Background: PKC is extremely important for a wide array of cellular processes. However, its inactivation is poorly understood.
Results: FADD deficiency or phosphoryl-mimicking mutation (FADD-D) leads to accumulation of phosphorylated PKC and sustained signaling.
Conclusion: The apoptotic adapter FADD is required for PKC dephosphorylation, degradation and signaling inactivation and may be regulated by its phosphorylation.
Significance: FADD is critical for PKC dephosphorylation, stability, and signaling termination.
Keywords: Cell Migration, Cell Signaling, PP2A, Protein Kinase C (PKC), Protein Phosphorylation, FADD
Abstract
Protein kinase C (PKC) plays important roles in diverse cellular processes. PKC has been implicated in regulating Fas-associated protein with death domain (FADD), an important adaptor protein involved in regulating death receptor-mediated apoptosis. FADD also plays an important role in non-apoptosis processes. The functional interaction of PKC and FADD in non-apoptotic processes has not been examined. In this study, we show that FADD is involved in maintaining the phosphorylation of the turn motif and hydrophobic motif in the activated conventional PKC (cPKC). A phosphoryl-mimicking mutation (S191D) in FADD (FADD-D) abolished the function of FADD in the facilitation of the turn motif and hydrophobic motif dephosphorylation of cPKC, suggesting that phosphorylation of Ser-191 negatively regulates FADD. We show that FADD interacts with PP2A, which is a major phosphatase involved in dephosphorylation of activated cPKC and FADD deficiency abolished PP2A mediated dephosphorylation of cPKC. We show that FADD deficiency leads to increased stability and activity of cPKC, which, in turn, promotes cytoskeleton reorganization, cell motility, and chemotaxis. Collectively, these results reveal a novel function of FADD in a non-apoptotic process by modulating cPKC dephosphorylation, stability, and signaling termination.
Introduction
Fas-associated protein with death domain (FADD),2 an important adaptor protein that couples death receptors with caspase-8 following extracellular death signals (1), is critical for death receptor-mediated apoptosis (2, 3). FADD has also been implicated in non-apoptotic processes. Embryonic death caused by FADD deficiency in mice is not attributable to impaired apoptosis (4). FADD-deficient thymocytes are blocked at the CD4-CD8 stage during T cell maturation (5, 6). FADD-deficient T cells were shown to have impaired proliferation in response to anti-CD3/anti-CD28 stimulation and disrupted cell cycle progression (6). Recently, FADD was reported to have critical roles in negatively regulating necroptosis (7–9).
PKC regulates many important physiological and pathological processes, including proliferation, differentiation, and migration (10–12). The PKC family of kinases can be divided into three subfamilies on the basis of their response to second messengers: conventional (cPKC) (α, βI, βII, and γ), novel (nPKC) (δ, ϵ, η, and θ), and atypical (ι/λ and ξ). In addition to regulation by intracellular second messengers, the maturation, stability, and activity of cPKC is regulated by three phosphorylation residues located in the activation loop, turn motif (TM) and hydrophobic motif (HM) (13). Newly synthesized PKC α or βII is phosphorylated in its activation loop by phosphoinositide-dependent kinase 1 (14, 15), which allows PKC to autophosphorylate the HM and TM (16). Recently, the mammalian target of rapamycin complex 2 was shown to mediate TM and HM phosphorylation of PKC α and βII in vivo (17, 18). Upon phosphorylation of these three residues, cPKCs are stabilized and ready to receive signals from second messengers.
PKC has been shown to modulate the recruitment of FADD to Fas (19–21). Specifically, PKCξ has been shown to promote FADD phosphorylation, which, in turn, has been proposed to be an important mechanism for Fas resistance (22). Phosphorylation of serine 191 (Ser-191) in the C terminus of FADD has been proposed to be critical for its non-apoptotic function. Mice bearing the mutation mimicking constitutive phosphorylation (S191D) of FADD are immunologically compromised, similar to FADD-deficient mice (23). FADD is highly phosphorylated in tumors with poor prognosis (24–26). Potential regulators of non-apoptotic activities of FADD, including CKIα, a kinase that mediates FADD phosphorylation, have been implicated in regulating non-apoptotic activities of FADD (27, 28). However, the functional consequence of FADD phosphorylation at cellular levels has yet to be elucidated.
The activation mechanisms of PKC are well documented, but the molecular mechanisms of PKC inactivation are less well understood. Dephosphorylation of TM and HM are critical for the inactivation and degradation of PKC α and βII (29–31). Several phosphatases are linked to TM and HM dephosphorylation. The heterotrimeric type 2A phosphatase (PP2A) physically associates with PKC and dephosphorylates PKCα and β upon phorbol 12-myristate 13-acetate (PMA) stimulation on the membrane (32, 33). Both PP2A and PP1 can dephosphorylate PKCα and βII in vitro (34, 35). A recent report showed that PH domain and Leucine rich repeat Protein Phosphatases (PHLPP), a novel Ser/Thr phosphatase, can destabilize cellular PKCα and βII by dephosphorylating the HM of PKC (36).
Here, we report that FADD is required for the PP2A catalytic subunit to recruit cPKC. In the absence of FADD, endogenous PKCα no longer interacted with PP2Ac, so was not dephosphorylated and resistant to degradation. More interestingly, the S191D mutation abolished the ability of FADD to promote PP2Ac recruiting PKCβII and promoted the resistance of PKCα and PKCβII to degradation. Both FADD deficiency and the S191D mutant enhanced cPKC phosphorylation, stability, and signaling. Moreover, up-regulated cPKC signaling promoted cytoskeleton remodeling and cell motility. Collectively, these findings suggest that FADD could regulate cPKC dephosphorylation and signaling termination.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
Full-length wild-type βII cDNA was kindly provided by Dr. Christer Larsson (37) and cloned into the pcDNA3.1 vector. PP2Ac was kindly provided by Dr. Tsuyoshi Ikehara (38) and cloned into the pRK5-FLAG vector. PKCβII-T634A/T638A/T641A, PKCβII-S660A, and PKCβII (S660E) were kindly provided by Dr. Alexandra Newton (36). Go6976 was purchased from Merck. PMA, MG132, protein inhibitor mixture, and CHX were from Sigma. Texas Red-X phalloidin was purchased from Invitrogen.
Immunoblotting
Cells were lysed in lysis buffer (50 mm Tris-HCl (pH 7.4), 250 mm NaCl, 0.5%Triton X-100, 50 mm NaF, 2 mm EDTA, 1 mm Na3VO4, and protein inhibitor mixture) for 30 min and then centrifuged (13,000 × g, 10 min, 4 °C). Anti-ERK, anti-Raf-1, anti-PKCα, and anti-PKCδ were purchased from BD Biosciences. Anti-phospho-HM of PKCα/β, anti-phospho-TM of PKCα/β, anti-phospho-(Ser) PKC substrate, anti-phospho-MARCKS (Ser-152/156), anti-phospho-MEK1, anti-phospho-Raf-1, anti-phospho-ERK1, and anti-phospho-FADD (Ser-191) were purchased from Cell Signaling Technology. Anti-PKCβII (c-18), anti-FADD, and anti-HA were purchased from Santa Cruz Biotechnology. Anti-PP2Ac was purchased from Millipore. Anti-FLAG was purchased from Stratagene. For preparation of soluble and insoluble fractions, the cells was lysed in lysis buffer and centrifuged (15,000 × g, 10 min, 4 °C), and the supernatant was used as soluble fraction. The pellet was sonicated with 1× sample buffer and used as in soluble fraction. All the immunoblotting experiments were repeated three to five times. One representative result for each experiment is shown.
Cell Culture, Stable Cell Line Construction via Retrovirus Infection, and Mice
293T and MEFs were cultured in DMEM (Hyclone) containing 10% FBS (Hyclone) with 50 units/ml penicillin/streptomycin. FADD mutant cell lines, FADD-A and FADD-D, were constructed on the basis of FADD−/− MEFs. The construction and validation of all cell lines were performed in the laboratory of Dr. Astar Winoto (University of California, Berkeley). Briefly, FADD-A and FADD-D mutant cDNAs were generated using PCR, as described previously (23), subcloned into the retroviral vector MSCV-Zeocin, and transfected into Bosc packing cells. Supernatant was used to infect FADD KO MEFs. Infected MEFs were selected with Zeocin for 1 month. The expression level of FADD and FADD mutants was examined using Western blot analyses and was similar across different MEFs. The FADD phosphorylation mutant mice (FADD-D in FADD−/− alleles) were generated as reported previously (23). Briefly, the FADD-D transgenic mice were generated and then mated to FADD+/− mice for at least two generations to obtain mice that express only FADD-D (with FADD−/− alleles). The mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care Jiangsu Province-accredited specific pathogen free (SPF) animal facility in Nanjing Drum Tower Hospital, and all animal protocols were approved by the Animal Care and Use Committee of the School of Life Sciences of Nanjing University.
Immunoprecipitation
Muscular tissues were homogenized in a lysis buffer containing 50 mm Tris-HCl (pH 7.4), 250 mm NaCl, 0.5%Triton X-100, 50 mm NaF, 2 mm EDTA, 1 mm Na3VO4, and protein inhibitor mixture and immunoprecipitated with anti-PKCβII antibody and protein G-agarose (Millipore). MEFs were directly lysed in the lysis buffer. The immunoprecipitates were washed six times with lysis buffer and subjected to Western blot analysis.
Phosphatase Activity Assay
Cells were lysed in lysis buffer without NaF and Na3VO4 and centrifuged for 20 min at 13,000 × g. PP2Ac was immunoprecipitated, and the activity was measured with the PP2A immunoprecipitation phosphatase assay kit (Millipore).
F-actin Staining, Wound Closure, Transwell Assays, and Chemotaxis
After the indicated treatments, cells were fixed with 4% formaldehyde for 1 h, penetrated with 0.5% Triton X-100 for 1 h, and then stained with Texas Red-X phalloidin for 1 h. F-actin was then visualized by microscopy (Carl Zeiss, Axioplan 2). For the wound closure assay, confluent cell monolayers were cultured in serum-free DMEM for 24 h and scraped manually with a pipette tip. The culture medium was replaced with fresh medium containing PMA or FBS. The transwell migration assay was performed using an 8-μm PET 24-well Millicell culture insert with hanging geometry (Millipore). Cells were cultured in serum-free medium overnight and treated with inhibitor for 2 h before trypsinization. 100-μl single-cell suspension at the concentration of 106 cells/ml was added to the inner side of the transwell. After 5 h, cells on the inner side of the membrane were wiped clean with a cotton swab. Cells on the outer side were fixed with 4% formaldehyde prior to staining with crystal violet. Images were captured with a ×10 objective lens. The average number of migrated cells in five to six randomly chosen fields of view per insert was taken to quantify the extent of migration. For the chemotaxis assay, splenocytes or mesenteric lymphocytes were isolated and cultured in serum-free medium for 2 h. A single-cell suspension was used for the transwell assay. After stimulation with SDF-1 or FBS, migrated cells were quantified using a QCMTM Chemotaxis cell migration assay (Millipore).
RESULTS
FADD Regulates cPKC Protein Levels
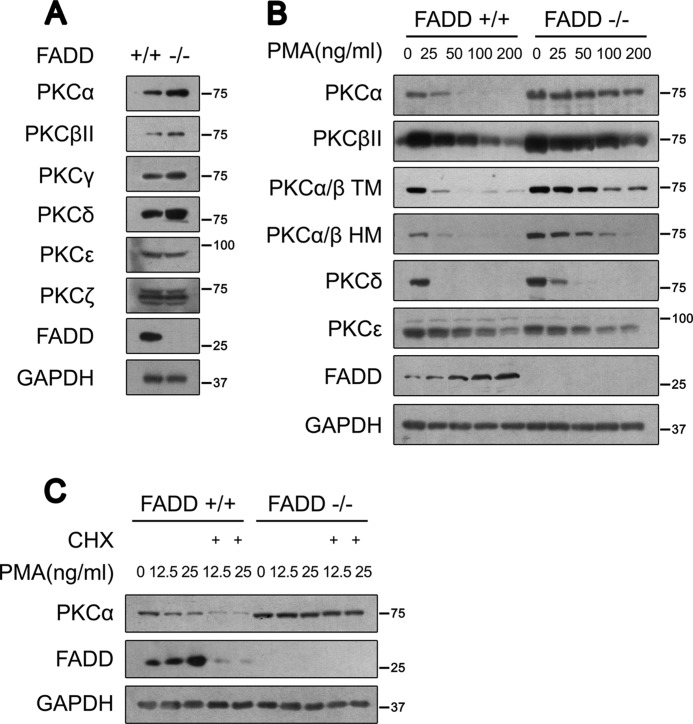
To study the effects of FADD on PKC, we examined FADD-deficient murine embryonic fibroblast cells (FADD−/− MEFs). Interestingly, we found that protein levels of all cPKCs and one nPKC, PKCδ, were increased in FADD−/− MEFs as compared with FADD+/+ MEFs (Fig. 1A). In contrast, the protein levels of other nPKCs, such as PKCϵ, and atypical PKCs, such as PKCξ, in FADD−/− MEFs were similar to levels in FADD+/+ MEFs (Fig. 1A). To examine whether the accumulation of cPKC and nPKC in FADD−/− MEFs might be attributable to increased stability, we treated cells with PMA in a long-term manner (12 h) with lower and milder concentrations that induced endogenous PKCs degradation. Compared with that of FADD+/+ MEFs, PKCα degradation was greatly reduced in FADD−/− MEFs upon PMA treatment (Fig. 1B). Likewise, the degradation of both PKCβII and PKCγ was also decreased in FADD−/− MEFs (Fig. 1B and data not shown). In contrast to cPKC, the degradation of PKCδ was reduced only at low PMA concentrations (Fig. 1B). On the other hand, the rate of degradation of the nPKC PKCϵ was comparable between FADD+/+ and FADD−/− MEFs (Fig. 1B). Interestingly, we found that the protein levels of FADD are induced in response to PMA treatment in a dose-dependent manner (Fig. 1B).
FIGURE 1.
Accumulation of cPKC and nPKC proteins in FADD−/− MEFs. A, expression levels of endogenous PKC isoforms in FADD+/+ and FADD−/− MEFs were monitored by Western blot analysis. B, degradation of endogenous PKCs in FADD+/+ and FADD−/− MEFs in response to PMA treatment at the indicated concentrations for 12 h. C, Western blot analysis of PKCα in FADD+/+ and FADD−/− MEFs upon CHX and PMA cotreatment. MEFs were treated with PMA plus CHX (20 μg/ml) for 12 h.
Because cPKCs such as PKCα and PKCβ are most affected by FADD deficiency, we concentrated our subsequent analysis of PKCα and PKCβ. We found that levels of both PKCα and PKCβ mRNA were comparable between FADD+/+ and FADD−/− MEFs after PMA stimulation (data not shown). Although inhibiting de novo protein synthesis with CHX significantly promotes the loss of PKCα in FADD+/+ MEFs in response to PMA, CHX had no significant effect on accumulated PKCα in FADD−/− MEFs (Fig. 1C). These data suggest that FADD is involved in posttranslational regulation of cPKC.
To confirm that the increased PKCα protein levels was a consequence of FADD deletion, we reintroduced a FADD expression plasmid into FADD−/− MEFs. Re-expression of FADD restored the PMA-induced PKCα degradation in FADD−/− MEFs (Fig. S1).
FADD Regulates cPKC TM and HM Phosphorylation
Previous studies have indicated that both TM and HM phosphorylation of PKCα/β are important for protein stability (29–31). Therefore, we analyzed TM and HM phosphorylation of cPKC in FADD−/− MEFs. Consistent with the elevated protein level, phospho-TM and phospho-HM of PKCα/β were significantly higher in FADD−/− MEFs than in FADD+/+ MEFs (Fig. 1B).
To differentiate the effect of FADD deficiency on the levels of PKCα protein versus that of phosphorylation, we treated cells with PMA in a short-term manner (3 h) with higher concentrations (500 ng/ml and 1000 ng/ml) to induce rapid phosphorylation loss without affecting protein level. Strikingly, TM dephosphorylation of PKCα/β was largely inhibited in FADD−/− MEFs, whereas HM dephosphorylation was comparable (Fig. 2A). These data suggest that FADD is required for TM dephosphorylation of PKCα/β but less important for HM dephosphorylation.
FIGURE 2.
FADD regulates cPKC TM and HM phosphorylation. A, MEFs were treated with the indicated concentrations of PMA for 3 h. Similar levels of PKCα were loaded to monitor TM and HM dephosphorylation. Phosphorylation of TM and HM was determined by Western blot analysis. B, MEFs were treated with 100 nm rapamycin or 1 μm 17-allylaminogeldanamycin for the time indicated. C, Western blot analysis of PKCα TM and HM phosphorylation after reintroduction of FADD into FADD−/− MEFs. After transfection with empty (-) or FADD (10 μg or 20 μg)-expressing plasmids for 24 h, FADD−/− MEFs were treated with 100 ng/ml PMA for 30 min.
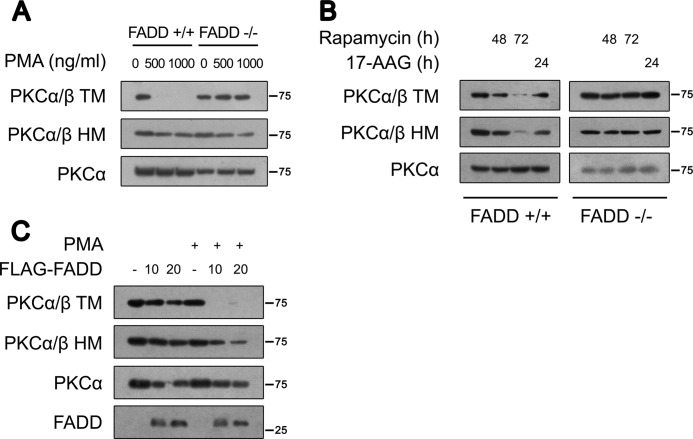
mTORC2 has been shown to be required for cPKC TM and HM phosphorylation (17, 18). Therefore, we evaluated whether TORC2 contributes to the increased TM and HM phosphorylation in FADD−/− MEFs. This is similar to other reports (17, 18), although inhibition of mTORC2 by prolonged rapamycin treatment showed an insignificant effect on total cPKC protein level. Indeed, it reduced TM and HM phosphorylation in FADD+/+ MEFs (Fig. 2B). In contrast, rapamycin had no effect on TM and HM phosphorylation in FADD−/− MEFs (Fig. 2B). In addition, heat shock protein 90 (HSP90) has been reported to protect dephosphorylated PKCα from degradation (39). The HSP90 inhibitor 17-AAG promoted TM and HM dephosphorylation in FADD+/+ MEFs, whereas it had no significant effects on phospho-TM and phospho-HM in FADD−/− MEFs (Fig. 2B). These experiments suggest that the persistent phosphorylation of cPKCs in FADD−/− MEFs is not due to increased phosphorylation.
To confirm the specific effects of FADD deletion on TM and HM phosphorylation in FADD−/− MEFs, we reintroduced a FADD plasmid into FADD−/− MEFs. Consistent with the abovementioned observations, restoration of FADD expression reduced PKCα protein levels. Furthermore, FADD expression reduced TM phosphorylation upon PMA treatment. Similarly, HM phosphorylation was also decreased in FADD-complemented FADD−/− MEFs (Fig. 2C).
FADD Regulates cPKC Stability
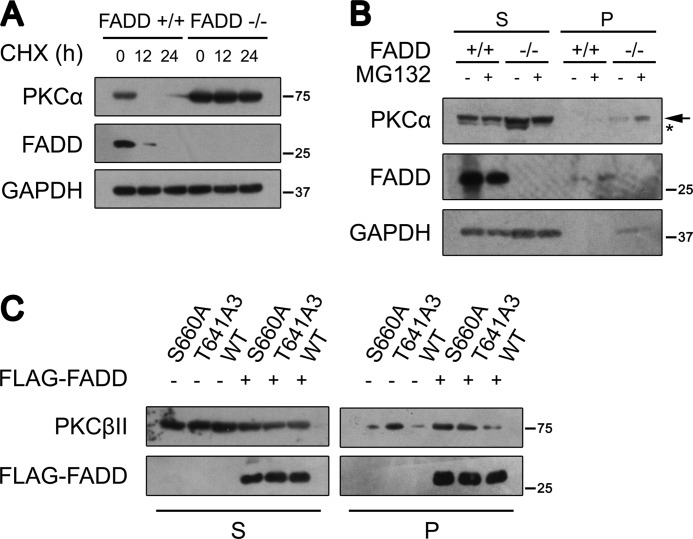
Phosphorylation of cPKC is known to provide resistance to degradation (40). Because cPKC is highly phosphorylated in FADD−/− MEFs, we explored whether the accumulated cPKC was due to increased protein stability in FADD−/− MEFs. We treated cells with CHX to monitor PKCα degradation. CHX reduced PKCα protein levels in FADD+/+ MEFs but had no significant effect on accumulated PKCα in FADD−/− MEFs (Fig. 3A). This result indicates that endogenous PKCα is resistant to degradation in the absence of FADD. It has been well established that mature PKCα is phosphorylated constitutively, stable, and localized in the detergent-soluble compartment. Upon dephosphorylation, PKCα became detergent-insoluble (36). Most of the accumulated endogenous PKCα was present in the detergent-soluble supernatant, consistent with the highly phosphorylated status of PKCα in FADD−/− MEFs (Fig. 3B).
FIGURE 3.
FADD regulates cPKC stability. A, MEFs were treated with 20 μg/ml CHX for the indicated times. PKCα protein level was determined using Western blot analysis. B, Western blot analysis of PKCα in subcellular fractions in FADD+/+ and FADD−/− MEFs. MEFs were fractionated into the detergent-soluble supernatant (S) and detergent-insoluble pellet (P) after treatment with 20 μg/ml MG132 for 12 h. The faster migrating band representing dephosphorylated PKCα is labeled with an asterisk. The slower migrating band representing phosphorylated PKCα is labeled with an arrow. PKCα distribution was determined using Western blot analysis. C, 293T cells were transfected with wild-type PKCβII, the TM mutation (PKCβII-T634A/T638A/T641A), and the HM mutation (PKCβII-S660A) plasmids with or without FADD. Expression of FADD was monitored by FLAG antibody. Cells were fractionated into the detergent-soluble supernatant and detergent-insoluble pellet.
We then determined the effect of FADD on the stability of the TM and HM phosphorylation mutants. We transfected wild-type PKCβII (WT), the TM mutation (PKCβII-T634A/T638A/T641A), and the HM mutation (PKCβII-S660A) into 293T cells in the absence or presence of FLAG-FADD. Consistent with previous studies (17, 18), the TM phosphorylation mutant accumulated more in the detergent-insoluble pellet than WT PKCβII and the HM mutant (Fig. 3C), suggesting a critical role of TM phosphorylation in cPKC stability. Indeed, FADD expression promoted a significant fraction of WT PKCβII and the HM mutant to be localized in the detergent-insoluble pellet (Fig. 3C).
FADD Regulates the Recruitment of PP2A to cPKC
A previous study has reported that TM dephosphorylation of cPKC is okadaic acid-sensitive, whereas HM dephosphorylation is okadaic acid-insensitive (36). Furthermore, PKCα dephosphorylation upon PMA treatment correlates with the presence of a membrane-associated PP2A and HM phosphorylation-defective PKCα can be dephosphorylated by PP2A in vitro (29, 34). To further study the regulation of cPKC by PP2A, we tested if PP2Ac had any effect on cPKC stability. Overexpressed PP2Ac in 293T decreased PKCβII distribution in the detergent-soluble supernatant and promoted its accumulation in the detergent-insoluble pellet (supplemental Fig. S2). PP2A showed a similar effect on PKCα (data not shown). These data indicate that PP2A promotes cPKC dephosphorylation.
To further study the effect of PP2A on TM dephosphorylation, a similar PKCβII protein level was immunoprecipitated from control and PP2Ac-overexpressed lysates. We found that overexpression of PP2Ac promoted significant TM dephosphorylation (supplemental Fig. S3). In addition, PP2Ac also induced HM dephosphorylation (supplemental Fig. S3).
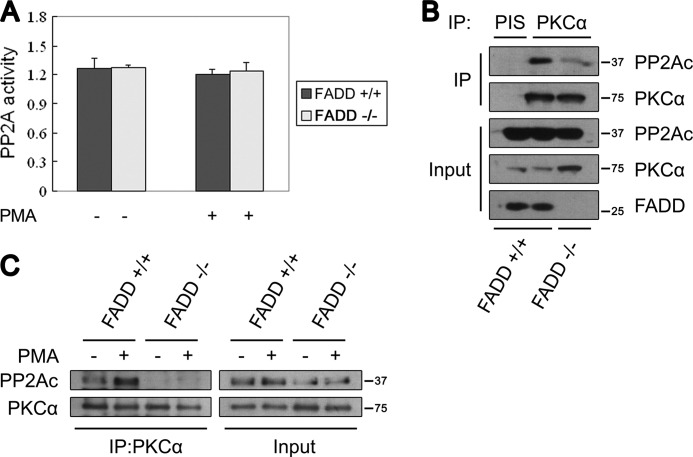
PP2A activity is mainly regulated by two mechanisms: phosphatase catalytic activity and substrate target specificity. Because no significant difference in PP2A catalytic activity was found between FADD+/+ and FADD−/− MEFs (Fig. 4A), we examined the possibility that FADD regulates the targeting specificity of PP2A. Interestingly, PP2Ac coprecipitated with PKCα in FADD+/+ MEFs, but much less PP2Ac coprecipitated with PKCα in FADD−/− MEFs with or without PMA (Fig. 4, B and C). These data indicate that FADD is required for PP2A to recruit cPKC.
FIGURE 4.
FADD regulates the interaction between PKCα and PP2A. A, no difference was seen in the PP2A activity in FADD+/+ and FADD−/− MEFs. PP2Ac was immunoprecipitated from 800 μg MEF lysate with an anti-PP2Ac monoclonal antibody, and a similar amount of PP2Ac was used for PP2A activity analysis. B, PP2Ac and PKCα coimmunoprecipitate in FADD+/+ but not FADD−/− MEFs. PKCα was immunoprecipitated (IP) from the cell lysate of FADD+/+ or FADD−/− MEFs. PIS, preimmune serum. C, PP2Ac and PKCα coimmunoprecipitate in FADD+/+ but not FADD−/− MEFs upon PMA stimulation. MEFs were treated with 1 μg/ml PMA for 30 min.
FADD Controls cPKC Signaling
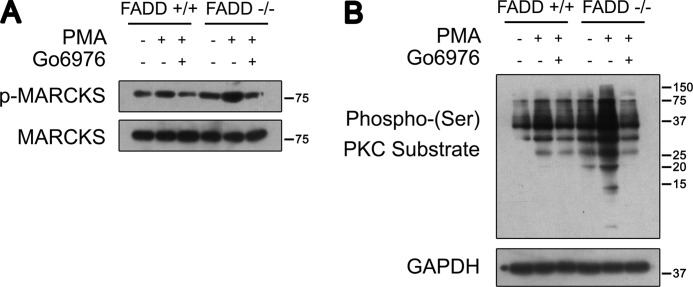
To examine whether enhanced cPKC phosphorylation in FADD−/− MEFs leads to increased phosphorylation of PKC substrates, we examined cPKC activity and signaling. One of the PKC substrates, MARCKS (41), was utilized to evaluate cPKC activity. MARCKS phosphorylation was induced upon a milder PMA treatment in FADD+/+ MEFs. The increased MARCKS phosphorylation was blocked by Go6976, a cPKC-specific inhibitor, in FADD−/− MEFs. Strikingly, significantly increased MARCKS phosphorylation under both basal and PMA-stimulated conditions was observed in FADD−/− MEFs in comparison with FADD+/+ MEFs (Fig. 5A). Consistently, enhanced serine phosphorylation of PKC substrates with or without PMA treatment was observed in FADD−/− MEFs when compared with FADD+/+ MEFs (Fig. 5B).
FIGURE 5.
FADD regulates cPKC activity and signaling. A, Western blot analysis of phosphorylated MARCKS in FADD+/+ and FADD−/− MEFs. MEFs were treated with 100 ng/ml PMA for a 30-min pretreatment with 5 μm Go6976 or not. B, Western blot analysis of phospho-(Ser) PKC substrate in FADD+/+ and FADD−/− MEFs. MEFs were treated with 100 ng/ml PMA for 1 h with or without pretreatment with 5 μm Go6976.
FADD Phosphorylation Regulates Its Novel Function
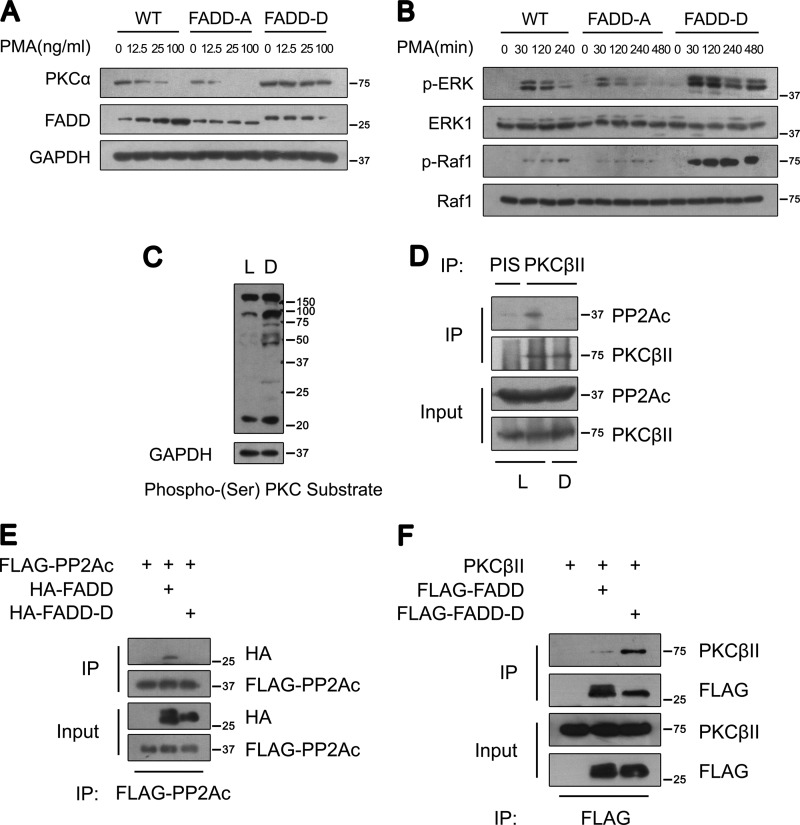
Phosphorylation of FADD at its C terminus has been shown to regulate its non-apoptotic function (23). Thus, we were interested in exploring whether phosphorylation of FADD at its C terminus had any effect on cPKC stability. Three types of MEFs, expressing wild-type FADD (WT) and the serine 191 to alanine (FADD-A) or to aspartic acid (FADD-D) FADD mutants, were utilized to examine the effect of FADD phosphorylation on PKCα expression and activity. Interestingly, PKCα was up-regulated in FADD-D MEFs compared with that in WT and FADD-A MEFs (Fig. 6A). Furthermore, PKCα degradation upon lower and milder PMA treatment overnight was also reduced significantly in FADD-D MEFs, whereas the expression and degradation patterns of PKCα in FADD-A MEFs were comparable with that in WT MEFs (Fig. 6A). Consistent with increased levels of PKCα, PMA-induced rapid phosphorylation of both Raf-1 and ERK in 30 min, which are known to be the downstream targets of PKC, was also significantly higher in FADD-D MEFs compared with that of the WT MEFs (Fig. 6B). Because the effect of FADD-D mutation mimics the null FADD mutation regarding PKC activation, these data suggest that phosphorylation of Ser-191 in FADD may negatively regulate the effect of FADD on PKC inactivation.
FIGURE 6.
Ser-191 phosphorylation of FADD regulates cPKC phosphorylation, stability, and signaling. A, Western blot analysis of PKCα in WT, FADD-A, and FADD-D MEFs. MEFs were treated with PMA at the indicated concentrations for 12 h. B, Western blot analysis of phosphorylated ERK and Raf-1 in WT, FADD-A, and FADD-D MEFs. MEFs were treated with 100 ng/ml PMA for the times indicated. C, Western blot analysis of phospho-(Ser) PKC substrates in cytosolic extracts of cardiac muscles from control littermates (L) and FADD-D mice (D). Representative results from five pairs of mice are shown. D, coimmunoprecipitation (IP) analysis of endogenous PKCβII and PP2Ac from cardiac muscles of control littermates (L) and FADD-D mice (D). Representative results from three pairs of mice are shown. E, coimmunoprecipitation analysis of FADD and PP2Ac in 293T cells. As indicated, lysates from the cells transfected with plasmids encoding FLAG-PP2Ac, HA-FADD, or HA-FADD-D were subjected to immunoprecipitation with an anti-FLAG antibody. FADD and FADD-D were detected using an anti-HA antibody. F, coimmunoprecipitation analysis of FADD and PKCβII in 293T cells. As indicated, lysates from the cells transfected with plasmids encoding FLAG-FADD, FLAG-FADD-D, or pcDNA-PKCβII were subjected to immunoprecipitation with an anti-FLAG antibody. PKCβII was visualized by anti-PKCβII.
To further extend our observation in MEFs, we compared the expression of cPKC and nPKC protein levels in FADD-D mice and wild-type littermates. Interestingly, FADD-D mice contained a higher amount of PKCα, βII, and δ in cardiac muscles than did control littermates (supplemental Fig. S4). A similar up-regulation was also observed in other tissues, such as skeletal muscles, lymphocytes, and thymocytes (data not shown). Consistent with elevated PKC activity in FADD-D mice, enhanced serine phosphorylation of PKC substrates was also observed (Fig. 6C).
To examine the interaction of FADD-D mutant with PP2Ac, we compared the interaction of PKCβII and PP2Ac in cardiac muscles of WT and FADD-D mice. We found that the interaction of PKCβII and PP2Ac found in WT was largely abolished in FADD-D mice (Fig. 6D). These results raise the interesting possibility that FADD phosphorylation regulates the interaction between cPKC and PP2Ac. Moreover, FADD-D failed to interact with PP2Ac (Fig. 6E). Paradoxically, the interaction between FADD-D and the PKCβII was enhanced compare with that of FADD (Fig. 6F), suggesting a critical role of phosphorylation in regulating the novel function of FADD.
FADD Regulates Cytoskeleton and Cell Motility
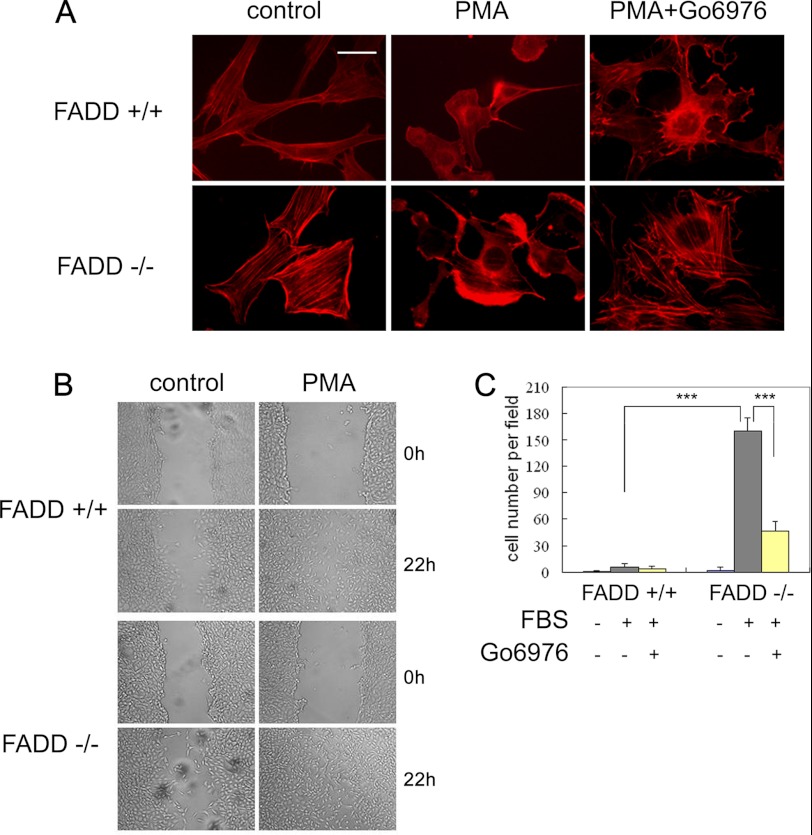
We explored the functional consequence of FADD phosphorylation on cell morphology and motility. Both FADD−/− MEFs and FADD-D lymphocytes have been reported to have abnormal cell morphology. They are bigger and more rounded than controls (42, 43). Because cPKC plays important roles in cytoskeleton regulation and cell migration, we hypothesized that deregulated cPKC might contribute to the abnormal morphology of FADD−/− MEFs. Indeed, FADD−/− MEFs had more rounded and non-aggregated F-actin morphology and more stress fibers than the FADD+/+ MEFs (Fig. 7A). F-actin disassembly is induced rapidly upon PMA treatment. Increased and enhanced lamellipodia accumulation was observed in FADD−/− compared with FADD+/+ MEFs treated with PMA, which was abolished by Go6976 (Fig. 7A).
FIGURE 7.
FADD modulates cell morphology, cytoskeleton reorganization, and cell motility. A, FADD+/+ and FADD−/− MEFs were cultured in serum-free medium for 24 h and then treated with 100 ng/ml PMA for 2 h or PMA plus 5 μm Go6976. F-actin was visualized with Texas Red-X phalloidin. Scale bar = 20 μm. B, wound closure analysis of FADD+/+ and FADD−/− MEFs. MEFs were cultured in serum-free medium for 24 h and then treated with 100 ng/ml PMA for 22 h. C, quantification of the Transwell assay of FADD+/+ and FADD−/− MEFs. Cells treated with 5% FBS for 5 h. MEFs were cultured in serum-free medium for 24 h, and 5 μm Go6976 was added 2 h before FBS treatment. Cells were counted from 5 or 6 equal areas. **, p < 0.01; ***, p < 0.005; Student's t test.
Next, we characterized the motility of FADD+/+ and FADD−/− MEFs using a wound closure assay. We found that PMA-stimulated FADD−/− MEFs but not WT MEFs were able to close the wound area after 24 h, suggesting that FADD−/− MEFs are more active in migration than that of WT MEFs (Fig. 7B). The highly active migrating behavior of FADD-deficient MEFs was further analyzed using a transwell assay that revealed that FADD−/− MEFs were approximately 10 times higher active in migration than WT MEFs. The increased cPKC activity is likely to play a major role in the highly migratory behavior of FADD−/− MEFs as the addition of Go6976 largely inhibited the increased migration (Fig. 7C and supplemental Fig. S5). Collectively, these results suggest that FADD is an important component of the signaling networks that regulate cytoskeleton and cell motility.
FADD Phosphorylation Regulates Cytoskeleton and Cell Mobility
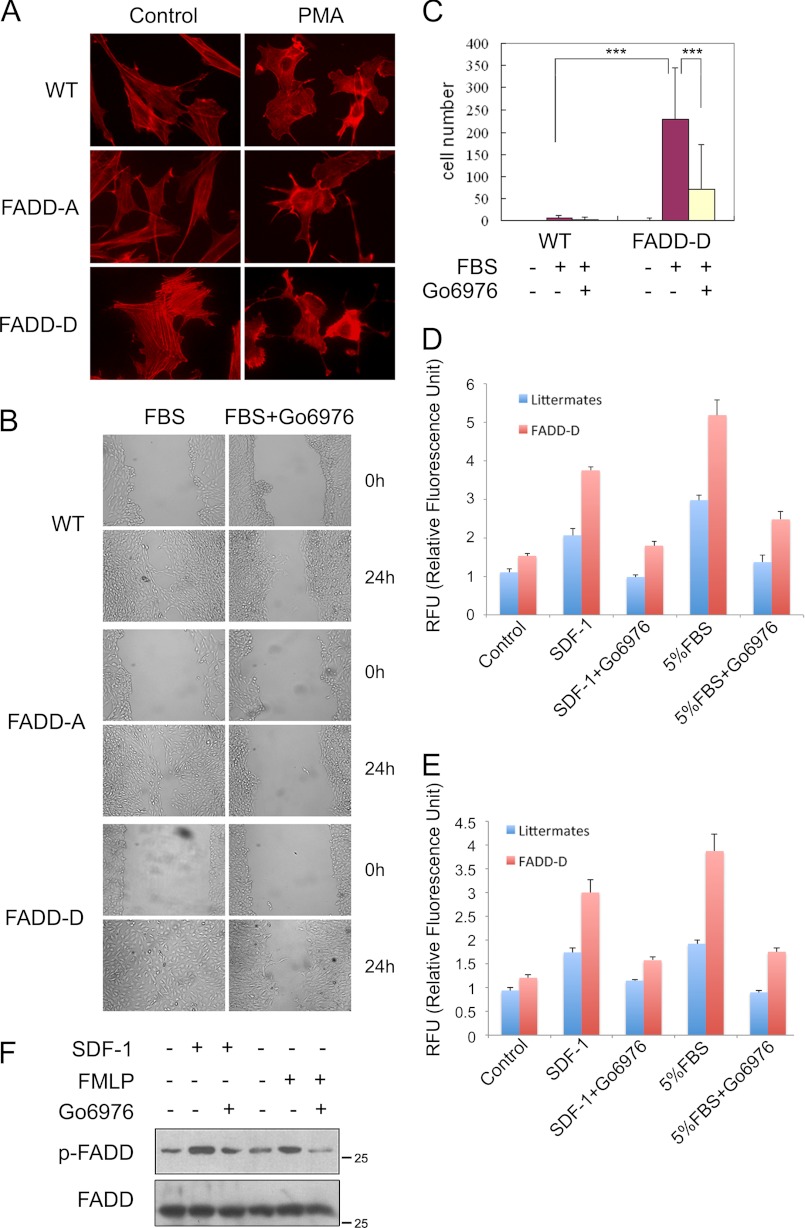
Similar to that of FADD−/− MEFs, FADD-D MEFs also had abnormal morphology and enhanced formation of stress fibers, whereas the morphology of FADD-A MEFs were comparable with that of WT MEFs (Fig. 8A). Enhanced lamellipodia formation upon PMA treatment was also observed in FADD-D MEFs compared with in FADD-A and WT MEFs (Fig. 8A).
FIGURE 8.
FADD-D regulates cytoskeleton and cell motility. A, WT, FADD-A or FADD-D MEFs were cultured in serum-free medium for 24 h and then treated with 100 ng/ml PMA for 2 h. F-actin was visualized with Texas Red-X phalloidin. B, wound closure analysis of WT, FADD-A or FADD-D MEFs treated with 10% FBS for 24 h. MEFs were cultured in serum-free medium for 24 h, and 5 μm Go6976 was added 2 h before FBS treatment. C, quantification of Transwell assay of WT, FADD-A, or FADD-D MEFs. Cells treated with 5% FBS for 5 h. MEFs were serum-starved for 24 h. MEFs were cultured in serum-free medium for 24 h, and 5 μm Go6976 was added 2 h before FBS treatment. Cells were counted from five or six equal areas. **, p < 0.01; ***, p < 0.005; Student's t test. D and E, mesenteric lymphocytes and splenocytes, respectively, from FADD-D mice and littermates were starved for 2 h. The transwell assay was carried out for 24 h with no treatment (CON), treatment with 150 ng/ml SDF-1, or with 5% FBS in the presence or absence of Go6976. Representative results from at least three pairs of mice are shown. Error bars represent mean ± S.D. from triplicate measures. (F) Splenocytes from C57/BL6 mice were treated with 100 μm formylmethionylleucylphenylalanine or 150 ng/ml SDF-1 for 30 min. Cells were preincubated with 5 μm Go6976 for 2 h. Phosphorylated FADD and total FADD were determined using Western blot analysis. Representative results from three mice are shown.
Next, we examined the effect of FADD-D mutation on cell motility. We found that FADD-D MEFs closed the wound much faster than those of WT or FADD-A MEFs (Fig. 8B). Furthermore, the accelerated migration was abolished by Go6976 (Fig. 8B). In the transwell assay, FADD-D MEFs also migrated significantly faster than WT MEFs (supplemental Fig. S6). Quantitative analysis of FADD-D MEFs revealed a 20-fold increase in cell migration (Fig. 8C).
Consistent with the results from MEFs, both mesenteric lymphocytes and splenocytes from FADD-D mice also demonstrated accelerated chemokine-induced migration (Fig. 8, D and E). A previous study indicated that cPKC promotes FADD phosphorylation in neutrophils treated with GM-CSF (28). We found that FADD phosphorylation was also induced by SDF-1 and FMLP (formyl-methionyl-leucyl-phenylalanine) in a cPKC-dependent manner (Fig. 8F). Collectively, these results suggest that FADD phosphorylation promotes cytoskeleton reorganization, cell migration, and chemotaxis.
DISCUSSION
The phosphorylation of three conserved sites (A-loop, TM, and HM) in PKC plays a critical role in its stability and activity (44). Endogenous PKC degradation is primed by dephosphorylation at these three sites. However, the molecular mechanisms that regulate dephosphorylation of PKCs remain largely unknown. In this study, we present that the apoptotic adaptor FADD is required for cPKC TM dephosphorylation. FADD also affected HM dephosphorylation. FADD is required for TM dephosphorylation induced by PMA, which mimics endogenous phobol esters such as diacylglycerol and Ca2+. PKCα and PKCβII are resistant to PMA-dependent degradation, which leads to their accumulation in FADD−/− cells. Because the expression of FADD is also induced by PMA (Fig. 1B), induction of FADD in response to PMA may serve to negatively regulate cPKCs after their activation.
Ser-191 phosphorylation of FADD is thought to have an insignificant effect on receptor-mediated apoptosis but to dramatically inhibit cell proliferation and differentiation (27). The molecular mechanisms of these effects are unknown. Here, we presented a molecular model for how Ser-191 phosphorylation regulates the novel function of FADD. The mutant mimicking constitutive phosphorylation (FADD-D) had a decreased interaction with PP2Ac, which then resulted in an impaired interaction between PP2Ac and PKCβII. These data suggest that Ser-191 phosphorylation abolishes the novel “adaptor” function of FADD. Interestingly, consistent with a previous report (28), Ser-191 phosphorylation of FADD was induced by chemokines in a cPKC-dependent manner, suggesting that Ser-191 is a potential target for cPKC to amplify its own signaling output.
Previous studies proved that caspase family proteases such as caspase-3, caspase-8, and caspase-11 regulate cell migration through distinct mechanisms (45–47). Our study revealed an interesting role of FADD and its phosphorylation in cell migration and chemotaxis. As an apoptotic adaptor protein, FADD is believed to be down-regulated in cancer cells to evade apoptosis. Paradoxically, FADD has been found overexpressed in many tumors, such as head and neck squamous cell carcinoma, oral squamous cell carcinoma, ovarian carcinoma, and lung adenocarcinomas (24, 48–50). Moreover, FADD phosphorylation has been demonstrated up-regulated in tumors such as oral squamous cell carcinoma, lung adenocarcinomas, and gastric cancer and is associated with a poor outcome (26, 49, 51). Our study indicates that FADD phosphorylation might promote tumor progression, such as metastasis, through inducing PKC signaling.
The results from this study could explain how FADD deficiency or constitutive phosphorylation result in disorders of cell morphology, cytoskeleton, and cell motility. cPKC plays critical roles in cytoskeleton organization via phosphorylating numerous substrates, such as MARCKS, adducin, and fascin, to regulate F-actin organization (10). F-actin formation is enhanced dramatically under FADD-deficient conditions or in the presence of a FADD mutant that mimics constitutive phosphorylation. We showed that this enhancement was inhibited by a cPKC inhibitor, suggesting that both FADD and its phosphorylation are crucial for cytoskeleton organization. Consistent with the observations that PKCα overexpression leads to larger cell sizes, enhanced stress fibers, more lamellipodia, and extensive actin fibers (52), similar changes were also observed in FADD−/− MEFs. These data indicate that FADD is important for cell morphology and the maintenance of F-actin organization by regulating cPKC.
A schematic framework of cPKC regulation by FADD is presented in supplemental Fig. S7. We propose that FADD acts to negatively regulate PKC dephosphorylation, stability, and signaling, ultimately controlling cytoskeleton organization and cell migration. Upon phosphorylation at Ser-191, FADD loses contact with PP2A. cPKC then dissociates from PP2A. The escape of cPKC from dephosphorylation and degradation by PP2A accelerates cytoskeleton turnover and enhances cell motility.
Supplementary Material
Acknowledgments
We thank Dr. Yuan Junying (Harvard University) for discussions and preparation of the manuscript. We also thank Dr. Astar Winoto (University of California, Berkeley) for providing the FADD mutant cell lines and mutant mice.
This study was supported in part by National Key Basic Research Project from the Chinese Ministry of Science and Technology Grants 2012CB967000 and 2011CB933502); Chinese National Nature Sciences Foundation Grants 30821006, 50973046, 31071196, 81072712, 30270291, 30330530, and 30425009; Specialized Research Fund for the Doctoral Program of Higher Education from the Chinese Ministry of Education Grants 200802840023 and 20030284040; Jiangsu Provincial Nature Science Foundation Grant BK2010046 and B22010074; and by the Scientific Research Foundation of the Graduate School of Nanjing University.

This article contains supplemental Figs. S1–S7.
- FADD
- fas-associated protein with death domain
- PKC
- protein kinase C
- cPKC
- conventional protein kinase C
- nPKC
- novel protein kinase C
- TM
- turn motif
- HM
- hydrophobic motif
- PP2A
- type 2A phosphatase
- MEF
- murine embryonic fibroblast
- PMA
- phorbol 12-myristate 13-acetate
- PHLPP
- PH domain and Leucine rich repeat Protein Phosphatases
- MARCKS
- Myristoylated alanine-rich C kinase substrate
- SPF
- specific-pathogen-free
- FMLP
- formyl-methionyl-leucyl-phenylalanine
- CHX
- cycloheximide.
REFERENCES
- 1. Zhang J., Winoto A. (1996) A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-induced apoptosis. Mol. Cell Biol. 16, 2756–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chinnaiyan A. M., O'Rourke K., Tewari M., Dixit V. M. (1995) FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, 505–512 [DOI] [PubMed] [Google Scholar]
- 3. Zhang J., Cado D., Chen A., Kabra N. H., Winoto A. (1998) Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 [DOI] [PubMed] [Google Scholar]
- 4. Yeh W. C., Pompa J. L., McCurrach M. E., Shu H. B., Elia A. J., Shahinian A., Ng M., Wakeham A., Khoo W., Mitchell K., El-Deiry W. S., Lowe S. W., Goeddel D. V., Mak T. W. (1998) FADD. Essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 [DOI] [PubMed] [Google Scholar]
- 5. Zhang J., Kabra N. H., Cado D., Kang C., Winoto A. (2001) FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J. Biol. Chem. 276, 29815–29818 [DOI] [PubMed] [Google Scholar]
- 6. Kabra N. H., Kang C., Hsing L. C., Zhang J., Winoto A. (2001) T cell-specific FADD-deficient mice. FADD is required for early T cell development. Proc. Natl. Acad. Sci. U.S.A. 98, 6307–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osborn S. L., Diehl G., Han S. J., Xue L., Kurd N., Hsieh K., Cado D., Robey E. A., Winoto A. (2010) Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc. Natl. Acad. Sci. U.S.A. 107, 13034–13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang H., Zhou X., McQuade T., Li J., Chan F. K., Zhang J. (2011) Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welz P. S., Wullaert A., Vlantis K., Kondylis V., Fernández-Majada V., Ermolaeva M., Kirsch P., Sterner-Kock A., van Loo G., Pasparakis M. (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 [DOI] [PubMed] [Google Scholar]
- 10. Larsson C. (2006) Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 18, 276–284 [DOI] [PubMed] [Google Scholar]
- 11. Parker P. J., Murray-Rust J. (2004) PKC at a glance. J. Cell Sci. 117, 131–132 [DOI] [PubMed] [Google Scholar]
- 12. Dempsey E. C., Newton A. C., Mochly-Rosen D., Fields A. P., Reyland M. E., Insel P. A., Messing R. O. (2000) Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L429–438 [DOI] [PubMed] [Google Scholar]
- 13. Parekh D. B., Ziegler W., Parker P. J. (2000) Multiple pathways control protein kinase C phosphorylation. EMBO J. 19, 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dutil E. M., Toker A., Newton A. C. (1998) Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr. Biol. 8, 1366–1375 [DOI] [PubMed] [Google Scholar]
- 15. Le Good J. A., Ziegler W. H., Parekh D. B., Alessi D. R., Cohen P., Parker P. J. (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 16. Edwards A. S., Newton A. C. (1997) Phosphorylation at conserved carboxyl-terminal hydrophobic motif regulates the catalytic and regulatory domains of protein kinase C. J. Biol. Chem. 272, 18382–18390 [DOI] [PubMed] [Google Scholar]
- 17. Ikenoue T., Inoki K., Yang Q., Zhou X., Guan K. L. (2008) Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 27, 1919–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Facchinetti V., Ouyang W., Wei H., Soto N., Lazorchak A., Gould C., Lowry C., Newton A. C., Mao Y., Miao R. Q., Sessa W. C., Qin J., Zhang P., Su B., Jacinto E. (2008) The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 27, 1932–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gómez-Angelats M., Cidlowski J. A. (2001) Protein kinase C regulates FADD recruitment and death-inducing signaling complex formation in Fas/CD95-induced apoptosis. J. Biol. Chem. 276, 44944–44952 [DOI] [PubMed] [Google Scholar]
- 20. Meng X. W., Heldebrant M. P., Kaufmann S. H. (2002) Phorbol 12-myristate 13-acetate inhibits death receptor-mediated apoptosis in Jurkat cells by disrupting recruitment of Fas-associated polypeptide with death domain. J. Biol. Chem. 277, 3776–3783 [DOI] [PubMed] [Google Scholar]
- 21. Kotone-Miyahara Y., Yamashita K., Lee K. K., Yonehara S., Uchiyama T., Sasada M., Takahashi A. (2004) Short-term delay of Fas-stimulated apoptosis by GM-CSF as a result of temporary suppression of FADD recruitment in neutrophils. Evidence implicating phosphatidylinositol 3-kinase and MEK1-ERK1/2 pathways downstream of classical protein kinase C. J. Leukocyte. Biol. 76, 1047–1056 [DOI] [PubMed] [Google Scholar]
- 22. de Thonel A., Bettaïeb A., Jean C., Laurent G., Quillet-Mary A. (2001) Role of protein kinase C ζ isoform in Fas resistance of immature myeloid KG1a leukemic cells. Blood 98, 3770–3777 [DOI] [PubMed] [Google Scholar]
- 23. Hua Z. C., Sohn S. J., Kang C., Cado D., Winoto A. (2003) A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity 18, 513–521 [DOI] [PubMed] [Google Scholar]
- 24. Chen G., Bhojani M. S., Heaford A. C., Chang D. C., Laxman B., Thomas D. G., Griffin L. B., Yu J., Coppola J. M., Giordano T. J., Lin L., Adams D., Orringer M. B., Ross B. D., Beer D. G., Rehemtulla A. (2005) Phosphorylated FADD induces NF-κB, perturbs cell cycle, and is associated with poor outcome in lung adenocarcinomas. Proc. Natl. Acad. Sci. U.S.A. 102, 12507–12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsuyoshi S., Shimada K., Nakamura M., Ishida E., Konishi N. (2006) FADD phosphorylation is critical for cell cycle regulation in breast cancer cells. Br. J. Cancer 94, 532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhojani M. S., Chen G., Ross B. D., Beer D. G., Rehemtulla A. (2005) Nuclear localized phosphorylated FADD induces cell proliferation and is associated with aggressive lung cancer. Cell Cycle 4, 1478–1481 [DOI] [PubMed] [Google Scholar]
- 27. Park S. M., Schickel R., Peter M. E. (2005) Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr. Opin. Cell Biol. 17, 610–616 [DOI] [PubMed] [Google Scholar]
- 28. Alappat E. C., Feig C., Boyerinas B., Volkland J., Samuels M., Murmann A. E., Thorburn A., Kidd V. J., Slaughter C. A., Osborn S. L., Winoto A., Tang W. J., Peter M. E. (2005) Phosphorylation of FADD at serine 194 by CKIα regulates its nonapoptotic activities. Mol. Cell 19, 321–332 [DOI] [PubMed] [Google Scholar]
- 29. Hansra G., Bornancin F., Whelan R., Hemmings B. A., Parker P. J. (1996) 12-O-Tetradecanoylphorbol-13-acetate-induced dephosphorylation of protein kinase Cα correlates with the presence of a membrane-associated protein phosphatase 2A heterotrimer. J. Biol. Chem. 271, 32785–32788 [DOI] [PubMed] [Google Scholar]
- 30. Hansra G., Garcia-Paramio P., Prevostel C., Whelan R. D., Bornancin F., Parker P. J. (1999) Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes. Biochem. J. 342, 337–344 [PMC free article] [PubMed] [Google Scholar]
- 31. Newton A. C. (2010) Protein kinase C. Poised to signal. Am. J. Physiol. Endocrinol. Metab. 298, E395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee I. H., Lim H. J., Yoon S., Seong J. K., Bae D. S., Rhee S. G., Bae Y. S. (2008) Ahnak protein activates protein kinase C (PKC) through dissociation of the PKC-protein phosphatase 2A complex. J. Biol. Chem. 283, 6312–6320 [DOI] [PubMed] [Google Scholar]
- 33. Boudreau R. T., Garduno R., Lin T. J. (2002) Protein phosphatase 2A and protein kinase Cα are physically associated and are involved in Pseudomonas aeruginosa-induced interleukin 6 production by mast cells. J. Biol. Chem. 277, 5322–5329 [DOI] [PubMed] [Google Scholar]
- 34. Gysin S., Imber R. (1997) Phorbol-ester-activated protein kinase C-α lacking phosphorylation at Ser-657 is down-regulated by a mechanism involving dephosphorylation. Eur. J. Biochem. 249, 156–160 [DOI] [PubMed] [Google Scholar]
- 35. Keranen L. M., Dutil E. M., Newton A. C. (1995) Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr. Biol. 5, 1394–1403 [DOI] [PubMed] [Google Scholar]
- 36. Gao T., Brognard J., Newton A. C. (2008) The phosphatase PHLPP controls the cellular levels of protein kinase C. J. Biol. Chem. 283, 6300–6311 [DOI] [PubMed] [Google Scholar]
- 37. Zeidman R., Pettersson L., Sailaja P. R., Truedsson E., Fagerström S., Påhlman S., Larsson C. (1999) Novel and classical protein kinase C isoforms have different functions in proliferation, survival and differentiation of neuroblastoma cells. Int. J. Cancer 81, 494–501 [DOI] [PubMed] [Google Scholar]
- 38. Ikehara T., Shinjo F., Ikehara S., Imamura S., Yasumoto T. (2006) Baculovirus expression, purification, and characterization of human protein phosphatase 2A catalytic subunits α and β. Protein Expr. Purif. 45, 150–156 [DOI] [PubMed] [Google Scholar]
- 39. Gao T., Newton A. C. (2002) The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J. Biol. Chem. 277, 31585–31592 [DOI] [PubMed] [Google Scholar]
- 40. Gould C. M., Newton A. C. (2008) The life and death of protein kinase C. Curr. Drug Targets 9, 614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. (1992) MARCKS is an actin filament cross-linking protein regulated by protein kinase C and calcium-calmodulin. Nature 356, 618–622 [DOI] [PubMed] [Google Scholar]
- 42. Osborn S. L., Sohn S. J., Winoto A. (2007) Constitutive phosphorylation mutation in Fas-associated death domain (FADD) results in early cell cycle defects. J. Biol. Chem. 282, 22786–22792 [DOI] [PubMed] [Google Scholar]
- 43. Shen H. M., Lin Y., Choksi S., Tran J., Jin T., Chang L., Karin M., Zhang J., Liu Z. G. (2004) Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol. Cell Biol. 24, 5914–5922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hauge C., Antal T. L., Hirschberg D., Doehn U., Thorup K., Idrissova L., Hansen K., Jensen O. N., Jørgensen T. J., Biondi R. M., Frödin M. (2007) Mechanism for activation of the growth factor-activated AGC kinases by turn motif phosphorylation. EMBO J. 26, 2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li J., Brieher W. M., Scimone M. L., Kang S. J., Zhu H., Yin H., von Andrian U. H., Mitchison T., Yuan J. (2007) Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat. Cell Biol. 9, 276–286 [DOI] [PubMed] [Google Scholar]
- 46. Zhao X., Wang D., Zhao Z., Xiao Y., Sengupta S., Xiao Y., Zhang R., Lauber K., Wesselborg S., Feng L., Rose T. M., Shen Y., Zhang J., Prestwich G., Xu Y. (2006) Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J. Biol. Chem. 281, 29357–29368 [DOI] [PubMed] [Google Scholar]
- 47. Stupack D. G., Teitz T., Potter M. D., Mikolon D., Houghton P. J., Kidd V. J., Lahti J. M., Cheresh D. A. (2006) Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature 439, 95–99 [DOI] [PubMed] [Google Scholar]
- 48. Brown L. A., Kalloger S. E., Miller M. A., Shih I. M., McKinney S. E., Santos J. L., Swenerton K., Spellman P. T., Gray J., Gilks C. B., Huntsman D. G. (2008) Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer 47, 481–489 [DOI] [PubMed] [Google Scholar]
- 49. Gibcus J. H., Menkema L., Mastik M. F., Hermsen M. A., de Bock G. H., van Velthuysen M. L., Takes R. P., Kok K., Alvarez Marcos C. A., van der Laan B. F., van den Brekel M. W., Langendijk J. A., Kluin P. M., van der Wal J. E., Schuuring E. (2007) Amplicon mapping and expression profiling identify the Fas-associated death domain gene as a new driver in the 11q13.3 amplicon in laryngeal/pharyngeal cancer. Clin. Cancer Res. 13, 6257–6266 [DOI] [PubMed] [Google Scholar]
- 50. Meredith S. D., Levine P. A., Burns J. A., Gaffey M. J., Boyd J. C., Weiss L. M., Erickson N. L., Williams M. E. (1995) Chromosome 11q13 amplification in head and neck squamous cell carcinoma. Association with poor prognosis. Arch. Otolaryngol. Head Neck Surg. 121, 790–794 [DOI] [PubMed] [Google Scholar]
- 51. Yoo N. J., Lee S. H., Jeong E. G., Lee J. W., Soung Y. H., Nam S. W., Kim S. H., Lee J. Y. (2007) Expression of nuclear and cytoplasmic phosphorylated FADD in gastric cancers. Pathol. Res. Pract. 203, 73–78 [DOI] [PubMed] [Google Scholar]
- 52. Rotenberg S. A., Sun X. G. (1999) Cell Growth & Differ 10, 343–352 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.