Background: The natural phenotype of mesenchymal stem cells (MSCs) has not been well characterized.
Results: MSCs from bone marrow naturally are CD44−; however, in vitro cultivation results in acquisition of CD44 expression on the cells.
Conclusion: Native MSCs in bone marrow lack CD44 expression.
Significance: Our findings highlight the natural phenotype of MSCs and open new possibilities for prospective isolation of MSCs from bone marrow.
Keywords: Bone Marrow, CD44, Cell Culture, Cell Surface Receptor, Flow Cytometry, Mesenchymal Stem Cells, Microarray
Abstract
Despite significant progress in our understanding of mesenchymal stem cell (MSC) biology during recent years, much of the information is based on experiments using in vitro culture-selected stromal progenitor cells. Therefore, the natural cellular identity of MSCs remains poorly defined. Numerous studies have reported that CD44 expression is one of the characteristics of MSCs in both humans and mice; however, we here have prospectively isolated bone marrow stromal cell subsets from both human and mouse bone marrow by flow cytometry and characterized them by gene expression analysis and function assays. Our data provide functional and molecular evidence suggesting that primary mesenchymal stem and progenitor cells of bone marrow reside in the CD44− cell fraction in both mice and humans. The finding that these CD44− cells acquire CD44 expression after in vitro culture provides an explanation for the previous misconceptions concerning CD44 expression on MSCs. In addition, the other previous reported MSC markers, including CD73, CD146, CD271, and CD106/VCAM1, are also differentially expressed on those two cell types. Our microarray data revealed a distinct gene expression profile of the freshly isolated CD44− cells and the cultured MSCs generated from these cells. Thus, we conclude that bone marrow MSCs physiologically lack expression of CD44, highlighting the natural phenotype of MSCs and opening new possibilities to prospectively isolate MSCs from the bone marrow.
Introduction
Mesenchymal stem cells (MSCs)2 were originally isolated from bone marrow (BM) by their capacity to generate colony-forming unit-fibroblast (CFU-F) in vitro (1). Although there has been significant progress in understanding of the biological features of MSCs, much of the information has been obtained from in vitro studies on culture-expanded cells, which may not represent the phenotype of MSCs in vivo (2–5). Multicolor fluorescence-activated cell sorting (FACS) has been fundamental for definition and prospective isolation of different cell populations of the hematopoietic system over the last 20 years. The recent development of FACS-based protocols for the isolation and characterization of MSCs directly from BM opens the possibility to better identify and characterize non-hematopoietic cell compartments in the BM. In mice, platelet-derived growth factor receptor α (PDGFRα), stem cell antigen-1 (SCA1), CD51, and Nestin are expressed on freshly isolated BM stromal cell populations enriched with MSCs (6–8). In humans, several surface proteins, including Stro-1, CD271, and CD146 may be used as markers for mesenchymal stem and progenitor cells (9–14). In addition, expression of markers, such as CD105, CD90, and CD49A, have been diversely reported to be characteristic of MSCs (15). Among those, CD44 has been reported to be highly expressed on in vitro expanded MSCs from both humans and mice (16–22). CD44 is an adhesion molecule existing in different isoforms that interact with multiple ligands, such as hyaluronan, selectins, collagen, and fibronectin (23). It is widely expressed in multiple cell types, including hematopoietic cells and cancer stem cells (24).
In the present study, by using multicolor FACS, microarray analysis, and a CFU-F assay, we have found that although freshly isolated MSCs from human and mouse BM express the surface markers previously reported to mark early mesenchymal progenitors, they lack expression of CD44. Further characterization of the cells revealed that the CD44+ cells displayed little or no CFU-F activity, whereas the CD44− cells contain almost all the clonogenic cells with multilineage differentiation potentials. However, in vitro culture of the CD44− MSCs and progenitor cells resulted in their conversion to a CD44-positive phenotype, providing an explanation for the previous observations suggesting CD44 as a marker for MSCs. Furthermore, the cultured MSCs derived from the fresh CD44− stromal cells display distinct gene expression profiles of cell adhesion molecules and growth factors as well as cytokines. These findings highlight the importance of in vivo/ex vivo analysis of mesenchymal cells for identifying their physiological properties and suggest that CD44 expression can be used as a negative rather than a positive marker for prospective isolation of MSCs from BM.
EXPERIMENTAL PROCEDURES
Subjects
BM aspirates were obtained from the iliac crest of normal young adult volunteers following informed consent according to procedures approved by the local ethics committee at Karolinska Institute (Stockholm, Sweden). Mouse bones were obtained from adult (3–4-month-old) normal FVB/N mice. Animal procedures were performed with approval from the ethics committee at Linköping University (Linköping, Sweden).
FACS Isolation and Analysis of Human BM MSCs
Mononuclear cells from BM aspirates of healthy adult volunteers were isolated by Ficoll-Hypaque (Lymphoprep, Axis-Shield PoC AS) density centrifugation. The CD45−CD235− cells were enriched by negative selection using CD45 and CD235 microbeads and magnetic-activated cell sorting (Miltenyi Biotec). The cells were then stained with anti-human CD271 CD146, CD105, CD106, CD73, STRO-1, CD29, CD45, and glycophorin A/CD235. Anti-human CD19 was included in the staining in order to exclude possible contamination of B cells in the sorted stromal cells. For information about the antibodies used in the study, see the supplemental material. Dead cells were excluded by propidium iodide staining. The cells were analyzed and sorted on a FACSAria II Sorp (BD Biosciences).
FACS Isolation and Analysis of Mouse MSCs
The BM mononuclear cells from femurs, tibias, and iliac crests of FVB/N mice were isolated using a standard protocol, which was tested in our laboratory without affecting cell surface marker expression. The bones were first crushed in PBS + 10% FBS (PAA) in order to obtain maximal cells in the BM endosteal region prior to enzyme treatment. The marrow cells were collected, and the bone fragments were then treated with 0.1% collagenase II (Worthington) and 0.05% trypsin-EDTA for 45 min at 37 °C. The tubes were shaken every 10 min during incubation. The treatment was stopped by adding ice-cold FBS to reach a final concentration of 20% FBS, and subsequently, the bones were washed with PBS + 10% FBS. The cells were collected and filtered via a 70-μm cell strainer (BD Biosciences). The bone and marrow cells were pooled and spun down at 300 × g for 10 min and then resuspended in PBS + 10% FBS. The stromal cells were first enriched by depleting hematopoietic cells using purified rat anti-mouse antibodies against CD45 and LIN (TER119, B220, CD4, CD8, GR1, and MAC1) and subsequently using sheep anti-rat Dynal beads (Invitrogen). The endothelial cells and the residual hematopoietic cells were visualized by CD31 and goat anti-rat tricolor antibody and/or CD45 and TER119. The dead cells were excluded by propidium iodide staining. The CD44+ and CD44− stromal cells were gated or analyzed based on fluorescent minus one (FMO) controls for CD44 expression on a FACSAria II Sorp sorter (BD Biosciences). For information about the antibodies used in the study, see the supplemental material.
CFU-F Assay
The stromal cells (CD45−LIN−CD31−CD44+ and CD45−LIN−CD31−CD44−) from normal mouse and human BM were sorted and plated into 96-well plates or 12-well plates containing complete Mesencult medium in the MesenCult® proliferation kit for mouse (catalog no. 05511) and human (catalog no. 05411), respectively (Stem Cell Technologies, Vancouver, Canada) in hypoxic condition (1% O2) for 10–12 days (mouse) or 12–14 days (human). The mouse cells were seeded at a density of 10, 40, 100, and 200 cells/well for the CD44− cells and 100, 200, 400, 1000, and 2000 cells/well, and the human cells were plated at 2, 5, and 10 cells/well for the CD44− cells and 10, 50, 100, and 1000 cells/well for the CD44+ cells. The complete MesenCult medium was prepared by mixing 1 part of MSC stimulatory supplements and 4 parts of Mesencult Basal Medium for mouse cells and 1 part of MSC stimulatory supplements and 9 parts of Mesencult Basal Medium for human cells (Stem Cell Technologies). The colonies were stained with Giemsa (Sigma) and scored under an inverted microscope (Leica DMIL, Leica Microsystems, Germany). A cluster of more than 50 cells was counted as one colony, and the images were taken using Leica Application Suite software.
Quantitative RT-PCR
Cells were sorted directly into buffer-RLT (Qiagen) and frozen at −80 °C. RNA extraction and DNase treatment were performed with the RNeasy microkit (Qiagen) according to the manufacturer's instructions for samples containing fewer than 105 cells. Eluted RNA samples were reverse transcribed using SuperScript III and random primers (Invitrogen) according to the protocol supplied by the manufacturer. Real-time quantitative PCRs (Q-PCRs) were performed by mixing 2× TaqMan universal PCR master mix, 20× TaqMan primer/probe mix, RNase-free H2O, and 2.5 μl of cDNA for a final reaction volume of 10 μl. For information about Assays-on-Demand probes, see below for the list of probes used for Q-PCR.
Cell Cycle Analysis
The analysis was performed as described (25). BM mononuclear cells from wild-type FVB/N mice were initially stained with antibodies against CD45, lineage cells, and CD44. After incubation with the cell surface antibodies, the cells underwent fixation with a Cytofix/Cytoperm kit (BD Biosciences) and staining of R-phycoerthrin-anti-KI67 and DAPI. Analysis was performed on a FACSAria II SORP (BD Biosciences).
In Vitro Differentiation Assays
The assay was performed on culture-expanded cells at passages 2–4. The cells were under stimulation with differentiation medium for 3–4 weeks. The medium was changed every 2–3 days. For osteoblast differentiation, the cells were cultured in complete α-minimum Eagle's medium or Dulbecco's modified eagle's medium containing 10% FBS, 10 nm HEPES (1 m), 100 units/ml of penicillin, 100 μg/ml streptomycin, 50 μg/ml ascorbic acid (Sigma), 1–5 × 10−7 m dexamethasone (Sigma), and 10 mm glycerol phosphate. The cells were fixed with 10% formalin or ice-cold methanol, and the calcium deposits were verified by using 1% alizarin red S (Sigma) (pH 4.1) or von Kossa staining (26). For the von Kossa silver nitrate staining method, cultures were fixed in cold methanol for 15–20 min. After rinsing, the fixed plates were incubated with 5% silver nitrate solution under UV light using a UV linker (UVitec, Cambridge, UK). Mineralized nodules were seen as dark brown to black spots. On the other hand, for alizarin red S (sodium alizarin sulfonate) staining, 1% alizarin red S (Sigma) was prepared in distilled water, and the pH was adjusted to 4.1–4.3 using 0.5% ammonium hydroxide. Cultures were stained with alizarin red S for 10–15 min after the fixation. After removal of unincorporated excess dye with distilled water, the mineralized nodules were labeled as red spots. For adipogenesis, the cultures were incubated in DMEM Glumax (Invitrogen) supplemented with 10% FBS, 10 nm HEPES, 100 units/ml penicillin, 100 μg/ml streptomycin, 5–10 μg/ml insulin (Sigma), 0.5 mm isobutylmethylxanthine (Sigma), 1–5 × 10−6 m dexamethasone (Sigma). The cells were fixed with 10% formalin and stained with 0.3% Oil Red O (Sigma) in methanol (Sigma). The chondrocyte differentiation was induced in monolayer culture, and the cells were cultured in 12-well plates in complete DMEM with high glucose (4.5 g/liter) containing 10−7 m dexamethasone, 1% ITS (Sigma), 2 mm sodium pyruvate (Sigma), 0.35 mm proline (Sigma), and 10 ng/ml TGF-β3 (R&D Systems) or complete chondrocyte differentiation medium (mixed by StemXVivo human/mouse chondrogenic supplement (catalogue no. CCM006), and StemXVivo human/mouse chondrogenic base medium (catalogue no. CCM005) in a 1:100 ratio (R&D Systems)). The chondrocyte differentiation was verified by staining of proteoglycan with both 0.1% toluidine blue (Sigma) (pH 2.0 to 2.5) and 1% Alcian blue in 3% acetic acid solution (pH 2.5). Then we removed the excess dye and washed the cells three times with distilled water. Then plates were mounted with Clear MountTM mounting solution (Invitrogen). The images were taken using bright field with Leica Application Suite software.
Microarray Analysis
RNA was extracted from the sorted CD45−LIN−CD44+ and CD44− subsets or culture-expanded MSCs at passages 1–3, labeled, and amplified according to the AffymetrixTM GeneChip expression analysis technical manual. Chips were scanned using a GeneChipTM Scanner 3000. Human genome U133 plus 2.0 chips were normalized using invariant set normalization, and probe level expression values were calculated using the PM-MM model provided by the dCHIP software for dCHIP analysis. For gene set enrichment analysis (GSEA), the data were normalized by RMAExpress software. GSEA of the microarray data was performed according to the instructions (available on the Broad Institute Web site). Gene sets tested included gene ontology (c5.all.v2.5.symbols.gmt), BioCarta (c2.biocarta.v2.5.symbols.gmt), and KEGG (c2.kegg.v2.5.symbols.gmt). After collapsing, there are 20,606 genes left from microarray data sets, and there are 1164 remaining gene sets after gene set size filtering (minimum = 15, maximum = 500). Gene sets with a nominal p value of <0.05 and false discovery rate of <0.25 were considered to be significantly enriched. Those genes occurring in the ranked list before the point at which a maximal GSEA enrichment score is achieved are referred to as the leading edge subset and thus are responsible for the core enrichment observed for a given gene set. Within each gene set, positions of a gene that are farther to the left (red) imply a higher correlation with the CD44-negative phenotype, and positions farther to the right (blue) imply a higher correlation with genes down-regulated upon CD44 expression.
Biochemical Pathway Analysis of Microarray Data
The lists of 2-fold changed genes from the microarray data were applied to the Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics resources version 6.7 for mapping KEGG pathways (27).
Statistical Analysis
The unpaired t test or Mann-Whitney test was used to compare the differences between the cell types based on the data distribution. All reported p values were obtained using GraphPad Prism version 4.0 software, and p < 0.05 was considered statistically significant. The frequencies of CFU-Fs were calculated by either Pearson in Excel or L-Calc software (Stem Cell Technologies).
RESULTS
Freshly Isolated Mesenchymal Stem and Progenitor Cells of Mouse BM Lack Expression of CD44
Because accumulated evidence has suggested that CD44 is a common positive marker on expanded MSCs (28), we wanted to investigate if this protein could be used to purify and characterize primary MSCs ex vivo. We first analyzed CD44 expression in BM stromal cells (CD45−LIN−CD31−) of mouse BM by FACS using an antibody recognizing all forms of CD44 (clone IM7) (Fig. 1A). This revealed that CD44 was expressed on a significant subfraction (45 ± 7%) of BM stromal cells. However, upon investigation of co-expression of CD44 with MSC-associated cell surface markers, including SCA1, PDGFRa/CD140a, and CD51/integrin αv (6, 7, 29), we found that the cells expressing these MSC markers were exclusively detected in the CD44− cell fraction (Fig. 1B). VCAM1/CD106 and CD105 have been reported to be expressed on cultured MSCs (30). However, analysis of the expression of these markers on freshly isolated BM cells suggested that although the CD44− stromal cells display high expression of VCAM1/CD106 and CD105, the expression of these surface molecules is also detected on the CD44+ stromal cells (Fig. 1B). These data suggest that the phenotypically defined MSCs are enriched in the CD44− cells of mouse BM.
FIGURE 1.
Clonogenic mesenchymal stem and progenitor cells lack expression of CD44 in mouse BM. A, one representative FACS profile shows analysis of CD44 expression in CD45−LIN−CD31− cells. The numbers in the panels are mean percentages of the CD44+/− cells, from 10 experiments. B, FACS analysis of expressions of SCA1, CD51, CD90.1, CD105, VCAM1/CD106, and PDGFRa/CD140a in CD45−LIN−CD44+/− cells. The numbers in the panels indicate mean percentages of the gated cells within CD45−LIN−CD31− cells. The data are from 3–10 experiments. C, limiting dilution of CFU-Fs in the CD44+/− cells. The cells were plated at densities of 10, 50, 100, 200 cells for the CD44− cells and 200, 500, 1000, and 2000 cells per well for the CD44+ cells in 96-well plates. The cell dose yielding 37.5% negative wells for CD44− cells was 167, indicated by a dashed line. The 95% confidence interval bands are shown as dotted lines. There were no CFU-Fs observed from the CD44+ cells at any of the doses. D, frequencies of CFU-Fs in the CD44+ and CD44− cells calculated by L-Calc (Stem Cell Technologies). Data were mean ± 95% confidence interval, from three experiments. nd, not detectable. E, morphology of Giemsa-stained CFU-Fs derived from the CD44− cells. F, Q-PCR analysis of expressions of MSC-associated genes. Data are for three independent sorting experiments. Each dot represents the mean of triplicate measurements in each experiment. MSC-associated genes include Fmod, Igf1, Nov, Nes, Col1a1, and Angptl1. The differences between the two cell types were compared by unpaired one-tailed t test. PI, propidium iodide.
To investigate the functional properties of the CD44+ and CD44− stromal cells, we sorted the cells (Fig. 1A) and evaluated their clonogenic potential by a limiting dilution CFU-F assay (Fig. 1, C and D). The frequency of CFU-Fs in the CD44− cells was 1 of 167, whereas no CFU-F could be detected in the CD44+ cells when plated at any of the indicated cell densities. The CFU-Fs generated from the CD44− cells were fibroblast-like, consistent with the immature phenotype of mesenchymal progenitor cells (Fig. 1E). In some of the experiments, CD45+LIN+ cells were sorted for the CFU-F assay as controls. However, no colonies were observed from 200,000 cells plated. These data indicate that the CD44− cells contain almost all CFU-Fs in mouse BM, whereas CD44 expression marks the cells lacking colony-forming capacity.
Nestin and fibromodulin (Fmod) have been reported to mark primary MSCs in mice (31) and humans (32). Consistent with the finding of enrichment of CFU-Fs in the CD44− cell fraction, Q-PCR analysis showed that Nestin and FmoD mRNAs were enriched in the CD44− cells and almost undetectable in the CD44+ cells (Fig. 1F). In addition, the CD44− cells expressed higher levels of mRNA encoding matrix protein and growth factors, including collagen type I (Col1a1), nephroblastoma overexpressed gene (Nov), angiopoietin like-1 (Angptl1), and insulin growth factor 1 (Igf1), all reported to be expressed in BM MSCs (7, 33, 34) (Fig. 1F). In all, these data provide molecular support for the idea that the CD44− cell population is enriched with MSCs and progenitors.
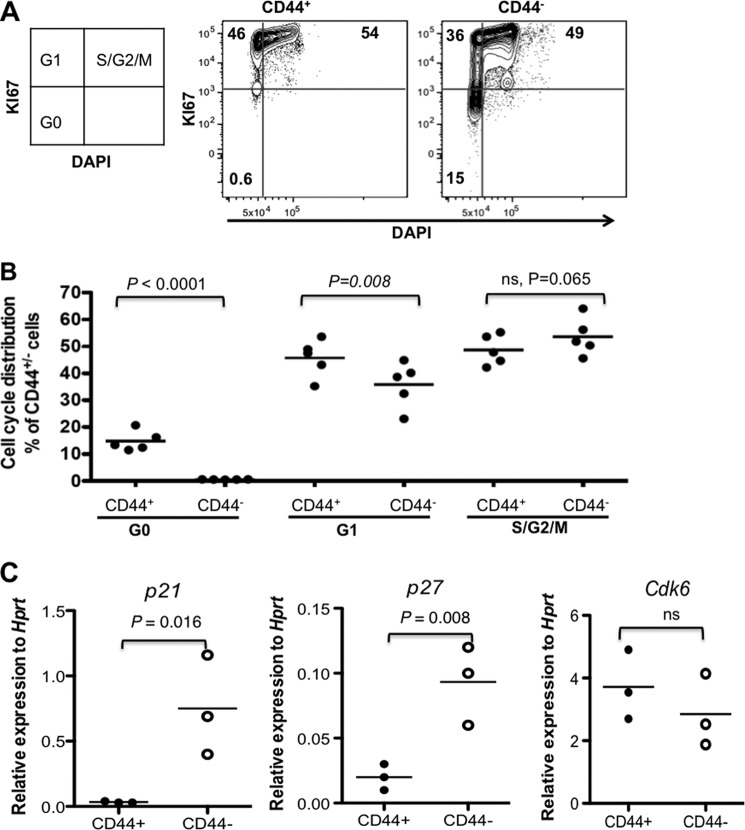
Another feature commonly associated with stem cell populations is quiescence under steady state conditions. In order to investigate the cell cycle status of the CD44+/− cells in mouse BM, we performed cell cycle analysis by simultaneous staining of KI67 and DAPI, revealing that although 0.6% of the CD44+ cells were in G0, around 15% of the CD44− cells resided in a dormant state (G0) (Fig. 2, A and B). Correspondingly, the absolute majority of the CD44+ cells accumulated in cycling (G1 and S/G2/M) stages (Fig. 2), indicating that a proportion of the CD44− cells remained quiescent, whereas most of the CD44+ cells were in the active cell cycle. This was supported by Q-PCR analysis showing up-regulation of the cell cycle inhibitor genes p21Cip and p27 in the CD44− cells, compared with the CD44+ cells (Fig. 2C). These data suggest that the physiologically quiescent cells reside in the CD44− and not the CD44+ cells. Taken together, the phenotypically, functionally, and molecularly defined BM mesenchymal stem and progenitor cells naturally do not express CD44.
FIGURE 2.
Quiescent mesenchymal cells reside in the CD44− stromal cell fraction. A, one representative FACS profile of cell cycle analysis of the CD45−LIN−CD44+ and CD44− cells by KI67 and DNA staining. Cell cycle status within the defined CD45−LIN−CD44+ and CD44− cells was determined by simultaneous two-parameter analysis with DNA content versus KI67 expression. Numbers in quadrants show the percentages of the gated CD44+ and CD44− cells in each of the cell cycle phases (G0, G1, and S/G2/M). B, mean cell cycle distribution of total CD44+ and CD44− cells. Data are from two experiments. C, Q-PCR analysis of cell cycle regulator genes p21, p27, and Cdk6 in the CD44+ and CD44− cells. The data were normalized to endogenous Hprt expression, from two independent sorting experiments. Each dot represents the mean of triplicate measurements for each gene. The differences between the two cell populations are indicated in the panels.
Human BM MSCs Are Enriched in CD44− Mesenchymal Cells
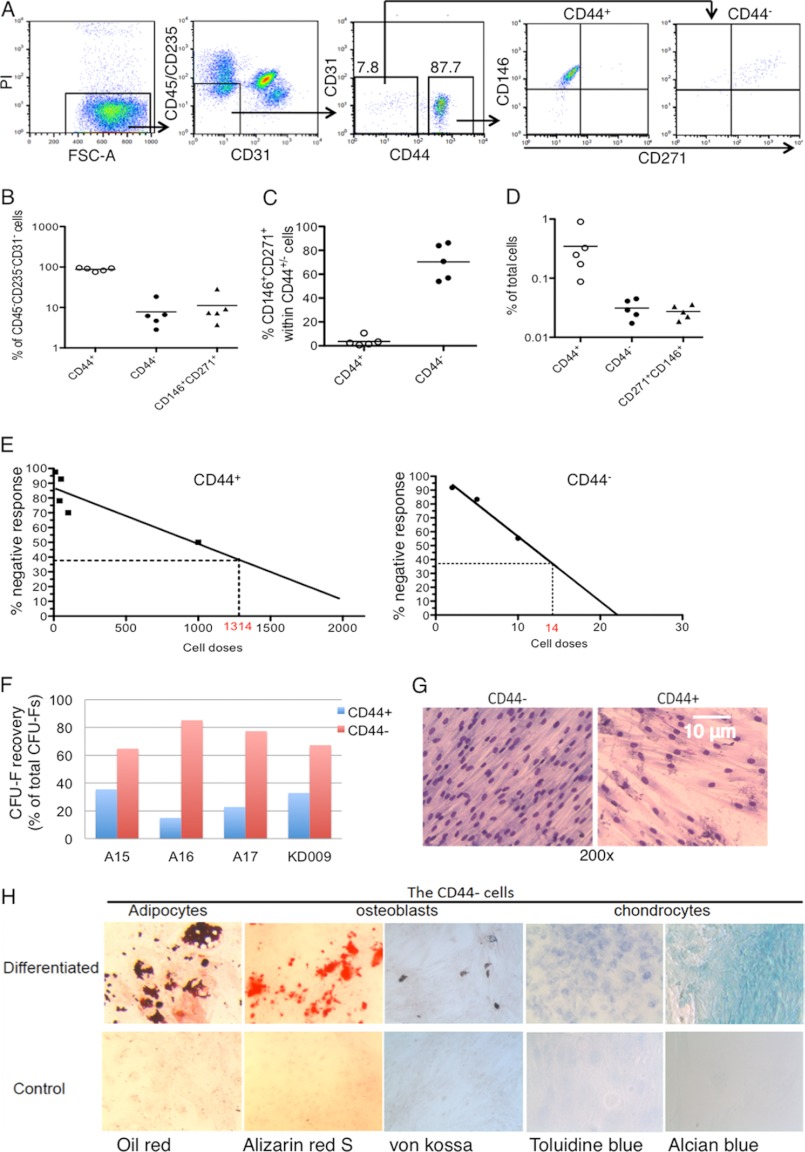
To investigate whether our findings from mouse studies hold true in humans, we analyzed CD44 expression in normal human BM stromal cells of healthy donors and characterized the CD44+/− cell subsets by multicolor FACS, colony assay, and global gene expression analysis. Within CD45−CD235−CD31− cells, the majority of the cells express CD44 on the cell surface, whereas less than 10% of them are CD44− (Fig. 3, A and B). Because CD146 and CD271 have been reported to be expressed on freshly isolated human BM MSCs (10, 11, 14, 35), we analyzed their expression in relation to that of CD44 in CD45−CD235−CD31− cells. Interestingly, although the majority of the CD44− cells are positive for both CD146 and CD271, only around 4% of the CD44+ cells express these markers (Fig. 3, A and B). Unique co-expression of CD146 and CD271 in the CD44− cells was also reflected in frequencies of the CD44− cells similar to that of the CD146+CD271+ cells in BM (Fig. 3, B–D). These data suggest that the absolute majority of the phenotypically defined MSCs reside in the CD44− cells in human BM.
FIGURE 3.
Human BM mesenchymal stem and progenitor cells primarily reside in CD44− stromal cell fraction. The data are from five analysis experiments with four healthy donors. A, FACS profiles show CD44, CD271, and CD146 expression in human BM CD45−CD235−CD31− cells and sorting of the CD44+/− cells. The stromal cells were first enriched by magnetic-activated cell sorting prior to staining of the antibodies. B, percentages of the CD44+ and CD44− cells within stromal cells in BM. C, expressions of CD146 and CD271 in the CD44+ and CD44− cells. D, the frequencies of CD146+CD271+ cells and CD44+ and CD44− cells in BM. E, limiting dilution of CFU-Fs in the CD44+/− cells. The cell doses yielding 37.5% negative wells are indicated by dashed lines on the x axis. F, CFU-F recovery from the CD44+/− cells of BM. G, morphology of Giemsa-stained CFU-Fs. H, in vitro differentiation of the CD44− cells. The adipocytes were identified by oil red stainings. The osteoblasts were confirmed by both Alizarin red and von Kossa stainings. The chondrocytes were identified by both toluidine blue and Alcian blue. PI, propidium iodide.
To test their clonogenic capacity, we sorted the CD44+/− subsets directly from human BM by FACS (Fig. 3A) and performed limiting dilution assays of CFU-Fs. We found that the CFU-F frequency in the CD44− cells reached 1:14, 94-fold higher than that (1:1314) in the CD44+ cells (Fig. 3E). Although the proportion of CD44− cells is much smaller than that of the CD44+ cells in the BM, the higher recovery of CFU-Fs from the CD44− cells suggests that they still contain the majority of the CFU-Fs (Fig. 3F). However, it is important to note that the most of the colonies formed from the CD44+ cells were observed when the cells were plated at higher, nonclonal density (2000 cells/cm2) (Fig. 3E). This could lead to an overestimation of the CFU-F frequency and the possibility that the CFU-F frequency may not reflect the frequency of the clonogenic cells in this cell population according to previous observations (2). Moreover, although the freshly sorted CD44− cells were highly proliferative and generated fibroblast-like cells when plated in culture (Fig. 3G), the colonies generated from the freshly sorted CD44+ cells displayed a dramatically reduced expansion capacity and could not be replated after the secondary culture. This, together with growth characteristics of the cells, suggests that most of the clonogenic cells in human BM are naturally CD44−.
Multipotency is a key stem cell feature for MSCs. We next performed an in vitro differentiation assay on expanded cells generated from freshly sorted CD44− cells to test their multilineage differentiation potentials. This revealed that the CD44− cells could generate adipocytes, osteoblasts, and chondrocytes in vitro (Fig. 3H). The differentiation assay could not be performed with the freshly sorted CD44+ cells because they could not be sufficiently expanded in culture. These data suggest that the CD44− cells contain a major part of the MSCs in human BM.
Furthermore, FACS analysis of the reported MSC-associated markers CD73, CD29, VCAM1, and STRO1 on BM cells indicated that the majority of the CD44− stromal cells from human BM expressed these surface antigens (supplemental Fig. S1). Surprisingly, the CD44− cells displayed low expression CD105, presenting a discrepancy from what has been reported for cultured MSCs (15).
Human CD44− Mesenchymal Cells Display MSC-associated Molecular Phenotype
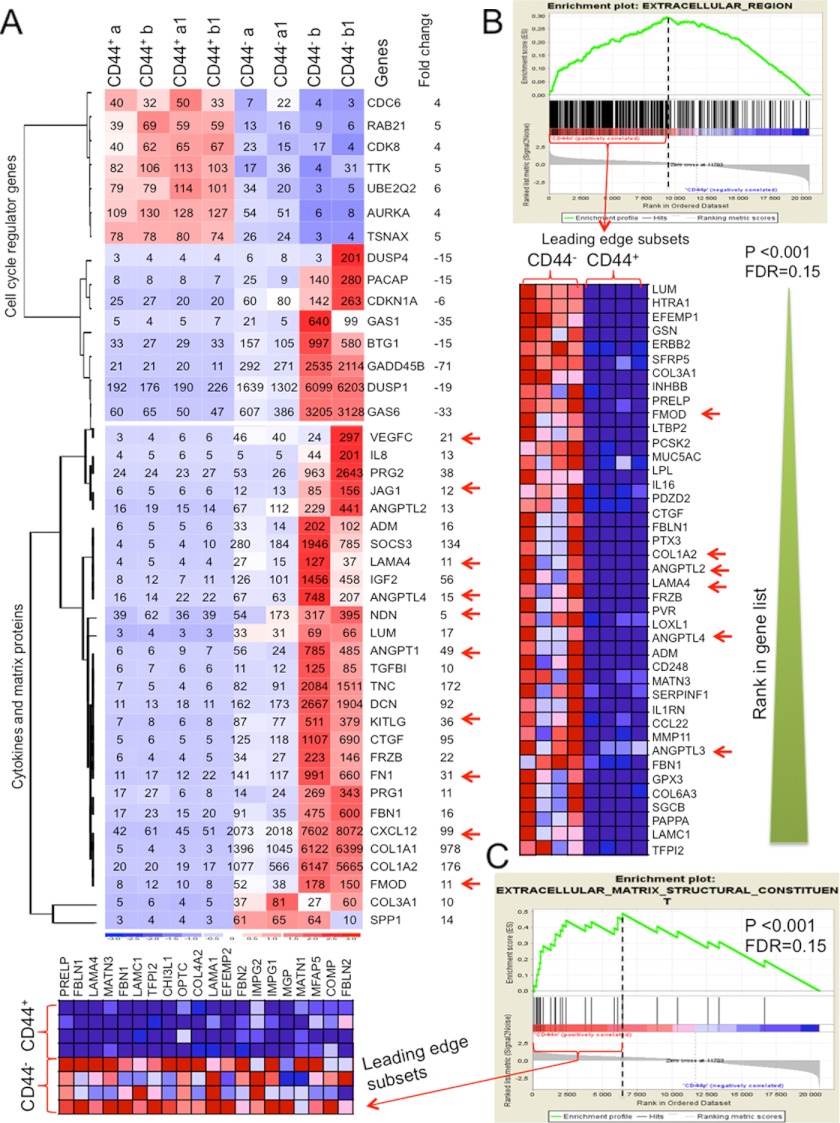
To further investigate molecular properties of the CD44− and CD44+ cells from human BM, we performed microarray analysis on freshly sorted human BM CD45−CD235−CD31−CD44+/− subsets. dCHIP analysis revealed 929 genes that are more than 4-fold differentially expressed in the CD44+ as compared with the CD44− cells. Among genes reported to be related to MSC properties, we noted down-regulation of cell cycle progression genes and up-regulation of the cell cycle inhibitor genes, including CDKN1A(p21), GAS1, and GAS6 in the CD44− cells as compared with CD44+ cells (Fig. 4A and supplemental Figs. S2 and S3), suggesting a relatively quiescent status of the CD44− cells. Importantly, the CD44− cells expressed higher levels of a set of cytokines and growth factors, including KIT ligand (KITLG), vascular endothelial growth factor (VEGFC), Jagget-1 (JAG1), angiopoietin-like 4 (ANGPTL4), ANGPT1, and CXCL12 as well as extracellular matrix proteins, such as laminin α4 (LAMA4), fibronectin (FN1), fibromodulin (FMOD), necdin (NDN), and collagen type I (COL1A1), all reported to be enriched in human BM MSCs (32–34, 36) and shown to be important for hematopoiesis (36, 37). On the contrary, expression of these genes is undetectable or low in the CD44+ cells (Fig. 4, A–C). The CD44− cell population also displays relatively higher expression of other MSC-related genes (supplemental Fig. S1). In addition, GSEA revealed that multiple genes sets for known biological functions/processes/pathways are significantly negatively or positively correlated with the CD44− phenotype (p < 0.001 and false discovery rate <0.25) (supplemental Tables S1–S3, Fig. 4, B and C, and supplemental Fig. S3). These include gene sets coding for extracellular molecules that are significantly enriched in the CD44− cells (Fig. 4, B and C, and supplemental Table S3). Furthermore, enrichment of cell cycle progression genes in the CD44+ cells (supplemental Fig. S3, B and C) confirms the relatively quiescent status of the CD44− cells. These data provide comprehensive views into potential functions of the CD44− and CD44+ subsets and important hints for further investigation of the role of human BM stromal cells in homeostasis and diseases.
FIGURE 4.
Microarray data provide molecular evidence for immature phenotype of the CD44− cells from human BM. A, dCHIP analysis of gene expressions of cytokines, growth factors, and extracellular matrix proteins in the CD44+ and CD44− cells from normal human BM. Clustering shows the genes being up-regulated more than 4-fold in the CD44− cells compared with the CD44+ cells. Red, high expression; blue, low expression. The numbers in the heat map are expression values of each indicated gene. B and C, GSEA analysis of significantly up-regulated gene sets in the CD44− cells. The green curves plot the enrichment score (ES). Black vertical dashed lines specify the maximum enrichment score. Significantly enriched data sets are defined according to GSEA default settings (p < 0.001 and false discovery rate of <0.25). The heat map shows expressions of the genes in the leading edge subsets (only the top 40 genes were shown for clarity if more than 40 genes in the leading edge subsets). B, enrichment of genes in the gene sets of the extracellular matrix region in the CD44− cells. C, enrichment of the gene set of extracellular matrix structural constituents in the CD44− cells. The data were from eight microarray platforms and two sorting experiments, normalized by RMAExpress software. See also supplemental Figs. S1 and S2 and Tables S1–S3.
Acquisition of CD44 Expression on Mesenchymal Stem and Progenitor Cells during in Vitro Culture
The finding that the CD44− stromal cells contain essentially all MSCs is completely contrary to what has been reported for culture-expanded MSCs. In order to test whether expression of CD44 was due to in vitro manipulation of the cells, we cultured the freshly isolated CD44− MSCs from mouse and human BM and analyzed CD44 expression on the cells after culture at different passages. As previously reported, these cells acquired CD44 expression on their surface after in vitro expansion early at the first passage and remained at a high level during the later passages (>98.7 ± 1.5%) (Fig. 5). We have developed multiple cell clones from single CFU-Fs generated from mouse CD44− cells in the limiting dilution assays, and all of the clones were positive for CD44 in culture at early and later passages (passages 5–14) (Fig. 5B). However, the acquisition of CD44 expression on the MSCs upon culture did not affect the proliferation capacity of the cells (Fig. 5D). These data suggested that CD44 was dramatically up-regulated on mesenchymal stem and progenitor cells during in vitro culture, and the CD44+ phenotype of expanded MSCs does not reflect the true cellular identity of the primary MSCs and progenitor cells.
FIGURE 5.
Acquisition of CD44 expression in CD45−LIN−CD44− MSCs and progenitor cells after culture. The freshly sorted CD44− cells from human and mouse BM were cultured, and the expanded cells were analyzed by FACS for CD44 expression. A, CD44 expression in the expanded mouse stromal cells after 8–10 days of culture. B, CD44 expressions in the cultured cells at later passages (passages 5–15) derived a single CD44− mouse cell clone. Red lines, expression of CD44; blue lines, isotype control stainings. C, CD44 expression in the cultured human BM stromal cells. The freshly sorted CD45−CD235−CD31−CD44− cells from BM of healthy humans were cultured for 14 days. A15, A16, and A17 indicate different donors. Red lines, expression of CD44; blue lines, isotype control stainings. D, -fold expansion of the cells generated from the freshly sorted CD44− cells during culture. A limited number (10–50 cells) of the sorted CD44− cells were plated in culture, the cells generated from the culture were counted, and -fold expansion was calculated. The data are mean ± S.E. (error bars) from three independent experiments on BM from three healthy volunteers. The x axis indicates the numbers of the passages (P).
In Vitro Cultivation Induces Extensive Changes in Gene Expression in Human MSCs
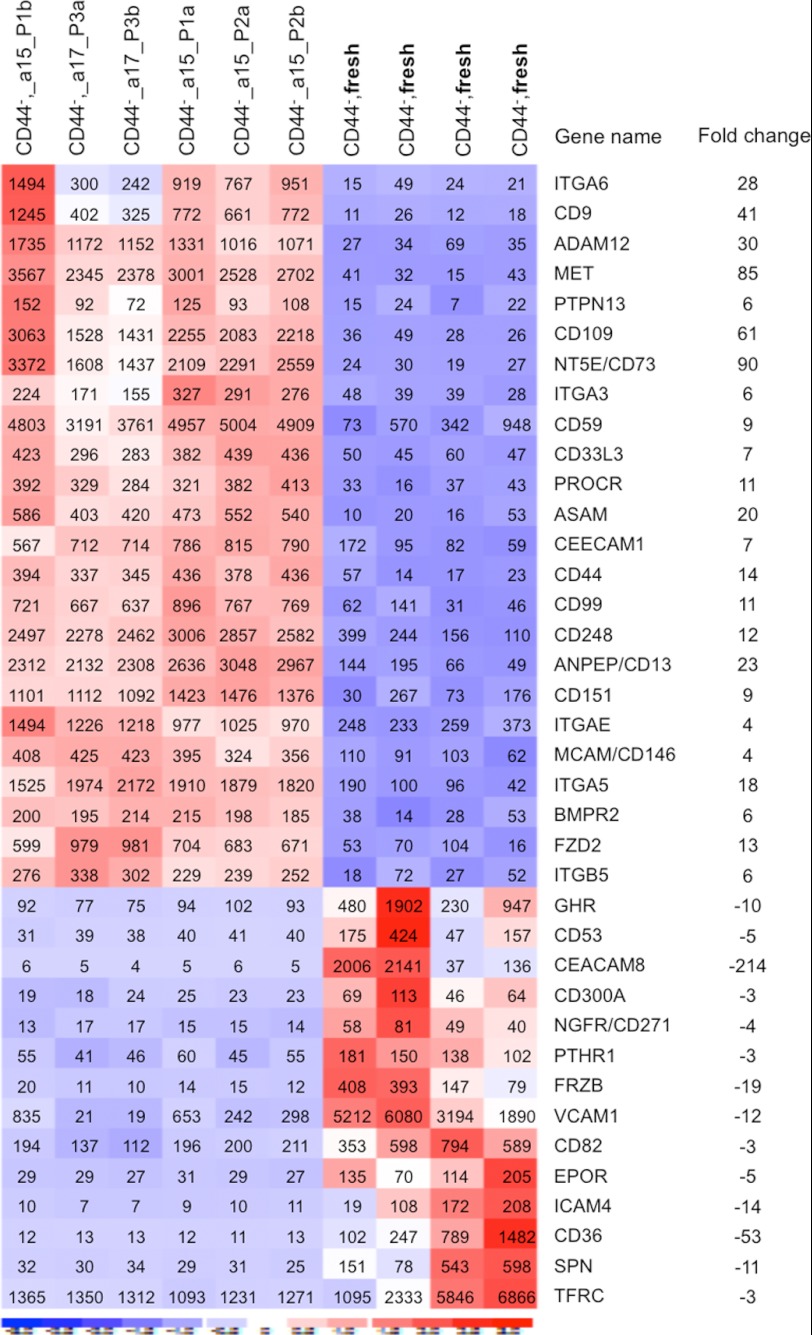
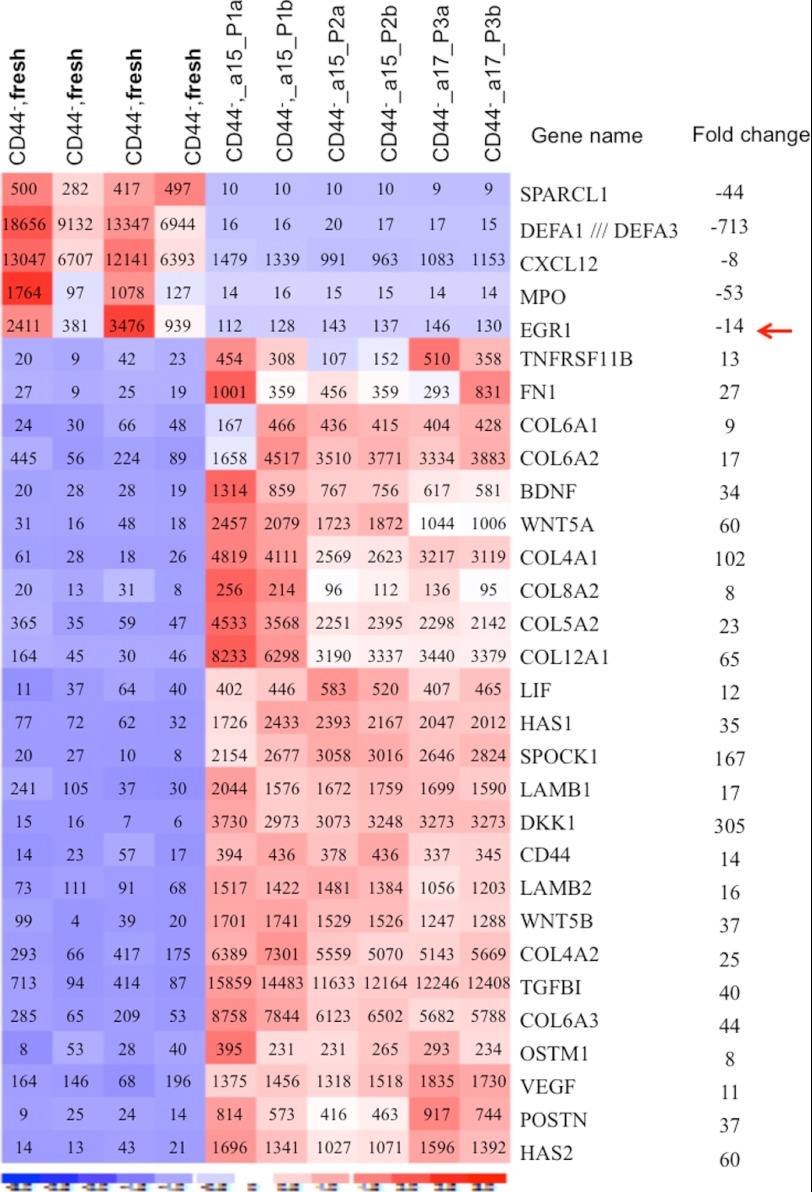
The induction of dramatic changes in CD44 expression and proliferation characteristics upon cultivation of the CD44− BM stromal cells prompted us to search for molecular mechanisms underlying culture-related changes in the MSCs. To this end, we performed microarray experiments to compare gene expression patterns in cultured MSCs at passages 1–3 with that of the freshly sorted CD44− mesenchymal cells (CD45−CD235−CD31−CD44−) from the same donors. dCHIP analysis of the microarray data revealed that 2708 genes were more than 2-fold differentially expressed in the CD44+ cultured MSCs as compared with the freshly sorted CD44− cells. Several of the changes appeared to be consistent between two different donors and over three passages. Among the differentially expressed genes, we identified a large number of surface antigens in addition to CD44 (Fig. 6). These included cell adhesion receptors, such as integrins (ITGA3, ITGAE, ITGB5, and ITGA6) and CD109, ADAM12, CD151, CD59, and CD248, some of which have been reported to be expressed on cultured MSCs (30, 38). Although MSC-associated markers, such as CD73 and CD146, are up-regulated, NGFR/CD271, VCAM1, CD36, and EPOR are down-regulated in the cultured MSCs compared with the fresh ex vivo analyzed CD44− cells. Importantly, in keeping with our FACS data, CD44 is dramatically up-regulated in the cultured MSCs. In addition, we observed dramatic up-regulation of hyaluronan synthase (HAS1 and HAS2); growth factor and matrix protein genes, including VEGF, WNT5A, WNT5B, FN1, LAMAB1, and BDGF; and collagens in the cultured MSCs (Fig. 7). Microarray data-based signal pathway mapping illustrated up-regulated (≥2-fold) genes for the WNT signal, focal adhesion, and MAPK signal pathways in the cultured MSCs expressing CD44 (supplemental Figs. S4 and S5). On the contrary, CXCL12, MPO, and EGR1 are significantly down-regulated in the cultured MSCs. Hence, we conclude that in vitro cultivation of human MSCs results in dramatic and consistent changes in gene expression patterns.
FIGURE 6.
Culture-induced alteration of cell adhesion molecule expression in MSCs from human BM. The microarray data on the freshly sorted CD44− mesenchymal cells and on the culture-expanded MSCs (acquired CD44 expression) derived from the fresh CD44− cells of the same donors were analyzed by dCHIP software. The microarray experiments on the culture-expanded MSCs were performed at passage (P) 1–3. a and b indicate replicate samples at each passage (P1–P3) from the indicated donors (a15 or a17). Clustering shows the genes being up-regulated more than 3-fold in the cultured cells compared with the freshly sorted CD44− cells. Red, high expression; blue, low expression. The numbers in the heat map are expression values of each indicated gene, and the -fold changes in expression of the receptors were calculated based on the mean expression values from each cell type. The data are from 10 microarray platforms (six arrays of the cultured cells and four arrays of the fresh cells) and two sorting experiments on two healthy volunteers, normalized by RMAExpress software.
FIGURE 7.
Differential expression of growth factor, matrix protein, and signaling molecules in freshly sorted MSCs and the cultured MSCs of human BM. Gene expressions of cytokines, growth factors, and extracellular matrix proteins in the freshly sorted CD44− mesenchymal cells and the culture-expanded MSCs (acquired CD44 expression) derived from the freshly sorted CD44− cells of the same donors were analyzed by dCHIP software. The microarray experiments on the culture-expanded MSCs were performed at passage (P) 1–3. a and b indicate replicate samples at each passage (P1–P3) from the indicated donors (a15 or a17). Clustering shows selected genes being up-regulated more than 8-fold in the cultured cells compared with the freshly sorted CD44− cells. For clarity, only part of the growth factors and signaling molecules are shown in the panel. Red, high expression; blue, low expression. The numbers in the heat map are expression values of each indicated gene, and the -fold changes were calculated based on the mean expression values from each cell type. The data are from 10 microarray experiments (six arrays of the cultured cells and four arrays of the fresh cells) and two sorting experiments on two healthy volunteers, normalized by RMAExpress software. See also supplemental Figs. S4 and S5.
DISCUSSION
In the present study, we have prospectively isolated the CD44+ and CD44− stromal cells from both human and mouse BM by multicolor FACS and characterized them phenotypically, functionally, and molecularly. In striking contrast to the previous finding of high expression of CD44 on culture-expanded MSCs, we have uncovered that native mesenchymal stem and progenitor cells lack CD44 expression. This finding is of great importance for the development of methods for isolation of BM MSCs both for experimental and clinical purposes.
SCA1, PDGFRα, and Nestin have recently been shown to be expressed on freshly isolated mouse BM MSCs (6, 31, 39). We here report that the BM stromal cells positive for those markers are enriched in the CD44− cells in mice. Similarly, the majority of the previously defined CD271+CD146+ MSCs (14) from human BM do not express CD44. Furthermore, we also show that the commonly used positive MSC markers CD73, CD106, CD29, and STRO1 are highly expressed on the freshly sorted CD44− cells. These data together with the finding of enrichment of CFU-F activities in the CD44− cells strongly support CD44 negative phenotype of the MSCs. This result can be further corroborated by the cell cycle analysis and molecular data showing enrichment of quiescent cells and the cells with multilineage differentiation potential in the CD44− cells. Another interesting finding in the present study is that the CD44− stromal cells express higher levels of hematopoiesis-regulating growth factors and matrix proteins, including CXCL12, KIT ligand, angiopoietin 1, VEGF, JAG1, LAMA4, and FN1, compared with the CD44+ cell fraction. Thus, this cell population might play an important role for maintenance of normal hematopoiesis in vivo.
Taken together, our data strongly suggest that CD44− stromal cells are enriched with multipotent mesenchymal stem and progenitor cells. Although MSCs are only a small fraction of the CD44− cells, given the fact that CD44 is widely expressed in hematopoietic cells and endothelial cells, using this marker alone or in combination with other negative markers, including CD45 and CD235, in humans and in combination with a positive marker, such as SCA1, in mice would significantly facilitate prospective isolation of MSCs from BM.
Most importantly, our data provide clear evidence for alteration of the MSC phenotype during in vitro manipulations of the cells, emphasizing the importance of prospective isolation of MSCs in order to uncover the nature and therapeutic potentials of the cells. The functional consequence for acquisition of CD44 expression on the expanded MSCs during therapeutic use is unclear. However, because the acquisition of CD44 expression already occurred at the first passage and remained on the cells at the later passages during in vitro expansion, it is likely that stem cell growth characteristics, such as sustainable expansion capacity of the MSCs, remained after acquiring CD44 expression. In addition, the cells expanded in culture from the CD44− cells could give rise to adipocytes, chondrocytes, and osteoblasts, suggesting the maintenance of multilineage differentiation potential of the cells after acquisition of CD44 expression on the surface. Hence, expression of CD44 per se does not appear to reduce the MSC potential of the cultured cells in vitro. However, it was reported that the culture-expanded MSCs displayed a reduction or total loss of homing capacity to BM although highly expressing CD44 (6). On the contrary, primary MSCs showed more efficient homing to BM compared with the cultured MSCs (40). Loss of BM homing capacity of the cultured MSCs might be due to culture-induced changes in expression of adhesion receptors, including CD44 and CXCR4 on the cells, which might lead to unwanted entrapment in other organs (6, 38). CD44 exists in multiple isoforms on different types of cells, and CD44 expression as well as its binding capacity toward its ligands are regulated by different cytokine stimulation and other environmental changes (41). It has been shown that Early growth response 1 (EGR1) regulates CD44 transcription via binding to the CD44 promoter in B cells (42). However, our gene expression data suggest that the level of EGR1 transcript is dramatically reduced (by 14-fold) in the cultured MSCs, whereas these cells acquire CD44 expression. Hence, apparently the functional linkage between those two molecules in the MSCs requires further investigation.
Comparing gene expression patterns and surface marker expression in the freshly isolated and cultured MSCs revealed that in vitro manipulation of MSCs resulted in dramatic overall changes in gene expression patterns. We here provided new evidence for changes in expression of adhesion receptors and signaling molecules. Whereas CD105, integrin α1/CD49A, α3/CD49C, α4/CD49D, α5/CD49E, α6/CD49F, CD151, and CD109 are up-regulated on the cultured MSCs, expression of other cell surface antigens, such as VCAM1, ICAM4, CD36, EPOR, and PTHGR, is reduced in the culture-expanded MSCs, as compared with the freshly sorted CD44− stromal cells. These changes could possibly result in changes in differentiation potential and other cellular processes of the cultured MSCs because those factors have been reported to be important for regulating lineage differentiation of human MSCs (reviewed in Ref. 43) (44–47). In addition, the changes in expression of cell surface receptors, including CD44, could result in the up-regulation of multiple signal molecules related to cell adhesion, growth factor pathways in the cultured MSCs; however, the latter could in turn lead to further changes of the cell surface receptor expression through inside-out signal transduction.
CD44 function is controlled by its posttranslational modifications such as sialofucosylations. It has been shown that the CD44 glycoform bearing α-2,3-sialyl modifications on the cultured MSCs is not reactive with BM vascular E-selectins, which resulted in poor osteotropism after systemic transplantation of the cells. However, this can be rescued by converting the glycoform to a selectin-binding glycoform of CD44 (48). On the other hand, it was reported that CD44 on the cultured MSCs contributed to migration of the cells into injured kidney via interaction with hyaluronic acid at sites of injury (17, 49). It is important to note that hyaluronic acid is also up-regulated in many solid cancers (24). Positive contribution of cultured MSCs to breast cancer development has been reported (50). Therefore, although MSCs hold great promise for cancer therapy (51), safety issues should be seriously considered before clinical use of culture-expanded MSCs for gene or drug delivery.
In summary, we have here provided phenotypic, functional, and molecular evidence that BM mesenchymal stem and progenitor cells physiologically do not express CD44 in both humans and mice. However, in vitro culture could result in acquisition of CD44 expression on their surface and changes in expression of cytokines, growth factors, matrix proteins, and other signaling molecules. These findings highlight the importance of ex vivo analysis of the cells and provide clear evidence for true cellular identity of MSCs and progenitor cells.
Supplementary Material
Acknowledgments
We thank Liselotte Lenner (Linköping University) for valuable advice and technical assistance and Professor Eva Hellström Lindberg (Karolinska University Hospital, Karolinska Institute) for bone marrow samples from healthy volunteers.
This work was supported by grants from the Swedish Cancer Society, the Swedish Research Council, the Swedish Childhood Cancer Foundation, and the Faculty of Medicine at Linköping University.

This article contains supplemental Tables S1–S3 and Figs. S1–S5.
- MSCs
- mesenchymal stem cells
- BM
- bone marrow
- CFU-F
- colony-forming unit-fibroblast
- FMO
- fluorescent minus one
- Q-PCR
- quantitative PCR.
REFERENCES
- 1. Friedenstein A. J., Gorskaja J. F., Kulagina N. N. (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 4, 267–274 [PubMed] [Google Scholar]
- 2. Bianco P., Robey P. G., Simmons P. J. (2008) Mesenchymal stem cells. Revisiting history, concepts, and assays. Cell Stem Cell 2, 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anjos-Afonso F., Bonnet D. (2007) Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood 109, 1298–1306 [DOI] [PubMed] [Google Scholar]
- 4. Gang E. J., Bosnakovski D., Figueiredo C. A., Visser J. W., Perlingeiro R. C. (2007) SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 109, 1743–1751 [DOI] [PubMed] [Google Scholar]
- 5. Jones E., McGonagle D. (2008) Human bone marrow mesenchymal stem cells in vivo. Rheumatology 47, 126–131 [DOI] [PubMed] [Google Scholar]
- 6. Morikawa S., Mabuchi Y., Kubota Y., Nagai Y., Niibe K., Hiratsu E., Suzuki S., Miyauchi-Hara C., Nagoshi N., Sunabori T., Shimmura S., Miyawaki A., Nakagawa T., Suda T., Okano H., Matsuzaki Y. (2009) Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 206, 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakamura Y., Arai F., Iwasaki H., Hosokawa K., Kobayashi I., Gomei Y., Matsumoto Y., Yoshihara H., Suda T. (2010) Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood 116, 1422–1432 [DOI] [PubMed] [Google Scholar]
- 8. Lundberg P., Allison S. J., Lee N. J., Baldock P. A., Brouard N., Rost S., Enriquez R. F., Sainsbury A., Lamghari M., Simmons P., Eisman J. A., Gardiner E. M., Herzog H. (2007) Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J. Biol. Chem. 282, 19082–19091 [DOI] [PubMed] [Google Scholar]
- 9. Lee R. H., Seo M. J., Pulin A. A., Gregory C. A., Ylostalo J., Prockop D. J. (2009) The CD34-like protein PODXL and α6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood 113, 816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Battula V. L., Treml S., Bareiss P. M., Gieseke F., Roelofs H., de Zwart P., Müller I., Schewe B., Skutella T., Fibbe W. E., Kanz L., Bühring H. J. (2009) Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica 94, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M., Bianco P. (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 [DOI] [PubMed] [Google Scholar]
- 12. Gronthos S., Zannettino A. C., Hay S. J., Shi S., Graves S. E., Kortesidis A., Simmons P. J. (2003) Molecular and cellular characterization of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 116, 1827–1835 [DOI] [PubMed] [Google Scholar]
- 13. Jones E. A., English A., Kinsey S. E., Straszynski L., Emery P., Ponchel F., McGonagle D. (2006) Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin. Cytom. 70, 391–399 [DOI] [PubMed] [Google Scholar]
- 14. Tormin A., Li O., Brune J. C., Walsh S., Schütz B., Ehinger M., Ditzel N., Kassem M., Scheding S. (2011) CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 117, 5067–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 [DOI] [PubMed] [Google Scholar]
- 16. Sun S., Guo Z., Xiao X., Liu B., Liu X., Tang P. H., Mao N. (2003) Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells 21, 527–535 [DOI] [PubMed] [Google Scholar]
- 17. Herrera M. B., Bussolati B., Bruno S., Morando L., Mauriello-Romanazzi G., Sanavio F., Stamenkovic I., Biancone L., Camussi G. (2007) Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 72, 430–441 [DOI] [PubMed] [Google Scholar]
- 18. Halfon S., Abramov N., Grinblat B., Ginis I. (2011) Markers distinguishing mesenchymal stem cells from fibroblasts are down-regulated with passaging. Stem Cells Dev. 20, 53–66 [DOI] [PubMed] [Google Scholar]
- 19. Sung J. H., Yang H. M., Park J. B., Choi G. S., Joh J. W., Kwon C. H., Chun J. M., Lee S. K., Kim S. J. (2008) Isolation and characterization of mouse mesenchymal stem cells. Transplant. Proc. 40, 2649–2654 [DOI] [PubMed] [Google Scholar]
- 20. Eslaminejad M. B., Nadri S., Hosseini R. H. (2007) Expression of Thy 1.2 surface antigen increases significantly during the murine mesenchymal stem cells cultivation period. Dev. Growth Differ. 49, 351–364 [DOI] [PubMed] [Google Scholar]
- 21. Both S. K., van der Muijsenberg A. J., van Blitterswijk C. A., de Boer J., de Bruijn J. D. (2007) A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 13, 3–9 [DOI] [PubMed] [Google Scholar]
- 22. Lu F. Z., Fujino M., Kitazawa Y., Uyama T., Hara Y., Funeshima N., Jiang J. Y., Umezawa A., Li X. K. (2005) Characterization and gene transfer in mesenchymal stem cells derived from human umbilical cord blood. J. Lab. Clin. Med. 146, 271–278 [DOI] [PubMed] [Google Scholar]
- 23. Lesley J., Hyman R., Kincade P. W. (1993) CD44 and its interaction with extracellular matrix. Adv. Immunol. 54, 271–335 [DOI] [PubMed] [Google Scholar]
- 24. Toole B. P. (2009) Hyaluronan-CD44 interactions in cancer. Paradoxes and possibilities. Clin Cancer Res. 15, 7462–7468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qian H., Buza-Vidas N., Hyland C. D., Jensen C. T., Antonchuk J., Månsson R., Thoren L. A., Ekblom M., Alexander W. S., Jacobsen S. E. (2007) Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1, 671–684 [DOI] [PubMed] [Google Scholar]
- 26. Wang Y. H., Liu Y., Maye P., Rowe D. W. (2006) Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol. Prog. 22, 1697–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 28. Chamberlain G., Fox J., Ashton B., Middleton J. (2007) Concise review. Mesenchymal stem cells. Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25, 2739–2749 [DOI] [PubMed] [Google Scholar]
- 29. Winkler I. G., Sims N. A., Pettit A. R., Barbier V., Nowlan B., Helwani F., Poulton I. J., van Rooijen N., Alexander K. A., Raggatt L. J., Lévesque J. P. (2010) Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828 [DOI] [PubMed] [Google Scholar]
- 30. da Silva Meirelles L., Caplan A. I., Nardi N. B. (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299 [DOI] [PubMed] [Google Scholar]
- 31. Méndez-Ferrer S., Michurina T. V., Ferraro F., Mazloom A. R., Macarthur B. D., Lira S. A., Scadden D. T., Ma'ayan A., Enikolopov G. N., Frenette P. S. (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tormin A., Brune J. C., Olsson E., Valcich J., Neuman U., Olofsson T., Jacobsen S. E., Scheding S. (2009) Characterization of bone marrow-derived mesenchymal stromal cells (MSC) based on gene expression profiling of functionally defined MSC subsets. Cytotherapy 11, 114–128 [DOI] [PubMed] [Google Scholar]
- 33. Xiao Y., Mareddy S., Crawford R., Dhaliwal N. (2010) Stem cell-related gene expression in clonal populations of mesenchymal stromal cells from bone marrow. Tissue Eng. Part A 16, 749–758 [DOI] [PubMed] [Google Scholar]
- 34. Tanabe S., Sato Y., Suzuki T., Suzuki K., Nagao T., Yamaguchi T. (2008) Gene expression profiling of human mesenchymal stem cells for identification of novel markers in early- and late-stage cell culture. J. Biochem. 144, 399–408 [DOI] [PubMed] [Google Scholar]
- 35. Quirici N., Soligo D., Bossolasco P., Servida F., Lumini C., Deliliers G. L. (2002) Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 30, 783–791 [DOI] [PubMed] [Google Scholar]
- 36. Battula V. L., Cabreira M. d. G., Wang Z., Ma W., Benito J., Ruvolo P. P., Davis R. E., Konopleva M., Andreeff M. (2010) Connective Tissue Growth Factor (CTGF) is Essential for Self-Renewal and Prolification of Mesenchymal Stromal Cells (MSCs) and Affects Leukemia-stroma Interactions. ASH Annual Meeting Abstracts 116, 3845 [Google Scholar]
- 37. Méndez-Ferrer S., Frenette P. S. (2007) Hematopoietic stem cell trafficking. Regulated adhesion and attraction to bone marrow microenvironment. Ann. N.Y. Acad. Sci. 1116, 392–413 [DOI] [PubMed] [Google Scholar]
- 38. Thankamony S. P., Sackstein R. (2011) Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc. Natl. Acad. Sci. U.S.A. 108, 2258–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Short B. J., Brouard N., Simmons P. J. (2009) Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol. Biol. 482, 259–268 [DOI] [PubMed] [Google Scholar]
- 40. Rombouts W. J., Ploemacher R. E. (2003) Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17, 160–170 [DOI] [PubMed] [Google Scholar]
- 41. Gee K., Kryworuchko M., Kumar A. (2004) Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Archivum Immunologiae et Therapiae Experimentalis 52, 13–26 [PubMed] [Google Scholar]
- 42. Maltzman J. S., Carman J. A., Monroe J. G. (1996) Role of EGR1 in regulation of stimulus-dependent CD44 transcription in B lymphocytes. Mol. Cell. Biol. 16, 2283–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gregory C. A., Gunn W. G., Reyes E., Smolarz A. J., Munoz J., Spees J. L., Prockop D. J. (2005) How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann. N.Y. Acad. Sci. 1049, 97–106 [DOI] [PubMed] [Google Scholar]
- 44. Lin H. Y., Tsai C. C., Chen L. L., Chiou S. H., Wang Y. J., Hung S. C. (2010) Fibronectin and laminin promote differentiation of human mesenchymal stem cells into insulin-producing cells through activating Akt and ERK. J. Biomed. Sci. 17, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martino M. M., Mochizuki M., Rothenfluh D. A., Rempel S. A., Hubbell J. A., Barker T. H. (2009) Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 30, 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sogo Y., Ito A., Matsuno T., Oyane A., Tamazawa G., Satoh T., Yamazaki A., Uchimura E., Ohno T. (2007) Fibronectin-calcium phosphate composite layer on hydroxyapatite to enhance adhesion, cell spread, and osteogenic differentiation of human mesenchymal stem cells in vitro. Biomed. Mater. 2, 116–123 [DOI] [PubMed] [Google Scholar]
- 47. Mayer H., Bertram H., Lindenmaier W., Korff T., Weber H., Weich H. (2005) Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells. Autocrine and paracrine role on osteoblastic and endothelial differentiation. J. Cell. Biochem. 95, 827–839 [DOI] [PubMed] [Google Scholar]
- 48. Sackstein R., Merzaban J. S., Cain D. W., Dagia N. M., Spencer J. A., Lin C. P., Wohlgemuth R. (2008) Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 14, 181–187 [DOI] [PubMed] [Google Scholar]
- 49. Poulsom R. (2007) CD44 and hyaluronan help mesenchymal stem cells move to a neighborhood in need of regeneration. Kidney Int. 72, 389–390 [DOI] [PubMed] [Google Scholar]
- 50. Karnoub A. E., Dash A. B., Vo A. P., Sullivan A., Brooks M. W., Bell G. W., Richardson A. L., Polyak K., Tubo R., Weinberg R. A. (2007) Mesenchymal stem cells within tumor stroma promote breast cancer metastasis. Nature 449, 557–563 [DOI] [PubMed] [Google Scholar]
- 51. Dai L. J., Moniri M. R., Zeng Z. R., Zhou J. X., Rayat J., Warnock G. L. (2011) Potential implications of mesenchymal stem cells in cancer therapy. Cancer Lett. 305, 8–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.