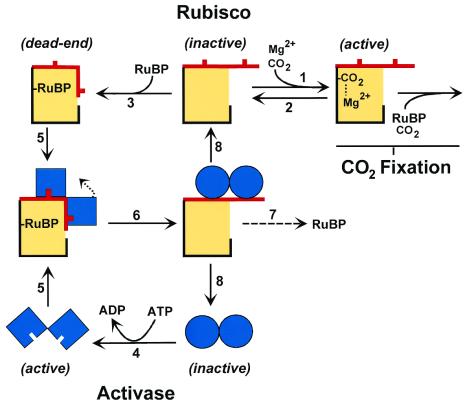
The enzyme Rubisco, short for ribulose-1,5-bisphosphate carboxylase/oxygenase, is the enzyme that incorporates CO2 into plants during photosynthesis. As it constitutes about 30% of the total protein in a plant leaf, Rubisco is probably the most abundant protein on earth and a major sink for plant nitrogen. Rubisco is widely accepted as the ultimate rate-limiting step in photosynthetic carbon fixation. Atmospheric oxygen competes with CO2 as a substrate for Rubisco, giving rise to photorespiration. When first purified, the enzyme appeared to have a poor affinity for CO2 [Km (CO2)] of 450 μM (1), whereas air in equilibrium with water, 25°C, is about 10 μM. Later, Lorimer et al. (2) showed that the active site of Rubisco must first be carbamylated by an activator CO2, separate from the substrate CO2, and must bind Mg2+ before binding the five-carbon substrate, ribulose-1,5-bisphosphate (RuBP). Indeed, on addition of RuBP, the measured Km(CO2) approached the concentrations of dissolved CO2 in water; however, the rate of the reaction was sustained for only 5 minutes and then declined rapidly. This decline was shown to be caused by the tight binding of RuBP to Rubisco that had lost the activator CO2 (Fig. 1). The missing ingredient needed to uncouple Rubisco and RuBP was found in the intact plant with a separate protein, called Rubisco activase (3). This enzyme acts on Rubisco and allows release of the bound RuBP so that the site can bind the activator CO2 and Mg2+. Rubisco activase itself requires ATP, and its activity is related to the energy charge of the chloroplast (4). Thus, the proportion of Rubisco that is active in a leaf (activation state) can vary depending on the effectiveness of Rubisco activase in removing bound RuBP. Regulation of Rubisco fine tunes the rate of CO2 fixation to the rate of photosynthetic electron transport, ensuring that chloroplast metabolites are always optimal for photosynthesis (5).
Figure 1.
Activase (blue) switches Rubisco (yellow) from an inactive to an active form by the ATP-dependent release of tight-binding sugar phosphates such RuBP. Only the active form of Rubisco is capable of catalyzing CO2 fixation, the first step in photosynthesis. In the scheme, a Rubisco active site that has spontaneously lost CO2 (decarbamylation) (step 2) binds RuBP (step 3). The binding of RuBP causes conformational changes that produce a dead-end complex consisting of inactive Rubisco and tightly bound RuBP. Activase physically interacts with Rubisco (step 5), changing the conformation of Rubisco (step 6) to one that binds RuBP less tightly. ATP hydrolysis by activase is required for these conformational changes, perhaps for priming activase (step 4). Because of the lower affinity, RuBP dissociates from the active site of Rubisco (step 7), which frees the site for subsequent carbamylation (step 1) or rebinding of RuBP (step 3), but probably after dissociation of activase (step 8). Crafts-Brandner and Salvucci (6) show that an accelerated rate of Rubisco deactivation occurs at high temperature (steps 2 and 3), which is not matched by a faster rate of activation by activase. ATP hydrolysis (step 4) and/or activase–Rubisco interactions (steps 5 and 6) may limit the rate of Rubisco activation at high temperature.
The paper by Crafts-Brandner and Salvucci (6) in this issue of PNAS provides evidence that, with plants under heat stress, the activation state of Rubisco and photosynthesis as measured by CO2 exchange is reduced. By duplicating the temperature response in the test tube under controlled conditions, and by using Rubisco and Rubisco activase isolated from tobacco, they were able to ascribe the limitation to a specific biochemical event, the inability of Rubisco activase to keep pace with a faster deactivation of Rubisco. Increased CO2 also decreased the activation state of Rubisco in leaves, and the authors conclude that the response could be explained by a decreased energy charge in the chloroplast that reduced the ATPase activity of Rubisco activase. By calculating photosynthesis on the basis of the kinetics of Rubisco and the amount of active enzyme in the leaf, the authors have shown that, under both high temperature and high CO2, photosynthesis was constrained by the activity of Rubisco activase.
Changes in the global environment since the beginning of the Industrial Revolution have raised concerns about the impact on natural and agroecosystems. Increasing CO2 levels and corresponding changes in temperature will directly affect the earth's carbon balance by altering photosynthetic carbon fixation. Thus, it is essential to develop an understanding of the mechanistic response of photosynthesis to environmental change. Models of photosynthesis (7, 8) have been widely adapted to predict how environmental changes will influence photosynthesis and to pinpoint biochemical limitations in the process. These models are based primarily on the kinetics of fully activated Rubisco, and they make a clear distinction between limitations attributable to the enzyme amount and those limiting the regeneration of the substrate, RuBP. The report by Crafts-Brandner and Salvucci (6) highlights the importance of Rubisco activation as a determinant of photosynthetic performance under conditions associated with a changing global climate. The clear conclusion of these experiments is that Rubisco activation is the major limitation to CO2 fixation during photosynthesis, certainly under high CO2, high temperature, and optimal light.
Although some of the major details are known, there is still much to be learned about the regulation of Rubisco. The structure of Rubisco is known from several plant sources (9). Structure determination has been a difficult feat: Rubisco from higher plants contains 16 subunits—8 large chloroplast-encoded subunits and 8 small nuclear-encoded subunits. Some details about the binding of activase are known (4), but how its binding disturbs the protein structure to allow release of RuBP is not understood. With the three-dimensional structure of Rubisco at hand, attempts are being made to improve the reaction kinetics of the enzyme. Whether it is possible to decrease the affinity of the activated Rubisco–RuBP complex for oxygen and thus increase carboxylation over oxygenation is still unknown. Attempts to alter specificity by mutagenesis or interspecific genetic replacement must consider interactions between Rubisco and Rubisco activase.
Footnotes
See companion article on page 13430.
References
- 1.Weissbach A, Horecker B L, Hurwitz J. J Biol Chem. 1956;218:795–810. [PubMed] [Google Scholar]
- 2.Lorimer G H, Badger M R, Andrews T J. Biochemistry. 1976;15:529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- 3.Salvucci M E, Portis A R, Jr, Ogren W L. Photosynthesis Res. 1985;7:193–201. doi: 10.1007/BF00037012. [DOI] [PubMed] [Google Scholar]
- 4.Portis A R., Jr J Exp Bot. 1995;46:1285–1291. [Google Scholar]
- 5.Perchorowicz J T, Raynes D A, Jensen R G. Proc Natl Acad Sci USA. 1981;78:2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crafts-Brandner S J, Salvucci M E. Proc Natl Acad Sci USA. 2000;97:13430–13435. doi: 10.1073/pnas.230451497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farquhar G D, von Caemmerer S, Berry J A. Planta. 1980;149:178–190. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey T D. Bot Rev. 1985;51:53–105. [Google Scholar]
- 9.Spreitzer R J. Photosynthesis Res. 1999;60:29–42. [Google Scholar]