Background: The role of HDAC3 in allergic skin inflammation remains unknown.
Results: HDAC3 interacts with FcϵRI and regulates expression of MCP1 through Sp1 and c-Jun to mediate allergic skin inflammation.
Conclusion: HDAC3 mediates allergic skin inflammation in relation with angiogenesis by regulating MCP1.
Significance: HDAC3 serves as a target for development of allergy therapeutics.
Keywords: Allergy, Angiogenesis, Histone Deacetylase, Histone Deacetylase Inhibitors, Rac1, Allergic Skin Inflammation, Fcepsilon Receptor, HDAC3, MCP1, FcϵRI
Abstract
We have shown the induction of histone deacetylase 3 (HDAC3) in antigen-stimulated rat basophilic leukemia cells via NF-κB. We investigated the role of HDAC3 in allergic skin inflammation. We used a BALB/c mouse model of triphasic cutaneous anaphylaxis (triphasic cutaneous reaction; TpCR) and passive cutaneous anaphylaxis (PCA) to examine the role of HDAC3 in allergic skin inflammation. Triphasic cutaneous reaction involved induction of HDAC3 and was mediated by HDAC3. HDAC3 showed an interaction with FcϵRIβ. Trichostatin A (TSA), an inhibitor of HDAC(s), disrupted this interaction. Cytokine array analysis showed that the down-regulation of HDAC3 led to the decreased secretion of monocyte chemoattractant protein 1 (MCP1). FcϵRI was necessary for induction of HDAC3 and MCP1. ChIP assays showed that HDAC3, in association with Sp1 and c-Jun, was responsible for induction of MCP1 expression. TSA exerted a negative effect on induction of MCP1. HDAC3 exerted a negative regulation on expression of HDAC2 via interaction with Rac1. The down-regulation of HDAC3 or inactivation of Rac1 induced binding of HDAC2 to MCP1 promoter sequences. TSA exerted a negative effect on HDAC3-mediated TpCR. The BALB/c mouse model of PCA involved induction of HDAC3 and MCP1. HDAC3 and MCP1 were necessary for PCA that involved ear swelling, enhanced vascular permeability, and angiogenesis. Recombinant MCP1 enhanced β-hexosaminidase activity and histamine release and also showed angiogenic potential. TSA exerted a negative effect on PCA. Our data show HDAC3 as a valuable target for the development of allergic skin inflammation therapeutics.
Introduction
The triphasic cutaneous reaction (TpCR)3 is a simple in vivo cutaneous reaction (1) and employs preformed IgE and chemical antigen, such as 2,4-dinitrofluorobenzene (DNFB). TpCR is accompanied by triphasic ear swelling after DNFB stimulation. The immediate phase is dependent on mast cells (1). However, the second phase is induced in mast cell-deficient WBB6F1-W/Wv mice (1). IL-1β and TNF-α seem to mediate the second phase of ear swelling (2, 3). Ear swelling is also observed 7–8 days after stimulation with DNFB (1). The third phase is reduced in mast cell-deficient WBB6F1-W/Wv mice (1). Molecular mechanisms associated with TpCR merit further investigation.
Passive cutaneous anaphylaxis (PCA) is IgE- and mast cell-dependent (4). IL-33 is produced by mast cells and mediates PCA (5). Histamine is the most prominent inducer of vascular permeability accompanied by PCA (6). Hypoxia-inducible factor (HIF), an angiogenic factor, is necessary for various allergic inflammatory diseases, including PCA (7). This suggests a close relationship between PCA and angiogenesis. Mast cells increase vascular permeability by heparin-initiated bradykinin formation (8). Heparin induces anaphylaxis (9). Anaphylaxis involves angiogenesis (10). Mast cells interact with endothelial cells to contribute to angiogenesis in multiple myelomas (11). These reports suggest that allergic inflammation involves vascular permeability and angiogenesis.
Glucocorticoid resistance in asthma is associated with the reduced HDAC2 (histone deacetylase 2) activity (12). Corticosteroid function is dependent on HDAC2 (13). The reduction of HDAC2 activity has been reported in various allergic diseases (14). The reduction of HDAC2 results from post-translational modification, such as tyrosine nitration (15). Conditional deletion of HDAC1 in T cells enhances Th2 cytokine expression in airway inflammation (16). Trichostatin A (TSA), an inhibitor of HDAC(s), attenuates airway inflammation in a mouse asthma model by decreasing expression of Th2 cytokines (17). HDAC3 expression is induced by antigen stimulation in RBL2H3 cells via NF-κB (18). NF-κB, activated by TNF-α, induces chronic inflammation in the adipose tissues by inhibiting expression of cytosolic phosphoenolpyruvate carboxykinase in adipocytes through activation of HDAC3 (19). This implies a role of HDAC3 in inflammation.
We examined the role of HDAC3 in allergic skin inflammation with respect to FcϵRI (Fcϵ receptor I) signaling. We investigated molecular mechanisms of HDAC3-mediated allergic skin inflammation and identified a downstream target of HDAC3. We investigated molecular mechanisms of expression regulation of this target gene by HDAC3. We examined whether HDAC3 activity and MCP1 (monocyte chemoattractant protein 1), a downstream target of HDAC3, were necessary for allergic skin inflammation in relation with angiogenesis.
EXPERIMENTAL PROCEDURES
Cell Lines and Cell Culture
RBL2H3 cells were obtained from the Korea Cell Line Bank (Seoul, Korea). Cells were grown in Dulbecco's modified Eagle's medium containing heat-inactivated fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cultures were maintained in 5% CO2 at 37 °C. Bone marrow-derived mouse mast cells were isolated and cultured according to the standard procedures (20).
Isolation of Mast Cells from Mice
Ears of BALB/c mice were cut into fragments and incubated in RPMI1640 medium supplemented with 25% fetal bovine serum, 1.5 mg/ml collagenase (Sigma-Aldrich), 0.5 mg/ml hyaluronidase (Sigma-Aldrich), 0.2 mg/ml protease (Sigma-Aldrich), and 0.5 mg/ml DNase I (Sigma-Aldrich) for 60 min at 37 °C. Dispersed cells were filtered sequentially through 70- and 40-μm cell strainers (BD Biosciences). The pelleted cells were resuspended in RPMI1640 medium containing 0.1% bovine serum albumin and submitted to a continuous isotonic Percoll gradient (72%) for mast cell isolation. Purified mast cells were resuspended in RPMI-FBS. The cell purity (>96%) and viability (>98%) were evaluated by toluidine blue and trypan blue exclusion staining, respectively.
Mice
Five-week-old female BALB/c mice were purchased from Nara Biotech (Seoul, Korea) and maintained in specific pathogen-free conditions. All animal experiments were approved by the institutional review board for animal studies of Kangwon National University.
Preparation of siRNA Duplexes
Construction of siRNA was carried out according to the instruction manual provided by the manufacturer (Ambion, Austin, TX).
IgE-dependent TpCR in the Mouse Ear
To induce IgE-dependent TpCR in the ear of female BALB/c mice, mice were sensitized by injecting mouse anti-DNP monoclonal IgE antibody (10 μg/kg) intravenously. Twenty-four hours later, a cutaneous reaction was evoked by painting with 25 μl of 0.15% DNFB acetone-olive oil (3:1) solution onto each surface of both ear lobes. Ear thickness was measured by using a digital gauge. In order to examine the effect of HDAC3 on TpCR, scrambled (100 nm) or HDAC3 siRNA (100 nm) was injected intravenously on the day of IgE sensitization. To examine the effect of MCP1 on TpCR, neutralizing MCP1 (nMCP1) antibody (10 μg/kg) or isotype-matched IgG (10 μg/kg) was injected intraperitoneally on the day of IgE sensitization and 2 days after DNFB stimulation.
Histological Analyses
Ear samples of BALB/c mice were fixed in 10% (v/v) buffered formalin, embedded in paraffin, sectioned at 4–6 μm, and then stained with hematoxylin and eosin to examine the extent of lymphocyte infiltration. Immunohistochemistry staining of ear or lung tissues was performed by using the avidin-biotin detection method (Vectastain ABC kit, Vector Laboratories Inc., Burlingame, CA). Briefly, 4–6-μm-thick sections of the paraffin-embedded tissue blocks were cut, mounted on positively charged glass slides, and dried in an oven at 56 °C for 30 min. The sections were deparaffinized in xylene and then rehydrated in graded ethanol and water. Endogenous peroxidase was blocked by incubation in 3% (v/v) hydrogen peroxide for 15 min. Antigen retrieval was accomplished by pretreatment of the sections with citrate buffer at pH 6.0 for 20 min at 56 °C in a microwave oven and then allowing the sections to cool for 30 min at room temperature. Nonspecific endogenous protein binding was blocked using 1% bovine serum albumin (BSA). The sections were then incubated with primary antibodies overnight at 4 °C. The following primary antibodies were used for detection of proteins: anti-HDAC3 (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-MCP1 (1:200; Santa Cruz Biotechnology, Inc.). Isotype-matched control IgG antibody (1:200; Santa Cruz Biotechnology, Inc.) was employed to serve as a negative control. After washing, biotinylated secondary antibodies were applied at 1:100 or 1:200 dilutions for 1 h. Color was developed with diaminobenzidine (Vector Laboratories Inc.). Sections were counterstained with Mayer's hematoxylin.
Immunofluorescence Staining
RBL2H3 cells were seeded onto glass coverslips in 24-well plates and were sensitized with DNP-specific IgE (100 ng/ml) for 16 h. After stimulation with DNP-HSA (100 ng/ml) for 1 h, cells were fixed with 4% paraformaldehyde (v/v) for 10 min and then permeabilized with 0.4% Triton X-100 for 10 min. Nonspecific antibody binding sites were blocked by incubation with 1% BSA in TBST for 30 min. Cells were then incubated with primary antibody specific to HDAC3 (1:200; BD Biosciences) or FcϵRIβ (1:200; Santa Cruz Biotechnology, Inc.) for 2 h, followed by washing with TBS-T three times. Anti-goat IgG-FITC (for detection of HDAC3) or anti-rabbit Alexa Fluor 586 (for detection of FcϵRIβ) secondary antibody (Molecular Probes) was added to cells and incubated for 1 h. Coverslips were then washed and mounted by applying Mount solution (Biomeda, Foster City, CA). Fluorescence images were acquired using a confocal laser-scanning microscope and software (Fluoview version 2.0) with a ×60 objective (Olympus FV300, Tokyo, Japan). To examine the effect of TSA on co-localization of HDAC3 with FcϵRIβ, IgE-sensitized RBL2H3 cells were incubated with TSA (50 nm) for 12 h prior to stimulation with DNP-HSA.
Passive Cutaneous Anaphylaxis
BALB/c mice were passively sensitized with an intradermal injection of DNP-specific IgE (0.5 μg/kg) into both ears. The mice were challenged 24 h later with an intravenous injection of DNP-HSA (250 μg/kg) plus 250 μl of PBS containing 2% (v/v) Evans blue solution. Thirty minutes after DNP-HSA challenge, the mice were euthanized, and the 2% (v/v) Evans blue dye was extracted from each dissected ear in 700 μl of acetone/water (7:3) at room temperature overnight. The absorbance of Evans blue in the extracts was measured with a spectrophotometer at 620 nm. In order to examine the effect of MCP1 on PCA, BALB/c mice were passively sensitized with an intradermal injection of DNP-specific IgE (0.5 μg/kg) along with neutralizing MCP1 antibody (10 μg/kg) or IgG (10 μg/kg). The mice were challenged 24 h later with an intravenous injection of DNP-HSA (250 μg/kg) plus 250 μl of PBS containing 2% (v/v) Evans blue solution.
Preparation of Tissue Lysates from Mouse Ears
Mouse ear tissues were dissected and flash frozen in liquid nitrogen and then crushed into a fine powder with a mortar and pestle. Total protein lysates were made by suspension of crushed tissue in modified radioimmune precipitation assay buffer (150 mm sodium chloride, 50 mm Tris-HCl, pH 7.4, 1 mm EDTA, 1 mm PMSF, 1% Triton X-100, 1% sodium deoxycholic acid, 0.1% sodium dodecyl sulfate) containing protease inhibitors and incubation at 4 °C for 30 min with constant agitation. Insoluble material was then removed by centrifugation at 13,000 × g for 15 min at 4 °C. Western blot analysis was performed using 20 μg of total protein.
Cytokine Array Analysis
Expression levels of cytokine/chemokines were determined by using a Proteom ProfilerTM mouse cytokine array kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Chromatin Immunoprecipitation (ChIP) Assay
Assays were performed according to the manufacturer's instructions (Upstate Biotechnology, Inc.). The antibody immunoprecipitates were reverse cross-linked. To examine the binding of HDAC3 or HDAC2 to MCP1 promoter sequences, PCR was done on the phenol/chloroform-extracted DNA with specific primers of MCP1 promoter-1 (5′-AGTGAGCAAGGTTGACAGGAAGCA-3′ (sense) and 5′-CTGTTCTCCACGGAGTTGCCCTTG-3′ (antisense)), MCP1 promoter-2 (5′-GCAT TGACCAAAGTCCATGGGCAG-3′ (sense) and 5′-AGCCTGGGAGGTCACCATTGGAAA-3′ (antisense)), and MCP1 promoter-3 (5′-GATGATGCTGCTCCTTGGCA-3′ (sense) and 5′-CCAACCCAAGCCCTTCTTTG-3′ (antisense)). To examine binding of HDAC3 to HDAC2 promoter sequences, PCR was done on the phenol/chloroform-extracted DNA with specific primers of HDAC2 promoter-1 (5′-ATGCAAGTGCTTAGAACTGTACC-3′ (sense) and 5′-GCCCCAGTGCCTGGTAGCCTA-3′ (antisense)), HDAC2 promoter-2 (5′-TAGGCTACCAGGCACTGGGGC-3′ (sense) and 5′-GGTGAACAACCTGCGCAAGG-3′ (antisense)) and HDAC2 promoter-3 (5′-CCTTGCGCAGGTTGTTCACC-3′ (sense) and 5′-CTCCTCCTCCTGCTGCTGCTG-3′ (antisense)).
Aortic Ring Formation Assays
In brief, 96-well plates were coated with 30 μl of Matrigel/well and polymerized in an incubator. Aortas isolated from 6-week-old male Sprague-Dawley rats were cleared of periadventitial fat and connective tissues in cold PBS and cut into rings of 1–1.5 mm in circumference. The aortic rings were randomized into wells and sealed with a 30-μl overlay of Matrigel. PBS or mouse recombinant MCP1 (100 ng/ml in PBS) was added. After 6 days, the extent of microvessel sprouting was determined by using an inverted microscope (magnification, 100×; Olympus). The assay was scored from 0 (negative) to 5 (most positive) in a double-blinded manner.
Whole Mount Staining
In order to examine effect of HDAC3 on angiogenesis, BALB/c mice were given an intravenous injection of scrambled (100 nm) or HDAC3 siRNA (100 nm) twice in a total of 5 days. Mouse ears were fixed in 4% (v/v) paraformaldehyde and blocked with TNB buffer (NEN Life Science Products) containing 0.3% Triton X-100. Primary antibody, rabbit anti-CD31 (PECAM-1) was diluted in TNB buffer, and ear samples were incubated with primary antibody overnight at 4 °C. The samples were then washed three times and subsequently incubated with the secondary antibody, anti-rabbit IgG Alex 488 (Molecular Probes, Inc.). In order to examine the relationship between PCA and angiogenesis, anti-DNP-specific IgE (0.5 μg) was injected into ears of BALB/c mice. The next day, the mice were challenged with intravenous injection of 250 μg of DNP-HSA in 250 μl of PBS. The mice were injected with DNP-HSA twice in a total of 6 days before whole mount staining employing anti-PECAM-1 antibody.
β-Hexosaminidase Secretion Assays
The β-hexosaminidase secretion assay was performed according to standard procedures (18). In order to examine the in vivo effect of HDAC3 or MCP1 on β-hexosaminidase, ear tissue lysates (20 μg) of a BALB/c mouse of each experimental group were used.
Histamine Release Assay
Serum histamine level was measured according to the manufacturer's instructions (SPI-Bio). For serum histamine levels, blood from each mouse was collected by cardiac puncture under anesthesia. To measure the cellular histamine level, culture supernatants were used.
Statistical Analysis
Data were analyzed and graphed using the GraphPad Prism statistics program (GraphPad Software). Results are presented as mean ± S.E. Statistical analysis was performed using t tests with differences between means considered significant when p was <0.05.
RESULTS
HDAC3 Is Necessary for TpCR
The induction of HDAC3 in antigen-stimulated RBL2H3 cells was reported (18). We examined a role of HDAC3 in allergic inflammation reaction. For this, we employed a BALB/c mouse model of TpCR. Ear swelling was seen at 1 h and 24 h after DNFB stimulation on ears (Fig. 1A). Increased ear swelling was also seen at 7 days after antigen stimulation (Fig. 1A). Western blot analysis of BALB/c mouse ear tissue lysates showed induction of HDAC3 (Fig. 1B). This increased expression of HDAC3 was sustained up to 7 days after antigen stimulation (Fig. 1B). The in vivo down-regulation of HDAC3 by siRNA exerted a negative effect on increased ear thickness by DNFB stimulation (Fig. 1C). BALB/c mice that received DNFB alone did not show changes in ear thickness (Fig. 1C). In vivo down-regulation of HDAC3 prevented antigen from inducing expression of C-Kit and tryptase, marker proteins for mast cell activation (Fig. 1D). Interestingly, we found an interaction between HDAC3 and FcϵRIβ by antigen stimulation (Fig. 1D). HDAC3 was necessary for an interaction between FcϵRIβ and Lyn, an essential molecule for FcϵRI signaling (Fig. 1D). Mast cells isolated from ear tissues of BALB/c mice showed an interaction between HDAC3 and FcϵRIβ and an interaction between HDAC3 and Lyn by DNFB stimulation (supplemental Fig. 1). Mast cells isolated from ear tissues of BALB/c mice also showed an increased expression of HDAC3 and Lyn by DNFB stimulation (supplemental Fig. 1). Taken together, these results suggest that HDAC3 is necessary for TpCR.
FIGURE 1.
HDAC3 mediates TpCR, an allergic skin inflammation. A, BALB/c mice were given an intravenous injection of DNP-specific IgE antibody (10 μg/kg). The next day, both ears of mice were painted with DNFB or DMSO. Ear thickness was measured for a total of 8 days. Means ± S.E. (error bars) of three independent experiments are shown. ***, p < 0.0005. Each experimental group consists of five BALB/c mice. B, at each time point after DNFB stimulation, ear tissue lysates from each BALB/c mouse were subjected to Western blot analysis. A representative blot of three independent experiments is shown. C, BALB/c mice were given an intravenous injection with DNP-specific IgE antibody along with scrambled or HDAC3 siRNA (each at 100 nm). The next day, both ears of mice were painted with DNFB or DMSO. Each experimental group consists of four BALB/c mice. Means ± S.E. of three independent experiments are depicted. ***, p < 0.0005, compared with IgE/scrambled; ##, p < 0.005, compared with IgE/SiHDAC3/DNFB (24 h); ###, p < 0.0005, compared with IgE/SiHDAC3/DNFB (1 h). D, ear tissue lysates prepared at the indicated time point were subjected to Western blot analysis (top). Tissue lysates prepared at the indicated time point were immunoprecipitated (IP) with anti-FcϵRIβ antibody (2 μg/ml), followed by Western blot analysis (IB) (bottom).
Interaction between HDAC3 and FcϵRIβ Requires HDAC3 Activity
Because HDAC3 showed an interaction with FcϵRIβ in a BALB/c mouse model of TpCR (Fig. 1D), we examined whether HDAC3 activity was required for this interaction. Antigen (DNP-HSA) stimulation induced an interaction between HDAC3 and FcϵRIβ and also induced an interaction between HDAC3 and Lyn in antigen-stimulated RBL2H3 cells and BMMCs (Fig. 2, A and B). TSA, an inhibitor of HDAC(s), prevented an interaction between HDAC3 and FcϵRIβ and also prevented interaction between HDAC3 and Lyn (Fig. 2, A and B). TSA did not affect expression of HDAC3, HDAC5, or HDAC2 in antigen-stimulated RBL2H3 cells (Fig. 2A) or in BMMCs (Fig. 2B). TSA did not affect expression of Lyn in these cell lines (data not shown). Immunofluorescence staining showed a partial co-localization of HDAC3 with FcϵRI in RBL2H3 cells (Fig. 2C). TSA inhibited co-localization of HDAC3 with FcϵRIβ (Fig. 2C). Sodium butyrate, an inhibitor of HDAC(s), inhibited an interaction between HDAC3 and FcϵRIβ and also inhibited an interaction between HDAC3 and Lyn (supplemental Fig. 2A). Sodium butyrate did not affect expression of HDAC3, HDAC5, or HDAC2 (supplemental Fig. 2A). Sodium butyrate inhibited co-localization of HDAC3 with FcϵRIβ (supplemental Fig. 2B). Taken together, HDAC3 activity is necessary for an interaction between HDAC3 and FcϵRIβ, and HDAC3 may mediate FcϵRI signaling.
FIGURE 2.
HDAC3 activity is required for an interaction between HDAC3 and FcϵRIβ. A, the IgE-sensitized RBL2H3 cells were incubated with or without TSA (50 nm) for 12 h prior to stimulation with or without DNP-HSA (100 ng/ml) for 1 h. Cell lysates prepared were immunoprecipitated (IP) with the indicated antibody (2 μg/ml), followed by Western blot analysis (top). Cell lysates prepared were subjected to Western blot analysis (IB) (bottom). B, the IgE-sensitized BMMCs were stimulated with DNP-HSA (100 ng/ml) for 1 h. Cell lysates prepared were immunoprecipitated with the indicated antibody, followed by Western blot analysis (top). Cell lysates prepared were subjected to Western blot analysis (bottom). C, the IgE-sensitized RBL2H3 cells were incubated with or without TSA (50 nm) for 12 h prior to stimulation with or without DNP-HSA for 1 h. Immunofluorescence staining employing the indicated antibody was performed.
HDAC3 Is Necessary for Induction of MCP1
In order to examine the mechanism of HDAC3-mediated TpCR, serum of BALB/c mice, obtained 24 h after painting of DNFB on ear, was subjected to cytokine array analysis. DNFB induced secretion of MCP1 (Fig. 3A). DNFB also induced secretion of C5a, well known stimulator of anaphylaxis (21). In vivo down-regulation of HDAC3 exerted a negative effect on secretion of MCP1, C5a, TIMP1, and sICAM1 in BALB/c mice stimulated with DNFB (Fig. 3A). Immunohistochemistry staining showed that HDAC3 was necessary for induction of MCP1 by antigen stimulation in a BALB/c mouse model of TpCR (Fig. 3B). TSA prevented induction of MCP1 by antigen stimulation (Fig. 3C), suggesting that HDAC3 activity is necessary for induction of MCP1. The down-regulation of HDAC3 prevented antigen from decreasing expression of HDAC2 and prevented antigen from inducing expression of MCP1 (Fig. 3D). However, the down-regulation of HDAC3 did not affect expression of Lyn (Fig. 3D). The down-regulation of FcϵRIβ prevented antigen from increasing expression of HDAC3 and MCP1 (Fig. 3E). The down-regulation of FcϵRIβ prevented antigen from decreasing expression of HDAC2 (Fig. 3E). The down-regulation of HDAC3 prevented an interaction between HDAC3 and FcϵRIβ and prevented an interaction between FcϵRIβ and Lyn (Fig. 3F). Taken together, HDAC3 acts downstream of FcϵRI and mediates the effect of FcϵRI on induction of MCP1.
FIGURE 3.
HDAC3 is necessary for induction of MCP1. A, serum of a BALB/c mice of each experimental group was obtained 24 h after DNFB stimulation and subjected to cytokine array analysis. Each BALB/c mouse was given an intravenous injection of DNP-specific IgE. On the same day, each siRNA (100 nm) was also injected via the tail vein. The next day, DNFB was painted on both ears of BALB/c mice. Each experimental group consists of four BALB/c mice. Representative array data are shown. B, paraffin section of the ear tissue of the indicated BALB/c mouse was subjected to immunohistochemistry staining employing anti-MCP1 antibody or isotype-matched control IgG. Representative images from four animals of each experimental group are shown (magnification, ×400; Olympus). C, the IgE-sensitized RBL2H3 cells or BMMCs were incubated with or without TSA (50 nm) for 12 h prior to stimulation with or without DNP-HSA (100 ng/ml) for 1 h. Cell lysates prepared were subjected to Western blot analysis. D, RBL2H3 cells were transiently transfected with the indicated siRNA (each at 10 nm). The next day, cells were sensitized with DNP-specific IgE (100 ng/ml) for 16 h, followed by stimulation with or without DNP-HSA for 1 h. Cell lysates were subjected to Western blot analysis. E, RBL2H3 cells were transiently transfected with the indicated siRNA (each at 10 nm). The next day, cells were sensitized with DNP-specific IgE (100 ng/ml) for 16 h, followed by stimulation with or without DNP-HSA for 1 h. Cell lysates were subjected to Western blot analysis. F, RBL2H3 cells were transiently transfected with the indicated siRNA (each at 10 nm). The next day, cells were sensitized with DNP-specific IgE (100 ng/ml) for 16 h, followed by stimulation with or without DNP-HSA for 1 h. Cell lysates were immunoprecipitated (IP) with the indicated antibody (2 μg/ml), followed by Western blot analysis.
HDAC3 Binds to Promoter Sequences of MCP1 and HDAC2
Because HDAC3 was necessary for induction of MCP1 (Fig. 3D), we examined the role of HDAC3 in expression regulation of MCP1. For this, ChIP assays were performed. MCP1 promoter sequences contain binding sites for transcription factors, such as AP1, Sp1, NF-κB, and Snail. Antigen stimulation induced binding of HDAC3, Sp1, and c-Jun to MCP1 promoter sequences (Fig. 4A). The down-regulation of HDAC3 prevented binding of Sp1 and c-Jun to MCP1 promoter sequences (Fig. 4A). TSA prevented binding of Sp1 and c-Jun to MCP1 promoter sequences (Fig. 4B). TSA prevented binding of HDAC3 to the AP1/Sp1 site of MCP1 promoter sequences (Fig. 4B). TSA did not affect binding of HDAC3 to the AP1/NF-κB or AP1/Sp1/Snail site of MCP1 promoter sequences (Fig. 4B). The down-regulation of HDAC3 (Fig. 4A) or TSA treatment (Fig. 4B) did not affect binding of NF-κB to MCP1 promoter sequences, suggesting that NF-κB may regulate expression of MCP1 independently of HDAC3. TSA inhibited an interaction between HDAC3 and Sp1 and also inhibited an interaction between HDAC3 and c-Jun (supplemental Fig. 3A). TSA inhibited induction of Sp1 and c-Jun by antigen stimulation (supplemental Fig. 3A). The down-regulation of HDAC3 showed the same effect as TSA on induction of Sp1 and c-Jun (supplemental Fig. 3B). The down-regulation of Sp1 or c-Jun inhibited induction of MCP1 and HDAC3 by antigen stimulation (supplemental Fig. 3C). These results suggest that HDAC3, through interaction with Sp1 or c-Jun, may be at least partially responsible for induction of MCP1. This is the first report that MCP1 expression is under the control of HDAC3. HDAC2 showed binding to MCP1 promoter sequences in the absence of antigen stimulation (Fig. 4C). The down-regulation of HDAC3 induced binding of HDAC2 to MCP1 promoter sequences (Fig. 4C). These results suggest that HDAC2 may act as a negative regulator of MCP1. The MCP1 promoter contains the binding site for YY1 (Fig. 4A). HDAC2 forms transcriptional repressor complexes by associating with many different proteins, including YY1, a mammalian zinc finger transcription factor (22). It is probable that HDAC2 interacts with YY1 to bind to the YY1 site of MCP1 promoter sequences. We examined the mechanism of negative regulation of HDAC2 by HDAC3. Sp1 and c-Jun showed the binding to HDAC2 promoter sequences (supplemental Fig. 4A). The down-regulation of HDAC3 (supplemental Fig. 4A) or TSA treatment (supplemental Fig. 4B) decreased binding of Sp1 and c-Jun to HDAC2 promoter sequences. HDAC3 showed binding to the Sp1 site of HDAC2 promoter sequences (supplemental Fig. 4A). TSA did not affect binding of HDAC3 to the Sp1 site of HDAC2 promoter sequences (supplemental Fig. 4B), suggesting that binding of HDAC3 to the HDAC2 promoter does not regulate HDAC2 expression because TSA did not affect expression of HDAC3 or HDAC2 (Fig. 2, A and B). Taken together, HDAC3 regulates expression of MCP1, through interaction with Sp1 or c-Jun, to mediate allergic inflammation.
FIGURE 4.
HDAC3 binds to promoter sequences of MCP1. A, RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA for 1 h. ChIP assays employing the indicated antibodies were performed. B, the IgE-sensitized RBL2H3 cells were incubated with or without TSA (50 nm) for 12 h prior to stimulation with or without DNP-HSA for 1 h. ChIP assays were performed. C, RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA for 1 h. ChIP assays employing the indicated antibodies were performed. IP, immunoprecipitation.
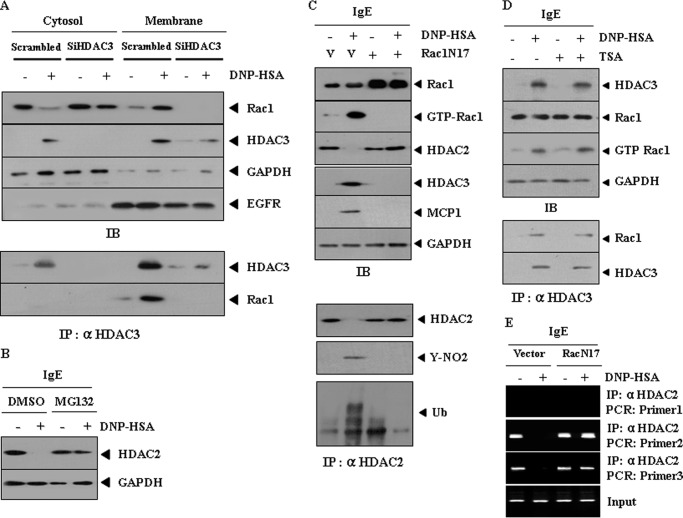
HDAC3-Rac1 Interaction Is Necessary for Down-regulation of HDAC2
It seems that HDAC3 does not exert transcriptional control over HDAC2 (supplemental Fig. 4, A and B). We examined the mechanism of expression regulation of HDAC2 in terms of post-translational modification. The role of reactive oxygen species in the down-regulation of HDAC(s) has been reported (23). We hypothesized that Rac1 would be involved in the down-regulation of HDAC2 by HDAC3. Antigen stimulation led to an interaction between HDAC3 and Rac1 at the membrane (Fig. 5A). MG132, an inhibitor of proteasomal degradation, prevented antigen from decreasing expression of HDAC2 in antigen-stimulated RBL2H3 cells (Fig. 5B), suggesting that down-regulation of HDAC2 may result from proteasomal degradation. The dominant negative Rac1 construct (Rac1N17) prevented antigen from decreasing expression of HDAC2 and prevented antigen from inducing ubiquitination and tyrosine nitration of HDAC2 (Fig. 5C). Rac1 activity was necessary for induction of HDAC3 and MCP1 (Fig. 5C). TSA did not affect Rac1 activity or an interaction between HDAC3 and Rac1 (Fig. 5D). It is reasonable that HDAC3-Rac1 interaction, but not HDAC3 activity, may be necessary for down-regulation of HDAC2. ChIP assays showed that the inactivation of Rac1 induced binding of HDAC2 to MCP1 promoter sequences (Fig. 5E). Reduced binding of HDAC2 is associated with the induction of MCP1 (24). It would be necessary to examine the mechanism of negative regulation of MCP1 by HDAC2.
FIGURE 5.
HDAC3-Rac1 interaction, but not HDAC3 activity, is necessary for down-regulation of HDAC2 by Rac1. A, RBL2H3 cells were transiently transfected with the indicated siRNA (10 nm each) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA for 1 h, followed by cellular fractionation. Cell lysates prepared from each fraction were immunoprecipitated (IP) with anti-HDAC3 antibody (2 μg/ml), followed by Western blot analysis (IB) (bottom). Cell lysates were also subjected to Western blot analysis (top). Cellular fractionation was performed as reported (18). B, the IgE-sensitized RBL2H3 cells were pretreated with or without MG132 (1 μm), an inhibitor of proteasomal degradation, for 1 h, prior to stimulation with DNP-HSA for 1 h. Cell lysates were subjected to Western blot analysis. C, control vector (1 μg) or dominant negative Rac1 construct (Rac1N17) (1 μg) was transfected into RBL2H3 cells prior to sensitization with DNP-specific IgE. Cell lysates prepared after stimulation with DNP-HSA for 1 h were subjected to Western blot analysis (top). Rac1 activity assay was also performed as reported (18). Cell lysates prepared were also immunoprecipitated with anti-HDAC2 antibody (2 μg/ml), followed by Western blot analysis (bottom). V, control vector. D, the IgE-sensitized RBL2H3 cells were incubated with or without TSA (50 nm) for 12 h prior to stimulation with or without DNP-HSA (100 ng/ml) for 1 h. Cell lysates prepared were subjected to Western blot analysis (top). Cell lysates prepared were also immunoprecipitated with anti-HDAC3 antibody (2 μg/ml), followed by Western blot analysis (bottom). E, RBL2H3 cells were transiently transfected with control vector (1 μg) or Rac1N17 (1 μg) prior to sensitization with DNP-specific IgE (100 ng/ml). The IgE-sensitized RBL2H3 cells were then stimulated with DNP-HSA for 1 h. ChIP assays employing the indicated antibodies were performed.
MCP1 Is Necessary for TpCR
Because HDAC3 was necessary for the induction of MCP1 (Fig. 3A), we examined the role of MCP1 in TpCR. The blocking of MCP1 by neutralizing antibody (nMCP1) exerted a negative effect on ear swelling by DNFB stimulation (Fig. 6A). MCP1 was necessary for both initial and late phases of allergic skin inflammation (Fig. 6A). The blocking of MCP1 prevented antigen from increasing expression of c-Kit and CCR2 (chemokine, CC motif, receptor 2), a receptor for MCP1 (Fig. 6A, right). MCP1 was necessary for activation of CCR2 and induction of c-Kit and tryptase by antigen stimulation (Fig. 6A, right). The blocking of MCP1 prevented antigen from increasing β-hexosaminidase activity in both RBL2H3 cells and BMMCs (Fig. 6B), suggesting that MCP1 may acts on mast cells and basophils to mediate TpCR. Recombinant MCP1 enhanced β-hexosaminidase activity in both RBL2H3 cells and BMMCs (Fig. 6C) and induced expression and activity of CCR2 in both RBL2H3 cells and BMMCs (Fig. 6D). Taken together, these results suggest that MCP1, induced by HDAC3, mediates TpCR.
FIGURE 6.
MCP1, regulated by HDAC3, is necessary for TpCR. A, BALB/c mice were given an intravenous injection of DNP-specific IgE (10 μg/kg). A BALB/c mice were also given an intraperitoneal injection of nMCP1 antibody (10 μg/kg) or isotype-matched IgG (10 μg/kg) the day before and 2 days after DNFB stimulation. ***, p < 0.0005; comparison was made between IgE/IgG and IgE/IgG/DNFB (1 h, 24 h, and 8 days). #, p < 0.005; comparison was made between IgE/IgG/DNFB and IgE/nMCP1Ab/DNFB (1 and 24 h). ###, p < 0.0005; comparison was made between IgE/IgG/DNFB and IgE/nMCP1 Ab/DNFB (8 days after DNFB stimulation). Western blot of ear tissue lysates, isolated 8 days after DNFB simulation, from a BALB/c mouse of each experimental group was performed (right). Ear tissue lysates were also subjected to immunoprecipitation (IP), followed by Western blot analysis (IB) (right). B, the IgE-sensitized RBL2H3 cells or IgE-sensitized BMMCs were preincubated with neutralizing nMCP1 antibody (2 μg/ml) or isotype-matched IgG (2 μg/ml) for 1 h, followed by stimulation with DNP-HSA for 1 h. The β-hexosaminidase activity assays were performed as described. Means ± S.E. of three independent experiments are depicted. *, p < 0.05; **, p < 0.005. C, RBL2H3 cells or BMMCs were treated with various concentrations of mouse recombinant MCP1 for 1 h. β-Hexosaminidase activity was measured as described. Means ± S.E. (error bars) of three independent experiments are depicted. **, p < 0.005; ***, p < 0.0005. D, RBL2H3 cells or BMMCs were treated with various concentrations of recombinant MCP1 for 1 h. Cell lysates were prepared and subjected to Western blot (top) or immunoprecipitation, followed by Western blot (bottom).
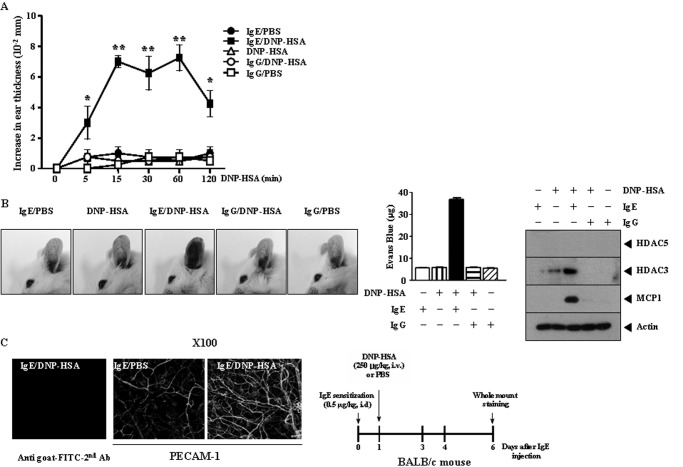
PCA Involves Induction of HDAC3 and MCP1
Just like TpCR, PCA involves ear swelling (25). HDAC3 was necessary for induction of C5a (Fig. 3A). Therefore, we examined the involvement of HDAC3 in PCA. For this, BALB/c mice were given an intradermal injection of DNP-specific IgE or DNP-specific IgG. Twenty-four hours later, BALB/c mice were given an intravenous injection of DNP-HSA. Ear swelling was evident 15 min after injection of DNP-HSA and decreased 120 min after DNP-HSA injection (Fig. 7A). DNP-specific IgG did not induce ear swelling (Fig. 7A). We examined whether PCA would accompany vascular permeability. For this, BALB/c mice were given an intradermal injection of DNP-specific IgE or DNP-specific IgG, Twenty-four hours later, BALB/c mice were given an intravenous injection of DNP-HSA or PBS along with 2% (v/v) Evans blue solution. PCA was accompanied by an enhanced vascular permeability (Fig. 7B). DNP-specific IgG did not enhance vascular permeability (Fig. 7B). Western blot analysis of ear tissue lysates of BALB/c mouse showed that PCA involved induction of HDAC3 and MCP1 (Fig. 7B, right). DNP-specific IgG did not induce expression of HDAC3 or MCP1 (Fig. 7B, right). We next examined whether PCA involved enhanced blood vessel formation. For this, BALB/c mice were given an intradermal injection of DNP-specific IgE. The next day, BALB/c mice were given an intravenous injection of DNP-HSA or PBS. Six days after injection of DNP-specific IgE, whole mount staining employing PECAM-1, an angiogenic marker, was performed. PCA involved enhanced blood vessel formation (Fig. 7C). TNP-specific IgE induced ear swelling (supplemental Fig. 5A). TNP-specific IgE also induced vascular permeability (supplemental Fig. 5B). PCA induced by TNP-specific IgE and TNP-BSA involved induction of HDAC3 and MCP1 and down-regulation of HDAC2 (supplemental Fig. 5B). These results suggest that PCA is accompanied by angiogenesis and involves induction of HDAC3 and MCP1.
FIGURE 7.
PCA involves induction of HDAC3 and MCP1. A, BALB/c mice were given an intradermal injection of DNP-specific IgE antibody (0.5 μg/kg) or DNP-specific IgG (0.5 μg/kg). The next day, BALB/c mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg). Ear thickness was measured for up to 120 min. Means ± S.E. (error bars) of three independent experiments are depicted. Each experimental group consists of five BALB/c mice. B, BALB/c mice were given an intradermal injection of DNP-specific IgE (0.5 μg/kg) or DNP-specific IgG (0.5 μg/kg). The next day, BALB/c mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg) along with 2% (v/v) Evans blue solution. Representative images from four animals of each experimental group are shown. Means ± S.E. of three independent experiments are depicted. Lysates from ears of BALB/c mice were subjected to Western blot analysis (right). A representative Western blot from four animals for each experimental group is shown. C, BALB/c mice were given an intradermal injection of DNP-specific IgE (0.5 μg/kg). The next day, mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg). Six days after injection of DNP-specific IgE, whole mount staining using anti-PECAM-1 antibody was performed (magnification, ×100; Olympus). Representative images from four animals of each experimental group are shown. As a control, ear tissue prepared from a BALB/c mice injected with DNP-specific IgE and DNP-HSA was subjected to immunofluorescence staining employing the indicated secondary antibody but not primary antibody.
HDAC3 Is Necessary for PCA
Because PCA involved induction of HDAC3 (Fig. 7B), we examined the role of HDAC3 in PCA. The in vivo down-regulation of HDAC3 exerted a negative effect on enhanced vascular permeability (Fig. 8A) and β-hexosaminidase activity and histamine release in a BALB/c mouse model of PCA (Fig. 8B). The in vivo down-regulation of HDAC3 prevented antigen from increasing expression of MCP1 and tryptase (Fig. 8C). PCA involved an interaction between HDAC3 and FcϵRIβ (Fig. 8C). The in vivo down-regulation of HDAC3 prevented an interaction between FcϵRIβ and Lyn (Fig. 8C). Immunohistochemistry staining showed that in vivo down-regulation of HDAC3 prevented antigen from inducing expression of MCP1 (Fig. 8D). The in vivo down-regulation of HDAC3 exerted a negative effect on ear swelling (supplemental Fig. 6A, left) and also exerted a negative effect on blood vessel formation in a BALB/c mouse model of PCA (supplemental Fig. 6B). Taken together, these results suggest the role of HDAC3 in PCA.
FIGURE 8.
HDAC3 is necessary for PCA. A, BALB/c mice were given an intradermal injection of DNP-specific IgE (0.5 μg/kg). On the same day, BALB/c mice were given an intravenous injection of scrambled (100 nm) or HDAC3 siRNA (100 nm). The next day, BALB/c mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg) along with 2% (v/v) Evans blue solution. One hour after injection of Evans blue solution, the dye was eluted from the ear in 700 μl of formamide at 63 °C. The absorbance was measured at 620 nm. Representative images from four animals of each experimental group are shown. *, p < 0.05; ***, p < 0.0005. B, ear tissue lysates prepared from BALB/c mouse of each experimental group were subjected to a β-hexosaminidase activity assay (left). Tissue lysates were prepared 1 h after injection of DNP-HSA. One hour after injection of DNP-HSA, serum of each BALB/c mouse was obtained and subjected to a histamine release assay (right). Each experimental group consists of four BALB/c mice. C, same as B except that Western blot analysis was performed. D, immunohistochemistry staining was performed using paraffin section prepared from ear tissue of a BALB/c mouse of each experimental group (magnification, ×400; Olympus).
HDAC3 Activity Is Required for in Vitro and in Vivo Allergic Inflammation
Because HDAC3 was shown to mediate allergic skin inflammation, we examined whether HDAC3 activity was necessary for allergic skin inflammation. Inhibition of HDAC3 by TSA prevented antigen from increasing histamine release and β-hexosaminidase activity in RBL2H3 cells (Fig. 9A, top) and BMMCs (Fig. 9A, bottom). A BALB/c mouse model showed that HDAC3 activity was necessary for ear swelling accompanied by TpCR (Fig. 9B). TSA exerted negative effects on histamine release and β-hexosaminidase activity in a BALB/c mouse model of TpCR (Fig. 9C). TSA did not affect in vivo expression of HDAC3 or HDAC2 (Fig. 9D). However, TSA exerted a negative effect on induction of MCP1 and tryptase (Fig. 9D). TSA inhibited an interaction between FcϵRIβ and HDAC3 and also inhibited an interaction between HDAC3 and Lyn (Fig. 9D). These results suggest that HDAC3 activity is required for in vitro and in vivo allergic inflammation.
FIGURE 9.
The HDAC3 activity is required for in vitro and in vivo allergic inflammation. A, IgE-sensitized RBL2H3 cells were incubated with various concentrations of TSA (1, 10, and 50 nm) for 12 h, followed by stimulation with DNP-HSA for 1 h (top). The IgE-sensitized BMMCs were incubated with TSA (50 nm) for 12 h, followed by stimulation with DNP-HSA for 1 h (bottom). Histamine release and β-hexosaminidase activity were measured as described. Means ± S.E. (error bars) of three independent experiments are depicted. *, p < 0.05; **, p < 0.005. Comparison was made between IgE/DNP-HSA and IgE/TSA/DNP-HSA. B, BALB/c mice were given an intravenous injection of DNP-specific IgE antibody (10 μg/kg). The next day, both ears of mice were painted with DNFB or DMSO along with TSA (500 nm). Ear thickness was measured for up to 6 h. Means ± S.E. of three independent experiments are shown. **, p < 0.005. Each experimental group consists of five BALB/c mice. C, at each time point after DNFB stimulation, histamine release and β-hexosaminidase activity were measured as described. Means ± S.E. of three independent experiments are depicted. *, p < 0.05; **, p < 0.005. D, at each time point after DNFB stimulation, ear tissue lysates were prepared and subjected to Western blot analysis (top). Cell lysates were also immunoprecipitated (IP) with the indicated antibody, followed by Western blot analysis (IB) (bottom).
MCP1 Is Necessary for Vascular Permeability and Angiogenesis Accompanied by PCA
Because PCA involved induction of MCP1 (Fig. 7B) and was accompanied by an enhanced angiogenesis (Fig. 7C), we examined whether MCP1 was necessary for PCA-induced angiogenesis. Neutralizing MCP1 antibody (nMCP1) exerted a negative effect on enhanced vascular permeability in a BALB/c mouse model of PCA (Fig. 10A). Mouse recombinant MCP1, injected intravenously, induced vascular permeability in BALB/c mice (Fig. 10B). MCP1 has potent histamine-releasing activity for basophils (26). Recombinant MCP1 enhances β-hexosaminidase activity and histamine release in BALB/c mice (Fig. 10C), suggesting that the MCP1/CCR2 signaling axis may induce mast cell degranulation in an IgE-independent manner. It would be interesting to examine whether recombinant MCP1 could activate FcϵRI signaling. Western blot analysis of ear tissue lysates of BALB/c mice showed that MCP1 induced expression and activation of CCR2, a receptor for MCP1 (Fig. 10D). Whole mount staining of BALB/c mouse ear tissue, 6 days after IgE sensitization, showed increased expression of PECAM-1 (Fig. 10E). The blocking of MCP1 exerted a negative effect on induction of PECAM-1 by DNP-HSA stimulation (Fig. 10E). Mouse recombinant MCP1 induced angiogenesis in BALB/c mice based on whole mount staining employing anti-PECAM-1 antibody (Fig. 10F) and aortic ring formation (Fig. 10G). Taken together, these results suggest that MCP1 is necessary for PCA-induced vascular permeability and angiogenesis.
FIGURE 10.
MCP1 is necessary for vascular permeability and angiogenesis accompanied by PCA. A, BALB/c mice were given an intradermal injection of DNP-specific IgE (0.5 μg/kg) along with an intravenous injection of IgG (10 μg/kg) or were given an intravenous injection of nMCP1 antibody (10 μg/kg) along with an intradermal injection of DNP-specific IgE (0.5 μg/kg). The next day, BALB/c mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg) along with 2% (v/v) Evans blue solution. Representative images from four animals of each experimental group are shown. *, p < 0.05; **, p < 0.005. B, BALB/c mice were given an intravenous injection of mouse recombinant MCP1 (rMCP1) protein (10 μg/kg) or PBS. One hour or 24 h after injection, BALB/c mice were given an intravenous injection of 2% (v/v) Evans blue solution. Representative images from four animals of each experimental group are shown. **, p < 0.005. C, BALB/c mouse ear tissue lysates prepared 1 h after injection of recombinant MCP1 were subjected to β-hexosaminidase activity assays (left). One hour after injection of recombinant MCP1, serum of each BALB/c mouse was obtained and subjected to a histamine release assay (right). **, p < 0.005. Each experimental group consists of four BALB/c mice. D, BALB/c mouse ear tissue lysates prepared at each time point were subjected to Western blot analysis (top). Ear tissue lysates were immunoprecipitated with the indicated antibody, followed by Western blot analysis (bottom). E, BALB/c mice were given an intradermal injection of DNP-specific IgE (0.5 μg/kg) along with an intravenous injection of IgG (10 μg/kg) or were given an intravenous injection of nMCP1 antibody (10 μg/kg) along with an intradermal injection of DNP-specific IgE (0.5 μg/kg). The next day, mice were given an intravenous injection of PBS or DNP-HSA (250 μg/kg). Whole mount staining was performed. Six days after sensitization with DNP-specific IgE, whole mount staining was performed. ***, p < 0.0005; **, p < 0.005. Each experimental group consists of four BALB/c mice. Representative images from four animals of each experimental group are shown. Area densities of blood vessels were measured. F, BALB/c mice were given an intravenous injection of mouse recombinant MCP1 protein (10 μg/kg) or PBS. Five days after the injection of MCP1 protein, ears of BALB/c mice were excised and subjected to whole mount staining employing anti-PECAM-1 antibody. **, p < 0.005. Representative images from four animals of each experimental group are shown (magnification, ×100; Olympus). Each experimental group consists of four BALB/c mice. Area densities of blood vessels were measured. G, recombinant MCP1 (10 ng) or PBS was added to rat aorta. After 6 days, the extent of microvessel sprouting was determined by using an inverted microscope (magnification, ×100; Olympus). Photographs are representative of endothelial cell sprouts formed from the margin of vessel segments. Error bars, S.E.
DISCUSSION
HDAC3 interacts with Src and is phosphorylated at its tyrosine residue by Src (27). Activation of the FcϵRI is followed by rapid phosphorylation of Src family kinases, such as Lyn (28), Syk (29), and Btk (30). This led us to hypothesize that antigen stimulation may induce an interaction between HDAC3 and FcϵRI. We found an interaction between FcϵRIβ and HDAC3 (Fig. 2A). TSA inhibited an interaction between HDAC3 and FcϵRIβ (Fig. 2A) and co-localization of HDAC3 with FcϵRIβ (Fig. 2C). It seems that HDAC3 acts downstream of FcϵRI (Fig. 2E). Therefore, it would be interesting to examine the effect of Lyn on expression and phosphorylation status of HDAC3. Characteristics of this novel interaction of HDAC3 and FcϵRIβ merit further investigation.
Human dermal microvascular endothelial cells are essential for development and maintenance of skin inflammation (31). Inflammatory skin disease displays prominent microvessel development (31). Sirtinol, a specific sirtuin (a class III HDAC) inhibitor, decreases monocyte adhesion on activated human dermal microvascular endothelial cells and diminishes inflammatory response to TNF-α (31). It would be interesting to examine expression of HDAC3 in human dermal microvascular endothelial cells and the role of HDAC3 in inflammatory response. Human psoriatic skin shows overexpression of HDAC1 (32). These reports suggest a potential role of HDAC(s) in human skin inflammatory diseases.
MCP1 is necessary for mast cell adhesiveness and migration and is necessary for the potential of most cells to recruit monocytes (33). MCP1 is necessary for type I hypersensitivity reaction, such as allergic conjunctivitis, and the blocking of MCP1 prevents allergen from stimulating mast cell degranulation (34). MCP1 expression is under the control of histone modifications (24). HDACs are considered as co-repressors of gene expression. However, HDAC5 plays an essential role of up-regulation of sodium/calcium exchange in cardiomyocytes (35). The inhibition of HDACs by FR276457 decreases MCP1 production (36). This suggests a role of HDAC(s) in up-regulation of MCP1 expression. We examined the possible involvement of MCP1 in expression regulation of HDAC3. The down-regulation of HDAC3 leads to the decreased secretion of MCP1 in a BALB/c mouse model of TpCR (Fig. 3A). The induction of MCP1 requires histone deacetylase activity (Fig. 3C). TNFα induces expression of MCP1 by NF-κB p65-dependent histone acetylation in NIH3T3 fibroblasts (37). PLCϵ cooperates with NF-κB to induce expression of MCP1 expression in human keratinocytes (38). Our result shows the binding of NF-κB to MCP1 promoter sequences (Fig. 4B).
Because HDAC2 expression is under the control of HDAC3, it is plausible that HDAC2 may serve as a negative regulator of allergic skin inflammation by regulating expression of MCP1. HDAC3 does not exert transcriptional control over HDAC2 (supplemental Fig. 4B). It is plausible that expression of HDAC2 may be under post-translational control. Rac1 is necessary for MCP1 production in vascular smooth muscle cells (39). Rac1 promotes proteasomal degradation of p21 (40) and is responsible for ubiquitination of the SWI/SNF protein BAF60b (41). HDAC3 interacts with Rac1 (Fig. 5A), and Rac1 is responsible for the down-regulation, tyrosine nitration, and ubiquitination of HDAC2 (Fig. 5C). Antigen stimulation induces Tip60, a histone acetyltransferase, in RBL2H3 and BMMCs.4 It will be also necessary to examine acetylation as a cause for ubiquitination of HDAC2. The down-regulation of HDAC3 prevents Rac1 from moving to the membrane (Fig. 5A), suggesting HDAC3 is necessary for translocation and activity of Rac1. Rac1 is necessary for induction of MCP1 (Fig. 5C). TSA does not affect Rac1 activity (Fig. 5D). It is plausible that HDAC3 activity is not necessary for Rac1 activity but is necessary for regulation of MCP1 expression. It will be interesting to identify domains of HDAC3 that are necessary for Rac1 activity and for translocation of Rac1 into the membrane. Overall, HDAC3-Rac1 interaction is necessary for expression regulation of MCP1 by HDAC3.
Allergic inflammation, such as PCA, involves vascular permeability resulting from cellular interaction (8). We show that a BALB/c mouse model of PCA involves enhanced vascular permeability and is accompanied by induction of HDAC3 and MCP1 (Fig. 7B).
Angiogenesis is necessary for allergic inflammation (42). Mast cell tryptase and chymase are angiogenic (43). VEGF secretion during hypoxia requires mast cells (44). Mast cells play an essential role of angiogenesis in cervical carcinogenesis (45). Vascular permeability is closely related with angiogenesis (46). We therefore hypothesized that PCA would be associated with angiogenesis. We show that a BALB/c mouse model of PCA involves angiogenesis (Fig. 7C). MCP1 promotes angiogenesis via MCP1-interacting protein (MCP1IP) (47) and mediates TGF-β-induced angiogenesis (48). The blocking of MCP1 exerts a negative effect on PCA-induced angiogenesis (Fig. 10E). It will be interesting to examine the effect of the MCP1/CCR2 signaling axis on expression of angiogenic marker proteins. Conditioned medium of antigen-stimulated RBL2H3 cells induces signaling changes in rat aortic endothelial cells (10). It is therefore plausible that MCP1 may mediate interaction between mast cells and endothelial cells. It would be interesting to identify downstream targets of MCP1. In conclusion, antigen stimulation induces interaction between HDAC3 and FcϵRIβ and interaction between HDAC3 and Lyn (Fig. 11). This interaction leads to interaction between HDAC3 and Rac1 (Fig. 11). HDAC3-Rac1 interaction is responsible for induction of Sp1 and c-Jun. Sp1 and c-Jun bind to MCP1 promoter sequences (Fig. 11). MCP1, induced by HDAD3, mediates allergic skin inflammation (Fig. 11). HDAC2, negatively regulated by FcϵRIβ and HDAC3, is under the control of proteasome-dependent ubiquitination (Fig. 11). HDAC2 is displaced from MCP1 promoter sequences upon degradation, and this leads to binding of c-Jun and Sp1 to MCP1 promoter sequences for induction of MCP1 expression (Fig. 11). HDAC2, Sp1, and c-Jun may compete for the sites on MCP1 (Fig. 11). Our study suggests that HDAC3 can serve as a target for the development of allergic skin inflammation therapeutics.
FIGURE 11.
Proposed mechanism of allergic skin inflammation mediated by HDAC3. Ub, ubiquitin; ROS, reactive oxygen species.
Supplementary Material
This work was supported by Korea Research Foundation Grants C1007988-01-02 and C1007565-01-02 and a grant from the Regional Innovation Center Program of the Ministry of Education, Science, and Technology.

This article contains supplemental Figs. 1–6.
Y. Kim, K. Kim, D. Park, E. Lee, H. Lee, Y.-S. Lee, J. Choe, and D. Jeoung, unpublished observations.
- TpCR
- triphasic cutaneous reaction
- BMMC
- bone marrow-derived mouse mast cell
- DNFB
- 2,4-dinitrofluorobenzene
- DNP
- dinitrophenyl
- HSA
- human serum albumin
- PCA
- passive cutaneous anaphylaxis
- PECAM-1
- platelet endothelial cell adhesion molecule
- RBL2H3
- rat basophilic leukemia
- TSA
- trichostatin A
- nMCP1
- neutralizing MCP1
- HIF
- hypoxia-inducible factor
- SPF
- specific pathogen-free
- PLC
- phospholipase C
- HDAC3
- histone deacetylase 3
- HDAC2
- histone deacetylase 2.
REFERENCES
- 1. Inagaki N., Nagai H. (2009) Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment. Mouse models for the development of remedies for human allergic dermatitis. J. Pharmacol. Sci. 110, 251–259 [DOI] [PubMed] [Google Scholar]
- 2. Sakurai T., Inagaki N., Nagai H. (1994) Life Sci. 54, 291–295 [DOI] [PubMed] [Google Scholar]
- 3. Inagaki N., Tsunematsu M., Sakurai T., Matsuo A., Nagai H. (1997) Effect of prednisolone on IgE-dependent biphasic cutaneous reaction in BALB/c mice. Gen. Pharmacol. 28, 93–97 [DOI] [PubMed] [Google Scholar]
- 4. Hsu C. L., Neilsen C. V., Bryce P. J. (2010) IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One 5, e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pushparaj P. N., Tay H. K., H'ng S. C., Pitman N., Xu D., McKenzie A., Liew F. Y., Melendez A. J. (2009) The cytokine interleukin-33 mediates anaphylactic shock. Proc. Natl. Acad. Sci. U.S.A. 106, 9773–9778 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Di Lorenzo A., Fernández-Hernando C., Cirino G., Sessa W. C. (2009) Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc. Natl. Acad. Sci. U.S.A. 106, 14552–14557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huerta-Yepez S., Baay-Guzman G. J., Bebenek I. G., Hernandez-Pando R., Vega M. I., Chi L., Riedl M., Diaz-Sanchez D., Kleerup E., Tashkin D. P., Gonzalez F. J., Bonavida B., Zeidler M., Hankinson O. (2011) Hypoxia-inducible factor promotes murine allergic airway inflammation and is increased in asthma and rhinitis. Allergy 66, 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oschatz C., Maas C., Lecher B., Jansen T., Björkqvist J., Tradler T., Sedlmeier R., Burfeind P., Cichon S., Hammerschmidt S., Müller-Esterl W., Wuillemin W. A., Nilsson G., Renné T. (2011) Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity 34, 258–268 [DOI] [PubMed] [Google Scholar]
- 9. Warkentin T. E., Greinacher A. (2009) Heparin-induced anaphylactic and anaphylactoid reactions. Two distinct but overlapping syndromes. Expert Opin. Drug Saf. 8, 129–144 [DOI] [PubMed] [Google Scholar]
- 10. Kim Y., Kim K., Park D., Eom S., Park H., Lee H., Lee Y. S., Choe J., Hahn J. H., Kim Y. M., Ro J. Y., Jeoung D. (2011) Integrin α5 interacts with EGFR, is necessary for FcγRI signaling, and is necessary for allergic inflammation in relation with angiogenesis. Mol. Immunol. 48, 1035–1045 [DOI] [PubMed] [Google Scholar]
- 11. Nico B., Mangieri D., Crivellato E., Vacca A., Ribatti D. (2008) Mast cells contribute to vasculogenic mimicry in multiple myeloma. Stem Cells Dev. 17, 19–22 [DOI] [PubMed] [Google Scholar]
- 12. Li L. B., Leung D. Y., Martin R. J., Goleva E. (2010) Inhibition of histone deacetylase 2 expression by elevated glucocorticoid receptor β in steroid-resistant asthma. Am. J. Respir. Crit. Care Med. 182, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meja K. K., Rajendrasozhan S., Adenuga D., Biswas S. K., Sundar I. K., Spooner G., Marwick J. A., Chakravarty P., Fletcher D., Whittaker P. (2008) Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 39, 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhavsar P., Ahmad T., Adcock I. M. (2008) The role of histone deacetylases in asthma and allergic diseases. J. Allergy Clin. Immunol. 121, 580–584 [DOI] [PubMed] [Google Scholar]
- 15. Adenuga D., Yao H., March T. H., Seagrave J., Rahman I. (2009) Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am. J. Respir. Cell Mol. Biol. 40, 464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grausenburger R., Bilic I., Boucheron N., Zupkovitz G., El-Housseiny L., Tschismarov R., Zhang Y., Rembold M., Gaisberger M., Hartl A. (2010) Conditional deletion of histone deacetylase 1 in T cells leads to enhanced airway inflammation and increased Th2 cytokine production. J. Immunol. 185, 3489–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi J. H., Oh S. W., Kang M. S., Kwon H. J., Oh G. T., Kim D. Y. (2005) Trichostatin A attenuates airway inflammation in mouse asthma model. Clin. Exp. Allergy 35, 89–96 [DOI] [PubMed] [Google Scholar]
- 18. Kim Y., Eom S., Kim K., Lee Y. S., Choe J., Hahn J. H., Lee H., Kim Y. M., Ha K. S., Ro J. Y., Jeoung D. (2010) Transglutaminase II interacts with Rac1, regulates production of reactive oxygen species, expression of snail, secretion of Th2 cytokines, and mediates in vitro and in vivo allergic inflammation. Mol. Immunol. 47, 1010–1022 [DOI] [PubMed] [Google Scholar]
- 19. Zhang J., Henagan T. M., Gao Z., Ye J. (2011) Inhibition of glyceroneogenesis by histone deacetylase 3 contributes to lipodystrophy in mice with adipose tissue inflammation. Endocrinology 152, 1829–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim D. Y., Jeoung D., Ro J. Y. (2010) Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J. Immunol. 185, 273–283 [DOI] [PubMed] [Google Scholar]
- 21. Albrecht E. A., Chinnaiyan A. M., Varambally S., Kumar-Sinha C., Barrette T. R., Sarma J. V., Ward P. A. (2004) C5a-induced gene expression in human umbilical vein endothelial cells. Am. J. Pathol. 164, 849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glenn D. J., Wang F., Chen S., Nishimoto M., Gardner D. G. (2009) Endothelin-stimulated human B-type natriuretic peptide gene expression is mediated by Yin Yang 1 in association with histone deacetylase 2. Hypertension 53, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito K., Lim S., Caramori G., Chung K. F., Barnes P. J., Adcock I. M. (2001) Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 15, 1110–1112 [PubMed] [Google Scholar]
- 24. Dje N'Guessan P., Riediger F., Vardarova K., Scharf S., Eitel J., Opitz B., Slevogt H., Weichert W., Hocke A. C., Schmeck B., Suttorp N., Hippenstiel S. (2009) Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 29, 380–386 [DOI] [PubMed] [Google Scholar]
- 25. Kanaoka Y., Maekawa A., Penrose J. F., Austen K. F., Lam B. K. (2001) Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 276, 22608–22613 [DOI] [PubMed] [Google Scholar]
- 26. Suzuki M., Hirai K., Kitani S., Takaishi T., Kihara H., Kasahara T., Ito K., Morita Y. (1996) Pharmacologic study of basophil histamine release induced by monocyte chemotactic protein-1 with kinase inhibitors. Int. Arch. Allergy Immunol. 111, 18–22 [DOI] [PubMed] [Google Scholar]
- 27. Longworth M. S., Laimins L. A. (2006) Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene 25, 4495–4500 [DOI] [PubMed] [Google Scholar]
- 28. Benhamou M., Feuillard J., Lortholary O., Bourgeois C., Michel L., LeGoff L., Michel A., Mencia-Huerta J. M., Lejeune F., Casassus P., Debré P., Arock M. (1996) Protein tyrosine kinases in activation signal of human basophils through the immunoglobulin E receptor type l. J. Leukoc. Biol. 59, 461–470 [DOI] [PubMed] [Google Scholar]
- 29. Jouvin M. H., Adamczewski M., Numerof R., Letourneur O., Vallé A., Kinet J. P. (1994) Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J. Biol. Chem. 269, 5918–5925 [PubMed] [Google Scholar]
- 30. Kawakami Y., Yao L., Miura T., Tsukada S., Witte O. N., Kawakami T. (1994) Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon Fc epsilon RI cross-linking. Mol. Cell Biol. 14, 5108–5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orecchia A., Scarponi C., Di Felice F., Cesarini E., Avitabile S., Mai A., Mauro M. L., Sirri V., Zambruno G., Albanesi C., Camilloni G., Failla C. M. (2011) Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS One 6, e24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tovar-Castillo L. E., Cancino-Díaz J. C., García-Vázquez F., Cancino-Gómez F. G., León-Dorantes G., Blancas-González F., Jiménez-Zamudio L., García-Latorre E., Cancino-Díaz M. E. (2007) Underexpression of VHL and overexpression of HDAC-1, HIF-1α, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int. J. Dermatol. 46, 239–246 [DOI] [PubMed] [Google Scholar]
- 33. Melgarejo E., Medina M. A., Sánchez-Jiménez F., Botana L. M., Domínguez M., Escribano L., Orfao A., Urdiales J. L. (2007) (−)-Epigallocatechin-3-gallate interferes with mast cell adhesiveness, migration, and its potential to recruit monocytes. Cell Mol. Life Sci. 64, 2690–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tominaga T., Miyazaki D., Sasaki S., Mihara S., Komatsu N., Yakura K., Inoue Y. (2009) Blocking mast cell-mediated type I hypersensitivity in experimental allergic conjunctivitis by monocyte chemoattractant protein-1/CCR2. Invest. Ophthalmol. Vis. Sci. 50, 5181–5188 [DOI] [PubMed] [Google Scholar]
- 35. Chandrasekaran S., Peterson R. E., Mani S. K., Addy B., Buchholz A. L., Xu L., Thiyagarajan T., Kasiganesan H., Kern C. B., Menick D. R. (2009) Histone deacetylases facilitate sodium/calcium exchanger up-regulation in adult cardiomyocytes. FASEB J. 23, 3851–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinugasa F., Noto T., Matsuoka H., Urano Y., Sudo Y., Takakura S., Mutoh S. (2010) Prevention of renal interstitial fibrosis via histone deacetylase inhibition in rats with unilateral ureteral obstruction. Transpl. Immunol. 23, 18–23 [DOI] [PubMed] [Google Scholar]
- 37. Boekhoudt G. H., Guo Z., Beresford G. W., Boss J. M. (2003) Communication between NF-κB and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J. Immunol. 170, 4139–4147 [DOI] [PubMed] [Google Scholar]
- 38. Harada Y., Edamatsu H., Kataoka T. (2011) PLCϵ cooperates with the NF-κB pathway to augment TNFα-stimulated CCL2/MCP1 expression in human keratinocyte. Biochem. Biophys. Res. Commun. 414, 106–111 [DOI] [PubMed] [Google Scholar]
- 39. Tölle M., Pawlak A., Schuchardt M., Kawamura A., Tietge U. J., Lorkowski S., Keul P., Assmann G., Chun J., Levkau B., van der Giet M., Nofer J. R. (2008) HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler. Thromb. Vasc. Biol. 28, 1542–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bao W., Thullberg M., Zhang H., Onischenko A., Strömblad S. (2002) Cell attachment to the extracellular matrix induces proteasomal degradation of p21CIP1 via Cdc42/Rac1 signaling. Mol. Cell Biol. 22, 4587–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lorès P., Visvikis O., Luna R., Lemichez E., Gacon G. (2010) FEBS Lett. 277, 1453–1464 [DOI] [PubMed] [Google Scholar]
- 42. Detoraki A., Staiano R. I., Granata F., Giannattasio G., Prevete N., de Paulis A., Ribatti D., Genovese A., Triggiani M., Marone G. (2009) Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 123, 1142–1149, 1149.e1–1149.e5 [DOI] [PubMed] [Google Scholar]
- 43. Ribatti D., Ranieri G., Nico B., Benagiano V., Crivellato E. (2011) Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int. J. Dev. Biol. 55, 99–102 [DOI] [PubMed] [Google Scholar]
- 44. García-Román J., Ibarra-Sánchez A., Lamas M., González Espinosa C. (2010) VEGF secretion during hypoxia depends on free radicals-induced Fyn kinase activity in mast cells. Biochem. Biophys. Res. Commun. 401, 262–267 [DOI] [PubMed] [Google Scholar]
- 45. Utrera-Barillas D., Castro-Manrreza M., Castellanos E., Gutiérrez-Rodríguez M., Arciniega-Ruíz de Esparza O., García-Cebada J., Velazquez J. R., Flores-Reséndiz D., Hernández-Hernández D., Benítez-Bribiesca L. (2010) The role of macrophages and mast cells in lymphangiogenesis and angiogenesis in cervical carcinogenesis. Exp. Mol. Pathol. 89, 190–196 [DOI] [PubMed] [Google Scholar]
- 46. Stoeltzing O., Ahmad S. A., Liu W., McCarty M. F., Wey J. S., Parikh A. A., Fan F., Reinmuth N., Kawaguchi M., Bucana C. D., Ellis L. M. (2003) Angiopoietin-1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumors. Cancer Res. 63, 3370–3377 [PubMed] [Google Scholar]
- 47. Niu J., Azfer A., Zhelyabovska O., Fatma S., Kolattukudy P. E. (2008) Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J. Biol. Chem. 283, 14542–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma J., Wang Q., Fei T., Han J. D., Chen Y. G. (2007) MCP-1 mediates TGF-β-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood 109, 987–994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.