Background: The Na,K-ATPase consists of one α (four isoforms) and one β (three isoforms) subunits.
Results: The α1 preferentially assembles with β1, whereas α2 preferentially binds to β2 isoform.
Conclusion: Assembly of α-β complexes is isoform-selective.
Significance: This selectivity is crucial for cell- and tissue-specific functions of the Na,K-ATPase.
Keywords: Cell Adhesion; ER Quality Control; ER Stress; Membrane Enzymes; Na,K-ATPase; Protein Assembly; Heterodimer Assembly; Isoform Selectivity; Stability
Abstract
To catalyze ion transport, the Na,K-ATPase must contain one α and one β subunit. When expressed by transfection in various expression systems, each of the four α subunit isoforms can assemble with each of the three β subunit isoforms and form an active enzyme, suggesting the absence of selective α-β isoform assembly. However, it is unknown whether in vivo conditions the α-β assembly is random or isoform-specific. The α2-β2 complex was selectively immunoprecipitated by both anti-α2 and anti-β2 antibodies from extracts of mouse brain, which contains cells co-expressing multiple Na,K-ATPase isoforms. Neither α1-β2 nor α2-β1 complexes were detected in the immunoprecipitates. Furthermore, in MDCK cells co-expressing α1, β1, and β2 isoforms, a greater fraction of the β2 subunits was unassembled with α1 as compared with that of the β1 subunits, indicating preferential association of the α1 isoform with the β1 isoform. In addition, the α1-β2 complex was less resistant to various detergents than the α1-β1 complex isolated from MDCK cells or the α2-β2 complex isolated from mouse brain. Therefore, the diversity of the α-β Na,K-ATPase heterodimers in vivo is determined not only by cell-specific co-expression of particular isoforms, but also by selective association of the α and β subunit isoforms.
Introduction
The Na,K-ATPase is universally expressed in all animal cells where it generates electrochemical ion gradients that are critical for many cellular processes (1–3). The Na,K-ATPase is composed of two essential subunits, α and β. There are four isoforms of the α subunits and three isoforms of the β subunits. In some tissues, the α-β heterodimer is associated with one of the seven members of the FXYD protein family (4, 5) that modulate kinetic properties of the enzyme.
The α1 and β1 isoforms are expressed ubiquitously, suggesting a housekeeping role for the α1-β1 Na,K-ATPase in most cells. In contrast, other Na,K-ATPase subunit isoforms are expressed in a tissue-specific manner. The α2 isoform is expressed mainly in muscle and nervous system (1–3, 6, 7); the α3 isoform is expressed mainly in neurons (8, 9), whereas the α4 isoform is found only in testis (10, 11). The β2 isoform is expressed predominantly in brain and muscle (3, 7), whereas the β3 isoform is mainly expressed in lung, testis, skeletal muscle, and liver (12, 13). The α2 isoform regulates contractility of cardiac, smooth, and skeletal muscle and plays a key role in the modulation of blood pressure in response to stress (3, 14, 15). In vivo human mutations in the α2 and α3 isoforms are associated with neurological diseases, familial hemiplegic migraine type 2, and rapid-onset dystonia-parkinsonism (16). The α4 isoform is required for sperm motility and fertility (10, 11). The β1 subunit plays an important role in intercellular adhesion in epithelia (17, 18), and the β2 subunit, or adhesion molecule on glia (AMOG),2 is important for adhesion and migration of neurons on glia (19). Decreased expression of the β1 subunit is associated with cancer (reviewed in Ref. 20), whereas abnormalities in expression and distribution of the β2 subunit are linked to glioma and epilepsy (21–24).
Therefore, it is clear that both α and β isoforms of the Na,K-ATPase have organ- and tissue-specific functions. However, very little is known about particular α-β heterodimers responsible for these roles. Transfection studies indicate that each of the four α subunit isoforms can assemble with each of the three β subunit isoforms and form a functional pump (6, 7, 25). These data imply that in cells co-expressing multiple Na,K-ATPase subunits isoforms, various α and β isoforms also assemble in different combinations, dependent on their relative cellular content. However, selective co-immunoprecipitation of the α2 subunit but not of the ubiquitously expressed α1 subunit, with the β2 subunit from mouse and rat brain (19, 26), as well as from heart and adrenal medullary cells of guinea pigs and rats (26, 27) suggest that the α2 subunit is the preferred binding partner of the β2 subunit. In support of this hypothesis, the tissue expression pattern of the β2 subunit, mainly in muscle and nervous system, is similar to that of the α2 subunit (3, 7).
Here, we show that not only does the β2 subunit preferentially assemble with the α2 subunit but also the α2 subunit is mostly associated with the β2 subunit in mouse brain. In addition, by analyzing the competition of the β1 or β2 subunits for binding to the α1 subunit in MDCK cells, we demonstrate that the β1 subunit is a greatly preferred binding partner of the α1 subunit compared with the β2 subunit. The results of co-immunoprecipitation of α and β subunits from various detergent extracts of native tissues and cultured cells indicate that α1-β1 and α2-β2 heterodimers are more stable than α1-β2 heterodimers. Therefore, there is selective assembly of the different α and β subunit isoforms with likely tissue-specific functional consequences.
EXPERIMENTAL PROCEDURES
Cell Lines
The Na,K-ATPase dog β1 or human β2 subunits linked with their N termini to YFP were constructed as described previously (28, 29). Stable MDCK cell lines expressing YFP-β1** and YFP-β2 were obtained and maintained as described previously (30).
Confocal Microscopy
Confocal microscopy images were acquired using the Zeiss LSM 510 laser scanning confocal microscope and LSM 510 software (version 3.2).
Primary Antibodies Used for Immunofluorescent Staining and Western Blot Analysis
For immunofluorescent staining, the monoclonal antibodies against the Na,K-ATPase α1 subunit, clone C464.6 (Millipore) and against the Na,K-ATPase β1 subunit, clone M17-P5-F11 (Affinity Bioreagents) and polyclonal antibodies against the Na,K-ATPase α2 subunit, (Millipore) and against the Na,K-ATPase β2 subunit (Millipore) were used. The polyclonal antibody against the Na,K-ATPase β1 subunit (31), which was a generous gift of Dr. W. James Ball, Jr. (University of Cincinnati), was used for Western blot analysis. Also, the following monoclonal antibodies were used for Western blot analysis: against GFP, clones 7.1 and 13.1, which also recognizes YFP (Roche Diagnostics), against the Na,K-ATPase α1 subunit, clone C464.6 (Millipore), against the Na,K-ATPase α3 subunit (Upstate), against the Na,K-ATPase β2 subunit, clone 35 (BD Bioscience Pharmingen), and against the Na,K-ATPase β3 subunit (Santa Cruz Biotechnology).
Extraction of Proteins from MDCK Cells and Mouse Brain Homogenates
Confluent MDCK cell monolayers grown in six-well plates were rinsed twice with ice-cold PBS and incubated with 200 μl/well of the extraction buffer at 4 °C for 30 min followed by scraping cells. Mouse brain homogenates containing 600 μg protein in 500 μl of 150 mm NaCl in 50 mm Tris, pH 7.5, were incubated with 500 μl of the extraction buffer at 4 °C for 30 min. The extraction buffer contained 150 mm NaCl in 50 mm Tris, pH 7.5, and the 2× concentration of the indicated detergent(s). When DOC was used alone, no NaCl was added to the extraction buffer. Prior to using, the extraction buffer was mixed with Complete protease inhibitor mixture (Roche Diagnostics), 1 tablet/50 ml. Cell extracts were clarified by centrifugation (15,000 × g, 10 min) at 4 °C. Where indicated, protein extracts were treated by PNGase F from Flavobacterium meningosepticum (New England Biolabs) or by Endo H from Streptomyces plicatus (Glyco-Prozyme Inc.) according to the manufacturer's instructions prior to loading on SDS-PAGE.
Immunoprecipitation
Protein extracts from MDCK cells or from mouse brain homogenates (100–300 μg protein) were incubated with 30 μl of the protein A-agarose suspension (Roche Diagnostics) in a total volume 1 ml of the extraction buffer at 4 °C with continuous rotation for at least 3 h (or overnight) to remove the components that non-specifically bind to protein A. The precleared cell extract was mixed with 2 μl of polyclonal antibodies against GFP, which also recognize YFP (Clontech), or 10 μl of polyclonal antibodies against the Na,K-ATPase α2 subunit (CHEMICON Intl.), or 10 μl of polyclonal antibodies against the Na,K-ATPase α subunit (32) and incubated with continuous rotation at 4 °C for 60 min. After addition of 30 μl of the protein A-agarose suspension, the mixture was incubated at 4 °C with continuous rotation overnight. The bead-adherent complexes were washed three times on the beads and then eluted as described previously (33).
Multi-round immunoprecipitation from MDCK cells expressing either YFP-β1 or YFP-β2 was performed as described above with the exception that, after the first round of immunoprecipitation using 30 μg protein in 1% CHAPS and 5 μl anti-α antibody, the unbound proteins in the supernatant were collected and incubated again with 5 μl of anti-α antibody in a second round of immunoprecipitation. A third round of immunoprecipitation was performed by using 4 μl of anti-GFP/YFP antibody from the supernatant after the second round of immunoprecipitation.
Where indicated, the bead-adherent proteins were treated with PNGase F or with Endo H. Deglycosylation by PNGase F was performed by incubation of the bead-adherent proteins with 1 μl of PNGase F in 30 μl of 50 mm sodium phosphate, pH 7.5, containing 1% Nonidet P-40 and at 37 °C for 1 h. Digestion by Endo H was performed by incubation of the bead-adherent proteins with 3 μl of Endo H in 30 μl of 50 mm sodium citrate/phosphate, pH 5.5, containing 1% Nonidet P-40 at 37 °C for 3 h. After incubation with glycosidases, the reaction mixture was separated from the beads. The adherent proteins were eluted from the beads by incubation in 30 μl of 2× SDS-PAGE sample buffer for 5 min at 80 °C. To account for possible dissociation of immunoprecipitated proteins from the beads during deglycosylation, the eluted proteins were combined with the reaction mixture. After separation by SDS-PAGE, the immunoprecipitated and co-immunoprecipitated proteins were analyzed by Western blot by using appropriate antibodies.
Isolation of Basolateral Plasma Membrane Proteins of MDCK Cells Using Surface-specific Biotinylation
Cells were maintained for 6 days after becoming confluent in Transwell inserts. Biotinylation and isolations of basolateral surface proteins was performed according to procedures described previously (34–36).
Western Blot Analysis
1–10 μg of proteins extracted from MDCK cells, microsomal membranes isolated from animal tissues in SDS-PAGE sample buffer, or 5–20 μl of proteins eluted from the protein A-conjugated agarose beads were loaded onto 4–12% gradient SDS-PAGE gels (Invitrogen). Proteins were separated by SDS-PAGE, transferred onto a nitrocellulose membrane (Bio-Rad), and detected by Western blot analysis as described previously (33). Immunoblots were quantified by densitometry using Zeiss LSM 510 software (version 3.2).
Immunofluorescent Staining
MDCK cells were fixed by incubation with 3.75% formaldehyde in PBS for 15 min at 37 °C and permeabilized by incubation with 0.1% Triton X-100 for 5 min. Fixed cells or frozen tissue sections on FDA standard frozen tissue rat or human arrays (BioChain) were incubated with Dako protein block serum-free solution (Dako Corp.) for 30 min. Immunostaining was performed by 1-h incubation with primary antibodies followed by 1-h incubation with Alexa Fluor 633- or Alexa Fluor 488-conjugated anti-mouse or anti-rabbit antibodies (Invitrogen).
Statistical Analysis
Statistical analysis was performed using Student's t test (GraphPad Prism 4 software and Microsoft Excel). Statistical significance and number of experiments are specified in the figure legends.
RESULTS
Distribution of Na,K-ATPase α1, α2, β1, and β2 Subunits in Rat and Human Tissues
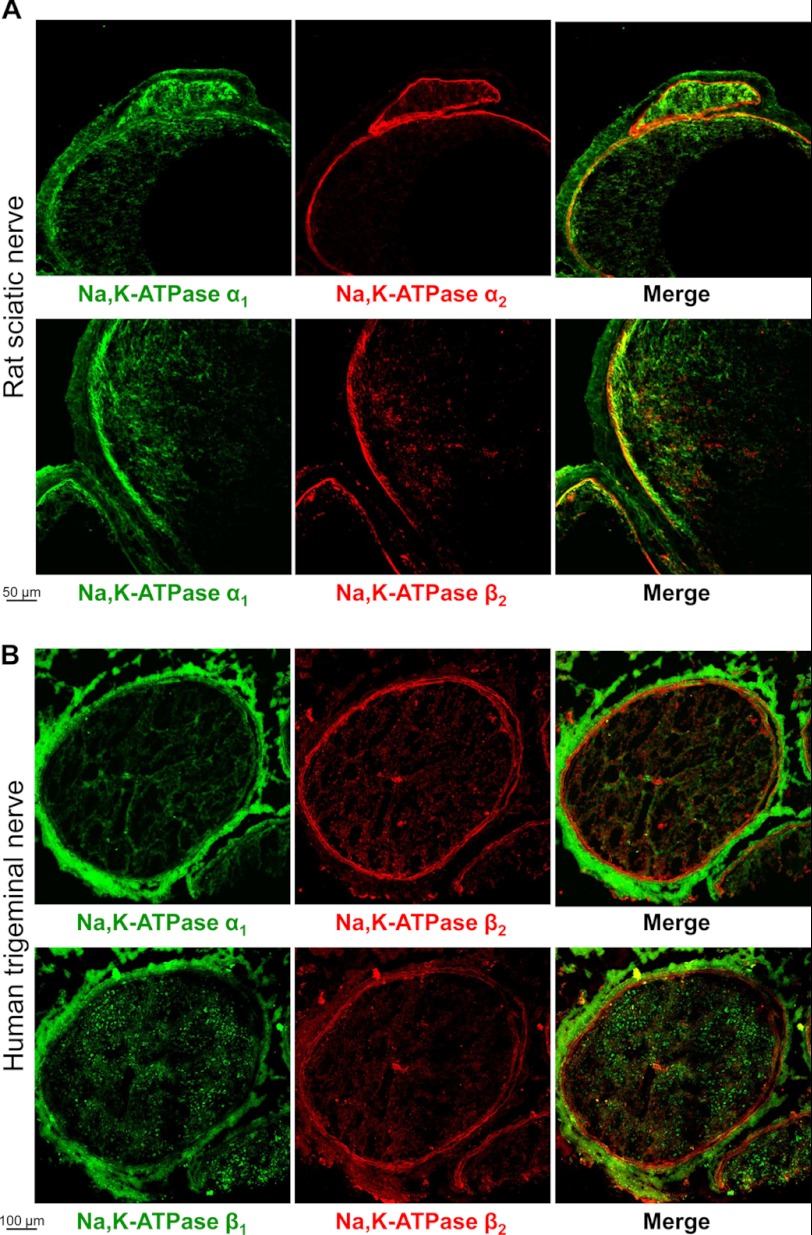
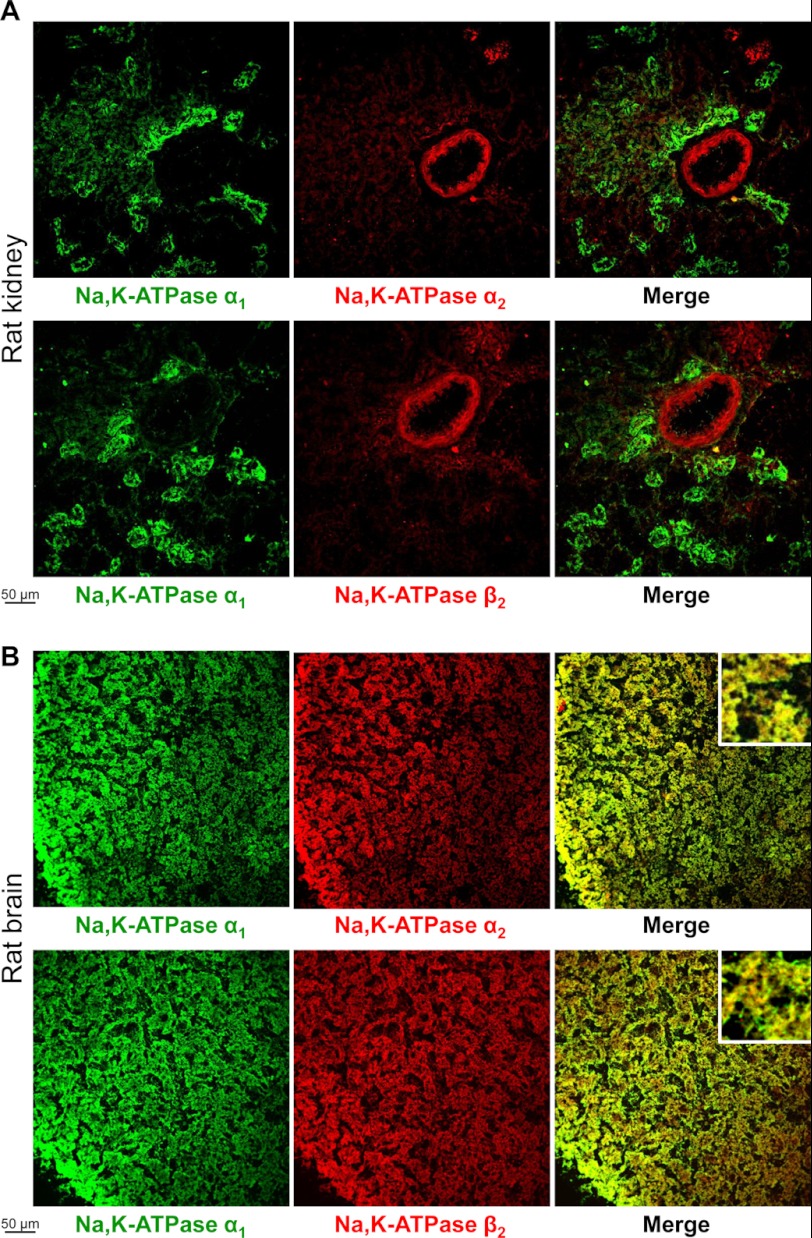
Normal rat or human frozen tissue arrays (BioChain) that contained sections of adrenal gland, brain, breast, colon, esophagus, heart, kidney, liver, skeletal muscle, nerve, and ovary were used to perform immunofluorescent double staining of the Na,K-ATPase α1 and α2 subunits. The Na,K-ATPase α1 and α2 subunits were differentially distributed in several organs, particularly in peripheral nerves and kidney, which are shown as examples (Figs. 1 and 2A). The α2 subunit was found predominantly in perineurium that surrounds fascicles, whereas the α1 subunit was also present in epineurium that surrounds nerves (Fig. 1). In the kidney, the α1 subunit was detected in tubules, whereas the α2 subunit was seen predominantly in renal arteriole and glomerulus (Fig. 2A). Double staining of the α1 and β2 subunits showed that tissue distribution of the β2 subunit is similar to that of the α2, but not of the α1 subunit in both peripheral nerve and kidney sections (Figs. 1 and 2A). Also, double staining of the β1 and β2 subunits showed that distribution of the β1 subunit in the nerve is similar to that of the α1 but not of the β2 subunit (Fig. 1B). Staining of the α1, α2, and β2 subunits in rat brain cortex showed both co-expression of these isoforms in the same cells and cell-specific expression (Fig. 2B). Co-expression of α1 and α2 or α1 and β2 is evident from co-localization of red and green fluorescence, producing a yellow color on the merged images, whereas the differential expression of these isoforms is seen from separated green and red spots on the merged images. Co-expression of the β1 isoform with both α2 and β2 isoforms in the same cells was also seen in human brain sections (data not shown).
FIGURE 1.
Localization of the Na,K-ATPase β2 subunit in nerve sections is similar to that of the Na,K-ATPase α2 subunit, but not of the Na,K-ATPase α1 subunit or β1 subunit. A, frozen sections of rat sciatic nerve were double stained by using mouse antibodies against α1 subunit (green) and either rabbit antibodies against α2 subunit (red) or rabbit antibodies against β2 subunit (red). B, frozen sections of human trigeminal nerve were double-stained by using either mouse antibodies against α1 subunit (green) or mouse antibodies against β1 subunit (green) and rabbit antibodies against β2 subunit (red). Anti-mouse Alexa Fluor 488-conjugated secondary antibodies were used to detect anti-α1 and anti-β1 primary antibodies, and anti-rabbit Alexa Fluor 633-conjugated secondary antibodies were used to detect anti-β2 and anti-α2 primary antibodies.
FIGURE 2.
Localization of the Na,K-ATPase α1, α2, and β2 subunits in rat kidney and brain sections. Frozen sections of rat kidney (A) and rat brain (B) were double-stained by using mouse antibodies against α1 subunit (green) and either rabbit antibodies against α2 subunit (red) or rabbit antibodies against β2 subunit (red). Anti-mouse Alexa Fluor 488-conjugated secondary antibodies were used to detect anti-α1 primary antibodies and anti-rabbit Alexa Fluor 633 conjugated secondary antibodies were used to detect anti-β2 and anti-α2 primary antibodies. Insets in the right panels (B) show images zoomed 5-fold.
Subunit Composition of Na,K-ATPase α-β Heterodimers in Mouse Brain
Western blot analysis detected the presence of six different isoforms of the Na,K-ATPase subunits in mouse brain extracts (Fig. 3), consistent with previously published results for rat brain (37). To validate the antibodies used to detect β subunits isoforms, we performed Western blot analysis of mouse brain extracts incubated with or without PNGase F that cleaves N-glycans attached to the β subunits. The bands at 33–35 kDa, which were expected for deglycosylated β1, β2, and β3 subunits, were detected in samples treated with PNGase F by using the antibodies listed under “Experimental Procedures” (Fig. 3A). In control samples, these antibodies detected bands at 45–50 kDa (Fig. 3A).
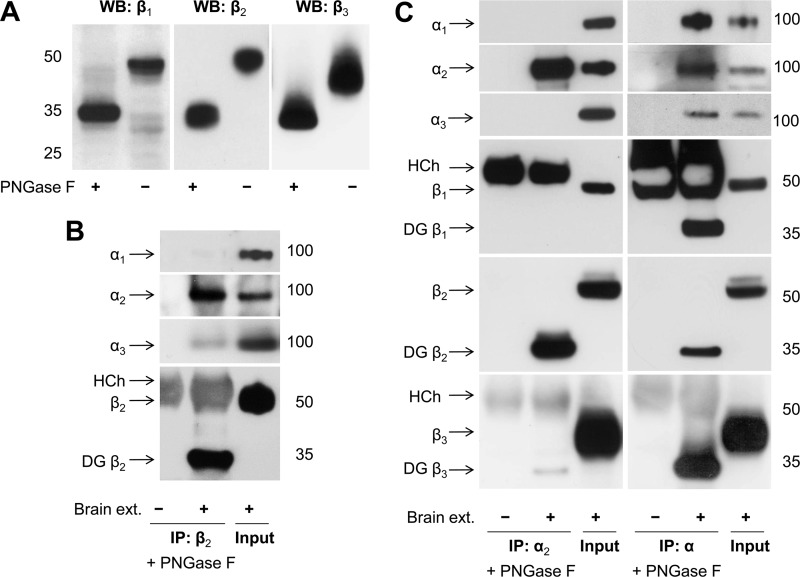
FIGURE 3.
The Na,K-ATPase α2 and β2 subunits are selectively co-immunoprecipitated from mouse brain extracts. Proteins were extracted from mouse brain homogenate by using 1% Nonidet P-40/0.5% DOC. A, the antibodies against the Na,K-ATPase β1, β2, and β3 isoforms were validated by Western blot analysis of mouse brain extracts preincubated with or without PNGase F that cleaves N-glycans from the β isoforms subunits and, hence, results in an increase in electrophoretic mobility of the subunits. B, Western blot analysis of the immunoprecipitated β2 subunit and co-immunoprecipitated α subunit isoforms shows that the α2 subunit is preferentially co-precipitated with the β2 subunit. Input lanes contain 4 and 10% of the extract used for immunoprecipitation on α and β blots, respectively. C, Western blot analysis of proteins immunoprecipitated and co-immunoprecipitated by using either the α2-specific antibodies (left panels) or the α-nonspecific antibodies (right panels) shows selective co-immunoprecipitation of the β2 subunit with the α2 subunit. Input lanes contain 10% of the extract (ext.) used for immunoprecipitation. To prevent an overlap of the β subunit bands with the heavy chain band of the antibodies used for immunoprecipitation, the immunoprecipitated proteins were treated with PNGase F prior to SDS-PAGE. HCh, heavy chain; IP, immunoprecipitation; WB, Western blot; DG, deglycosylated.
Immunoprecipitation of the β2 subunit from 1% Nonidet P-40/0.5% DOC extracts of mouse brain homogenates resulted in co-precipitation of the α2 isoform and of a minor amount of the α3 isoform but not of the α1 subunit (Fig. 3B). These results are consistent with detection of the α2 subunit but not of the α1 subunit in the fraction isolated from mouse brain by immunoaffinity chromatography using the antibody against the β2 subunit (38).
Immunoprecipitation of the Na,K-ATPase α2 subunit resulted in co-precipitation of the β2 subunit but not of the β1 subunit (Fig. 3C). A minor amount of the β3 subunit was detected in the immunoprecipitated fraction (Fig. 3C). No α1 or α3 subunits were detected in the immunoprecipitated fraction, confirming specificity of the anti-α2 antibody. In contrast, the antibody raised against the 4/5 loop of the Na,K-ATPase α1 subunit that is relatively homologous in other α isoforms (32) immunoprecipitated all three α isoforms, α1, α2, and α3 and all three β subunit isoforms from mouse brain extracts (Fig. 3C), confirming its non-selectivity, as expected.
To evaluate stability of the various Na,K-ATPase α-β complexes, we compared co-immunoprecipitates of the β subunits with α subunits from mouse brain extracts obtained by using increasing concentrations of the non-ionic detergent, DDM. The amount of the α2 subunit immunoprecipitated with specific anti-α2 antibodies was similar in 0.5%, 1 and 2% DDM (Fig. 4A, left panel). In contrast, the amount of the β2 subunits, which were co-precipitated with the α2 subunit, gradually decreased with increasing detergent concentration (Fig. 4, A, left panel, and B), showing a partial dissociation of the α2-β2 complex by DDM. No co-precipitation of the β1 or β3 subunit was found at any DDM concentration tested.
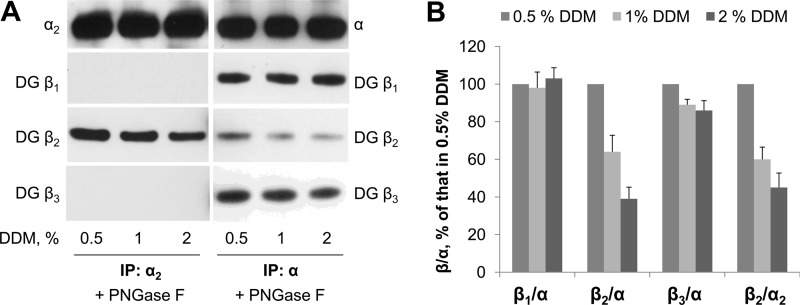
FIGURE 4.
The Na,K-ATPase α2-β2 complex is less stable than the Na,K-ATPase α-β1 or α-β3 complexes in detergent extracts obtained from mouse brain membranes. A, various concentrations of DDM were used to extract proteins from mouse brain homogenate. Western blot analysis of proteins immunoprecipitated (IP) and co-immunoprecipitated by using either the α2-specific antibodies (left panels) or the α-nonspecific antibodies (right panels) shows a stepwise decrease in the amount of β2 subunits co-immunoprecipitated by using both antibodies, but not in the amount of β1 or β3 subunits co-immunoprecipitated by using α-nonspecific antibodies, with increasing detergent concentrations. B, densitometric quantification of the results shown in A was performed by dividing the signal from the β antibody by the corresponding signal of the α antibody. A comparative graph shows these ratios as a percentage of the ratio obtained in 0.5% DDM. IP, immunoprecipitation; DG, deglycosylated by PNGase F prior to SDS-PAGE.
The total amount of all three α subunit isoforms immunoprecipitated by nonspecific anti-α antibodies was similar in 0.5%, 1, and 2% DDM extracts (Fig. 4A, right panel). The amount of the β2 subunit co-precipitated by nonspecific anti-α antibodies gradually decreased with increasing detergent concentration (Fig. 4, A, right panel, and B). This decrease was similar to that observed with the specific anti-α2 antibodies (Fig. 4, A, left panel, and B), strongly suggesting that precipitation of the β2 subunit by nonspecific anti-α antibodies reflects α2-β2 complexes. This conclusion is consistent with co-precipitation of the α2 but not of the α1 subunit, with the β2 subunit (Fig. 3C). The amount of the β1 subunit that was co-precipitated with α subunits by the nonspecific anti-α antibodies was not affected by detergent concentration (Fig. 4A, right panel). Only a slight decrease in the amount of the β3 subunit co-precipitated with α subunits was observed with an increase in DDM concentration (Fig. 4, A, right panel, and B). These results indicate that complexes of the α1 or α3 subunit with either the β1 or β3 subunit are preserved at all tested DDM concentrations. Therefore, both α2 and β2 subunits predominantly associate with each other but not with other partner subunits in mouse brain.
Plasma Membrane and Intracellular Distribution of Na,K-ATPase α1, β1, and β2 in MDCK Cells
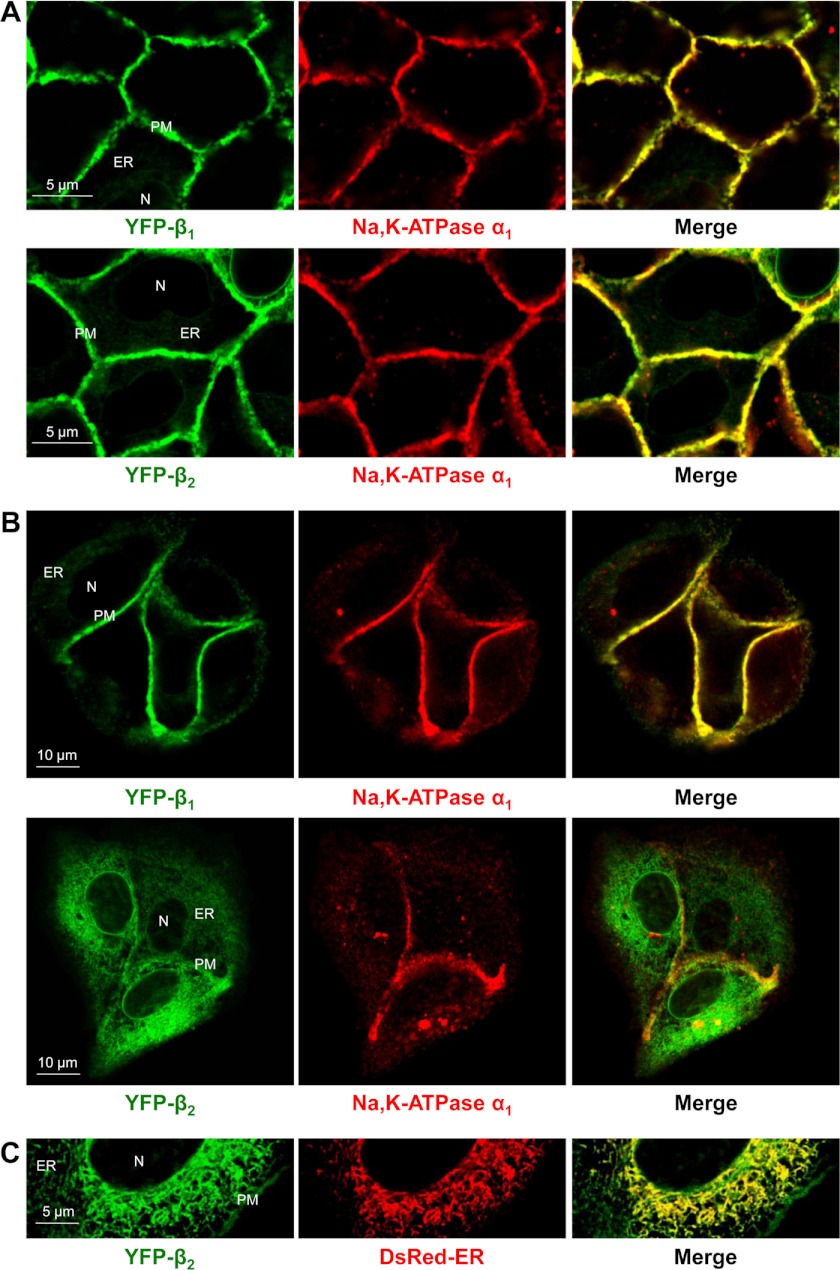
To test whether the α1 subunit preferentially assembles with the β1 or β2 isoform, the content of α1-assembled and α1-unbound β subunits was compared in stable MDCK cell lines expressing either YFP-β1 or YFP-β2. The major endogenous Na,K-ATPase isoforms in these cells are α1 and β1. Both YFP-β1 and YFP-β2 were co-localized with the endogenous α1 subunit in the lateral membranes, as detected by immunofluorescence (Fig. 5A), consistent with previously reported results (29). In addition, both YFP-β1 and YFP-β2 but not the α1 subunit were found inside the cells (Fig. 5A), indicating that these intracellular forms of YFP-β1 and YFP-β2 are not assembled with the α1 subunit. This intracellular retention was greater in dispersed cells than in confluent monolayers (Fig. 5B), and it showed co-localization with the ER marker (Fig. 5C). The fraction of YFP-β2 localized in the ER was greater than that of YFP-β1 (Fig. 5, A and B).
FIGURE 5.
The amount of α1-unbound YFP-β2 retained in the ER of MDCK cells is greater than that of YFP-β1. Horizontal confocal microscopy sections of confluent monolayers (A) or dispersed colonies (B and C) of MDCK cells expressing either YFP-β1 or YFP-β2. Both YFP-β1 and YFP-β2 (green) are co-localized with the endogenous α1 subunit (red) in the lateral membranes, but not inside the cells as detected by immunostaining of fixed cells using the monoclonal antibody against the Na,K-ATPase α1 subunit (A and B). The intracellular retention of α1-unassembled YFP-β2 is more prominent than that of YFP-β1 and more evident in dispersed colonies than in confluent monolayers. This intracellular fraction of YFP-β2 (green) shows co-localization with the ER (red) as detected by transient expression of the fluorescent ER marker, DsRed2-ER (C). N, nucleous; PM, plasma membrane.
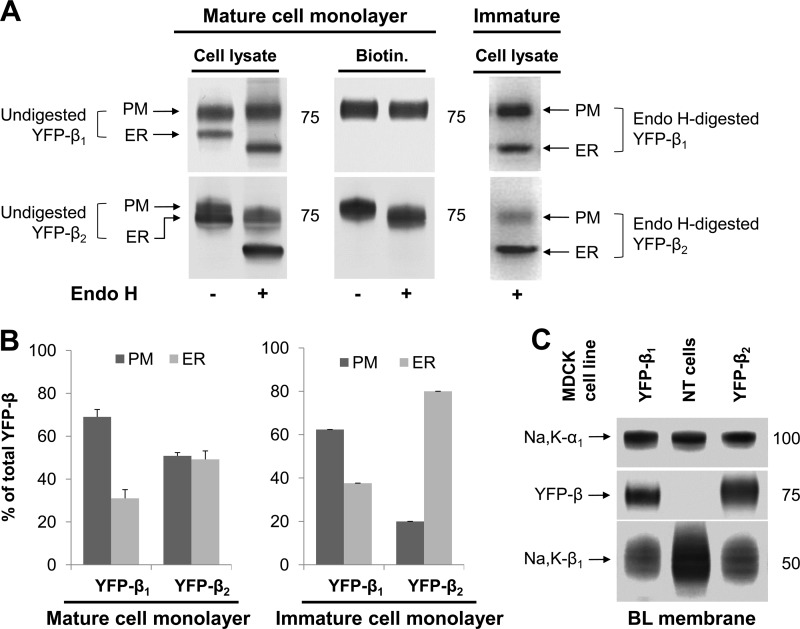
To quantify the levels of the endogenous and exogenous Na,K-ATPase subunits in the ER and the plasma membrane in the two cell lines, we analyzed total cell lysates and plasma membrane fractions by Western blot analysis. In both cell lines, the YFP-linked β subunit was detected as two bands in total lysates (Fig. 6A). These two bands represent differentially N-glycosylated species of fusion proteins. Only a single band, which corresponded to the upper band in cell lysate, was found in the basolateral membrane (Fig. 6A), indicating that the lower band in cell lysate corresponds to the intracellular fraction of YFP-β1 or YFP-β2. As shown previously, this intracellular form of either YFP-β1 or YFP-β2 mostly represents the ER-resident fraction of each fusion protein (34). The relative amount of this ER-resident fraction appears to be greater for YFP-β2 than for YFP-β1 (Fig. 6A, left lanes). However, direct densitometric quantification of the two fractions of YFP-β2 was not possible because of a significant overlap between two bands on SDS-PAGE (Fig. 6A, top panel, left lane).
FIGURE 6.
The greater intracellular retention of YFP-β2 than YFP-β1 is not associated with its higher level in the plasma membrane. A and B, the comparative Western blot analysis (A) of total cell lysates and basolateral biotinylated proteins (BL membrane) of MDCK cells stably expressing either YFP-β1 or YFP-β2 shows that the upper band found in either cell lysate represents the mature plasma membrane fraction (PM), whereas the lower band corresponds to the intracellular fraction ER-resident fraction (ER). Treatment with Endo H resulted in a slight increase in electrophoretic mobility of BLM YFP-β2, but not of BLM YFP-β1, and a major increase in electrophoretic mobility of ER YFP-β1 and ER YFP-β2. This allows a better separation of PM and ER fractions of YFP-β1 or YFP-β2 on SDS-PAGE and their densitometric quantification (B). C, Western blot analysis of proteins isolated by basolateral surface-selective biotinylation show that stable expression of either YFP-β1 or YFP-β2 in MDCK cells resulted in a significant decrease in the amount of the endogenous Na,K-ATPase β1 subunits in the basolateral membranes but did not change the level of the α1 subunits, as compared with non-transfected cells (NT cells). YFP-β1 and YFP-β2 are present in the basolateral membrane at similar levels in the two transfected cell lines. PM, basolateral plasma membrane.
To separate these two bands, we used Endo H, which is known to remove high-mannose and hybrid, but not the complex-type N-glycans, from glycoproteins. Treatment of total cell lysates or biotinylated proteins with Endo H resulted in a slight increase in electrophoretic mobility of the plasma membrane fraction of YFP-β2 but not of YFP-β1 (Fig. 6A). The ER-resident form in cell lysates was completely deglycosylated by Endo H, producing a band at ∼60 kDa that corresponds to the protein core molecular mass of YFP-β (Fig. 6A). As a result, a better separation of the plasma membrane and ER fractions on SDS-PAGE was observed in Endo H-treated cell lysates, allowing densitometric quantification (Fig. 6B).
This quantification confirmed the greater ER retention of YFP-β2 (49% of total cellular content) as compared with YFP-β1 (31% of total cellular content) in mature cell monolayers. The difference in the relative content of the ER form of YFP-β2 and YFP-β1 was more prominent in immature cell monolayers, 80 and 38% of total cellular content, respectively (Fig. 6, A and B). In contrast, the levels of YFP-β1 and YFP-β2 in the basolateral membrane were similar in the two transfected cell lines (Fig. 6C). Also, the levels of the endogenous α1 and β1 subunits were similar in YFP-β1- and YFP-β2-expressing MDCK cell lines (Fig. 6C).
Therefore, the amount of α1-unassembled YFP-β2 in the ER is significantly greater than that of YFP-β1, whereas the quantities of α1-assembled YFP-β1 and YFP-β2 in the plasma membrane are similar (Fig. 5 and see Fig. 8), suggesting preferential assembly of the α1 subunit with the β1 isoform rather than with the β2 isoform.
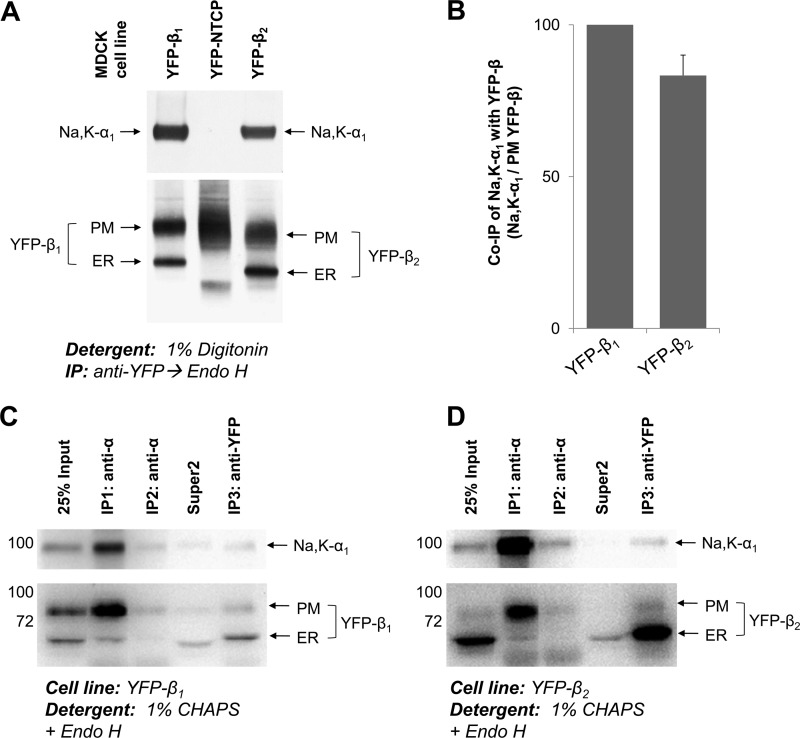
FIGURE 8.
The Na,K-ATPase α1-β2 complex is preserved in digitonin and CHAPS extracts from MDCK cells. A, proteins were extracted from MDCK cells expressing YFP-β1, or YFP-β2, or YFP-linked bile acid transporter (YFP-NTCP), with the extraction buffer containing 1% digitonin. YFP-linked β1 or β2 subunits were immunoprecipitated and treated with Endo H followed by elution of proteins from the beads. Co-immunoprecipitation of the Na,K-ATPase α1 subunit was detected with YFP-β1 and with YFP-β2, but not with YFP-NTCP, indicating that there is no nonspecific precipitation of the α1 subunit. B, densitometric quantification of the results presented in A was performed by dividing the density of the α1 subunit band by the density of the corresponding PM YFP-β band. C and D, proteins were extracted from MDCK cells expressing either YFP-β1 (C) or YFP-β2 (D) using extraction buffer containing 1% CHAPS and subject to successive rounds of immunoprecipitation (IP) using anti-α antibody followed by a final round of immunoprecipitation using anti-GFP/YFP antibody. Both extracted and immunoprecipitated proteins were treated with Endo H prior to SDS-PAGE. The majority of YFP-β subunits co-immunoprecipitated with α1 were complex-type glycosylated. Almost all of the β subunits not assembled with α1 were Endo H-sensitive and immunoprecipitated in the final anti-GFP/YFP immunoprecipitation. PM, plasma membrane; Super2, supernatant after the second round of immunoprecipitation.
Stability of Na,K-ATPase α1-β1 and α1-β2 Complexes Isolated from MDCK Cells
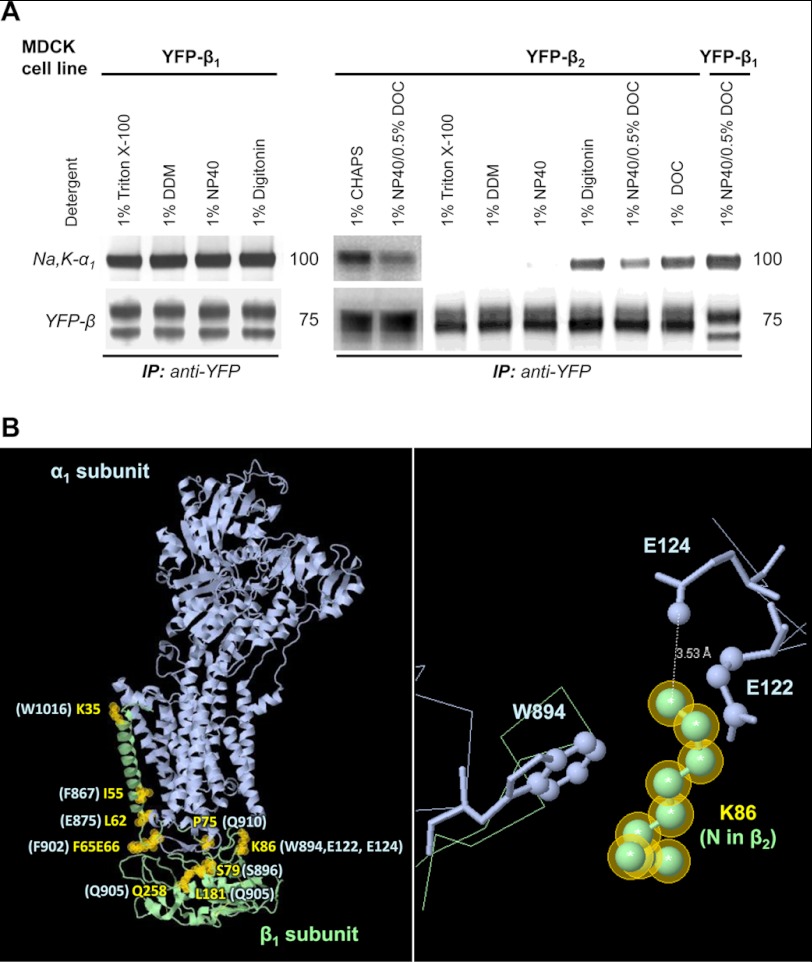
Co-immunoprecipitation of the Na,K-ATPase α1 subunit with YFP-β1 was detected in 1% Triton X-100, 1% DDM, 1% Nonidet P-40, 1% digitonin, and also in a mixture of 1% Nonidet P-40 and 0.5% DOC (Fig. 7A). The amount of co-precipitated α1 subunit was similar in all tested detergents. In contrast, co-immunoprecipitation of the Na,K-ATPase α1 subunit with YFP-β2 was observed only in a few selected detergents, 1% digitonin, 1% DOC, 1% CHAPS, and a mixture of % Nonidet P-40 and 0.5% DOC (Fig. 7A). The maximal amount of the Na,K-ATPase α1 subunit was co-immunoprecipitated with YFP-β2 in digitonin, DOC, and CHAPS. No co-precipitation was seen in 1% Triton X-100, 1% DDM, or 1% Nonidet P-40 (Fig. 7A). These results indicate that the Na,K-ATPase α1-β2 complexes are less stable than the α1-β1 complexes.
FIGURE 7.
The Na,K-ATPase α1-β1 complex is more stable than the Na,K-ATPase α1-β2 complex in detergent extracts from MDCK cells. A, MDCK cells stably expressing either YFP-β1 or YFP-β2 were lysed by incubation with the extraction buffer containing an appropriate detergent (as indicated). After scraping the cells and removing non-extracted material by centrifugation, YFP-linked β1 or β2 subunits were immunoprecipitated. Immunoprecipitated YFP-β1 or YFP-β2 and co-immunoprecipitated α1 subunit were analyzed by Western blot. Co-immunoprecipitation of the Na,K-ATPase α1 subunit with YFP-β1 was detected in all tested detergents. In contrast, co-immunoprecipitation of the Na,K-ATPase α1 subunit with YFP-β2 was observed only in selected detergents. B, a model of the Na,K-ATPase α1 and β1 subunits based on the crystal structure of the sodium-potassium pump at 2.4 A resolution (Protein Data Bank code 2ZXE) (39) shows the α1-interacting residues in the β1 subunit that are different in the β2 subunit (yellow labels and yellow shaded circles). The corresponding β1-interacting residues in the α1 subunit are indicated in light blue. A close-up view of Lys-86 of the β1 subunit and its interacting residues in the α1 subunit is shown in the right panel.
Alignment of the β1 subunit with the β2 subunit shows that 10 α1-interacting residues in the β1 subunit predicted by the high resolution structure of the α1-β1 Na,K-ATPase (39) differ from the corresponding residues in the β2 subunit (Fig. 7B). These differences are expected to weaken the α-β interaction. For example, the presence of an asparagine residue in the β2 subunit instead of Lys-86 would not allow effective interaction with Glu-122, Glu-124, and Trp-894 of the α1 subunit (Fig. 7B), consistent with the lower stability of α1-β2 complexes as compared with that of the α1-β1 complexes.
To determine whether co-precipitation of α1 subunits with YFP-β1 and YFP-β2 was specific, YFP-linked sodium-taurocholate co-transporting polypeptide (NTCP), the integral basolateral membrane protein that does not interact with the Na,K-ATPase, was used as a negative control. YFP-NTCP stably expressed in MDCK cells (28) was immunoprecipitated from 1% digitonin cell extracts under the same conditions that were used to immunoprecipitate YFP-β1 and YFP-β2 (Fig. 8A). The Na,K-ATPase α1 subunits were co-precipitated with YFP-β1 and YFP-β2, but not with YFP-NTCP, indicating that co-precipitation of α1 subunits with YFP-β1 and YFP-β2 was exclusively due to specific α-β interactions.
The α1 subunits were predominantly located in the plasma membrane, whereas both YFP-β1 and YFP-β2 were also found in the ER (Fig. 5A). Therefore, the amount of co-precipitated α1 subunits should be compared with the amount of the plasma membrane fraction of YFP-β proteins, but not to the total amount of immunoprecipitated YFP-β. To better separate the plasma membrane and ER-resident fractions, the immunoprecipitated proteins were treated with Endo H prior to SDS-PAGE (Fig. 8A). Western blot analysis followed by densitometric quantification, which allowed calculation of the amount of co-precipitated α1 subunits relative to the amount of the plasma membrane fractions of immunoprecipitated YFP-β1 or YFP-β2. This quantification showed that the amount of the YFP-β2-bound α1 subunits was 15% less than the amount of YFP-β1-bound α1 subunits (Fig. 8B). Similar results were obtained in 1% CHAPS (data not shown).
If the lower amount of YFP-β2-bound α1 subunit was related to partial disruption of the α1-β2 complex by detergent, the detergent extract must contain the α1-unbound plasma membrane forms of YFP-β2 subunits. To determine the presence of α1-unbound β subunits in 1% CHAPS extracts of both YFP-β1- and YFP-β2-expressing MDCK cells, multiround immunoprecipitation using anti-α antibody was performed. The first round of immunoprecipitation pulled down a vast majority of the α1 subunits. Co-precipitated fractions of YFP-β1 or YFP-β2 contained predominantly mature forms and minor amounts of the ER-resident immature forms (Fig. 8, C and D). The second round of immunoprecipitation using anti-α antibody precipitated the rest of the α1 subunits and mature YFP-β1 or YFP-β2. The third round of immunoprecipitation using anti-GFP antibody pulled down almost exclusively the immature forms of YFP-β1 or YFP-β2 and no α1 subunits (Fig. 8, C and D). These results confirm that both YFP-β1 and YFP-β2 are assembled with the α1 subunits in the plasma membrane, whereas the majority of the ER-resident YFP-β1 and YFP-β2 are not assembled with the α1 subunits (Fig. 8, C and D). In addition, the data demonstrate that the (YFP-β2)-α1 complex is fully preserved in 1% CHAPS extracts.
DISCUSSION
β1, but Not β2 Isoform, Is Preferred Binding Partner of α1 Subunit
YFP-β1 and YFP-β2 stably expressed in MDCK cells are distributed predominantly between the basolateral plasma membrane and ER (Fig. 5). All of the β subunits present in the basolateral membrane are α1-assembled, as we showed previously for the endogenous β1 subunits (40) and now for both exogenous YFP-β1 and YFP-β2 (Fig. 8). In contrast, the majority of the ER-resident β subunits are not bound to α1 subunits (Fig. 5B and Fig. 8, C and D) (34, 40). These results are consistent with the previous finding showing that both YFP-β1 and YFP-β2 compete with the endogenous β1 subunits for binding to the limited amount of the endogenous α1 subunits (34). Only the α1-assembled β subunits exit the ER, whereas the unassembled subunits are retained in the ER and rapidly degraded (34). As a result of this competition, a fraction of α1-β1 heterodimers exported from the ER is replaced by α1-(YFP-β) heterodimers, explaining the decrease in the amount of the mature endogenous β1 subunit in the basolateral membrane in both YFP-β1- and YFP-β2-expressing cell lines as compared with non-transfected MDCK cells (Fig. 6C). YFP-β1- and YFP-β2-expressing cell lines have similar quantities of exogenous and endogenous Na,K-ATPase subunits in the plasma membrane (Fig. 6C), whereas the abundance of the ER-located α1-unbound YFP-β2 is greater than that of YFP-β1 (Fig. 5 and Fig. 6, A and B). Because the ER retention of YFP-β1 or YFP-β2 is due to their competition with the same number of endogenous β1 subunits for binding to the α1 subunit, these results imply that the β2 subunit has lower affinity for the α1 subunit than does the β1 subunit.
This interpretation, however, is complicated by the fact that α1-unassembled β subunits and β-unassembled α1 subunits are not freely floating in the ER. Instead, the orphan α and β subunits are bound to ER chaperones (33, 41), which facilitate normal folding of the subunits and possibly the assembly process per se. The β2 but not the β1 subunit persistently binds calnexin in the ER, suggesting that it undergoes repeated calnexin-assisted folding prior to its assembly with the α subunit (29, 33). Persistent calnexin binding to glycoproteins is dependent on repeated cycles of de- and reglucosylation of glycoprotein N-glycans by the ER glucosidase and UGGT1, respectively (42). It is possible that the β2 subunit is not completely folded by the time when calnexin is dissociated from deglucosylated β2 subunit and thus is recognized by the folding sensing enzyme, UGGT1 (43). UGGT1 reglucosylates the β2 subunit N-glycans, which results in repeated calnexin binding. However, it cannot be excluded that calnexin-free β2 subunits bind UGGT1 not because they are misfolded, but because they fail to assemble with the α1 subunits due to their lower α1-binding affinity as compared with that of the β1 subunits. The UGGT1-mediated reglucosylation of the β2 subunit would then induce its re-binding to calnexin. Therefore, both longer association with calnexin and greater accumulation in the ER of the β2 subunit as compared with those of the β1 subunit could result from either the longer time required for folding of the β2 subunit or its lower affinity to the α1 subunit. The lower binding affinity of the β2 subunit toward the α1 subunit is anticipated from the differences between the α1-interacting residues of the β1 subunit and the corresponding residues of the β2 subunit (Fig. 7B).
The α1-β2 heterodimers are less resistant to the disruptive effect of various detergents than the α1-β1 complexes. Co-immunoprecipitation of the Na,K-ATPase α1 subunit with YFP-β2 was observed only in selected detergents, and the amount of co-precipitated α1 subunit varied in these detergents (Fig. 7), showing that the α1-β2 complex is partially or completely disrupted by the majority of tested detergents. On the other hand, the amount of the Na,K-ATPase α1 subunit that co-precipitated with YFP-β1 is the same in all tested detergents, indicating that the α1-β1 complex is fully preserved in these detergents (Fig. 7). These results are in agreement with previously reported disruption of α1-β2, but not of the α1-β1 or α2-β2 complexes formed in Xenopus oocytes by Triton X-100 (44, 45).
Selective Formation of α2-β2 Na,K-ATPase in Mouse Brain
The β2 subunit of the Na,K-ATPase was first discovered as AMOG (19). Immunoaffinity purification of AMOG from mouse brain by using AMOG-specific antibody resulted in co-purification of a 100-kDa protein that later was identified as the Na,K-ATPase α2 subunit (and possibly α3 subunit), but not the α1 subunit (38). Consistent with these data, we found that immunoprecipitation of the β2 subunit from mouse brain resulted in co-immunoprecipitation of the α2, but not of the α1 subunit (Fig. 3B). Conversely, immunoprecipitation of the α2 subunit selectively co-precipitated the β2 subunit (Fig. 3C and Fig. 4). Six isoforms of the Na,K-ATPase are expressed in the brain (9, 37). Both α2 and β2 subunits are expressed predominantly in glial cells (37), so the formation of the α2-β2 complexes is in part due to the cell-specific co-expression of the two isoforms. However, both α2 and β2 subunits are also found in subsets of neurons (37). Similarly, the α1 and β1 isoforms are expressed in both glial cells and neurons. With the exception of the neuron-specific α3 subunit, other isoforms are expressed in both neurons and glial cells (37, 46–48). Even though there are cell- and region-specific differences in expression of various isoforms, many cell types in brain contain multiple Na,K-ATPase subunit isoforms (9, 37, 46). Accordingly, co-expression of the α1, α2, and β2 subunits in the same cells in rat brain cortex is detected here by immunofluorescence (Fig. 2B). Therefore, the preferential formation of the α2-β2 complexes in the brain is determined not only by cell-specific co-expression of these isoforms, but also by their binding preferences. Preferential formation of the α2-β2 was also detected in heart and adrenal medullary cells, where the α1 subunit is more abundant than the α2 subunit (26, 27), emphasizing preferential β2 subunit binding to the α2 subunit.
Interestingly, the α2-β2 complexes are less stable than complexes of the α1 or α3 subunit with either β1 or β3 subunit (Fig. 4). Recent studies have demonstrated that α2-β1 complexes are less stable to heat and detergents than α1-β1 or α3-β1 complexes perhaps due to weaker interactions of the α2 subunit with phosphatidylserine, which stabilizes the protein (49). Thus, it is possible that detergent-mediated disruption of α2-β2 complexes (Fig. 4) is the result of displacement of selectively bound phosphatidylserine.
Preferential assembly of α2 and β2 isoforms in the brain may have several implications. The β subunits are known to modify the kinetic properties of the α isoforms. The α2-β2 heterodimer has the lowest K+ affinity among nine different α-β heterodimers formed by each of the three α subunit isoforms (α1-α3) and each of the three β subunit isoforms (β1-β3) (25). Thus, assembly of the α2 subunit preferentially with the β2 isoform may be crucial to restore external K+ homeostasis after a series of action potentials in the nervous system because the α2-β2 heterodimer would respond to an increase in external K+ because of its low K+ affinity (25). In astrocytes, the Na,K-ATPase α2 subunits form complexes with different glutamate transporters, and glutamate inward transport is inhibited by ouabain, suggesting a specific role of the Na,K-ATPase α2 subunit in reuptake of glutamate from the synaptic cleft (50). Because the activity of the least K+-sensitive α2-β2 isoform would increase more at the elevated external K+ concentration, assembly with the β2 subunit may be important for the specific role of the Na,K-ATPase α2 subunit in glutamate clearance.
Considerable evidence exists for the presence of endogenous ouabain-like molecules in mammalian tissues that may serve to regulate Na,K-ATPase activity (3, 14, 51). Particularly, a signaling role of the Na+/K+-ATPase has been demonstrated in regulating synaptic plasticity and dendritic growth in cortical neurons (52). The human α1, α2, and α3 isoforms have similar ouabain affinities (25). However, the lowest K+ affinity of the α2-β2 isoform implies that this heterodimer has the lowest K+/ouabain antagonism as compared with other α-β heterodimer isoforms (25). So, at physiological K+ concentrations, ouabain and endogenous ouabain-like compounds may bind predominantly to the α2-β2 isoform and to a lesser extent to other complexes and thus specifically regulate the α2-dependent signaling pathways.
Natural in vivo mutations in the α2 subunit are associated with familial hemiplegic migraine and epilepsy (16). Most of these mutations cause functional defects in active Na+ and K+ transport and impaired clearance of extracellular K+ or glutamate due to either the impairment of maturation and hence plasma membrane delivery of the enzyme, or the loss of the catalytic activity (16, 53). It is known that neurological diseases, particularly epilepsy, are closely associated with the ER stress-related retention of essential ion transporters in the ER (54–57). We showed recently that the β2 isoform is much more sensitive to the ER stress than the β1 isoform (33). Because the β subunit is essential for maturation of the Na,K-ATPase α-β heterodimers (58) and the α2 selectively forms a complex with the β2 isoform in the brain, it is possible that stress-induced impairment of the β2 subunit folding in the ER increases the ER retention of the α2 subunit, which decreases the Na,K-ATPase ion transport activity of the α2β2 Na,K-ATPase and thus contributes to epilepsy. Consistent with this hypothesis, abnormalities in distribution of the β2 subunit are linked to epilepsy (21, 22).
Therefore, the selectivity of α-β assembly, which is determined both by cell-specific expression and by isoform-specific binding preferences of α and β subunits, is crucial for cell- and tissue-specific functions of the Na,K-ATPase.
Acknowledgment
We thank Dr. W. James Ball, Jr. (University of Cincinnati) for providing the antibodies against the Na,K-ATPase β1 subunit.
This work was supported, in whole or in part, by National Institutes of Health Grants DK077149 (to O. V.), DK058333 (to G. S.), and GM39500 (to J. H. K.).
- AMOG
- adhesion molecule on glia
- YFP-β1 and YFP-β2
- the fusion proteins between the yellow fluorescent protein and the Na,K-ATPase β1 subunit and β2 subunit, respectively
- PNGase F
- peptide:N-glycosidase F
- Endo H
- Endo-β-N-acetylglucosaminidase H
- ER
- endoplasmic reticulum
- DOC
- sodium deoxycholate
- DDM
- n-dodecyl β-d-maltoside
- NTCP
- sodium-taurocholate cotransporting polypeptide
- MDCK
- Madin-Darby canine kidney
- UGGT1
- UDP-glucose glycoprotein glucosyltransferase 1.
REFERENCES
- 1. Sweadner K. J. (1989) Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta 988, 185–220 [DOI] [PubMed] [Google Scholar]
- 2. Kaplan J. H. (2002) Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 [DOI] [PubMed] [Google Scholar]
- 3. Lingrel J. B. (2010) The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 72, 395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sweadner K. J., Rael E. (2000) The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68, 41–56 [DOI] [PubMed] [Google Scholar]
- 5. Geering K. (2008) Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 17, 526–532 [DOI] [PubMed] [Google Scholar]
- 6. Blanco G., Mercer R. W. (1998) Isozymes of the Na,K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–650 [DOI] [PubMed] [Google Scholar]
- 7. Blanco G. (2005) Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 25, 292–303 [DOI] [PubMed] [Google Scholar]
- 8. McLean W. J., Smith K. A., Glowatzki E., Pyott S. J. (2009) Distribution of the Na,K-ATPase α subunit in the rat spiral ganglion and organ of corti. J. Assoc. Res. Otolaryngol. 10, 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dobretsov M., Stimers J. R. (2005) Neuronal function and α3 isoform of the Na/K-ATPase. Front Biosci. 10, 2373–2396 [DOI] [PubMed] [Google Scholar]
- 10. Woo A. L., James P. F., Lingrel J. B. (2000) Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J. Biol. Chem. 275, 20693–20699 [DOI] [PubMed] [Google Scholar]
- 11. Jimenez T., McDermott J. P., Sánchez G., Blanco G. (2011) Na,K-ATPase α4 isoform is essential for sperm fertility. Proc. Natl. Acad. Sci. U.S.A. 108, 644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malik N., Canfield V. A., Beckers M. C., Gros P., Levenson R. (1996) Identification of the mammalian Na,K-ATPase 3 subunit. J. Biol. Chem. 271, 22754–22758 [DOI] [PubMed] [Google Scholar]
- 13. Arystarkhova E., Sweadner K. J. (1997) Tissue-specific expression of the Na,K-ATPase β3 subunit. The presence of β3 in lung and liver addresses the problem of the missing subunit. J. Biol. Chem. 272, 22405–22408 [DOI] [PubMed] [Google Scholar]
- 14. Blaustein M. P., Zhang J., Chen L., Song H., Raina H., Kinsey S. P., Izuka M., Iwamoto T., Kotlikoff M. I., Lingrel J. B., Philipson K. D., Wier W. G., Hamlyn J. M. (2009) The pump, the exchanger, and endogenous ouabain: Signaling mechanisms that link salt retention to hypertension. Hypertension 53, 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rindler T. N., Dostanic I., Lasko V. M., Nieman M. L., Neumann J. C., Lorenz J. N., Lingrel J. B. (2011) Knock-out of the Na,K-ATPase α-isoform in the cardiovascular system does not alter basal blood pressure but prevents ACTH-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 301, H1396–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bøttger P., Doğanli C., Lykke-Hartmann K. (2012) Migraine- and dystonia-related disease mutations of Na+/K+-ATPases: Relevance of behavioral studies in mice to disease symptoms and neurological manifestations in humans. Neurosci. Biobehavioral Rev. 36, 855–871 [DOI] [PubMed] [Google Scholar]
- 17. Vagin O., Dada L. A., Tokhtaeva E., Sachs G. (2012) The Na,K-ATPase α1β1 heterodimer as a cell adhesion molecule in epithelia. Am. J. Physiol. Cell Physiol. 302, C1271-C1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cereijido M., Contreras R. G., Shoshani L., Larre I. (2012) The Na+,K+-ATPase as self-adhesion molecule and hormone receptor. Am. J. Physiol. Cell Physiol. 302, C473–481 [DOI] [PubMed] [Google Scholar]
- 19. Antonicek H., Persohn E., Schachner M. (1987) Biochemical and functional characterization of a novel neuron-glia adhesion molecule that is involved in neuronal migration. J. Cell Biol. 104, 1587–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rajasekaran S. A., Rajasekaran A. K. (2009) Na,K-ATPase and epithelial tight junctions. Front Biosci. 14, 2130–2148 [DOI] [PubMed] [Google Scholar]
- 21. Boer K., Spliet W. G., van Rijen P. C., Jansen F. E., Aronica E. (2010) Expression patterns of AMOG in developing human cortex and malformations of cortical development. Epilepsy Res. 91, 84–93 [DOI] [PubMed] [Google Scholar]
- 22. Boer K., Troost D., Timmermans W., van Rijen P. C., Spliet W. G., Aronica E. (2010) PI3K-mTOR signaling and AMOG expression in epilepsy-associated glioneuronal tumors. Brain Pathol. 20, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Boom J., Wolter M., Blaschke B., Knobbe C. B., Reifenberger G. (2006) Identification of novel genes associated with astrocytoma progression using suppression subtractive hybridization and real-time reverse transcription-polymerase chain reaction. Int. J. Cancer 119, 2330–2338 [DOI] [PubMed] [Google Scholar]
- 24. Senner V., Schmidtpeter S., Braune S., Püttmann S., Thanos S., Bartsch U., Schachner M., Paulus W. (2003) AMOG/β2 and glioma invasion: Does loss of AMOG make tumor cells run amok? Neuropathol. Appl. Neurobiol. 29, 370–377 [DOI] [PubMed] [Google Scholar]
- 25. Crambert G., Hasler U., Beggah A. T., Yu C., Modyanov N. N., Horisberger J. D., Lelièvre L., Geering K. (2000) Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J. Biol. Chem. 275, 1976–1986 [DOI] [PubMed] [Google Scholar]
- 26. Harada K., Lin H., Endo Y., Fujishiro N., Sakamoto Y., Inoue M. (2006) Subunit composition and role of Na+,K+-ATPases in ventricular myocytes. J. Physiol. Sci. 56, 113–121 [DOI] [PubMed] [Google Scholar]
- 27. Lin H., Ozaki S., Fujishiro N., Takeda K., Imanaga I., Prestwich G. D., Inoue M. (2005) Subunit composition and role of Na+,K+-ATPases in adrenal chromaffin cells. J. Physiol. 564, 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vagin O., Tokhtaeva E., Sachs G. (2006) The role of the β1 subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion. J. Biol. Chem. 281, 39573–39587 [DOI] [PubMed] [Google Scholar]
- 29. Tokhtaeva E., Munson K., Sachs G., Vagin O. (2010) N-glycan-dependent quality control of the Na,K-ATPase β2 subunit. Biochemistry 49, 3116–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vagin O., Turdikulova S., Sachs G. (2005) Recombinant addition of N-glycosylation sites to the basolateral Na,K-ATPase β1 subunit results in its clustering in caveolae and apical sorting in HGT-1 cells. J. Biol. Chem. 280, 43159–43167 [DOI] [PubMed] [Google Scholar]
- 31. Sun Y., Ball W. J., Jr. (1992) Determination of Na+-K+-ATPase α- and β-isoforms and kinetic properties in mammalian liver. Am. J. Physiol. 262, C1491–1499 [DOI] [PubMed] [Google Scholar]
- 32. Gatto C., Wang A. X., Kaplan J. H. (1998) The M4M5 cytoplasmic loop of the Na,K-ATPase, overexpressed in Escherichia coli, binds nucleoside triphosphates with the same selectivity as the intact native protein. J. Biol. Chem. 273, 10578–10585 [DOI] [PubMed] [Google Scholar]
- 33. Tokhtaeva E., Sachs G., Vagin O. (2010) Diverse pathways for maturation of the Na,K-ATPase β1 and β2 subunits in the endoplasmic reticulum of Madin-Darby canine kidney cells. J. Biol. Chem. 285, 39289–39302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tokhtaeva E., Sachs G., Vagin O. (2009) Assembly with the Na,K-ATPase α1 subunit is required for export of β1 and β2 subunits from the endoplasmic reticulum. Biochemistry 48, 11421–11431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gottardi C. J., Dunbar L. A., Caplan M. J. (1995) Biotinylation and assessment of membrane polarity: Caveats and methodological concerns. Am. J. Physiol. 268, F285–295 [DOI] [PubMed] [Google Scholar]
- 36. Kroepfl J. F., Gardinier M. V. (2001) Identification of a basolateral membrane targeting signal within the cytoplasmic domain of myelin/oligodendrocyte glycoprotein. J. Neurochem 77, 1301–1309 [DOI] [PubMed] [Google Scholar]
- 37. Peng L., Martin-Vasallo P., Sweadner K. J. (1997) Isoforms of Na,K-ATPase α and β subunits in the rat cerebellum and in granule cell cultures. J. Neurosci. 17, 3488–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gloor S., Antonicek H., Sweadner K. J., Pagliusi S., Frank R., Moos M., Schachner M. (1990) The adhesion molecule on glia (AMOG) is a homologue of the β subunit of the Na,K-ATPase. J. Cell Biol. 110, 165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009) Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature 459, 446–450 [DOI] [PubMed] [Google Scholar]
- 40. Clifford R. J., Kaplan J. H. (2008) β-Subunit overexpression alters the stoicheometry of assembled Na,K-ATPase subunits in MDCK cells. Am. J. Physiol. Renal Physiol. 295, F1314–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beggah A. T., Geering K. (1997) α and β subunits of Na,K-ATPase interact with BiP and calnexin. Ann. N.Y. Acad. Sci. 834, 537–539 [DOI] [PubMed] [Google Scholar]
- 42. Molinari M., Galli C., Vanoni O., Arnold S. M., Kaufman R. J. (2005) Persistent glycoprotein misfolding activates the glucosidase II/UGT1-driven calnexin cycle to delay aggregation and loss of folding competence. Mol. Cell 20, 503–512 [DOI] [PubMed] [Google Scholar]
- 43. Ritter C., Quirin K., Kowarik M., Helenius A. (2005) Minor folding defects trigger local modification of glycoproteins by the ER folding sensor GT. EMBO J. 24, 1730–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmalzing G., Ruhl K., Gloor S. M. (1997) Isoform-specific interactions of Na,K-ATPase subunits are mediated via extracellular domains and carbohydrates. Proc. Natl. Acad. Sci. U.S.A. 94, 1136–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geering K., Crambert G., Yu C., Korneenko T. V., Pestov N. B., Modyanov N. N. (2000) Intersubunit interactions in human X,K-ATPases: Role of membrane domains M9 and M10 in the assembly process and association efficiency of human, nongastric H,K-ATPase α subunits (ATP1al1) with known β subunits. Biochemistry 39, 12688–12698 [DOI] [PubMed] [Google Scholar]
- 46. McGrail K. M., Phillips J. M., Sweadner K. J. (1991) Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: Both neurons and glia can express more than one Na,K-ATPase. J. Neurosci. 11, 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cameron R., Klein L., Shyjan A. W., Rakic P., Levenson R. (1994) Neurons and astroglia express distinct subsets of Na,K-ATPase α and β subunits. Brain Res. Mol. Brain Res. 21, 333–343 [DOI] [PubMed] [Google Scholar]
- 48. Watts A. G., Sanchez-Watts G., Emanuel J. R., Levenson R. (1991) Cell-specific expression of mRNAs encoding Na+,K+-ATPase α- and β-subunit isoforms within the rat central nervous system. Proc. Natl. Acad. Sci. U.S.A. 88, 7425–7429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kapri-Pardes E., Katz A., Haviv H., Mahmmoud Y., Ilan M., Khalfin-Penigel I., Carmeli S., Yarden O., Karlish S. J. (2011) Stabilization of the α2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket. J. Biol. Chem. 286, 42888–42899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rose E. M., Koo J. C., Antflick J. E., Ahmed S. M., Angers S., Hampson D. R. (2009) Glutamate transporter coupling to Na,K-ATPase. J. Neurosci. 29, 8143–8155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bagrov A. Y., Shapiro J. I. (2008) Endogenous digitalis: Pathophysiologic roles and therapeutic applications. Nat. Clin. Pract. Nephrol. 4, 378–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Desfrere L., Karlsson M., Hiyoshi H., Malmersjö S., Nanou E., Estrada M., Miyakawa A., Lagercrantz H., El Manira A., Lal M., Uhlén P. (2009) Na,K-ATPase signal transduction triggers CREB activation and dendritic growth. Proc. Natl. Acad. Sci. U.S.A. 106, 2212–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leo L., Gherardini L., Barone V., De Fusco M., Pietrobon D., Pizzorusso T., Casari G. (2011) Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 7, e1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamamoto A., Murphy N., Schindler C. K., So N. K., Stohr S., Taki W., Prehn J. H., Henshall D. C. (2006) Endoplasmic reticulum stress and apoptosis signaling in human temporal lobe epilepsy. J. Neuropathol. Exp. Neurol. 65, 217–225 [DOI] [PubMed] [Google Scholar]
- 55. Hirose S. (2006) A new paradigm of channelopathy in epilepsy syndromes: Intracellular trafficking abnormality of channel molecules. Epilepsy Res. 70, S206–217 [DOI] [PubMed] [Google Scholar]
- 56. Paschen W. (2003) Endoplasmic reticulum: A primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium 34, 365–383 [DOI] [PubMed] [Google Scholar]
- 57. Doyle K. M., Kennedy D., Gorman A. M., Gupta S., Healy S. J., Samali A. (2011) Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J. Cell. Mol. Med. 15, 2025–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Geering K. (2001) The functional role of beta subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 33, 425–438 [DOI] [PubMed] [Google Scholar]