Background: The effects of extracorporeal shock wave (ESW) on adhesion and migration of osteoblasts have not been reported until now.
Results: Optimal intensity shock wave promotes osteoblast adhesion and migration.
Conclusion: ESW promotes the adhesion and migration of osteoblasts via integrin β1-mediated expression of phosphorylated FAK.
Significance: This provides a mechanistic basis for improving the effectiveness of ESW treatment in fracture healing and tissue engineering.
Keywords: Bone, Cell Adhesion, Cell Migration, Integrins, Osteoblasts, Focal Adhesion Kinase, Shock Wave
Abstract
To search for factors promoting bone fracture repair, we investigated the effects of extracorporeal shock wave (ESW) on the adhesion, spreading, and migration of osteoblasts and its specific underlying cellular mechanisms. After a single period of stimulation by 10 kV (500 impulses) of shock wave (SW), the adhesion rate was increased as compared with the vehicle control. The data from both wound healing and transwell tests confirmed an acceleration in the migration of osteoblasts by SW treatment. RT-PCR, flow cytometry, and Western blotting showed that SW rapidly increased the surface expression of α5 and β1 subunit integrins, indicating that integrin β1 acted as an early signal for ESW-induced osteoblast adhesion and migration. It has also been found that a significant elevation occurred in the expression of phosphorylated β-catenin and focal adhesion kinase (FAK) at the site of tyrosine 397 in response to SW stimulation after the increasing expression of the integrin β1 molecule. When siRNAs of integrin α5 and β1 subunit were added, the level of FAK phosphorylation elevated by SW declined. Interestingly, the adhesion and migration of osteoblasts were decreased when these siRNA reagents as well as the ERK1/2 signaling pathway inhibitors, U0126 and PD98059, were present. Further studies demonstrated that U0126 could inhibit the downstream integrin-dependent signaling pathways, such as the FAK signaling pathway, whereas it had no influence on the synthesis of integrin β1 molecule. In conclusion, these data suggest that ESW promotes the adhesion and migration of osteoblasts via integrin β1-mediated expression of phosphorylated FAK at the Tyr-397 site; in addition, ERK1/2 are also important for osteoblast adhesion, spreading, migration, and integrin expression.
Introduction
Extracorporeal shock wave (ESW)3 is indicated as an alternative, non-invasive but promising method for treatment of bone fractures, effective even in delayed fracture healing or nonunion (1–6). The cure rate of the above disorders after ESW therapy has been reported to be 75–91% (7). The ESW used is generated by an electron hydraulic shock wave generator, which can cause an explosive evaporation of water and produce high energy acoustic waves (8). The acoustic waves are focused on a fluid-filled head with a silicone-type membrane and therefore can be transmitted into a specific site (8).
Fracture healing is a complex physiologic process that involves the coordinated participation of several cell types. The osteoblasts play a crucial role in this process. For bone formation to occur, osteoblast precursor cells must migrate from the bone marrow compartment to bone surfaces, where they adhere, differentiate, and deposit the bone matrix. After transient or long term treatment of ESW, a positive effect on osteogenic activation has been shown according to data from studies on osteoblast proliferation and differentiation either in vitro or in vivo (9, 10). The promotion of osteoblast proliferation and differentiation is well documented to be through significant inductions of numerous cellular factors, such as bone morphogenetic proteins (11), transforming growth factor (TGF-β1) (12), and vascular endothelial growth factor (VEGF) (12, 13), as well as osteocalcin (14). However, no previous reports have focused on the effect of shock waves on the adhesion and migration of osteoblasts. It is unknown whether the optimal intensity energy of ESW can promote osteoblast adhesion and migration.
The cell membrane can be altered by low intensity shock waves and is reported to be the most sensitive part of the cell (15). We hypothesized that the influence on the adhesion and migration of osteoblasts may also be the result of the effects of ESW on cell membranes. As we know, specific transmembrane proteins, called integrins, mediate the interactions between the cells and the extracellular matrix proteins (ECMs). These integrins consist of an α subunit and a β subunit (16). Many researchers have stated that integrin β1 is the major subunit in osteoblasts (17–19). For the attachment of fibronectin or collagen type I, osteoblasts express integrin β1 (17). Once cells have attached, the integrin is also involved in passing information from the ECMs to the cell and from the cell toward the ECMs (“outside-in signaling” and “inside-out signaling”) (20). Blocking the integrin β1 with specific antibody had an inhibitory effect on the initial attachment to the implants and thus delayed migration, proliferation, and differentiation of osteoblasts (21, 22). Based on flow cytometric analysis, the expression of integrin α5 and β1 subunits in the cell membrane was transiently increased in response to ultrasound stimulation (23). Focal adhesion kinase (FAK) has been established as a key component of the signal transduction pathways triggered by integrins (24).
However, there has been no prior evidence showing that ESW promotes osteoblast adhesion and migration. In this study, we found that an optimal intensity ESW treatment promoted osteoblast adhesion and migration via the induction of the integrin β1 molecule, which mediated the phosphorylation of FAK and cross-talk with other specific signal transduction pathways. We also sought to investigate the effects of ESW on adhesion, spreading, and migration ex vivo in a cell culture model, aiming to clarify the underlying specific molecular mechanisms.
EXPERIMENTAL PROCEDURES
Animals
3-day-old Sprague-Dawley rats (male or female) were obtained from the Experimental Animal Center of Shantou University Medical College (Shantou, China). Care of rats in this investigation conformed to National Institutes of Health guidelines (67) and followed the rules of the National Animal Protection Law of China. The study was approved by the Institutional Animal Care and Use Committee of Shantou University Medical College.
Reagents and Antibodies
Dulbecco's minimal essential medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone. Collagen type II and fibronectin were purchased from Sigma-Aldrich. 0.25% trypsin, 0.02% EDTA, 1% penicillin/streptomycin, TRIzol reagent, and LipofectamineTM RNAiMax were purchased from Invitrogen. Plastic culture dishes, cell culture plates, and Transwell inserts with polycarbonate membrane containing 8.0-μm pores were obtained from Costar (Cambridge, MA) or Nunc (Nalge, Denmark) unless stated otherwise. Mitogen-activated protein kinase (MAPK)/ERK kinase (MEK) inhibitor (PD98059/U0126), p38 MAPK inhibitor (SB203580), and C-Jun N-terminal kinase (JNK) inhibitor (SP600125) were purchased from Promega (Madison, WI). Phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002), protein kinase A (PKA) inhibitor (H-89), and JAK-2 inhibitor (AG490) were products of Calbiochem. The PCR primers for integrin α5/β1 subunits and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were synthesized by Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China). The PCRMix kits were from Tiangen (Beijing, China). The effective small interfering RNA of integrin α5 and β1 subunits were designed and synthesized by GenePharma Co., Ltd. (Shanghai, China). For flow cytometry, phycoerythrin (PE)-conjugated anti-rat CD29 and CD49e together with PE-conjugated IgG (isotype control) were purchased from E-Biosciences. For Western blotting, polyclonal primary antibodies against integrin α5 and β1 subunits were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Monoclonal antibodies against FAK, phospho-FAKTyr-397, phospho-FAKTyr-576/577, phospho-FAKTyr-925, β-catenin (6B3), phospho-β-catenin (Ser-33/37/Thr-41), ERK1/2, and phospho-ERK1/2 were purchased from Cell Signaling Technology, Co., Ltd. Primary monoclonal antibody for β-actin and horseradish peroxidase-conjugated secondary antibody were also from Cell Signaling Technology. The SuperSignal Western blotting detection kit was obtained from Pierce. Other chemicals and reagents were of molecular biology grade and were purchased from local commercial stores.
Cell Cultures
Primary rat osteoblasts were isolated from calvaria of 3-day-old Sprague-Dawley rats and cultured using methods described previously (25, 26). Briefly, the newborn rats were sacrificed by decapitation and immersed in 75% alcohol for 5 min. The calvaria were cleaned of any extraneous tissue and then cut into small pieces. The bone chips were recovered with a bone curette and were digested in DMEM with 0.1% collagenase (w/v) for 4 h, containing 10% (v/v) FBS in a humidified atmosphere of 95% air, 5% CO2 at 37 °C. Then the solution with bone chips was pooled and filtered through 70-μm nylon filters (Falcon, BD Biosciences). A preplating step was included to reduce the number of contaminating non-osteoblasts. The dispersed cells were plated in DMEM containing 10% heat-inactivated fetal bovine serum for 30 min to remove the non-osteoblasts, and then 2 × 106 cells/ml (10 ml/dish) were placed in 100-mm culture dishes and maintained in a humidified atmosphere of 95% air, 5% CO2 at 37 °C. The medium was replaced every other day. When the cells covered 90% of the bottom of dish, they were detached with 0.25% trypsin and passaged. The purified osteoblasts were identified using alkaline phosphatase assays, cells containing dark brown particles were considered to be osteoblasts, and the positive rate of osteoblasts was calculated (supplemental Fig. 1, C and D). Alizarin red staining of calcified nodule was also performed (supplemental Fig. 1B). The cells used in the experiments were of the fourth passage, whereas in the siRNA transfection experiments, the third passage subcultured cells were used.
ESW Treatment of Rat Osteoblasts in Vitro
In our experiments, ESW was generated by Huikang type IV shock wave equipment (Huikang, Shengzhen, China) with a focus spot about 25 mm in diameter. Osteoblasts were washed and resuspended with complete medium. Cell suspensions were subjected to ESW treatment with modification as described before (27, 28). Cells were suspended in 15-ml sterile polystyrene tubes at a concentration of 1 × 106/ml. First of all, to identify the optimal intensity of ESW, ESW treatment with 250, 500, 750, and 1000 impulses at different energy levels (5, 10, 15, and 20 kV) was applied to the cell suspensions. After ESW treatment, cells were cultured for 24 and 48 h for cell proliferation assays while stained with trypan blue to assess cell survival at 1 h. Once an optimal intensity of ESW was determined, ESW treatment of osteoblast suspensions (samples with or without inhibitors/siRNAs) lasted 10 min. Then the cells were placed onto plastic dishes or culture plates for different times as required. Osteoblasts without ESW treatment were run as controls.
Blocking with Specific Inhibitors of Signal Transduction Pathways
In studies of the ESW-induced signal transduction of cell adhesion and migration, cells were treated with 50 μm PD98059, a MEK1 inhibitor; 20 μm U0126, a MEK1/2 inhibitor; 25 μm LY294002, a PI3K signal pathway inhibitor; 15 μm SB203580, a p38/MAPK inhibitor; 20 μm SP600125, a JNK inhibitor; 25 μm H-89, a protein kinase A inhibitor; and 50 μm AG490, a JAK signal pathway inhibitor, for 60 min prior to ESWT. Cells were washed and resuspended before they were subjected to the optimal intensity of ESW treatment as described above. After the treatment, cells were cultured until the time when a maximum elevation of integrin α5 and β1 subunits would be reached without adding specific inhibitors, as indicated. Then we identified the specific signal transduction pathways involved in ESW-induced adhesion and migration by Western blotting analysis.
Transient Transfection with Small Interfering RNA
According to the gene sequences of integrin α5 (NM_001108118) and β1 (NM_017022) and the principles of siRNAs design, double strand siRNA oligonucleotides targeting both genes (sense (5′-CCGCAUCCUGGAGUCUUCATT-3′) and antisense (5′-UGAAGACUCCAGGAUGCGGTT-3′) for integrin α5 oligonucleotide and sense (5′-GAUCAGGAGAACCACAGAATT-3′) and antisense (5′-UUCUGUGGUUCUCCUGAUCTT-3′) for integrin β1 oligonucleotide) were synthesized by GenePharma Technology Co., Ltd. (Shanghai, China), respectively. A pair of negative control siRNAs were also designed. For transfection, cells were plated onto culture plates of six wells and grown to 70–80% confluence in the complete medium without antibiotics. Then the cells were transfected with integrin α5/β1 siRNAs or a negative control siRNA using LipofectamineTM RNAiMAX reagent according to the manufacturer's recommendations. Briefly, we prepared RNAi duplex-LipofectamineTM RNAiMAX complexes by the following steps for each well of the osteoblast sample: (a) diluted 10 μl of RNAi duplex in 240 μl of Opti-MEM® I reduced serum medium; (b) diluted 5 μl of LipofectamineTM RNAiMAX in 245 μl of Opti-MEM® I reduced serum medium; (c) combined the diluted RNAi duplex with the diluted LipofectamineTM RNAiMAX and incubated for 10–20 min by mixing gently at room temperature. Cells were incubated with the oligonucleotide duplexes in serum-free conditions for 6 h at 37 °C. Then the cells were harvested and resuspended with the complete medium. Cell suspensions were given the optimal intensity of ESW treatment as described above. After ESW treatment, cells were incubated for a certain additional period of time. The effects of siRNAs on the expression of integrin α5/β1 and its downstream signal pathway proteins were assessed using RT-PCR or Western blotting.
Cell Survival and Viability Assays
To determine the optimal intensity of ESW, we performed cell survival and viability assays. After different doses of ESW treatment, the cell survival was determined with a hemocytometer by staining with 0.4% trypan blue in ammonium chloride. Osteoblast viability was determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cells treated with either vehicle or ESW treatment were cultured in 96-well culture plates (200 μl/well). After 24 or 48 h, they were supplemented with 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 g/liter, Fluka Co. product) and incubated for another 4 h. Then the supernatant was discarded by aspiration, and the osteoblast preparation was shaken with 150 μl of DMSO for 10 min, before the OD value was measured at 490 nm by using a microplate reader (Thermo Scientific, Beijing, China).
Cell Adhesion and Spreading Assays
Osteoblasts with or without ESW treatment were concentrated by centrifugation (1000 rpm, 5 min) and resuspended in the complete medium. Cells were again counted with a hemocytometer and seeded on plastic culture dishes and incubated for 2, 4, 6, 8, and 10 h at 37 °C, 95% O2, 5% CO2 in a humidified incubator at the same concentration of 1 × 104 cells/cm2. Non-adherent cells were removed by rinsing with PBS, and adherent cells were released with 0.25% trypsin/EDTA and counted by independent blinded investigators. Then the adherence rates for the shock wave group and the control group were calculated. Cells plated onto fibronectin-coated culture dishes were run as positive controls. All of the samples were routinely observed with inverted phase-contrast microscopy (Nikon, Japan) to confirm the procedure of cell adhesion.
Cell Migration Assay
The migration of osteoblasts was evaluated by using a modified transwell insert assay. In brief, osteoblasts with or without ESW treatment in the presence or absence of integrin α5/β1 siRNAs were rendered into single-cell suspensions. 2 × 104 osteoblasts in 200 μl of complete medium were transferred into the upper chamber of transwell inserts (8-μm pore size; Costar). An aliquot of 0.6 ml of complete medium containing VEGF (50 ng/ml) was placed in the bottom wells. After incubation for 12 and 24 h at 37 °C, the non-migrated cells on the upper side of the membranes were wiped gently with ice-cold PBS-soaked cotton swabs. Then the migrated cells on the bottom side of the membranes were washed with PBS, fixed with methanol, and stained with 4′,6-diamidino-2-phenylindole (DAPI) solution. For quantification, cells migrating into the lower chamber were counted manually in five even high power (×100) microscopic fields by using fluorescent microscopy (Nikon), and the average numbers of cells/field were determined.
A wound healing test was also performed to measure osteoblast migration. Briefly, single cell suspensions obtained as described above were inoculated at 1 × 104/well in 6-well plates. When the cells became fully confluent, a scar was made along a straight line using a 200-μl pipette, and the floating cells were rinsed off with PBS. The scar was marked in the plate, and the cells were grown in serum-free medium. Five viewpoints were photographed at 12 and 24 h after the scar was made under a microscope at ×40 magnification, and the mean migration distance was determined and analyzed using Image Pro Plus version 6.0 software.
Reverse Transcription and Polymerase Chain Reaction (RT-PCR)
The gene expression of integrin α5 and β1 subunits was examined. Total RNA was isolated from cells using TRIzol reagent as instructed by the manufacturer. After the isolation of the RNA, the concentration of RNA was determined based on the optical density of the sample, which was measured at 260 nm. 1 μg of RNA was used in the reverse transcriptase (RT) reaction. The first strand cDNA from the RT reaction was used as a template in the PCR. To perform PCR, 12.5 μl of PCR mixture, 1 μl of cDNA, 9.5 μl of RNase-free water, 1 μl of reverse primer, and 1 μl of forward primer were added and vortexed together. The PCR was performed using a thermocycler (Bio-Rad). DNA was PCR-amplified under the following conditions: 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 60 s for a total of 36 cycles. Primer sequences used were as follows: integrin α5 (Itga5, NM_001108118), 5′- ACCAAGACGGCTACAATGATG-3′ (sense) and 5′-TGAGGCAGAAGCTAAGGTTGA-3′ (antisense); integrin β1 (Itgb1, NM_017022), 5′-GGAGGAATGTAACACGACTGC-3′ (sense) and 5′-CAGATGAACTGAAGGACCACC-3′ (antisense); glyceraldehyde-3-phosphatase (GAPDH, NM_017008), 5′-ACCACAGTCCATGCCATCAC-3′ (sense) and 5′-CCACCACCCTGTTGCTGTA-3′ (antisense). The reference housekeeping gene used to normalize the amount of mRNA in the cultures was GAPDH. All RT-PCR products were electrophoresed in 1.5% agarose gel and visualized under a UV transilluminator after staining with Goldview and photographed. The relative expression was quantified densitometrically by using Quantity One software (version 4.5.2; Bio-Rad).
Flow Cytometric Analysis
To elucidate whether integrin α5 and β1 subunits expressed on the cell membranes were involved in the ESW-enhanced cell adhesion and migration, we determined the quantity of both subunits via flow cytometry, respectively, according to the manufacturer's protocols. After ESW treatment for 0.5, 1, 2, 4, 8, and 12 h, osteoblasts were washed with ice-cold PBS and then resuspended in 400 μl of binding buffer at 1 × 106 cells/ml. Aliquots of 390-μl suspensions with a single cell were incubated with 10 μl of PE-conjugated anti-rat CD29 and CD49e (0.2 mg/ml; E-Biosciences), respectively, or PE-conjugated IgG (isotype control; E-Biosciences) for 30 min at 4 °C in the dark. Osteoblasts without ESW treatment as control groups were treated according to the same procedure. Fluorescence was measured using a FACSort flow cytometer (BD Biosciences). Approximately 10,000 cells were counted in each sample, and data were analyzed with the use of WinMDI software (version 2.9; Bio-Soft Net).
Protein Extraction and Concentration Assays
Osteoblasts were washed twice in ice-cold PBS and allowed to sit on ice for 30 min in 200 μl of lysis buffer (25 mm Tris-HCl, pH 7.4, 0.5 mm EDTA, 0.5 mm EGTA, 0.05% Triton X-100, 10 mm β-mercaptoethanol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mm NaF, 5 mm Na3VO4). Then the samples were centrifuged at 12,000 × g for 10 min at 4 °C, and the supernatants of the respective samples were collected and stored at −20 °C. Proteins were denatured by boiling for 5 min after measurement of the concentration in the BCA assays (kits were from Beyotime (Jiangsu, China)). According to the instruction provided with the kit, samples were incubated with BCA reagent for 30 min, and the absorbance of each was read at 560 nm using a microplate reader. BSA ranging from 0 to 500 μg/ml was used as a standard.
Determination of Integrin α5 and β1 Subunits
To detect the expression of integrin α5 and β1 subunit protein, Western blotting was taken into consideration. Equal amounts of proteins were separated on 8% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membrane (Millipore). The membrane was blocked by a 1-h incubation at room temperature in a Tris-buffered saline solution (TBS-T; 20 mm Tris, pH 7.6, 135 mm NaCl, and 0.05% Tween) containing 5% nonfat dry milk. Membranes were probed with anti-integrin α5 and β1 antibodies (1:600 dilution; Santa Cruz Biotechnology, Inc.) at 4 °C overnight. After the primary antibody incubation, the membrane was washed three times with TBS-T. The appropriate secondary antibody, horseradish peroxidase-labeled goat anti-rabbit IgG, was then added to the membrane according to the vendor's recommendation (1:8000 dilution; Cell Signaling Technology) and incubated for 1 h at room temperature. The membrane was again washed three times with TBS-T. The bound antibodies were detected by use of SuperSignal Western blotting kits (Pierce). Densitometric analysis of Western blots involved the use of Quantity One software (version 4.5.2; Bio-Rad). The expression of β-actin was shown as a control for equal protein loading.
Determination of FAK Phosphorylation and β-Catenin Activation
As described above, the procedures of detecting phospho-FAK, FAK, phospho-β-catenin, and β-catenin were the same as described above except that 10% SDS-polyacrylamide gels were used, and the blocking solution was changed to TBST with 5% BSA. The monoclonal primary antibodies (Cell Signaling Technology products) worked at dilutions of 1:1000.
Determination of ERK1/2 Phosphorylation
The data from studies on blocking the signal transduction pathways with specific inhibitors above indicated that the ERK/MAPK pathway was involved in the ESW-induced integrin expression. We did research on how the ERK1/2 phosphorylation level changed with either ESW treatment or not through Western blotting detection using 12% SDS-polyacrylamide gels. The procedure was similar to that noted above.
Statistical Analysis
The data presented are from one of three separate sets of experiments, of which yielded comparable results. All data are expressed as means ± S.D. Student's unpaired t test was used to compare differences between two groups. One-way analysis of variance followed by Student-Newman-Keuls test was used to compare the differences among more than two groups. A probability (p) value less than 0.05 was considered statistically significant.
RESULTS
ESW with 10 kV for 500 Impulses Was Definite as the Optimal Intensity
The cell survival was determined with a hemocytometer by staining with 0.4% trypan blue in ammonium chloride. We found that the osteoblast survival rate was not affected by energy lower than 10 kV for 500 impulses, whereas osteoblasts receiving 15 or 20 kV of shock wave showed significantly reduced survival as compared with the control group (p < 0.01) (supplemental Fig. 2). Cell proliferation assays showed that at 24 h after shock wave treatment, there were no statistically significant differences between osteoblasts receiving 5 or 10 kV and the control cells (p > 0.05). Furthermore, at 48 h, shock wave at 5 kV induced statistically significant differences in the proliferation of osteoblasts compared with the controls (p < 0.05), whereas shock wave at 10 kV for 500 impulses caused a marked increase in the proliferation of osteoblasts as compared with the control (p < 0.01) (supplemental Fig. 3). These findings indicate that shock wave caused a dose-dependent effect on the survival and growth of osteoblasts and that 10 kV for 500 impulses was an optimal level for shock wave treatment for osteoblasts. Therefore, ESW at 10 kV for 500 impulses was used in the subsequent experiments.
ESW Promotes Osteoblast Adhesion and Migration, Which Were Mediated by Integrins
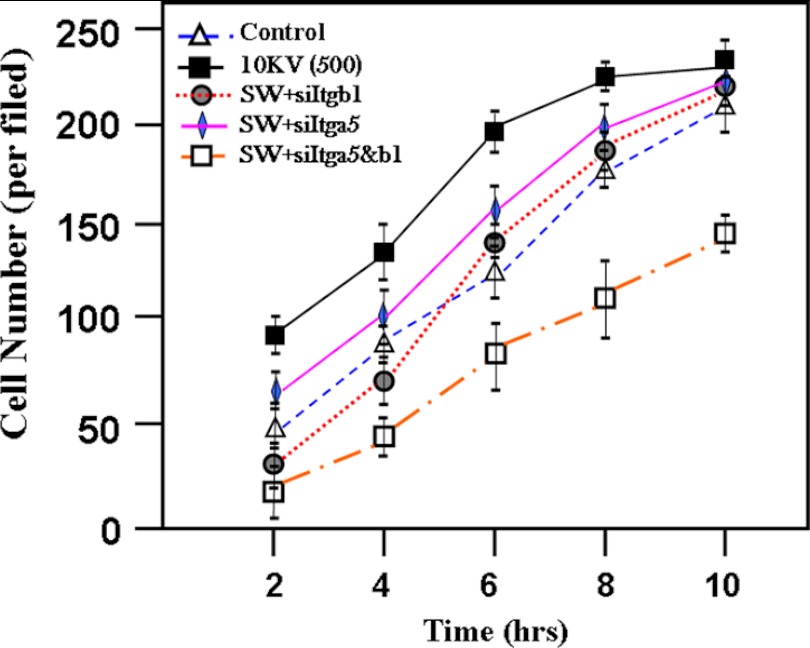
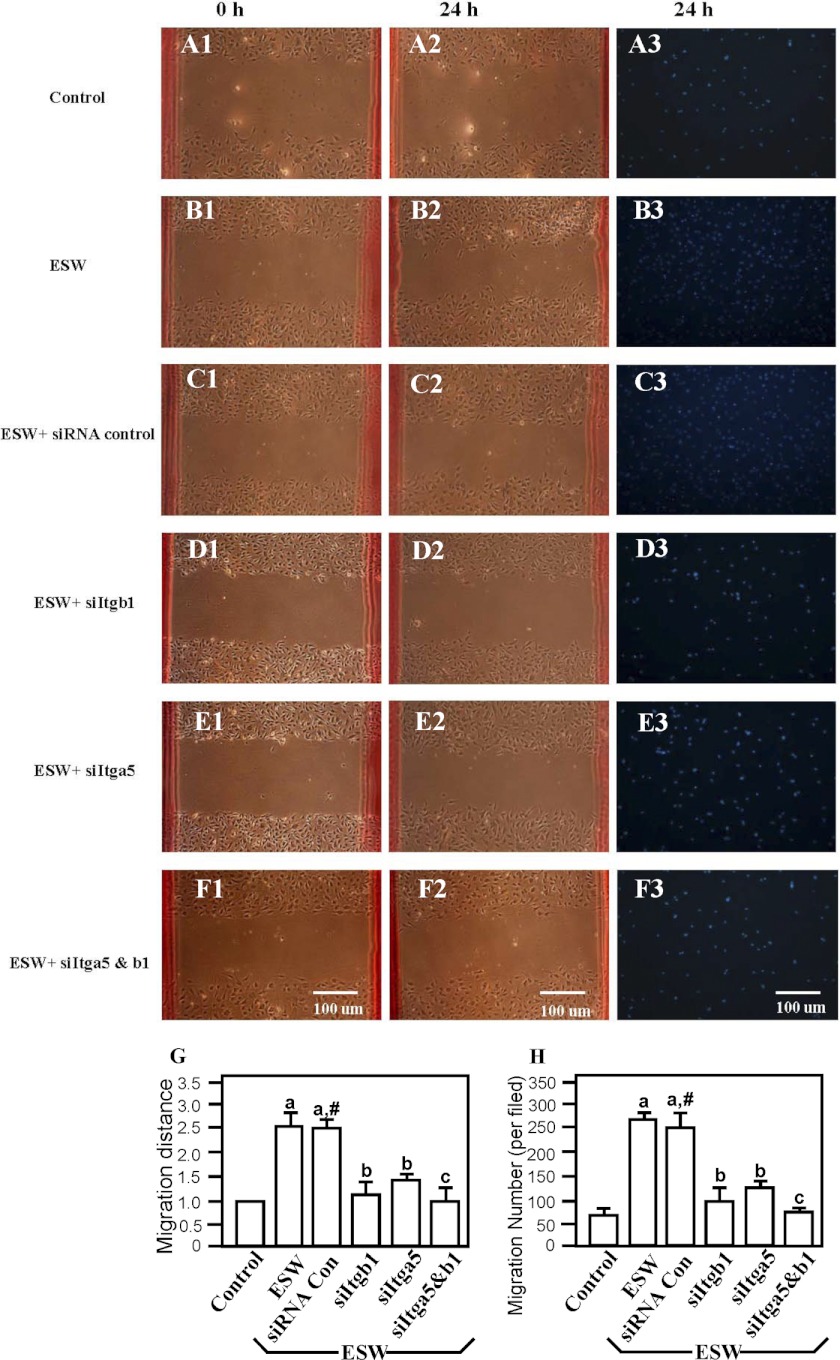
Adhesion, spreading property, and migratory capacity of cells were also important aspects of bone cell behavior, whereas there was no report focusing on the effect of ESW on the adhesion and migration of osteoblasts. In the present study, the effect of ESW on osteoblasts adhesion was evaluated in an adhesion assay. The SW at 10 kV for 500 impulses enhanced osteoblast adhesion significantly. The adhesion rates of osteoblasts were significantly higher at 2, 4, 6, 8, and 10 h after ESWT (Fig. 1). Dynamic observation under an inverted microscope showed that osteoblasts were more fully spread after ESWT (data not shown). The migration of osteoblasts was analyzed in modified transwell tests and wound healing assays. Results from the wound healing assays were consistent with the transwell tests. ESW accelerated osteoblast migration. After ESWT for 24 h, the average migration distance was increased by ∼3-fold, and the migrated cell number was increased by ∼4-fold (Fig. 2). In addition, the adhesion and migration of osteoblasts after ESW were impaired in the absence of α5 and β1 integrin (Figs. 1 and 2). We found that cell adhesion to matrices was primarily mediated by integrins under the condition of ESWT. These results prompted us to investigate whether or not shock wave could induce expression of integrins (shown in Figs. 3 and Fig. 4). Adding signal transduction pathway inhibitor U0126 could inhibit the ESW-augmented adhesion and migration of osteoblasts, whereas no influence was observed from several other inhibitors (data not shown). This encouraged us to perform more experiments to evaluate if the ERK1/2 signaling pathway could be activated by ESW (results shown in Fig. 7).
FIGURE 1.
Optimal intensity of ESW (10 kV for 500 impulses) accelerated osteoblast adhesion. Data are presented as the mean ± S.D. (error bars) in triplicate independent experiments (n = 3). The data show that at the times of 2, 4, 6, 8, and 10 h after ESW treatment, the number of adhesive osteoblasts was significant higher than the number without ESW treatment. p < 0.01 as compared with the control group at the same period. When the siItgb1 was added prior to ESW treatment, the promotion of adhesion of osteoblasts by ESW was inhibited. p < 0.01 as compared with the ESW group at the same period. p > 0.05 as compared with the control group at the same period. It was observed that siItga5 also inhibited the ESW-induced adhesion although not as significantly as did siItgb1. The promotion of adhesion induced by ESW was abrogated, whereas integrin α5 and β1 subunits were silenced. p < 0.01 as compared with the ESW group at the same period.
FIGURE 2.
ESW promoted migration of osteoblasts as shown in transwell tests and wound healing assays. The promotion could be inhibited by both siItgb1 and siItga5. Primary cultured osteoblasts were divided into six groups randomly; those were cells with (SW) (B1–B3) or without 10 kV for 500 impulses of ESWT (Control) (A1–A3) and with negative siRNA control (SW + siRNA control) (C1–C3), siItga5 (SW + siItga5) (E1–E3), siItgb1 (SW + siItgb1) (D1–D3), or both siItga5 and siItgb1 (F1–F3) for 6 h prior to ESWT. Results from the wound healing assays (G) and transwell tests (H) were consistent. Data are presented as the mean ± S.D. (error bars) (n = 6). a, p < 0.01; b, p < 0.05; c, p > 0.05 as compared with the control group. #, p > 0.05 as compared with the ESWT group. Scale bars, 100 μm.
FIGURE 3.
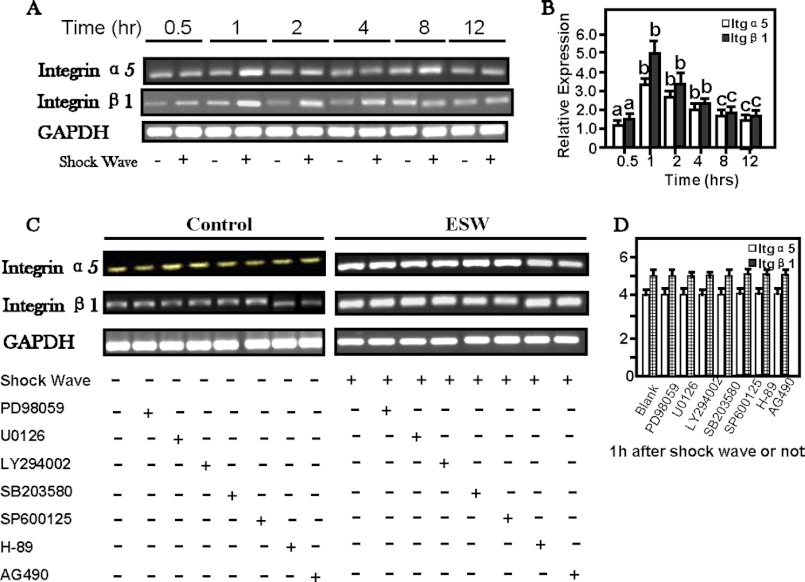
A–D, ESW-induced elevations of mRNA level of α5 and β1 integrin of osteoblasts, peaking at 1 h (B). The specific inhibitors for signal transduction pathways had no influence on the integrin expression (C). A and C, representative electrophoretic images. The osteoblasts were harvested to extract total RNA 0.5, 1, 2, 4, 8, and 12 h after 500 impulses of 10-kV shock wave treatment. The cells without ESWT were run as control groups. After standardization of housekeeping gene expression, equal amounts of cDNA from each sample were subjected to 36 cycles to amplify Itga5 and Itgb1 mRNA expression. The values of the control group were normalized to 100%. a, p > 0.05; b, p < 0.01; c, p < 0.05 as compared with the control group at certain time periods. In addition, several signal transduction pathway inhibitors were added to the samples for 1 h prior to ESWT. 2 h after ESWT, the samples were collected to extract RNA and to analyze whether the Itga5 and Itgb1 mRNA were influenced by signal pathway inhibitors listed above. Our data showed that no influence on the expression of Itga5 or Itgb1 mRNA was observed under the conditions with or without inhibitors (p > 0.05). Error bars, S.D.
FIGURE 4.
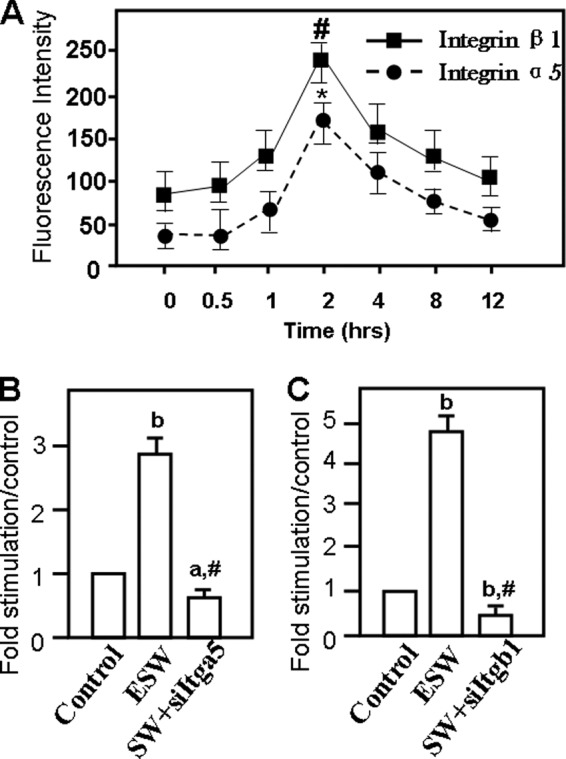
ESW enhanced integrin α5 and β1 subunit protein production in 2 h according to the data from flow cytometry analysis and Western blotting. For flow cytometry, osteoblasts from experimental groups and the control group were stained with PE-conjugated anti-rat Itga5 or Itgb1 antibody under the guidance of the manufacturer. It is shown that both Itga5 and Itgb1 proteins increased significantly in 2 h in the experimental group (A). *, p = 0.021; #, p < 0.01 as compared with the blank control group. Samples with or without ESWT and those associated with siItga5 and/or siItgb1 prior to ESW were subjected to radioimmune precipitation assay lysis. Western blotting was applied to analyze whether Itga5 and Itgb1 expression levels changed in the protein extractions. The same results are shown as those indicated by flow cytometry (B and C). In addition, the data from the ESW plus siRNA groups indicated that the siRNA reagents for both Itga5 and Itgb1 were effective (B and C). a, p < 0.05; b, p < 0.01 as compared with the blank control group. #, p < 0.01 as compared with ESW group. Results are presented with mean values ± S.E. (error bars) calculated from four paired triplicate experiments.
FIGURE 7.
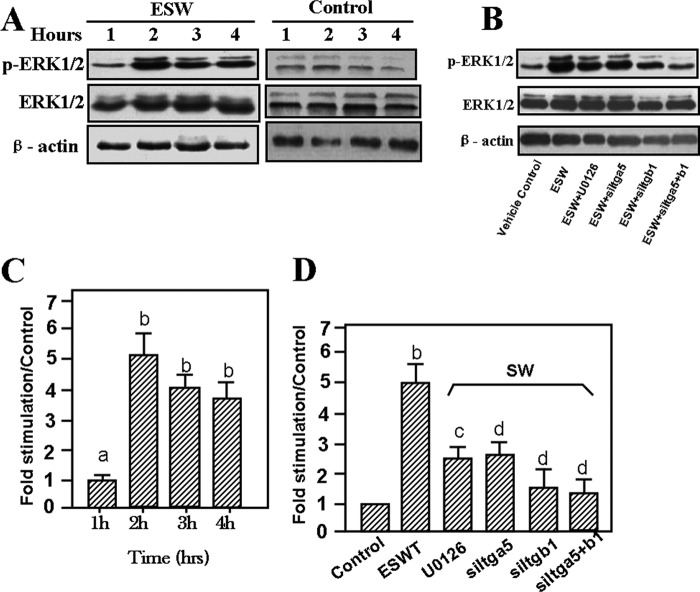
ESW activated ERK phosphorylation after treatment. ERK1/2 phosphorylation was increased in 2 h (A and C). The higher production of phosphorylated ERK1/2 persisted for 4 h (A and C). Osteoblasts were serum-deprived for 12 h before treatment with 10 kV for 500 impulses in the absence or presence of U0126 and siRNAs for the indicated time. Western blotting analysis was performed with antibodies against ERK and its phosphorylated forms (p-ERK) (B). C and D, summary of the results (mean ± S.E. (error bars), n = 4, triplicate in each experiment). a, p > 0.05 as compared with the control at the same period. b, p < 0.01 as compared with the control at the same period. c, p > 0.05 as compared with the control group. d, p < 0.01 as compared with the ESW group.
ESW Promotes Integrin α5 and β1 Subunit mRNA Expression
Primary osteoblasts were treated with ESW at an intensity of 10 kV for 500 impulses. There were no significant differences in cell viability between the ESW and control groups. Results from RT-PCR indicated that both integrin α5 and β1 subunit mRNAs were significantly elevated, peaking at 1 h after ESW, by ∼5-fold and then returning to the base level after 12 h (Fig. 3, A and B). Although multiple signaling pathway inhibitors of working concentrations were applied to pretreat the osteoblasts prior to ESWT, respectively, our results indicated that ESW-augmented integrin α5 and β1 subunit expression was not attenuated (Fig. 3, C and D). Moreover, ESW and inhibitors used in this study did not affect the mRNA expression of the housekeeping gene GAPDH (Fig. 3).
ESW Promotes Integrin α5 and β1 Protein Expression
The background expression of integrin α5 and β1 subunit protein was determined in osteoblasts (Fig. 4). During the period from 30 min to 12 h after ESWT, flow cytometry analysis showed that synchronous elevation of both integrin α5 and β1 protein, peaking at 2 h, as compared with the control groups (Fig. 4A). Western blotting results also demonstrated that osteoblasts subjected to ESWT significantly increased integrin α5 and β1 protein levels at 2 h by ∼3-fold and ∼5-fold, respectively (Fig. 4, B and C). What is more, the effects of ESW on integrin α5 and β1 expressions were abrogated by small interfering RNA pretreatment (Fig. 4, B and C). These findings suggest that integrin α5 and β1 expression was promoted in an early period after ESWT.
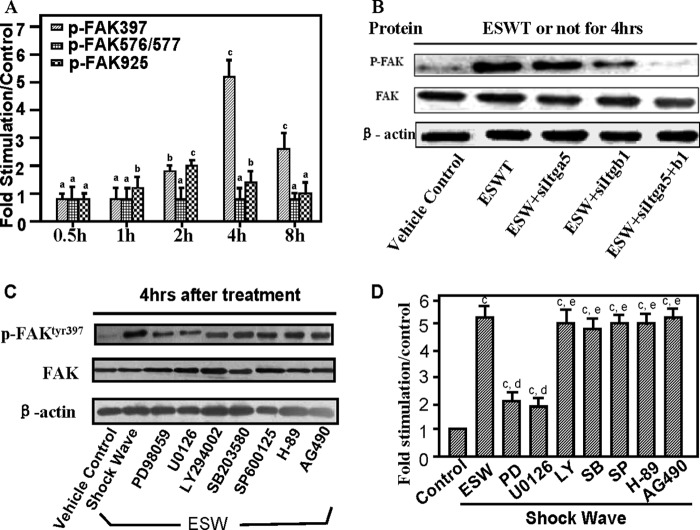
ESW Treatment Induces Phosphorylation of FAK, Which Is Mediated by Integrin α5 and β1
To determine the change of phosphorylation level of FAK at different sites caused by ESW at 10 kV for 500 impulses, osteoblasts were harvested at 0.5, 1, 2, 4, and 8 h after ESWT. We found a marked elevation of FAK phosphorylation at Tyr-397 in 2 h, peaking at 4 h, by ∼5-fold, and also a slight increase of phosphorylation of FAK at Tyr-925 in 2 h after ESWT, by ∼2-fold, whereas ESW could not affect the expression of FAK with phosphorylation at Tyr-576/577 (Fig. 5A). Moreover, to explore whether the FAK activation was associated with the elevated integrin α5 and β1 proteins, 4 h after the optimal dose of ESWT, the expression of phosphorylation of FAK at Tyr-397 declined in cells pretreated with integrin siRNAs (Fig. 5B). As shown in Fig. 5B, the decline was most significant when both siItga5 and siItgb1 were present. This indicated that integrin- FAK signaling (interactions with integrin α5β1 and FAK activation) played an important role in ESW-induced adhesion and migration of osteoblasts.
FIGURE 5.
Evaluations of phosphorylation levels of focal adhesion kinase surrounding Tyr-397, Tyr-576/577, and Tyr-925. After experimental groups were subjected to direct exposure to 10 kV for 500 impulses of ESWT, we collected the extracts at 0.5, 1, 2, 4, or 8 h. Samples without ESWT were set as the control group. Then the extracts were quantified in triplicate using Western blotting and normalized by β-actin expression (A). A marked elevation of FAK phosphorylation (p-FAK) at Tyr-397 peaking at 4 h was observed, and we also observed a slight increase of FAK phosphorylation at Tyr-925 in 2 h after ESWT. ESW had no influence on the expression of FAK phosphorylation at Tyr-576/577 (Fig. 5A). A representative electrophoretic image of the study on FAK phosphorylation at Tyr-397 influenced by siRNAs of integrins is also depicted (B). 4 h after the optimal dose of ESWT, a decline in the expression of phosphorylated FAK at Tyr-397 was observed in the group with siRNA pretreatment. However, total protein expression levels of FAK were not affected by silencing of Itga5 and/or Itgb1 (Fig. 5B). Moreover, several specific cell signal pathway inhibitors, namely PD98059, U0126, LY294002, SB203580, SP600125, H-89, and AG490, were also added to the osteoblasts for 1 h, respectively, before ESWT. Both bands of total FAK and β-actin showed equal amounts of proteins subjected to protein electrophoresis (C). The data indicated that both PD98059 and U0126, unlike other inhibitors listed, inhibited ESW-induced FAK phosphorylation at Tyr-397 (D). Data represent the mean ± S.E. (error bars) in triplicate independent experiments (n = 3). The values of the control group were normalized to 100%. a, p > 0.05; b, p < 0.05; c, p < 0.01 as compared with the control group at the same time. d, p < 0.01; e, p > 0.05 versus ESW group.
ERK1/2 Up-regulates Integrin-mediated FAK Phosphorylation
We sought to investigate whether certain mediators were involved in the ESW promotion of the integrin-FAK signal pathway. After adding specific signal pathway inhibitors to osteoblasts for 60 min prior to ESWT, we found that both 50 μm PD98059 and 20 μm U0126 (MEK1/2 inhibitors) significantly suppressed ESW-induced expression of phosphorylated FAK at the tyrosine 397 site, by ∼2-fold (Fig. 5, C and D). Inhibition of p38 activity by 15 μm SB203580 and JNK by 20 μm SP600125 did not affected ESW-promoted FAK activation. 25 μm H-89 (PKA inhibitor) and 50 μm AG490 (JAK inhibitor) had no influence on FAK production (Fig. 5, C and D). These findings suggest that ERK1/2, but not p38, JNK, PKA, or JAK, was essential for ESW-induced phosphorylation of FAK at Tyr-397 site.
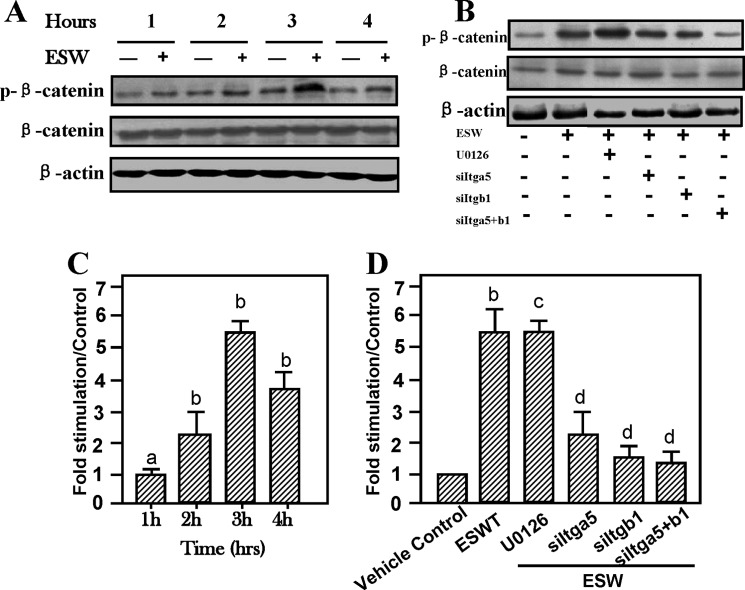
ESW Enhances the Activation of Wnt/β-catenin Mediated by Integrin α5 and β1
We investigated another important bioactive molecule that might be responsible for ESW-induced cell adhesion and migratory capacity. Immunoblotting indicated significantly increased β-catenin activation as demonstrated by phosphorylated β-catenin expression, peaking at 3 h after ESWT (Fig. 6A), by ∼5-fold (Fig. 6C). Pretreatment with integrin siRNAs significantly reduced ESW-promoted phosphorylation of β-catenin expression (Fig. 6D). Nevertheless, U0126 did not affect ESW-promoted activation of β-catenin (Fig. 6D). We believe that Wnt/β-catenin was another signal pathway activated by ESW, possibly responsible for resisting the inhibitory effect of U0126, to maintain the integrin-mediated ERK-dependent phosphorylation of FAK (Fig. 5D).
FIGURE 6.
Enhancement of β-catenin activity by ESW (500 impulses at 10 kV) stimulation after elevation of integrin α5 and β1 expression. ESW raised β-catenin phosphorylation in 3 h (A). ERK1/2 inhibitor U0126 did not alter the activation of β-catenin (B). Cytosolic extracts of osteoblasts treated with ESW in the presence of U0126 (with a final concentration of 20 μm) for 60 min prior to ESW were subjected to Western blotting. Phosphorylated β-catenin and β-catenin were probed with anti-phospho-β-catenin and β-catenin primary monoclonal antibodies, respectively. C, note that in comparison with the control, ESW exposure markedly elevated the activation of β-catenin. Further studies on the relationship between expression of integrins and β-catenin by transfection have shown that knocking out integrins led to base-line level expression of activation of β-catenin (B and D). D, summary of the results (mean ± S.E. (error bars), n = 4, triplicate in each experiment). a, p > 0.05 as compared with the control at the same period. b, p < 0.01 as compared with the control at the same period. c, p > 0.05 as compared with the ESW group. d, p < 0.01 as compared with the ESW group.
ESW Promotion of ERK Phosphorylation Was Mediated by Integrin α5 and β1
ERK became activated in osteoblasts under ESW induction and inhibited when integrin siRNAs were present. ERK1/2 phosphorylation was increased in 2 h, and the higher production of phosphorylated ERK1/2 persisted for 4 h (Fig. 7, A and C). Integrin siRNAs could markedly attenuate ESW-induced ERK activation (Fig. 7, B and D). Also, U0126 abrogated the expression of phosphorylated ERK1/2 (Fig. 7, B and D). These findings indicate that ERK activation was integrin-dependent under ESWT.
DISCUSSION
In the present study, we found that (a) an optimal extracorporeal shock wave (10 kV for 500 impulses) promoted primary culture osteoblast adhesion and migration, (b) α5β1 integrins played critical roles in ESW-induced osteoblast adhesive and migratory capacity mostly by up-regulating the phosphorylation of FAK, and (c) ERK1/2 was essential for ESW-augmented osteoblast behaviors as one of the most important mediators for the integrin α5β1-dependent FAK signaling pathway triggered by the exposure to ESW.
Interest in the application of ESWT to orthopedic diseases was initially stimulated by the finding that after the modality was focused on the ureter, bone remodeling of the pelvis occurred (28). Since the 1990s, shock waves have been used to treat musculoskeletal diseases, especially delayed fracture healing and nonunion (5, 10, 29, 30). Bone formation is the most important procedure during the bone regeneration period. Osteoblasts play a central role in bone formation by synthesizing multiple bone matrix proteins and by differentiation into osteocytes. Many investigators had committed to studying the effects of ESW on osteoblast differentiation and proliferation, and the underlying mechanisms included the involvement of growth factors, gene expressions, and several signal transduction pathways (15, 17, 23, 31). Although the adhesion behavior as well as migration was also important for osteoblasts in ESW-induced fracture healing, there have been no investigations focused on these two aspects before. We focused on the effects of ESWT on the adhesion and migration of osteoblasts while aiming to reveal the underlying specific biochemical signals triggered by ESW.
Different shock waves could induce different outcomes (15, 32). In our experiments, dose-dependent complications were observed, and it was determined that optimal intensity of energy was 500 impulses at 10 kV. Osteoblast adhesion refers to cell-cell adhesion and cell-ECM adhesion. After ESWT, the cell adhesion rate was much higher (80.24 ± 2.3% versus 43.77 ± 2.11% at 6 h) than without ESWT. The migration activity of osteoblasts was also significantly enhanced. Using the small interfering RNA technology, we knocked down the integrin α5 and β1 and observed significant inhibitions of adhesion, spreading, and migration in ESWT cells. These data indicated that ESW-induced adhesion, spreading, and migration partly depended on the expression of integrins.
Integrins are the cell surface receptors that comprise an expanding family of transmembrane heterodimers of an α subunit and a β subunit (33). They primarily mediate cell adhesion and migration, as well as being involved in cell proliferation, programmed cell death, and differentiation (34). It was reported that impairment of the integrin gene led to decreased expression of OPG (osteoprotegerin), PGE2 (prostaglandin E2), and TGF-β1 (22). It has also been shown that ultrasound treatment at 125 milliwatt/cm2 for 10 min transiently increased the surface expression of α5 and β1 integrin in both MC3T3-E1 and primary osteoblasts (23). Integrin α5β1 is the classical fibronectin receptor and mediates critical interactions between osteoblasts and fibronectin (35). Flow cytometry and Western blotting revealed that ESW increased the cell surface expression of α5 and β1 integrin. The α5β1 integrin knockdown in osteoblasts was insensitive to ESW stimulation and characterized by poor adhesion to plastic culture dishes and significantly impaired migratory capacity, which caused severe defects in wound healing and transwell tests. Herein, in our study, the rapid increase in the expression of integrin α5 and β1 subunit mRNA after shock wave stimulation might be the initial step in the accelerated adhesion and migration of osteoblasts.
A number of important biological processes, including cell motility, are controlled by integrin-dependent signals, and FAK has been implicated in these processes as an important component of an integrin-dependent signaling pathway, which transmits signals from the extracellular matrix into the cytoplasm (24, 36–38). Previous studies suggested that integrin β1-mediated expression of FAK regulated osteoblast behaviors (39), which played an important role in cell cycle progress and migration (34). Inhibition of endogenous FAK signaling resulted in reduced motility (40). Conversely, enhancing FAK signaling increases cell migration (38). The major autophosphorylation site of FAK, tyrosine 397, is absolutely required for enhancing cell migration (41, 42). Also, the Src recruitment into a complex with FAK is required for cell migration by augmenting the other sites of phosphorylation on FAK (43). In our study, we observed a marked elevation of FAK phosphorylation at Tyr-397, peaking at 4 h following ESW-induced expression of integrin α5 and β1, and a slight increase of FAK phosphorylation at Tyr-925 in 2 h after ESWT. ESW could not influence the expression of FAK phosphorylation at Tyr-576/577. What is more, a significant decline in FAK phosphorylation after ESWT was observed after knocking down integrin genes. Thus, we believe that FAK as a downstream reactor of the integrin-mediated signaling pathway plays a crucial role in ESW-induced osteoblast adhesion and migration.
A short pulse of mechanical force induced gene expression and growth in MC3T3-E1 osteoblasts via an ERK 1/2 pathway (44). By transducing human osteoblasts with a pseudotyped retrovirus encoding a mutated ERK1 protein with a dominant negative action against both ERK1 and ERK2 (ERK1DN cells), cell adhesion, spreading, and migration were inhibited, and expression of the integrins was decreased (45). Although there were also reports showing that ERK1/2 of the MAPK signaling family was downstream of FAK (43, 46), in our study, with the optimal intensity of SW (500 impulses at 10 kV), we found that ERK1/2 inhibitor U0126 did not influence the expression of integrin α5 and β1. In contrast, we found that both integrin α5 and β1 could trigger the phosphorylation of ERK1/2, which also played an important role in cell-adhesive and migratory function as mediators. A possible explanation for the paradoxical findings in the present study is that shock wave as a mechanical wave with critical biomechanical properties can change the spreading capacity of osteoblasts and also alter integrin expression (23). Furthermore, α5β1 integrin had been verified to be involved in mechanotransduction pathways (47). Another possible explanation for the discrepancy is that ESW could activate Shc first (Fig. 8) and enhance the ERK1/2 phosphorylation (48, 49). Additional studies would be required to confirm these speculations.
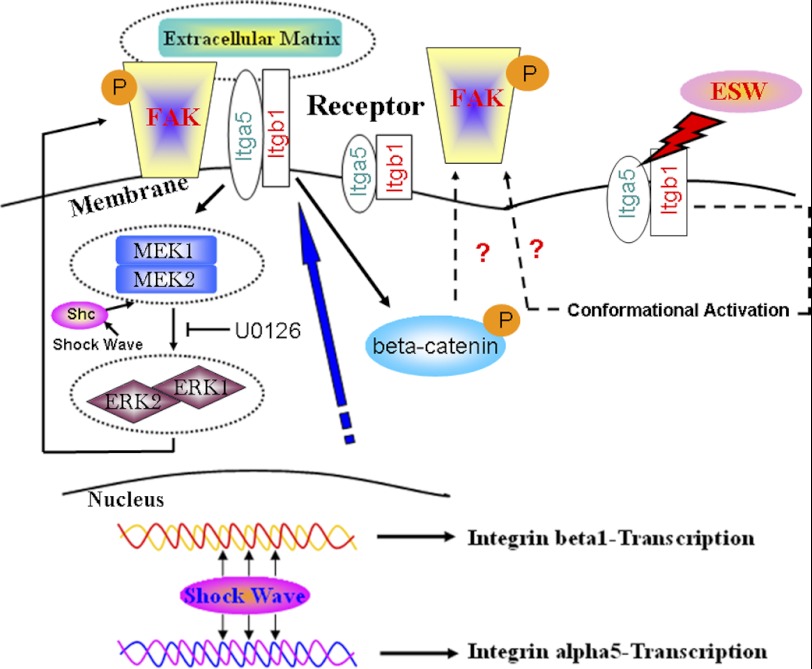
FIGURE 8.
Hypothetical model elucidating the regulation of phosphorylated FAK expression through an integrin α5 and β1-mediated MEK-ERK1/2-dependent pathway after ESWT. ESW directly stimulates integrin α5 and β1 mRNA expression inside the cell nucleus, and then the integrin protein expression is increased. Integrins induce MEK1/2 to phosphorylate ERK1/2, and then the activated ERK1/2 phosphorylates FAK and enhances its binding to the corresponding sites located in the adhesion sites, finally resulting in the enhancement of adhesion and migration. The Wnt/β-catenin signal pathway may also be involved in ESW-induced integrin-FAK signaling. The conformational activation of existing integrin α5/β1 complexes at the osteoblast surfaces may also be an additional potential mechanism that requires our further study.
The downstream signaling in the integrin pathway may involve the activation of β-catenin (47). It has been found that cyclical pressure-induced strain resulted in rapid phosphorylation of β-catenin in human articular chondrocytes (47). There are also studies showing that mechanical loading up-regulates Wnt/β-catenin genes (50, 51). In our study, we found that β-catenin phosphorylation level significantly increased at 3 h after ESW-induced expression of integrin α5 and β1. The activation of β-catenin was abolished by knocking down integrin α5 and/or β1 rather than with U0126. It was consistent with the previous study indicating that β-catenin was activated by integrin (47). As described above, U0126 inhibited the major phosphorylation site of FAK but not the whole. We therefore speculate that Wnt/β-catenin might be another mediator in the integrin-FAK signaling pathway in response to ESW (Fig. 8). The available evidence therefore suggests that the Wnt/β-catenin signaling pathway provides an important contribution to bone cell adaptive responses to mechanical stimulation (52–54).
A large amount of data suggests that integrin activation is one, if not the primary, early event. As a kind of mechanosensor, the existing integrin α5β1 at the cell surface becomes activated via conformational changes, which could be induced by mechanical stimuli (55–57). The conformational activation of integrin α5β1 might also play a crucial role in the process of ESW-induced adhesion and migration of osteoblasts. Batra et al. (55) have demonstrated that PI3K signaling was responsible for the shear stress-induced conformational activation of integrin α5β1, leading to the opening of the hemichannels. Nevertheless, the mechanism of how integrins become activated, which is induced by ESW, requires our further study (Fig. 8).
Many studies have shown that effective physical stimulation could induce a series of biological changes in osteoblasts, such as osteogenic effect (58, 59), alteration of gene expression (44, 60), and related signal pathway changes (44, 61). The beneficial effects for enhanced osteoblast adhesion and migration may result from reorganization of actin cytoskeleton to a certain degree (23). The regulation of cell function by actin cytoskeleton is complex and may also involve integrin-mediated matrix binding and signal pathways (62–66). In addition, cells need to spread fully during migration (45), and the enhanced ability of osteoblasts to spread would promote cell migration.
In summary, for the first time, to our knowledge, we have found that extracorporeal shock wave treatment promoted primary osteoblast adhesion, spreading property, and migratory capacity. ESW-induced functional activity of osteoblasts was via elevating both mRNA level and protein expression of integrin α5 and β1 subunits, particularly the β1 subunit involved in FAK signal pathway activation (Fig. 8). We have also further demonstrated that the ERK1/2 signal pathway was essential for FAK signal cascades. Our findings provide additional insight into the mechanism by which ESW stimulated fracture healing. In addition, by specifically controlling cell and extracellular matrix organization using ESWT, this technology holds great potential for advancing the fabrication of complex engineered tissues in vitro. Although our understanding of ESW-induced adhesion and migration of osteoblasts is increasing, the picture is still far from clear. There is still much to be learned before we fully understand the mechanisms induced by ESWT.
Supplementary Material
Acknowledgments
These experiments were mainly carried out in the Laboratory of Molecular Cardiology, First Affiliated Hospital, Shantou University Medical College. We thank Yan-lin She for expertise in use of the extracorporeal shock wave generator and for the use of equipment. We are grateful to Yu-long Lin for expert technical assistance with use of the flow cytometer.

This article contains supplemental Figs. 1–3.
- ESW
- extracorporeal shock wave
- SW
- shock wave
- ESWT
- ESW treatment
- SD
- Sprague-Dawley
- FAK
- focal adhesion kinase
- ECM
- extracellular matrix protein
- FAK
- focal adhesion kinase
- PE
- phycoerythrin
- CD29/Itgb1
- integrin β1
- CD49e/Itga5
- integrin α5.
REFERENCES
- 1. Cacchio A., Giordano L., Colafarina O., Rompe J. D., Tavernese E., Ioppolo F., Flamini S., Spacca G., Santilli V. (2009) Extracorporeal shock-wave therapy compared with surgery for hypertrophic long-bone nonunions. J. Bone Joint Surg. Am. 91, 2589–2597 [DOI] [PubMed] [Google Scholar]
- 2. Moretti B., Notarnicola A., Garofalo R., Moretti L., Patella S., Marlinghaus E., Patella V. (2009) Shock waves in the treatment of stress fractures. Ultrasound Med. Biol. 35, 1042–1049 [DOI] [PubMed] [Google Scholar]
- 3. Beutler S., Regel G., Pape H. C., Machtens S., Weinberg A. M., Kremeike I., Jonas U., Tscherne H. (1999) [Extracorporeal shock wave therapy for delayed union of long bone fractures. preliminary results of a prospective cohort study]. Unfallchirurg 102, 839–847 [DOI] [PubMed] [Google Scholar]
- 4. Ikeda K., Tomita K., Takayama K. (1999) Application of extracorporeal shock wave on bone. Preliminary report. J. Trauma 47, 946–950 [DOI] [PubMed] [Google Scholar]
- 5. Wang C. J., Chen H. S., Chen C. E., Yang K. D. (2001) Treatment of nonunions of long bone fractures with shock waves. Clin. Orthop. Relat. Res. 387, 95–101 [DOI] [PubMed] [Google Scholar]
- 6. Wang C. J., Huang H. Y., Chen H. H., Pai C. H., Yang K. D. (2001) Effect of shock wave therapy on acute fractures of the tibia. A study in a dog model. Clin. Orthop. Relat. Res. 387, 112–118 [DOI] [PubMed] [Google Scholar]
- 7. Birnbaum K., Wirtz D. C., Siebert C. H., Heller K. D. (2002) Use of extracorporeal shock wave therapy (ESWT) in the treatment of non-unions. A review of the literature. Arch. Orthop. Trauma Surg. 122, 324–330 [DOI] [PubMed] [Google Scholar]
- 8. McClure S., Dorfmüller C. (2003) Extracorporeal shock wave therapy. Theory and equipment. Clin. Techniques Equine Pract. 2, 348–357 [Google Scholar]
- 9. Rompe J. D., Rosendahl T., Schöllner C., Theis C. (2001) High-energy extracorporeal shock wave treatment of nonunions. Clin. Orthop. Relat. Res. 387, 102–111 [DOI] [PubMed] [Google Scholar]
- 10. Rivilis I., Milkiewicz M., Boyd P., Goldstein J., Brown M. D., Egginton S., Hansen F. M., Hudlicka O., Haas T. L. (2002) Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am. J. Physiol. Heart. Circ. Physiol. 283, H1430–H1438 [DOI] [PubMed] [Google Scholar]
- 11. Wang F. S., Yang K. D., Kuo Y. R., Wang C. J., Sheen-Chen S. M., Huang H. C., Chen Y. J. (2003) Temporal and spatial expression of bone morphogenetic proteins in extracorporeal shock wave-promoted healing of segmental defect. Bone 32, 387–396 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y. J., Wurtz T., Wang C. J., Kuo Y. R., Yang K. D., Huang H. C., Wang F. S. (2004) Recruitment of mesenchymal stem cells and expression of TGF-β1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J. Orthop. Res. 22, 526–534 [DOI] [PubMed] [Google Scholar]
- 13. Wang F. S., Wang C. J., Chen Y. J., Chang P. R., Huang Y. T., Sun Y. C., Huang H. C., Yang Y. J., Yang K. D. (2004) Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1α and VEGF-A expression in shock wave-stimulated osteoblasts. J. Biol. Chem. 279, 10331–10337 [DOI] [PubMed] [Google Scholar]
- 14. Jun H., Da X., Aibin Z., Jiangnan Z. (2006) Extracorporeal shock wave promotes postnatal human bone marrow stromal cells osteogenesis in vivo. Prog. Biochem. Biophys. 33, 452–457 [Google Scholar]
- 15. Steinbach P., Hofstädter F., Nicolai H., Rössler W., Wieland W. (1992) In vitro investigations on cellular damage induced by high energy shock waves. Ultrasound Med. Biol. 18, 691–699 [DOI] [PubMed] [Google Scholar]
- 16. Tuckwell D. S., Humphries M. J. (1993) Molecular and cellular biology of integrins. Crit. Rev. Oncol. Hematol. 15, 149–171 [DOI] [PubMed] [Google Scholar]
- 17. Schneider G. B., Whitson S. W., Cooper L. F. (1999) Restricted and coordinated expression of β3-integrin and bone sialoprotein during cultured osteoblast differentiation. Bone 24, 321–327 [DOI] [PubMed] [Google Scholar]
- 18. Clover J., Dodds R. A., Gowen M. (1992) J. Cell Sci. 103, 267–271 [DOI] [PubMed] [Google Scholar]
- 19. Gronowicz G. A., McCarthy M. B. (1995) Glucocorticoids inhibit the attachment of osteoblasts to bone extracellular matrix proteins and decrease β1-integrin levels. Endocrinology 136, 598–608 [DOI] [PubMed] [Google Scholar]
- 20. Hynes R. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 21. Siebers M. C., Walboomers X. F., van den Dolder J., Leeuwenburgh S. C., Wolke J. G., Jansen J. A. (2008) The behavior of osteoblast-like cells on various substrates with functional blocking of integrin-β1 and integrin-β3. J. Mater. Sci. Mater. Med. 19, 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L., Zhao G., Olivares-Navarrete R., Bell B. F., Wieland M., Cochran D. L., Schwartz Z., Boyan B. D. (2006) Integrin β1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxyvitamin D3. Biomaterials 27, 3716–3725 [DOI] [PubMed] [Google Scholar]
- 23. Yang R. S., Lin W. L., Chen Y. Z., Tang C. H., Huang T. H., Lu B. Y., Fu W. M. (2005) Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone 36, 276–283 [DOI] [PubMed] [Google Scholar]
- 24. Guan J. L. (1997) Focal adhesion kinase in integrin signaling. Matrix Biol. 16, 195–200 [DOI] [PubMed] [Google Scholar]
- 25. Tang C. H., Yang R. S., Liou H. C., Fu W. M. (2003) Enhancement of fibronectin synthesis and fibrillogenesis by BMP-4 in cultured rat osteoblast. J. Bone. Miner. Res. 18, 502–511 [DOI] [PubMed] [Google Scholar]
- 26. Cheng S. L., Lai C. F., Fausto A., Chellaiah M., Feng X., McHugh K. P., Teitelbaum S. L., Civitelli R., Hruska K. A., Ross F. P., Avioli L. V. (2000) Regulation of αVβ3 and αVβ5 integrins by dexamethasone in normal human osteoblastic cells. J. Cell. Biochem. 77, 265–276 [DOI] [PubMed] [Google Scholar]
- 27. Wang F. S., Wang C. J., Huang H. J., Chung H., Chen R. F., Yang K. D. (2001) Physical shock wave mediates membrane hyperpolarization and Ras activation for osteogenesis in human bone marrow stromal cells. Biochem. Biophys. Res. Commun. 287, 648–655 [DOI] [PubMed] [Google Scholar]
- 28. Uslu M. M., Bozdogan O., Güney S., Bilgili H., Kaya U., Olcay B., Korkusuz F. (1999) The effect of extracorporeal shock wave treatment (ESWT) on bone defects. An experimental study. Bull. Hosp. Joint Dis. 58, 114–118 [PubMed] [Google Scholar]
- 29. Schaden W., Fischer A., Sailler A. (2001) Extracorporeal shock wave therapy of nonunion or delayed osseous union. Clin. Orthop. Relat. Res. 387, 90–94 [DOI] [PubMed] [Google Scholar]
- 30. Wang C. J., Weng L. H., Chou W. Y., Hsu S. L., Ko J. Y., Ko S. F., Huang C. C. (2012) Extracorporeal shock wave therapy enhances early tendon-bone healing and reduces bone tunnel enlargement in hamstring autograft anterior cruciate ligament reconstruction. Am. J. Sports. Med., in press [DOI] [PubMed] [Google Scholar]
- 31. Hofmann A., Ritz U., Hessmann M. H., Alini M., Rommens P. M., Rompe J. D. (2008) Extracorporeal shock wave-mediated changes in proliferation, differentiation, and gene expression of human osteoblasts. J. Trauma 65, 1402–1410 [DOI] [PubMed] [Google Scholar]
- 32. Seidl M., Steinbach P., Wörle K., Hofstädter F. (1994) Induction of stress fibers and intercellular gaps in human vascular endothelium by shock waves. Ultrasonics 32, 397–400 [DOI] [PubMed] [Google Scholar]
- 33. Brakebusch C., Bouvard D., Stanchi F., Sakai T., Fässler R. (2002) Integrins in invasive growth. J. Clin. Invest. 109, 999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Damsky C. H., Ilić D. (2002) Integrin signaling. It's where the action is. Curr. Opin. Cell Biol. 14, 594–602 [DOI] [PubMed] [Google Scholar]
- 35. Moursi A. M., Globus R. K., Damsky C. H. (1997) J. Cell Sci. 110, 2187–2196 [DOI] [PubMed] [Google Scholar]
- 36. Schlaepfer D. D., Hauck C. R., Sieg D. J. (1999) Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71, 435–478 [DOI] [PubMed] [Google Scholar]
- 37. Parsons J. T., Martin K. H., Slack J. K., Taylor J. M., Weed S. A. (2000) Focal adhesion kinase. A regulator of focal adhesion dynamics and cell movement. Oncogene 19, 5606–5613 [DOI] [PubMed] [Google Scholar]
- 38. Schaller M. D. (2001) Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta 1540, 1–21 [DOI] [PubMed] [Google Scholar]
- 39. Nakayamada S., Okada Y., Saito K., Tamura M., Tanaka Y. (2003) β1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor κB ligand on osteoblasts and osteoclast maturation. J. Biol. Chem. 278, 45368–45374 [DOI] [PubMed] [Google Scholar]
- 40. Ilić D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 [DOI] [PubMed] [Google Scholar]
- 41. Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. (1999) Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19, 4806–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sieg D. J., Hauck C. R., Schlaepfer D. D. (1999) J. Cell Sci. 112, 2677–2691 [DOI] [PubMed] [Google Scholar]
- 43. Chaturvedi L. S., Marsh H. M., Basson M. D. (2007) Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells. Am. J. Physiol. Cell. Physiol. 292, C1701–C1713 [DOI] [PubMed] [Google Scholar]
- 44. Hatton J. P., Pooran M., Li C. F., Luzzio C., Hughes-Fulford M. (2003) A short pulse of mechanical force induces gene expression and growth in MC3T3-E1 osteoblasts via an ERK1/2 pathway. J. Bone Miner. Res. 18, 58–66 [DOI] [PubMed] [Google Scholar]
- 45. Lai C. F., Chaudhary L., Fausto A., Halstead L. R., Ory D. S., Avioli L. V., Cheng S. L. (2001) ERK is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J. Biol. Chem. 276, 14443–14450 [DOI] [PubMed] [Google Scholar]
- 46. Huang D., Khoe M., Befekadu M., Chung S., Takata Y., Ilic D., Bryer-Ash M. (2007) Focal adhesion kinase mediates cell survival via NF-κB and ERK signaling pathways. Am. J. Physiol. Cell. Physiol. 292, C1339–C1352 [DOI] [PubMed] [Google Scholar]
- 47. Lee H. S., Millward-Sadler S. J., Wright M. O., Nuki G., Salter D. M. (2000) Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and β-catenin in human articular chondrocytes after mechanical stimulation. J. Bone Miner. Res. 15, 1501–1509 [DOI] [PubMed] [Google Scholar]
- 48. Yee K. L., Weaver V. M., Hammer D. A. (2008) Integrin-mediated signaling through the MAP kinase pathway. IET Syst. Biol. 2, 8–15 [DOI] [PubMed] [Google Scholar]
- 49. Lee D. Y., Yeh C. R., Chang S. F., Lee P. L., Chien S., Cheng C. K., Chiu J. J. (2008) Integrin-mediated expression of bone formation-related genes in osteoblast-like cells in response to fluid shear stress. Roles of extracellular matrix, Shc, and mitogen-activated protein kinase. J. Bone Miner. Res. 23, 1140–1149 [DOI] [PubMed] [Google Scholar]
- 50. Lau K. H., Kapur S., Kesavan C., Baylink D. J. (2006) Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J. Biol. Chem. 281, 9576–9588 [DOI] [PubMed] [Google Scholar]
- 51. Robinson J. A., Chatterjee-Kishore M., Yaworsky P. J., Cullen D. M., Zhao W., Li C., Kharode Y., Sauter L., Babij P., Brown E. L., Hill A. A., Akhter M. P., Johnson M. L., Recker R. R., Komm B. S., Bex F. J. (2006) Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 281, 31720–31728 [DOI] [PubMed] [Google Scholar]
- 52. Drees F., Pokutta S., Yamada S., Nelson W. J., Weis W. I. (2005) α-Catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin filament assembly. Cell 123, 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamada S., Pokutta S., Drees F., Weis W. I., Nelson W. J. (2005) Deconstructing the cadherin-catenin-actin complex. Cell 123, 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Faux M. C., Coates J. L., Kershaw N. J., Layton M. J., Burgess A. W. (2010) Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions. PLoS One 5, e14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Batra N., Burra S., Siller-Jackson A. J., Gu S., Xia X., Weber G. F., DeSimone D., Bonewald L. F., Lafer E. M., Sprague E., Schwartz M. A., Jiang J. X. (2012) Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. U.S.A. 109, 3359–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Katsumi A., Naoe T., Matsushita T., Kaibuchi K., Schwartz M. A. (2005) Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J. Biol. Chem. 280, 16546–16549 [DOI] [PubMed] [Google Scholar]
- 57. Tzima E., del Pozo M. A., Shattil S. J., Chien S., Schwartz M.A. (2001) Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20, 4639–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Warden S. J., Favaloro J. M., Bennell K. L., McMeeken J. M., Ng K. W., Zajac J. D., Wark J. D. (2001) Low-intensity pulsed ultrasound stimulates a bone-forming response in UMR-106 cells. Biochem. Biophys. Res. Commun. 286, 443–450 [DOI] [PubMed] [Google Scholar]
- 59. Rubin C. T., Gross T. S., McLeod K. J., Bain S. D. (1995) Morphologic stages in lamellar bone formation stimulated by a potent mechanical stimulus. J. Bone Miner. Res. 10, 488–495 [DOI] [PubMed] [Google Scholar]
- 60. Chen Y. J., Wang C. J., Yang K. D., Chang P. R., Huang H. C., Huang Y. T., Sun Y. C., Wang F. S. (2003) Pertussis toxin-sensitive Gαi protein and ERK-dependent pathways mediate ultrasound promotion of osteogenic transcription in human osteoblasts. FEBS Lett. 554, 154–158 [DOI] [PubMed] [Google Scholar]
- 61. Plotkin L. I., Mathov I., Aguirre J. I., Parfitt A. M., Manolagas S. C., Bellido T. (2005) Mechanical stimulation prevents osteocyte apoptosis. Requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell. Physiol. 289, C633–C643 [DOI] [PubMed] [Google Scholar]
- 62. Humphries M. J., Travis M. A., Clark K., Mould A. P. (2004) Mechanisms of integration of cells and extracellular matrices by integrins. Biochem. Soc. Trans. 32, 822–825 [DOI] [PubMed] [Google Scholar]
- 63. Tarone G., Hirsch E., Brancaccio M., De Acetis M., Barberis L., Balzac F., Retta S. F., Botta C., Altruda F., Silengo L. (2000) Integrin function and regulation in development. Int. J. Dev. Biol. 44, 725–731 [PubMed] [Google Scholar]
- 64. Klingbeil C. K., Hauck C. R., Hsia D. A., Jones K. C., Reider S. R., Schlaepfer D. D. (2001) Targeting Pyk2 to β1-integrin-containing focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J. Cell Biol. 152, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hsia D. A., Lim S. T., Bernard-Trifilo J. A., Mitra S. K., Tanaka S., den Hertog J., Streblow D. N., Ilic D., Ginsberg M. H., Schlaepfer D. D. (2005) Integrin α4β1 promotes focal adhesion kinase-independent cell motility via α4 cytoplasmic domain-specific activation of c-Src. Mol. Cell. Biol. 25, 9700–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schlaepfer D. D., Jones K. C., Hunter T. (1998) Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase. Summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol. Cell. Biol. 18, 2571–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. National Institutes of Health (1996) Guide for the Care of Use of Laboratory Animals, NIH Publication 85-23, National Institutes of Health, Bethesda, MD [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.