Background: The role of long noncoding RNA (lncRNA) highly up-regulated in liver cancer (HULC) in hepatocarcinogenesis mediated by hepatitis B virus X protein (HBx) remains unclear.
Results: Up-regulation of HULC by HBx promotes hepatoma cell proliferation via down-regulating p18.
Conclusion: HULC contributes to HBx-related hepatocarcinogenesis through suppressing p18.
Significance: The finding provides insight into the roles of lncRNAs in HBx-associated hepatocarcinogenesis.
Keywords: Cell Proliferation, Hepatocellular Carcinoma, Transcription Regulation, Tumor Suppressor Gene, Viral protein, Highly Up-regulated in Liver Cancer (HULC), Long Noncoding RNA
Abstract
Long noncoding RNAs (lncRNAs) play crucial roles in human cancers. It has been reported that lncRNA highly up-regulated in liver cancer (HULC) is dramatically up-regulated in hepatocellular carcinoma (HCC). Hepatitis B virus X protein (HBx) contributes importantly to the development of HCC. However, the function of HULC in HCC mediated by HBx remains unclear. Here, we report that HULC is involved in HBx-mediated hepatocarcinogenesis. We found that the expression levels of HULC were positively correlated with those of HBx in clinical HCC tissues. Moreover, we revealed that HBx up-regulated HULC in human immortalized normal liver L-O2 cells and hepatoma HepG2 cells. Luciferase reporter gene assay and chromatin immunoprecipitation (ChIP) assay showed that HBx activated the HULC promoter via cAMP-responsive element-binding protein. We further demonstrated that HULC promoted cell proliferation by methyl thiazolyl tetrazolium, 5-ethynyl-2′-deoxyuridine, colony formation assay, and tumorigenicity assay. Next, we hypothesized that HULC might function through regulating a tumor suppressor gene p18 located near HULC in the same chromosome. We found that the mRNA levels of p18 were inversely correlated with those of HULC in the above clinical HCC specimens. Then, we validated that HULC down-regulated p18, which was involved in the HULC-enhanced cell proliferation in vitro and in vivo. Furthermore, we observed that knockdown of HULC could abolish the HBx-enhanced cell proliferation through up-regulating p18. Thus, we conclude that the up-regulated HULC by HBx promotes proliferation of hepatoma cells through suppressing p18. This finding provides new insight into the roles of lncRNAs in HBx-related hepatocarcinogenesis.
Introduction
Hepatocellular carcinoma (HCC)3 is the fifth most common cancer in the world. It is also a leading cause of cancer death in many countries, mainly in Asia and Africa (1). Chronic infection with hepatitis B virus (HBV) has been proved to directly link with the development of HCC (2). Among the four proteins that originate from the HBV genome, the HBV X protein (HBx) has been detected with high frequency in liver cells from patients suffering from chronic hepatitis, cirrhosis, and liver cancer (3). HBx locates both in cell cytoplasm and in nucleus, acting as a multifunctional protein in the development of HBV-related liver cancer, including modulation of cell growth regulatory gene, regulation of apoptosis, and inhibition of nucleotide excision repair of damaged cellular DNA (4–6). Overwhelming findings indicate that HBx may cause a growth stimulus for hepatocytes, and thus, contribute to HCC development (7–9).
Regulatory noncoding RNAs (ncRNAs), such as microRNAs, small interfering RNAs, and various classes of long noncoding RNAs (lncRNAs), play increasingly important roles in the development of human diseases (10–13). lncRNAs, ranging from 300 nucleotides to over 10 kb, are abundantly transcribed by the mammalian genome (14–16). The data gathered to date strongly implicate lncRNAs in the basal regulation of protein-coding genes at both the transcriptional and the post-transcriptional levels (17). The dysregulation of lncRNAs has gradually become known as a primary feature of human cancers, such as colon cancer (18), breast cancer (19, 20) and HCC (21). Highly up-regulated in liver cancer (HULC) was first identified as a novel mRNA-like lncRNA up-regulated dramatically in HCC by Panzitt et al. (22), using an HCC-specific cDNA microarray platform. Wang et al. (23) reported that HULC was activated by the transcription factor cAMP-responsive element-binding protein (CREB). However, whether HULC is involved in the development of HBV-related HCC has not been elucidated so far.
In this study, we are interested in the roles of lncRNA HULC in the development of HCC mediated by HBx. Our results show that HULC contributes to the proliferation of hepatoma cells mediated by HBx through down-regulating tumor suppressor gene p18. Our finding provides new insight into the roles of lncRNAs in the development of HCC mediated by HBx.
EXPERIMENTAL PROCEDURES
Cell Culture
Human immortalized normal liver L-O2 cells and L-O2-X (L-O2 stably transfected with the HBx gene) cells were cultured in RPMI 1640 medium (Invitrogen) (24). Human hepatoma HepG2 cells and HepG2-X (HepG2 stably transfected with the HBx gene) cells were cultured in DMEM (Invitrogen) (25). Human hepatoma cells Hep3B and PLC/PRF/5, containing integrated HBV genome, were cultured in minimum essential medium (Invitrogen). The medium was supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 100 units/ml penicillin, and 100 units/ml streptomycin. Cultures were incubated at 37 °C in a humidified atmosphere with 5% CO2.
Tissue Samples
Tissue samples of human liver cancer were obtained during surgery at the Tianjin First Center Hospital (Tianjin, China). The adjacent nontumorous liver tissues obtained from normal parts of the surgical specimens were used as control samples. 33 clinical HCC tissue samples, in which 21 HCC samples were paired with their adjacent nontumorous liver tissues, were immediately snap-frozen in liquid nitrogen and stored at −80 °C. The detailed information of the 33 patients is shown in supplemental Table 1. Informed consent was obtained from each patient, and the study was approved by the Institute Research Ethics Committee at Nankai University.
Total RNA Isolation, Reverse Transcription-PCR, and Real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen), and cDNA was synthesized with PrimeScript reverse transcriptase (TaKaRa, Dalian, China) and oligo(dT) following the manufacturer's instructions. Reverse transcription-PCR (RT-PCR) or real-time PCR was performed to analyze mRNA expression. RT-PCR programs were as follows: 94 °C for 5 min, 94 °C for 30 s, 50 °C annealing for 30 s, 72 °C for 30 s followed by 35 cycles. Real-time PCR was performed using SYBR Premix Ex TaqTM II kit (TaKaRa). The conditions of real-time PCR were as follows: 94 °C for 10 s, 94 °C for 5 s, 52 °C for 30 s to anneal, 72 °C for 15 s followed by 40 cycles. We used specific primers for HULC (forward primer, 5′-ATC TGC AAG CCA GGA AGA GTC-3′, and reverse primer, 5′-CTT GCT TGA TGC TTT GGT CTGT-3′) and for p18 (forward primer, 5′-CGG GAG GTT CTT GTT CTG-3′, and reverse primer, 5′-TTT GTT GGC TTG CTT GAC-3′). As a control, GAPDH was amplified with specific primers (forward primer, 5′-CAT CAC CAT CTT CCA GGA GCG-3′, and reverse primer, 5′-TGA CCT TGC CCA CAG CCTT-3′).
Western Blot Analysis
Methods for Western blot were described previously (26). Briefly, proteins were prepared with radio-immunoprecipitation assay lysis buffer and quantified by Bradford assay (Bio-Rad). Equal amounts of protein were subjected to SDS-PAGE. The primary antibodies used for Western blot were polyclonal anti-AIMP3/p18 (Abcam, Cambridge, UK), monoclonal anti-CREB (Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal anti-HBx (Abcam), and monoclonal anti-human β-actin (Sigma). All experiments were repeated three times.
Plasmid Construction
The promoter region of HULC was amplified by PCR using specific primers according to previous research (forward primer, 5′-CGG GGT ACC CTT TGT CCC TTG GTT AGG-3′, and reverse primer, 5′-CCG CTC GAG TAA AGG CTC CAA TTC CAT-3′) (23). Mutant construct of HULC promoter was carried out using specific primers that deleted the CREB binding site (forward primer, 5′-CGG GGT ACC CTT TGT CCC TTG GTT AGG-3′, and reverse primer, 5′-CCG CTC GAG CCA AGC CCA GTT ACCT-3′). The 5′-flanking region of p18 (from −1003 to +349 bp) was amplified from the genomic DNA of HepG2 using specific primers (forward primer, 5′-CCG CTC GAG TGG GAT AGG GAA GGT GTA-3′, and reverse primer, 5′-CCC AAG CTT CAT AAT GGG ATG ATG ACA AT-3′). The target fragments were inserted upstream of the firefly luciferase gene in the pGL3-Basic vector (Promega, Madison, WI), making transcription of the firefly luciferase gene under the control of each fragment. The resulting vectors were sequenced and named pGL3-HULC-Pro-WT, pGL3-HULC-Pro-MUT, and pGL3-p18-Pro.
The full-length HULC was amplified from cDNA of HepG2 cells using primers (forward primer, 5′-CTA GCT AGC ATG GGG GTG GAA CTC ATG ATGG-3′, and reverse primer, 5′-CCC AAG CTT AAG AAT GGA CAT CAT TTT ATT TCA-3′) and then cloned into pcDNA3.1 vector. The resulting vector was sequenced and named pcDNA3.1-HULC. Plasmids constructed previously were used, such as pSilencer-HBx (pSi-HBx, producing small interfering RNAs targeting HBx), pSilencer-control (pSi-Ctrl, negative control), pCMV-HBx, and pCMV (negative control) (24).
Small Interfering RNAs and Transfection
Small interfering RNAs (siRNAs) targeting human HULC mRNA (si-HULC) (target sequence: 5′-AAC CTC CAG AAC TGT GAT CCA-3′ (22)); targeting human p18 mRNA (si-p18) (target sequence: 5′-TAC AGT GCT CAG GGC GAG CGA-3′ (27)); and targeting human CREB mRNA (si-CREB) (target sequence: 5′-GAG AGA GGT CCG TCT AATG-3′ (28)) and negative control siRNA (si-Ctrl) were synthesized by RiboBio (Guangzhou, China). Transfections were performed in 6-, 24-, or 96-well plates after seeded cells were cultured for 24 h. siRNA reagents or/and different doses of plasmids were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Luciferase Reporter Gene Assay
Luciferase activity was determined using a luciferase reporter assay system (Promega) on a luminometer (TD-20/20; Turner Designs, Sunnyvale, CA) according to the manufacturer's instructions. Cells were seeded at a density of 3 × 104 per well into 24-well plates. After 12 h, 200 ng of plasmid encoding firefly luciferase under the control of HULC or p18 promoter was co-transfected with 100 ng of pRL-TK encoding Renilla luciferase. Regulating factors, such as 200 ng of pSilencer-HBx (or pCMV-HBx) or siRNAs (100 nm si-HULC or si-Ctrl), were co-transfected with luciferase plasmids. The pSilencer-control vector, pCMV vector, negative control siRNA (si-Ctrl), and pGL3-Basic plasmid were used as controls. Cells were lysed with passive lysis buffer 48 h after transfection, and firefly as well as Renilla luciferase expression was assessed. The luciferase readings of each sample were normalized against the Renilla luciferase levels (29).
Chromatin Immunoprecipitation (ChIP) Assay
The ChIP assay was carried out using the EpiQuikTM chromatin immunoprecipitation kit from Epigentek Group Inc. (Brooklyn, NY). Briefly, HepG2 cells transfected with si-CREB or si-Ctrl were fixed with 1% formaldehyde. Protein-DNA complexes were immunoprecipitated with HBx antibody or CREB antibody. Normal mouse IgG was used as a negative control antibody. DNA from these samples was subjected to PCR analyses with HULC promoter-specific primers (forward primer, 5′-GAA ACC CTA ATC TCC AGT GTG AT-3′, and reverse primer, 5′-GGT CTG GTT CTC GTG ACG ACT CTTC-3′). Amplification of soluble chromatin prior to immunoprecipitation was used as an input control.
Cell Proliferation Assay in Vitro
Cell proliferation was determined by methyl thiazolyl tetrazolium (MTT) assay and 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay. MTT assay was performed by using MTT reagent (Sigma) as described previously (26). EdU incorporation assay was carried out using the Cell-LightTM EdU imaging detection kit (RiboBio) according to the manufacturer's instructions.
Colony Formation Assay
HepG2-X cells were transfected with 100 nm HULC siRNA or control siRNA, respectively. 48 h after transfection, 1000 cells were placed into each well of 6-well plates and kept in complete medium for 2 weeks. Colonies were fixed with methanol and stained with methylene blue.
In Vivo Tumorigenicity Assay
Cells were trypsinized 48 h after transfection, washed twice, and resuspended with sterile phosphate-buffered saline. 200 μl of the cell suspensions (4 × 106 cells) was injected subcutaneously into the right flank of female BALB/c athymic nude mice (4–6 weeks of age), six mice per group. On the 5th day after injection, tumors began to develop, and their volumes were measured routinely using a caliper. Tumor volume was calculated with the formula 0.5× (L × W2) (where L indicates length, and W indicates width). 30 days after injection, all mice were killed. Tumors weight and the expression of HULC RNA were measured. The mouse experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication Number 80-23) and were conducted according to the institutional ethical guidelines for animal experiment.
Immunohistochemistry (IHC)
Immunohistochemical staining of samples was performed as reported previously (24). The primary antibodies used were rabbit anti-Ki67 (Thermo Fisher Scientific, Ely, UK) and rabbit anti-BrdU (Genomapping Technology, Tianjin, China).
In Vivo BrdU Labeling
Methods for in vivo BrdU labeling were described previously (30). Briefly, 24 and 4 h before sacrificing, athymic mice bearing tumors were injected with 350 μl of bromodeoxyuridine (BrdU; 10 mg/ml, Sigma) or PBS as control twice. The tumor tissues were fixed, and BrdU incorporation was detected by IHC using the primary antibody of rabbit anti-BrdU (Genomapping Technology, Tianjin, China).
Statistical Analysis
Statistical significance was assessed by comparing mean values (± S.D.) using the two-tailed Student's t test for independent groups. The probability value p < 0.05 was considered to be statistically significant. The expression levels of HULC, p18, and HBx in clinical HCC tissues and their corresponding adjacent nontumorous tissues were compared using a Wilcoxon signed-rank test. Correlation between the expression levels of HBx and HULC (or HULC and p18) in the tissue specimens was explored by Pearson's correlation coefficient.
RESULTS
HBx Up-regulates HULC in Liver Cells and Hepatoma Cells
It has been reported that lncRNA HULC is up-regulated in HCC (22, 23). To confirm the elevation of HULC, we examined the expression levels of HULC in 21 pairs of clinical HCC specimens and their adjacent nontumorous liver tissues. We found that the expression levels of HULC were remarkably increased in HCC tissues relative to their adjacent nontumorous liver specimens (Fig. 1A and supplemental Fig. 1). To further investigate the relationship between HBx and HULC, we examined the mRNA levels of HBx and HULC in the 33 clinical HCC specimens (including the above 21 clinical HCC tissues) by quantitative real-time PCR. We found that the relative mRNA levels of HBx and HULC were positively correlated in the HCC tissues (Fig. 1B). Then, we measured the mRNA levels of HBx in 21 pairs of clinical HCC specimens and their adjacent nontumorous liver tissues by real-time PCR. The results showed that HBx mRNA could be detected in all of the 21 HCC tissues and 19 out of 21 nontumorous liver specimens. We found that the mRNA levels of HBx in nontumorous liver specimens were lower relative to HCC tissues (supplemental Fig. 2), which was consistent with our previous study (28). We also revealed that the mRNA levels of HBx were positively correlated with those of HULC in the 19 HBx-positive clinical nontumorous liver tissues (Fig. 1C). Therefore, we speculated that HBx might up-regulate HULC in the cells.
FIGURE 1.
HBx up-regulates HULC in liver cells and hepatoma cells. A, real-time PCR analysis of HULC expression in 21 paired HCC tissues and their corresponding nontumorous tissues. Data were analyzed using 2−ΔΔCt. Statistical significance of HULC expression difference between HCC and normal liver was determined by Wilcoxon signed-rank test (***, p < 0.001, versus nontumorous tissues). B, correlation between the relative mRNA levels of HBx and HULC in 33 HCC tissue specimens. Statistical analysis was performed using Pearson's correlation coefficient (r = 0.8173, p < 0.0001, Pearson's correlation). C, correlation between HBx and HULC expression in the 19 HBx-positive nontumorous liver tissues. Statistical analysis was performed using Pearson's correlation coefficient (r = 0.7925, p < 0.0001, Pearson's correlation). The data presented were from three independent experiments. D, the relative expression levels of HULC were quantified by real-time PCR in L-O2 and HepG2 cells, as well as their corresponding HBx stably transfected cells (L-O2-X and HepG2-X), respectively. E, the relative mRNA levels of HULC were measured by real-time PCR in L-O2 cells transfected with pCMV-HBx plasmid (upper portion). Western blot analysis was performed to determine the efficiency of overexpressing HBx (lower portion). F, the relative expression levels of HULC were detected by real-time PCR in HepG2-X transfected with pSi-HBx plasmid (upper portion). Western blot analysis was performed to measure the efficiency of silencing HBx (lower portion). Data are reported as mean ± S.D. for three independent experiments (*, p < 0.05, **, p < 0.01, Student's t test).
Our data revealed that the expression levels of HULC were increased in L-O2-X/HepG2-X cells relative to L-O2/HepG2 cells (Fig. 1D). Real-time PCR showed that overexpressing HBx led to a significant increase of HULC in L-O2 cells in a dose-dependent manner (Fig. 1E, upper portion). Moreover, silencing HBx in HepG2-X cells resulted in the down-regulation of HULC dose-dependently (Fig. 1F, upper portion). In parallel, the efficiencies of overexpressing or silencing HBx were measured by Western blot assays, respectively (Fig. 1E and F, lower portions). Thus, we conclude that HBx is able to up-regulate HULC in liver cells and hepatoma cells.
HBx Activates the HULC Promoter via CREB
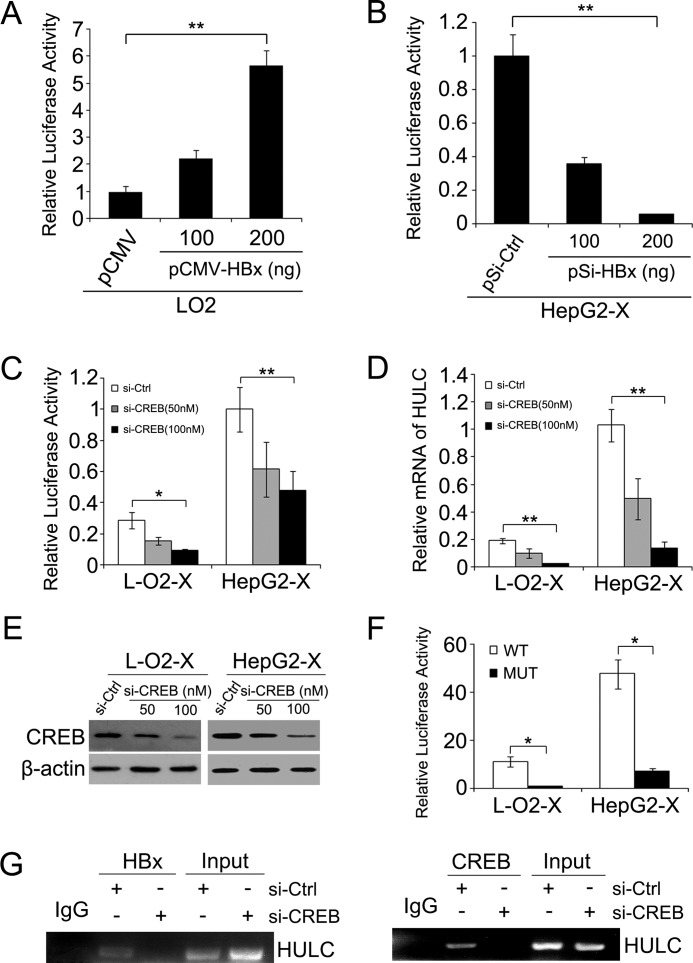
To investigate the mechanism of up-regulation of HULC mediated by HBx, we cloned the promoter region of HULC according to the previous research (23). Luciferase reporter gene assays showed that the overexpression of HBx could increase the HULC promoter activity in L-O2 cells (Fig. 2A), whereas the knockdown of HBx led to an obvious decrease of HULC promoter activity in HepG2-X cells dose-dependently (Fig. 2B), suggesting that the HBx is able to activate the promoter of HULC. It has been reported that the transcription factor CREB contributes to the activation of HULC promoter containing the CREB binding site in hepatoma cells (23). HBx regulates transcription of CREB-dependent promoters through interacting with CREB (31, 32). Therefore, we supposed that CREB might be involved in the regulation of HULC mediated by HBx. Luciferase reporter gene assays showed that silencing CREB markedly decreased the promoter activities of HULC in L-O2-X/HepG2-X cells in a dose-dependent manner (Fig. 2C). The relative mRNA levels of HULC and the protein levels of CREB in the cells were decreased by CREB siRNA (Fig. 2, D and E). Meanwhile, HBx failed to work when the CREB binding site was deleted in the HULC promoter (Fig. 2F). To further determine whether HBx could interact with CREB to bind to the HULC promoter, we performed ChIP assays with HBx or CREB antibody. As shown in Fig. 2G, both anti-HBx and anti-CREB antibodies specifically enriched the HULC promoter region containing the CREB binding site in HepG2-X cells treated with control siRNA, whereas they failed to enrich the HULC promoter in the cells treated with CREB siRNA. Thus, we conclude that HBx is able to activate the HULC promoter through interacting with CREB.
FIGURE 2.
HBx activates the HULC promoter via CREB. A, the promoter activities of HULC were examined by luciferase reporter gene assay in L-O2 cells overexpressing HBx. B, the promoter activities of HULC were measured by luciferase reporter gene assay in HepG2-X cells silencing HBx. Renilla luciferase vector was used as an internal control. Normalized luciferase activity in negative control (pCMV or pSi-Ctrl) transfected cells was set to 1. C, luciferase activity of HULC promoter was determined by luciferase reporter gene assay after silencing CREB. D, the relative mRNA levels of HULC were determined by real-time PCR in L-O2-X/HepG2-X transfected with si-CREB. E, Western blot analysis was performed to determine the expression level of CREB. F, the effect of HBx on the promoter activity of HULC was detected by luciferase reporter gene assays when the CREB binding site in the promoter region of HULC was deleted. MUT, mutant. G, the interaction between HBx and the promoter region of HULC was examined by ChIP analysis. Data are reported as mean ± S.D. for three independent experiments (*, p < 0.05, **, p < 0.01, Student's t test).
HULC Promotes Proliferation of Hepatoma Cells in Vitro and in Vivo
To investigate the function of HULC in hepatocarcinogenesis, we examined cell proliferation by MTT assay, EdU incorporation assay, and colony formation analysis. First, we selected HBx stably transfected cell lines (L-O2-X and HepG2-X), with a relatively high level of HULC, to estimate the effect of decreased HULC on cell proliferation. We found that silencing HULC resulted in a significant decrease of cell proliferation of L-O2-X/HepG2-X cell lines in a time-dependent manner by MTT assay (Fig. 3, A and B). Additionally, the overexpression of HULC was able to enhance the proliferation of L-O2 cells with a relatively low level of HULC (Fig. 3C). Then, we used human hepatoma cells Hep3B and PLC/PRF/5, naturally producing HBx, to analyze the effect of HULC on cell proliferation. MTT assay revealed that the knockdown of HULC inhibited the proliferation of Hep3B and PLC/PRF/5 cell lines (Fig. 3D), which was consistent with the data that the knockdown of HULC inhibited the proliferation of HBx stably transfected cells L-O2-X and HepG2-X. Moreover, EdU incorporation assay also showed that HULC promoted proliferation of HepG2-X cells (Fig. 3E). The colony formation assay indicated that silencing HULC resulted in a more than 50% decrease in colony numbers in HepG2-X cells relative to control (Fig. 3F). These results suggest that HULC enhances the proliferation of hepatoma cells in vitro.
FIGURE 3.
HULC promotes proliferation of hepatoma cells in vitro. After silencing or overexpressing HULC, cell proliferation was determined by (A–D) MTT assay, (E) EdU incorporation assay, and (F) colony formation assay. Data are reported as mean ± S.D. for three independent experiments. Statistically significant differences are indicated: *, p < 0.05, **, p < 0.01, ***, p < 0.001, Student's t test. OD Value, optical density value.
We further observed the proliferation enhancement effect of HULC in vivo. We found that silencing HULC reduced the tumor volume, average weight of tumors, and frequency of tumor formation (si-Ctrl versus si-HULC transfectants, 6/6 versus 5/6) (Fig. 4, A–C). Real-time PCR confirmed that the expression levels of HULC were down-regulated in the tumor tissues of the si-HULC group relative to the si-Ctrl group (Fig. 4D). Then, we tested the expression of Ki-67, a cell proliferation marker, by IHC. Our results showed that the expression levels of Ki-67 in si-HULC xenografts were significantly decreased when compared with those in si-Ctrl xenografts (Fig. 4E). Therefore, we conclude that HULC is able to promote the proliferation of hepatoma cells in vitro and in vivo.
FIGURE 4.
HULC promotes proliferation of hepatoma cells in vivo. A, the growth curves of tumors derived from HepG2-X cells transfected with 100 nm si-HULC or si-Ctrl (**, p < 0.01, versus control, Student's t test). B, the average weight of tumors (**, p < 0.01, versus control, Student's t test). C, the images of tumors from nude mice. D, the relative mRNA levels of HULC in the tumor tissues from mice were detected by real-time PCR. Statistically significant differences are indicated: ***, p < 0.001, Student's t test. Data are reported as mean ± S.D. for three independent experiments. E, the expression levels of Ki-67 were measured by immunohistochemistry assay (IHC) in the tumor tissues from mice. Results are representative of three independent experiments.
HULC Down-regulates p18 in Hepatoma Cells
As a regulator, HULC itself cannot directly promote cell proliferation. Therefore, we tried to find its possible target genes. It has been reported that lncRNAs overlapping or antisense to protein-coding gene promoters may contribute to regulating the expression of their neighboring protein-coding genes through epigenetic modifications (33). We retrieved information from the National Center for Biotechnology Information (NCBI) Gene database (www.ncbi.nlm.nih.gov/gene) and found a tumor suppressor gene p18 located near HULC (Fig. 5A). p18 is known as eukaryotic translation elongation factor 1, ϵ-1 (EEF1E1) or AIMP3. It was first identified as a factor associated with a multi-tRNA synthetase complex (34). Park et al. (27) reported that p18 could act as a tumor suppressor by translocating to the nucleus to activate p53 through the interaction with ataxia telangiectasia-mutated (ATM) in response to DNA damage, and the expression level of p18 was decreased in HCC. In our study, we confirmed that the expression levels of p18 were decreased in 21 paired HCC tissues when compared with their corresponding nontumorous liver tissues by real-time PCR (Fig. 5B). Then, we further investigated the correlation between HULC and p18 in 33 clinical HCC tissues and found that the expression levels of HULC and p18 were inversely correlated (Fig. 5C). Interestingly, we found that silencing HULC by si-HULC resulted in the up-regulation of p18 in HepG2 cells at the mRNA and protein levels in a dose-dependent manner (Fig. 5D). Then, we cloned the 5′-flanking region of p18 (from −1003 to +349 bp) into pGL3-Basic vector (termed pGL3-p18-Pro). Luciferase reporter gene assays showed that the luciferase activities of pGL3-p18-Pro were approximately 15 times stronger than those of the promoter-less plasmid pGL3-Basic (data not shown), suggesting that the fragment of −1003 to ∼+349 bp may be the promoter region of p18. Then, we observed that the overexpression of HULC could decrease the promoter activity of p18 in L-O2 cells, whereas the knockdown of HULC led to an obvious increase of p18 promoter activity in HepG2 cells dose-dependently (Fig. 5E). The efficiencies of overexpressing or silencing HULC were confirmed by real-time PCR (supplemental Fig. 3, A and B). Moreover, we detected p18 protein levels in tumor tissues of mice, which were obtained from tumorigenicity assay shown in Fig. 4C. Western blot analysis revealed that p18 protein expression levels were higher in the tumor tissues derived from the si-HULC group relative to the si-Ctrl group (Fig. 5F). Thus, we conclude that HULC is able to down-regulate p18 in hepatoma cells.
FIGURE 5.
HULC down-regulates p18 in hepatoma cells. A, a diagram of genes located around HULC. B, relative mRNA levels of p18 in 21 paired HCC tissues and their corresponding nontumorous tissues were detected by real-time PCR analysis. C, correlation between the relative mRNA levels of HULC and p18 in 33 HCC tissue specimens (r = −0.5229, p = 0.0018, Pearson's correlation). D, the mRNA and protein levels of p18 were assessed by RT-PCR and Western blot in HepG2 cells transfected with si-HULC, respectively. E, the promoter activity of p18 was determined by luciferase reporter gene assay in L-O2 cells overexpressing HULC (left). The promoter activity of p18 was measured by luciferase reporter gene assay in HepG2 cells silencing HULC (right). Normalized luciferase activity in negative control (pcDNA3.1 or si-Ctrl) transfected cells was set to 1. F, the protein levels of p18 in tumors from mice shown in Fig. 4C were examined by Western blot analysis. Data are reported as mean ± S.D. for three independent experiments (**, p < 0.01, ***, p < 0.001, Student's t test).
HULC Promotes Proliferation of Hepatoma Cells through Down-regulating p18 in Vitro and in Vivo
It has been reported that p18 is capable of inhibiting cell growth in a transgenic mouse model (27). In this study, we observed that silencing p18 increased cell viability in L-O2 cells by MTT assay (Fig. 6A, upper portion), and the protein levels of p18 were confirmed by Western blot analysis (Fig. 6A, lower portion). Next, we detected whether p18 was required for the HULC-enhanced cell proliferation of HepG2 cells. MTT assay showed that the decreased cell proliferation ability by si-HULC was partially rescued by si-p18 in vitro (Fig. 6B). We further detected the effect of p18 on the HULC-promoted HepG2 cell proliferation in vivo. Tumorigenicity assay showed that the proliferation inhibitory function of si-HULC was partially attenuated by si-p18 (Fig. 6, C–E). Moreover, immunohistochemical staining in the tumor tissues showed that the decrease of Ki-67 expression levels by silencing HULC was partially rescued by the knockdown of p18 (Fig. 6F, upper portion). Moreover, IHC assays showed that the number of HepG2 cells incorporated BrdU was decreased by silencing HULC and that the reduction of BrdU incorporation was partially attenuated by the knockdown of p18 (Fig. 6F, lower portion). Thus, we conclude that HULC promotes the proliferation of hepatoma cells through down-regulating p18 in vitro and in vivo.
FIGURE 6.
HULC promotes proliferation of hepatoma cells through down-regulating p18 in vitro and in vivo. A, the proliferation of L-O2 cells treated with si-p18 was measured by MTT assay, and the protein levels of p18 were confirmed by Western blot analysis. OD Value, optical density value. B, cell proliferation of HepG2 cells co-transfected with si-HULC and si-p18 was determined by MTT assay. C, the average weight of tumors derived from HepG2 cells transfected with 100 nm si-HULC or/and si-p18. D, the growth curves of tumors. E, the image of tumors from nude mice. F, immunohistochemical staining for Ki-67 and BrdU in the tumor tissues from mice. Data are reported as mean ± S.D. for three independent experiments (**, p < 0.01, ***, p < 0.001, Student's t test).
Decrease of HULC Abolishes HBx-enhanced Cell Proliferation through Up-regulating p18
Western blot revealed that p18 was down-regulated in L-O2-X (or HepG2-X) cells relative to L-O2 (or HepG2) cells and that the treatment with pSilencer-HBx (pSi-HBx) resulted in the increase of p18 in L-O2-X (or HepG2-X) cells (Fig. 7A), suggesting that HBx is able to down-regulate p18 in liver cells and hepatoma cells. Interestingly, the knockdown of HULC was able to rescue the p18 expression levels, which were decreased by HBx in L-O2-X (or HepG2-X) cells (Fig. 7A), suggesting that HBx down-regulates p18 through HULC. Moreover, we found that the knockdown of HULC partially blocked the HBx-enhanced cell proliferation in HepG2-X cells (Fig. 7B), whereas it rescued the HBx-decreased p18 expression level (Fig. 7A, right), suggesting that the capability of cell proliferation is inversely associated with the expression level of p18. Thus, we conclude that elevated HULC by HBx promotes the proliferation of hepatoma cells through down-regulation of p18.
FIGURE 7.
Decrease of HULC abolishes HBx-enhanced cell proliferation through up-regulating p18. A, the protein levels of p18 were determined by Western blot in L-O2/HepG2, L-O2-X/HepG2-X, and L-O2-X/HepG2-X treated by pSi-HBx or si-HULC, respectively. B, cell proliferation was detected by MTT in HepG2, HepG2-X, and HepG2-X treated by pSi-HBx or si-HULC. Data are reported as mean ± S.D. for three independent experiments (**, p < 0.05, ***, p < 0.01, Student's t test). OD Value, optical density value.
DISCUSSION
Chronic HBV infection is a major cause of HCC, and the multifunctional oncoprotein HBx plays a crucial role in the development of HCC (35). Although regulatory lncRNAs were previously disregarded as transcriptional “noise,” growing evidence suggests that they contribute to the development of liver cancer (36). Previous studies showed that lncRNA high expression in HCC (HEIH) and H19 were deregulated in HCC and facilitated cell growth in the development of HCC (37, 38). HULC was identified as an lncRNA, which was up-regulated dramatically in liver cancer (22, 23). However, whether HULC is involved in the hepatocarcinogenesis mediated by HBx is poorly understood. In this study, we focused on investigating the role of HULC in HBx-associated HCC.
According to the report that HULC is up-regulated in HCC tissues (22), we are interested in the correlation between HBx and HULC in HBV-associated HCC. Interestingly, we found that the mRNA levels of HBx were positively correlated with those of HULC in 33 clinical HCC tissues. Then, we observed that HBx was able to up-regulate HULC in liver cells and hepatoma cells. Thus, we conclude that HBx up-regulates HULC in the cells. Next, we tried to identify the underlying mechanism for how HBx up-regulated HULC. Given that CREB up-regulates the expression of HULC (23) and that HBx interacts with CREB to regulate the transcription of CREB-dependent promoters (39), therefore, it is reasonable to hypothesize that HBx may regulate HULC through interacting with CREB. Our data showed that HBx was able to activate the HULC promoter via interacting with CREB.
Up to now, the function of HULC in HCC has not been elucidated. It has been reported that prostate cancer-associated ncRNA PCGEM1 promotes cell proliferation and colony formation, as well as regulates apoptosis of prostate cancer cells (40, 41). Yang et al. (37) found that lncRNA HEIH promoted tumor growth through enhancer of zeste homolog 2. lncRNA HOX transcript antisense RNA (HOTAIR) was reported to be associated with poor prognosis in colorectal cancers (13) and had been found to reprogram chromatin state to promote cancer metastasis (42). This suggests that lncRNAs may be involved in multiple processes, such as cell proliferation, apoptosis, and cancer metastasis. In this study, we report that HULC is able to enhance the proliferation of hepatoma cells in vitro and in vivo. Intriguingly, IHC assays showed that the expression levels of Ki-67 were decreased in the tumor tissues treated with si-HULC, further confirming that HULC contributes to cell proliferation in vivo.
The mechanisms of how lncRNAs contribute to diseases are complicated and diversified. lncRNAs have a variety of ways to regulate their target genes, such as epigenetic regulation of protein-coding gene expression and direct modulation of gene transcription. We are interested in the potential targets of HULC and the mechanism of how HULC promotes proliferation of hepatoma cells. An lncRNA HOTAIR transcribed from the HOXC locus regulated the chromatin methylation state of the HOXD locus through directly binding to the polycomb repressive complex PRC2 (43, 44). Feng et al. (45) reported that lncRNA Evf2 activated the transcription factor Dlx2 and recruited it to an ultraconserved genomic element to induce transcription of Dlx5, which played as a Dlx2 transcriptional coactivator. lncRNAs located near protein-coding genes may contribute to regulating the expression of their neighboring protein-coding genes. Yu et al. (46) found that an lncRNA antisense to the p15 tumor suppressor gene regulated the chromatin and DNA methylation status of the p15 locus. An lncRNA antisense to p21 was also shown to behave similarly (47). Accordingly, being interested in the neighboring genes located around HULC, We retrieved information from the NCBI Gene database (www.ncbi.nlm.nih.gov/gene) and found a tumor suppressor gene p18 (also termed EEF1E1) located near HULC. p18 has been reported to regulate cell cycle and to work in the signal pathways including ATM/ATR (ATM and Rad3-related) and p53 (27). We speculated that HULC might regulate cell proliferation via p18. Interestingly, we found that the mRNA levels of p18 were inversely correlated with those of HULC in 33 clinical HCC tissues. Moreover, we found that HULC could down-regulate p18 at the mRNA and protein levels and activate the promoter activity of p18. Moreover, we observed that p18 was involved in the HULC-promoted proliferation of hepatoma cells in vitro and in vivo. Therefore, HULC is able to promote proliferation of hepatoma cells through suppressing its target gene p18.
Taken together, we report the biological function of HULC in hepatocarcinogenesis mediated by HBx. Our finding suggests that the up-regulation of HULC by HBx promotes the proliferation of hepatoma cells through down-regulating the tumor suppressor gene p18. Our data provide new insights into the roles of lncRNAs in HBx-associated hepatocarcinogenesis.
Supplementary Material
This work was supported in part by National Basic Research Program of China (973 Program, Grants 2009CB521702 and 2011CB512113) and the Natural Scientific Foundation of China (Grants 81071624 and 81071623).

This article contains supplemental Table 1 and Figs. 1–3.
- HCC
- hepatocellular carcinoma
- HBV
- hepatitis B virus
- HBx
- hepatitis B virus X protein
- HULC
- highly up-regulated in liver cancer
- lncRNA
- long noncoding RNA
- ncRNA
- noncoding RNA
- CREB
- cAMP-responsive element-binding protein
- MTT
- methyl thiazolyl tetrazolium
- EdU
- 5-ethynyl-2′-deoxyuridine
- IHC
- immunohistochemistry
- Ctrl
- control
- ATM
- ataxia telangiectasia-mutated.
REFERENCES
- 1. El-Serag H. B., Rudolph K. L. (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 [DOI] [PubMed] [Google Scholar]
- 2. Liu Q., Chen J., Liu L., Zhang J., Wang D., Ma L., He Y., Liu Y., Liu Z., Wu J. (2011) The X protein of hepatitis B virus inhibits apoptosis in hepatoma cells through enhancing the methionine adenosyltransferase 2A gene expression and reducing S-adenosylmethionine production. J. Biol. Chem. 286, 17168–17180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim C. M., Koike K., Saito I., Miyamura T., Jay G. (1991) HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature 351, 317–320 [DOI] [PubMed] [Google Scholar]
- 4. Lee S., Tarn C., Wang W. H., Chen S., Hullinger R. L., Andrisani O. M. (2002) Hepatitis B virus X protein differentially regulates cell cycle progression in X-transforming versus nontransforming hepatocyte (AML12) cell lines. J. Biol. Chem. 277, 8730–8740 [DOI] [PubMed] [Google Scholar]
- 5. Wang W. H., Hullinger R. L., Andrisani O. M. (2008) Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis. J. Biol. Chem. 283, 25455–25467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin-Lluesma S., Schaeffer C., Robert E. I., van Breugel P. C., Leupin O., Hantz O., Strubin M. (2008) Hepatitis B virus X protein affects S phase progression leading to chromosome segregation defects by binding to damaged DNA-binding protein 1. Hepatology 48, 1467–1476 [DOI] [PubMed] [Google Scholar]
- 7. Kew M. C. (1997) Increasing evidence that hepatitis B virus X gene protein and p53 protein may interact in the pathogenesis of hepatocellular carcinoma. Hepatology 25, 1037–1038 [DOI] [PubMed] [Google Scholar]
- 8. Xu J., Yun X., Jiang J., Wei Y., Wu Y., Zhang W., Liu Y., Wang W., Wen Y., Gu J. (2010) Hepatitis B virus X protein blunts senescence-like growth arrest of human hepatocellular carcinoma by reducing Notch1 cleavage. Hepatology 52, 142–154 [DOI] [PubMed] [Google Scholar]
- 9. Yoo Y. G., Lee M. O. (2004) Hepatitis B virus X protein induces expression of Fas ligand gene through enhancing transcriptional activity of early growth response factor. J. Biol. Chem. 279, 36242–36249 [DOI] [PubMed] [Google Scholar]
- 10. Malone C. D., Hannon G. J. (2009) Small RNAs as guardians of the genome. Cell 136, 656–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carthew R. W., Sontheimer E. J. (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moazed D. (2009) Small RNAs in transcriptional gene silencing and genome defense. Nature 457, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kogo R., Shimamura T., Mimori K., Kawahara K., Imoto S., Sudo T., Tanaka F., Shibata K., Suzuki A., Komune S., Miyano S., Mori M. (2011) Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 71, 6320–6326 [DOI] [PubMed] [Google Scholar]
- 14. Bertone P., Stolc V., Royce T. E., Rozowsky J. S., Urban A. E., Zhu X., Rinn J. L., Tongprasit W., Samanta M., Weissman S., Gerstein M., Snyder M. (2004) Global identification of human transcribed sequences with genome tiling arrays. Science 306, 2242–2246 [DOI] [PubMed] [Google Scholar]
- 15. Kapranov P., Drenkow J., Cheng J., Long J., Helt G., Dike S., Gingeras T. R. (2005) Examples of the complex architecture of the human transcriptome revealed by RACE and high-density tiling arrays. Genome Res. 15, 987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinn J. L., Euskirchen G., Bertone P., Martone R., Luscombe N. M., Hartman S., Harrison P. M., Nelson F. K., Miller P., Gerstein M., Weissman S., Snyder M. (2003) The transcriptional activity of human chromosome 22. Genes Dev. 17, 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taft R. J., Pang K. C., Mercer T. R., Dinger M., Mattick J. S. (2010) Noncoding RNAs: regulators of disease. J. Pathol 220, 126–139 [DOI] [PubMed] [Google Scholar]
- 18. Gibb E. A., Brown C. J., Lam W. L. (2011) The functional role of long noncoding RNA in human carcinomas. Mol. Cancer 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Askarian-Amiri M. E., Crawford J., French J. D., Smart C. E., Smith M. A., Clark M. B., Ru K., Mercer T. R., Thompson E. R., Lakhani S. R., Vargas A. C., Campbell I. G., Brown M. A., Dinger M. E., Mattick J. S. (2011) SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 17, 878–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maruyama R., Shipitsin M., Choudhury S., Wu Z., Protopopov A., Yao J., Lo P. K., Bessarabova M., Ishkin A., Nikolsky Y., Liu X. S., Sukumar S., Polyak K. (2012) Altered antisense-to-sense transcript ratios in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 109, 2820–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin R., Maeda S., Liu C., Karin M., Edgington T. S. (2007) A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene 26, 851–858 [DOI] [PubMed] [Google Scholar]
- 22. Panzitt K., Tschernatsch M. M., Guelly C., Moustafa T., Stradner M., Strohmaier H. M., Buck C. R., Denk H., Schroeder R., Trauner M., Zatloukal K. (2007) Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology 132, 330–342 [DOI] [PubMed] [Google Scholar]
- 23. Wang J., Liu X., Wu H., Ni P., Gu Z., Qiao Y., Chen N., Sun F., Fan Q. (2010) CREB up-regulates long noncoding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 38, 5366–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang X., Dong N., Yin L., Cai N., Ma H., You J., Zhang H., Wang H., He R., Ye L. (2005) Hepatitis B virus X protein up-regulates survivin expression in hepatoma tissues. J. Med. Virol. 77, 374–381 [DOI] [PubMed] [Google Scholar]
- 25. Wang Q., Zhang W., Liu Q., Zhang X., Lv N., Ye L. (2010) A mutant of hepatitis B virus X protein (HBxΔ127) promotes cell growth through a positive feedback loop involving 5-lipoxygenase and fatty acid synthase. Neoplasia 12, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shan C., Xu F., Zhang S., You J., You X., Qiu L., Zheng J., Ye L., Zhang X. (2010) Hepatitis B virus X protein promotes liver cell proliferation via a positive cascade loop involving arachidonic acid metabolism and p-ERK1/2. Cell Res. 20, 563–575 [DOI] [PubMed] [Google Scholar]
- 27. Park B. J., Kang J. W., Lee S. W., Choi S. J., Shin Y. K., Ahn Y. H., Choi Y. H., Choi D., Lee K. S., Kim S. (2005) The haploinsufficient tumor suppressor p18 up-regulates p53 via interactions with ATM/ATR. Cell 120, 209–221 [DOI] [PubMed] [Google Scholar]
- 28. Shan C., Zhang S., Cui W., You X., Kong G., Du Y., Qiu L., Ye L., Zhang X. (2011) Hepatitis B virus X protein activates CD59 involving DNA binding and let-7i in protection of hepatoma and hepatic cells from complement attack. Carcinogenesis 32, 1190–1197 [DOI] [PubMed] [Google Scholar]
- 29. Hu N., Zhang J., Cui W., Kong G., Zhang S., Yue L., Bai X., Zhang Z., Zhang W., Zhang X., Ye L. (2011) miR-520b regulates migration of breast cancer cells by targeting hepatitis B X-interacting protein and interleukin-8. J. Biol. Chem. 286, 13714–13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwak Y. T., Radaideh S. M., Ding L., Li R., Frenkel E., Story M. D., Girard L., Minna J., Verma U. N. (2011) Cells lacking IKKα show nuclear cyclin D1 overexpression and a neoplastic phenotype: role of IKKα as a tumor suppressor. Mol. Cancer Res. 9, 341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maguire H. F., Hoeffler J. P., Siddiqui A. (1991) HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science 252, 842–844 [DOI] [PubMed] [Google Scholar]
- 32. Bouchard M. J., Schneider R. J. (2004) The enigmatic X gene of hepatitis B virus. J. Virol. 78, 12725–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martens J. A., Laprade L., Winston F. (2004) Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429, 571–574 [DOI] [PubMed] [Google Scholar]
- 34. Ko Y. G., Park H., Kim S. (2002) Novel regulatory interactions and activities of mammalian tRNA synthetases. Proteomics 2, 1304–1310 [DOI] [PubMed] [Google Scholar]
- 35. Zhang X., Zhang H., Ye L. (2006) Effects of hepatitis B virus X protein on the development of liver cancer. J. Lab. Clin. Med. 147, 58–66 [DOI] [PubMed] [Google Scholar]
- 36. Struhl K. (2007) Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat. Struct. Mol. Biol. 14, 103–105 [DOI] [PubMed] [Google Scholar]
- 37. Yang F., Zhang L., Huo X. S., Yuan J. H., Xu D., Yuan S. X., Zhu N., Zhou W. P., Yang G. S., Wang Y. Z., Shang J. L., Gao C. F., Zhang F. R., Wang F., Sun S. H. (2011) Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 54, 1679–1689 [DOI] [PubMed] [Google Scholar]
- 38. Matouk I. J., DeGroot N., Mezan S., Ayesh S., Abu-lail R., Hochberg A., Galun E. (2007) The H19 noncoding RNA is essential for human tumor growth. PLoS One 2, e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Waris G., Siddiqui A. (2003) Regulatory mechanisms of viral hepatitis B and C. J. Biosci. 28, 311–321 [DOI] [PubMed] [Google Scholar]
- 40. Petrovics G., Zhang W., Makarem M., Street J. P., Connelly R., Sun L., Sesterhenn I. A., Srikantan V., Moul J. W., Srivastava S. (2004) Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene 23, 605–611 [DOI] [PubMed] [Google Scholar]
- 41. Fu X., Ravindranath L., Tran N., Petrovics G., Srivastava S. (2006) Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell Biol. 25, 135–141 [DOI] [PubMed] [Google Scholar]
- 42. Gupta R. A., Shah N., Wang K. C., Kim J., Horlings H. M., Wong D. J., Tsai M. C., Hung T., Argani P., Rinn J. L., Wang Y., Brzoska P., Kong B., Li R., West R. B., van de Vijver M. J., Sukumar S., Chang H. Y. (2010) Long noncoding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rinn J. L., Kertesz M., Wang J. K., Squazzo S. L., Xu X., Brugmann S. A., Goodnough L. H., Helms J. A., Farnham P. J., Segal E., Chang H. Y. (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsai M. C., Manor O., Wan Y., Mosammaparast N., Wang J. K., Lan F., Shi Y., Segal E., Chang H. Y. (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng J., Bi C., Clark B. S., Mady R., Shah P., Kohtz J. D. (2006) The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 20, 1470–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu W., Gius D., Onyango P., Muldoon-Jacobs K., Karp J., Feinberg A. P., Cui H. (2008) Epigenetic silencing of tumor suppressor gene p15 by its antisense RNA. Nature 451, 202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morris K. V., Santoso S., Turner A. M., Pastori C., Hawkins P. G. (2008) Bidirectional transcription directs both transcriptional gene activation and suppression in human cells. PLoS Genet 4, e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.