Background: Human CYP4F2 metabolizes vitamin E, but its role in vivo is unknown.
Results: Disruption of Cyp4f14, a murine ortholog of CYP4F2, severely alters vitamin E metabolism and status.
Conclusion: CYP4F14 is an important regulator of vitamin E status, and its absence reveals counterbalancing mechanisms.
Significance: An understanding of the fate of vitamin E is needed to predict and interpret its biological activities.
Keywords: Antioxidants, Cytochrome P450, Homologous Recombination, Metabolism, Vitamin E, Tocopherol
Abstract
Vitamin E is a family of naturally occurring and structurally related lipophilic antioxidants, one of which, α-tocopherol (α-TOH), selectively accumulates in vertebrate tissues. The ω-hydroxylase cytochrome P450–4F2 (CYP4F2) is the only human enzyme shown to metabolize vitamin E. Using cDNA cloning, cell culture expression, and activity assays, we identified Cyp4f14 as a functional murine ortholog of CYP4F2. We then investigated the effect of Cyp4f14 deletion on vitamin E metabolism and status in vivo. Cyp4f14-null mice exhibited substrate-specific reductions in liver microsomal vitamin E-ω-hydroxylase activity ranging from 93% (γ-TOH) to 48% (γ-tocotrienol). In vivo data obtained from metabolic cage studies showed whole-body reductions in metabolism of γ-TOH of 90% and of 68% for δ- and α-TOH. This metabolic deficit in Cyp4f14−/− mice was partially offset by increased fecal excretion of nonmetabolized tocopherols and of novel ω-1- and ω-2-hydroxytocopherols. 12′-OH-γ-TOH represented 41% of whole-body production of γ-TOH metabolites in Cyp4f14−/− mice fed a soybean oil diet. Despite these counterbalancing mechanisms, Cyp4f14-null mice fed this diet for 6 weeks hyper-accumulated γ-TOH (2-fold increase over wild-type littermates) in all tissues and appeared normal. We conclude that CYP4F14 is the major but not the only vitamin E-ω-hydroxylase in mice. Its disruption significantly impairs whole-body vitamin E metabolism and alters the widely conserved phenotype of preferential tissue deposition of α-TOH. This model animal and its derivatives will be valuable in determining the biological actions of specific tocopherols and tocotrienols in vivo.
Introduction
Vitamin E is a family of hydrophobic 6-hydroxychromanols synthesized by plants whose function is thought to be the scavenging of lipophilic free radicals in cell membranes or lipid stores. Of nine known family members, several, particularly the tocopherols with saturated side chains, are common in the diet. However, while all possess roughly similar radical scavenging activity in vitro (1, 2), one, α-tocopherol (α-TOH),2 preferentially accumulates in animal tissue. Although the typical North American diet contains two to four times as much γ-TOH as compared with α-TOH due to the prevalence of soybean and corn oils (3, 4), α-TOH is present in serum at levels five to six times that of γ-TOH (5). This preferential accumulation of α-TOH (the “α-TOH phenotype”) is widespread throughout the animal kingdom; however, the biological advantage of this conservation remains an enigma. The preferential accumulation of α-TOH is apparently facilitated by two activities as follows: preferential retention of α-TOH by the hepatic tocopherol transfer protein (α-TTP) (6, 7), and extensive post-absorptive catabolism of vitamers other than α-TOH by vitamin E-ω-hydroxylase (8). The relative contribution of these two activities in vivo has not been investigated. Vitamin E-ω-hydroxylase activity has been observed in Drosophila, with similar substrate specificity to that of the human activity, suggestive of a biological importance and evolutionary advantage of this highly conserved activity (9).
We previously identified cytochrome P450 4F2 (CYP4F2) as a human vitamin E-ω-hydroxylase (10). CYP4F2 catalyzes the ω-hydroxylation of the terminal methyl group of the 16-carbon branched side chain of tocopherols and tocotrienols (T3s, with three double bonds in the side chain). This oxidation is the first and rate-limiting step in the ω-oxidation pathway of vitamin E catabolism, and it represents the only reported enzyme-mediated pathway of vitamin E metabolism. Following the initial ω-hydroxylation event, tocopherols and tocotrienols undergo a series of side chain shortening steps, analogous to fatty acid β-oxidation, yielding short chain carboxychromanol metabolites that are excreted in urine (11–13). In the human, CYP4F2 is expressed predominantly in the liver (14) and was initially identified as a ω-hydroxylase of leukotriene B4 (LTB4). Whereas several human CYP enzymes other than CYP4F2 have been shown to ω-hydroxylate LTB4, including CYP4F3A (leukocyte) and CYP4F3B (liver) (15), CYP4F2 remains the only human enzyme demonstrated to metabolize vitamin E.
We hypothesized that TOH-ω-hydroxylase activity is essential for establishing and maintaining the α-TOH phenotype. To test this hypothesis, we sought to identify the ortholog of CYP4F2 in mice, disrupt its expression by homologous recombination, and determine the consequences of its ablation on tocopherol metabolism and status.
EXPERIMENTAL PROCEDURES
Materials
TRIzol reagent, Superscript First Strand synthesis kit, pcDNA3.1/Hygro+, and Platinum PCR Supermix High Fidelity were purchased from Invitrogen, and primers were from Integrated DNA Technologies (Coralville, IA). QIA Shredder spin columns, RNeasy mini kit, and RNase-free DNase were purchased from Qiagen (Valencia, CA). High Capacity cDNA reverse transcription kit, Taqman Universal Master Mix, and all Taqman assays were obtained from Applied Biosystems (Foster City, CA). Rabbit anti-human CYP4F2 antibody and preimmune (control) IgG were purchased from Research Diagnostics (Concord, MA). Tocopherols were obtained from Matreya, LLC (Pleasant Gap, PA). γ-T3 was a gift from Volker Berl (BASF Global, Schwarzheide, Germany). The internal standards d9-α-TOH and d9-α-CEHC were custom-synthesized by J. Swanson (Cornell University, Ithaca, NY). Pyridine and N,O-bis-[trimethylsilyl]trifluoroacetamide containing 1% trimethylchlorosilane were purchased from Pierce. COS-7 cells were obtained from American Type Culture Collection (Manassas, VA). Sesamin was acquired from Cayman Chemical (Ann Arbor, MI), and bovine serum albumin, NADPH, β-glucuronidase (from Escherichia coli), and sulfatase (from Aerobacter aerogenes) were purchased from Sigma. Wild-type C57B/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Rodent chow (Teklad 7912) was purchased from Teklad Diets (Madison, WI), and modified AIN-93G rodent diet was manufactured by DYETS Inc. (Bethlehem, PA).
Immunoinhibition of Mouse Liver Microsomal TOH-ω-Hydroxylase by Anti-human CYP4F Antibody
Microsomes were prepared from fresh mouse liver by standard differential centrifugation. Liver was minced and homogenized using a Teflon/glass homogenizer in 5 volumes of ice-cold 15 mm HEPES buffer containing 0.25 m sucrose, 1 mm EDTA, and 1 mm PMSF, pH 7.4, and then centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant was centrifuged at 100,000 × g for 1 h at 4 °C. The microsomal pellet was resuspended in 0.1 mm sodium phosphate (NaP) buffer containing 1 mm EDTA, 0.1 mm DTT, and 20% glycerol. Microsomal protein concentration was determined by a Bradford-based Bio-Rad assay using bovine serum albumin (BSA) as the standard. Microsomes were preincubated with 1, 8, or 25 μg of anti-human CYP4F2 IgG antibody or 25 μg of preimmune (control) IgG for 30 min on ice. TOH-ω-hydroxylase activity was determined as described previously (10), using 60 μm δ-TOH-BSA complex as substrate.
BLAST Comparison of Human CYP4F2 with Murine CYP Sequences
A comparison of the protein sequence of human CYP4F2 (NCBI accession number AAC27730.1) with that of all reported murine proteins was conducted using the BLASTP program (blast.ncbi.nlm.nih.gov) and the RefSeq protein database. A BLAST comparison of the amino acid sequence of the putative substrate binding domain of CYP4F2 (residues 69–115 (16)) was also conducted. Two CYP enzymes reported to be expressed in murine liver, CYP4F14 and CYP4F15 (17), were selected for further investigation based on high levels of homology with human CYP4F2.
Cloning, Expression, and Assessment of TOH-ω-Hydroxylase Activity of CYP4F14 and CYP4F15
Total RNA was extracted from mouse liver using TRIzol reagent and reverse-transcribed using Superscript First Strand synthesis kit. CYP4F14 and CYP4F15 cDNA was amplified from mouse liver RNA by one-step RT-PCR using primers based on the published sequences of murine liver CYP4F14 cDNA (18) (GenBankTM accession number AB037541) and CYP4F15 (GenBankTM accession number BC021377). The cDNA was restricted and ligated into pCDNA3.1/Hygro+ vector using the HindIII and XhoI restriction enzymes, and correct sequences were confirmed by sequencing. The Cyp4f14 and Cyp4f15 genes (in the pCDNA3.1/Hygro+) were transfected into COS-7 cells. Forty eight hours post-transfection, cells were exposed to hygromycin (200 μg/ml). Discrete colonies were picked after about 3 weeks in the selection media. Expression of CYP4F14 and CYP4F15 protein was verified by Western blotting using anti-CYP4F2 antibody, which cross-reacts with other CYP4F (human) and CYP4F (mouse) enzymes due to the high sequence homology, according to the manufacturer.
Total cell membrane fractions from homogenates of COS-7 cultures were prepared by ultracentrifugation (100,000 × g). TOH-ω-hydroxylase activity was assessed in aliquots of COS-7 cell membranes using δ-TOH as substrate as described previously (10). Preparations were compared at similar CYP4F14/4F15 protein expression levels as determined by Western blot.
TOH-ω-Hydroxylase Activity and Sesamin Sensitivity of Murine CYP4F14 in COS-7 Cells
A COS-7 clone expressing CYP4F14 was incubated with a mixture of 20 μm each α-TOH, γ-TOH, and δ-TOH, with or without 1 μm sesamin, for 48 h. Media were extracted and assayed for the presence of ω-oxidation metabolites of the three substrate tocopherols by GC-MS as described previously (19).
Generation of Cyp4f14-null Mice
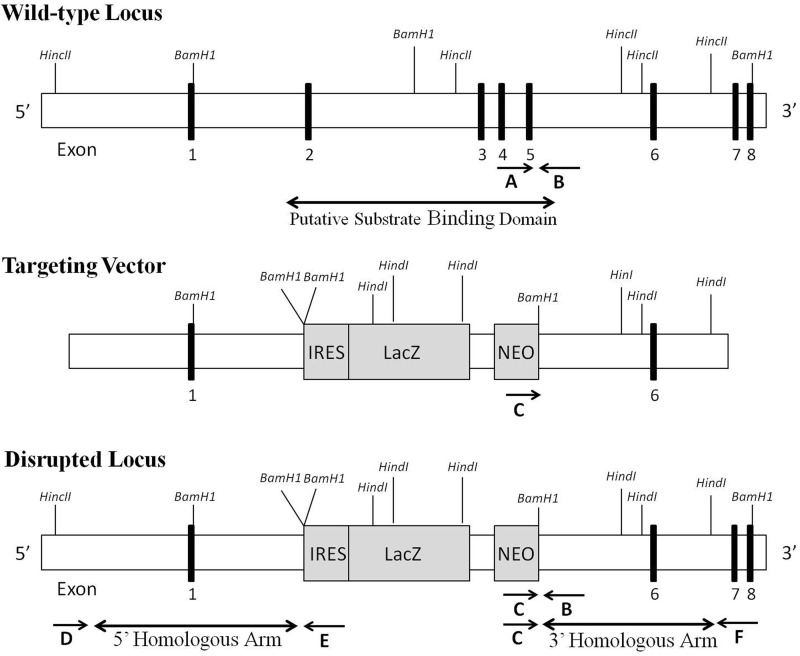
The gene targeting vector was designed to target exons 2–5, which includes the putative substrate binding domain of CYP4F14 (16), with the insertion of an IRES/LacZ/Neo/poly(A) cassette to allow the tracing of gene expression (Fig. 1). Murine 129/SvEv embryonic stem cells were transfected, and integration of the genomic construct at the predicted site within the Cyp4f14 locus was confirmed by long distance PCR using probes external to the targeted region (3′ homologous arm and 5′ homologous arm). Following ES cell expansion and karyotyping, selected clones were microinjected into C57BL/6 blastocysts and implanted into pseudopregnant female mice. Male germ line chimeras carrying a Cyp4f14 mutant allele were backcrossed with wild-type C57BL/6J females. Heterozygous F1 progeny were intercrossed to obtain F2 mice that were homozygous wild-type (Cyp4f14+/+), heterozygous null (Cyp4f141+/−), and homozygous null (Cyp4f14−/−) for the targeted allele. Use of mice was in accordance with protocols approved by the Cornell Institutional Animal Care and Use Committee and following the National Institutes of Health guidelines for laboratory animal use.
FIGURE 1.
Strategy of disruption of Cyp4f14 in the mouse. Genomic structure of Cyp4f14 locus (top panel), the Cyp4f14 gene targeting construct containing a IRES/LacZ/Neo/Poly(A) cassette (middle panel), and the disrupted locus in which exons 2–5 have been replaced (bottom panel). The arrows marked A–C show the location of primers used for confirmation of germ line transmission and tail snip genotyping. The arrows marked C–F indicate the location of primers used for long distance PCR analysis, confirming the integration of the targeting construct into the Cyp4f14 locus.
Genotyping of Cyp4f14-deficient Mice
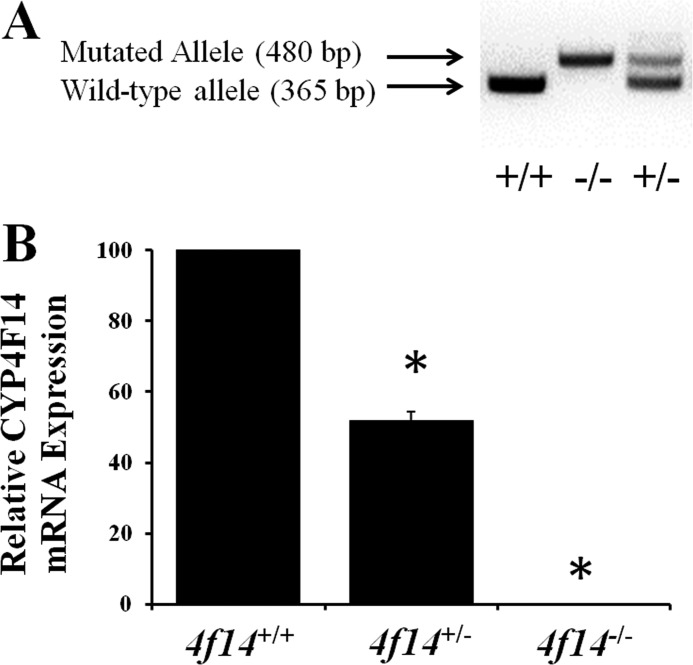
Genomic DNA was prepared from 0.2-cm tail snips using the HotSHOT method (20). Genomic DNA (50–200 ng) was used directly for PCR amplification with Platinum PCR SuperMix High Fidelity. Wild-type primers CTGGACATGTTTGAGCACG (forward; primer A) and GAACCACAGGCTAAGTCCATC (reverse; primer B) and a primer specific for the neomycin insert TCTGGATTCATCGACTGTGG (forward; primer C) were used for PCR amplification. The cycling protocol was as follows: 94 °C, 2 min (1 cycle); 94 °C, 15 s; 60 °C, 30 s; 68 °C, 1 min (35 cycles), and 68 °C, 7 min (1 cycle). The PCR products were separated on a 2% agarose gel yielding a 365-bp band for wild-type mice and a 480-bp band for Cyp4f14-null mice (Fig. 3A).
FIGURE 3.
Cyp4f14 gene expression in mouse liver. A, PCR genotyping of Cypf14 animals. Tail DNA was extracted from F2 littermates of a heterozygous cross and subjected to PCR using primers specific for the wild-type (365 bp) and mutated (480 bp) alleles. B, relative CYP4F14 mRNA in liver of Cyp4f14+/+, Cyp4f14+/−, and Cyp4f14−/− mice. CYP4F14 mRNA in Cyp4f14+/− mice was ∼50% of wild-type mice and undetectable in Cyp4f14−/− mice (p < 0.05). Asterisks indicate significant differences from wild type (p < 0.05).
Experimental Diets
Initial studies were conducted in mice fed Teklad 7912, a commercial chow-type diet containing low concentrations of δ- and γ-TOH. Subsequent studies utilized a modified AIN-93G semipurified diet designed to be rich in γ-TOH in the form of soybean oil, the most prevalent vegetable oil in North America. Samples of diets were ground using a mortar and pestle, and lipids were extracted using hexane/methyl tert-butyl ether (MTBE) (2:1). Lipid extracts were dried and derivatized using pyridine and N,O-bis(trimethylsilyl)trifluoroacetamide, 1% trimethylsilyl group, and tocopherols were quantified by GC-MS using d9-α-TOH as internal standard. Tocopherol content of the chow and soybean oil diets, hereafter referred to as γT-10 and γT-165 denoting their respective concentration of γ-TOH, is presented in Table 1.
TABLE 1.
Dietary tocopherol composition
Lipids were extracted from each diet, and vitamin E was quantified by GC-MS using d9-α-TOH as internal standard.
| Diet | Vitamin E |
|||
|---|---|---|---|---|
| δ-TOH | γ-TOH | α-TOH | α-T acetate | |
| mg/kg | ||||
| γT-10 diet | 9 | 10 | 10 | 150 |
| γT-165 diet | 83 | 165 | 12 | 0 |
Feeding Studies
Mice of all three genotypes weaned onto the γT-10 diet at 21 days of age were fed the diet for 3 weeks. Cyp4f14+/+ and Cyp4f14−/− mice weaned onto the γT-165 diet were fed the diet for 6 weeks. At the end of the feeding periods, mice were deeply anesthetized by isoflurane inhalation and exsanguinated via cardiac puncture. Heparinized blood was centrifuged at 6,000 × g for 5 min and serum frozen at −80 °C until analysis. Liver tissue samples were flash-frozen in liquid nitrogen for RT-PCR and vitamin E analysis. The remainder of the liver was immediately used for microsomal preparation as described above. Samples of additional tissues (kidney, lung, heart, testis, muscle (psoas major), and abdominal fat) were flash-frozen for vitamin E analysis.
24-Hour Urine and Fecal Collections
Cyp4f14+/+ and Cyp4f14−/− mice fed γT-10 or γT-165 diets were placed in wire bottom polycarbonate metabolic cages in which urine and fecal pellets could be collected separately. Mice were afforded access to food and water, and collections were made over 24 h following a 24-h acclimation period.
CYP4F14 Expression
Total RNA was extracted from flash-frozen tissue liver samples (10–30 mg). Tissue samples were homogenized in 600 μl of RLT containing (1% v/v) β-mercaptoethanol using a rotor-stator homogenizer. Homogenized samples were centrifuged at 10,000 rpm for 3 min to remove debris, and the supernatants were loaded onto QIAshredder spin columns followed by RNA isolation using the RNeasy mini kit, including an on-column DNA digestion step. RNA was reverse-transcribed using High Capacity cDNA Reverse transcription kit according to the manufacturer's instructions. The cDNA levels were detected using Taqman Universal Master Mix using LightCycler 480 II (Roche Applied Science) with pre-designed TaqMan assays for CYP4F14 (Mm00491623_m1) and CYP3All (Mm00731567_m1), using eukaryotic 18 S RNA (Hs99999901_s1) as the reference gene. Amplification conditions were as follows: 50 °C, 2 min; 95 °C, 10 min (1 cycle); 95 °C, 15 s; 60 °C, 1 min (40 cycles); 37 °C, 10 s (1 cycle). The percent remaining expression of each target mRNA relative to 18 S mRNA was calculated based on the threshold cycle (Ct) as 2−Δ(ΔCt), where ΔCt = Cttarget − Ct18 S and Δ(ΔCt) = ΔCthet − ΔCtwt or ΔCtko − ΔCtwt, where het is heterozygote.
Microsomal Vitamin E-ω-Hydroxylase Activity
Microsomal vitamin E-ω-hydroxylase activity was assayed using γ-TOH, δ-TOH, α-TOH, and γ-T3 as substrates complexed with BSA as described (8). The reaction system (0.5 ml) consisted of 0.1 m NaP buffer (containing 0.1 mmol/liter EDTA, pH 7.4), 1.0 mm NADPH, and 25 μg of microsomal protein. Substrate concentrations were 80 μmol/liter δ-TOH, 80 μmol/liter γ-TOH, 250 μmol/liter α-TOH, and 20 μmol/liter γ-T3 to account for differences in microsomal uptake of each form of vitamin E (8). Following incubation at 37 °C for 15 and 30 min, hydroxylation products were extracted, derivatized, and quantitated by GC-MS as described previously (8), using d9-α-TOH as internal standard. Product formation for all substrates was linear over the 0–30-min period, the slope of which was used to compare enzyme activity between treatments.
Urine and Fecal Analysis of Tocopherols and Metabolites
24-Hour urine and fecal samples from Cyp4f14+/+ and Cyp4f14−/− mice fed the γT-10 diet (n = 4) and mice fed the γT-165 diet (n = 6) were incubated with β-glucuronidase (800 units for urine and 1,600 units for feces dissolved in NaP buffer, pH 6.8) and sulfatase (0.4 units for urine, 0.8 units for feces) for 2 h at 37 °C. Samples were acidified to pH 2 with 3 n HCl, extracted with hexane/MTBE (3:1), and derivatized as above. Tocopherols and their metabolites were quantified by GC-MS using d9-α-TOH and d9-α-carboxyethylhydroxychroman as internal standards.
Tocopherol Quantification in Tissue and Plasma
Solid tissue samples were homogenized in 1 ml of 0.9% NaCl, 2 ml of isopropyl alcohol, and 1 ml of MTBE using a Polytron homogenizer, after which 3 ml of hexane was added, and the mixture was shaken and then centrifuged to separate organic and aqueous layers. The organic layer was evaporated to dryness using N2 gas and derivatized as described above. Plasma (40 μl) was extracted with 80 μl of ethanol, 100 μl of MTBE, and 1 ml of hexane. Tocopherols were quantified using GC-MS with d9-α-TOH as internal standard.
GC-MS Quantification of Tocopherols and Metabolites
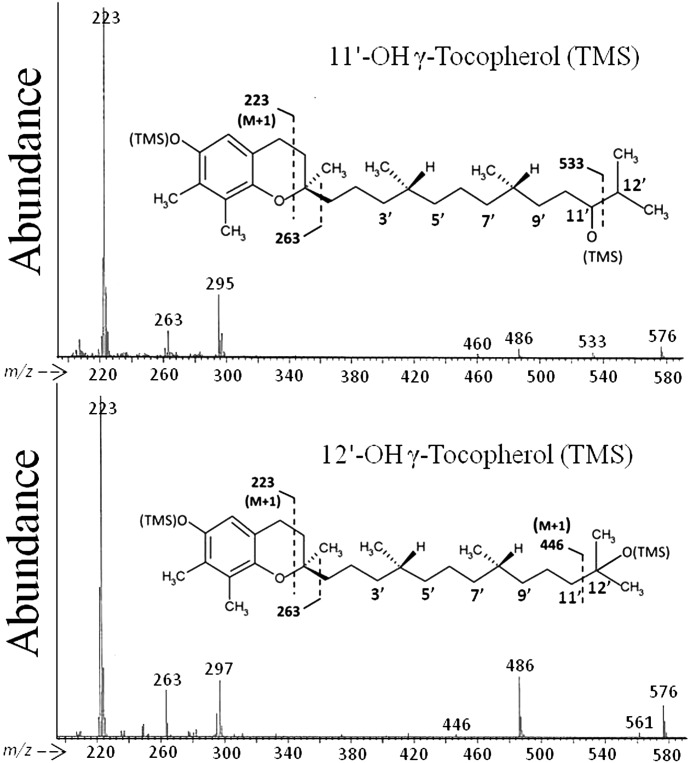
A Hewlett-Packard 6890 gas chromatograph (GC) coupled to a Hewlett-Packard 5872 mass selective detector, operated in selected ion mode, was used for quantification of the various forms of vitamin E and their metabolites. The gas chromatograph was fitted with a Hewlett-Packard HP-1 methylsiloxane capillary column (30 m × 0.25 μm) and operated in split injection mode using helium as the carrier gas. Plasma tocopherols and microsomal ω-oxidation metabolites were resolved isothermally at 280 °C. Analysis of diet, tissue, and fecal tocopherols and urinary and fecal tocopherol metabolites was conducted using a temperature program of 200 °C for 2 min, ramped to 250 °C at 7 °C/min, held 6 min, then ramped to 280 °C at 25 °C/min, and then held for 16 min. Novel 11′-OH and 12′-OH metabolites of γ- and δ-TOH were identified by their mass spectra using the same temperature program with the instrument operated in scan mode.
Statistical Analysis
A goodness of fit χ2 test was performed to test whether offspring of Cyp4f14+/− breeding pairs were produced in a Mendelian ratio. For RT-PCR data, the average 18 S-normalized threshold cycle (ΔCt) for each target gene was compared between the wild-type, heterozygote, and knock-out samples using Wilcoxon 2 group test, which yielded the estimation of ΔΔCt. Because of the small sample size, a nonparametric test followed by a Bonferonni correction for multiple comparisons was used to compare ΔΔCt between genotype groups. Microsomal enzyme activity data (pmol of 13′-OH product/min/mg of protein) and in vivo total ω-oxidation metabolite excretion (urine + fecal) were log-transformed to meet the assumptions of the statistical tests used. Differences in enzyme activity and ω-oxidation metabolite excretion between genotypes were tested using one-way analysis of variance for each tocopherol substrate, with post hoc multiple comparison corrections using a Tukey's correction.
Mean percentage reductions in enzyme activity and total ω-oxidation metabolite excretion were calculated by comparing individual Cyp4f14−/− values with the mean Cyp4f14+/+ value. These percentage reductions were compared between each tocopherol substrate using a nonparametric Wilcoxon 2 group test, followed by Bonferonni correction for multiple comparisons. 24-Hour urine and fecal metabolite and tocopherol excretion data were log-transformed as necessary. Means or transformed means were compared between genotypes using Student's t test. As necessary, nontransformed data were compared using a nonparametric Wilcoxon two-group test. Plasma and tissue α-, γ-, and δ-TOH data were log-transformed for comparison between genotypes using one-way ANOVA, using Tukey's post hoc multiple comparison correction when all three genotypes were compared. All tests were two-sided, and a p value of <0.05 was considered statistically significant. Analyses were performed using JMP version 8 (SAS Institute).
RESULTS
Identification of CYP4F14 as a Murine TOH-ω-Hydroxylase
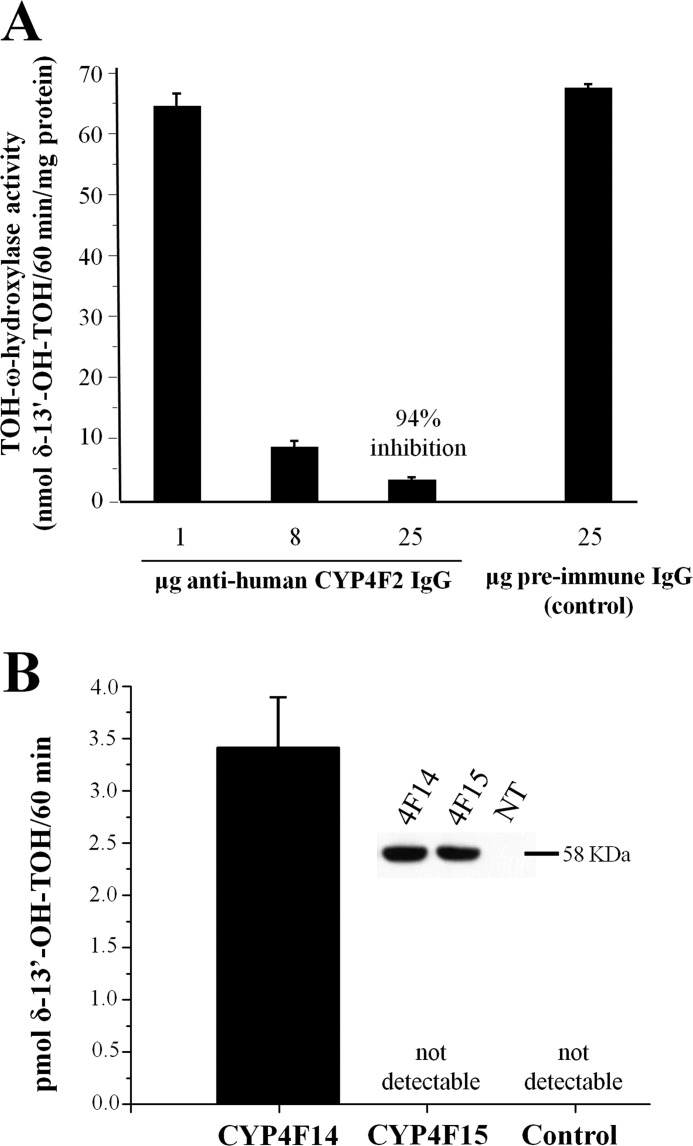
Treatment of mouse liver microsomes with anti-human CYP4F2 antibodies inhibited TOH-ω-hydroxylase activity by 94% compared with control (Fig. 2A), indicating that virtually all TOH-ω-hydroxylase activity in mouse liver microsomes resulted from CYP4F activity. An ortholog search revealed two murine proteins with >80% sequence identity to that of human CYP4F2 protein, namely CYP4F15 and CYP4F14. Both genes were reported to be expressed in murine liver (17). Therefore, both genes were amplified from mouse liver cDNA, cloned into a mammalian expression vector, and transfected into COS-7 cells that exhibit no detectable endogenous TOH-ω-hydroxylase activity. Cell lines that stably express CYP4F15 and CYP4F14 were then selected and propagated. Anti-CYP4F2 Western blotting of the stably transfected cells showed immunoreactive bands at the expected molecular mass of 58 kDa that was not observed in nontransfected controls (Fig. 2B, inset). These data confirmed that the selected cell lines expressed the two CYP enzymes at similar levels.
FIGURE 2.
Evidence that CYP4F14 is a functional murine vitamin E ω-hydroxylase. A, inhibition of mouse microsomal tocopherol-ω-hydroxylase activity by anti-4F antibody. Mouse liver microsomes were incubated with the indicated concentrations of anti-human CYP4F2 IgG or preimmune (control) IgG and 60 μm δ-TOH. Formation of 13′-OH-δ-TOH was quantified by GC-MS and normalized to microsomal protein content. B, assessment of TOH-ω-hydroxylase activity in COS-7 cell membranes. Total cell membrane preparations from COS-7 cells expressing CYP4F14 or CYP4F15 were incubated with 60 μm δ-TOH, and production of 13′-OH-δ-TOH was quantified by GC-MS. The inset shows an anti-CYP4F2 Western blot of the two preparations and of untransfected control (NT).
Membrane preparations from the stably transfected cell lines were assayed for TOH-ω-hydroxylase activity. Membranes from COS-7/Cyp4f14 cells exhibited robust TOH-ω-hydroxylase activity, whereas those prepared from COS-7/Cyp4f15 and nontransfected control cultures did not (Fig. 2B). In addition, ω-hydroxylase activity in COS-7/Cyp4f14 cultures exhibited a similar substrate selectivity (δ-TOH > γ-TOH > α-TOH) and inhibition by sesamin (data not shown) as human HepG2 and A549 cells, both of which express CYP4F2 and exhibit TOH-ω-hydroxylase activity (21, 22). Based on these findings, we concluded that CYP4F14 is a murine TOH-ω-hydroxylase, and we proceeded to examine its function in vivo by targeted disruption of its expression in the mouse.
Generation and Initial Characterization Cyp4f14-disrupted Mice
F1 Cyp4f14+/− mice were bred, generating a total of 90 F2 offspring whose genotype segregated in a Mendelian fashion (29% Cyp4f14−/−, 19% Cyp4f14+/+, and 52% Cyp4f14+/−). Cyp4f14−/− mice exhibited no overt anatomic abnormalities, and body weights of the three genotypes were similar irrespective of age or diet. Expression analysis using RT-PCR confirmed the absence of CYP4F14 mRNA in livers of Cyp4f14−/− mice and 50% reduction in Cyp4f14+/− mice (Fig. 3B). Hepatic expression of CYP3A11 was similar between genotypes in both experimental diet groups (data not shown).
Hepatic Vitamin E-ω-Hydroxylase Activity in Cyp4f14-disrupted Mice
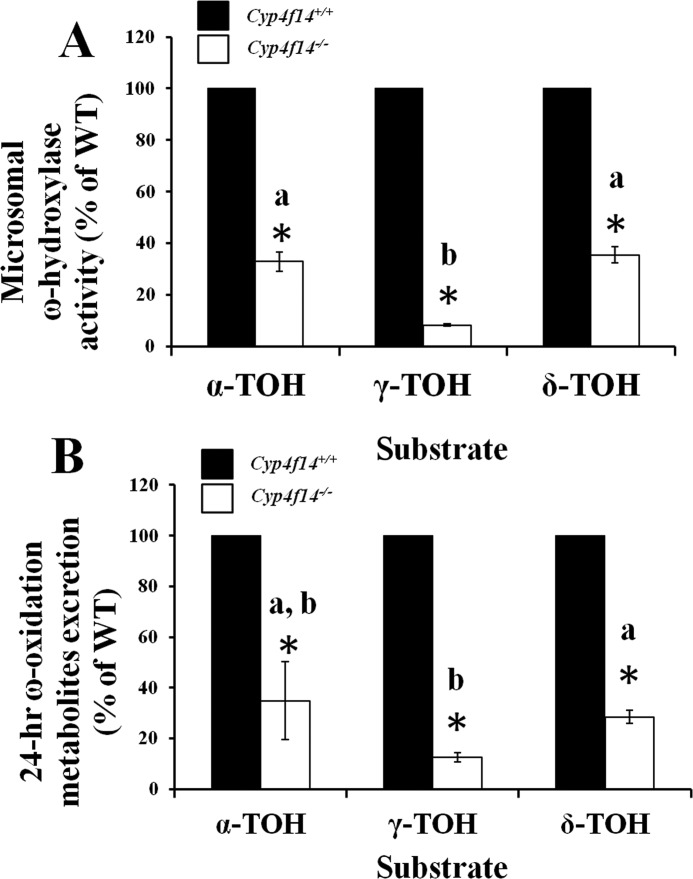
Hepatic microsomal vitamin E-ω-hydroxylase activity in mice fed the γT-10 diet was substantially reduced in Cyp4f14−/− mice relative to Cyp4f14+/+ mice. The extent of reduction varied among tocopherol substrates, with the largest reduction seen with γ-TOH (93%); activity toward α- and δ-TOH was 66 and 63% reduced, respectively. ω-Hydroxylation of γ-T3 was reduced by 48% in Cyp4f14−/− mice, and reductions in activity in Cyp4f14+/− mice were approximately half that of Cyp4f14−/− mice (data not shown). Similar results were obtained using microsomes from livers of mice fed the γT-165 diet (Fig. 4A).
FIGURE 4.
Impact of Cyp4f14 disruption on liver microsomal vitamin E-ω-hydroxylase activity and on whole-body excretion of ω-oxidation metabolites (urine + fecal). A, mouse liver microsomes from wild-type (solid bar) and Cyp4f14−/− (open bar) mice fed the γT-165 diet were incubated with the indicated tocopherol substrates (250 μmol/liter α-TOH and 80 μmol/liter γ- and δ-TOH) and the corresponding 13′-OH metabolites quantified by GC-MS. Values are percentage of wild-type preparations (n = 6). Wild-type activity was 21.0 ± 1.64, 88.1 ± 7.20, and 161.2 ± 11.7 pmol of 13′-OH product/min/mg protein toward α-TOH, γ-TOH, and δ-TOH, respectively. Reduction in activity was substrate-dependent, with the greatest impact (>90%) on ω-hydroxylation of γ-TOH. Asterisks indicate significant differences from wild type; different letters indicate significant differences in percent reduction between substrates (p < 0.05). B, 24-h urinary plus fecal excretion of total ω-oxidation metabolites (13′-OH, 13′-COOH, and all chain shortened forms) was quantified by GC-MS. Metabolite levels are expressed as percentages of values observed in wild-type mice (n = 6). Asterisks represent significant differences from wild type; different letters indicate significant differences in percent reduction between substrates (p < 0.05). Wild-type ω-hydroxy metabolite excretion was 0.39 ± 0.10, 109.3 ± 8.82, and 62.5 ± 7.99 nmol/24 h toward α-TOH, γ-TOH, and δ-TOH, respectively. The pattern of substrate-dependent impact of Cyp4f14 disruption was similar to that observed in microsomes (A).
Effect of Cyp4f14 Disruption on Urinary and Fecal Excretion of TOH-ω-Oxidation Metabolites
Metabolic cages were utilized to obtain 24-h urine and fecal samples from Cyp4f14+/+ and Cyp4f14−/− mice. In urine, only short chain metabolites (3′- and 5′-carboxychromanols) of dietary tocopherols were detected. In mice fed the γT-165 diet, urinary excretion of these two γ-TOH metabolites was reduced by about 90% in Cyp4f14−/− mice relative to their wild-type counterparts (Table 2). Smaller but still significant reductions in urinary output of short chain metabolites of α- and δ-TOH were observed in the Cyp4f14−/− mice. Similar results were obtained from mice on the γT-10 diet (data not shown).
TABLE 2.
24-Hour urinary metabolite excretion in Cyp4f14+/+ and Cyp4f14−/− mice
Mice were fed the γT-165 diet for 6 weeks after weaning. Values represent mean ± S.E. (n = 6).
| Urinary metabolitesa | |||||
|---|---|---|---|---|---|
| α-3′-COOH | γ-3′-COOH | γ-5′-COOH | δ-3′-COOH | δ-5′-COOH | |
| Cyp4f14+/+ | 0.39 ± 0.10 | 21.3 ± 2.79 | 19.9 ± 4.77 | 23.1 ± 1.47 | 16.4 ± 3.99 |
| Cyp4f14−/− | 0.14 ± 0.06b | 2.47 ± 0.32b | 1.58 ± 0.13b | 7.71 ± 1.02b | 3.45 ± 0.87b |
| % Reduction | 65% | 88% | 92% | 67% | 79% |
a Values are nmol/24 h.
b Data are significantly different from Cyp4f14+/+ mice (p < 0.04). The 5′-COOH metabolite of α-TOH was not detected.
Analysis of 24-h fecal samples revealed the presence of all six possible carboxychromanol metabolites and the 13′-OH metabolites of γ- and δ-TOH formed via the ω-oxidation pathway in mice fed the γT-165 diet. Disruption of Cyp4f14 expression resulted in significant reductions in all seven ω-oxidation metabolites of γ-TOH and the reduction of many δ-TOH metabolites (Table 3). Fecal levels of δ- and γ-TOH metabolites from mice fed the γT-10 diet were low and similar among genotypes (data not shown). The 13′-OH metabolite of α-TOH was the only appreciable α-TOH metabolite in the feces of both diet groups, and it was found at trace concentrations in both genotypes (data not shown).
TABLE 3.
24-Hour fecal metabolite excretion in Cyp4f14+/+ and Cyp4f14−/− mice
Mice were fed the γT-165 diet for 6 weeks after weaning. Values represent mean ± S.E. (n = 6).
| Cyp4f14 genotype | ω-Hydroxylase metabolite productsa |
ω-1,2 Metabolitesa |
Total metabolitesa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3′-COOH | 5′-COOH | 7′-COOH | 9′-COOH | 11′-COOH | 13′-COOH | 13′-OH | 11′-OH | 12′-OH | |||
| γ-TOH | +/+ | 6.1 ± 3.1 | 8.9 ± 2.0 | 0.9 ± 0.3 | 15.9 ± 1.9 | 5.2 ± 0.8 | 23.4 ± 1.8 | 7.8 ± 1.0 | 1.1 ± 0.2 | 17.6 ± 2.3 | 86.8 ± 8.0 |
| −/− | 0.8 ± 0.2b | 2.5 ± 0.9b | 0.2 ± 0.1b | 1.7 ± 0.3b | 1.7 ± 0.4b | 1.6 ± 0.2b | 1.2 ± 0.3b | 3.3 ± 0.9b | 41.3 ± 9.1b | 54.2 ± 11.6b | |
| δ-TOH | +/+ | 2.0 ± 1.3 | 5.2 ± 2.0 | 0.3 ± 0.1 | 2.2 ± 0.4 | 1.0 ± 0.1 | 6.5 ± 1.1 | 5.8 ± 1.0 | 2.3 ± 0.2 | 14.9 ± 2.7 | 37.3 ± 7.4 |
| −/− | 1.1 ± 0.4 | 2.0 ± 0.4 | 0.1 ± 0.0 | 0.5 ± 0.1b | 0.4 ± 0.1b | 0.5 ± 0.1b | 2.1 ± 0.4b | 3.2 ± 0.7 | 17.9 ± 3.0 | 27.7 ± 4.6 | |
a Values are nmol/24 h.
b Data are significantly different from Cyp4f14+/+ mice (p < 0.05). Metabolites of α-TOH were undetectable or at trace levels.
Whole-body 24-h excretion of total tocopherol metabolites generated via the ω-oxidation pathway (urinary plus fecal) in Cyp4f14+/+ and Cyp4f14−/− mice fed the γT-165 diet was calculated. Excretion of ω-oxidation metabolites of the three dietary tocopherols by Cyp4f14−/− mice was reduced to approximately the same extents (88, 72, and 65%, respectively, for γ-, δ-, and α-TOH) as those observed in the in vitro ω-hydroxylation assays (Fig. 4, A and B).
Fecal Excretion of Novel ω-1- and ω-2-Hydroxylation Products of γ- and δ-TOH
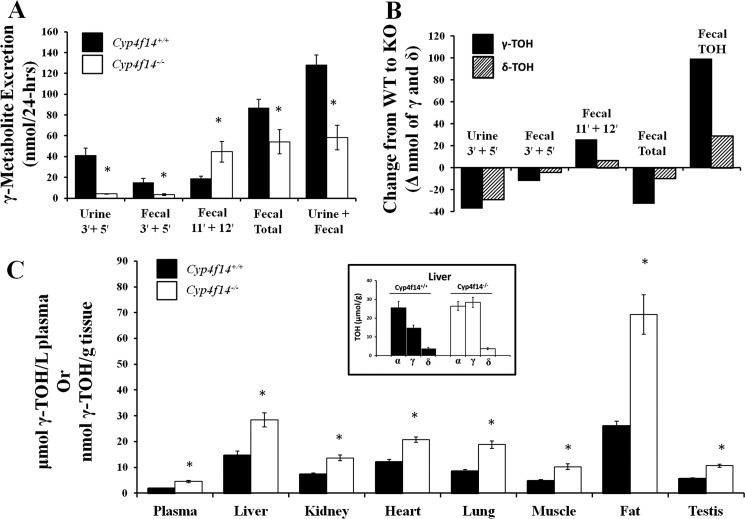
Two metabolites not previously reported to occur in vivo, 12′-OH and 11′-OH oxidation products of γ- and δ-TOH, were also observed in fecal samples. The structures of these metabolites were determined by mass spectrometry analysis (Fig. 5). These represent products of ω-1 and ω-2 hydroxylation activities, respectively. 12′-OH-γ-TOH was the most abundant γ-TOH metabolite in fecal samples of Cyp4f14−/− mice fed the γT-165 diet, representing ∼80% of total fecal γ-TOH metabolites, and was the second most abundant of all excreted γ-TOH metabolites in Cyp4f14+/+ mice. Interestingly, fecal excretion of 12′-OH-γ-TOH was significantly greater in Cyp4f14−/− mice (41.3 ± 9.1 nmol/day) than in Cyp4f14+/+ mice (7.6 ± 2.3 nmol/day, Table 3). Consequently, 24-h fecal output of total metabolites (sum of ω, ω-1, ω-2 metabolites of α-, γ-, and δ-TOHs) was reduced by only 34% in Cyp4f14−/− mice fed the γT-165 diet relative to Cyp4f14+/+ mice, whereas the analogous reduction in total urinary metabolites (3′- and 5′-COOH metabolites only) was 81% (Fig. 6A). In contrast, the excretion of the ω-1 and ω-2 metabolites of δ-TOH was not significantly increased in Cyp4f14−/− mice compared with Cyp4f14+/+ mice (Fig. 6B).
FIGURE 5.
Mass spectra of novel fecal ω-1- and ω-2-hydroxytocopherol metabolites. Lipid extracts of 24-h fecal samples from mice fed a soybean oil diet were derivatized and subjected to electron impact GC-MS analysis. Upper panel, 11′-OH-γ-tocopherol (trimethylsilyl derivative). Lower panel, 12′-OH-γ-tocopherol (trimethylsilyl derivative).
FIGURE 6.
Impact of Cyp4f14 disruption on whole-body TOH metabolism and on concentrations of γ-TOH in plasma and tissues. A, metabolites of γ-TOH in 24-h urine and fecal collections from wild-type mice (solid bar) and Cyp4f14−/− mice (open bar) were quantified by GC-MS. Asterisks indicate significant differences from wild-type mice. B, change (nanomoles) from wild-type mice to Cyp4f14−/− mice in 24-h urinary and fecal excretion of metabolites of γ-TOH (solid bar) and δ-TOH (hatched bar) or the parent tocopherols. Cyp4f14−/− mice counterbalance the loss of ω-oxidation capacity by increased fecal excretion of 11′-OH- and 12′-OH metabolites and parent tocopherols. C, concentration of γ-TOH in plasma and tissues of wild-type (solid bar) and Cyp4f14−/− mice (open bar) fed the γT-165 (soybean oil) diet for 6 weeks. Inset compares tocopherol composition of liver of wild-type and Cyp4f14-null mice. Asterisks indicate significant differences from wild-type mice (p < 0.05).
Fecal Excretion of Parent Tocopherols in Cyp4f14+/+ and Cyp4f14−/− Mice
Unmetabolized tocopherols were present in all 24-h fecal samples. In mice fed the γT-165 diet for 6 weeks, 24-h fecal tocopherol excretion was in the order of γ-TOH > δ-TOH > α-TOH, similar to their prevalence in the diet (Table 1). Fecal excretion of γ- and δ-TOHs by Cyp4f14−/− mice was approximately twice that of Cyp4f14+/+ mice (p < 0.05, Fig. 6B). Fecal excretion of γ-TOH increased from 137 nmol/24 h in wild-type mice to 239 nmol/24 -h in Cyp4f14−/− mice. The analogous values for δ-TOH were 32 and 63 nmol/24 h in wild-type and Cyp4f14−/− mice, respectively.
Effect of Cyp4f14 Disruption on Plasma and Tissue Tocopherol Concentrations
In Cyp4f14−/− mice fed the γT-165 diet for 6 weeks, concentrations of γ-TOH in plasma and tissues were approximately twice those of Cyp4f14+/+ mice (Fig. 6C). In the liver, concentrations of γ-TOH were similar, or in some mice higher, than those of α-TOH. Concentrations of δ-TOH were significantly elevated only in fat tissue, and those of α-TOH were significantly lower in heart tissue (Table 4). In Cyp4f14−/− mice fed the γT-10, liver γ-TOH concentrations, although low, were double those of Cyp4f14+/+ mice (p < 0.05); there were no differences between genotypes of concentrations of any tocopherol in other tissues (data not shown).
TABLE 4.
Tocopherol concentration of plasma and tissue of Cyp4f14+/+ and Cyp4f14−/− mice
Mice were fed the γT-165 diet for 6 weeks after weaning. Values represent mean ± S.E. (n = 6). Plasma, μmol/liter; tissues, nmol/g.
| Tissue | Genotype | Tocopherol concentration |
||
|---|---|---|---|---|
| α-TOH | γ-TOH | δ-TOH | ||
| Plasma | CYP4f14+/+ | 8.78 ± 0.34 | 1.89 ± 0.13 | 0.36 ± 0.07 |
| CYP4f14−/− | 9.55 ± 0.60 | 4.49 ± 0.39a | 0.45 ± 0.13 | |
| Liver | CYP4f14+/+ | 25.5 ± 3.27 | 14.7 ± 1.60 | 3.64 ± 0.69 |
| CYP4f14−/− | 26.4 ± 2.30 | 28.4 ± 2.75a | 3.70 ± 0.49 | |
| Kidney | CYP4f14+/+ | 28.4 ± 2.81 | 7.27 ± 0.50 | 1.41 ± 0.16 |
| CYP4f14−/− | 24.0 ± 1.88 | 13.7 ± 1.04a | 1.62 ± 0.29 | |
| Heart | CYP4f14+/+ | 53.6 ± 4.04 | 12.1 ± 1.06 | 3.21 ± 0.41 |
| CYP4f14−/− | 42.3 ± 1.03a | 20.8 ± 1.07a | 4.44 ± 1.17 | |
| Lung | CYP4f14+/+ | 41.5 ± 2.54 | 8.45 ± 0.70 | 1.49 ± 0.17 |
| CYP4f14−/− | 40.0 ± 2.28 | 18.8 ± 1.37a | 1.90 ± 0.19 | |
| Muscle | CYP4f14+/+ | 16.7 ± 1.29 | 4.79 ± 0.39 | 1.55 ± 0.30 |
| CYP4f14−/− | 17.0 ± 1.12 | 10.3 ± 1.17a | 1.62 ± 0.23 | |
| Fat | CYP4f14+/+ | 65.6 ± 6.68 | 26.0 ± 1.98 | 14.8 ± 1.03 |
| CYP4f14−/− | 85.7 ± 8.77 | 69.2 ± 7.67a | 22.1 ± 3.20 | |
| Testis | CYP4f14+/+ | 21.6 ± 1.16 | 5.58 ± 0.40 | 0.76 ± 0.05 |
| CYP4f14−/− | 20.9 ± 1.65 | 10.6 ± 0.53a | 0.98 ± 0.16 | |
a Data are significantly different from Cyp4f14+/+ mice (p < 0.02).
DISCUSSION
Tocopherols and tocotrienols undergo ω-hydroxylation at the terminal methyl groups of their hydrophobic side chains, the first step in their catabolism to short chain carboxychromanols that are excreted in the urine. In the human, TOH-ω-hydroxylation is catalyzed by CYP4F2, which selectively catabolizes forms of vitamin E other than α-TOH. The ω-hydroxylation pathway therefore likely contributes to the α-TOH phenotype, a highly conserved trait in animals. The preferential retention of α-TOH additionally arises from the selection of α-TOH by the tocopherol transfer protein, the absence of which results in severe vitamin E deficiency (23, 24). As all forms of vitamin E exhibit similar radical trapping antioxidant activity in vitro (1), the evolutionary advantage of the preferential accumulation of α-TOH and the physiological consequences of accumulation of other forms of vitamin E are unknown. In an effort to investigate these issues, we sought to identify the functional murine ortholog of CYP4F2 and determine the consequences of its deletion on vitamin E metabolism and status.
CYP4F14, one of five functional murine CYP4F enzymes, exhibited vitamin E-ω-hydroxylase activity when expressed in COS-7 cells. Like CYP4F2, CYP4F14 is expressed predominantly in the liver. CYP4F15, a highly homologous murine CYP also expressed in liver, had no detectable ω-hydroxylase activity. Disruption of Cyp4f14 by homologous recombination substantially reduced, but did not eliminate, vitamin E-ω-hydroxylase activity in murine liver microsomes. The reduction in activity was substrate-dependent, being greatest for γ-TOH (92%). These results indicate that at least one other vitamin E-ω-hydroxylase is expressed in the mouse liver. Plasma and tissue tocopherol concentrations in Cyp4f14−/− mice fed a diet low in γ- and δ-TOH were similar to those of wild-type mice except for the liver, where γ-TOH levels were slightly but significantly elevated. However, in mice fed a diet rich in γ-TOH (soybean oil), the metabolic deficit caused by Cyp4f14 deletion resulted in substantial elevation (2-fold) of γ-TOH concentrations in all tissues and in plasma. Notably, concentrations of γ-TOH in the liver were similar to or higher than those of α-TOH thereby disrupting the α-TOH phenotype in this tissue. Tissue and plasma levels of δ-TOH remained low and unchanged, presumably due to residual δ-TOH-ω-hydroxylase activity sufficient to catabolize the modest amount of δ-TOH in the diet. Accumulation of γ-TOH in Cyp4f14−/− mice did not result in any apparent physiologically abnormal phenotype. A 2–3-fold increase in tissue γ-TOH levels significantly reduced protein nitration and ascorbic acid oxidation in an experimental model of inflammation (25). Therefore, a model in which non-α-TOH forms of vitamin E accumulate to a greater degree than normal will allow for the investigation of the biological activities of these compounds in regard to disease development, prevention, and treatment.
The impact of Cyp4f14 disruption on whole-body tocopherol metabolism was investigated by analysis of 24-h urine and fecal samples. Urine contained only the shortest chain (3′ and 5′) carboxychromanol metabolites, whereas the feces contained the full spectrum of known metabolites of δ- and γ-TOHs formed via the ω-hydroxylase pathway, with the longer chain metabolites predominating. These results demonstrate that short chain carboxychromanols are directed to secretion from the liver into the bloodstream and filtered by the kidney, whereas the longer and more hydrophobic metabolites are directed to biliary secretion and excretion via the feces. One major impact of Cyp4f14 disruption was to dramatically alter the proportion of total metabolites excreted via urinary versus fecal routes. In wild-type mice, 60% of total metabolite output was eliminated via the feces, whereas in Cyp4f14−/− mice, in which urinary short chain carboxychromanols were drastically reduced, this proportion rose to 85%.
Novel ω-1- (12′) and ω-2 (11′)-hydroxyl metabolites of δ- and γ-TOH were identified in fecal pellets of mice fed the γT-165 diet. These apparently arise in liver and are secreted in bile, as they were detected in the few bile samples able to be obtained from animals fed this diet (data not shown). The 12′-OH metabolite is a terminal metabolite in that it cannot undergo oxidation to the corresponding 12′-keto form, and therefore cannot undergo side chain truncation. The metabolic fate of the 11′-OH form remains uncertain. Because we have been unable to confirm the presence of the corresponding 11′-keto form, we assume that it does not materially contribute to the formation of shorter chain carboxychromanols excreted in either feces or urine. Formation of these novel metabolites constituted a significant alternative pathway of metabolism of γ- and δ-TOHs, increasing from 30% of total fecal metabolites in wild-type mice to 80% in Cyp4f14-null mice.
Fecal excretion of 11′-OH and 12′-OH metabolites of γ- TOH was increased substantially in Cyp4f14−/− mice, thereby partially counterbalancing the loss of CYP4F14 activity. This additionally contributed to the impact of Cyp4f14 disruption on the shift of tocopherol metabolite excretion from the urinary route to the fecal route and from short chain metabolites to longer chain metabolites. At present it is not known whether the increase in excretion of these ω-1 and ω-2 metabolites resulted from increased activity of a yet unidentified enzyme or from increased availability of substrate secondary to the loss of CYP4F14. This increase apparently did not involve the induction of CYP3A11 because its expression was not altered by disruption of Cyp4f14. Cyp4f14−/− mice also exhibited increased fecal excretion of parent δ- and γ-TOHs, representing a second means of counterbalancing for the decrement in catabolic capacity. Whether this results from decreased intestinal absorption or increased biliary secretion of tocopherols is unknown as few animals presented with sufficient gallbladder bile for collection. Nevertheless, the combination of these counterbalancing effects was insufficient to prevent substantial tissue accumulation of γ-TOH in mice fed the soybean oil diet.
Given the importance of α-TTP in the trafficking of α-TOH in the liver, we quantified α-TTP expression by Western blotting as a function of both diet and genetic background. α-TTP was visualized by Western blotting using a rabbit polyclonal antibody raised against the full-length human protein, purified from overexpressing E. coli. Neither variable influenced hepatic α-TTP expression (data not shown).
These studies revealed the heretofore unappreciated quantitative importance of fecal excretion of tocopherols and tocopherol metabolites as a mechanism for preventing elevation of tocopherol concentrations in serum and tissues. In wild-type mice, up to 80% of whole-body output of vitamin E (sum of intact tocopherols and their metabolites) occurred via the feces. This value rose to 96% in the Cyp4f14-null animals. Whereas Zhao et al. (26) reported the existence of many tocopherol metabolites in feces, to our knowledge there are no reports of the quantitative contribution of the fecal route to vitamin E status. In addition to the novel ω-1 and ω-2 metabolites reported here, this is the first report of the presence of significant amounts of 13′-OH tocopherol metabolites in feces. How these latter metabolites escape further side chain oxidation is unclear, but it suggests that yet-unknown mechanisms are present in the liver that direct these long chain metabolites to biliary secretion.
These studies investigated the effects of Cyp4f14 deletion on vitamin E metabolism from two perspectives, in vitro liver microsomal P450-mediated tocopherol hydroxylation and in vivo metabolic capacity as measured by 24-h excretion of tocopherol metabolites in urine and feces. The substrate-dependent decrement resulting from Cyp4f14 deletion was remarkably similar between microsomal and whole-body measurements of tocopherol ω-oxidation (Fig. 4). This observation indicates that the liver plays a pivotal role in whole-body vitamin E ω-oxidation that can be qualitatively predicted by microsomal ω-hydroxylation activity. Interestingly, only small amounts of the 12′-OH metabolites of γ- and δ-TOHs were produced in the microsomal system (data not shown), although they composed a major fraction of tocopherol metabolites in vivo. The reason for this discrepancy is presently unknown.
It is unknown whether the redundancy in the catabolic oxidation of vitamin E evident in mice also exists in humans. The human CYP3A4 has been hypothesized to be involved in vitamin E metabolism based on the finding that supplemental dietary α-TOH in mice leads to increased mRNA expression of its putative ortholog, CYP3A11, but not that of mouse CYP4F enzymes (27). However, evaluation of all of the major human liver CYP enzymes, including CYP3A4, showed that CYP4F2 was the only CYP enzyme to exhibit ω-hydroxylase activity toward vitamin E (10). Additionally, conditions expected to alter CYP4F2 activity result in changes in vitamin E status. For example, short term ingestion of sesame seeds, which contain sesamin, an inhibitor of CYP4F2 (28), results in elevation of serum γ-TOH (29). A recent genome-wide association study reported that the V433M variant of CYP4F2 was positively associated with plasma vitamin E concentration (30). We previously reported that this variant exhibited a reduction in enzyme-specific activity by 40–60% depending on the substrate (31). Our heterozygous mice, which display a 50% reduction in vitamin E-ω-hydroxylase activity, exhibit a 50% increase in tissue γ-TOH concentrations. Taken together, these findings suggest the importance of CYP4F2 as a major regulator of vitamin E composition of human plasma and tissues.
In summary, our data demonstrate that CYP4F14 is the major, but not the only, vitamin E-ω-hydroxylase in mice and has critical function in regulating body-wide vitamin E status. Disruption of Cyp4f14 expression resulted in hyper-accumulation of γ-TOH in plasma and tissues in mice fed a diet similar in tocopherol composition to that consumed by many human populations. This accumulation occurred despite the combined counterbalancing effects of increased fecal excretion of novel ω-1- and ω-2-tocopherol oxidation metabolites and increased fecal excretion of parent tocopherols. Redundancy in vitamin E-ω-hydroxylase activity and the existence of counterbalancing mechanisms imply a fundamental biological advantage of the α-TOH phenotype that remains elusive.
Acknowledgments
We thank Francoise Vermeylen of the Cornell Statistical Consulting Unit for providing valuable statistical support and Dr. Lynn Ulatowski for α-TTP Western blotting.
This work was supported, in whole or in part, by National Institutes of Health Grants DK067494 (to D. M.) and DK007158 (to S. A. B.).
- TOH
- tocopherol
- CYP4F2
- cytochrome P450 4F2
- α-TTP
- α-tocopherol transfer protein
- T3
- tocotrienol
- NaP
- sodium phosphate
- MTBE
- methyl tert-butyl ether
- LTB4
- leukotriene B4.
REFERENCES
- 1. Yoshida Y., Niki E., Noguchi N. (2003) Comparative study on the action of tocopherols and tocotrienols as antioxidant. Chemical and physical effects. Chem. Phys. Lipids 123, 63–75 [DOI] [PubMed] [Google Scholar]
- 2. Saito Y., Yoshida Y., Akazawa T., Takahashi K., Niki E. (2003) Cell death caused by selenium deficiency and protective effect of antioxidants. J. Biol. Chem. 278, 39428–39434 [DOI] [PubMed] [Google Scholar]
- 3. Dial S., Eitenmiller R. (1995) in Nutrition, Lipids, Health, and Disease (Ong A. S. H., Niki E., Packer L., eds) pp. 327–342, AOCS Press, Champaign, IL [Google Scholar]
- 4. Bieri J. G., Evarts R. P. (1973) Tocopherols and fatty acids in American diets. The recommended allowance for vitamin E. J. Am. Diet. Assoc. 62, 147–151 [PubMed] [Google Scholar]
- 5. Ford E. S., Schleicher R. L., Mokdad A. H., Ajani U. A., Liu S. (2006) Distribution of serum concentrations of α-tocopherol and γ-tocopherol in the United States population. Am. J. Clin. Nutr. 84, 375–383 [DOI] [PubMed] [Google Scholar]
- 6. Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., Inoue K. (1997) Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 409, 105–108 [DOI] [PubMed] [Google Scholar]
- 7. Traber M. G., Sokol R. J., Burton G. W., Ingold K. U., Papas A. M., Huffaker J. E., Kayden H. J. (1990) Impaired ability of patients with familial isolated vitamin E deficiency to incorporate α-tocopherol into lipoproteins secreted by the liver. J. Clin. Invest. 85, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sontag T. J., Parker R. S. (2007) Influence of major structural features of tocopherols and tocotrienols on their ω-oxidation by tocopherol-ω-hydroxylase. J. Lipid Res. 48, 1090–1098 [DOI] [PubMed] [Google Scholar]
- 9. Parker R. S., McCormick C. C. (2005) Selective accumulation of α-tocopherol in Drosophila is associated with cytochrome P450 tocopherol-ω-hydroxylase activity but not α-tocopherol transfer protein. Biochem. Biophys. Res. Commun. 338, 1537–1541 [DOI] [PubMed] [Google Scholar]
- 10. Sontag T. J., Parker R. S. (2002) Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 277, 25290–25296 [DOI] [PubMed] [Google Scholar]
- 11. Schönfeld A., Schultz M., Petrizka M., Gassmann B. (1993) A novel metabolite of RRR-α-tocopherol in human urine. Nahrung 37, 498–500 [DOI] [PubMed] [Google Scholar]
- 12. Wechter W. J., Kantoci D., Murray E. D., Jr., D'Amico D. C., Jung M. E., Wang W. H. (1996) A new endogenous natriuretic factor, LLU-α. Proc. Natl. Acad. Sci. U.S.A. 93, 6002–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swanson J. E., Ben R. N., Burton G. W., Parker R. S. (1999) Urinary excretion of 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman is a major route of elimination of γ-tocopherol in humans. J. Lipid Res. 40, 665–671 [PubMed] [Google Scholar]
- 14. Nishimura M., Yaguti H., Yoshitsugu H., Naito S., Satoh T. (2003) Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high sensitivity real time reverse transcription PCR. Yakugaku Zasshi 123, 369–375 [DOI] [PubMed] [Google Scholar]
- 15. Kikuta Y., Kusunose E., Kusunose M. (2002) Prostaglandin and leukotriene ω-hydroxylases. Prostaglandins Other Lipid Mediat. 68, 345–362 [DOI] [PubMed] [Google Scholar]
- 16. Christmas P., Jones J. P., Patten C. J., Rock D. A., Zheng Y., Cheng S. M., Weber B. M., Carlesso N., Scadden D. T., Rettie A. E., Soberman R. J. (2001) Alternative splicing determines the function of CYP4F3 by switching substrate specificity. J. Biol. Chem. 276, 38166–38172 [DOI] [PubMed] [Google Scholar]
- 17. Christmas P., Tolentino K., Primo V., Berry K. Z., Murphy R. C., Chen M., Lee D. M., Soberman R. J. (2006) Cytochrome P-450 4F18 is the leukotriene B4 ω-1/ω-2-hydroxylase in mouse polymorphonuclear leukocytes. Identification as the functional ortholog of human polymorphonuclear leukocyte CYP4F3A in the down-regulation of responses to LTB4. J. Biol. Chem. 281, 7189–7196 [DOI] [PubMed] [Google Scholar]
- 18. Kikuta Y., Kasyu H., Kusunose E., Kusunose M. (2000) Expression and catalytic activity of mouse leukotriene B4 ω-hydroxylase, CYP4F14. Arch. Biochem. Biophys. 383, 225–232 [DOI] [PubMed] [Google Scholar]
- 19. Parker R. S., Swanson J. E. (2000) A novel 5′-carboxychroman metabolite of γ-tocopherol secreted by HepG2 cells and excreted in human urine. Biochem. Biophys. Res. Commun. 269, 580–583 [DOI] [PubMed] [Google Scholar]
- 20. Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., Warman M. L. (2000) Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). BioTechniques 29, 52, 54 [DOI] [PubMed] [Google Scholar]
- 21. You C. S., Sontag T. J., Swanson J. E., Parker R. S. (2005) Long-chain carboxychromanols are the major metabolites of tocopherols and tocotrienols in A549 lung epithelial cells but not HepG2 cells. J. Nutr. 135, 227–232 [DOI] [PubMed] [Google Scholar]
- 22. Yamane M., Abe A. (2000) ω-Hydroxylation activity toward leukotriene B4 and polyunsaturated fatty acids in the human hepatoblastoma cell line, HepG2, and human lung adenocarcinoma cell line, A549. J. Biochem. 128, 827–835 [DOI] [PubMed] [Google Scholar]
- 23. Manor D., Morley S. (2007) The α-tocopherol transfer protein. Vitam. Horm. 76, 45–65 [DOI] [PubMed] [Google Scholar]
- 24. Traber M. G., Arai H. (1999) Molecular mechanisms of vitamin E transport. Annu. Rev. Nutr. 19, 343–355 [DOI] [PubMed] [Google Scholar]
- 25. Jiang Q., Lykkesfeldt J., Shigenaga M. K., Shigeno E. T., Christen S., Ames B. N. (2002) γ-Tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation, Free Radic. Biol. Med. 33, 1534–1542 [DOI] [PubMed] [Google Scholar]
- 26. Zhao Y., Lee M. J., Cheung C., Ju J. H., Chen Y. K., Liu B., Hu L. Q., Yang C. S. (2010) Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans. J. Agric. Food Chem. 58, 4844–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mustacich D. J., Gohil K., Bruno R. S., Yan M., Leonard S. W., Ho E., Cross C. E., Traber M. G. (2009) α-Tocopherol modulates genes involved in hepatic xenobiotic pathways in mice. J. Nutr. Biochem. 20, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parker R. S., Sontag T. J., Swanson J. E. (2000) Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 277, 531–534 [DOI] [PubMed] [Google Scholar]
- 29. Cooney R. V., Custer L. J., Okinaka L., Franke A. A. (2001) Effects of dietary sesame seeds on plasma tocopherol levels. Nutr. Cancer 39, 66–71 [DOI] [PubMed] [Google Scholar]
- 30. Major J. M., Yu K., Wheeler W., Zhang H., Cornelis M. C., Wright M. E., Yeager M., Snyder K., Weinstein S. J., Mondul A., Eliassen H., Purdue M., Hazra A., McCarty C. A., Hendrickson S., Virtamo J., Hunter D., Chanock S., Kraft P., Albanes D. (2011) Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 20, 3876–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bardowell S. A., Stec D. E., Parker R. S. (2010) Common variants of cytochrome P450 4F2 exhibit altered vitamin E-ω-hydroxylase specific activity. J. Nutr. 140, 1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]