Background: HSP27 phosphorylation plays pivotal roles on F-actin polymerization and actin cytoskeleton organization.
Results: The loss of hTid-1S expression was observed in lesional human psoriatic skin.
Conclusion: The binding of hTid-1S with MK5 inhibits HSP27 phosphorylation and attenuates F-actin polymerization.
Significance: The lack of hTid-1S expression correlates with the aberrant actin cytoskeleton organization in psoriatic human skin.
Keywords: Cell Migration, Cytoskeleton, Keratinocytes, p38 MAPK, Psoriasis, F-actin, HSP27, MK5, Epidermis, hTid-1
Abstract
The biochemical mechanism by which the human tumorous imaginal disc1S (hTid-1S) interferes with actin cytoskeleton organization in keratinocytes of human skin epidermis was investigated. We found that hTid-1, specifically hTid-1S, interacts with MK5, a p38-regulated/activated protein kinase, and inhibits the protein kinase activity of MK5 that phosphorylates heat shock protein HSP27 in cultured HeLa cells. Thus, hTid-1S expression inhibits the phosphorylation of HSP27 known to play important roles in F-actin polymerization and actin cytoskeleton organization. The interplay between MK5/HSP27 signaling and hTid-1S expression was supported by the inhibition of HSP27 phosphorylation and MK5 activity in HeLa cells in response to hypoxia during which hTid-1S expression was down-regulated. We also found that overexpression of hTid-1S results in the inhibition of HSP27 phosphorylation, F-actin polymerization, and actin cytoskeleton organization in transduced HaCaT keratinocytes. This study further proposes that the loss of hTid-1S expression in the basal layer of skin epidermis correlates with the enhanced HSP27 phosphorylation, keratinocyte hyperproliferation, and excess actin cytoskeleton organization in lesional psoriatic skin.
Introduction
hTid-12 was first identified as a human homologue of Drosophila tumor suppressor protein Tid56 (1). hTid-1 belongs to the DnaJA3 family of proteins known to interact with HSP70 family proteins (2). At least two isoforms of hTid-1, namely, hTid-1L and hTid-1S, have been reported. Both isoforms of hTid-1 containing amino-terminal mitochondrial signal sequence reside mainly in the mitochondrial matrix. However, these two isoforms of hTid-1 can interact with many cytoplasmic proteins when they are transiently retained in the cytoplasm before they transport to the mitochondria (3). The two alternative splice variants have opposing effects on the decision of cell fate. It has been reportedly known that proapoptotic hTid-1L stimulates cytochrome c release from the mitochondria whereas antiapoptotic hTid-1S inhibits cytochrome c release resulting in the inactivation of caspase-3 and subsequent inhibition of apoptosis in human osteosarcoma cells (4).
Using yeast two-hybrid screening (5), we have identified hTid-1S as a new binding protein that interacts with mitogen-activated protein kinase (MAPK)-activated protein kinase 5 (MK5). MK5 and its human homologue p38-regulated/activated kinase (PRAK) belong to a Ca2+/calmodulin-dependent protein kinase and display approximately 40% amino acid sequence identity with other p38 MAPK-activated protein kinases MK2 and MK3 (6–8). In response to cellular stresses such as heat shock, UV light, oxidation, and proinflammatory cytokines, the activation phosphorylation site of MK5 at Thr-182 is phosphorylated by p38 MAPK and extracellular signal-regulated kinases (ERKs) (9–12). Studies have identified substrate proteins for MK5 including tumor suppressor p53 (13), human small heat shock protein HSP27, and its murine HSP25 homologue. As the phosphorylation of HSP27 is required for F-actin polymerization, MK5-induced HSP27 phosphorylation participates in actin filament dynamics and stabilization of actin cytoskeleton (14–16).
The epidermis of skin maintains its barrier function through continuous self-renewal and terminal differentiation processes. During these processes, keratinocytes leave the basal cell layer, proliferate, lose metabolic activities, and finally differentiate into dead cornified cells in outmost layer of the epidermis (17). To control the balance between self-renewal and terminal differentiation in the epidermis of skin, cytoskeleton remodeling should accompany the cellular events such as cell-cell and cell-matrix adhesion, cell proliferation, and cell migration. Psoriasis is a common chronic inflammatory skin disease that is characterized by a marked hyperproliferation and altered differentiation of keratinocytes. This disease affecting ∼1–3% of the US population (18) is commonly believed to have strong genetic components with altered expression of >1,300 genes in lesions of psoriatic skin (19, 20). This study delineates a biochemical mechanism by which the absence of hTid-1S expression leads to aberrant actin filament dynamics in lesional psoriatic skin. Here, we propose that the absence of a hTid-1S expression (3) stimulates the activity of MK5, increases the phosphorylation of HSP27, and enhances actin cytoskeleton organization in the hyperthickened epidermis of psoriatic skin.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
TNF-α and antibodies against tubulin and FLAG epitope were obtained from Sigma. Polyclonal rabbit antibody directed against MK5 (H-180) and monoclonal mouse antibody against hTid-1 (RS-11) or HSP27 (F-4) were supplied by Santa Cruz Biotechnology. Polyclonal rabbit antibody against phospho-HSP27 (Ser-82) was from Stressgen. [γ-32P]ATP and protein G-Sepharose Fast Flow Resin were provided by Amersham Biosciences. Rhodamine-conjugated phalloidin was purchased from Invitrogen.
DNA Constructs
cDNA encoding MK5 was cloned into BamHI and EcoRV sites of pcDNA3-HA cloning vector (14) to generate HA-MK5 expression vector. A full-length cDNA encoding hTid-1S or hTid-1L was cloned into HindIII and BamHI sites of pFLAG-CMV2 cloning vector (Sigma) to generate expression vector for FLAG-hTid-1S or FLAG-hTid-1L. To generate recombinant adenoviral vector for FLAG-hTid-1S, cDNA encoding FLAG-hTid-1S was PCR-amplified and cloned into pAd/CMV/V5-DEST adenoviral vector (Invitrogen Gateway System). The resulting Ad FLAG-hTid-1S expression vector was linearized by digestion with PacI, transfected, and amplified in 293A cells. The viral particles were purified by centrifugation in cesium chloride gradients.
Cell Culture, Transfection, Cell Migration, and Cell Quantification
Human cervical cancer HeLa cells or immobilized human keratinocyte HaCaT cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen) in a humidified atmosphere of 5% CO2 at 37 °C. HeLa cells were transfected with indicated vector constructs using Lipofectamine reagent (Invitrogen). HaCaT keratinocytes was infected with adenoviruses harboring recombinant adenoviral vector encoding FLAG-hTid-1S for 48 h. Cell migration assay was carried out employing a QCMTM Cell Migration Assay (Chemicon). The growth of HaCaT keratinocytes was measured by crystal violet staining according to Kueng et al. (21).
Immunoprecipitation
HeLa cells (∼107) were lysed in EBC lysis buffer (120 mm NaCl, 0.5% Nonidet P-40, 50 mm Tris-Cl, pH 8.0, 100 mm NaF, 200 mm sodium orthovanadate) containing 1 mm PMSF. Cell preparations were centrifuged at 4 °C for 10 min at 10,000 × g, after which the supernatant constituted the cell lysate. The cell lysate was incubated with MK5 antibody for 4 h at 4 °C before the addition of 30 μl of 50% protein G-Sepharose equilibrated with EBC lysis buffer. After additional incubation for 12 h at 4 °C, beads were washed four times with the lysis buffer. Immunocomplexes were eluted from the resin by boiling in a sodium dodecyl sulfate (SDS) sample buffer (250 mm Tris-Cl, pH 6.8, 40% glycerol, 8% SDS, 4% 2-mercaptoethanol, 0.002% bromphenol blue) for 10 min. hTid-1 bound to MK5 was analyzed by 12% SDS-PAGE followed by immunoblot analysis using anti-hTid-1 antibody.
RNA Interference
To construct an expression vector for small hairpin RNA (hTid-1-shRNA), hTid-1-specific sequence containing a hairpin loop (5-GATCCCCAGCTACGGCTACGGAGACTTCAAGAGAGTCTCCGTAGCCGTAGCTGTTTTTGGAAA-3) was cloned into the BglII and HindIII sites of p3in1 vector (22). HeLa cells (2 × 106) were transfected with indicated amount of hTid-1-shRNA or p3in1 vector using Lipofectamine reagent. Experiments were carried out 24 h after hTid-1-shRNA transfection.
Immunoprecipitation MK5 Kinase Assay
Control or transfected HeLa cells overexpressing FLAG-hTid-1S were grown for 24 h under normal or hypoxic conditions. Cells were washed twice in PBS and lysed in EBC lysis buffer. Lysates were centrifuged for 10 min at 10,000 × g at 4 °C. MK5 protein kinase in the supernatant was immunoprecipitated using anti-MK5-protein G-Sepharose. Beads were mixed for 4 h at 4 °C and washed three times with the lysis buffer. Human recombinant HSP27 substrate protein (Abcam) (3 μg) and beads containing immunoprecipitated MK5 were added to a total volume of 30 μl of reaction mixture containing 50 mm Tris-HCl, pH 7.5, 10 mm MgCl2,0.1 mm ATP, 1 μg/μl BSA, and 10 μCi of [γ-32P]ATP. The reaction mixture was incubated for 20 min with shaking at 30 °C. The reaction was stopped by heating at 95 °C for 5 min after the addition of 10 μl of 4× SDS sample buffer. The activity of MK5 as a protein kinase was evaluated by measuring the transfer of 32P moiety from [γ-32P]ATP to MK5 and HSP27 substrate protein. Autophosphorylation of MK5 and HSP27 phosphorylation was detected by autoradiography followed by 12% SDS-PAGE.
Staining of Actin Cytoskeleton
F-actin staining for immunofluorescence microscopy was performed as described previously (14). In brief, HeLa or HaCaT cells were fixed with 3.7% formaldehyde in PBS at room temperature, permeabilized with PBS containing 0.1% Triton X-100 at −20 °C, and blocked with 1% bovine serum albumin (BSA) in PBS. F-actin was stained with rhodamine-conjugated phalloidin (3 μg/ml) in PBS containing 1% BSA for 20 min. F-actin polymerization was examined by monitoring stress fiber formation under a confocal scanning microscope.
Biopsies
Psoriasis patients and normal controls participated voluntarily and gave their written informed consents for this study. This study was approved by Institutional Review Board of each respective institution. Biopsies from the lesinonal plaque-type psoriatic skin were taken from the center of the plaque from psoriasis patients diagnosed and selected at the Department of Dermatology, Chungnam National University Hospital, South Korea, as described by Johansen et al. (23). Normal skin was obtained from skin transplants of patients. All epidermal tissue samples included the dermis and the epidermis. For immunofluorescence analysis, biopsies were fixed and embedded in paraffin. Alternatively, samples were frozen in liquid nitrogen and used for Western blot analysis.
Immunohistochemistry
Immunohistochemistry was performed using Chem-Mate EnVision Detection Kit (DAKO, Carpinteria, CA). Tissue sections were deparaffinized and rehydrated. After incubation with 0.3% H2O2 for 20 min, sections were placed in 10 mm EDTA, pH 8.0, and heated in a microwave for 5 min. Slides were stained with anti-hTid-1, anti-HSP27, or anti-phospho-HSP27 (Ser-82) antibody at room temperature for 1 h followed by the incubation with the secondary antibody for 30 min. F-actin was visualized by staining slides with rhodamine-conjugated phalloidin for 1 h. Tissue sections were developed with 3,3′-diaminobenzidine and counterstained with Mayor's hematoxylin.
RESULTS
hTid-1S Interacts with MK5 to Inhibit HSP27 Phosphorylation
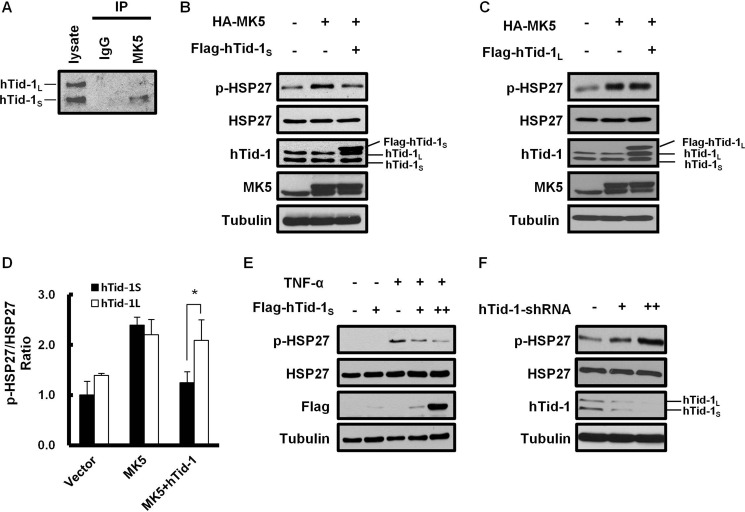
A potential interaction between MK5 and hTid-1S by the yeast two-hybrid assay using MK5 as a bait prompted us to verify their interaction in cultured human cells. Intracellular interaction between the two endogenous proteins in HeLa cells was examined by coimmunoprecipitation analysis. A human DnaJ protein hTid-1S was coimmunoprecipitated with MK5 (Fig. 1A). However, the hTid-1L isoform, which differs from hTid-1S only at carboxyl-terminal tail (4), fails to interact with MK5. We explored the possibility that the binding of hTid-1S with MK5 interferes with the catalytic activity of MK5. Because HSP27 is a prominent MK5 substrate protein, we attempted to explain the biological role of hTid-1S interaction with MK5 by examining the effect of hTid-1S on the phosphorylation of HSP27. Immunoblot analysis demonstrated that FLAG-hTid-1S expression does not affect the intracellular level of HSP27 but inhibits the phosphorylation of HSP27 induced by the expression of HA-MK5 (Fig. 1, B and D). In addition, data in Fig. 1, C and D, indicate that the ability of MK5 to phosphorylate HSP27 was inhibited specifically by hTid-1S isoform, which interacts with MK5, but not by hTid-1L isoform. The phosphorylation of HSP27 induced by TNF-α, a proinflammatory cytokine known to activate MK5 (9), was also inhibited by the expression of hTid-1S (Fig. 1E). Transfection of hTid-1-shRNA suppressed the expressions of both hTid-1L and hTid-1S and increased HSP27 phosphorylation in HeLa cells, supporting the negative role of hTid-1S expression on the kinase activity of MK5 that phosphorylates HSP27 (Fig. 1F).
FIGURE 1.
hTid-1S interacts with MK5 to inhibit its kinase activity. A, coimmunoprecipitation was performed to prove intracellular interaction between MK5 and hTid-1S in HeLa cells. Cell lysate proteins were mixed with anti-MK5 antibody. Immunoprecipitated proteins were separated in 12% SDS-PAGE and detected by immunoblot analysis using anti-hTid-1 antibody, which recognizes both hTid-1S and hTid-1L. B and C, HeLa cells (2 × 106) were transfected with expression vector for HA-MK5 (1 μg) alone or together with expression vector for FLAG-hTid-1S or FLAG-hTid-1L (1 μg each). After incubation for 24 h, cell lysates were resolved in 12% SDS-PAGE. Cellular level of phosphorylated HSP27 (pHSP27) or HSP27 was analyzed by immunoblot analysis using respective antibodies. Expression of FLAG-hTid-1S, HA-MK5, or tubulin in cell lysates was also monitored by immunoblot analysis. D, relative band intensities in Western blot in B and C were quantified by using ImageJ software and the pHSP27/HSP27 ratio in transfected HeLa cells was determined. The band intensity of pHSP27 of untreated cells was set to 1.0. Values represent means ± S.D. (error bars; n ≥ 3 experiments). Statistical significance was determined by Student's t test. *, p < 0.05. E, HeLa cells (2 × 106) were transfected with expression vector for FLAG-hTid-1S (1 or 2 μg). After incubation for 24 h, transfected cells were further incubated in serum-free medium for 3 h and treated with 100 ng/ml TNF-α for 20 min. Cellular level of endogenous HSP27, pHSP27, tubulin, or ectopically expressed FLAG-hTid-1S was examined by immunoblot analysis. F, HeLa cells (2 × 106) were treated with hTid-1-shRNA (1 or 2 μg). After incubation for 24 h, cell lysate proteins were resolved in 12% SDS-PAGE. Cellular level of endogenous HSP27, pHSP27, hTid-1, or tubulin was examined by immunoblot analysis using respective antibodies.
hTid-1S Inhibits Actin Cytoskeleton Organization
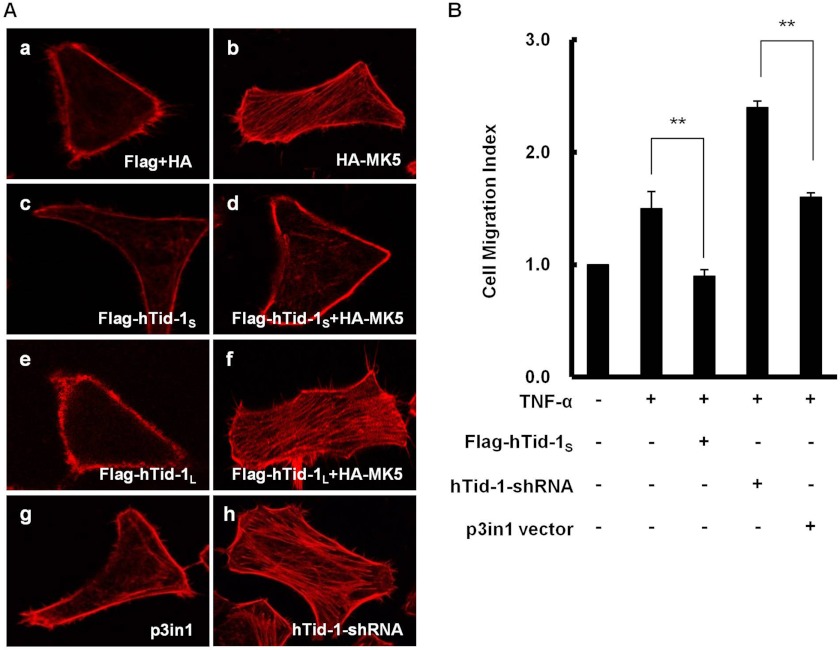
The phosphorylation of HSP27 has been known to confer stabilization of actin cytoskeleton as well as F-actin polymerization in cultured mammalian cells under stress conditions induced by reactive oxygen metabolites, tumor necrosis factors, growth factors, or heat shock (24, 25). The expression of hTid-1S, which interacts with MK5, is anticipated to interfere with HSP27 phosphorylation by MK5. We examined whether the expression of hTid-1S plays negative roles on MK5-mediated HSP27 phosphorylation and subsequent actin cytoskeleton organization in HeLa cells coexpressing HA-MK5 and FLAG-hTid-1S. Actin cytoskeleton organization leading to stress fiber formation was increased by MK5 expression. Although the expression of hTid-1S decreased MK5-induced stress fiber formation, the expression of hTid-1L did not influence actin cytoskeleton organization in HeLa cells coexpressing MK5 and hTid-1L. The ability of hTid-1S to inactivate MK5/HSP27 signaling was further supported by showing the increased stress fiber formation in HeLa cells transfected with hTid-1-shRNA. The silencing of hTid-1S by hTid-1-shRNA treatment led to the increase in actin cytoskeleton organization in HeLa cells (Fig. 2A). Cell migration is a cellular process that requires F-actin polymerization and actin cytoskeleton stabilization (26). Therefore, we examined whether the expression of hTid-1S that disrupts actin cytoskeleton organization can affect cell migration. Overexpression of hTid-1S resulted in the inhibition of TNF-α-induced cell migration, whereas the silencing of hTid-1S by hTid-1-shRNA stimulated cell migration in HeLa cells treated with TNF-α (Fig. 2B).
FIGURE 2.
Effects of hTid-1S expression on actin cytoskeleton organization and cell migration. A, disruption of MK5-mediated actin cytoskeleton organization by hTid-1S expression. a, HeLa cells (5 × 105) were cotransfected with expression vectors for HA and FLAG (1 μg each). b and c, cells were transfected with expression vector for HA-MK5 or FLAG-hTid-1S (1 μg each). d, cells were cotransfected with expression vectors for HA-MK5 and FLAG-hTid-1S (1 μg each). e, cells were transfected with expression vector for FLAG-hTid-1L (1 μg). f, cells were cotransfected with expression vectors for HA-MK5 and FLAG-hTid-1L (1 μg each). g, and h, cells were transfected with p3in1 vector or hTid-1-shRNA (1 μg each). Transfected or cotransfected HeLa cells were incubated for 24 h, fixed, permeabilized, blocked, stained with rhodamine-conjugated phalloidin for F-actin, and imaged by confocal microscopy. B, inhibition of TNF-α-induced cell migration by hTid-1S. HeLa cells (2 × 106) were transfected with expression vector for FLAG-hTid-1S, hTid-1-shRNA, or p3in1 vector (1 μg each). At 24 h after transfection, cells were starved for 24 h. Prior to cell migration experiment, cells were left untreated or treated with 100 ng/ml TNF-α for 20 min. After incubation for 12 h in 6.5-mm Transwell plates, cell migration was measured colorimetrically on a standard microplate reader. Cell migration was expressed as a Migration Index (Chemicon). Cell migration of untreated HeLa cells was set to 1.0. Data represent results from three independent experiments means ± S.D. (error bars; n ≥ 3 experiments). **, p < 0.01.
Activation of MK5/HSP27 Signaling under Hypoxic Conditions
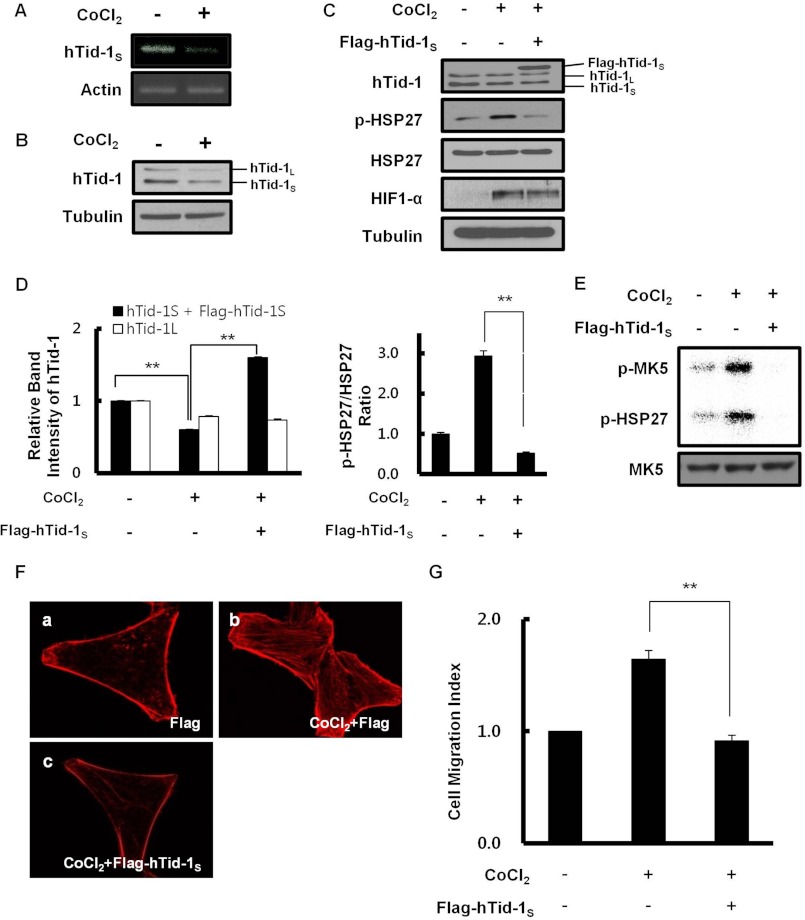
Transcriptional and translational down-regulation of hTid-1L in cultured human cells under hypoxic conditions has been described previously (27). In this study, we examined mRNA and protein levels of hTid-1S in HeLa cells grown under hypoxic conditions. CoCl2 was chosen as a hypoxia-mimicking agent to maintain hypoxic signal throughout the experiments (28, 29). Intracellular levels of hTid-1S mRNA and protein were reduced in HeLa cells treated with 200 μm CoCl2 for 24 h (Fig. 3, A and B). Our data also illustrate that phosphorylation of HSP27 was elevated by hypoxia. However, the phosphorylation of HSP27 was severely inhibited by the expression of hTid-1S in transfected HeLa cells grown under hypoxic conditions (Fig. 3, C and D). Moreover, immunoprecipitation kinase assay in Fig. 3E strongly supports that the increased HSP27 phosphorylation under hypoxic conditions is attributable to the activation of MK5 protein kinase activity through the activation phosphorylation of MK5. MK5 phosphorylation as well as HSP27 phosphorylation was elevated in HeLa cells grown under hypoxic conditions. However, the expression of hTid-1S completely inhibited the phosphorylation of HSP27 and autophosphorylation of MK5 in transfected HeLa cells under hypoxic conditions. Increased stress fiber formation in HeLa cells under hypoxic conditions also implicates that down-regulation of hTid-1S during hypoxia increases MK5-dependent HSP27 phosphorylation and facilitates actin cytoskeleton organization (Fig. 3F). In addition to the stimulation of actin cytoskeleton organization, cell migration was enhanced in HeLa cells by down-regulating hTid-1S expression during hypoxia (Fig. 3G).
FIGURE 3.
Down-regulation of hTid-1S expression under hypoxic conditions enhances actin cytoskeleton organization. A, HeLa cells (2 × 106) were treated with 200 μm CoCl2 for 24 h. Reverse transcription-PCR analysis was carried out using specific primer for hTid-1S (forward, 5′-TCAGGGTGCAGAAAAGCCCT-3′; reverse, 5′-CTAGTTTCCAGTGGATCTTTTTC-3′). β-Actin served as a loading control. B, intracellular level of hTid-1S or hTid-1L was examined by immunoblotting using anti-hTid-1 antibody. Tubulin was used as a loading control. C, HeLa cells (2 × 106) were transiently transfected with expression vector for FLAG-hTid-1S (2 μg). After incubation for 24 h, transfected cells were treated with 200 μm CoCl2 for 24 h. Cellular level of endogenous HSP27, phosphorylated HSP27, hTid-1, HIF1-α, or tubulin was examined by immunoblot analysis. D, relative band intensities in Western blot were quantified, and the pHSP27/HSP27 ratio in HeLa cells is presented. The band intensity of hTid-1S plus FLAG-hTid-1S or hTid-1L in untreated cells was, respectively, set to 1.0 (left). The pHSP27/HSP27 ratio of untreated cells was set to 1.0 (right). Data represent mean ± S.D. (error bars; n ≥ 3 experiments). **, p < 0.01. E, HeLa cells (∼107) were left untreated or treated with 200 μm CoCl2 for 24 h. Cell lysate proteins were immunoprecipitated using anti-MK5 antibody. MK5 kinase activity was determined by measuring the transfer of 32P-labeled phosphate moiety from [γ-32P]ATP to MK5 and HSP27. Activation phosphorylation of MK5 or HSP27 phosphorylation was visualized by autoradiography (upper). Cellular level of MK5 present in total cell lysates was examined by immunoblot analysis using anti-MK5 antibody (lower). F, effect of hTid-1S expression on hypoxia-induced actin cytoskeleton organization was evaluated. a and b, HeLa cells (5 × 105) were left untreated (a) or treated with 200 μm CoCl2 for 24 h (b). c, HeLa cells (5 × 105) were transfected with expression vector for FLAG-hTid-1S (1 μg). After incubation for 24 h, cells were treated with 200 μm CoCl2 for 24 h. F-actin was visualized after staining cells with rhodamine-conjugated phalloidin. G, effect of hTid-1S expression on hypoxia-induced cell migration is shown. HeLa cells were transfected with expression vector for hTid-1S. After incubation for 24 h, control or transfected HeLa cells were treated with 200 μm CoCl2 for 24 h. Cell migration was expressed as a Migration Index as in Fig. 2B. Data represent mean ± S.D. (error bars; n ≥ 3 experiments). **, p < 0.01.
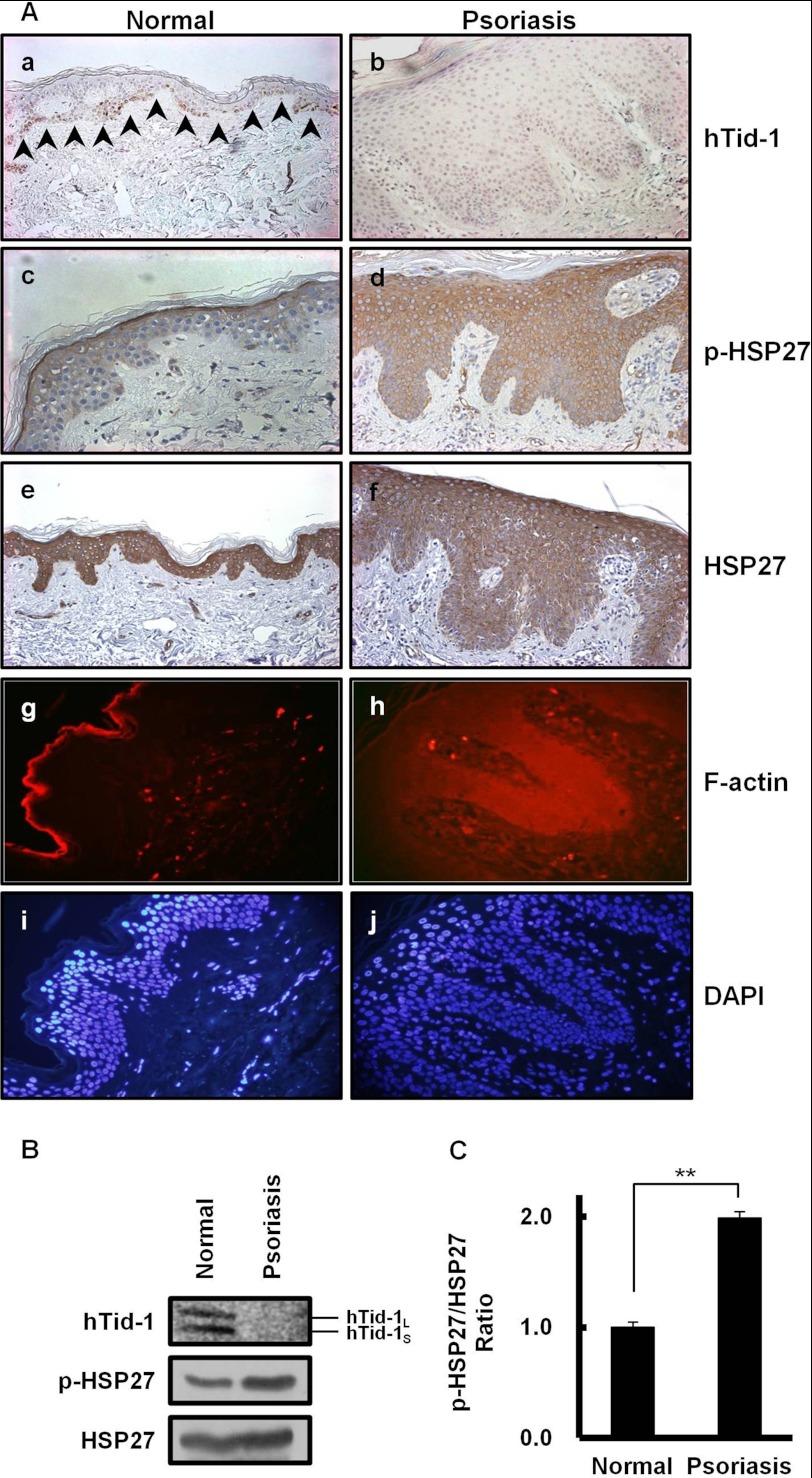
Absence of hTid-1S Expression and Aberrant Actin Cytoskeleton Organization in Psoriatic Human Skin
There have been studies showing the involvement of activated MAPK p38 (30) and MAPK-activated protein kinase that phosphorylates HSP27 (31) in hyperproliferative epidermis of psoriatic skin. Our experimental results in Fig. 4 imply that hTid-1S expression negatively regulates HSP27 phosphorylation and actin cytoskeleton organization in human skin epidermis. A comparative analysis of hTid-1 expression between lesional psoriatic and normal skin was carried out. Monoclonal mouse antibody employed in this study recognizes both hTid-1S and hTid-1L. In normal skin, hTid-1 expression was focal in the basal layer of epidermis (Fig. 4Aa). On the contrary, hTid-1 expression was not detected in the epidermis of psoriatic skin (Fig. 4Ab). HSP27 was expressed in both epidermis of normal and hyperthickened psoriatic skin (Fig. 4A, e and f). The localization of phosphorylated HSP27 that stabilizes actin cytoskeleton (24, 25) markedly differs in normal and lesional psoriatic skin. Phosphorylated HSP27 was restricted to the granular layer of normal skin, whereas phosphorylated HSP27 was localized in the entire hyperthickened epidermis of lesional psoriatic skin (Fig. 4A, c and d). In normal skin, actin filament formation was observed extensively in the granular layer of epidermis where phosphorylated HSP27 is present (Fig. 4Ag). In psoriatic skin, F-actin polymerization and stress fiber formation were intensively stimulated throughout the whole hyperthickened epidermis including basal layer, stratum spinosum, and granular layer (Fig. 4Ah). To further support the reciprocal relationship between hTid-1S expression and HSP27 phosphorylation, the levels of HSP27 and its phosphorylation form in the epidermal tissue from lesional psoriatic skin were compared with those from normal controls. To evaluate the important role of hTid-1S expression on HSP27 phosphorylation, the pHSP27/HSP27 ratio in the epidermis from psoriatic human skin was compared with that from normal control by immunoblotting using HSP27 as a loading control. Western blot analysis ascertained the loss of hTid-1S expression together with the enhanced HSP27 phosphorylation in the epidermis from lesional psoriatic skin (Fig. 4B).
FIGURE 4.
Absence of hTid-1 expression and enhanced actin cytoskeleton organization in lesional psoriatic human skin. A, sections of skin epidermis from normal controls (a, c, e, and g) and lesional psoriatic skin (b, d, f, and i) were immunostained with anti-hTid-1, anti-HSP27, or anti-phospho-HSP27 antibody. Arrowheads indicate positive staining of cytosolic hTid-1 in the basal layer of the normal epidermis (a). Sections of normal (g) and lesional psoriatic skin (h) were immunostained with rhodamine-conjugated phalloidin for F-actin. The cyan color corresponds to DAPI staining of nuclei of cell in g (i) and h (j) (original magnification, ×400). B, HSP27 phosphorylation is significantly enhanced in lesional psoriatic skin lacking hTid-1S expression. Immunoblotting was performed on cell extracts prepared from the epidermis of keratome biopsies from normal (three different subjects) or lesional psoriatic skin (three different psoriatic patients). Data are the representative Western blots of hTid-1, HSP27, and phosphorylated HSP27 in human keratinocytes. C, band intensities in Western blots were quantified. The pHSP27/HSP27 ratio in the epidermis from psoriatic skin was compared with that from normal controls. The pHSP27/HSP27 band intensity ratio in the epidermis from normal skin was set to 1.0. Values represent means ± S.D. (error bars; n ≥ 3 samples). **, p < 0.01.
Inhibition of F-actin Polymerization by hTid-1S Expression in Human HaCaT Keratinocytes
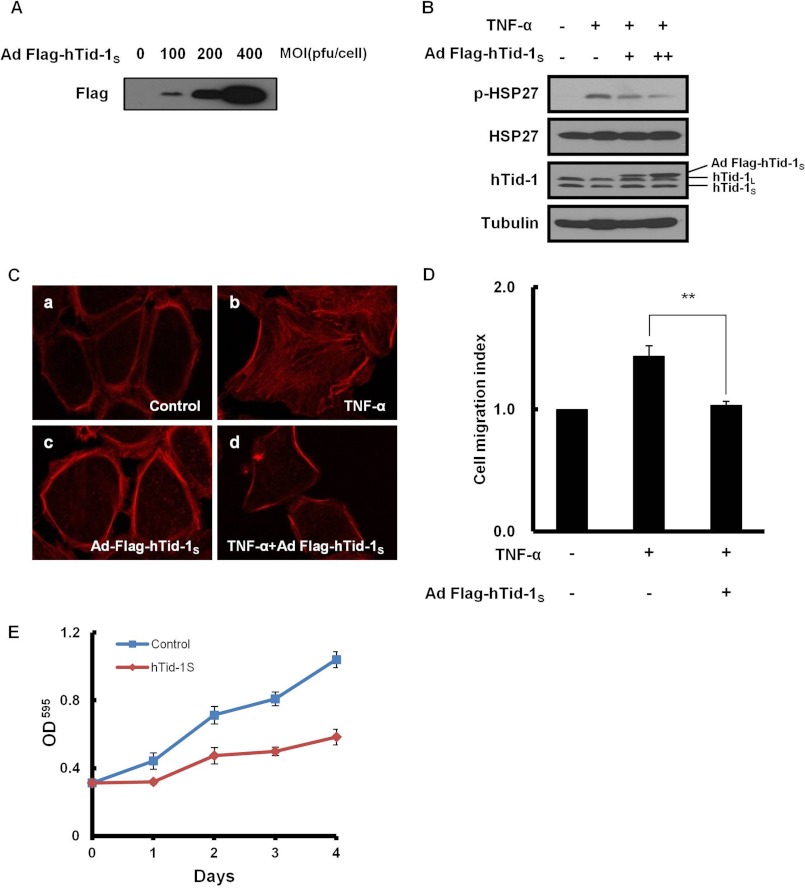
To examine whether hTid-1S expression also attenuates actin cytoskeleton organization in the epidermis of human skin, we evaluated the effect of hTid-1S expression on MK5/HSP27 signaling in cultured human HaCaT keratinocytes. Ectopic expression of hTid-1S in HaCaT cells infected with recombinant adenoviruses was confirmed by immunoblotting (Fig. 5A). The expression of hTid-1S in HaCaT keratinocytes interfered with the phosphorylation of HSP27 induced by TNF-α (Fig. 5B). Overexpression of hTid-1S inhibited F-actin polymerization, resulting in reduced stress fiber formation in transduced HaCaT cells treated with TNF-α (Fig. 5C). Cell migration induced by TNF-α was also inhibited by hTid-1S expression in HaCaT cells (Fig. 5D). As actin cytoskeleton rearrangement is required for cell proliferation as well as cell motility (32), we examined whether hTid-1S expression affects proliferation of HaCaT keratinocytes. The retarded keratinocyte proliferation was accompanied by the reduced stress fiber formation in transduced HaCaT keratinocytes overexpressing hTid-1S (Fig. 5E).
FIGURE 5.
Inactivation of MK5/HSP27 signaling by hTid-1S expression in human HaCaT keratinocytes. A, ectopic expression of hTid-1S in HaCaT keratinocytes infected with recombinant adenoviruses expressing FLAG-hTid-1S (100, 200, or 400 pfu/cell) examined by immunoblotting using antibody for FLAG epitope. B, inhibition of HSP27 phosphorylation by hTid-1S overexpression in HaCaT keratinocytes treated with TNF-α (100 ng/ml). Cell extracts prepared 48 h after viral infection (100 or 200 pfu/cell) were subjected to immunoblot analysis for the level of HSP27 or pHSP27. C, transduced HaCaT keratinocytes examined for the effect of hTid-1S overexpression on TNF-α-induced actin cytoskeleton organization. D, inhibition of TNF-α-induced cell migration in HaCaT keratinocytes. HaCaT keratinocytes (2 × 106) were infected with either recombinant adenoviral vector for FLAG-hTid1S (100 pfu/cell each). At 24 h after infection, cells were starved for 24 h. Cell migration was examined as in Fig. 2B. E, retarded HaCaT keratinocyte proliferation by hTid-1S expression. HaCaT cells seeded on 9-well plates (5 × 105) were infected with recombinant adenoviral vector for FLAG-hTid-1S (100 pfu/cell). After incubation for the stated amount of time, cell number was determined colorimetrically using the crystal violet viability assay. Data represent mean ± S.D. (error bars; n ≥ 3 experiments).
DISCUSSION
The p38 MAPK signal pathway has been shown to participate in F-actin polymerization, actin cytoskeleton organization, and actin filament dynamics in various types of cells (33, 34). F-actin organization by activated p38 MAPK has been known to be largely mediated by MK2, which phosphorylates HSP27 (35, 36). Recently, we and others reported that MK5 is also involved in F-actin polymerization and actin cytoskeleton organization (14, 15). Both MK5 and MK2 phosphorylate their target protein HSP27, which plays important roles in the regulation of F-actin polymerization. Here, we propose that hTid-1S is a new regulator that controls HSP27 phosphorylation. A coimmunoprecipitation assay provides evidence for the intracellular binding of hTid-1S with MK5. We found that hTid-1S interacts with MK5 and inhibits HSP27 phosphorylation in HeLa cells (Fig. 1). Despite functional and structural similarities between MK5 and MK2, hTid-1S failed to interact with MK2 (data not shown). The binding ability of hTid-1S with MK5 seems to be important for the modulation of MK5 kinase activity because the phosphorylation of HSP27 in transfected HeLa cells was not affected by the expression of hTid-1L, which lacks the binding ability with MK5 (Fig. 1C).
In addition to ATP-dependent chaperonin activity (37, 38), HSP27 has been known as an actin filament-capping protein that modulates actin polymerization by its state of phosphorylation. Unphosphorylated form of HSP27 inhibits F-actin polymerization. During cellular stress and growth, HSP27 undergoes rapid phosphorylation and promotes F-actin polymerization leading to actin cytoskeleton organization and stress fiber formation (35, 39–41). Immunofluorescence analysis of actin stress fiber formation in HeLa cells coexpressing hTid-1S and MK5 illustrates the inhibition of MK5-induced F-actin polymerization by hTid-1S. Enhanced actin cytoskeleton organization by silencing of hTid-1S using hTid-1-shRNA supports the negative role of hTid-1S on F-actin polymerization (Fig. 2A). Because cell migration is a multistep cellular process initiated by forming protrusive structures like filopodia, lamellipodia, and invadopodia/podosomes that require F-actin polymerization (42), it is reasonable to anticipate that the inhibition of HSP27 phosphorylation by hTid-1S expression may well interfere with cell migration. The expression of hTid-1S in HeLa cells inhibited TNF-α-induced cell migration, but the silencing of hTid-1S by hTid-1-shRNA treatment enhanced cell migration (Fig. 2B), supporting the negative role of hTid-1S on cell migration.
Previous studies have shown that the organization of actin cytoskeleton and the formation of stress fiber are activated (43), but the expression of hTid-1L is down-regulated in response to hypoxia in cultured human cells (27). However, our data demonstrate that hTid-1S is involved in the modulation of actin cytoskeleton organization during hypoxia. Cellular levels of hTid-1S mRNA and protein were decreased after the treatment of HeLa cells with hypoxia-mimicking agent CoCl2 (Fig. 3, A and B). Our results imply that the decreased hTid-1S expression under hypoxic conditions enhances HSP27 phosphorylation and mediates the activation of MK5/HSP27 signal pathway (Fig. 3, C and D) leading to the enhanced actin cytoskeleton organization. MK5 is a protein kinase that requires autophosphorylation at Thr-182 in the catalytic domain for its activation (9). We found that the activity of MK5 as a protein kinase becomes activated in response to hypoxia, during which hTid-1S expression is repressed. The activation phosphorylation of MK5 at Thr-182 was proven to be completely inhibited by hTid-1S expression in transfected HeLa cells treated with CoCl2 (Fig. 3E). Inhibition of MK5 protein kinase activity by hTid-1S expression in cells under hypoxic conditions strongly supports our hypothesis that hTid-1S binding to MK5 inhibits MK5 activity and subsequent HSP27 phosphorylation resulting in the down-regulation of F-actin polymerization. As F-actin polymerization is inhibited by ectopic expression of hTid-1S during hypoxia (Fig. 3F), cell migration under hypoxic conditions was also affected by hTid-1S expression as shown in Fig. 3G.
In this study, we attempted to interpret the biological roles of hTid-1S expression on MK5/HSP27 signaling in association with the etiology of psoriasis skin disease. Rationales for the involvement of hTid-1 signaling in the etiology of psoriasis disease are as follows. (i) The activity of transcription factor NF-κB is up-regulated in the epidermis of psoriatic lesions (44), but hTid-1 expression represses NF-κB activity (45, 46). (ii) The expression of HIF1-α, which mediates oxygen homeostasis and angiogenesis under hypoxic conditions, is up-regulated in psoriatic skin (47); however, the expression of hTid-1 inhibits angiogenesis by destabilizing HIF1-α (27). (iii) Th1-derived IFNγ is increased greatly in the epidermis of psoriatic lesions (48), where hTid-1L and hTid-1S interact with IFNγ to inhibit its transcriptional competence (49).
The direct bearing of actin cytoskeleton organization on the induction of psoriasis was first raised by Wanger and Sundqvist (50), who showed excess F-actin polymerization in the epidermis of lesional skin from patients with psoriasis. More recently, proteomic analysis has demonstrated that concentrations of cytoskeleton proteins including actin are increased in the plasma samples from patients with psoriasis compared with normal controls, emphasizing the relevance of actin filament dynamics to the etiology of psoriasis (51). The important role of hTid-1S expression on actin filament dynamics through F-actin polymerization in the epidermis of human skin is exemplified in Fig. 4. Immunohistochemistry data disclose the absence of hTid-1 expression, enhanced HSP27 phosphorylation, and the stimulation of actin cytoskeleton organization in hyperthickened psoriatic epidermis (Fig. 4A). Western blot analysis showing the loss of hTid-1S expression together with the increased pHSP27/HSP27 ratio in lesional epidermis from patients with psoriasis disease strongly suggests that the aberrant actin cytoskeleton organization correlates with the absence of hTid-1S expression in the epidermis of psoriatic skin (Fig. 4, B and C). These demonstrations make a fine agreement with the experimental results showing the inhibition of HSP27 phosphorylation and reduced actin cytoskeleton organization by hTid-1S expression in HeLa cells (Fig. 3). From the in vitro study using fibroblastic HeLa cells, can it be concluded that the loss of hTid-1S expression stimulates HSP27 phosphorylation and F-actin polymerization in the epidermis? The correlation between the loss of hTid-1S expression and the enhancement of HSP27 phosphorylation was also investigated in cultured human keratinocyte HaCaT cells. In accordance with the inactivation of MK5/HSP27 signaling by hTid-1S expression, actin cytoskeleton organization was inhibited in HaCaT keratinocytes expressing FLAG-hTid-1S (Fig. 5, A–C). Inhibition of cell migration (Fig. 5D) and antiproliferative potential by hTid-1S in HaCaT keratinocytes (Fig. 5E) also support that the loss of hTid-1S expression is involved in the aberrant actin cytoskeleton organization and keratinocyte hyperproliferation in lesional psoriatic skin. Inordinate actin cytoskeleton organization may disrupt cytoskeletal plasticity in the hyperthickened epidermis of psoriatic skin (Fig. 6). Here, we propose hTid-1S as one of potential targets in understanding the etiology of psoriatic skin disease.
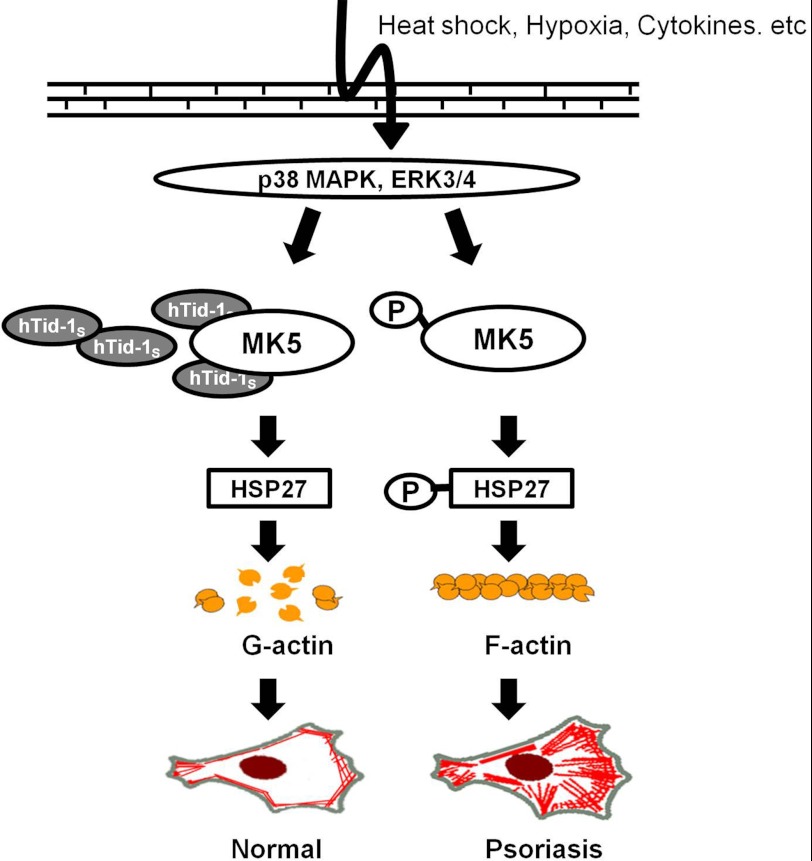
FIGURE 6.
Proposed model for the aberrant actin cytoskeleton organization in the epidermis of psoriatic skin. p38 MAPK and ERK activate MK5. The expression of hTid-1S inhibits the kinase activity of MK5 which phosphorylates HSP27 and attenuates F-actin polymerization in keratinocytes of normal skin. However, the loss of hTid-1 expression in the hyperthickened epidermis of psoriatic skin fails to inhibit MK5 activity, leading to the increased HSP27 phosphorylation, aberrant actin cytoskeleton organization, and hyperproliferation of keratinocytes.
This work was supported by National Research Foundation of Korea Grants 2010-0011268 and 2009-0085835 (to C. O. J.).
- hTid-1
- human tumorous imaginal disc1
- MK5
- MAPK-activated kinase protein 5.
REFERENCES
- 1. Schilling B., De-Medina T., Syken J., Vidal M., Münger K. (1998) A novel human DnaJ protein, hTid-1, a homolog of the Drosophila tumor suppressor protein Tid56, can interact with the human papillomavirus type 16 E7 oncoprotein. Virology 247, 74–85 [DOI] [PubMed] [Google Scholar]
- 2. Qiu X. B., Shao Y. M., Miao S., Wang L. (2006) The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 63, 2560–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu B., Garrido N., Spelbrink J. N., Suzuki C. K. (2006) Tid1 isoforms are mitochondrial DnaJ-like chaperones with unique carboxyl termini that determine cytosolic fate. J. Biol. Chem. 281, 13150–13158 [DOI] [PubMed] [Google Scholar]
- 4. Syken J., De-Medina T., Münger K. (1999) TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions. Proc. Natl. Acad. Sci. U.S.A. 96, 8499–8504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu H. Y., Meakin S. O. (2002) ShcB and ShcC activation by the Trk family of receptor tyrosine kinases. J. Biol. Chem. 277, 26046–26056 [DOI] [PubMed] [Google Scholar]
- 6. Gaestel M. (2006) MAPKAP kinases: MKs, two's company, three's a crowd. Nat. Rev. Mol. Cell Biol. 7, 120–130 [DOI] [PubMed] [Google Scholar]
- 7. Ronkina N., Kotlyarov A., Gaestel M. (2008) MK2 and MK3: a pair of isoenzymes? Front. Biosci. 13, 5511–5521 [DOI] [PubMed] [Google Scholar]
- 8. Ronkina N., Kotlyarov A., Dittrich-Breiholz O., Kracht M., Hitti E., Milarski K., Askew R., Marusic S., Lin L. L., Gaestel M., Telliez J. B. (2007) The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol. Cell. Biol. 27, 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. New L., Jiang Y., Zhao M., Liu K., Zhu W., Flood L. J., Kato Y., Parry G. C., Han J. (1998) PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 17, 3372–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schumacher S., Laass K., Kant S., Shi Y., Visel A., Gruber A. D., Kotlyarov A., Gaestel M. (2004) Scaffolding by ERK3 regulates MK5 in development. EMBO J. 23, 4770–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seternes O. M., Mikalsen T., Johansen B., Michaelsen E., Armstrong C. G., Morrice N. A., Turgeon B., Meloche S., Moens U., Keyse S. M. (2004) Activation of MK5/PRAK by the atypical MAP kinase ERK3 defines a novel signal transduction pathway. EMBO J. 23, 4780–4791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kant S., Schumacher S., Singh M. K., Kispert A., Kotlyarov A., Gaestel M. (2006) Characterization of the atypical MAPK ERK4 and its activation of the MAPK-activated protein kinase MK5. J. Biol. Chem. 281, 35511–35519 [DOI] [PubMed] [Google Scholar]
- 13. Sun P., Yoshizuka N., New L., Moser B. A., Li Y., Liao R., Xie C., Chen J., Deng Q., Yamout M., Dong M. Q., Frangou C. G., Yates J. R., 3rd, Wright P. E., Han J. (2007) PRAK is essential for ras-induced senescence and tumor suppression. Cell 128, 295–308 [DOI] [PubMed] [Google Scholar]
- 14. Tak H., Jang E., Kim S. B., Park J., Suk J., Yoon Y. S., Ahn J. K., Lee J. H., Joe C. O. (2007) 14-3-3ϵ inhibits MK5-mediated cell migration by disrupting F-actin polymerization. Cell. Signal. 19, 2379–2387 [DOI] [PubMed] [Google Scholar]
- 15. Kostenko S., Johannessen M., Moens U. (2009) PKA-induced F-actin rearrangement requires phosphorylation of Hsp27 by the MAPKAP kinase MK5. Cell. Signal. 21, 712–718 [DOI] [PubMed] [Google Scholar]
- 16. Perander M., Keyse S. M., Seternes O. M. (2008) Does MK5 reconcile classical and atypical MAP kinases? Front. Biosci. 13, 4617–4624 [DOI] [PubMed] [Google Scholar]
- 17. Green H. (1980) The keratinocyte as differentiated cell type. Harvey Lect. 74, 101–139 [PubMed] [Google Scholar]
- 18. Lebwohl M. (2003) Psoriasis. Lancet 361, 1197–1204 [DOI] [PubMed] [Google Scholar]
- 19. Valdimarsson H., Thorleifsdottir R. H., Sigurdardottir S. L., Gudjonsson J. E., Johnston A. (2009) Psoriasis: as an autoimmune disease caused by molecular mimicry. Trends Immunol. 30, 494–501 [DOI] [PubMed] [Google Scholar]
- 20. Bowcock A. M., Krueger J. G. (2005) Getting under the skin: the immunogenetics of psoriasis. Nat. Rev. Immunol. 5, 699–711 [DOI] [PubMed] [Google Scholar]
- 21. Kueng W., Silber E., Eppenberger U. (1989) Quantification of cells cultured on 96-well plates. Anal. Biochem. 182, 16–19 [DOI] [PubMed] [Google Scholar]
- 22. Kim S. W., Hayashi M., Lo J. F., Fearns C., Xiang R., Lazennec G., Yang Y., Lee J. D. (2005) Tid1 negatively regulates the migratory potential of cancer cells by inhibiting the production of interleukin-8. Cancer Res. 65, 8784–8791 [DOI] [PubMed] [Google Scholar]
- 23. Johansen C., Flindt E., Kragballe K., Henningsen J., Westergaard M., Kristiansen K., Iversen L. (2005) Inverse regulation of the nuclear factor-κB binding to the p53 and interleukin-8 κB response elements in lesional psoriatic skin. J. Invest. Dermatol. 124, 1284–1292 [DOI] [PubMed] [Google Scholar]
- 24. Huot J., Houle F., Spitz D. R., Landry J. (1996) HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 56, 273–279 [PubMed] [Google Scholar]
- 25. Huot J., Lambert H., Lavoie J. N., Guimond A., Houle F., Landry J. (1995) Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur. J. Biochem. 227, 416–427 [DOI] [PubMed] [Google Scholar]
- 26. Samstag Y., Eibert S. M., Klemke M., Wabnitz G. H. (2003) Actin cytoskeletal dynamics in T lymphocyte activation and migration. J. Leukoc. Biol. 73, 30–48 [DOI] [PubMed] [Google Scholar]
- 27. Bae M. K., Jeong J. W., Kim S. H., Kim S. Y., Kang H. J., Kim D. M., Bae S. K., Yun I., Trentin G. A., Rozakis-Adcock M., Kim K. W. (2005) Tid-1 interacts with the von Hippel-Lindau protein and modulates angiogenesis by destabilization of HIF-1α. Cancer Res. 65, 2520–2525 [DOI] [PubMed] [Google Scholar]
- 28. Wang G. L., Semenza G. L. (1995) Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270, 1230–1237 [DOI] [PubMed] [Google Scholar]
- 29. Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 30. Johansen C., Kragballe K., Westergaard M., Henningsen J., Kristiansen K., Iversen L. (2005) The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br. J. Dermatol. 152, 37–42 [DOI] [PubMed] [Google Scholar]
- 31. Johansen C., Funding A. T., Otkjaer K., Kragballe K., Jensen U. B., Madsen M., Binderup L., Skak-Nielsen T., Fjording M. S., Iversen L. (2006) Protein expression of TNF-α in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J. Immunol. 176, 1431–1438 [DOI] [PubMed] [Google Scholar]
- 32. Kubler M. D., Watt F. M. (1993) Changes in the distribution of actin-associated proteins during epidermal wound healing. J. Invest. Dermatol. 100, 785–789 [DOI] [PubMed] [Google Scholar]
- 33. Rousseau S., Houle F., Landry J., Huot J. (1997) p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15, 2169–2177 [DOI] [PubMed] [Google Scholar]
- 34. Hedges J. C., Dechert M. A., Yamboliev I. A., Martin J. L., Hickey E., Weber L. A., Gerthoffer W. T. (1999) A role for p38MAPK/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem. 274, 24211–24219 [DOI] [PubMed] [Google Scholar]
- 35. Guay J., Lambert H., Gingras-Breton G., Lavoie J. N., Huot J., Landry J. (1997) Regulation of actin filament dynamics by p38 MAP kinase-mediated phosphorylation of heat shock protein 27. J. Cell Sci. 110, 357–368 [DOI] [PubMed] [Google Scholar]
- 36. Pichon S., Bryckaert M., Berrou E. (2004) Control of actin dynamics by p38 MAP kinase-Hsp27 distribution in the lamellipodium of smooth muscle cells. J. Cell Sci. 117, 2569–2577 [DOI] [PubMed] [Google Scholar]
- 37. Ferns G., Shams S., Shafi S. (2006) Heat shock protein 27: its potential role in vascular disease. Int. J. Exp. Pathol. 87, 253–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jakob U., Gaestel M., Engel K., Buchner J. (1993) Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268, 1517–1520 [PubMed] [Google Scholar]
- 39. Benndorf R., Hayess K., Ryazantsev S., Wieske M., Behlke J., Lutsch G. (1994) Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem. 269, 20780–20784 [PubMed] [Google Scholar]
- 40. Huot J., Houle F., Marceau F., Landry J. (1997) Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ. Res. 80, 383–392 [DOI] [PubMed] [Google Scholar]
- 41. Lavoie J. N., Lambert H., Hickey E., Weber L. A., Landry J. (1995) Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol. Cell. Biol. 15, 505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamaguchi H., Condeelis J. (2007) Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773, 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kayyali U. S., Pennella C. M., Trujillo C., Villa O., Gaestel M., Hassoun P. M. (2002) Cytoskeletal changes in hypoxic pulmonary endothelial cells are dependent on MAPK-activated protein kinase MK2. J. Biol. Chem. 277, 42596–42602 [DOI] [PubMed] [Google Scholar]
- 44. Lizzul P. F., Aphale A., Malaviya R., Sun Y., Masud S., Dombrovskiy V., Gottlieb A. B. (2005) Differential expression of phosphorylated NF-κB/RelA in normal and psoriatic epidermis and down-regulation of NF-κB in response to treatment with etanercept. J. Invest. Dermatol. 124, 1275–1283 [DOI] [PubMed] [Google Scholar]
- 45. Cheng H., Cenciarelli C., Nelkin G., Tsan R., Fan D., Cheng-Mayer C., Fidler I. J. (2005) Molecular mechanism of hTid-1, the human homolog of Drosophila tumor suppressor l(2)Tid, in the regulation of NF-κB activity and suppression of tumor growth. Mol. Cell. Biol. 25, 44–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng H., Cenciarelli C., Tao M., Parks W. P., Cheng-Mayer C. (2002) HTLV-1 Tax-associated hTid-1, a human DnaJ protein, is a repressor of IκB kinase β subunit. J. Biol. Chem. 277, 20605–20610 [DOI] [PubMed] [Google Scholar]
- 47. Rosenberger C., Solovan C., Rosenberger A. D., Jinping L., Treudler R., Frei U., Eckardt K. U., Brown L. F. (2007) Up-regulation of hypoxia-inducible factors in normal and psoriatic skin. J. Invest. Dermatol. 127, 2445–2452 [DOI] [PubMed] [Google Scholar]
- 48. Kryczek I., Bruce A. T., Gudjonsson J. E., Johnston A., Aphale A., Vatan L., Szeliga W., Wang Y., Liu Y., Welling T. H., Elder J. T., Zou W. (2008) Induction of IL-17+ T cell trafficking and development by IFN-γ: mechanism and pathological relevance in psoriasis. J. Immunol. 181, 4733–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sarkar S., Pollack B. P., Lin K. T., Kotenko S. V., Cook J. R., Lewis A., Pestka S. (2001) hTid-1, a human DnaJ protein, modulates the interferon signaling pathway. J. Biol. Chem. 276, 49034–49042 [DOI] [PubMed] [Google Scholar]
- 50. Wanger L., Sundqvist K. G. (1978) Detection of immunoglobulin-bearing lymphocytes in humans with special reference to patients with contact dermatitis. Acta Derm. Venereol. Suppl. 58, 73–76 [PubMed] [Google Scholar]
- 51. Plavina T., Hincapie M., Wakshull E., Subramanyam M., Hancock W. S. (2008) Increased plasma concentrations of cytoskeletal and Ca2+-binding proteins and their peptides in psoriasis patients. Clin. Chem. 54, 1805–1814 [DOI] [PubMed] [Google Scholar]