Background: TAZ overexpression is implicated in cancer development.
Results: The N-terminal phosphodegron is phosphorylated by GSK3, which creates a binding site for β-TrCP, resulting in TAZ ubiquitylation and degradation.
Conclusion: The N-terminal phosphodegron regulates TAZ stability in response to the dysregulated PI3K pathway.
Significance: Our study reveals the underlying mechanism of TAZ N-terminal phosphodegron regulation in elevated TAZ cancers with a dysregulated PTEN/PI3K pathway.
Keywords: EMT, Glycogen Synthase Kinase 3, Phosphorylation, PI 3-Kinase (PI3K), Protein Degradation, PTEN, Hippo Pathway, TAZ
Abstract
The Hippo tumor suppressor pathway plays a major role in development and organ size control, and its dysregulation contributes to tumorigenesis. TAZ (transcriptional co-activator with PDZ-binding motif; also known as WWTR1) is a transcription co-activator acting downstream of the Hippo pathway, and increased TAZ protein levels have been associated with human cancers, such as breast cancer. Previous studies have shown that TAZ is inhibited by large tumor suppressor (LATS)-dependent phosphorylation, leading to cytoplasmic retention and ubiquitin-dependent degradation. The LATS kinase, a core component of the Hippo pathway, phosphorylates the C-terminal phosphodegron in TAZ to promote its degradation. In this study, we have found that the N-terminal phosphodegron of TAZ also plays a role in TAZ protein level regulation, particularly in response to different status of cellular PI3K signaling. GSK3, which can be inhibited by high PI3K via AKT-dependent inhibitory phosphorylation, phosphorylates the N-terminal phosphodegron in TAZ, and the phosphorylated TAZ binds to β-TrCP subunit of the SCFβ-TrCP E3 ubiquitin ligase, thereby leading to TAZ ubiquitylation and degradation. We observed that the TAZ protein level is elevated in tumor cells with high PI3K signaling, such as in PTEN mutant cancer cells. This study provides a novel mechanism of TAZ regulation and suggests a role of TAZ in modulating tissue growth and tumor development in response to PI3K signaling.
Introduction
How tissue and organ size are controlled by coordinating cell proliferation and apoptosis remains largely unknown. The Hippo pathway, a signaling pathway initially discovered by genetic screens in Drosophila over the last decade, regulates organ size by controlling both cell proliferation and apoptosis (1–3). This pathway is conserved from Drosophila to mammals. In mammals, the Hippo pathway plays an essential role in development and also regulates organ size. Dysregulation of the Hippo pathway is associated with tumor growth. For example, the neurofibromatosis tumor suppressor gene, NF2, acts upstream of the Hippo pathway. Merlin, the gene product of NF2, activates LATS to increase TAZ phosphorylation (4). In addition, other genes in the Hippo pathway, such as SAV1 and MOB1, are known to be mutated in human cancer cell lines (5, 6). Moreover, knockout of Hippo pathway genes causes organ hypertrophy and tumor growth in mouse models. These observations established a key role of the mammalian Hippo pathway in organ growth and tumor development.
The mammalian LATS1/2 and MST1/2 protein kinases are the homologues of Drosophila Warts and Hippo, respectively (7). They constitute the core components of the Hippo pathway and act in a kinase cascade. YAP, a transcription co-activator, is the mammalian homologue of Drosophila Yorkie. YAP is phosphorylated and inhibited by LATS (8). TAZ, first identified as a 14-3-3-binding protein, shares ∼50% sequence identity with YAP and has also been shown to function as a transcriptional co-activator downstream of the Hippo pathway (4, 9). YAP and TAZ represent the major function output of the Hippo pathway to regulate gene expression, cell proliferation, apoptosis, and organ size. TAZ is involved in the development of multiple organs, such as lung, fat, muscle, bone, limb, and heart, as well as many cellular processes, including stem cell differentiation, cell proliferation, and epithelial mesenchymal transition (EMT)3 (4, 10–15). Taz knock-out mice develop two severe abnormalities: polycystic kidney disease and emphysema (16, 17).
TAZ has been implicated in human tumorigenesis. Similar to YAP, TAZ is inhibited by the Hippo pathway due to the inhibitory phosphorylation by the LATS kinase. Overexpression of TAZ in MCF10A cells promotes cell proliferation, EMT, and oncogenesis (4, 15, 18). Notably, elevated TAZ expression is observed in more than 20% of breast cancers, especially invasive ductal carcinomas (15). TAZ is also implicated in papillary thyroid carcinoma and non-small cell lung cancer (19, 20). Recently, studies have demonstrated that TAZ plays an important role in breast cancer stem cell self-renewal and mesenchymal differentiation in glioma (21, 22). Together, these findings suggest an oncogenic activity of TAZ and the importance of controlling TAZ activity during normal development.
LATS-dependent phosphorylation of TAZ S89 results in 14-3-3 binding and cytoplasmic location, therefore inhibiting TAZ function by sequestration from cell nucleus. Moreover, TAZ protein levels can be regulated by ubiquitylation and proteasome degradation. We have recently shown that a C-terminal phosphodegron mediates TAZ degradation (23). Phosphorylation of TAZ at Ser-311 by LATS primes for sequential phosphorylation of TAZ at Ser-314 by CK1. The Ser-311 and Ser-314 doubly phosphorylated TAZ binds to and is ubiquitylated by the SCF E3 ubiquitin ligase, thereby resulting in proteasome degradation and functional inhibition. Interestingly, we found that the sensitivity of TAZ protein level to MG132, a proteasome inhibitor, treatment is different in different breast cancer cell lines (23). Notably, TAZ contains another phosphodegron located in the N-terminal region, and the N-terminal phosphodegron is unique in TAZ but not shared by YAP (24). This study investigates the mechanism of the N-terminal phosphodegron in regulating TAZ degradation.
In this report, we showed that the N-terminal phosphodegron is phosphorylated by GSK3, a protein kinase that is inhibited by the PI3K pathway. Phosphorylation of TAZ Ser-58/62 by GSK3 creates a binding site for β-TrCP, thus resulting in the recruitment of the SCFβ-TrCP E3 ubiquitin ligase. SCF promotes TAZ ubiquitylation and degradation. The N-terminal phosphodegron regulates TAZ stability in response to PI3K activation or PTEN mutation. TAZ is stabilized by high PI3K activity or PTEN mutation, revealing a possible molecular link of TAZ accumulation in tumor cells with abnormal AKT activation and a role of TAZ in tissue growth control in response to PI3K signaling. Therefore, the N-terminal and C-terminal phosphodegrons regulate the biological functions of TAZ in response to different signaling pathways.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
HEK293T, NIH3T3 and HeLa cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (HyClone) and 100 units/ml penicillin and streptomycin (Invitrogen). MCF10A cells were maintained in DMEM/F-12 medium (Invitrogen) supplemented with 5% horse serum (Invitrogen), 20 ng/ml epidermal growth factor, 0.5 μg/ml hydrocortisone, 10 μg/ml insulin, 100 ng/ml cholera toxin, and 100 units/ml penicillin and streptomycin (Invitrogen). Cell transfection was performed using Lipofectamine 2000 (Invitrogen) or calcium phosphate methods. Cells were harvested at 24 h post-transfection for protein analysis. To establish stable TAZ-expressing cells, pBabe-TAZ retroviruses were generated and used to infect MCF10A cells or NIH3T3, and stable pools were selected with puromycin for 5 days.
Western Blotting Analysis
Protein lysates were prepared from HeLa, NIH3T3, MCF10A, and stable pool cells in a buffer containing 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Nonidet P-40, 1 mm EDTA, 1 mm PMSF, 25 mm NaF, and a mixture of protease inhibitors (Roche Applied Science). Tissue lysate (40 μg) was resolved by SDS-PAGE, followed by Western blotting analysis. Antibodies to FLAG (catalog no. A00170 from GenScript or catalog no. A2220 from Sigma), TAZ (generated or from BD Biosciences), GAPDH(6C5) (SC32233, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), HA(F7) (SC7392, Santa Cruz Biotechnology, Inc.), β-catenin (catalog no. 9562, Cell Signaling), and β-actin (13E5, catalog no. 4970, Cell Signaling) were purchased commercially.
For immunoprecipitation experiments, 500 μg of cell lysate was incubated with anti-FLAG M2-agarose for 3 h at 4 °C. Beads were washed three times with lysis buffer and centrifuged at 5,000 × g for 5 min between each wash. Protein was eluted from beads with 50 μl of Laemmli sample buffer (Bio-Rad). Lysates were resolved on 8–10% SDS-polyacrylamide gels and transferred onto nitrocellulose (Bio-Rad) for Western blotting.
Immunoprecipitation and Kinase Assay
For the LATS2/GSK3β kinase assays, 293T cells were transfected with HA-LATS2 or HA-GSK3β. 36 h post-transfection, cells were lysed with lysis buffer (50 mm HEPES (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 50 mm NaF, 1.5 mm Na3VO4, protease inhibitor mixture (Roche Applied Science), 1 mm DTT, 1 mm PMSF) and immunoprecipitated with anti-HA antibodies. The immunoprecipitates were washed three times with lysis buffer, followed once with wash buffer (40 mm HEPES, 200 mm NaCl) and once with kinase assay buffer (30 mm HEPES, 50 mm potassium acetate, 5 mm MgCl2). The immunoprecipitated LATS2 was subjected to a kinase assay in the presence of 500 μm cold ATP, and 1 μg of His-TAZ and mutants was expressed and purified from E. coli as substrate. The reaction mixtures were incubated at 25 °C for 50 min, terminated with SDS sample buffer, and subjected to SDS-PAGE and ECL development.
Visualization of F-actin
Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 30 min, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 10 min, washed with phosphate-buffered saline, and then stained with rhodamine-conjugated phalloidin in phosphate-buffered saline.
Wound Healing Assay
Monolayer cells were wounded with a sterile plastic tip. Cell migration was observed 16 or 20 h later by microscopy.
RESULTS
TAZ Protein Level Is Negatively Regulated by PTEN
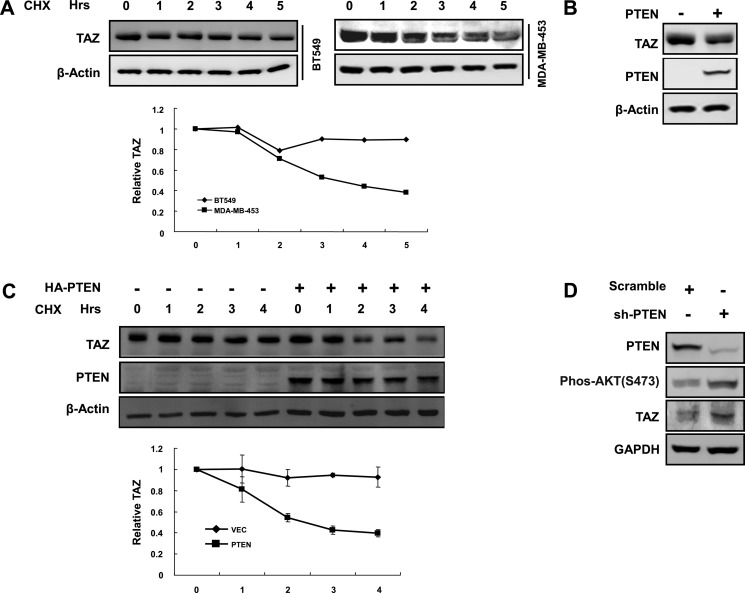
TAZ is overexpressed in around 20% of human breast cancer tissues (15). Interestingly, we previously found that the sensitivity of TAZ protein level to MG132 treatment was different in different breast cancer cell lines (23). MG132 treatment had a minor effect on TAZ protein levels in BT549 and Hs578T cells, indicating that TAZ is rather stable in these breast cancer cell lines. Notably, BT549 has no expression of PTEN, which is a tumor suppressor inhibiting the PI3K pathway. We speculated that PTEN may play an important role in TAZ expression. We compared TAZ stability between BT549 and another breast cancer cell line, MDA-MB-453, which has wild type PTEN. We observed that TAZ was more stable in the PTEN null BT549 cell line than in the PTEN wild type MDA-MB-453 cell line (Fig. 1A). To further test a role of PTEN in TAZ stability regulation, PTEN was re-expressed in the BT549 cells. Interestingly, PTEN expression reduced the steady state level of TAZ protein (Fig. 1B) and decreased TAZ stability (Fig. 1C). Furthermore, we reduced PTEN expression in MDA-MB-231 cells using shRNA. We found that knockdown of PTEN increased the TAZ protein level (Fig. 1D). Collectively, our data show that TAZ stability (and hence protein level) is negatively regulated by PTEN.
FIGURE 1.
TAZ protein level is negatively regulated by PTEN. A, PTEN is inversely correlated with TAZ stability in breast tumor cell lines. Both BT549 and MDA-MB-453 cells were treated with cycloheximide (CHX) (20 μg/ml) for the indicated times. Endogenous TAZ protein levels were determined. Relative TAZ levels were normalized against β-actin. B, PTEN overexpression decreases TAZ protein level. HA-PTEN was transfected into BT549 for WB analysis. TAZ levels were normalized against β-actin. C, ectopic expression of PTEN in BT549 destabilizes TAZ. HA-PTEN was transfected into BT549 cells, as indicated, and cells were treated with cycloheximide (20 μg/ml) for the indicated times. Endogenous TAZ protein levels were determined. Relative TAZ levels were quantified and normalized against β-actin. D, PTEN knockdown increases TAZ protein. Scramble and shPTEN (purchased from Sigma-Aldrich; clone ID: NM_000314.x-3001s1c1) plasmids were transfected into MDA-MB-231 cells. Protein levels and AKT phosphorylation were determined by Western blotting. Error bars, S.D.
TAZ Protein Level Is Regulated by PI3K and AKT
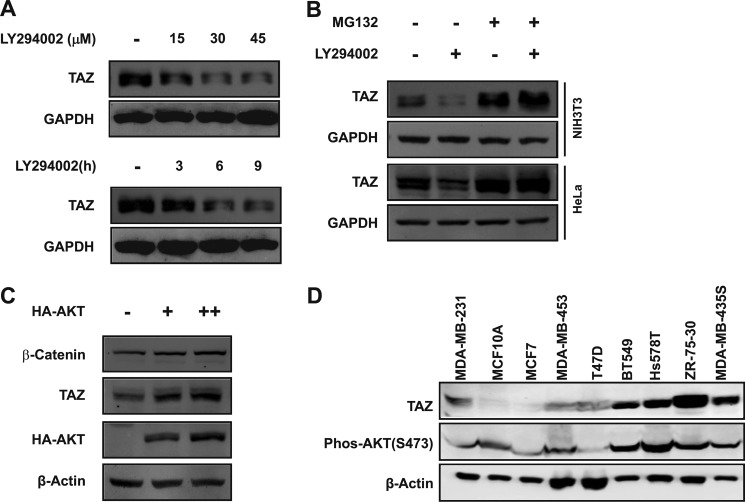
The PI3K pathway is the key signaling pathway affected by PTEN. PTEN inactivation results in accumulation of PI3K product and AKT activation. We hypothesized that PI3K and AKT might regulate the TAZ protein level. To this end, we treated NIH3T3 cells with LY294002 (a PI3K inhibitor) and found that LY294002 treatment resulted in a decrease of TAZ expression in a dose- and time-dependent manner (Fig. 2A). Given the fact that PTEN regulates TAZ protein stability (Fig. 1), a logical speculation is that PI3K also regulates TAZ protein degradation. If so, the effect of LY294002 on TAZ protein level should be blocked by MG132. We treated cells with MG132 and found that the MG132 indeed blocked the reduction of TAZ protein by LY294002 (Fig. 2B), indicating that LY294002 promotes proteasome-dependent TAZ degradation. We also determined the effect of overexpression of AKT on TAZ protein level and found that overexpression of AKT increased the TAZ protein level in a dose-dependent manner (Fig. 2C). Moreover, we examined TAZ protein levels and AKT activity, which is a physiological indicator of PI3K signaling, in several breast cancer cell lines. AKT activity was indirectly determined by Western blotting for Ser-473 phosphorylation, which contributes to AKT activation. We found that breast cancer cell lines with a high AKT Ser-473 phosphorylation generally displayed a high level of TAZ protein (Fig. 2D), indicating a positive correlation between AKT activity and TAZ protein levels. These observations support a physiological role of PI3K in TAZ stability regulation.
FIGURE 2.
TAZ protein level is regulated by PI3K and AKT. A, PI3K inhibitor LY294002 decreases TAZ protein levels. NIH3T3 cells were treated with PI3K inhibitor LY294002 at the indicated concentrations for 6 h (top) or as 30 μm for indicated times (bottom). Cell lysates were analyzed by WB. B, LY294002-induced TAZ degradation is blocked by MG132. HeLa and NIH3T3 cells were treated with or without PI3K inhibitor (LY294002) or MG132 as indicated for 6 h. Endogenous TAZ protein levels were determined by WB along with the GAPDH control. C, overexpression of AKT increases TAZ protein level in a dose-dependent manner. NIH3T3 cells were transfected with vector or HA-AKT as indicated. Endogenous TAZ protein levels were determined by WB along with β-actin control. β-Catenin was included as a positive control. D, correlation between TAZ protein levels and AKT phosphorylation. Eight breast cancer cell lines and one “normal” breast epithelial cell line (MCF10A) were examined for TAZ protein and AKT phosphorylation.
GSK3 Destabilizes TAZ
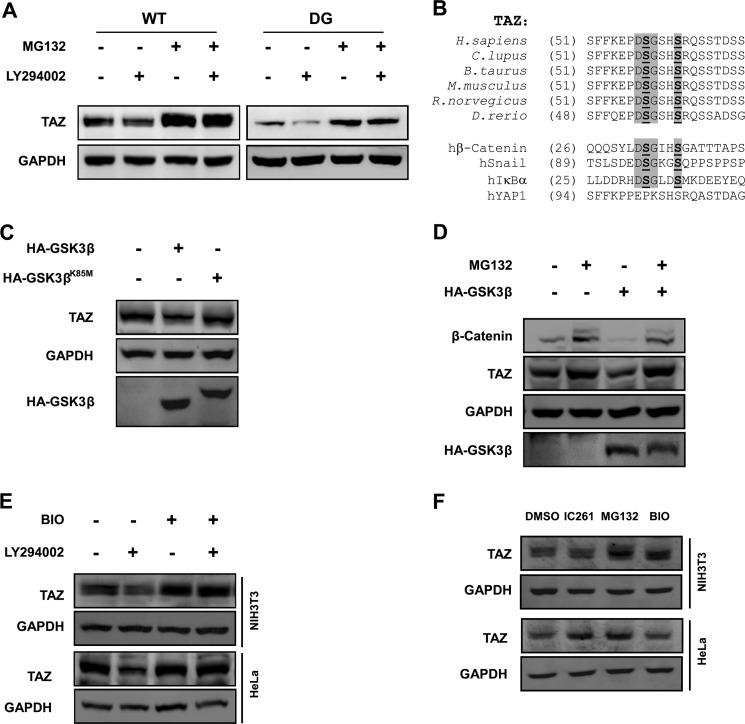
We previously found that the C-terminal phosphodegron plays an important role in TAZ degradation. The functional importance of the C-terminal phosphodegron in LY294002-promoted TAZ degradation was investigated. Surprisingly, the C-terminal phosphodegron DG mutant had little effect in conferring TAZ resistance to LY294002-promoted degradation (Fig. 3A). These data indicate that the C-terminal phosphodegron is not responsible for LY294002-induced TAZ degradation.
FIGURE 3.
GSK3 destabilizes TAZ. A, C-terminal phosphodegron is not responsible for LY294002-induced TAZ degradation. NIH3T3 cells stably expressing TAZWT and TAZDG (C-terminal phosphodegron DG mutant) were treated with or without LY294002 and MG132, as indicated. TAZ protein levels were determined by WB along with the GAPDH control. B, alignment of N-terminal phosphodegron in TAZ from different organisms. The phosphodegrons of β-catenin, Snail, and hIκBα are included for comparison with the DSGXXS motif. C, kinase activity of GSK3β is required for TAZ degradation. The WT or kinase-dead K85M mutant of GSK3β was transfected into HeLa cells, and endogenous TAZ protein levels were determined. D, MG132 blocks GSK3β overexpression-induced decrease of TAZ protein level. HA-GSK3β was transfected into HeLa cells as indicated, followed by treatment with MG132 (10 μm) for 6 h as indicated. Endogenous TAZ protein levels were determined along with the GAPDH control. β-Catenin was included as a positive control. E, PI3K inhibitor-induced TAZ degradation is blocked by GSK-3 inhibitor. HeLa and NIH3T3 cells were treated with PI3K inhibitor (LY294002) or GSK3 inhibitor (BIO) as indicated for 6 h. Endogenous TAZ protein levels were determined by WB along with the GAPDH control. F, regulation of TAZ protein levels in a cell type-dependent manner. HeLa and NIH3T3 cells were treated with casein kinase 1δ/1ϵ inhibitor (IC261), MG132, and GSK-3 inhibitor (BIO) as indicated, and endogenous TAZ protein levels were determined by WB along with the GAPDH control.
TAZ contains another phosphodegron in its N-terminal region that also matches the β-TrCP recognition sequence DSGXXS and is adjacent to the LATS phosphorylation site Ser-66 (Fig. 3B). Phosphorylation of Ser-66 by LATS could provide the priming phosphorylation for subsequent phosphorylation of Ser-62 and Ser-58 by GSK3β in the phosphodegron. This would be analogous to the mechanism of SCFβ-TrCP-mediated β-catenin degradation regulated by the sequential phosphorylation first by CK1 and then by GSK3β. Therefore, we tested the effect of GSK3 in TAZ degradation. Indeed, we observed that ectopic expression of GSK3β decreased the steady state level of endogenous TAZ (Fig. 3C). In contrast, the kinase-dead K85M mutant of GSK3β had no effect on TAZ protein level compared with WT GSK3β (Fig. 3C). Furthermore, GSK3β overexpression resulted in a decrease of TAZ protein levels, which is significantly blocked by MG132, showing the same pattern as the positive control, β-catenin (Fig. 3D). Because GSK3 is known to be phosphorylated and inhibited by AKT, which functions downstream of PI3K, we proposed that PI3K might regulate TAZ protein level via GSK3. To test the above hypothesis, we treated cells with the GSK3 inhibitor BIO and found that BIO could block LY294002-induced TAZ degradation in both HeLa and NIH3T3 cells (Fig. 3E). Because we have previously shown that a CK1ϵ/δ-specific inhibitor, IC261, can block TAZ degradation via C-terminal phosphodegron (23), we treated HeLa and NIH3T3 with DMSO, IC261, MG132, and BIO individually. We found that BIO had an obvious effect on TAZ protein level in NIH3T3 cells but a minor effect in HeLa cells (Fig. 3F). In contrast, IC261 had a minor effect on TAZ protein level in NIH3T3 cells but an obvious effect in HeLa cells (Fig. 3F). These observations suggest that the N-terminal and C-terminal phosphodegrons play a different role in TAZ degradation in different cell lines. Taken together, our data suggest that GSK3 plays a physiological role in mediating the effect of PI3K on TAZ stability.
GSK3 Interacts with and Phosphorylates TAZ
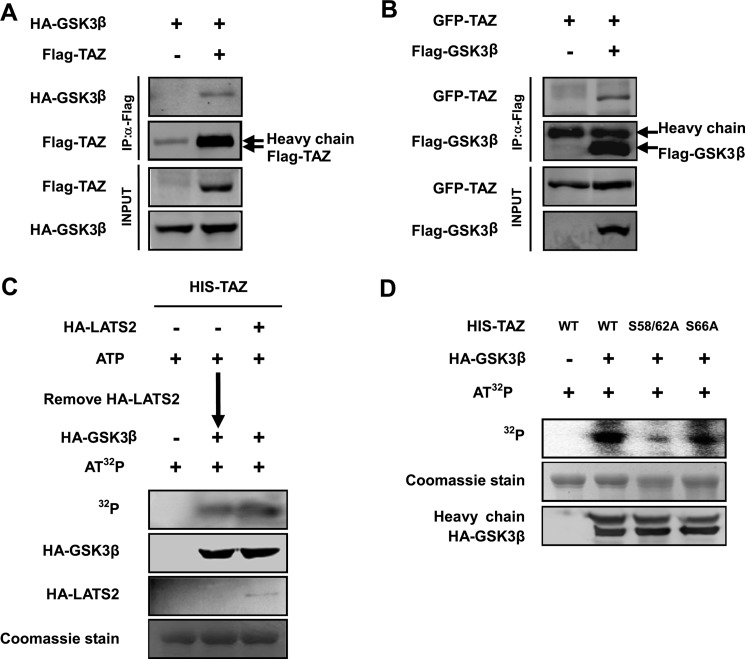
A direct interaction between a kinase and its target proteins often occurs. To characterize whether GSK3 could bind with TAZ, we performed co-immunoprecipitation experiments with transfected proteins. We found that HA-GSK3β could be readily pulled down by TAZ (Fig. 4A). Similarly, reciprocal co-immunoprecipitation experiments also showed that TAZ was co-precipitated with FLAG-GSK3β (Fig. 4B). These results show a physical interaction between GSK3 and TAZ, consistent with the notion of TAZ as a GSK3 substrate.
FIGURE 4.
GSK3 interacts with and phosphorylates TAZ. A and B, TAZ binds to GSK3β. GSK3β was co-transfected with TAZ into HEK293T cells as indicated. GSK3β and TAZ associations were examined by reciprocal co-immunoprecipitation (IP) as indicated. C and D, GSK3β directly phosphorylates the TAZ N-terminal phosphodegron in vitro. His-TAZ WT or mutants were expressed and purified from E. coli. HA-LATS2 and HA-GSK3β were immunoprecipitated from transfected 293T cells. In vitro kinase assay was performed using purified His-TAZ as a substrate. C, the purified TAZ was incubated with HA-LATS2 immunoprecipitated from transfected HEK293T cells in the presence of cold ATP. HA-LATS2 was removed from the kinase reaction. The LATS-treated His-TAZ was then incubated with HA-GSK3 immunoprecipitated from transfected HEK293T cells in the presence of radioactive ATP. D, phosphorylation of His-TAZ WT, S58A/S62A, and S66A mutants was also performed by adding GSK3β without the LATS2 pretreatment. Phosphorylation of TAZ was detected by a 32P autoradiograph. His-TAZ used in the in vitro reaction was shown by Coomassie Blue staining.
Phosphorylation of a protein by GSK3 normally requires a priming phosphorylation at the +4-position (25). As we described above, phosphorylation of Ser-66 by LATS could prime for phosphorylation of Ser-62 and Ser-58 by GSK3β (Fig. 3B). In order to characterize this, in vitro sequential phosphorylation of TAZ by LATS2 and GSK3β was examined. Purified TAZ was preincubated with LATS2 in the presence of cold ATP. LATS2 was removed from the reaction, and TAZ was then incubated with GSK3β in the presence of [32P]ATP. We found that TAZ was indeed phosphorylated by GSK3. Surprisingly, TAZ phosphorylation by GSK3β did not require LATS prior phosphorylation because preincubation with LATS did not increase the phosphorylation of TAZ by GSK3 (Fig. 4C). We then mutated the putative LATS phosphorylation site Ser-66 and the GSK3β phosphorylation residues Ser-58 and Ser-62. TAZS58A/S62A but not the TAZS66A mutant diminished phosphorylation by GSK3β, suggesting that Ser-58 and Ser-62 are important for GSK3β phosphorylation, whereas the Ser-66 is not (Fig. 4D). Our data confirms that the N-terminal phosphodegron can indeed be phosphorylated by GSK3, but priming phosphorylation is not required.
PI3K and GSK3 Modulates TAZ Stability via the N-terminal Phosphodegron
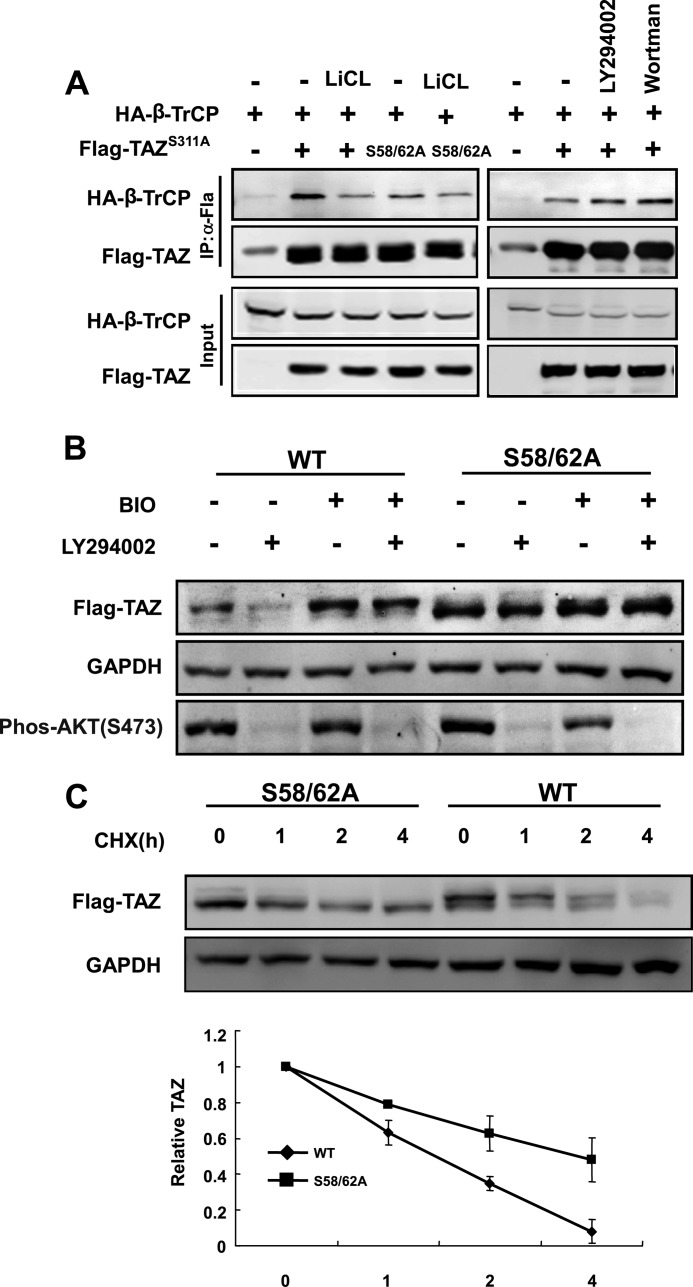
We have observed that mutation of the C-terminal phosphodegron decreased β-TrCP binding. However, the TAZS311A mutant still retained some β-TrCP binding as indicated by a longer exposure of the Western blotting (Fig. 5A). Consistent with a role of GSK3 in promoting the interaction between β-TrCP and the N-terminal phosphodegron, LiCl, which is a GSK3 inhibitor, decreased the co-immunoprecipitation between β-TrCP and TAZS311A. As expected, mutation of Ser-58 and Ser-62 also diminished the interaction between β-TrCP and the TAZS311A. These data shows that the N-terminal phosphodegron has a phosphorylation dependent β-TrCP binding activity although much weaker than that of the C-terminal phosphodegron. Finally, we tested the functional significance of the N-terminal phosphodegron in LY294002-induced TAZ degradation. LY294002 decreased the protein levels of WT TAZ, BIO blocked LY294002-induced TAZ degradation in 3T3 cells (Fig. 5B). In contrast, the TAZS58A/S62A mutant was resistant to LY294002-induced reduction in TAZ protein level, and BIO had no effect in TAZ accumulation (Fig. 5B), indicating that PI3K and GSK3 act through the N-terminal phosphodegron to regulate TAZ protein levels. We also tested the functional significance of the Ser-58/62 phosphodegron in TAZ stability regulation and observed that mutation of S58A/S62A stabilized TAZ (Fig. 5C). Collectively, our data demonstrates that the N-terminal phosphodegron is required for TAZ degradation in response to PI3K inhibition. Furthermore, phosphorylation of the N-terminal phosphodegron by GSK3β promotes TAZ ubiquitylation and degradation, probably by recruiting β-TrCP, and GSK3β is critically important for PI3K-AKT to modulate TAZ protein levels.
FIGURE 5.
AKT and GSK3 modulate TAZ stability via the N-terminal phosphodegron. A, interaction between the N-terminal phosphodegron and β-TrCP. The indicated plasmids were transfected into 293T cells. Co-immunoprecipitation was performed to determine the interaction between TAZS311A and β-TrCP. The TAZS311A mutant displayed a low level interaction with β-TrCP, which was further decreased by mutation of the N-terminal phosphodegron or GSK3 inhibition by LiCl. Treatment with PI3K inhibitor (LY294002 or wortmannin) or GSK3 inhibitor (LiCl) is indicated. B, TAZS58A/S62A mutant is resistant to PI3K inhibitor-induced degradation. NIH3T3 cells stably expressing vector, TAZ, and TAZS58A/S62A were treated as indicated, and cell lysates were analyzed by WB. C, TAZ was stabilized by mutation of phosphorylation sites in N-terminal phosphodegron. The half-lives of TAZ mutants in NIH3T3 cells stably expressing TAZ and TAZS58A/S62A were analyzed by WB. Relative TAZ levels were quantified by the ratio between TAZ and GAPDH. Error bars, S.D.
Mutation of the N-terminal Phosphodegron Increases TAZ Activity
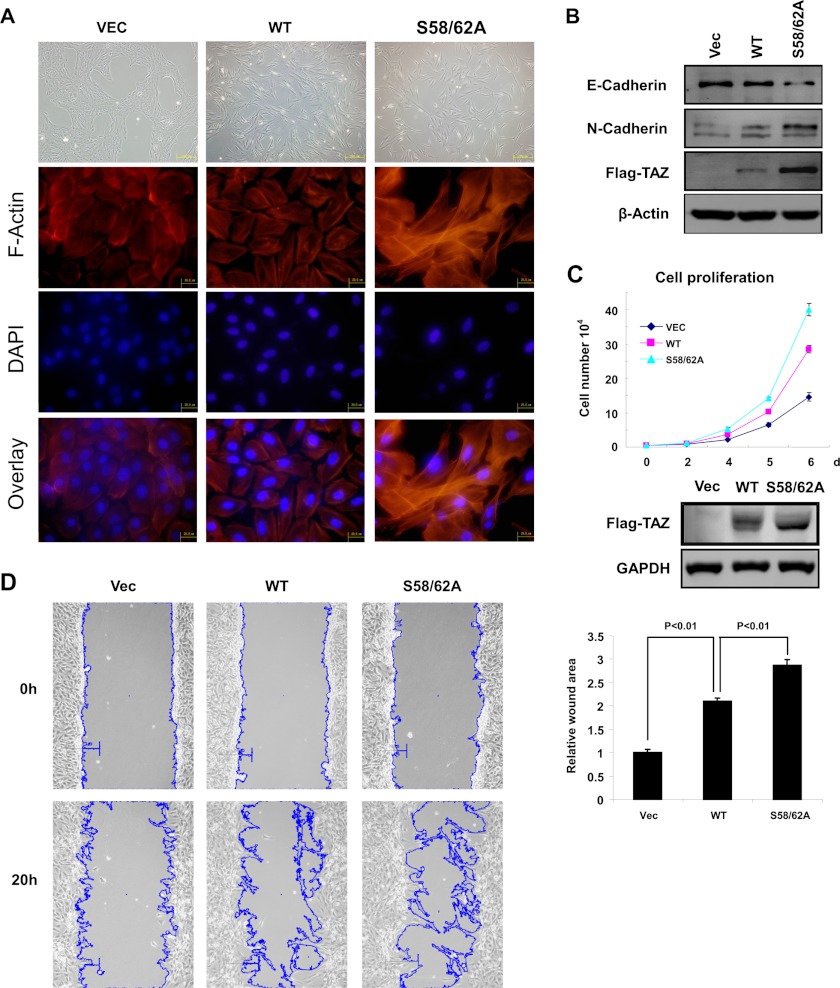
TAZ is known to induce EMT and promote cell proliferation and cell migration. To determine the functional significance of TAZ N-terminal phosphodegron, we established MCF10A and NIH3T3 cells stably expressing vector, TAZWT, and TAZS58A/S62A mutant. The control MCF10A cells grew in clusters, typical for epithelial cells, whereas the TAZ expressing cells displayed a loss of cell-cell contact and were scattered (Fig. 6A). TAZ expression also induced stress fiber formation. We found that S58A/S62A was more potent than the TAZWT to promote EMT morphological changes in MCF10A cells (Fig. 6A). The TAZS58A/S62A mutant displayed very strong stress fibers. Western blotting for EMT markers also showed that the phosphorylation mutant TAZ caused a stronger induction of N-cadherin, a marker for mesenchymal cells, and a stronger reduction of E-cadherin, a marker for epithelial cells (Fig. 6B). We also determined cell proliferation of cells expressing wild type and S58A/S62A mutant. The TAZS58A/S62A mutant caused a stronger enhancement in cell proliferation in NIH3T3 cells (Fig. 6C). Furthermore, cell migration was determined by wound healing assays. Expression of the TAZ phosphodegron mutant stimulated cell migration more potently than expression of the TAZWT did (Fig. 6D, left), and the calculated wound area showed statistical significance (p < 0.01) (Fig. 6D, right). These data show that the N-terminal phosphodegron negatively regulates TAZ biological functions because mutation of the N-terminal phosphodegron increases TAZ activity.
FIGURE 6.
Mutation of the N-terminal phosphodegron enhances TAZ function. A, TAZS58A/S62A mutant induces a stronger morphological change (top) and altered F-actin organization in MCF10A cells (bottom). Phase-contrast images of MCF10A cells expressing vector (Vec), TAZ, and TAZS58A/S62A are shown. Cells were stained with rhodamine-conjugated phalloidin. B, TAZS58A/S62A mutant is more potent in inducing EMT in MCF10A cells. Cell lysates from MCF10A cells expressing vector, TAZ, and TAZS58A/S62A were probed for epithelial markers and mesenchymal markers as indicated. C, TAZS58A/S62A mutant is more potent at promoting cell growth. Growth curves of NIH3T3 cells stably expressing vector, TAZ, and TAZS58A/S62A were determined. D, TAZS58A/S62A mutant stimulates cell migration. NIH3T3 cells expressing vector, TAZ, and TAZS58A/S62A were analyzed for migration by a wound healing assay. The outlines of the wound areas were generated by ImageJ (National Institutes of Health). Error bars, S.D.
DISCUSSION
As the major functional output of the Hippo pathway, both YAP and TAZ protein levels are tightly regulated (23, 26). High levels of YAP and TAZ can cause organ hypertrophy and eventually tumorigenesis (27). On the contrary, depletion of YAP and TAZ results in proliferation inhibition and apoptosis. In this report, we uncovered a new mechanism of TAZ stability regulation via the N-terminal phosphodegron. Importantly, this mechanism of regulation couples TAZ protein levels to PI3K signaling via GSK3-dependent phosphorylation, which is recognized by the F-box protein, β-TrCP, and subsequently ubiquitylated by the SCFβ-TrCP E3 ligase. Although the same SCF E3 is involved in recognition of both the N-terminal and C-terminal phosphodegrons, the regulation of the two phosphodegrons is different. The N-terminal phosphodegron can act independently of C-terminal phosphodegron because phosphorylation of either is sufficient to induce TAZ degradation. The data in this report also suggest that elevated TAZ protein may contribute to PTEN mutation/deletion-induced cell proliferation and tumorigenesis.
Our data indicate that GSK3 is responsible for phosphorylation of the N-terminal phosphodegron. GSK3 kinase normally requires priming phosphorylation at the +4-position in the motif (S/T)XXX(S/T) (25). Ser-66 adjacent to the N-terminal phosphodegron is a LATS phosphorylation site (Fig. 3B). Surprisingly, in vitro phosphorylation of the N-terminal phosphodegron by GSK3β does not require priming phosphorylation by LATS. Consistently, mutation of the LATS phosphorylation site Ser-66 does not affect TAZ destabilization in response to PI3K inhibition, indicating that LATS may not be involved in the regulation of the N-terminal phosphodegron. The presence of N-terminal phosphodegron in TAZ provides an additional layer of regulation. Our data also suggest a LATS-independent mechanism of TAZ regulation.
GSK3 kinase was initially identified as a key enzyme in glycogen metabolism and has been shown to regulate various biological processes, such as insulin response, nutrient signaling, and cell fate determination (25). GSK3 is known to destabilize growth-stimulating proteins, such as cyclin D, cyclin E, and β-catenin, by phosphorylation (25, 28–31). We propose that a common mechanism of GSK3 in growth inhibition is to phosphorylate and destabilize growth-promoting proteins. TAZ represents one such GSK3 target protein. GSK3 is inhibited by AKT (32); therefore, phosphorylation of TAZ N-terminal phosphodegron by GSK3β may provide a molecular mechanism for TAZ destabilization in response to PI3K inhibition. Consistent with this model, PTEN expression or PI3K inhibition induced TAZ destabilization is blocked by GSK3 inhibitors. It is worth noting that the N-terminal phosphodegron is not conserved in YAP, whereas the C-terminal phosphodegron is conserved, indicating a difference in YAP and TAZ regulation. We propose that the N-terminal and C-terminal phosphodegrons in TAZ receive different signals to regulate protein stability.
PI3K signaling has been implicated in promoting tissue and organ growth (33, 34). We speculate that activation of TAZ (by increasing protein level) may mediate part of the physiological function of PI3K in stimulating tissue growth. Moreover, activation of the PI3K pathway is frequently observed in human cancers (35, 36). This can be achieved by an activating mutation in growth factor receptors and PI3K or an inactivating mutation in PTEN. High PI3K signaling results in constitutive AKT activation and GSK3 inhibition. Therefore, phosphorylation of the TAZ N-terminal phosphodegron by GSK3 may provide a molecular mechanism for TAZ stabilization by PI3K activation. Consistent with this model, we observed a positive correlation between AKT phosphorylation and TAZ protein levels in human breast cancer cell lines. Moreover, re-expression of PTEN in the PTEN null cancer BT549 cells also decreased TAZ protein stability. TAZ is a potent transcription factor that stimulates cell proliferation and inhibits apoptosis. TAZ may mediate the growth-promoting signals downstream of the PI3K. When PI3K is activated, TAZ protein levels would be accumulated, therefore contributing to the mitogenic activity of the PI3K pathway. Conversely, when PI3K signaling activity is low, TAZ protein level would be decreased, thus leading to apoptosis and growth inhibition. TAZ overexpression can induce epithelial cells to undergo EMT, which is associated with tumor metastasis. Moreover, TAZ expression induces oncogenic transformation in NIH3T3 cells in vitro (37). It has been reported that TAZ protein levels are elevated in human cancers (15). Our data suggest a model in which, under pathological conditions, elevated TAZ protein levels may contribute to tumorigenesis in cancer cells with dysregulated PI3K signaling, as caused by an activating mutation in PI3K or inactivating mutation in PTEN.
Supplementary Material
Acknowledgments
We thank the members of the Fudan Molecular and Cell Biology laboratory for discussions throughout this study.
This work was supported by 973 Program Grants 2009CB918401, 2011CB910600, and NCET-09-0315; National Science Foundation of China (NSFC) Grants 31071192 and 30972937; NSFC-NIH Grant 81110313; 100 Talents Program of Shanghai Health Grant XBR2011041; and the “Scholar of Dawn” Program of the Shanghai Education Commission (to Q. Y. L.). This work was also supported by the 985 Program and the Shanghai Leading Academic Discipline Project (Project B110). This work is also supported by National Institutes of Health Grants CA132809 and CIRM RB2–01547 (to K. L. G.).

This article contains supplemental Figs. S1 and S2.
- EMT
- epithelial mesenchymal transition
- WB
- Western blot.
REFERENCES
- 1. Harvey K., Tapon N. (2007) The Salvador-Warts-Hippo pathway. An emerging tumor suppressor network. Nat. Rev. Cancer 7, 182–191 [DOI] [PubMed] [Google Scholar]
- 2. Pan D. (2007) Hippo signaling in organ size control. Genes Dev. 21, 886–897 [DOI] [PubMed] [Google Scholar]
- 3. Saucedo L. J., Edgar B. A. (2007) Filling out the Hippo pathway. Nat. Rev. Mol. Cell Biol. 8, 613–621 [DOI] [PubMed] [Google Scholar]
- 4. Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., Pei X. H., Zhao S., Xiong Y., Guan K. L. (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the Hippo pathway. Mol. Cell. Biol. 28, 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai Z. C., Wei X., Shimizu T., Ramos E., Rohrbaugh M., Nikolaidis N., Ho L. L., Li Y. (2005) Control of cell proliferation and apoptosis by Mob as tumor suppressor, Mats. Cell 120, 675–685 [DOI] [PubMed] [Google Scholar]
- 6. Tapon N., Harvey K. F., Bell D. W., Wahrer D. C., Schiripo T. A., Haber D. A., Hariharan I. K. (2002) Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 [DOI] [PubMed] [Google Scholar]
- 7. Edgar B. A. (2006) From cell structure to transcription. Hippo forges a new path. Cell 124, 267–273 [DOI] [PubMed] [Google Scholar]
- 8. Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., Guan K. L. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanai F., Marignani P. A., Sarbassova D., Yagi R., Hall R. A., Donowitz M., Hisaminato A., Fujiwara T., Ito Y., Cantley L. C., Yaffe M. B. (2000) TAZ: a novel transcriptional co-activator regulated by interactions with 14–3-3 and PDZ domain proteins. EMBO J. 19, 6778–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong J. H., Hwang E. S., McManus M. T., Amsterdam A., Tian Y., Kalmukova R., Mueller E., Benjamin T., Spiegelman B. M., Sharp P. A., Hopkins N., Yaffe M. B. (2005) TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309, 1074–1078 [DOI] [PubMed] [Google Scholar]
- 11. Hong J. H., Yaffe M. B. (2006) TAZ. A β-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle 5, 176–179 [DOI] [PubMed] [Google Scholar]
- 12. Mahoney W. M., Jr., Hong J. H., Yaffe M. B., Farrance I. K. (2005) The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem. J. 388, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murakami M., Nakagawa M., Olson E. N., Nakagawa O. (2005) A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc. Natl. Acad. Sci. U.S.A. 102, 18034–18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park K. S., Whitsett J. A., Di Palma T., Hong J. H., Yaffe M. B., Zannini M. (2004) TAZ interacts with TTF-1 and regulates expression of surfactant protein-C. J. Biol. Chem. 279, 17384–17390 [DOI] [PubMed] [Google Scholar]
- 15. Chan S. W., Lim C. J., Guo K., Ng C. P., Lee I., Hunziker W., Zeng Q., Hong W. (2008) A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 68, 2592–2598 [DOI] [PubMed] [Google Scholar]
- 16. Makita R., Uchijima Y., Nishiyama K., Amano T., Chen Q., Takeuchi T., Mitani A., Nagase T., Yatomi Y., Aburatani H., Nakagawa O., Small E. V., Cobo-Stark P., Igarashi P., Murakami M., Tominaga J., Sato T., Asano T., Kurihara Y., Kurihara H. (2008) Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am. J. Physiol. Renal Physiol. 294, F542–F553 [DOI] [PubMed] [Google Scholar]
- 17. Hossain Z., Ali S. M., Ko H. L., Xu J., Ng C. P., Guo K., Qi Z., Ponniah S., Hong W., Hunziker W. (2007) Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc. Natl. Acad. Sci. U.S.A 104, 1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang H., Liu C. Y., Zha Z. Y., Zhao B., Yao J., Zhao S., Xiong Y., Lei Q. Y., Guan K. L. (2009) TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J. Biol. Chem. 284, 13355–13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Cristofaro T., Di Palma T., Ferraro A., Corrado A., Lucci V., Franco R., Fusco A., Zannini M. (2011) TAZ/WWTR1 is overexpressed in papillary thyroid carcinoma. Eur. J. Cancer 47, 926–933 [DOI] [PubMed] [Google Scholar]
- 20. Zhou Z., Hao Y., Liu N., Raptis L., Tsao M. S., Yang X. (2011) TAZ is a novel oncogene in non-small cell lung cancer. Oncogene 30, 2181–2186 [DOI] [PubMed] [Google Scholar]
- 21. Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C., Inui M., Montagner M., Parenti A. R., Poletti A., Daidone M. G., Dupont S., Basso G., Bicciato S., Piccolo S. (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 [DOI] [PubMed] [Google Scholar]
- 22. Bhat K. P., Salazar K. L., Balasubramaniyan V., Wani K., Heathcock L., Hollingsworth F., James J. D., Gumin J., Diefes K. L., Kim S. H., Turski A., Azodi Y., Yang Y., Doucette T., Colman H., Sulman E. P., Lang F. F., Rao G., Copray S., Vaillant B. D., Aldape K. D. (2011) The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 25, 2594–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu C. Y., Zha Z. Y., Zhou X., Zhang H., Huang W., Zhao D., Li T., Chan S. W., Lim C. J., Hong W., Zhao S., Xiong Y., Lei Q. Y., Guan K. L. (2010) The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J. Biol. Chem. 285, 37159–37169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tian Y., Kolb R., Hong J. H., Carroll J., Li D., You J., Bronson R., Yaffe M. B., Zhou J., Benjamin T. (2007) TAZ promotes PC2 degradation through a SCFβ-Trcp E3 ligase complex. Mol. Cell. Biol. 27, 6383–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen P., Frame S. (2001) The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2, 769–776 [DOI] [PubMed] [Google Scholar]
- 26. Zhao B., Li L., Tumaneng K., Wang C. Y., Guan K. L. (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(β-TRCP). Genes Dev. 24, 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao B., Li L., Guan K. L. (2010) Hippo signaling at a glance. J. Cell Sci. 123, 4001–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welcker M., Singer J., Loeb K. R., Grim J., Bloecher A., Gurien-West M., Clurman B. E., Roberts J. M. (2003) Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol. Cell 12, 381–392 [DOI] [PubMed] [Google Scholar]
- 29. Hart M. J., de los Santos R., Albert I. N., Rubinfeld B., Polakis P. (1998) Down-regulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3 β. Curr. Biol. 8, 573–581 [DOI] [PubMed] [Google Scholar]
- 30. Diehl J. A., Cheng M., Roussel M. F., Sherr C. J. (1998) Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12, 3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alt J. R., Cleveland J. L., Hannink M., Diehl J. A. (2000) Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14, 3102–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 33. Leevers S. J., Weinkove D., MacDougall L. K., Hafen E., Waterfield M. D. (1996) The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 15, 6584–6594 [PMC free article] [PubMed] [Google Scholar]
- 34. Verdu J., Buratovich M. A., Wilder E. L., Birnbaum M. J. (1999) Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1, 500–506 [DOI] [PubMed] [Google Scholar]
- 35. Zhao J. J., Roberts T. M. (2006) PI3 kinases in cancer. From oncogene artifact to leading cancer target. Sci. STKE 2006, pe52. [DOI] [PubMed] [Google Scholar]
- 36. Vogt P. K., Kang S., Elsliger M. A., Gymnopoulos M. (2007) Cancer-specific mutations in phosphatidylinositol 3-kinase. Trends Biochem. Sci. 32, 342–349 [DOI] [PubMed] [Google Scholar]
- 37. Chan S. W., Lim C. J., Huang C., Chong Y. F., Gunaratne H. J., Hogue K. A., Blackstock W. P., Harvey K. F., Hong W. (2011) WW domain-mediated interaction with Wbp2 is important for the oncogenic property of TAZ. Oncogene 30, 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.