogen) according to the manufacturer's instructions by using 0.2, 0.5, 1, 5, and 10 μm antisense or sense in serum-starved medium for 24 h.
Electron Microscopy
Transmission electron microscopy was performed as previously described (50). Briefly, tenocytes cultures were fixed for 1 h in Karnovsky's fixative and then post-fixed in 1% OsO4 solution. After dehydration, pellets were embedded in Epon, and ultrathin cuts were made on a Reichert-Ultracut E. and contrasted with a mixture of 2% uranyl acetate/lead citrate. A transmission electron microscope (Zeiss, Jena, Germany) was used to examine the cultures. To quantify apoptosis or cells with mitochondrial changes, the number of cells exhibiting typical morphological features of apoptotic cell death was determined by scoring 100 cells from 20 different microscopic fields per culture, and the number of apoptotic cells was expressed as an indicator of tenocyte culture degradation.
Immunofluorescence Analysis of Sirt-1
The effect of specific Sirt-1 ASO or SO on the Sirt-1 expression was investigated by an immunocytochemical method as previously described in detail (51). Briefly, the tenocytes were cultured in 4-well glass plates and incubated for 24 h. Serum-starved cells were treated with 0.5 μm ASO or SO and 10 μl/ml Lipofectin for 24 h in serum-starved medium. Glass plates were rinsed 3 times in Hanks' solution (PBS) before methanol fixation and permeabilization of the cells for 1 h at ambient temperature (AT). Cells were rinsed 3 times with a mixture of protease-free bovine serum albumin (BSA) and PBS for 10 min at AT and then incubated with primary antibodies (Sirt-1, 1:30 in PBS/BSA) in a humid chamber overnight at 4 °C. They were gently washed several times with PBS/BSA before incubation with rhodamine-red conjugated secondary antibody for 1.5 h at AT and finally washed again 3 times with aqua dest. Counter-staining was performed with DAPI to visualize the cell nuclei. Samples were evaluated under light microscope (Leica, Germany), and photomicrographs were digitally stored.
Immunoprecipitation and Immunoblotting
A detailed description of the technique used for the following experiments has been previously published (52, 53). Briefly, tenocytes monolayer cultures were rinsed in PBS, and the proteins were extracted with lysis buffer (50 mm Tris/HCl (pH 7.2), 150 mm NaCl, l% (v/v) Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium pyrophosphate, 100 mm sodium fluoride, 0.01% (v/v) aprotinin, pepstatin A (4 μg/ml), leupeptin (10 μg/ml), and 1 mm phenylmethylsulfonyl fluoride (PMSF)) for 30 min on ice. After adjusting the total protein concentration, samples were separated by SDS-PAGE (5, 7.5, or 12% gels) under reducing conditions. For immunoprecipitation, the extracts were precleared by incubating them first with 25 μl of either normal rabbit IgG serum or normal mouse IgG-serum and Staphylococcus aureus cells, then with primary antibodies diluted in wash buffer (0.1% Tween 20, 150 mm NaCl, 50 mm Tris-HCl (pH 7.2), 1 mm CaCl2, 1 mm MgCl2 and 1 mm PMSF) for 2h at 4 °C, and finally with S. aureus cells for 1h at 4 °C. Control immunoprecipitation experiments were performed by incubating the samples with non-immune rabbit anti-mouse IgG alone. S. aureus cells were washed 5 times with wash buffer and once with 50 mm Tris-HCl (pH 7.2) and then boiled in SDS-PAGE sample buffer. Separated proteins were transferred to nitrocellulose membranes and incubated in blocking buffer (5% (w/v) skimmed milk powder in PBS, 0.1% Tween 20) for 1 h at AT. Membranes were incubated overnight with the first antibody diluted in blocking buffer at 4 °C on a shaker, washed 3 times with blocking buffer, and then incubated with the secondary antibody conjugated with alkaline phosphatase for 90 min at AT. Membranes were rinsed with blocking buffer and then washed 3 times in 0.1 m Tris (pH 9.5) containing 0.05 m MgCl2 and 0.1 m NaCl. Specific antigen-antibody complexes were rendered visible using nitro blue tetrazolium and 5-bromo-4-chloro-3-indoylphosphate (p-toluidine salt; Pierce) as the substrates for alkaline phosphatase. Total protein concentration was determined according to the bicinchoninic acid system (Pierce) using bovine serum albumin as a standard. Specific binding was quantified by densitometry using “quantity one” (Bio-Rad).
Cell Viability Assay
Tenocytes were seeded in 96-well plates, allowed to attach for 24 h, and transfected with various concentrations of SO or ASO for another 24 h. Cell viability was determined by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT kit from Sigma) uptake method as previously described (54). Briefly, for the cell viability and proliferation assay, 5000 tenocytes were cultured for 24 h and then treated with 10 ng/ml IL-1β, 10 μl/ml Lipofectin, 0.2, 0.5, 1, 5, and 10 μm SO or ASO for 24 h or left untreated and evaluated after 24 h at 37 °C. Final samples were adjusted in a final volume of 0.1 ml and incubated for 24 h at 37 °C, then examined for cell number. One hundred microliters of medium and 10 μl of MTT solution (5 mg/ml PBS, sterile) were added to each well and incubated for 4 h at 37 °C, 5% CO2. Subsequently, 100 μl of 10% SDS (w/v) in 0.01 m HCl was added, and the samples were further incubated for 18 h. The absorbance was measured at 570/630 nm with a 96-well plate reader (Bio-Rad). Absorbance values were normalized to the values obtained from untreated cells as a base-line value. The assay was performed in triplicate, and the results are provided as the mean values with S.D. from three independent experiments.
Apoptotic Assay
Serum-starved tenocytes were exposed to 10 ng/ml IL-1β alone, 10 μl/ml Lipofectin, 0.2, 0.5, 1, 5, and 10 μm SO or ASO for 24 h or left untreated and evaluated after 24 h at 37 °C. Ultrathin sections of the samples were prepared and evaluated with an electron microscope (TEM 10, Zeiss, Jena, Germany). For statistical analysis, the number of cells with morphological features of apoptotic cell death was determined by scoring 100 cells from 20 different microscopic fields.
Immune Complex Kinase Assay
To examine the effect of resveratrol on IL-1β-, NA-, SO-, and ASO-induced IKK activation, immune complex kinase assays were performed as previously described (22). Briefly, the IKK complex was immunoprecipitated from cell lysates with antibodies against IKK-α and IKK-β and subsequently incubated with protein A/G-agarose beads (Pierce). The beads were washed with lysis buffer and resuspended in a kinase assay solution containing 50 mm HEPES (pH 7.4), 20 mm MgCl2, 2 mm dithiothreitol, 10 μm unlabeled ATP, and 2 mg of the IKK substrate GST-IκBα (amino acids 1–54) and incubated at 30 °C for 30 min. Enzymatic activity of IKK was assessed by phosphorylation of GST-IκBα using a specific antibody against phospho-specific IκBα. To demonstrate the total amounts of IKK-α and IKK-β in each sample, whole cell lysates were transferred to a nitrocellulose membrane after SDS-PAGE. Detection of IKK-α and IKK-β was performed by immunoblotting with either anti-IKK-α or anti-IKK-β antibodies.
Statistical Analysis
Numerical data are expressed as the mean values (±S.D.) for a representative experiment performed in triplicate. The means were compared using Student's t test assuming equal variances. Differences were considered to be statistically significant if the p value was less than 0.05.
RESULTS
Specific ASO against Sirt-1 in Human Tenocytes Leads to Decreased Sirt-1 Expression in Monolayer Culture as Revealed by Immunofluorescence Microscopy
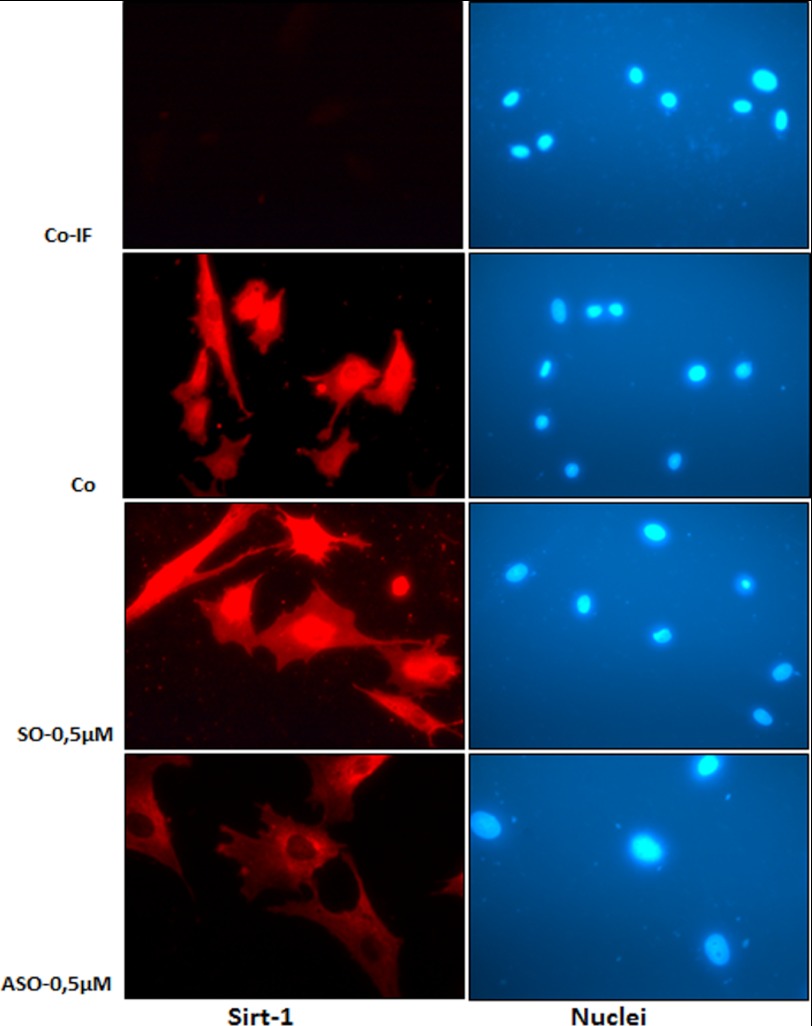
Human tenocytes either served as controls or were transfected with 0.5 μm SO or ASO in the presence of Lipofectin for 24 h, and resistant colonies were collected. The collected tenocytes were subjected to immunolabeling with Sirt-1 antibodies and rhodamine-coupled secondary antibodies. Counterstaining was performed with DAPI to visualize the cell nuclei. Immunofluorescence microscopy showed that treatment of human tenocytes with Sirt-1 ASO (Fig. 1) but not with control SO (Fig. 1) specifically knocked down Sirt-1 protein levels in the nuclei of the cells. Images shown are representative of three independent experiments.
FIGURE 1.
Sirt-1 expression and localization in human tenocytes in monolayer culture after treatment with specific ASO against Sirt-1 examined by immunofluorescence microscopy. Human tenocytes were either transfected with 0.5 μm SO or ASO in the presence of transmembranous carrier Lipofectin (10 μl/ml) for 24 h or were left untreated (Co-IF, without primary antibody; Co, with primary antibody). For immunolabeling, cells were incubated with antibodies against Sirt-1 followed by incubation with rhodamine-coupled secondary antibodies and counterstaining with DAPI to visualize the cell nuclei. Images shown are representative of three independent experiments. a–h, ×160. Co-IF, co-immunofluorescence.
Inhibition of Sirt-1 by ASO Specifically Reduces Sirt-1 Protein Levels in Human Tenocytes
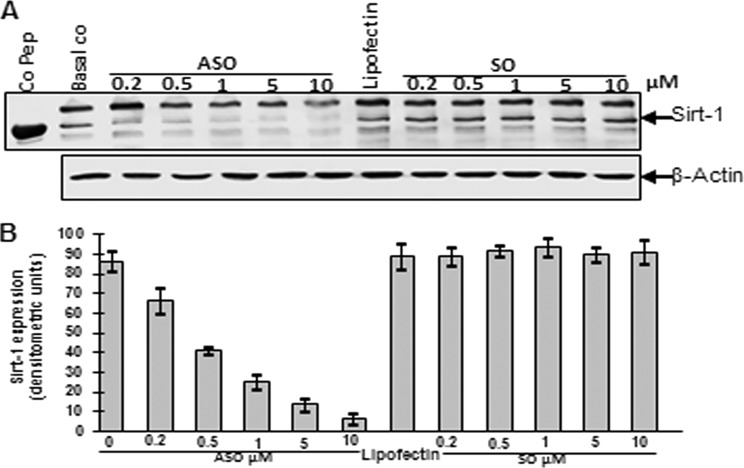
Cells were treated with different concentrations of Sirt-1 SO or ASO (0.2, 0.5, 1, 5, 10 μm) in the presence of Lipofectin for 24 h. Whole cell lysates (500 ng/lane) were fractionated and analyzed by immunoblotting using anti-Sirt-1 and anti-β-actin antibodies (Fig. 2A). Sirt-1 control peptide (Co Pep) was used as a control for antibody specificity. Relative density of Sirt-1 is presented in Fig. 2B. Densitometric evaluation was performed in triplicate, and the results are provided as the mean values with S.D. from three independent experiments. As demonstrated in both Western blot and densitometry, treatment with ASO clearly down-regulated levels of Sirt-1 protein in a dose-dependent manner. The dosage of 10 μm ASO almost completely eliminated Sirt-1 protein in the tenocytes. Contrarily, Lipofectin and Sirt-1 SO treatment had no effect on Sirt-1 protein levels. They remained comparable with the untreated basal control, thereby highlighting the specificity of ASO. Expression of the housekeeping protein β-actin was unaffected.
FIGURE 2.
Western blot analysis and densitometric evaluation of Sirt-1 expression in human tenocytes in monolayer culture after treatment with different concentrations of specific ASO against Sirt-1. Human tenocytes were either transfected with different concentrations of SO or ASO (0.2, 0.5, 1, 5, 10 μm) in the presence of transmembranous carrier Lipofectin (10 μl/ml) for 24 h or left untreated as basal control (Basal co) or treated with Lipofectin (10 μl/ml) alone. A, cells were lysed, and Western blot analysis was performed with antibodies against Sirt-1 protein and housekeeping protein β-actin. Sirt-1 control peptide (Co Pep) served as a control for antibody specificity. B, densitometric evaluation of Sirt-1 expression as revealed by Western blot analysis was performed in triplicate. The results are provided as mean values with S.D. from at least three independent experiments.
Down-regulation of Sirt-1 with ASO Induces p53 Acetylation and Bax Expression
Sirt-1 deacetylates and inactivates p53 protein and through that inhibits p53-dependent apoptosis in response to stress (23, 24). Co-immunoprecipitation studies were carried out to determine the relationship between Sirt-1 and p53 in tenocytes and whether Sirt-1 may either promote or inhibit p53 by deacetylation in tenocytes.
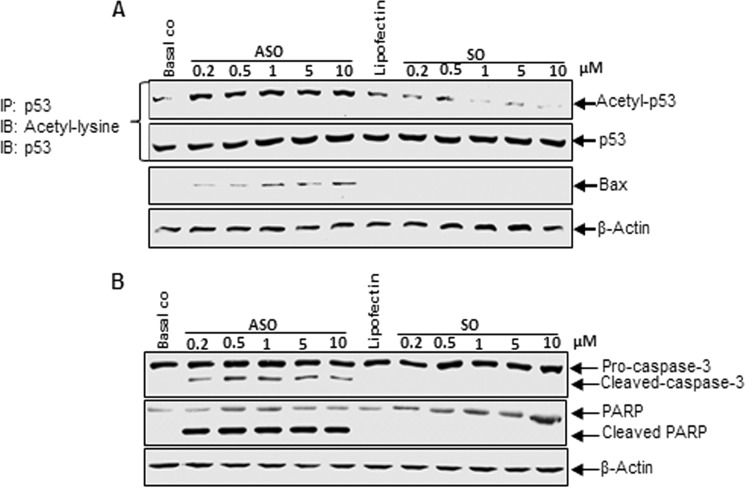
We next examined whether specific antisense oligonucleotides against Sirt-1 induced p53 acetylation, tenocytes were transfected with SO or ASO (0.2, 0.5, 1, 5, 10 μm) derived from the nucleotide sequence coding upstream part of the catalytic domain of Sirt-1 protein in the presence of Lipofectin for 24 h. Immunoprecipitation with anti-p53 antibody and subsequent Western blotting with anti-acetyl lysine and anti-p53 antibodies revealed that ASO induced p53 acetylation in a dose-dependent manner with the strongest effect at 10 μm in the tenocytes (Fig. 3A). In contrast, Lipofectin and Sirt-1 SO treatment had no effect on p53 protein acetylation, suggesting the higher acetylated content of p53 protein is related to down-regulated Sirt-1 expression and p53 could be a substrate for Sirt-1 deacetylase in tenocytes.
FIGURE 3.
Effects of specific ASO against Sirt-1 on activation of tumor suppressor protein p53 and pro-apoptotic proteins Bax, caspase-3m and PARP in human tenocytes in monolayer culture. Human tenocytes were either transfected with different concentrations of SO or ASO (0.2, 0.5, 1, 5, 10 μm) in the presence of Lipofectin (10 μl/ml) for 24 h or left untreated as basal control (Basal co) or treated with Lipofectin (10 μl/ml) alone. Whole cell lysates were immunoprecipitated (IP) for p53 and submitted to Western blot analysis (IB) for detection of acetyl-lysine and p53 or analyzed by Western blotting (IB) with antibodies against Bax and β-actin (A) or caspase-3, PARP and β-actin (B).
To further examine the effect of down-regulation of Sirt-1 on p53 downstream gene products, the expression of the pro-apoptotic Bax protein was probed by Western blot. Bax expression was examined after treatment of the cells with different concentrations of Sirt-1 SO or ASO (0.2, 0.5, 1, 5, 10 μm) in the presence of Lipofectin for 24 h. As shown in Fig. 3A, the level of Bax increased steadily with increasing ASO concentration, but no Bax was detected in Lipofectin- or SO-treated cells. Taken together, these results suggest that down-regulation of Sirt-1 in tenocytes can activate p53 and up-regulate the expression of p53 downstream target genes. Furthermore, the increase in p53 acetylation levels in response to Sirt-1 knockdown may enhance apoptosis in tenocytes.
Down-regulation of Sirt-1 with ASO Leads to Cleavage of Caspase-3 and PARP
To examine whether the increase in acetylated p53 and downstream pro-apoptotic protein Bax by down-regulation of Sirt-1 with ASO leads to further apoptotic signaling via caspase-3 and PARP cleavage, human tenocytes in monolayer were treated with different concentration of SO or ASO (0.2, 0.5, 1, 5, 10 μm) as described above. The cells were lysed and submitted to immunoblotting with antibodies against activated caspase-3 and PARP as well as β-actin. Fig. 3B demonstrates cleavage of caspase-3 and PARP in cells treated with Sirt-1 ASO at all concentrations, whereas there were no signs notable in basal control, Lipofectin- and SO-treated cells. Expression of the housekeeping protein β-actin remained unaffected. These results indicate that Sirt-1 down-regulation by ASO in tenocytes induces apoptosis via caspase-3 and PARP activation.
Down-regulation of Sirt-1 with ASO Inhibits Sirt-1/p53 Interaction
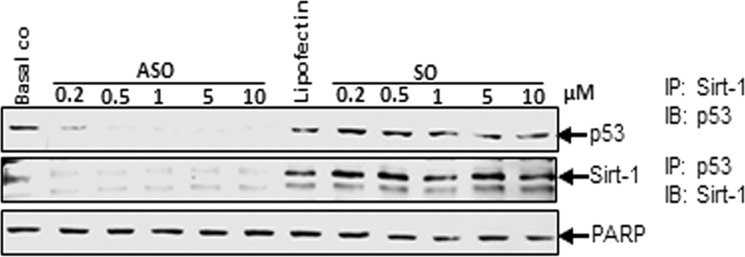
Based on our results that down-regulation of Sirt-1 with ASO led to increased acetylation of tumor suppressor protein p53, we next wanted to prove if p53 is a direct substrate for Sirt-1 deacetylation in tenocytes. Monolayer cultures were treated with different concentrations of SO or ASO (0.2, 0.5, 1, 5, 10 μm) in the presence of Lipofectin for 24 h. Whole cell lysates were immunoprecipitated for Sirt-1 and then probed for p53 by Western blotting or vice versa (Fig. 4). Interestingly, in samples immunoprecipitated for Sirt-1, virtually no p53 could be detected if cells had been treated with ASO. In contrast, control and Lipofectin- and SO-treated cells revealed considerable amounts of p53, suggesting that p53 is directly connected to Sirt-1. Similarly, in samples immunoprecipitated for p53, Sirt-1 protein was strongly diminished if the tenocytes had been treated with ASO compared with levels of Sirt-1 in control or Lipofectin- or SO-treated cells. These results clearly demonstrate that the two proteins directly interact with each other and that p53 is a substrate for Sirt-1 deacetylation in tenocytes.
FIGURE 4.
Effects of specific ASO against Sirt-1 on direct interaction between Sirt-1 and p53. Monolayer cultures of human tenocytes were either transfected with different concentrations of SO or ASO (0.2, 0.5, 1, 5, 10 μm) in the presence of Lipofectin (10 μl/ml) for 24 h or left untreated as basal control or treated with Lipofectin (10 μl/ml) alone. Whole cell lysates were immunoprecipitated (IP) for Sirt-1 and subsequently analyzed by Western blotting (IB) with antibodies against p53 or were immunoprecipitated for p53 and probed for Sirt-1. PARP was examined as a loading control.
Down-regulation of Sirt-1 with ASO Induces Mitochondrial Changes and Apoptosis in Human Tenocytes in Vitro
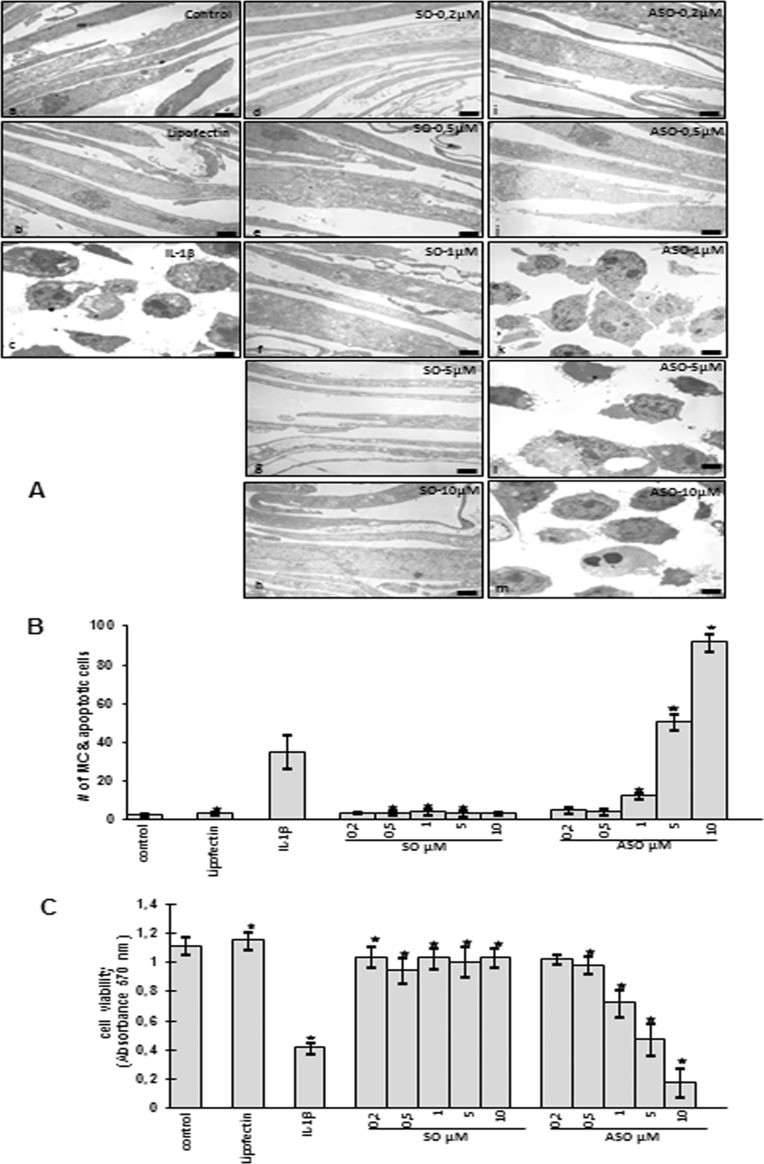
To see whether the down-regulation of Sirt-1 mRNA and protein expression leads to increased cell damage and death of tenocytes after ASO treatment, we examined cell morphology by transmission electron microscopy for cell viability and apoptosis. Untreated (Fig. 5Aa) or Lipofectin-treated (Fig. 5Ab) tenocytes in monolayer cultures showed a typically fibroblast-like shape with small cytoplasmic processes, a large mostly euchromatic nucleus with nucleoli and a well structured cytoplasm. Monolayer cultured tenocytes treated with SO in various concentrations (0.2, 0.5, 1, 5, 10 μm) in the presence of Lipofectin for 24 h equally exhibited a flattened shape with numerous microvilli-like cytoplasmic processes and a well organized cytoplasm comparable with control cells (Fig. 5A, d–h). In contrast, treatment with the inflammatory cytokine IL-1β as a positive control revealed substantial degenerative changes in tenocyte morphology. The cell shapes became rounded, typical microvilli-like processes disappeared, and multiple vacuoles as well as swollen and degenerated cell organelles, especially mitochondria, could be observed. The cell nuclei contained more condensed heterochromatin, and many cells became apoptotic (Fig. 5Ac). To test whether the IL-1β-induced cell degeneration, particularly mitochondrial changes and apoptosis, is mediated via a Sirt-1-dependent pathway, serum-starved tenocytes were transfected with ASO against Sirt-1 in various concentrations (0.2, 0.5, 1, 5, 10 μm) in the presence of Lipofectin for 24 h. The results clearly showed a dose-dependent increase in morphological signs of degeneration and degradation (Fig. 5A, i–m). Treatment with 1 μm AS led to changes such as multiple vacuoles, swelling of rough endoplasmic reticulum, and clustering of swollen mitochondria as well as degeneration of other cell organelles (Fig. 5Ak). These included areas of condensed heterochromatin in the cell nuclei and multiple autophagic cytoplasmic vacuoles. Tenocytes exposed to 5 and 10 μm of ASO became more and more rounded, lost their microvilli-like processes, and underwent apoptosis (Fig. 5A, l–m).
FIGURE 5.
Dose-dependent effects of ASO against Sirt-1 on mitochondrial changes, apoptosis, and cell viability in human tenocytes in vitro. A, shown is transmission electron microscopic evaluation of serum-starved human tenocytes in monolayer cultures treated with different concentrations (0.2, 0.5, 1, 5, or 10 μm) of sirtuin SO (d–h) or ASO (i–m) and Lipofectin (10 μl/ml) as a transmembranous carrier for 24 h. Control cultures were left untreated (a) or treated with 10 μl/ml Lipofectin alone as a negative control (b) for 24 h. As a positive control, cells were stimulated with proinflammatory cytokine IL-1β (10 ng/ml) for 24 h (c). a–m, ×5000; bar = 1 μm. B, to quantify degenerative changes, tenocyte monolayer cultures of A were examined for apoptosis and mitochondrial changes (MC) by counting 100 cells from 20 microscopic fields. The examination was performed in triplicate, and the results are provided as the mean values with S.D. from three independent experiments. Values were compared with the control and statistically significant values with p < 0.05 were designated by an asterisk (*). C, cell viability of monolayer-cultured human tenocytes treated as mentioned above were evaluated by using the MTT method, and the absorbance was measured at 570/630 nm. Absorbance values were normalized to the values obtained from untreated cells as a baseline value. Statistically significant values with p < 0.05 were designated by an asterisk (*). The assay was performed in triplicate, and the results are provided as mean values with S.D. from three independent experiments.
To confirm the morphological findings and quantify them, cells displaying severe mitochondrial changes or apoptosis were counted among 100 cells from 20 microscopic fields. As shown in (Fig. 5B), Sirt-1-specific ASO compounds effectively induced cell death in a dose-dependent manner with 92% apoptotic cells at 10 μm, 50% at 5 μm, and 12% at 1 μm ASO compared with untreated cells. Numbers of apoptotic cells treated with 5 and 10 μm ASO even exceeded those of the positive control treated with IL-1β by 15 and 57%, respectively. Mitochondrial changes and apoptotic cells in control and Lipofectin- and Sirt-1 SO-treated tenocytes as well as in cultures treated with 0.2 and 0.5 μm of ASO did not change compared with controls. These results indicate that the down-regulation of Sirt-1 by ASO can induce severe mitochondrial damage and apoptotic cell death in a dose-dependent manner.
Down-regulation of Sirt-1 with ASO Affects Cell Viability and Proliferation of Human Tenocytes in Vitro
Given the specific activity of ASO on the level of Sirt-1 mRNA and protein, we next tested the effects of Sirt-1 down-regulation on tenocyte viability by using the MTT method. The absorbance was measured at 570/630 nm, and absorbance values were normalized to the values obtained from untreated cells as a base-line value. Fig. 5C demonstrates a severe decrease of 20, 50, and 80% in cell viability of tenocytes treated with 1, 5, and 10 μm of ASO, respectively. Cell viability in IL-1β-treated cells was decreased by 60%, whereas treatment with Lipofectin alone or Sirt-1 SO, similar to treatment with ASO at concentrations of 0.2 and 0.5 μm, had no or only little effects on cell viability of the tenocytes.
Knock-down of Sirt-1 with ASO Modulates NF-κB/Akt-mediated Inflammation and Apoptosis in Human Tenocytes in Vitro
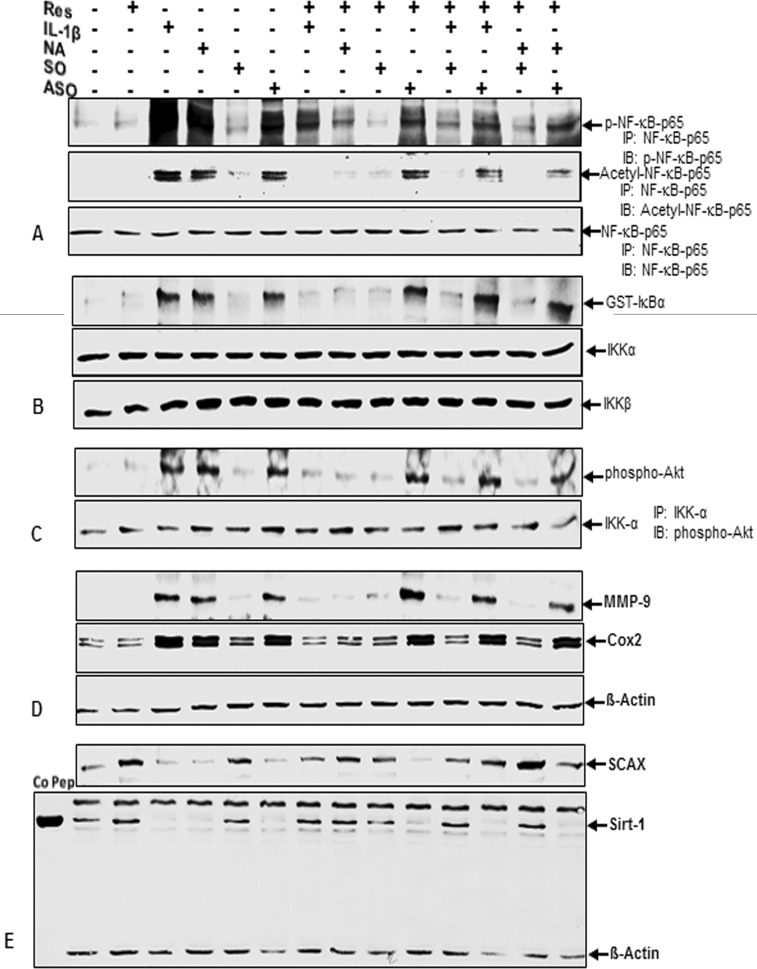
Tenocytes in monolayer culture were either left untreated or treated with resveratrol (5 μm), NA (10 mm), IL-1β (10 ng/ml), or with Sirt-1 SO or ASO (0.5 μm) in the presence of Lipofectin for 24 h, or cells were pretreated with resveratrol (5 μm) for 1 h followed by co-treatment with NA (10 mm), IL-1β (10 ng/ml), Sirt-1 SO, or ASO (0.5 μm) in the presence of Lipofectin for 24 h, or tenocyte cultures were pretreated with resveratrol (5 μm) for 1 h followed by a 1-h treatment with NA (10 mm) or IL-1β (10 ng/ml) and then transfected with either SO or ASO in the presence of Lipofectin for 24 h.
Specific ASO against Sirt-1 Induces Phosphorylation and Acetylation of NF-κB
Whole cell lysates were immunoprecipitated for the p65 subunit of NF-κB and subjected to Western blotting with antibodies against p-p65, anti-acetyl-lysine, and p65 (Fig. 6A). Although NF-κB subunit p65 was continuously detected in all samples, thereby verifying successful immunoprecipitation, phosphorylated and acetylated p65 were markedly up-regulated in tenocytes treated with IL-1β, NA, or ASO alone. Interestingly, this activation of the NF-κB pathway was of equal intensity regardless of which agent was applied. This suggests that down-regulation of Sirt-1 on protein levels by Sirt-1 inhibitor NA or on mRNA levels by ASO leads to the same NF-κB activation mechanisms as pro-inflammatory cytokine IL-1β. Co-treatment with Sirt-1 activator resveratrol reduced NF-κB activation in IL-1β- and NA-, but not in ASO-treated cells, indicating that Sirt-1 suppression on mRNA levels is not reversible by resveratrol and highlighting the crucial role of Sirt-1 in inhibiting NF-κB pathway.
FIGURE 6.
Effects of resveratrol, IL-1β, NA, and ASO against Sirt-1 on NF-κB/Akt signaling pathway and tenocyte transcription factor SCAX in human tenocytes. Serum-starved human tenocytes in monolayer culture (2 × 105 cells/dish) were either left untreated or treated with 5 μm resveratrol (Res), 10 mm NA, 10 ng/ml IL-1β, or 0.5 μm Sirt-1 SO or ASO in the presence of Lipofectin (10 μl/ml) for 24 h or cells were pretreated with resveratrol (5 μm) for 1 h followed by co-treatment with NA (10 mm), IL-1β (10 ng/ml), Sirt-1 SO, or ASO (0.5 μm) in the presence of Lipofectin (10 μl/ml) for 24 h. Other tenocyte cultures were pretreated with resveratrol (5 μm) for 1 h followed by a 1-h treatment with NA (10 mm) or IL-1β (10 ng/ml) and were then transfected with either SO or ASO (0.5 μm) in the presence of Lipofectin (10 μl/ml) for 24 h. Cells were harvested, and whole cell lysates were immunoprecipitated for p65 subunit of NF-κB and then subjected to Western blotting with antibodies against p-p65, acetyl-lysine, and p65 (A), or for IKK and analyzed by an immune complex kinase assay as described under “Experimental Procedures” (B). The level of activation of IKK proteins was then determined by Western blotting using anti-IKK-α, anti-IKK-β, and anti-phospho-specific IκBα antibodies. In another approach, cell lysates were immunoprecipitated for IKK-α and then examined by Western blot analysis with antibodies against phosphorylated Akt (C) or were fractionated (500 ng of protein/lane) on SDS-PAGE and analyzed by Western blotting with antibodies against MMP-9, Cox-2, and housekeeping protein β-actin (D). E, whole cell extracts were prepared, separated by SDS-PAGE, and subjected to Western blot analysis using antibodies against tenocyte transcription factor SCAX, Sirt-1 protein, and housekeeping protein β-actin. Sirt-1 control peptide (Co Pep) was used as a control for antibody specificity. The results shown are representative of three independent experiments.
Resveratrol Inhibits IκB-α Kinase Activity Induced by IL-1β and NA but Not by ASO against Sirt-1
Whole cell extracts were immunoprecipitated with an antibody against IκB kinase (IKK) and then analyzed by an immune complex kinase assay as described under “Experimental Procedures.” To examine the level of activation of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKK-α, anti-IKK-β, and anti-phospho-specific IκBα antibodies. As shown in Fig. 6B, IKK-α and IKK-β were equally detected in all samples, proving that immunoprecipitation was successful. Although in basal control and resveratrol- and Sirt-1 SO-treated tenocytes, practically no phospho-specific IκBα was found, levels were clearly accelerated in IL-1β-, NA-, and ASO-treated cells. Combined treatment with resveratrol fully inhibited IL-1β- and NA-induced activation of IκBα comparable with control levels but had no influence on activation by ASO treatment. These results indicate that Sirt-1 inhibits IκBα phosphorylation and activation of IKK and that resveratrol enhances this.
Resveratrol Inhibits Akt Phosphorylation Induced by IL-1β and NA but Not by ASO against Sirt-1
Whole cell extracts were immunoprecipitated with anti-IKK-α antibody followed by Western blot analysis using anti-phospho-specific Akt and anti-IKK-α antibodies. Fig. 6C clearly demonstrates a strong up-regulation of phosphorylated Akt in IL-1β-, NA-, and ASO-treated tenocytes compared with basal control, resveratrol, and Sirt-1 SO treatment. In contrast, a combination of the agents with resveratrol completely inhibited the effect of IL-1β and NA treatment comparable with controls but did not abolish Akt phosphorylation by ASO treatment, suggesting an essential role for Sirt-1 in inhibiting of upstream kinase Akt pathway. Protein levels of IKK-α remained equal in all samples.
Resveratrol Inhibits NF-κB-dependent Proinflammatory and Matrix Degrading Gene Products in Tenocytes Induced by IL-1β and NA but Not by ASO against Sirt-1
Equal amounts of total proteins were separated by SDS-PAGE and analyzed by immunoblotting using antibodies raised against Cox-2, MMP-9, and β-actin. The results shown in Fig. 6D are representative of three independent experiments. Although housekeeping protein β-actin stayed unaffected, there was a clear increase of Cox-2 and MMP-9 expression in tenocytes treated with IL-1β, NA, and ASO, compared with basal control, resveratrol, and Sirt-1 SO treatment (Fig. 6D). Co-treatment with resveratrol down-regulated NF-κB-dependent gene products to control levels in all treatment groups, except in cells treated with Sirt-1 ASO. These results demonstrate that down-regulation of Sirt-1 on the protein level by Sirt-1 inhibitor NA and on the mRNA level by ASO equally produces an inflammatory response as by stimulation with proinflammatory cytokine IL-1β and that resveratrol can inhibit this inflammation signaling through activation of Sirt-1 if down-regulation of Sirt-1 is induced at the protein level and not at the gene level.
Resveratrol Inhibits Down-regulation of Tenocyte Transcription Factor SCAX and Sirt-1 Protein in Tenocytes Induced by IL-1β and NA but Not by ASO against Sirt-1
Whole cell lysates were fractionated and analyzed by immunoblotting using antibodies raised against SCAX, Sirt-1, and β-actin (Fig. 6E). Sirt-1 control peptide (Co Pep) was used as a control for antibody specificity. Synthesis of the housekeeping protein β-actin remained unaffected. Sirt-1 protein as well as tenocyte transcription factor SCAX both were substantially down-regulated by treatment with IL-1β, NA, and ASO, whereas in cotreated tenocytes resveratrol reinforced Sirt-1 and SCAX proteins up to control levels in all combinations of the agents, except with Sirt-1 ASO. These findings strongly suggest that there is a connection between Sirt-1 protein and tenocyte transcription factor SCAX and that resveratrol is able to enhance tenocyte differentiation and proliferation by up-regulating SCAX via Sirt-1.
DISCUSSION
The goal of this study was to investigate the effect of ASO against Sirt-1 on NF-κB and p53 signaling pathways and NF-κB/p53-regulated gene products that modulate inflammation and apoptosis.
IL-1β is a proinflammatory cytokine that plays an important role in various cellular responses such as inflammation, apoptosis, and proliferation. Many kinases such as MAPKs, Akt, and Src can be activated by IL-1β (25). Acetylation of NF-κB lysine residues is an important process that affects both the DNA binding ability and transcriptional activity of the protein (26, 27). Sirt-1, a NAD-dependent deacetylase for a number of histone and non-histone substrates, belongs to the class III histone deacetylases (HDACs) (23, 28, 29). Members of the class I HDACs regulate the transcriptional activity of NF-κB. HDACs deacetylate NF-κB, resulting in increased IκBα association or loss of transactivation potential of the protein (26, 30, 31). Deacetylation of NF-κB subunit p65 leads to a decrease in NF-κB transcription activity, thereby reducing production of proinflammatory cytokines and anti-apoptotic genes (29).
Resveratrol is a phytoalexin stilbene produced naturally in response to environmental stress in plants and has been reported to have therapeutic potential for the treatment of chronic diseases, such as cardiovascular and pulmonary diseases, diabetes, autoimmune disease, osteoarthritis, and osteoporosis (32–34). Although the mechanisms of resveratrol stimulatory effects in cells are not fully understood, it is known to be a potent activator of Sirt-1 deacetylase activity and modulates Sirt-1 signaling (11, 16, 35).
In this study we found that ASO against Sirt-1, like NA or IL-1β, down-regulated the expression and activation of Sirt-1 protein in human tenocytes. We observed that tenocytes treated with ASO against Sirt-1 exhibited an increased activation and acetylation of the tumor suppressor p53 and high expression or cleavage of p53 downstream gene products Bax, caspase-3, and PARP, suggesting that Sirt-1 must act at a common step to all of these signaling proteins. Tumor suppressor p53 is stimulated by various stress signals and plays an important role in modulating the cell cycle, apoptosis, and DNA repair (36, 37). The activation of p53 occurs posttranslationally, including phosphorylation and acetylation. Acetylation of the lysine residues in p53 increases its DNA binding activity, co-activator recruitment, and cellular senescence (38). p53 has been identified and characterized as one of many substrates for Sirt-1 including transcription factors for chromatin modification, DNA repair, insulin signaling, NF-κB, MyoD, HMG I, E2F, and FOXO (11, 26, 39–41). Consistent with these studies, we demonstrated a direct interaction between Sirt-1 and p53 through immunoprecipitation.
Based on the above data, we speculated that activation of p53 by ASO-induced down-regulation of Sirt-1 could increase protein expression and activity of Bax. The activation of pro-apoptotic protein Bax initiates the release of cytochrome c from mitochondrial membranes and thereby triggers apoptosis (42). Bax can be activated directly by p53 during apoptotic signaling pathway (43) and is also tightly associated with the DNA repair factor ku70 (44). Sirt-1 can deacetylate ku70 and causes inhibition of Bax expression (45). Electron microscopy and Western blot analysis confirmed that knockdown of Sirt-1 by ASO led to an increase in apoptosis and of Bax protein, suggesting that down-regulation of Sirt-1 is involved in Bax expression.
In addition, we showed that knockdown of Sirt-1 by ASO caused NF-κB activation. This activation was mediated through the stimulation of IKK, which led to up-regulated phosphorylation of IκBα. It is known that phosphorylation and degradation of IκBα leads to activation and release of NF-κB (46). We also investigated how knockdown of Sirt-1 activates IKK by examining its effects on kinases that function upstream of IKK, such as Akt (47). The results showed that Akt-induced NF-κB activation is at least in part activated by Sirt-1 ASO, IL-1β, and NA. This suggests that Akt is one of the main upstream stimulatory kinases that is modulated by Sirt-1. We and other laboratories have shown that Akt has been implicated in the phosphorylation of the p65 subunit of NF-κB (22, 48).
Sirt-1 inhibition stimulated activation of IKK, phosphorylation of IκB-α, and p65 phosphorylation and acetylation. This correlated with up-regulation of NF-κB-regulated gene products involved in cell proliferation, inflammation, and apoptosis. Resveratrol reversed the IL-1β or NA-induced up-regulation of various gene products that mediate inflammation, matrix degradation, and apoptosis, all known to be regulated by NF-κB, but not the effects of ASO against Sirt-1. These results suggest that down-regulation of Sirt-1 by mRNA interference abrogates its suppressive effects on p53 and NF-κB, thus highlighting the importance of Sirt-1 for cell survival. To our knowledge this is the first investigation that describes the effects of ASO against Sirt-1 on NF-κB activation in human tenocytes.
The tendon-specific transcription factor SCXA is required for expression of tendon-specific ECM genes (49). We found that ASO against Sirt-1, NA, and IL-1β down-regulated the expression of SCXA in tenocytes, whereas resveratrol up-regulated it. Thus, resveratrol stimulates tenocytes at least in part through activation of the tenogenic transcription factor scleraxis, enhancing transcription of tendon-associated genes in a SCXA-dependent fashion.
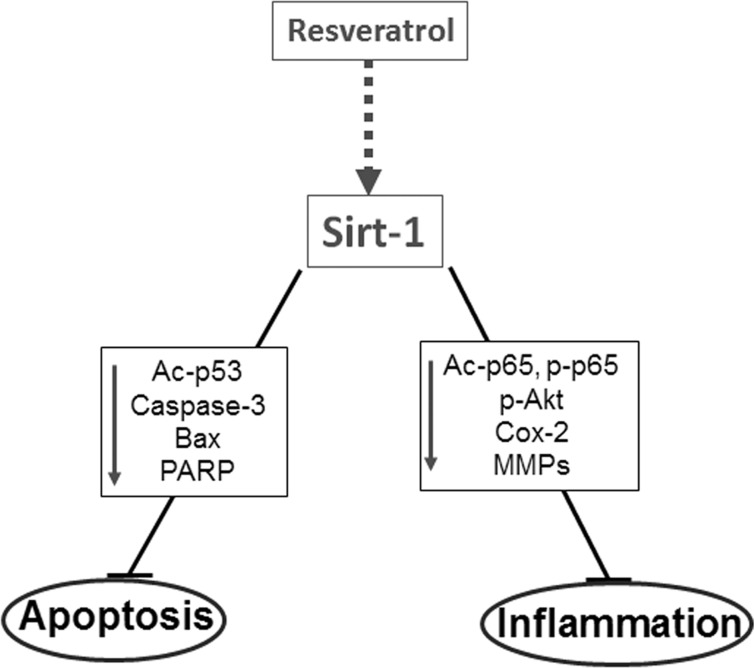
In conclusion, we have shown that down-regulation of Sirt-1 by ASO leads to similar effects as caused by stimulation with IL-1β or NA such as enhanced inflammatory signaling, reduced cell survival, and activation of NF-κB in human tenocytes. In contrast, Sirt-1 activation by resveratrol suppresses NA- and IL-1β-induced inflammatory signaling and apoptosis through deacetylation of NF-κB subunit p65 and tumor suppressor p53, thereby inhibiting the activation pathway of NF-κB- and p53-mediated apoptosis (Fig. 7). Furthermore, resveratrol did not suppress the negative cellular effects of ASO against Sirt-1, hereby highlighting the crucial role of this enzyme. Our results suggest that up-regulation of Sirt-1 appears to be an important consequence of resveratrol on tenocytes and may be useful in the development of future therapies for the treatment of tendinitis.
FIGURE 7.
Schematic diagram shows resveratrol anti-apoptotic and anti-inflammatory mechanism of action in tenocytes.
Acknowledgments
We gratefully acknowledge Christina Pfaff, Ursula Schwikowski, Katharina Sperling, and Kawsar Bhuiyan for excellent technical assistance.
Footnotes
- ASO
- antisense oligonucleotide
- SO
- sense oligonucleotide
- PARP
- poly(ADP-ribose)polymerase
- SCXA
- scleraxis
- AT
- ambient temperature
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IKK
- IκB kinase
- HDAC
- histone deacetylase
- NA
- nicotinamide
- MC
- mitochondrial changes.
REFERENCES
- 1. Favata M., Beredjiklian P. K., Zgonis M. H., Beason D. P., Crombleholme T. M., Jawad A. F., Soslowsky L. J. (2006) Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J. Orthop. Res. 24, 2124–2132 [DOI] [PubMed] [Google Scholar]
- 2. Anitua E., Andía I., Sanchez M., Azofra J., del Mar Zalduendo M., de la Fuente M., Nurden P., Nurden A. T. (2005) Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop. Res. 23, 281–286 [DOI] [PubMed] [Google Scholar]
- 3. Järvinen T. A., Kannus P., Maffulli N., Khan K. M. (2005) Achilles tendon disorders. Etiology and epidemiology. Foot Ankle Clin. 10, 255–266 [DOI] [PubMed] [Google Scholar]
- 4. Lian O. B., Engebretsen L., Bahr R. (2005) Prevalence of jumper's knee among elite athletes from different sports. A cross-sectional study. Am. J. Sports Med. 33, 561–567 [DOI] [PubMed] [Google Scholar]
- 5. Scott A., Khan K. M., Duronio V. (2005) IGF-I activates PKB and prevents anoxic apoptosis in Achilles tendon cells. J. Orthop. Res. 23, 1219–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding G. J., Fischer P. A., Boltz R. C., Schmidt J. A., Colaianne J. J., Gough A., Rubin R. A., Miller D. K. (1998) Characterization and quantitation of NF-κB nuclear translocation induced by interleukin-1 and tumor necrosis factor-α. Development and use of a high capacity fluorescence cytometric system. J. Biol. Chem. 273, 28897–28905 [DOI] [PubMed] [Google Scholar]
- 7. Tak P. P., Gerlag D. M., Aupperle K. R., van de Geest D. A., Overbeek M., Bennett B. L., Boyle D. L., Manning A. M., Firestein G. S. (2001) Inhibitor of nuclear factor κB kinase β is a key regulator of synovial inflammation. Arthritis Rheum. 44, 1897–1907 [DOI] [PubMed] [Google Scholar]
- 8. Tang J. B., Xu Y., Wang X. T. (2004) Tendon healing in vitro. Activation of NIK, IKKα, IKKβ, and NF-κB genes in signal pathway and proliferation of tenocytes. Plast Reconstr. Surg. 113, 1703–1711 [DOI] [PubMed] [Google Scholar]
- 9. Tsuzaki M., Guyton G., Garrett W., Archambault J. M., Herzog W., Almekinders L., Bynum D., Yang X., Banes A. J. (2003) IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res. 21, 256–264 [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal B. B., Bhardwaj A., Aggarwal R. S., Seeram N. P., Shishodia S., Takada Y. (2004) Role of resveratrol in prevention and therapy of cancer. Preclinical and clinical studies. Anticancer Res. 24, 2783–2840 [PubMed] [Google Scholar]
- 11. Shakibaei M., Buhrmann C., Mobasheri A. (2011) Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 286, 11492–11505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shishodia S., Chaturvedi M. M., Aggarwal B. B. (2007) Role of curcumin in cancer therapy. Curr. Probl. Cancer 31, 243–305 [DOI] [PubMed] [Google Scholar]
- 13. Das S., Das D. K. (2007) Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targets 6, 168–173 [DOI] [PubMed] [Google Scholar]
- 14. Jang M., Cai L., Udeani G. O., Slowing K. V., Thomas C. F., Beecher C. W., Fong H. H., Farnsworth N. R., Kinghorn A. D., Mehta R. G., Moon R. C., Pezzuto J. M. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220 [DOI] [PubMed] [Google Scholar]
- 15. Joe A. K., Liu H., Suzui M., Vural M. E., Xiao D., Weinstein I. B. (2002) Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 8, 893–903 [PubMed] [Google Scholar]
- 16. Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 17. Holmes-McNary M., Baldwin A. S., Jr. (2000) Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. Cancer Res. 60, 3477–3483 [PubMed] [Google Scholar]
- 18. Manna S. K., Mukhopadhyay A., Aggarwal B. B. (2000) Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κ B, activator protein-1, and apoptosis. Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 164, 6509–6519 [DOI] [PubMed] [Google Scholar]
- 19. Scherer L. J., Rossi J. J. (2003) Approaches for the sequence-specific knockdown of mRNA. Nat. Biotechnol. 21, 1457–1465 [DOI] [PubMed] [Google Scholar]
- 20. Pan W. H., Clawson G. A. (2006) Antisense applications for biological control. J. Cell. Biochem. 98, 14–35 [DOI] [PubMed] [Google Scholar]
- 21. Pirollo K. F., Rait A., Sleer L. S., Chang E. H. (2003) Antisense therapeutics. From theory to clinical practice. Pharmacol. Ther. 99, 55–77 [DOI] [PubMed] [Google Scholar]
- 22. Boring C. C., Squires T. S., Tong T. (1991) Cancer statistics, 1991. CA Cancer J. Clin. 41, 19–36 [DOI] [PubMed] [Google Scholar]
- 23. Luo J., Nikolaev A. Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. (2001) Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 [DOI] [PubMed] [Google Scholar]
- 24. Vaziri H., Dessain S. K., Ng Eaton E., Imai S. I., Frye R. A., Pandita T. K., Guarente L., Weinberg R. A. (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 [DOI] [PubMed] [Google Scholar]
- 25. Dinarello C. A. (1997) Interleukin-1. Cytokine Growth Factor Rev. 8, 253–265 [DOI] [PubMed] [Google Scholar]
- 26. Chen L. f., Fischle W., Verdin E., Greene W. C. (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 27. Kiernan R., Brès V., Ng R. W., Coudart M. P., El Messaoudi S., Sardet C., Jin D. Y., Emiliani S., Benkirane M. (2003) Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 278, 2758–2766 [DOI] [PubMed] [Google Scholar]
- 28. Guarente L., Picard F. (2005) Calorie restriction. The SIR2 connection. Cell 120, 473–482 [DOI] [PubMed] [Google Scholar]
- 29. Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., Mayo M. W. (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ashburner B. P., Westerheide S. D., Baldwin A. S., Jr. (2001) The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 21, 7065–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhong H., May M. J., Jimi E., Ghosh S. (2002) The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Mol. Cell 9, 625–636 [DOI] [PubMed] [Google Scholar]
- 32. Cao Z., Li Y. (2004) Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes. Protection against oxidative and electrophilic injury. Eur. J. Pharmacol. 489, 39–48 [DOI] [PubMed] [Google Scholar]
- 33. Mizutani K., Ikeda K., Kawai Y., Yamori Y. (1998) Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 253, 859–863 [DOI] [PubMed] [Google Scholar]
- 34. Savouret J. F., Quesne M. (2002) Resveratrol and cancer. A review. Biomed. Pharmacother 56, 84–87 [DOI] [PubMed] [Google Scholar]
- 35. Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E. A., Caldwell S. D., Napper A., Curtis R., DiStefano P. S., Fields S., Bedalov A., Kennedy B. K. (2005) Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 280, 17038–17045 [DOI] [PubMed] [Google Scholar]
- 36. Ryan K. M., Phillips A. C., Vousden K. H. (2001) Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13, 332–337 [DOI] [PubMed] [Google Scholar]
- 37. Vogelstein B., Lane D., Levine A. J. (2000) Surfing the p53 network. Nature 408, 307–310 [DOI] [PubMed] [Google Scholar]
- 38. Sakaguchi K., Herrera J. E., Saito S., Miki T., Bustin M., Vassilev A., Anderson C. W., Appella E. (1998) DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12, 2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gu W., Roeder R. G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 [DOI] [PubMed] [Google Scholar]
- 40. Haigis M. C., Guarente L. P. (2006) Mammalian sirtuins. Emerging roles in physiology, aging, and calorie restriction. Genes Dev. 20, 2913–2921 [DOI] [PubMed] [Google Scholar]
- 41. Martínez-Balbás M. A., Bauer U. M., Nielsen S. J., Brehm A., Kouzarides T. (2000) Regulation of E2F1 activity by acetylation. EMBO J. 19, 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levine A. J. (1997) p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 [DOI] [PubMed] [Google Scholar]
- 43. Chipuk J. E., Kuwana T., Bouchier-Hayes L., Droin N. M., Newmeyer D. D., Schuler M., Green D. R. (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, 1010–1014 [DOI] [PubMed] [Google Scholar]
- 44. Matsushita N., Takami Y., Kimura M., Tachiiri S., Ishiai M., Nakayama T., Takata M. (2005) Role of NAD-dependent deacetylases SIRT1 and SIRT2 in radiation and cisplatin-induced cell death in vertebrate cells. Genes Cells 10, 321–332 [DOI] [PubMed] [Google Scholar]
- 45. Cohen H. Y., Miller C., Bitterman K. J., Wall N. R., Hekking B., Kessler B., Howitz K. T., Gorospe M., de Cabo R., Sinclair D. A. (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392 [DOI] [PubMed] [Google Scholar]
- 46. Sakurai H., Miyoshi H., Toriumi W., Sugita T. (1999) Functional interactions of transforming growth factor β-activated kinase 1 with IκB kinases to stimulate NF-κB activation. J. Biol. Chem. 274, 10641–10648 [DOI] [PubMed] [Google Scholar]
- 47. Gustin J. A., Maehama T., Dixon J. E., Donner D. B. (2001) The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor κ B activity. J. Biol. Chem. 276, 27740–27744 [DOI] [PubMed] [Google Scholar]
- 48. Simeonidis S., Stauber D., Chen G., Hendrickson W. A., Thanos D. (1999) Mechanisms by which IκB proteins control NF-κB activity. Proc. Natl. Acad. Sci. U.S.A. 96, 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schweitzer R., Chyung J. H., Murtaugh L. C., Brent A. E., Rosen V., Olson E. N., Lassar A., Tabin C. J. (2001) Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 [DOI] [PubMed] [Google Scholar]
- 50. Shakibaei M., De Souza P. (1997) Differentiation of mesenchymal limb bud cells to chondrocytes in alginate beads. Cell Biol. Int. 21, 75–86 [DOI] [PubMed] [Google Scholar]
- 51. Shakibaei M., John T., Schulze-Tanzil G., Lehmann I., Mobasheri A. (2007) Suppression of NF-kB activation by curcumin leads to inhibition of expression of cyclooxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes. Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 73, 1434–1445 [DOI] [PubMed] [Google Scholar]
- 52. Shakibaei M., John T., De Souza P., Rahmanzadeh R., Merker H. J. (1999) Signal transduction by ß1 integrin receptors in human chondrocytes in vitro: collaboration with IGF-I. Biochem. J. 342, 615–623 [PMC free article] [PubMed] [Google Scholar]
- 53. Shakibaei M., Csaki C., Nebrich S., Mobasheri A. (2008) Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes. Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 76, 1426–1439 [DOI] [PubMed] [Google Scholar]
- 54. Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. (2008) Regulation of inflammation signaling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol, 75, 677–687 [DOI] [PubMed] [Google Scholar]
- 55. Chalk A. M., Sonnhammer E. L. (2002) Computational antisense oligo prediction with a neural network model. Bioinformatics 18, 1567–1575 [DOI] [PubMed] [Google Scholar]