Background: TRAF2 function in the EGF pathway and colon cancer development is unclear.
Results: Knockdown TRAF2 blocked EGF- induced cell transformation and EGF signaling pathway through RSK2 ubiquitination.
Conclusion: TRAF2 has an important function in the EGF pathway and is overexpressed and required in colon cancer development.
Significance: TRAF2 regulates the EGF pathway through RSK2/CREB/c-Fos and is important for colon cancer development.
Keywords: AP1 Transcription Factor, Epidermal Growth Factor (EGF), RSK, TRAF, Transformation, EGF Signaling
Abstract
TRAF2 has an important function in mediating the TNF-R signaling pathway toward activation of NF-κB and JNKs. Here we reveal a novel function of TRAF2 in the epidermal growth factor (EGF) signaling pathway. Knockdown of TRAF2 blocked EGF-induced AP-1 activity and anchorage- independent cell transformation. Notably, we showed that EGF induces ribosomal S6 kinase 2 (RSK2) ubiquitination, and knocking down TRAF2 suppresses ubiquitination of RSK2 induced by EGF. We also found that TRAF2 affects RSK2 activity through RSK2 ubiquitination. RSK2 plays a critical role in AP-1 activity mediated through CREB and c-Fos, which regulates anchorage-independent cell transformation. In addition, TRAF2 is overexpressed in colon cancer and required for colon cancer development, suggesting that TRAF2 might be a potential molecular target for cancer prevention and treatment.
Introduction
TNF-R-associated factor family protein 2 (TRAF2)2 is a well known protein in the tumor necrosis factor receptor superfamily, member 1A (TNF-R1) signaling pathway. In the presence of TNF-α, the receptor forms a trimer, and the intracellular domain is exposed and recognized by TNF receptor-associated death domain (TRADD). TRADD, a scaffold protein, recruits TRAF2, TRAF5, and receptor interacting protein (RIP) (1). The complex activates different downstream signaling pathways, such as NF-κB (2), AP-1 (3), or the caspase cascades (4). TRAF2 was proposed to act as an E3 ligase for Lys-63 polyubiquitination of RIP1 or itself, a function that is critical to activate the IKK/NF-κB or JNK/AP-1 pathway. TRAF2 has a typical ring domain in its N terminus, and overexpression of TRAF2 leads to ubiquitination of RIP1 and itself through UBC13/UEV1A (5). In TRAF2/TRAF5 double knock-out cells, both JNKs and NF-κB activation are deficient (6). In contrast, only JNK activation and not NF-κB activation, is defective in TRAF2 knock-out cells, which is likely due to the redundant role of TRAF2 and TRAF5 in TNF-α-induced NF-κB activation (7).
Whether TRAF2 functions as an E3 ligase is controversial because TRAF2 E3 ligase activity is not observed in vitro (8). In contrast, TRAF6, another TRAF family member, exerts E3 ligase activity in vitro (9). In addition, c-IAP1/2 was reported to act as an E3 ligase for RIP1 in vitro and in vivo, and c-IAP1/2 binding to the TNF-R complex requires TRAF2 (10). These data led investigators to conclude that TRAF2 serves as an adaptor protein rather than an E3 ligase in the TNF-induced signaling pathway. However, Alvarez et al. (11) recently provided evidence showing that TRAF2 actually is an E3 ligase for RIP1 Lys-63 polyubiquitination in vitro.
Ribosomal S6 kinases (RSKs) comprise a family of serine/threonine kinases consisting of four (RSK1-RSK4) human isoforms that are downstream of the mitogen-regulated Ras/ERK-MAPK pathway. EGF induces extracellular signal-regulated kinase 1/2 (ERK1/2) activation, which directly phosphorylate and activate ribosomal S6 kinase 2 (RSK2). RSK2 then phosphorylates substrates like CREB (cAMP-responsive element-binding protein) (12), c-Fos (13), ATF4 (activating transcription factor 4) (14), and ATF1 (activating transcription factor 1) (15), which promotes their cellular functions.
We found that EGF-induced cell transformation and signaling are dramatically abrogated in knockdown TRAF2 HaCaT cells and TRAF2 knock-out murine embryonic fibroblasts (MEFs). Based on current knowledge, to explain how TRAF2 regulates the EGF signal transduction pathway is difficult. Here, we present a novel function of TRAF2 in mediating the EGF-induced signal transduction pathway. We showed that TRAF2 directly affects RSK2 activity but has no effect on phosphorylation of ERK1/2. We showed that EGF induces RSK2 ubiquitination, and knocking down TRAF2 abrogates ubiquitination of RSK2 induced by EGF. We also found that TRAF2 is required for RSK2 ubiquitination at Lys-345 and -364, which is related to RSK2 activity. The Ras-MAPK pathway is frequently activated in colorectal cancer, and we also showed that TRAF2 and RSK2 are overexpressed in different colorectal cancer cell lines and colon cancer tissues. Knockdown of TRAF2 expression dramatically blocked the malignant phenotype, including cell proliferation, colony growth in soft agar, and the ability to form tumors in athymic nude mice mediated through RSK2/CREB. This indicates that TRAF2 plays a critical role in colorectal tumorigenesis.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Chemical reagents including Tris, NaCl, and SDS for molecular biology and buffer preparation were purchased from Sigma. Cell culture media and other supplements were purchased from Invitrogen. Antibodies to detect TRAF2, phosphorylated CREB (Ser-133), phosphorylated RSK (Ser-380), cIAP1/2, and P4D1 were obtained from Cell Signaling Technology, Inc. (Beverly, MA). The antibodies against c-Fos, RSK2, TRAF2, β-actin, and HA were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-FLAG was purchased from Sigma. The Xpress antibody was from Invitrogen, and anti-RSK1 and active RSK2 were purchased from Upstate Biotechnology, Inc. (Charlottesville, VA). The GST-CREB protein was purchased from SignalChem, Inc. (Richmond, BC, Canada).
Construction of Expression Vectors
The expression constructs including RSK2 and truncated RSK2 (16), FLAG-TRAF2-WT, and FLAG-TRAF2-DN were generous gifts (17) and were amplified and used for expression in HEK293 and HeLa cells. RSK1-HA, HA-Lys-48-ubiquitin, and HA-Lys-63-ubiquitin were purchased from Addgene (Cambridge, MA). GST-IκBα was purified as described previously (16). pCDNA4.0-RSK2-K334R, -K345R, -K364R, -K367R and K345R,K364R mutants were constructed from pCDNA4.0-RSK2-WT using a site-directed mutagenesis kit (Stratagene, La Jolla, CA). RSK2 was subcloned into the pBabe vector by Xba1,BamH1 from the pCDNA4.0-RSK2. The pBabe-RSK2-K345R,K364R mutant was constructed as described above. Lentivirus plasmids containing sh-TRAF2 (#1, TRCN0000004572;#2, TRCN0000004574), sh-cIAP1 (TRCN0000003783), and sh-cIAP2 (TRCN0000003778) were purchased from Thermo Scientific (Huntsville, AL).
Cell Culture and Transfection
TRAF2+/+ and TRAF2−/− MEFs were a generous gift (17). They were cultured with DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotics at 37 °C in a 5% CO2 incubator. Human embryonic kidney (HEK293) cells, 293T cells, and HaCaT cells were grown in Dulbecco's modified Eagle's medium (Hyclone, San Diego, CA) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA), 100 units/ml penicillin, and 100 mg/ml streptomycin and cultured at 37 °C in a humidified incubator with 5.0% CO2. HeLa cells (human cervix adenocarcinoma) were grown in Eagle's minimum essential medium (MEM) supplemented with 10% FBS, 100 units/ml penicillin, and 100 mg/ml streptomycin and cultured at 37 °C in a humidified incubator with 5% CO2. The cells were maintained by splitting at 90% confluence, and media were changed every 3 days. When cells reached 50–60% confluence, transfection was performed using JetPEI (Polyplus-transfection Inc., New York, NY) following the manufacturer's suggested protocol. The cells were cultured for 36–48 h, and then proteins were extracted for further analysis.
Lentiviral and Retroviral Infection
To construct knockdown TRAF2, RSK2, and cIAP1/2 cells, the lentivirus plasmid of TRAF2, RSK2, and cIAP1/2 was co-transfected into 293T cells together with PSPAX2 and PMD2-G. Viral supernatant fractions were collected at 48 h after transfection and filtered through a 0.45-μm filter. The viral supernatant fractions were infected into the appropriate cells together with 10 μg/ml Polybrene. At 16 h after infection, the medium was replaced with fresh medium containing the appropriate concentration of puromycin. The appropriate experiments were performed with these cells until the control cells (without infection) completely died (usually 2–3 days) in the puromycin medium. For generation of double knockdown TRAF2/RSK2 cells, stable knockdown RSK2 cells were first generated as described above. Then these stable knockdown cells were infected with sh-TRAF2 viral supernatant fraction. At 3–4 days after infection, the appropriate experiments were performed using these cells. For generation of stable HaCaT cells expressing RSK2-WT or RSK2-K354R,K364R, the pBabe-mock, pBabe-RSK2-WT, or pBabe-RSK2-K354R,K364R plasmid was co-transfected into 293T cells together with pCI-VSVG and pCI-GPZ. Viral supernatant fractions were collected at 48 h after transfection and filtered through a 0.45-μm filter. The pBabe-mock, pBabe-RSK2-WT, and pBabe-RSK2-K354R,K364R viral supernatant fractions were infected into HaCaT cells together with 10 μg/ml Polybrene. At 16 h after infection, the medium was replaced with fresh medium containing 1 μg/ml puromycin, and cells were incubated for 6 days.
Immunoblotting and Immunoprecipitation
Protein samples from cells were extracted with Nonidet P-40 cell lysis buffer (50 mm Tris-Cl, pH 8.0, 150 mm NaCl, 0.5% Nonidet P-40, and protease inhibitor mixture). For immunoblotting, 30 μg of protein were used with appropriate specific antibodies and an alkaline phosphatase (AP)-conjugated secondary antibody, and proteins were detected by the STORM machine using the fluorescence/chemiluminescence mode (Amersham Biosciences). For immunoprecipitation, the extractions were combined with agarose A/G beads (50% slurry) by rocking at 4 °C overnight. The beads were washed, mixed with 6× SDS sample buffer, boiled, and then resolved by 10% SDS-PAGE. The proteins were detected using the appropriate specific antibodies and an AP-conjugated secondary antibody. For immunoprecipitation (IP) under denaturing conditions, proteins were extracted using regular IP buffer plus 1% SDS and heated at 95 °C for 5 min. The samples were diluted 1:10 in regular IP buffer before IP. The beads were washed, mixed with 6× SDS sample buffer, boiled, and then resolved by 10% SDS-PAGE. The proteins were visualized by immunoblotting.
Immunofluorescence Staining
Appropriate cells were fixed in 4% paraformaldehyde and permeabilized in 0.5% Triton X-100 for 30 min. Fixed cells were then incubated with an RSK2 mouse monoclonal antibody (Santa Cruz Biotechnology) and TRAF2 rabbit polyclonal antibody (Cell Signaling Biotechnology, Inc.) overnight followed by incubation with red fluorescent Alexa Fluor 568 dye-labeled anti-mouse IgG or green fluorescent Alexa Fluor 488 dye-labeled anti-rabbit IgG (Invitrogen). Nuclei were stained with DAPI. Samples were viewed with a confocal fluorescence microscope system (NIKON C1si confocal spectral imaging system, NIKON Instruments Co., Melville, NY).
Flow Cytometry Analysis
Cells were seeded into 6 wells and cultured for 48 h, and then cells were fixed in ethanol and stained with propidium iodide before flow cytometry analysis of cell cycle.
Anchorage-independent Cell Growth
For EGF-induced cell transformation, cells (8 × 103/ml/well) were exposed to EGF in 1 ml of 0.3% basal medium Eagle agar containing 10 or 20% FBS. The cultures were maintained in a 37 °C, 5% CO2 incubator for the appropriate number of days, and cell colonies were scored using a microscope and the Image-Pro PLUS (v. 6) computer software program (Media Cybernetics). For cancer cell anchorage-independent growth, cells (8.0 × 103/well) were seeded into 6-well plates with 0.3% basal medium Eagle agar containing 10% FBS and cultured for the appropriate number of days. Colonies were scored using a microscope and the Image-Pro PLUS (v.6) computer software program (Media Cybernetics).
Immunoprecipitation in Vitro Kinase Assay
For the IP kinase assay, RSK1-HA, RSK2-Xpress, or RSK2 were immunoprecipitated with anti-HA, anti-Xpress, or anti-RSK2. The immunoprecipitates were incubated with purified GST-IκBα or the GST-CREB protein as substrate for the in vitro kinase assay. Reactions were carried out at 30 °C for 30 min in a mixture containing 50 μm unlabeled ATP or 32P-labeled γ-ATP and then were stopped by adding 6×SDS sample buffer. Samples were boiled and visualized by Western blotting or Coomassie Blue staining.
Luciferase Assays
Cells were transfected with pAP-1-Luc and SV-40-Renilla-Luc (Promega, Madison, WI) in the presence of JetpeI. At 20 h after transfection, cells were starved for 16 h and then were treated with EGF (20 ng/ml) for various times and disrupted in passive lysis buffer. Lysates were analyzed for firefly and Renilla luciferase activities using the dual luciferase assay kit (Promega).
Immunohistochemical Analysis of Tissue Array
A human colon tissue array (CO801) was purchased from US Biomax, Inc. (Rockville, MD). The tissue array includes matched normal tissues that were biopsied from the adjacent tissue of each cancer tissue from 40 individual patients. A Vectastain Elite ABC Kit obtained from Vector Laboratories (Burlingame, CA) was used for immunohistochemical staining according to the protocol recommended by the manufacturer. Briefly, the slide was baked at 60 °C for 2 h, deparaffinized, and rehydrated. To exposure antigens, the slide was unmasked by submerging it into boiling sodium citrate buffer (10 mm, pH 6.0) for 10 min, and then the slide was treated with 3% H2O2 for 10 min. The slides were blocked with 50% goat serum albumin in 1× PBS in a humidified chamber for 1 h at room temperature and then with the RSK2 and TRAF2 antibodies (1:100 dilution in 50% goat serum with PBS) at 4 °C in a humidified chamber overnight. The slide was washed and hybridized with the secondary antibody obtained from Vector Laboratories (Burlingame, CA) (anti-rabbit, anti-mouse 1:200) for 1 h at room temperature. Slides were stained using the Vectastain Elite ABC kit. The intensity was estimated using the Image-Pro PLUS (v.6) computer software program and ImageJ (NIH) computer program. Statistical analyses were performed using Prism 5.0 statistics software.
In Vivo Tumor Growth
Athymic nude mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The mice were divided into two groups (n = 15) and injected in the right flank with sh-Mock or sh-TRAF2 HCT116 cancer cells (1 × 106). Tumors were measured by caliper twice a week. All studies were performed in accordance with the guidelines approved by the University of Minnesota Institutional Animal Care and Use Committee.
RESULTS
TRAF2 Blocks EGF-induced Anchorage-independent Cell Transformation Mediated through RSK2
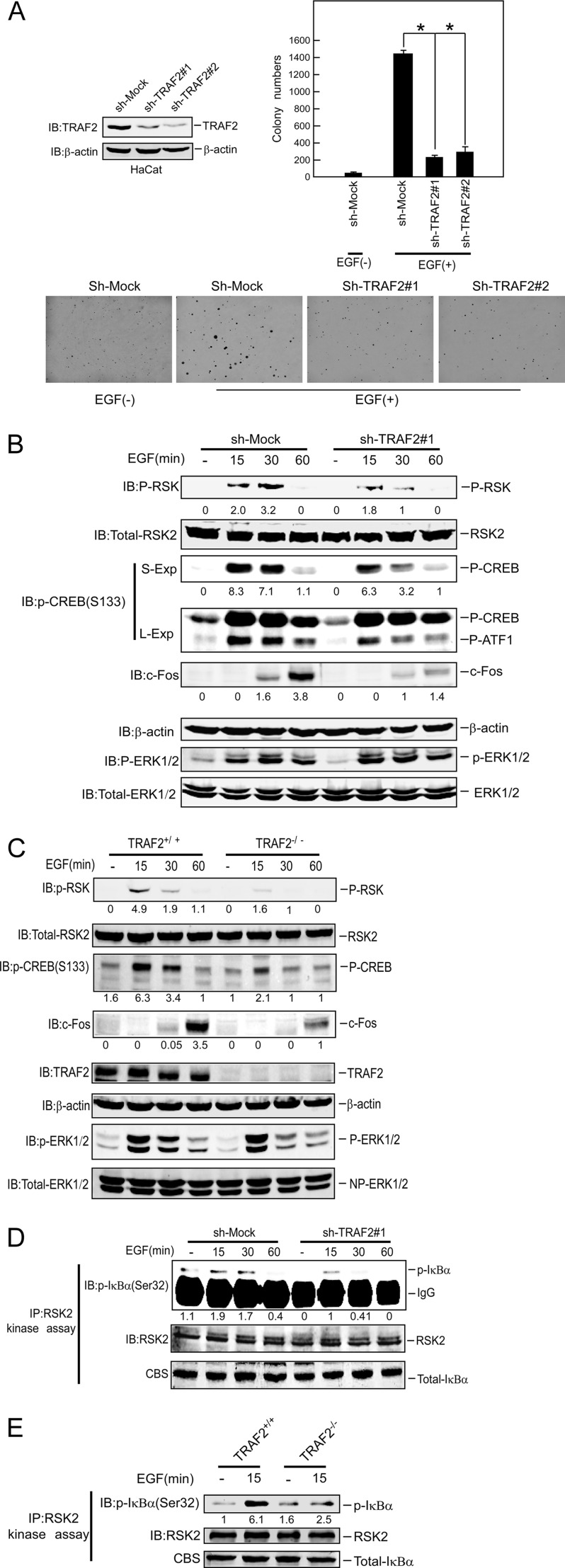
We generated stable knockdown TRAF2 HaCaT cells in which TRAF2 was dramatically decreased by two different sh-TRAF2 sequences (Fig. 1A, left panel). The soft agar assay was performed to assess the effect of knockdown TRAF2 on cell transformation. Notably, EGF-induced anchorage-independent colony formation was markedly attenuated in knockdown TRAF2 HaCaT cells (Fig. 1A, right lower panels, and supplemental Fig. 1C). We also noticed that cell proliferation was blocked (supplemental Fig. 1A) and cell cycle progress was inhibited in G1-S phase by knockdown TRAF2 (supplemental Fig. 1B). TRAF2 has a critical function in the TNFR1 pathway mediated through the RIP1-Ikkα/β pathway. However, based on current knowledge regarding TRAF2 function, to explain the role of TRAF2 in the EGF pathway is challenging. To investigate the role of TRAF2 in the EGF signaling pathway, we assessed the activation of the Ras-MAPK pathway in knockdown TRAF2 HaCaT cells after EGF treatment. Results showed that knockdown TRAF2 expression impaired the phosphorylation of RSK and CREB and also inhibited EGF-induced c-Fos expression (Fig. 1B). However, the phosphorylation of ERK1/2 was not affected (Fig. 1B). Similar results were observed after knocking down TRAF2 cells using another sh-TRAF2 sequence (supplemental Fig. 1D) and in TRAF2- deficient (TRAF2−/−) MEFs (Fig. 1C). IKKα/β is downstream of TRAF2 in the TNF-R1 pathway, but its role in the EGF pathway is unclear. Our result showed that IKKα/β cannot be activated by EGF treatment (supplemental Fig. 1E). In addition, TRAF2 had no effect on the interaction between ERK1/2 and RSK2 (supplemental Fig. 1F). CREB (12) and ATF1 (15) are well known or novel substrates, respectively, for RSK2. Both CREB and ATF1 contribute to the induction of c-Fos expression (18). Our results showing that phosphorylated CREB or c-Fos expression was decreased in TRAF2 knockdown or TRAF2 deficient cells indicate that TRAF2 might directly regulate RSK2 activity. To determine the direct effect of TRAF2 on RSK2 activity, endogenous RSK2 was immunoprecipitated from knockdown TRAF2 cells or TRAF2-deficient MEFs treated with EGF for various times (Fig. 1, D and E). The precipitated immunocomplexes were incubated with GST-IκBα as substrate for the RSK2 IP kinase assay. The results indicated that RSK2 activity was decreased in TRAF2 knockdown (Fig. 1D) or deficient cells (Fig. 1E). RSK1 has a high homology and similar function with RSK2 in the EGF pathway. To test for a possible effect of TRAF2 on RSK1, RSK1-HA and RSK2-Xpress were co-transfected with TRAF2 into 293 cells. At 36 h after transfection, RSK1 and RSK2 were immunoprecipitated with anti-HA and anti-Xpress, respectively. The immunocomplexes were then subjected to an IP kinase assay by incubating with GST-IκBα, which is a substrate for RSK1 (19) and RSK2. The result showed that RSK1 activity is slightly increased, whereas RSK2 activity was dramatically increased by TRAF2, which indicated that RSK2 is a major molecule mediated by TRAF2 (supplemental Fig. 1G). In addition, overexpression of TRAF2 did not effect phosphorylation of ERK1/2 (supplemental Fig. 1G) even though RSK2 activity is increased after overexpression of TRAF2. Taken together, these results showed that TRAF2 has an important role in the EGF-induced signaling pathway, and TRAF2 directly affects RSK2 activity.
FIGURE 1.
TRAF2 plays a critical role in EGF-induced anchorage-independent neoplastic cell transformation mediated by RSK2. A, knockdown of TRAF2 blocks EGF-induced cell transformation. Knockdown of TRAF2 in HaCaT cells was performed as described under “Experimental Procedures,” and cells were exposed to EGF (20 ng/ml) in 0.3% basal medium Eagle agar containing 20% FBS. The cultures were maintained in a 37 °C, 5% CO2 incubator for 15 days, and then colonies were counted using a microscope and the Image-Pro PLUS (v.6) computer software program. Representative photos are shown, and the graph shows data from multiple experiments expressed as the means ± S.D., and the asterisks (*) indicate a significant difference (p < 0.05 Student's t test). IB, immunoblot. B and C, shown is the effect of TRAF2 on the EGF signaling pathway. B, knockdown TRAF2 HaCaT cells induced by sh-TRAF2#1 were starved for 36 h and then treated with EGF (20 ng/ml) for various times. Immunoblotting was used to detect phosphorylation of RSK, CREB, ATF1, ERK1/2, and RSK2 and c-Fos expression as indicated. β-Actin was used to verify equal loading of protein. C, TRAF2 knock-out MEFs were starved for 16 h and then treated with EGF (20 ng/ml) for various times. Immunoblotting was used to detect phosphorylation of RSK, CREB, ERK1/2, and RSK2 and TRAF2 and c-Fos expression as indicated. D, RSK2 activity was decreased in TRAF2 knockdown cells. Knockdown TRAF2 HaCaT cells were starved for 36 h and then treated with EGF (20 ng/ml) for various times. RSK2 was immunoprecipitated from cell lysates. The immunocomplexes were incubated with GST-IκBα as the substrate for RSK2 in an in vitro kinase assay as described under “Experimental Procedures.” Phosphorylated IκBα was visualized by Western blot using the phosphorylation IκBα (Ser-32) antibody to demonstrate RSK2 activity. E, RSK2 activity is decreased in TRAF2 knock-out cells. TRAF2 knock-out MEFs were starved for 16 h and then treated with EGF (20 ng/ml) for 15 min. RSK2 was immunoprecipitated from cell lysates for an in vitro kinase assay as described for D.
TRAF2 Regulates AP-1 Activity
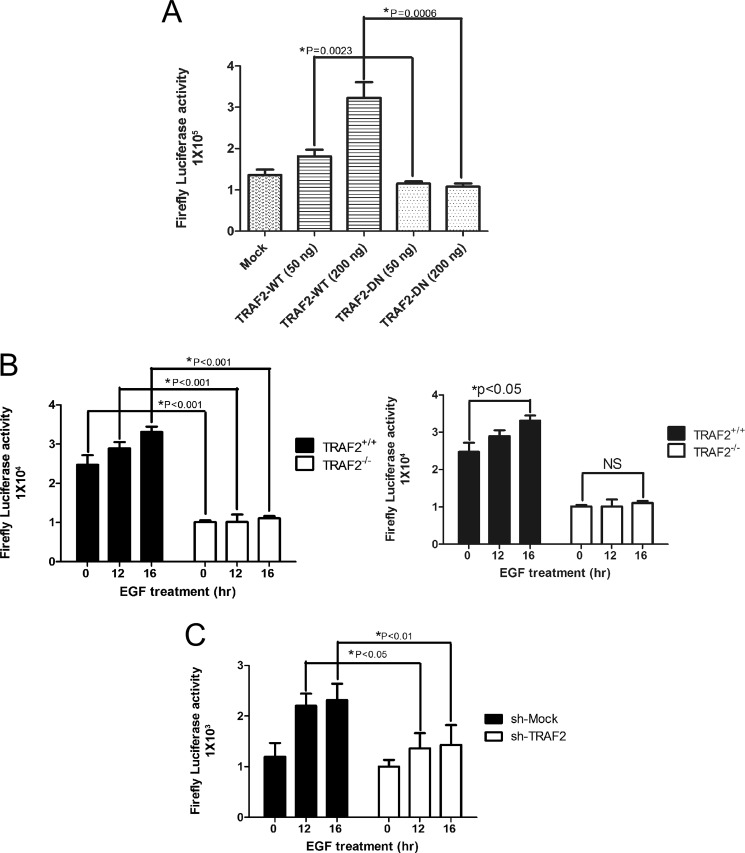
AP-1 is an important mediator of EGF-induced anchorage-independent cell transformation and tumor development (20) and comprises a dimeric complex that can includes Jun, Fos, ATF1, and other family members. To determine whether TRAF2 can regulate AP-1 activity, the AP-1 luciferase reporter gene, Renilla luciferase gene, and TRAF2-WT or TRAF2-DN (ring domain deletion) were co-transfected into HEK293 cells. The data showed that AP-1 transcriptional activity increased dose-dependently in cells expressing TRAF2-WT but not in cells expressing TRAF2-DN (Fig. 2A). We also co-transfected the AP-1 luciferase reporter and Renilla luciferase genes into TRAF2-deficient MEFs or TRAF2 knockdown HaCaT cells. The results indicated that AP-1 transcriptional activity was also decreased in TRAF2−/− or TRAF2 knockdown cells (Fig. 2, B and C).
FIGURE 2.
TRAF2 regulates AP-1 activity. A, overexpression of TRAF2 increases AP-1 activity. Various amounts of FLAG-TRAF2-WT or FLAG-TRAF2-DN were transfected with a plasmid mixture containing the AP-1-luciferase reporter gene (100 ng) and the Renilla luciferase gene (20 ng) for normalization. At 24 h after transfection, the firefly luciferase activity was determined in cell lysates and normalized against Renilla luciferase activity. Significant differences were evaluated using Student's t test, and the respective asterisks indicate a significant difference (p < 0.05). B, AP-1 activity is decreased in TRAF2 knock-out MEFs. TRAF2 knock-out MEFs were co-transfected with a plasmid mixture containing the AP-1 luciferase reporter gene (0.4 μg) and the Renilla luciferase gene (0.1 μg) for normalization. At 20 h after transfection, cells were starved for 16 h and then treated with EGF (20 ng/ml) for various times as indicated. Firefly luciferase activity was determined in cell lysates and normalized against Renilla luciferase activity. Significant differences were evaluated using Student's t test, and the respective asterisks indicate a significant difference (p < 0.05). C, AP-1 activity is decreased in TRAF2 knockdown HaCaT cells. TRAF2 knockdown cells were co-transfected with a plasmid mixture containing the AP-1 luciferase reporter gene (0.8 μg) and the Renilla luciferase gene (0.2 μg) for normalization. At 20 h after transfection, cells were starved for 16 h and then treated with EGF (20 ng/ml) for various times as indicated. Firefly luciferase activity was determined in cell lysates and normalized against Renilla luciferase activity. Significant differences were evaluated using Student's t test, and the respective asterisks indicate a significant difference (p < 0.05).
TRAF2 Interacts with RSK2
We showed above that TRAF2 directly affects RSK2 activity. Here, we also report that TRAF2 is a novel protein binding partner with RSK2. RSK2-Xpress and TRAF2-FLAG were co-transfected into HEK293 cells. RSK2-Xpress was immunoprecipitated with anti-Xpress, and TRAF2-FLAG was detected in the IP complex (supplemental Fig. 2A). TRAF5 and TRAF2 have similar functions in the TNF-R1 pathway. Therefore, we also determined whether TRAF5 could interact with RSK2, and the result showed that TRAF2, but not TRAF5, was detected in the RSK2 immunoprecipitated complex (Fig. 3A). Endogenous RSK2 or TRAF2 in HaCaT cells was detected in the complex immunoprecipitated with a TRAF2 or RSK2 antibody but not in the extract immunoprecipitated with the IgG control antibody (Fig. 3, B and C). In addition, TRAF2 binding with RSK2 is increased after EGF treatment as well as TNF treatment (Fig. 3D). The endogenous TRAF2 interaction with endogenous RSK2 was also observed in HT29 colon cancer cells (supplemental Fig. 2, B and C), and immunofluorescence staining results showed that TRAF2 co-localized with RSK2 only in the cytoplasm (supplemental Fig. 2D).
FIGURE 3.
TRAF2 binds to RSK2. A, TRAF2, but not TRAF5, interacts with RSK2. TRAF2-FLAG or TRAF5-HA with RSK2-Xpress was co-transfected into HEK239 cells. At 36 h after transfection, TRAF2-FLAG or TRAF1-HA was immunoprecipitated with anti-FLAG or anti-HA. Western blotting (IB) was performed using the indicated antibodies. B and C, endogenous RSK2 or TRAF2 interacts with endogenous TRAF2 or RSK2 in HaCaT cells. Cell extracts were used for immunoprecipitation with a TRAF2 or RSK2 antibody and control IgG. The immunoprecipitated complex was detected by Western blotting with an RSK2 or TRAF2 antibody. D, shown is the effect of TNF and EGF treatment on RSK2 binding to TRAF2. HaCaT cell extracts were treated with TNF or EGF and harvested at 5 or 10 min and then was used for immunoprecipitation with a TRAF2 antibody or control IgG. The immunoprecipitated complex was detected by Western blotting with the indicated antibody.
TRAF2 Is Required for Lys-63 Polyubiquitination of RSK2 at Lys-345 and -364
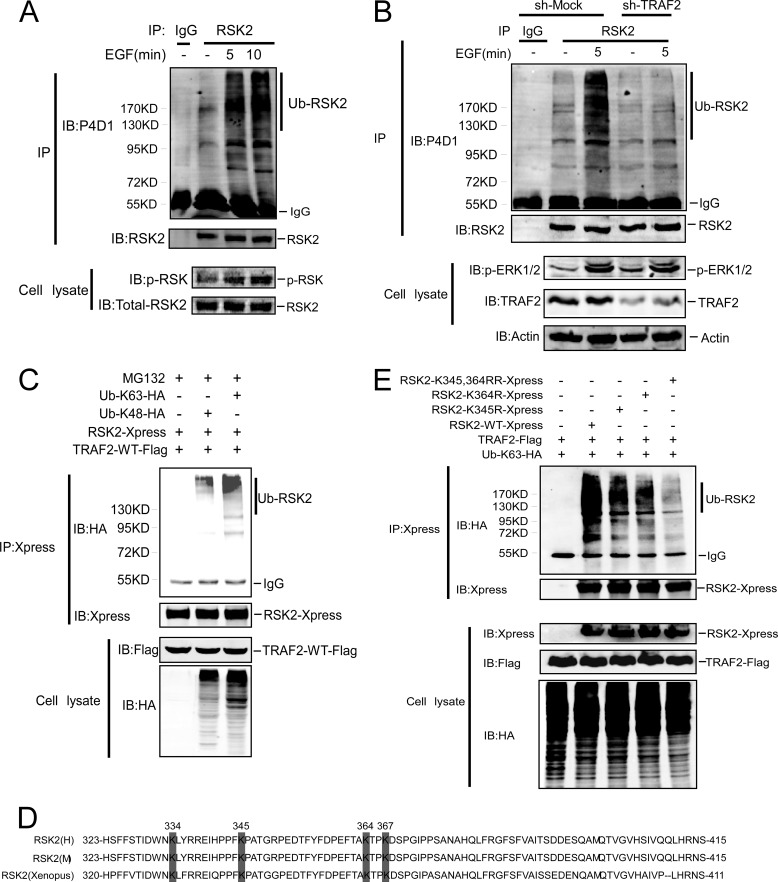
The results above showed that TRAF2 is a novel RSK2 interacting protein, and knocking down TRAF2 blocks EGF-induced anchorage-independent cell transformation, which was associated with decreased RSK2 and AP-1 activity. The question as to how TRAF2 regulates RSK2 activity was addressed. Lys-48-linked ubiquitin is known to be related to proteasome degradation, whereas Lys-63-linked ubiquitin chains play a role in DNA repair (21), act as protein adaptors (22–23), and influence protein kinase activity (24–25). To determine whether ubiquitination of RSK2 requires TRAF2, TRAF2-WT or TRAF2-DN (ring domain deletion) along with RSK2 and HA-Ub were co-transfected into HEK293 cells. Compared with Mock or TRAF2-DN cells, ubiquitinated RSK2 was increased in cells expressing TRAF2-WT (supplemental Fig. 3A). Notably, we found that EGF induces endogenous RSK2 ubiquitination (Fig. 4A), and knockdown TRAF2 abrogates ubiquitination of RSK2 induced by EGF treatment (Fig. 4B). Consistent with our observation that TRAF2 is required for ubiquitination of RSK2, knockdown of TRAF2 in HCT116 colorectal cancer cells substantially diminished ubiquitinated RSK2 (Supplemental Fig. 3B), but RSK2 protein expression was not changed in knockdown TRAF2 cells even though efficient ubiquitination was observed (supplemental Fig. 3B), suggesting that the major effect of RSK2 ubiquitination is not related to protein degradation. Next, to identify the lysine residue (or residues) in the ubiquitin molecule that is involved in forming the polyubiquitin chains on RSK2, Lys-48 ubiquitin or Lys-63 ubiquitin along with RSK2 and TRAF2 were co-expressed in HEK293 cells. We also treated cells with the proteasome inhibitor MG132 to prevent degradation of Lys-48-linked RSK2. The results indicated that RSK2 was more heavily coupled to Lys-63 ubiquitin (Fig. 4C) even when treated with MG132. Similar results were also observed in the absence of MG132 treatment (supplemental Fig. 3C). To identify the ubiquitin acceptor sites in RSK2, Lys-63-Ub and TRAF2 along with individual RSK2 deletion mutants (supplemental Fig. 3D) were co-transfected into HEK293 cells. The result showed that Lys-63-linked polyubiquitin was detectable in full-length RSK2, RSK2-D1 (D1–68), and RSK2-D2 (D69–323) but not in RSK2-D3 (D1–415) (supplemental Fig. 3E), suggesting that the ubiquitin acceptor sites in RSK2 are located between residues 323 and 415. Four potential ubiquitin sites (Lys-334, -345, -364, -367) are located in this area (Fig. 4D). We replaced each lysine with an arginine residue to determine the effect on ubiquitination. The result showed that K345R and K364R partially reduced RSK2 ubiquitination (supplemental Fig. 3F), but these two single mutations did not affect RSK2 function (supplemental Fig. 3G). We generated a double RSK2 mutant (K345R,K364R), which dramatically reduced RSK2 ubiquitination compared with RSK2 full-length or either of the two single K345R or K364R mutants (Fig. 4E). These results suggested that both lysine residues (Lys-345 and -364) contribute to Lys-63-linked polyubiquitination of RSK2.
FIGURE 4.
TRAF2 is required for Lys-63 polyubiquitination of RSK2 at Lys-345 and -364. A, EGF induces endogenous RSK2 ubiquitination. Endogenous RSK2 was immunoprecipitated from HaCaT cells with an RSK2 antibody. Cells were treated with EGF for different times as indicated. Endogenous ubiquitinated RSK2 was detected by a P4D1 antibody. IB, immunoblot. B, knockdown TRAF2 abrogates endogenous RSK2 ubiquitination. Knockdown of TRAF2 in HaCaT cells was performed as described under “Experimental Procedures.” RSK2 was immunoprecipitated from TRAF2 knockdown EGF-treated HaCaT cells with an RSK2 antibody. Western blot was performed using the indicated antibodies. C, Lys-63-linked polyubiquitination occurs in RSK2. HEK293 cells were co-transfected with an HA-tagged single lysine-containing mutant as indicated along with TRAF2-FLAG and RSK2-Xpress. At 30 h after transfection, cells were treated with MG132 (20 μm) for 2 h and then immunoprecipitated with anti-Xpress. Ubiquitinated RSK2-Xpress was visualized by Western blot using anti-HA. D, structure and schematic diagrams of a potential ubiquitination site(s) in RSK2. E, Lys-345 and -364 are major ubiquitination sites in RSK2. Individual RSK2-Xpress mutants (as indicated), FLAG-TRAF2, and HA-Lys-63-Ub were co-transfected into HEK293 cells. At 36 h after transfection, the protein extracts were used for immunoprecipitation with anti-Xpress, and ubiquitinated RSK2-Xpress was detected by Western blotting with anti-HA.
Ubiquitination of RSK2 Is Required for RSK2 Activity
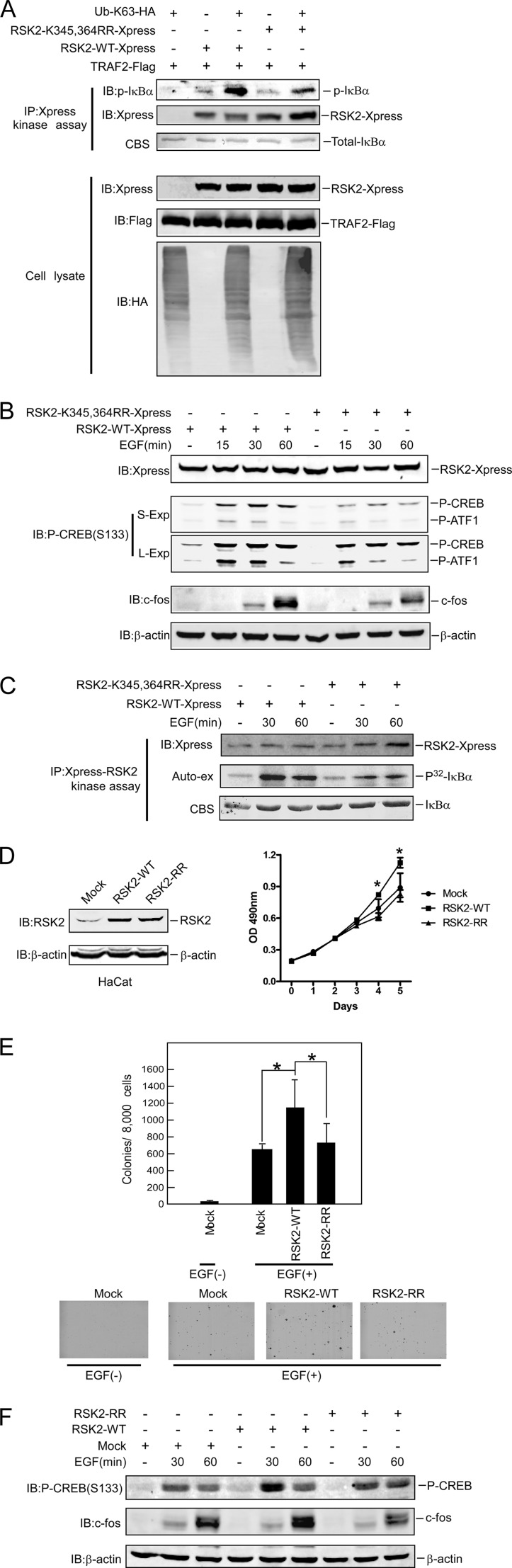
To investigate whether ubiquitination of RSK2 affects its activity, the RSK2-WT or RSK2-K345R,K364R plasmid with Ub-Lys-63 and TRAF2 was transfected into 293 cells. An RSK2-Xpress IP kinase assay was conducted, and results showed that RSK2-WT activity was dramatically increased after co-transfection with Ub-Lys-63-HA compared with RSK2-WT alone or RSK2-K345R,K364R with Ub-Lys-63-HA (Fig. 5A). This result indicated that Ub-Lys-63 has a critical role for mediating RSK2 activity. To investigate the effect of EGF on RSK2-K345R,K364R activity, RSK2-WT and RSK2-K345R,K364R were transfected into HeLa cells. At 20 h after transfection, cells were starved for 16 h and then treated with EGF for various times as indicated. Compared with RSK2-WT, phosphorylation of CREB or ATF1 was decreased, and EGF-induced c-Fos expression was attenuated in cells expressing RSK2-K345R,K364R (Fig. 5B). Furthermore, a 32P-labeled RSK2 IP kinase assay was performed to directly assess RSK2-K345R,K364R activity. Results showed that RSK2-K354R,K364R activity was decreased compared with wild type (Fig. 5C). A similar result was observed in another non-32P-labeled RSK2 IP kinase assay (supplemental Fig. 4), suggesting that the double mutation (K345R,K364R) directly affects RSK2 activity. Ectopic expression of RSK2 reportedly increases cell growth and induces anchorage-independent cell transformation (26). To study the physiological role of ubiquitinated RSK2, we generated HaCaT cells stably expressing RSK2-WT or RSK2-K345R,K364R (Fig. 5D, right panel). Growth (Fig. 5D, left panel) and EGF-induced anchorage-independent colony formation (Fig. 5E) were both attenuated in the RSK2-K345R,K364R-expressing cells. Phosphorylation of CREB- and EGF-induced c-Fos expression was also decreased in cells expressing RSK2-K345R,K364R (Fig. 5F). Taken together, these data suggest that ubiquitination of RSK2 at Lys-345 and -364 is required for RSK2 kinase activity to induce cell transformation.
FIGURE 5.
Ubiquitination of RSK2 is required for RSK2 activity. A, ubiquitin is required for RSK2 activity. RSK2-WT or RSK2-K345R,K364R with Ub-Lys-63 and TRAF2 were transfected into 293 cells. At 36 h after transfection, RSK2-Xpress was immunoprecipitated by anti-Xpress for an in vitro kinase assay using GST-IκBα as substrate as described under “Experimental Procedures.” Phosphorylated IκBα was detected using the phosphorylation IκBα (Ser-32) antibody to demonstrate RSK2 activity. Immunoblotting (IB) was performed using the indicated antibodies. B, mutant RSK2-K345R,K364R attenuates RSK2 activity. Xpress-RSK2-WT and Xpress-RSK2- K345R,K364R were transfected into HeLa cells. At 20 h after transfection, cells were starved for 16 h and then treated with EGF (50 ng/ml) for various times as indicated. Immunoblotting was performed using the indicated antibodies. C, Xpress-RSK2-WT and Xpress-RSK2- K345R,K364R were transfected into HeLa cells. At 20 h after transfection, cells were starved for 16 h and then treated with EGF (50 ng/ml) for various times as indicated. RSK2-Xpress was immunoprecipitated with anti-Xpress for a 32P-labeled γ-ATP in vitro kinase assay using GST-IκBα as substrate as described under “Experimental Procedures.” Phosphorylated IκBα was detected by auto-exposure (auto-ex) to show RSK2 activity. CBS, Coomassie Blue Staining. D, overexpression of RSK2-K345R,K364R attenuates cell proliferation. Stable HaCaT cells overexpressing RSK2-WT or RSK2-K345R,K364R (left panel) were constructed as described under “Experimental Procedures.” HaCaT cells expressing Mock, RSK2-WT, or RSK2-RR were seeded (1 × 103 per well/in 100 μl) into 96-well plates, and proliferation was assessed using the CellTiter96 Aqueous One Solution detection kit. Cell viability was estimated by reading the absorbance (A490). The graph shows data from multiple experiments expressed as the means ± S.D. The asterisks (*) indicate a significant difference (p < 0.05 Student's t test) (right panel). E, overexpression of mutant RSK2-K345R,K364R attenuates EGF-induced anchorage-independent cell transformation. HaCaT cells expressing Mock, RSK2-WT, or RSK2-RR was exposed to EGF (20 ng/ml) in 0.3% BME agar containing 10% FBS. The cultures were maintained in a 37 °C, 5% CO2 incubator for 15 days, and then colonies were counted using a microscope and the Image-Pro PLUS (v.6) computer software program. The graph shows data from multiple experiments expressed as the means ± S.D. The asterisks (*) indicate a significant difference (p < 0.05 Student's t test). F, the effect of mutant RSK2-K345R,K364R on the EGF signaling pathway is shown. HaCaT cells expressing Mock, RSK2-WT, or RSK2-RR were starved for 36 h and treated with EGF (20 ng/ml) for various times as indicated. Immunoblotting was performed using the indicated antibodies.
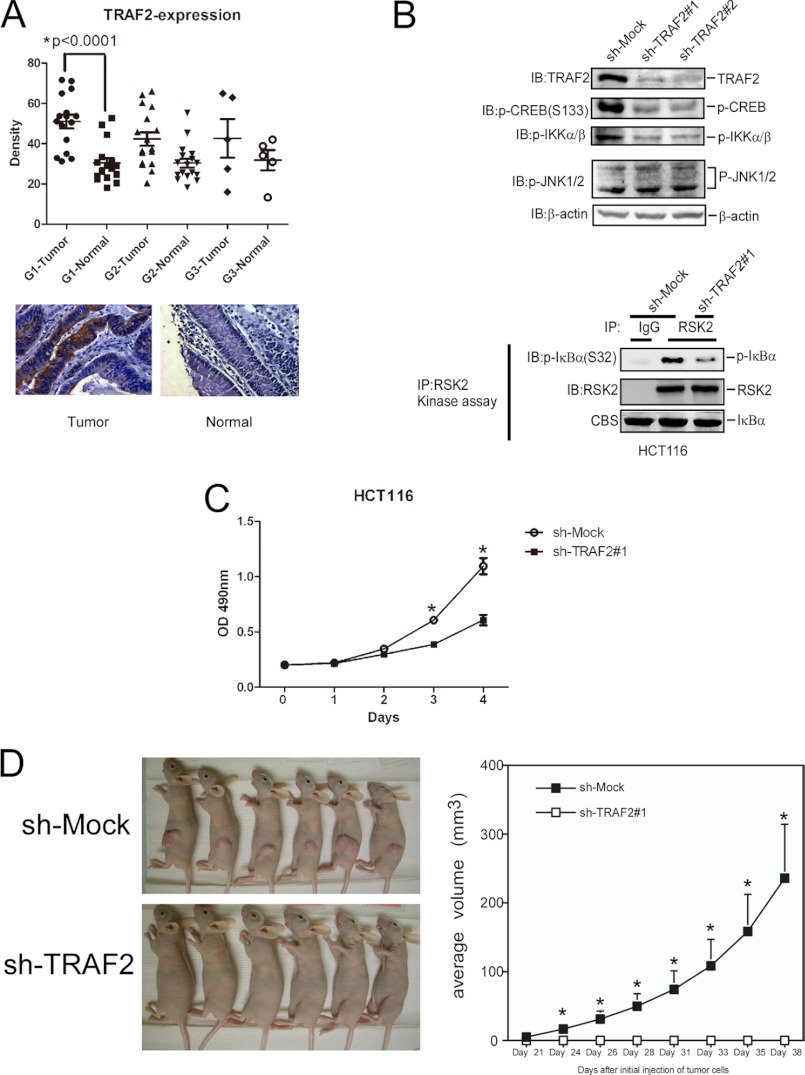
TRAF2 Is Overexpressed in Colon Cancer and Required for Tumor Growth
Ras-MAPK is highly activated in colorectal cancer, but the role of TRAF2 in colorectal tumorigenesis has not been addressed. We searched the Gene Expression Atlas data base to learn about TRAF2 and RSK2 expression in colon cancer, and the results indicated that TRAF2 and RSK2 are both highly expressed in colon cancer (data not shown). We also examined TRAF2 and RSK2 expression in different colorectal cancer cell lines, and these results showed that, compared with a normal colon cell line (HCEC), TRAF2 and RSK2 are both overexpressed in the eight colorectal cancer cell lines examined (supplemental Fig. 5A). This result is consistent with a previous study that reported high expression of RSK2 in HCT116 and HT29 colon cancer cells (27). Furthermore, we also analyzed a human colon tissue array containing 40 colon tumor samples and 40 matched normal samples. The mean score for staining of TRAF2 in tumor tissue (grade 1) was significantly higher than that in normal tissue (p < 0.0001; Fig. 6A). A statistically significant difference was observed for RSK2 expression for grade 1 tumor samples (supplemental Fig. 5B), suggesting that TRAF2 and RSK2 might play a role in the early stage of colon tumorigenesis. To determine whether overexpression of TRAF2 is required for tumor cell growth, TRAF2 expression was knocked down by specific shRNA in HCT116 cells. Results showed that phosphorylation of CREB and IKKα/β was decreased, but phosphorylation of ERK1/2 and JNK1/2 was not changed in TRAF2 knockdown cells (Fig. 6B, upper panel, and supplemental Fig. 5C). An IP kinase assay showed that RSK2 activity was also decreased in knockdown TRAF2 cells (Fig. 6B, lower panel). Because cIAP1/2 is well known as a TRAF2 binding partner, we also tested the effect of knocking down cIAP1/2 on phosphorylation of CREB (supplemental Fig. 6, upper panel) and ubiquitination of RSK2 (supplemental Fig. 6, lower panel). The results showed that no apparent changes in the phosphorylation of CREB or ubiquitination of RSK2 in knockdown cIAP1/2 HCT116 cells. In addition, HCT116 cancer cell growth (Fig. 6C), anchorage-independent growth (supplemental Fig. 7) and HCT116 tumor development in a xenograft mouse model (Fig. 6D) were all attenuated in knockdown TRAF2 cells. Furthermore, individual knockdown of TRAF2 or RSK2 or double knockdown of both TRAF2 and RSK2 dramatically decreased anchorage-independent growth in HCT116 colon cancer cells (supplemental Fig. 7). In addition, overexpression RSK2 slightly increased AP-1 activity in TRAF2−/− cells (supplemental Fig. 8A) and anchorage-independent growth in TRAF2 knockdown cells (supplemental Fig. 8B), which indicated that RSK2 activity has a dependent and independent manner with TRAF2. To confirm the role of TRAF2 on colorectal cancer, we tested the effect of TRAF2 on malignant phenotype in HT29 cell line. The results showed that cell growth and anchorage-independent growth were significantly decreased in cells expressing knockdown TRAF2 (supplemental Fig. 9, A–C). Phosphorylation of CREB and ATF1 was also decreased, but phosphorylation of ERK1/2 was not changed (supplemental Fig. 9D). Overall, the data indicate that TRAF2 is required for colon tumor cell growth in both cell culture and xenograft models.
FIGURE 6.
TRAF2 is overexpressed in colon cancer and required for tumor growth. A, expression of TRAF2 in a colon cancer tissue array is shown. Immunohistochemical staining was performed using a TRAF2 antibody as described under “Experimental Procedures.” The intensity was estimated by Image-Pro PLUS (v.6) computer software program and ImageJ (NIH) computer program. Statistical analyses were performed using Prism 5.0 statistics software. The density of each individual sample is shown in the upper panels. The asterisk (*) indicates a significant difference as indicated. B, knockdown of TRAF2 decreases RSK2 activity. HCT116 colorectal cancer cells were infected with sh-TRAF2 lentivirus as described under “Experimental Procedures.” RSK2 was immunoprecipitated from cell lysates. The immunocomplexes were incubated with GST-IκBα as a substrate for RSK2 in an in vitro kinase assay as described under “Experimental Procedures.” Phosphorylation of IκBα (Ser-32) was used to show RSK2 activity. Western blotting (IB) was performed using the indicated antibodies. C, knockdown of TRAF2 attenuates proliferation of HCT116 colorectal cancer cells. Sh-mock and sh-TRAF2#1 cells were seeded (1 × 103 per well/100 μl) into 96-well plates, and proliferation was assessed using the CellTiter96 Aqueous One Solution detection kit and estimated by reading the absorbance (A490). The graph shows data from multiple experiments expressed as the means ± S.D. The asterisks (*) indicate a significant difference (p < 0.05 Student's t test). D, knockdown of TRAF2 blocks growth of HCT116 colorectal cancer cells in a xenograft mouse model. sh-Mock or sh-TRAF2 #1 cells (1 × 106) were injected into the right flank of female athymic nude mice. Growth of tumors was monitored as described under “Experimental Procedures.” Results are shown as mean tumor volume ± S.E., and the asterisks (*) indicate a significant difference (p < 0.05 one way analysis of variance).
DISCUSSION
Most studies regarding TRAF2 have focused on the TNF-R1 pathway, and therefore, the role of TRAF2 in other signaling pathways is not known. We generated HaCaT cells stably expressing knockdown of TRAF2. Surprisingly, EGF-induced cell transformation was markedly attenuated in TRAF2 knockdown cells (Fig. 1A), and phosphorylation of CREB, ATF1, RSK, and expression of the c-Fos protein were also decreased in TRAF2 knockdown cells and TRAF2 knock-out MEFs (Fig. 1, B and C) after EGF treatment. Activation of JNK and IKKα/β is known to be dependent on TRAF2 in the presence of TNF treatment. However, with EGF treatment, phosphorylation of IKKα/β and IκBα were not detectable (supplemental Fig. 1D). In addition, phosphorylation of JNK was not detectable as reported previously (15). This indicated that TRAF2 regulates the EGF pathway through other molecules but not IKKα/β or JNK.
RSK2, a member of the p90 ribosomal S6 kinase (RSK) family of proteins, is a serine/threonine kinase that is activated downstream of the ERKs cascade. RSK2 has a broad range of functions that are involved in protein translation (28), cell proliferation, cell cycle (29), cell transformation (26, 30), and cell survival (30–32). RSK2, as a protein kinase, directly phosphorylates CREB and ATF1. We determined RSK2 activity in TRAF2-deficient cells. RSK2 IP kinase results showed that RSK2 activity was down-regulated in both TRAF2 knockdown and TRAF2−/− cells (Fig. 1, D and E). We also found that TRAF2 interacts with RSK2, and EGF treatment increases TRAF2 binding with RSK2 (Fig. 3). Based on these results, we concluded that TRAF2 directly regulates RSK2 activity.
Because RSK1 has high homology with RSK2, we tested the effect of TRAF2 on RSK1, and results showed that RSK1 activity was only slightly increased compared with RSK2 activity mediated by TRAF2 (supplemental Fig. 1F). These results led us to conclude that RSK2, but not RSK1, is a major molecule mediated by TRAF2. Even though to some degree RSK1 and RSK2 have redundant roles, differences in the mediation of RSK1 and RSK2 by TRAF2 are not surprising. For example, only mutations in the human rsk2 gene are associated with Coffin-Lowry syndrome (33), and RSK2, but not RSK1, is required for invasion and metastasis in head and neck tumors (34).
AP-1 activity is required for EGF-induced anchorage-independent cell transformation and tumor development. We also found that AP-1 activity was decreased in both TRAF2 knockdown and TRAF2−/− cells (Fig. 2, B and C). In the TNF-R1 pathway, TRAF2 regulates AP-1 activity through JNKs, whereas in the EGF pathway EGF treatment does not activate JNKs, and TRAF2 regulates AP-1 activity through the RSK2/CREB/c-Fos axis.
Ubiquitination, like phosphorylation or acetylation, is an important protein modification that involves various biological processes. Lys-48-linked poly ubiquitination leads to protein degradation, whereas Lys-63-linked poly-ubiquitination is related to protein trafficking and signaling activation. Here, we found EGF can induce endogenous RSK2 ubiquitination (Fig. 4A), and knock down TRAF2 abrogates this process (Fig. 4B). Furthermore, we found that RSK2 is coupled with Lys-63-mediated ubiquitination. These results implied that ubiquitination of RSK2 is not involved in protein degradation. To investigate the role of ubiquitination in RSK2, we found that Lys-345 and -364 are major ubiquitin sites in RSK2. Lysine point mutations at Lys-345 and -364 dramatically reduced RSK2 ubiquitination (Fig. 4E) and kinase activity (Fig. 5, A–C). Cell growth and anchorage-independent transformation were decreased in mutant RSK2-K345R,K364R HaCaT cells (Fig. 5, D–F), which showed that ubiquitination of RSK2 is associated with its protein kinase activity and cellular function. That ubiquitination controls protein kinase activity is not surprising because TRAF2 was reported to regulate MLK3 activity through ubiquitin (35). Free Lys-63 polyubiquitin chains were reported to directly activate TAK1 and IKK (36) even though the exact mechanism is unclear. Others showed that Lys-63 poly-ubiquitination of Akt affects Akt membrane recruitment, which is required for its activation (24). We showed that ubiquitination of RSK2 is required for its activity, but details regarding the mechanisms of the effect of ubiquitin on RSK2 kinase activity provide an interesting direction for our future studies.
Although TRAF2 was reported to function in pancreatic and lung cancer through NF-κB (17, 37), the role of TRAF2 in tumorigenesis mediated through the Ras-MAPK pathway is not yet clear. We found that TRAF2 and RSK2 are overexpressed in eight different colon cancer cell lines compared with a normal colon cell line (supplemental Fig. 5A). Tissue array analysis data showed that TRAF2 and RSK2 are both overexpressed in colorectal tumor tissue, especially in grade 1 tumors, compared with normal tissue (Fig. 6A; supplemental Fig. 5B). Furthermore, the results showed that TRAF2 is required for the malignant phenotype of HCT116 cells, including proliferation, colony growth in soft agar, and ability to form tumors in athymic nude mice (Fig. 6, C and D). RSK2 activity and phosphorylated CREB were also decreased in knockdown TRAF2 HCT116 cells (Fig. 6B). Previous results showed that EGF treatment could not activate IKKα/β, but we also observed that phosphorylation of IKKα/β, but not phosphorylation of JNKs, was decreased in knockdown TRAF2 cells under normal conditions (Fig. 6B). This suggested that TRAF2 might regulate phosphorylation of IKKα/β through other growth factor pathways. This result indicated that TRAF2 is required for colon tumorigenesis through both the Ras-MAPK and NF-κB pathways.
Taken together, our study reveals that in addition to TRAF2 function in the TNF-R1 pathway, TRAF2 is required for RSK2 activity mediated through RSK2 ubiquitination. RSK2 activity plays a critical role in AP-1 activation mediated through CREB/c-Fos, which regulates EGF-induced anchorage-independent cell transformation and signaling transduction pathway. We also showed that TRAF2 is overexpressed in colon cancer and is required for colon tumorigenesis and, therefore, might be a good molecular target for tumor prevention or treatment.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA007646, CA111536, CA120388, R37CA081064, and ES016548. This work was also supported by The Hormel Foundation.

This article contains supplemental Figs. 1–9.
- TRAF2
- TNF-R-associated factor family protein 2
- TNF-R1
- TNF receptor superfamily, member 1A
- RIP
- receptor interacting protein
- RSK
- ribosomal S6 kinase
- CREB
- cAMP-responsive element-binding protein
- ATF
- activating transcription factor
- MEF
- murine embryonic fibroblast
- AP
- alkaline phosphatase
- IP
- immunoprecipitation
- IKK
- IκB kinase.
REFERENCES
- 1. Hsu H., Xiong J., Goeddel D. V. (1995) The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81, 495–504 [DOI] [PubMed] [Google Scholar]
- 2. Yang J., Lin Y., Guo Z., Cheng J., Huang J., Deng L., Liao W., Chen Z., Liu Z., Su B. (2001) The essential role of MEKK3 in TNF-induced NF-κB activation. Nat. Immunol. 2, 620–624 [DOI] [PubMed] [Google Scholar]
- 3. Lee Z. H., Kwack K., Kim K. K., Lee S. H., Kim H. H. (2000) Activation of c-Jun N-terminal kinase and activator protein 1 by receptor activator of nuclear factor κB. Mol. Pharmacol. 58, 1536–1545 [PubMed] [Google Scholar]
- 4. Micheau O., Tschopp J. (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 [DOI] [PubMed] [Google Scholar]
- 5. Newton K., Matsumoto M. L., Wertz I. E., Kirkpatrick D. S., Lill J. R., Tan J., Dugger D., Gordon N., Sidhu S. S., Fellouse F. A., Komuves L., French D. M., Ferrando R. E., Lam C., Compaan D., Yu C., Bosanac I., Hymowitz S. G., Kelley R. F., Dixit V. M. (2008) Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134, 668–678 [DOI] [PubMed] [Google Scholar]
- 6. Bradley J. R., Pober J. S. (2001) Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20, 6482–6491 [DOI] [PubMed] [Google Scholar]
- 7. Tada K., Okazaki T., Sakon S., Kobarai T., Kurosawa K., Yamaoka S., Hashimoto H., Mak T. W., Yagita H., Okumura K., Yeh W. C., Nakano H. (2001) Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-κB activation and protection from cell death. J. Biol. Chem. 276, 36530–36534 [DOI] [PubMed] [Google Scholar]
- 8. Yin Q., Lamothe B., Darnay B. G., Wu H. (2009) Structural basis for the lack of E2 interaction in the RING domain of TRAF2. Biochemistry 48, 10558–10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin Q., Lin S. C., Lamothe B., Lu M., Lo Y. C., Hura G., Zheng L., Rich R. L., Campos A. D., Myszka D. G., Lenardo M. J., Darnay B. G., Wu H. (2009) E2 interaction and dimerization in the crystal structure of TRAF6. Nat. Struct. Mol. Biol. 16, 658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertrand M. J., Milutinovic S., Dickson K. M., Ho W. C., Boudreault A., Durkin J., Gillard J. W., Jaquith J. B., Morris S. J., Barker P. A. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 [DOI] [PubMed] [Google Scholar]
- 11. Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., Milstien S., Spiegel S. (2010) Sphingosine 1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xing J., Ginty D. D., Greenberg M. E. (1996) Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273, 959–963 [DOI] [PubMed] [Google Scholar]
- 13. Swanson K. D., Taylor L. K., Haung L., Burlingame A. L., Landreth G. E. (1999) Transcription factor phosphorylation by pp90(rsk2). Identification of Fos kinase and NGFI-B kinase I as pp90(rsk2). J. Biol. Chem. 274, 3385–3395 [DOI] [PubMed] [Google Scholar]
- 14. Yang X., Matsuda K., Bialek P., Jacquot S., Masuoka H. C., Schinke T., Li L., Brancorsini S., Sassone-Corsi P., Townes T. M., Hanauer A., Karsenty G. (2004) ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology. Implication for Coffin-Lowry syndrome. Cell 117, 387–398 [DOI] [PubMed] [Google Scholar]
- 15. Liu K., Cho Y. Y., Yao K., Nadas J., Kim D. J., Cho E. J., Lee M. H., Pugliese A., Zhang J., Bode A. M., Dong Z., Dong Z. (2011) Eriodictyol inhibits RSK2-ATF1 signaling and suppresses EGF-induced neoplastic cell transformation. J. Biol. Chem. 286, 2057–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng C., Cho Y. Y., Zhu F., Xu Y. M., Wen W., Ma W. Y., Bode A. M., Dong Z. (2010) RSK2 mediates NF-κB activity through the phosphorylation of IκBα in the TNF-R1 pathway. FASEB J. 24, 3490–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng M., Morgan-Lappe S. E., Yang J., Bockbrader K. M., Pamarthy D., Thomas D., Fesik S. W., Sun Y. (2008) Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 68, 7570–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kako K., Wakamatsu H., Ishida N. (1996) c-fos CRE-binding activity of CREB/ATF family in the SCN is regulated by light but not a circadian clock. Neurosci. Lett. 216, 159–162 [DOI] [PubMed] [Google Scholar]
- 19. Schouten G. J., Vertegaal A. C., Whiteside S. T., Israël A., Toebes M., Dorsman J. C., van der Eb A. J., Zantema A. (1997) IκBα is a target for the mitogen-activated 90-kDa ribosomal S6 kinase. EMBO J. 16, 3133–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong Z., Birrer M. J., Watts R. G., Matrisian L. M., Colburn N. H. (1994) Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc. Natl. Acad. Sci. U.S.A. 91, 609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang B., Elledge S. J. (2007) Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc. Natl. Acad. Sci. U.S.A. 104, 20759–20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Z. H., Wong E. T., Shi Y., Niu J., Chen Z., Miyamoto S., Tergaonkar V. (2010) ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol. Cell 40, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinz M., Stilmann M., Arslan S. Ç., Khanna K. K., Dittmar G., Scheidereit C. (2010) A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-κB activation. Mol. Cell 40, 63–74 [DOI] [PubMed] [Google Scholar]
- 24. Yang W. L., Wang J., Chan C. H., Lee S. W., Campos A. D., Lamothe B., Hur L., Grabiner B. C., Lin X., Darnay B. G., Lin H. K. (2009) The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 325, 1134–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Békés M., Salvesen G. S. (2009) The CULt of caspase-8 ubiquitination. Cell 137, 604–606 [DOI] [PubMed] [Google Scholar]
- 26. Cho Y. Y., Yao K., Kim H. G., Kang B. S., Zheng D., Bode A. M., Dong Z. (2007) Ribosomal S6 kinase 2 is a key regulator in tumor promoter induced cell transformation. Cancer Res. 67, 8104–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho Y. Y., Yao K., Pugliese A., Malakhova M. L., Bode A. M., Dong Z. (2009) A regulatory mechanism for RSK2 NH2-terminal kinase activity. Cancer Res. 69, 4398–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shahbazian D., Roux P. P., Mieulet V., Cohen M. S., Raught B., Taunton J., Hershey J. W., Blenis J., Pende M., Sonenberg N. (2006) The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 25, 2781–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willard F. S., Crouch M. F. (2001) MEK, ERK, and p90RSK are present on mitotic tubulin in Swiss 3T3 cells. A role for the MAP kinase pathway in regulating mitotic exit. Cell Signal 13, 653–664 [DOI] [PubMed] [Google Scholar]
- 30. Kang S., Dong S., Gu T. L., Guo A., Cohen M. S., Lonial S., Khoury H. J., Fabbro D., Gilliland D. G., Bergsagel P. L., Taunton J., Polakiewicz R. D., Chen J. (2007) FGFR3 activates RSK2 to mediate hematopoietic transformation through tyrosine phosphorylation of RSK2 and activation of the MEK/ERK pathway. Cancer Cell 12, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eisinger-Mathason T. S., Andrade J., Groehler A. L., Clark D. E., Muratore-Schroeder T. L., Pasic L., Smith J. A., Shabanowitz J., Hunt D. F., Macara I. G., Lannigan D. A. (2008) Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival. Mol. Cell 31, 722–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peng C., Cho Y. Y., Zhu F., Zhang J., Wen W., Xu Y., Yao K., Ma W. Y., Bode A. M., Dong Z. (2011) Phosphorylation of caspase-8 (Thr-263) by ribosomal S6 kinase 2 (RSK2) mediates caspase-8 ubiquitination and stability. J. Biol. Chem. 286, 6946–6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trivier E., De Cesare D., Jacquot S., Pannetier S., Zackai E., Young I., Mandel J. L., Sassone-Corsi P., Hanauer A. (1996) Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature 384, 567–570 [DOI] [PubMed] [Google Scholar]
- 34. Kang S., Elf S., Lythgoe K., Hitosugi T., Taunton J., Zhou W., Xiong L., Wang D., Muller S., Fan S., Sun S. Y., Marcus A. I., Gu T. L., Polakiewicz R. D., Chen Z. G., Khuri F. R., Shin D. M., Chen J. (2010) p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J. Clin. Invest. 120, 1165–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korchnak A. C., Zhan Y., Aguilar M. T., Chadee D. N. (2009) Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell. Signal. 21, 1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xia Z. P., Sun L., Chen X., Pineda G., Jiang X., Adhikari A., Zeng W., Chen Z. J. (2009) Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trauzold A., Röder C., Sipos B., Karsten K., Arlt A., Jiang P., Martin-Subero J. I., Siegmund D., Müerköster S., Pagerols-Raluy L., Siebert R., Wajant H., Kalthoff H. (2005) CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB J. 19, 620–622 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.