Background: The PDE6 γ-subunit serves multiple functions during visual transduction.
Results: Several regions of Pγ that interact with PDE6 or transducin were identified.
Conclusion: Multiple interacting sites of Pγ with PDE catalytic dimer, transducin, and the transducin/RGS9 complex coordinate the activation and deactivation of PDE6.
Significance: This work contributes to understanding how defects in PDE6 structure/function lead to retinal disease.
Keywords: Allosteric Regulation, Cyclic GMP (cGMP), Cyclic Nucleotides, Heterotrimeric G Proteins, Phosphodiesterases, Photoreceptors, RGS Proteins, Vision, PDE6, Visual Transduction
Abstract
The cGMP phosphodiesterase (PDE6) involved in visual transduction in photoreceptor cells contains two inhibitory γ-subunits (Pγ) which bind to the catalytic core (Pαβ) to inhibit catalysis and stimulate cGMP binding to the GAF domains of Pαβ. During visual excitation, interaction of activated transducin with Pγ relieves inhibition. Pγ also participates in a complex with RGS9–1 and other proteins to accelerate the GTPase activity of activated transducin. We studied the structural determinants for these important functions of Pγ. First, we identified two important sites in the middle region of Pγ (amino acids 27–38 and 52–54) that significantly stabilize the overall binding affinity of Pγ with Pαβ. The ability of Pγ to stimulate noncatalytic cGMP binding to the GAF domains of PDE6 has been localized to amino acids 27–30 of Pγ. Transducin activation of PDE6 catalysis critically depends on the presence of Ile54 in the glycine-rich region of Pγ in order to relieve inhibition of catalysis. The central glycine-rich region of Pγ is also required for transducin to increase cGMP exchange at the GAF domains. Finally, Thr-65 and/or Val-66 of Pγ are critical residues for Pγ to stimulate GTPase activity of transducin in a complex with RGS9–1. We propose that the glycine-rich region of Pγ is a primary docking site for PDE6-interacting proteins involved in the activation/inactivation pathways of visual transduction. This functional mapping of Pγ with its binding partners demonstrates the remarkable versatility of this multifunctional protein and its central role in regulating the activation and lifetime of visual transduction.
Introduction
Rod and cone photoreceptors respond to light by triggering a biochemical cascade leading to the activation of the cGMP-specific phosphodiesterase (PDE6).2 Because of its dominant role in controlling cGMP levels (and hence membrane conductance), the extent and duration of PDE6 activation must be precisely regulated. Catalytic activity of the rod PDE6 catalytic heterodimer (Pαβ) is directly regulated by its inhibitory γ-subunits (Pγ) that tightly bind to Pαβ to inhibit catalysis in the dark-adapted photoreceptor cell (1). During the first steps in vision, photoisomerized rhodopsin activates transducin, which binds GTP and releases its activated α-subunit (Tα*-GTP) to activate the PDE6 holoenzyme (αβγγ) by relieving the inhibition by Pγ at the active sites of the enzyme. The recovery of the dark-adapted state requires inactivation of Tα*-GTP by its intrinsic GTPase activity which is the rate-limiting step for recovery of the photoresponse. The GTPase rate of Tα*-GTP is modulated by the Regulator of G-protein Signaling 9–1 (RGS9–1) to which Pγ binds to potentiate the GTPase-accelerating function of RGS9–1 (2). The importance of the proper functioning and regulation of these proteins is underscored by the fact that genetic disruptions of PDE6 or the proteins with which it interacts often result in a loss of visual function, photoreceptor degeneration, and/or blindness (3–5).
The 87-amino acid Pγ subunit (localized to the signal-transducing outer segment compartment of rod photoreceptors) is remarkable for the variety of regulatory functions it performs as well as the multitude of proteins with which it interacts in addition to the catalytic subunits of PDE6 (6). The primary regulatory role of Pγ is to regulate access of substrate to the catalytic pocket of PDE6 and thereby control cGMP hydrolytic rates. This function is carried out by the last few C-terminal residues of Pγ interacting with the PDE6 catalytic domains in the immediate vicinity of the active site (7–9). An allosterically mediated inhibition of catalysis that occurs in the absence of the C-terminal residues of Pγ has also been identified (10). Pγ also enhances the affinity with which cGMP binds to noncatalytic binding sites within the regulatory domain of the PDE6 catalytic dimer (11, 12); the region of Pγ responsible for this effect is in the central region of the Pγ sequence, which is known to have high affinity for the catalytic dimer (13–15). In addition to these two distinct functional regions, chemical cross-linking studies support the idea that Pγ binds in an extended conformation along the entire surface of the catalytic subunits (16, 17), including both regulatory domains (GAFa and GAFb, named for their widespread occurrence in cGMP PDEs, certain adenylate cyclases, and the Escherichia coli Fh1a protein (18)) and the catalytic domains.
The molecular mechanism by which activated transducin α-subunit interacts with Pγ to de-inhibit catalysis of the PDE6 holoenzyme is not well understood. Biochemical, structural, and physiological studies support a model in which Tα*-GTP binds not only to the C-terminal tail of Pγ (to displace Pγ from occluding the PDE6 catalytic pocket), but also to several additional sites (most notably Trp-70 and Leu-76) within the last third of the Pγ sequence (19–26). However, Pαβ reconstituted with Pγ63–87 (i.e. the C-terminal fragment of Pγ consisting of amino acids 63 to 87) could not be activated by Tα*-GTPγS (10), indicating that additional sites of interaction of activated transducin with Pγ are required for activation of the PDE6 holoenzyme. The N-terminal half of Pγ (specifically amino acids 24–45) has been reported to interact with transducin α-subunit (27–30), and the greater efficiency with which cone versus rod PDE6 can be activated by transducin has been attributed to differences in the GAF binding interactions with Pγ (31). However, cross-linking and pull-down experiments suggest that Tα*-GTP interactions are weaker with the N-terminal half of Pγ than with the C-terminal region (26), raising questions about the functional significance of these interactions.
The recovery of the dark-adapted state following cessation of a light stimulus requires the inactivation of Tα-GTP by its intrinsic GTPase activity; this reaction has been shown to be rate-limiting for the recovery of the rod photoresponse (32). This GTPase rate is determined by a complex of proteins that include Tα*-GTP, RGS9–1, and other proteins (33); Pγ serves to facilitate the formation of a tighter complex of these proteins to potentiate the GTPase accelerating function of RGS9–1 (34–37). However, the interaction surface of Pγ with RGS9–1 and the functional significance of the interactions are unclear. Whereas biochemical and structural evidence shows that the C-terminal region of Pγ can bind to RGS9–1 (20, 23, 38), cross-linking and interaction assays have implicated the N-terminal half of Pγ in binding to the transducin/RGS9 complex (39). Furthermore, transgenic animals expressing a phosphorylation-incompetent mutation at Thr-35 of Pγ show altered photoresponse kinetics consistent with a disruption of the Pγ-mediated acceleration of GTPase activity by RGS9–1 (40).
In this report, we used functional interaction assays to demonstrate that the intrinsically disordered Pγ subunit forms multiple stabilizing interactions with Pαβ that extend from the N-terminal region of Pγ (interacting with the cGMP binding site in the GAFa domain) to the last several C-terminal residues of Pγ (serving to occlude the active site in the catalytic domain), and including a newly discovered interaction region in the glycine-rich central portion of Pγ. We also localized the Pγ residues directly responsible enhancing the ability of cGMP to bind to the noncatalytic binding sites on the PDE6 catalytic dimer, and identified neighboring residues that stabilize this effect. Finally, we identified the structural requirements for Pγ to effectively interact with activated transducin to activate PDE6 catalysis (at the enzyme active site), to increase cGMP exchange (with noncatalytic binding sites in the regulatory GAFa domain), and to bind to the transducin/RGS9–1 complex (to accelerate the GTPase rate of the transducin α-subunit). Together, these results provide a framework for understanding the sequential interactions of Pγ with PDE6 catalytic subunits and with its other binding partners that allow for precise temporal control of PDE activation and inactivation during visual transduction.
EXPERIMENTAL PROCEDURES
Materials
Bovine retinas were purchased from W. L. Lawson, Inc. Synthetic peptides Pγ10–30, Pγ19–30, Pγ21–30, Pγ63–87, Pγ65–87, and Pγ68–87 were purchased from New England Peptide. Ultima Gold scintillation fluid was from PerkinElmer Life & Analytical Sciences. Filtration membranes were from Millipore, the bicinchoninic acid protein assay reagents and immobilized glutathione were from Thermo Scientific/Pierce. All other chemicals were from Sigma-Aldrich. [3H]cGMP and [γ-32P]GTP were from PerkinElmer Life & Analytical Sciences. The primers for constructing Pγ mutants were obtained from Invitrogen. The plasmid purification kits were from Qiagen.
Construction of Pγ Mutants
Mutants lacking specific regions of the N-terminal sequence were constructed by PCR using primers designed to amplify various portions of the bovine rod Pγ sequence. The PCR products were inserted into the NotI and BamHI sites of pGEX-6P-1 vector, and followed by transformation into the E. coli BL21/(DE3) strain. The sequence of all Pγ mutants was confirmed by DNA sequencing at the Hubbard Center for Genome Studies (University of New Hampshire).
Purification of Pγ Mutants
Following expression of recombinant Pγ mutants in E. coli BL21(DE3), the bacterial extract was purified by immobilized glutathione. The affinity-purified protein was treated with HRV3C protease to remove the glutathione S-transferase fusion protein. Immobilized glutathione beads were added to the cleavage mix to remove any un-cleaved protein and the cleaved glutathione S-transferase. The Pγ mutants (containing five additional N-terminal amino acids derived from the fusion partner) were then further purified by C18 reverse-phase high pressure liquid chromatography. The purity (> 95%) and size of these proteins were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein concentrations were determined by the bicinchoninic acid protein assay (41) using bovine γ-globulin as a standard. In those Pγ constructs that were directly compared (e.g. full-length Pγ, Pγ67–87), we failed to observe an effect of the five additional N-terminal amino acids on Pγ inhibitory potency for Pαβ.
PDE6 and Pαβ Purification and Functional Assays
Bovine rod PDE6 was purified from bovine retinas as described (42). Pαβ catalytic dimers lacking Pγ were prepared by limited trypsin proteolysis and re-purified by Mono Q anion exchange chromatography prior to use (42). PDE6 catalytic activity was measured in 20 mm Tris, 10 mm MgCl2, 0.5 mg/ml bovine serum albumin, using a colorimetric assay (43). The PDE6 concentration was estimated based on the rate of cGMP hydrolysis of trypsin-activated PDE6 and the knowledge of the kcat of the enzyme (5600 mol cGMP hydrolyzed per mol Pαβ per s (44)).
The inhibition potency (IC50) of synthetic peptides and Pγ truncation mutants was determined using 0.2 nm Pαβ and 2 mm cGMP as substrates. Under these conditions, wild-type Pγ and N-terminal mutants up to Pγ21–87 inhibited Pαβ in a stoichiometric manner, behavior consistent with previous studies (44, 45).
[3H]cGMP binding to PDE6 was measured with a filter binding assay (46). The maximum binding of [3H]cGMP to nucleotide-depleted Pαβ was typically between 1 to 2 mol cGMP per mol Pαβ in the presence of wild-type Pγ. For measurements of cGMP dissociation kinetics, Pαβ reconstituted with Pγ or Pγ mutants were first incubated with 1 μm [3H]cGMP for 5 min at room temperature, and at time 0 1 mm unlabeled cGMP was added, and samples were filtered at various times thereafter.
Preparation of Bovine Rod Outer Segments (ROS) and GTPase Assay
Bovine rod outer segment (ROS) were prepared from commercial frozen bovine retinas on a step sucrose gradient using the standard method under dark condition (42) and stored at −80 degree. GTPase activity of transducin was determined by a single-turnover technique described in Ref. 47. In brief, ROS membrane was washed with assay buffer in dark (10 mm Tris, pH 7.8, 100 mm NaCl, 8 mm MgCl2, 1 mm DTT), the pellet was exposed to light for 1 min and then re-suspended in assay buffer. The reaction was initiated by mixing 20 μl of ROS membrane (20 μm rhodopsin final concentration) and 20 μl of 0.2 μm [γ-32P]GTP. The reaction was stopped by addition of 100 μl of 6% perchloric acid. [32P]Pi was separated by activated charcoal and determined by liquid scintillation counting.
Purification of Persistently Activated Transducin α-Subunit (Tα*-GTPγS) and Transducin Activation of Reconstituted Pαβ and Pγ Mutants
Transducin α-subunits were extracted from the PDE6-depleted ROS membranes by addition of 50 μm GTPγS. The extracted Tα*-GTPγS was purified on a Blue-Sepharose column as described (48, 49), followed by gel filtration chromatography to completely remove PDE6. The concentration of Tα*-GTPγS was determined by a colorimetric protein assay. Purified Tα*-GTPγS was stored at 4 °C and used within a few weeks. For the transducin activation measurement, purified Pαβ was pre-incubated with Pγ mutants or Pγ peptides at the indicated concentration to inhibit PDE activity (see figure legends). Ten micro molar-activated transducin (supplemented with 50 μm GTPγS) was added to above mixture and incubated for 5 min. The PDE activity was measured using 2 mm cGMP as substrate.
Data Analysis
All experiments were repeated at least three times, and averages are reported as the mean ± S.E. Curve fitting was performed using Sigmaplot (SPSS, Inc.).
RESULTS AND DISCUSSION
Multiple Regions of Pγ Contribute to the High Affinity with Which Pγ Inhibits Catalysis
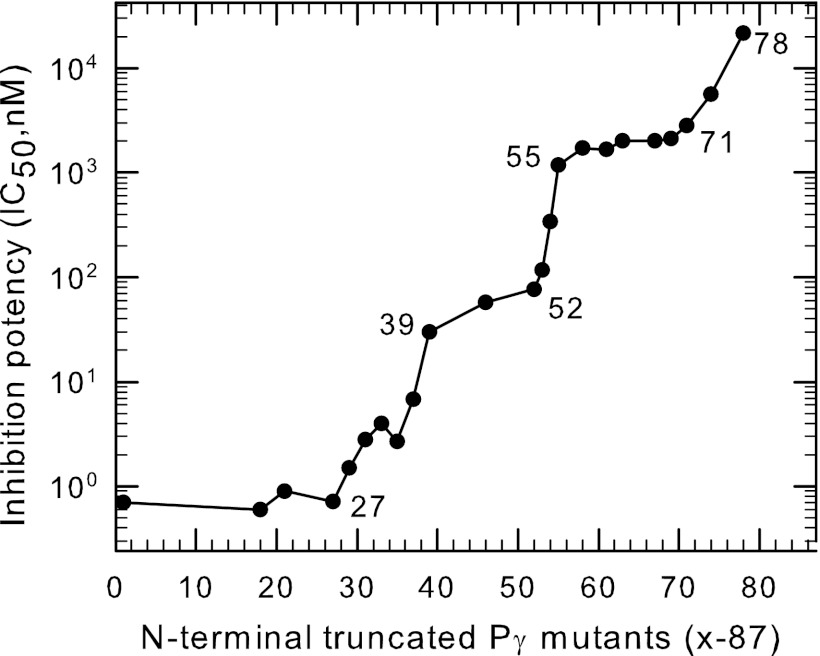
Previous studies have defined two distinct regions of Pγ that interact with PDE6 catalytic dimer, but did not account for the very high affinity of Pγ for the PDE6 catalytic dimer (see Introduction). We hypothesized that Pγ must contain as-yet undiscovered interacting sites, which are responsible for stabilizing Pγ binding to Pαβ. To test this, we generated a series of N-terminal truncated Pγ mutants, all of which contain the last ten amino acids as a reporter of the ability to inhibit catalysis. By comparing the relative binding affinity of various N-terminal truncated peptides, we discovered two new interaction “hotspots” for Pγ with Pαβ. Fig. 1 shows that Pγ mutants lacking amino acid residues 27 to 38 showed a progressive, ∼20-fold loss of binding affinity. A second region that stabilized Pγ binding to Pαβ by ∼15-fold was identified as Asp52-Asp53-Ile54 within the glycine-rich region of Pγ (Fig. 1). (A third set of stabilizing residues (Leu78-His79-Glu80) was previously identified in the C-terminal region (10).) In contrast, amino acid residues 1–26, 39–51, and 55–62 of Pγ showed little ability to stabilize the inhibition of catalysis at the active site (Fig. 1). We conclude from this analysis that three discrete regions of the Pγ sequence (i.e. polycationic region, glycine-rich region, and C-terminal region) account for almost all of the favorable interactions that contribute to the high overall affinity of Pγ for the catalytic dimer of PDE6. An important physiological implication of these multiple Pγ-Pαβ stabilizing regions is that the Pγ subunit is very unlikely to interact with binding partners other than PDE6 in the dark-adapted state of the photoreceptor cell.
FIGURE 1.
Multiple regions of Pγ stabilize its interaction with PDE6 catalytic dimer to inhibit catalysis. Purified Pαβ (0.2 nm) was pre-incubated with the indicated N-terminal truncated Pγ mutants (Pγx-87) for 20 min, followed by addition of 2 mm cGMP substrate. Catalytic activity was measured by the phosphate release assay. The inhibition potency (IC50) was calculated from curve fitting the results to a 3-parameter logistic equation. The data represent the mean of at least three experiments; error bars (coefficient of variation < 10% in all cases) were omitted for clarity. The abscissa represents the position number of the starting amino acid of the N-terminal truncated Pγ mutant, with position 1 being the wild-type sequence. Data for Pγ63–87, Pγ71–87, Pγ74–87, and Pγ78–87 were taken from Ref. 10.
The Ability of N-terminal Pγ Fragments to Augment Inhibition of Catalysis by the C-terminal Region of Pγ
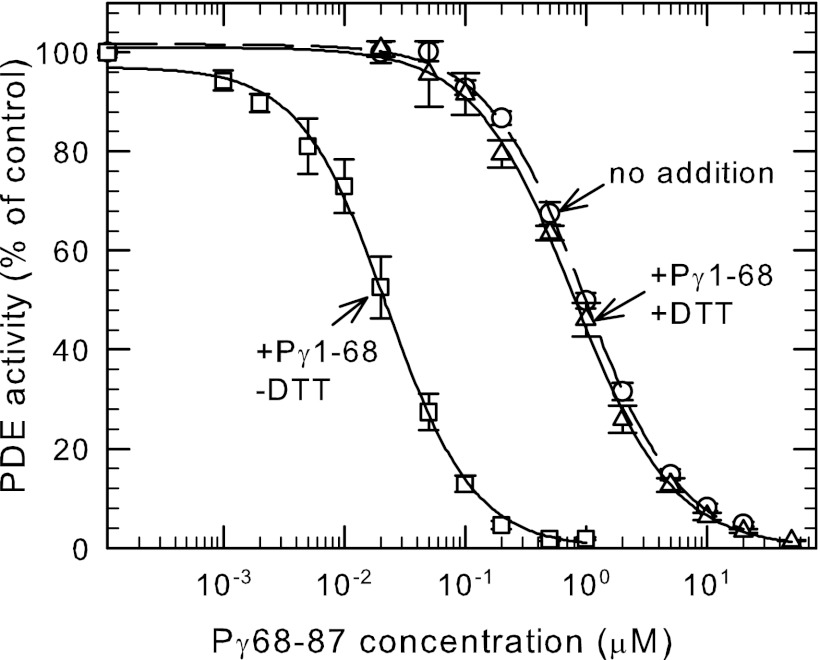
Previously we reported that a Pγ truncation mutant lacking the last 17 amino acids at its C terminus (Pγ1–70) enhanced 100-fold the ability of a synthetic peptide (Pγ63–87) to inhibit catalysis; shorter truncation mutants (e.g. Pγ1–60) or smaller C-terminal peptides failed to exhibit this effect (10). To pinpoint the amino acids of Pγ responsible for this effect, we constructed several synthetic peptides (Pγ69–87, Pγ68–87, and Pγ67–87) and truncation mutants (Pγ1–67 and Pγ1–68). Testing various combinations of N-terminal and C-terminal fragments of Pγ, we found that Pγ1–68 in combination with Pγ68–87 was able to exhibit the same enhancement of inhibitory effectiveness as reported in the earlier study. This mixture of two Pγ fragments overlapping only at Cys-68 have an overall binding affinity (IC50 = 20 nm; Fig. 2) that is 100-fold less than the value for wild-type Pγ under the same experimental conditions.
FIGURE 2.
An intermolecular disulfide bond between an N-terminal and C-terminal Pγ fragment enhances the inhibitory potency of the C-terminal region of Pγ. Purified Pαβ (0.2 nm) was pre-incubated with 1 μm Pγ1–68 and increasing concentrations of Pγ68–87 in the presence (△) or absence (□) of 1 mm DTT. The concentration dependence of Pγ68–87 was also assayed in the absence of Pγ1–68 (○). PDE catalytic activity was measured following addition of 2 mm cGMP. The data are the mean ± S.E. (n = 4). The lines represent the fit to a 3-parameter logistic equation with IC50 values of: Pγ68–87 alone = 1.0 ± 0.03 μm; +Pγ1–68 plus DTT = 0.8 ± 0.03 μm, and; +Pγ1–68 minus DTT = 0.02 ± 0.001 μm.
To study the importance of the Cys-68 residue that is shared by both Pγ fragments, we constructed site-directed mutants in which serine or alanine replacing the naturally occurring Cys-68 terminal residue in the Pγ1–68 truncation mutant. We found that neither the Pγ1–68Ser nor the Pγ1–68Ala were able to enhance the effectiveness of the C-terminal Pγ68–87 peptide to inhibit Pαβ (data not shown). This led us to carefully evaluate the possibility that disulfide bond formation between the two Pγ fragments might be responsible for the enhanced inhibitory potency when Pγ1–68 and Pγ68–87 were incubated with Pαβ. To test this, we compared the behavior of identical mixtures of Pαβ and the two Pγ fragments in the presence or absence of 1 mm DTT. As shown in Fig. 2, Pγ1–68 failed to enhance the potency of Pγ68–87 in the presence of DTT, indicating that disulfide bond formation between the two fragments was responsible for the enhancement of inhibitory potency. Control experiments confirmed that 1 mm DTT had no effects on the apparent inhibitory potency of Pγ68–87 in the absence of the N-terminal Pγ fragment (data not shown). These results fail to support the idea that allosteric communication induced by the N-terminal fragment alters the conformation of the catalytic domain (10).
The ability of the N-terminal half of Pγ to enhance 50-fold the binding of Pγ68–87 when the two fragments are tethered to each other by a disulfide bond implies that substantial conformational flexibility likely exists between different regions of Pγ. Under this artificial circumstance, the N-terminal region anchors Pγ to the catalytic subunit, and brings the C-terminal region into proximity of the active site to permit inhibition of catalysis. The fact that the overall effectiveness of inhibition is 100-fold less than the wild-type protein implies that disruption of the local structure of Pγ caused by linking the two fragments by a disulfide bond impairs the ability of the C-terminal inhibitory residues to bind to the active site of the enzyme.
Important Pγ Interaction Sites with Pαβ to Enhance cGMP Binding to the GAF Domain of PDE6
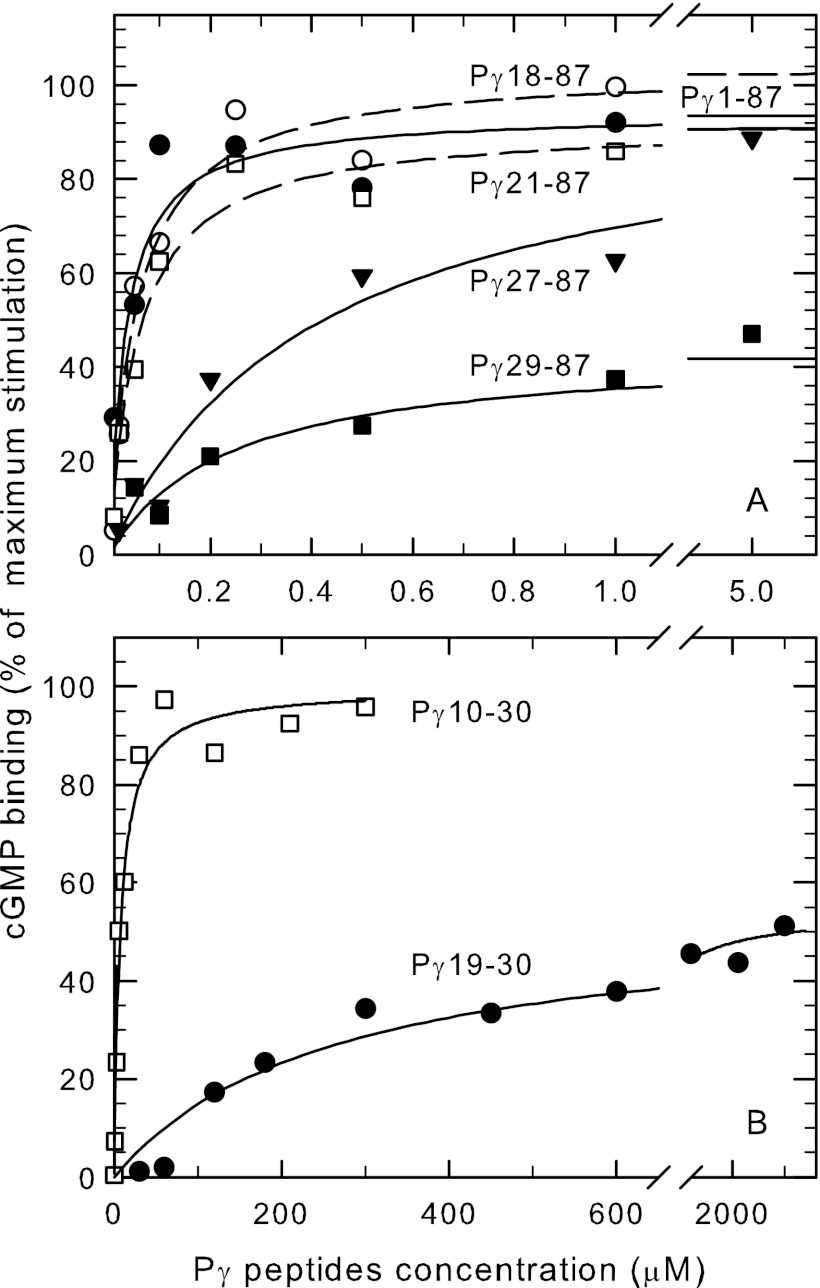
Having identified the important interacting residues of Pγ with PDE6 catalytic dimer to increase the inhibition potency, we next questioned to what extent these interaction sites contributed to the ability of Pγ to stabilize cGMP binding to the GAF domains of PDE6. To test this, we first measured the ability of a series of N-terminal truncated mutations to stabilize cGMP binding to the GAF domain of Pαβ. As shown in Fig. 3A, Pγ18–87 and Pγ21–87 were able to stimulate cGMP binding to the same extent as wild type Pγ and with a similar binding affinity (K1/2 = 30–50 nm). Although the Pγ27–87 mutant was able to stimulate cGMP binding to Pαβ to the same maximum extent as wild type Pγ, the affinity was reduced 7-fold compared with Pγ21–87 (Fig. 3A). Removing two additional residues (Pγ29–87) reduced to one-half the maximum extent of stimulation of cGMP binding (Fig. 3A), while Pγ31–87 was ineffective (<20% stimulation; data not shown). We conclude from these N-terminal Pγ truncation mutants that amino acids 27–30 of Pγ are required to maximally stimulate cGMP binding to the GAFa domains of PDE6. Furthermore, neighboring amino acids (amino acids 21 through 26) enhance the local interactions between Pγ and the GAFa domain of Pαβ that result in stimulation of cGMP binding.
FIGURE 3.
Amino acids in the N-terminal portion of Pγ stabilize cGMP binding to the GAF domains of Pαβ. Purified Pαβ (20 nm) was pre-incubated with 10 mm EDTA, 20 mm dipicolinic acid, and 50 μm sildenafil for 2 h at 22 °C. [3H]cGMP binding was measured in the presence of increasing amount of the indicated N-terminal truncated Pγ mutants (A) and synthetic peptides (B), and is reported as the percent stimulation of cGMP binding when comparing Pαβ (0% stimulation) to Pαβ incubated with 1 μm wild-type Pγ (100% stimulation, Bmax). The data are the average of at least three experiments, with the curves representing the fit of the data to a hyperbolic function. A, N-terminal truncated Pγ mutants: Pγ1–87 (●), K1/2 = 31 ± 10 nm, Bmax = 94%; Pγ18–87 (○), K1/2 = 52 ± 13 nm, Bmax = 104%; Pγ21–87 (□), K1/2 = 56 ± 11 nm, Bmax = 91%; Pγ27–87 (▾), K1/2 = 409 ± 85 nm, Bmax = 98%; Pγ29–87 (■), K1/2 = 457 ± 180 nm, Bmax = 44%. B, synthetic Pγ peptides: Pγ10–30 (□), K1/2 = 10 ± 4.3 μm, Bmax = 107%; Pγ19–30 (●), K1/2 = 280 ± 70 μm, Bmax = 47%.
To better define the region of Pγ responsible for stimulating cGMP binding to the GAFa domains of Pαβ, we resorted to using small synthetic peptides of this region of Pγ. Although the shortest peptide we tested, Pγ21–30, contained the residues identified above as being important for stimulating cGMP binding (Fig. 3A), this 10-residue oligopeptide lacked sufficient affinity for Pαβ to effectively induce this effect (≤ 20% stimulation of cGMP binding; data not shown). Full stimulation of cGMP binding by Pγ could be achieved if ten additional amino acids were present (Pγ10–30) to help stabilize peptide binding to Pαβ (K1/2 of 10 μm; Fig. 3B, see also Ref. (15)). Partial (47%) restoration of Pγ-mediated cGMP binding was observed with a peptide of intermediate length, Pγ19–30, but with substantially lower peptide binding affinity (K1/2 = 280 μm; Fig. 3B). These results with Pγ synthetic peptides reveal for the first time that amino acid residues 10–18 within the N-terminal region of Pγ can play a local, stabilizing role in the ability of Pγ to stimulate cGMP binding to the GAF domains of Pαβ.
We conclude from these results that four amino acids (Pro27-Pro28-Lys29-Phe30) bordering the pro-rich and polycationic regions of Pγ are required to enhance cGMP binding affinity to the GAFa domains of Pαβ. Neighboring residues on either side of this tetrapeptide provide local stabilizing interactions between Pγ and the Pαβ GAF domains that enhance the effectiveness of this four-amino acid segment, consistent with chemical cross-linking studies showing interactions of Val-21, Pro-23, and Phe-30 with the GAF domains of the PDE6 catalytic subunits (16, 17, 50).
Pγ Residue Ile-54 Is Important for Tα*-GTPγS to Relieve Inhibition of PDE6 Catalysis
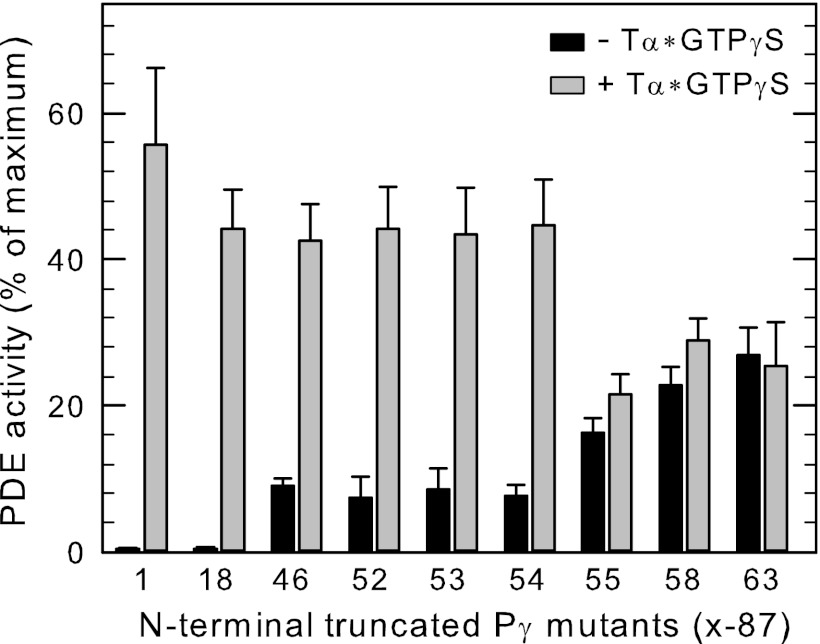
Previous work demonstrated that activated transducin was incapable of relieving inhibition of catalysis resulting from binding of the C-terminal fragment (Pγ63–87) reconstituted with Pαβ, suggesting that activated transducin required additional sites of interaction with Pγ to effectively de-inhibit PDE6 catalysis (10). To identify additional regions of Pγ responsible for the favorable interactions with Tα*-GTPγS leading to de-inhibition, we created a series of N-terminal truncation mutants that span the entire amino acid sequence of Pγ. These purified peptides were reconstituted with Pαβ at concentrations sufficient to inhibit catalysis by 80% or greater (Fig. 4, solid bars). As expected, a short truncation of the N-terminal region (Pγ18–87) that retains the GAF-interacting domain of Pγ was capable of effectively interacting with Tα*-GTPγS to relieve inhibition of PDE6 catalysis (Fig. 4, gray bars). Unexpectedly, when Pαβ was reconstituted with Pγ46–87 (which lacks the entire GAF-interacting region), addition of activated transducin resulted in activation of PDE6 catalysis (Fig. 4.). This demonstrates that the GAF interacting region of Pγ is not a requirement for transducin activation of PDE6. Transducin also successfully de-inhibited Pαβ reconstituted with Pγ52–87, Pγ53–87, and Pγ54–87 to the same maximum extent as wild type Pγ. However, Pαβ reconstituted with Pγ55–87 was unable to be fully activated by Tα*-GTPγS, and shorter peptides (e.g. Pγ58–87) were also ineffective (<10% activation, Fig. 4). We conclude that Ile54 in the glycine-rich region of Pγ is essential for transducin to effectively interact with Pγ to displace the C-terminal region to fully activate PDE6 catalysis.
FIGURE 4.
Isoleucine-54 of Pγ is critical for transducin to effectively bind to Pγ to relieve inhibition of catalysis. Purified Pαβ (1 nm) was incubated with the indicated N-terminal truncated Pγ mutants (x-87, where x is the first amino acid position number) to suppress 80% or greater of the catalytic activity: 5 nm Pγ1–87; 10 nm Pγ18–87; 0.5 μm Pγ46–87; 1 μm Pγ52–87; and Pγ53–87; 2 μm Pγ54–87; 5 μm Pγ55–87; and Pγ58–87; data for Pγ63–87 was taken from Ref. 10. Following 10 min of incubation at room temperature, activated transducin (10 μm) was added to one portion of each reconstituted PDE6 preparation, followed by addition of 2 mm cGMP to measure PDE6 catalytic activity. The data are the mean ± S.E. for three individual experiments and are reported as the percent of Pαβ activity when no additional Pγ was present.
Ile54 of Pγ has been visualized in proximity to the Trp-70 residue (important in the ability of Tα* to activate PDE6) in the crystal structure of a complex of a C-terminal Pγ fragment, a chimeric form of transducin α-subunit, and the RGS domain of RGS9–1 (23). Chemical cross-linking studies have shown much more favorable interactions of the glycine-rich region of Pγ with PDE6 catalytic subunits compared with transducin (26). Together with our work, these results support the hypothesis that the glycine-rich region of Pγ plays a critical role in the transducin activation mechanism of PDE6. We propose that in the transition from the dark-adapted to light-activated state, the glycine-rich region of Pγ relays its interactions from the Pαβ catalytic dimer to Tα* as it binds to the PDE6 holoenzyme. This “docking” of Tα* to the glycine-rich region of Pγ (centering around Ile-54) is necessary for Tα* to form additional interactions with the C-terminal region of Pγ (to displace the C-terminal region of Pγ from its binding sites in the catalytic pocket to de-inhibit catalysis) as well as the N-terminal half of Pγ (see next section).
Interaction of Tα*-GTPγS with the Glycine-rich Region of Pγ Increases the Rate of cGMP Dissociation from PDE6 GAFa Domains
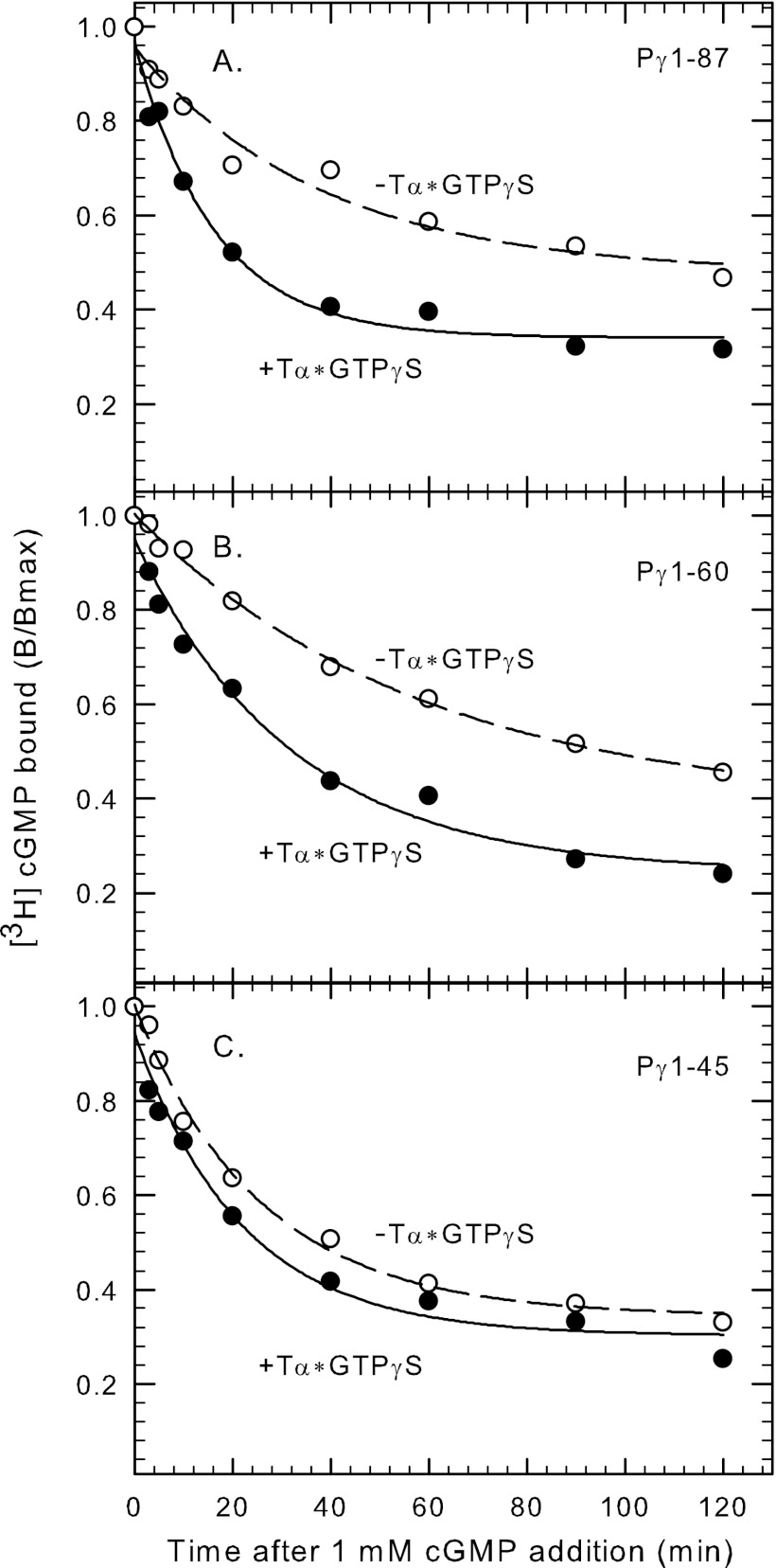
The region of Pγ that stabilizes cGMP binding to the GAFa domains of Pαβ (amino acids 21 to 30; see above) have also been reported to interact with transducin α-subunit based on binding assays of Pγ21–45 with Tα*-GTPγS (28, 29). To directly test whether Tα*-GTPγS can alter cGMP binding to the GAFa domain through disrupting Pγ binding to Pαβ, we measured the ability of Tα*-GTPγS to accelerate the rate of cGMP dissociation from Pαβ reconstituted with various Pγ fragments. As shown in Fig. 5A, addition of Tα*-GTPγS to Pαβ reconstituted with full-length Pγ increased 2.2 ± 0.2-fold (n = 4) the rate at which cGMP exchange occurs at the noncatalytic cGMP binding sites. Pγ1–60 was equally effective (2.2 ± 0.4-fold; n = 3) as wild-type in this regard (Fig. 5B). In contrast, Pγ1–45 reconstituted with Pαβ showed virtually no stimulation of cGMP dissociation rate (1.2 ± 0.1-fold; n = 5) upon Tα*-GTPγS addition (Fig. 5C). We conclude that Tα*-GTPγS requires interactions with Pγ in the glycine-rich region (specifically amino acids 46–60) in order to weaken the interactions of Pγ in the region of amino acids 21–30 that are responsible for modulating cGMP affinity to the GAFa domains of PDE6. The ability of activated transducin α-subunit to accelerate cGMP dissociation may represent a negative feedback mechanism operating during light adaptation (see “Conclusions”).
FIGURE 5.
Interaction of transducin with the glycine-rich region of Pγ increases the rate of cGMP dissociation from PDE6. Nucleotide-depleted Pαβ (20 nm) was pre-incubated with 10 mm EDTA, 20 mm dipicolinic acid, and 50 μm vardenafil for 2 h at 22 °C. [3H]cGMP was added in the presence of 40–80 nm full-length Pγ (A), Pγ1–60 (B), or Pγ1–45 (C), and the samples incubated for 5 min. For each condition, addition of Pγ or truncated mutants stimulated binding 1.5-fold compared with Pαβ dimer alone; the maximum extent of binding (Bmax) just prior to initiating dissociation was Pγ1–87, 1.7 ± 0.2 mol cGMP/mol Pαβ; Pγ1–60, 1.7 ± 0.1 mol cGMP/mol Pαβ; Pγ1–45, 1.6 ± 0.1 mol cGMP/mol Pαβ. [3H]cGMP dissociation was induced by addition of unlabeled cGMP supplemented with or without 2 μm activated transducin (Tα*-GTPγS), and the amount bound (B) assayed at various times thereafter. The data shown in the figure are from one representative experiment, with the results fitted to a single-exponential decay process with t1/2 values as follows: A, −Tα* = 26.3 min, +Tα* = 11.2 min; B, −Tα* = 40.5 min, +Tα* = 22.1 min; C, −Tα* = 17.7 min, +Tα* = 15.0 min.
Characterization of the Regions of Pγ Important for Facilitating Deactivation of the Complex of PDE6/Transducin/RGS9–1
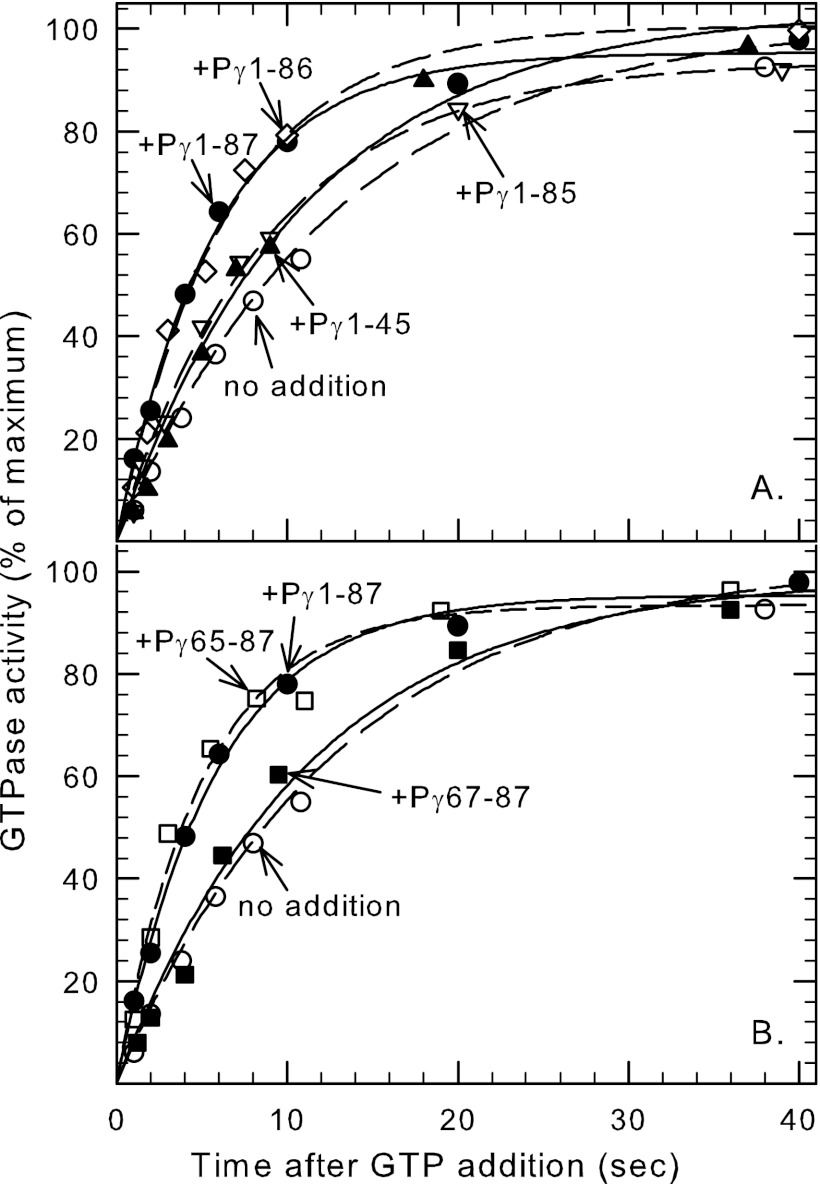
Another important role of Pγ is to form a protein complex with RGS9–1, transducin, and other accessory proteins to accelerate the GTPase activity of activated transducin during deactivation of PDE6 (38). However, there is conflicting evidence regarding the sites of interaction of Pγ with this complex (see Introduction). To precisely define the Pγ residues required for GTPase acceleration, we first utilized a set of Pγ mutants truncated to various extents at the C terminus. These Pγ mutants were incubated with ROS membranes and tested for their ability to accelerate the GTPase activity of transducin above the intrinsic activity of this membrane preparation. As shown in Fig. 6A, wild-type Pγ (Pγ1–87) and a mutant lacking the terminal Ile-87 (Pγ1–86) both stimulated the GTPase rate by ∼2.5-fold compared with the control lacking any exogenous Pγ. Removal of an additional amino acid (Pγ1–85) slowed the GTPase rate by 60% compared with full-length Pγ, consistent with a previous study (20). We conclude that a single amino acid, Ile-86, is critical for the ability of Pγ to potentiate GTPase acceleration.
FIGURE 6.
Two major regions of Pγ are critical for the acceleration of GTPase activity of activated transducin. Bovine ROS membranes (containing PDE6, transducin, and RGS9–1) were incubated with Pγ truncation mutants for 20 min at room temperature. GTPase activity was then measured by addition of 0.1 μm [γ-32P]GTP. The reaction was stopped at the indicated time by addition of perchloric acid. GTPase activity is reported as a percent of maximum activity (1 h incubation; >98% substrate hydrolyzed). The data shown in the figure are representative of at least three different experiments, in which the lines represent the fit of the data to a single exponential rise to maximum. A, C-terminal truncated Pγ mutants (2 μm final concentration): no addition (○), t1/2 = 8.0 ± 0.8 s; Pγ1–45 (▴), t1/2 = 6.2 ± 0.9 s; Pγ1–85 (▿), t1/2 = 5.7 ± 0.7 s; Pγ1–86 (♢), t1/2 = 3.7 ± 0.4 s; Pγ1–87(●), t1/2 = 3.1 ± 0.8 s. B, N-terminal truncation mutants of Pγ (tested at 10 μm final concentration): no addition (○), t1/2 = 8.0 ± 0.8 s; Pγ65–87 (□), t1/2 = 3.1 ± 0.3 s; and Pγ67–87 (■), t1/2 = 6.8 ± 0.4 s. In all cases except for Pγ67–87, the t1/2 values for the indicated Pγ mutants were statistically significant (p < 0.01) compared with the control (no Pγ added).
Larger C-terminal truncations of Pγ (e.g. Pγ1–70 (not shown) or Pγ1–45 (Fig. 6A)) caused no further disruption of its ability to stimulate the GTPase rate. To determine the potential participation of the N-terminal half of Pγ in GTPase acceleration, we tested the ability of Pγ46–87 to accelerate GTPase activity. We found a modest (2-fold) decrease in overall affinity of Pγ46–87 with the transducin/RGS9–1 complex (K1/2 = 0.35 ± 0.04 μm) compared with wild-type Pγ (K1/2 = 0.14 ± 0.02 μm). This indicates no significant role for the N-terminal half of Pγ to participate in GTPase acceleration in the Tα*-GTPγS/RGS9–1 complex.
To identify more precisely which regions of Pγ interact with the transducin/RGS9–1 complex to stimulate GTPase activity, we next tested the ability of C-terminal synthetic peptides and N-terminal truncation mutants of Pγ to maximally accelerate the GTPase activity of ROS membrane preparations. We first determined that Pγ65–87 was the minimum C-terminal fragment sufficient to cause maximal stimulation of GTPase activity, since removal of two additional amino acids (Pγ67–87) abolished the potentiating effect entirely (Fig. 6B). To quantitatively define the regions of Pγ that enhance its effectiveness to accelerate GTPase activity, we examined the concentration dependence of this acceleration effect. Whereas Pγ67–87 was completely ineffective at highest concentrations tested (500 μm), adding Thr-65 and Val-66 enhanced >100-fold the ability of Pγ to potentiate the GTPase activity of the transducin/RGS9–1 complex (Pγ65–87, K1/2 = 2.5 ± 1.4 μm). Addition of two more amino acids (Pγ63–87; K1/2 = 2.2 ± 0.3 μm) had no effect on the affinity, while inclusion of an additional eight amino acids (Pγ55–87; K1/2 = 0.6 ± 0.2 μm) stabilized the interaction 4-fold. Further elongation of Pγ had little effect on the ability to stimulate GTPase activity.
Two conclusions arise from these results: 1) Ile-86 in conjunction with Thr-65 and/or Val-66 of Pγ are required to maximally accelerate the GTPase rate of the Tα*/RGS9–1 complex, consistent with previous evidence (20, 23); 2) stabilizing interactions in the region of amino acids 55–62 of Pγ may help anchor Pγ to the Tα*/RGS9–1 complex even though they are not required for maximal potentiation of the GTPase rate.
CONCLUSIONS
This comprehensive analysis of the functionally important regions of the inhibitory γ-subunit that interact with the PDE6 catalytic dimer, with activated transducin α-subunit, and with the Tα*/RGS9–1 complex (summarized in Fig. 7) reveals the complexity of the regulatory mechanisms mediated by multiple regions of this small protein. The ability of the N-terminal and C-terminal regions of Pγ to span the surface of the Pαβ dimer from the GAFa domain to the active site of the catalytic domain underscores the extended linear structure that Pγ must assume when associated with the Pαβ catalytic dimer. Since Pγ free in solution is a natively unfolded protein (6, 51, 52), its extended conformation is a consequence of binding to the Pαβ dimer at each of the six distinct regions of the Pγ linear structure (defined in Fig. 7). Because Pγ seldom (if ever) completely dissociates from Pαβ during mammalian visual transduction (53–55), it is likely that the structural elements important for interaction of Pγ with Tα*, RGS9–1, or other putative binding partners (e.g. GARP2 (56)) occur while Pγ is associated with the catalytic subunits and involve a disruption of Pγ-Pαβ interactions at the same time as new interactions form between Pγ and its other binding partners.
FIGURE 7.
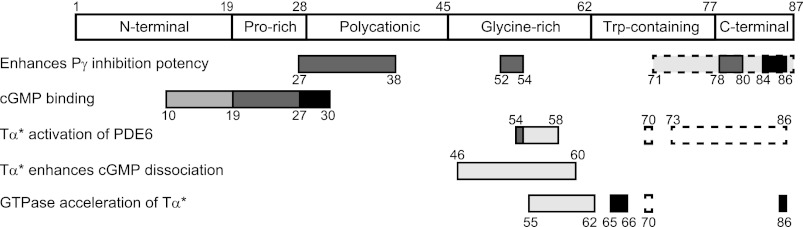
Functionally important interaction sites of the inhibitory Pγ subunit with PDE6, transducin, and the transducin/RGS9–1 complex. The 87 amino acid Pγ subunit is defined in terms of six structurally distinct domains: N-terminal region (amino acids 1–19); proline-rich region (amino acids 20–28); polycationic region (amino acids 29–45); glycine-rich region (amino acids 46–62); tryptophan-containing region (amino acids 63–77); and the C-terminal region (amino acids 78–87). The sites that are required for any of the five given functions are shown with solid black boxes. Amino acid residues having major stabilizing effects on Pγ binding to its binding partner are shown in dark gray, while additional, weaker sites of interaction are shown in light gray. The dashed boxes represent critical functional regions of Pγ identified previously (10, 20, 22, 24).
Our work reveals the central importance of the glycine-rich region of Pγ as a primary “docking site” with Pαβ (amino acids 52–54), with Tα* (amino acids 54–55), and with the Tα*/RGS9–1 complex (amino acids 55–62; Fig. 7). We hypothesize that this stretch of amino acids stabilizes binding of Tα* to position it to develop additional interactions with the C-terminal “hinge” (amino acids 71–77) and “blocking” (amino acids 78–87) regions of Pγ (8, 24) that lead to de-inhibition of PDE6 catalysis. A subset of residues within the glycine-rich region of Pγ (specifically amino acids 55–62) is also implicated in facilitating the potentiating role of Pγ to accelerate GTPase activity of the Tα*/RGS9–1 complex. Further, this same region of Pγ is implicated in regulating cGMP exchange kinetics at the noncatalytic cGMP binding sites of Pαβ. This latter effect may result from another hinge-like mechanism whereby docking of Tα* to the glycine-rich region of Pγ enables Tα* to form additional, previously identified, interactions with the polycationic region of Pγ (27–30) which could, in turn, counteract the stabilizing effects of the Pro-rich region (amino acids 27–30) of Pγ on cGMP binding to the GAFa domains of Pαβ.
These multiple interactions of Pγ with Pαβ and with other PDE6 binding partners likely occur in a exquisitely controlled temporal sequence that begins with the immediate response to light stimulation of a dark-adapted photoreceptor (i.e. visual excitation leading to PDE6 activation by Tα*). This leads to the subsequent acceleration of photoresponse recovery during light adaptation (RGS9–1-catalyzed acceleration of Tα* GTPase activity being the rate-limiting step; (32)). Finally, slowly developing aspects of photoreceptor desensitization (reviewed in Ref. (2)) may relate to the ability of Tα*-activated PDE6 to increase cGMP dissociation from noncatalytic cGMP binding sites on PDE6 when cytosolic cGMP levels remain low for an extended time. All of these above-mentioned interactions are mediated by changes in Pγ interactions with Pαβ, Tα*, RGS9–1, and other proteins that form a large multiprotein signaling complex on the photoreceptor disk membrane (57). Future efforts will be directed to mapping the individual regions of Pγ that serve to relay information about the state of light activation from Pαβ to the other members of this signaling complex, furthering our understanding of the mechanistic basis for visual dysfunction and photoreceptor degeneration that can result from genetic defects in Pγ or its binding partners (4).
Acknowledgments
We thank Sue Matte, Karyn Cahill, and Christina Loporcaro for help with construction of some of the Pγ mutants, protein purification, and manuscript review.
This work was supported, in whole or in part, by National Institutes of Health Grant EY-05798. Partial funding was provided by the New Hampshire Agricultural Experiment Station. This is Scientific Contribution Number 2479.
- PDE
- cyclic nucleotide phosphodiesterase
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- Pαβ
- catalytic dimer of PDE6 α- and β-subunits
- Pγ
- inhibitory γ subunit of PDE6
- GAF
- regulatory domain of PDE6 named for their presence in cGMP-regulated PDEs, certain adenylate cyclases, and the transcription factor Fh1A of bacteria
- Tα*
- activated transducin α-subunit.
REFERENCES
- 1. Cote R. H. (2006) in Photoreceptor phosphodiesterase (PDE6): a G-protein-activated PDE regulating visual excitation in rod and cone photoreceptor cells. (Beavo J. A., Francis S. H., Houslay M. D., eds) Cyclic Nucleotide Phosphodiesterases in Health and Disease, pp. 165–193, CRC Press, Boca Raton, FL [Google Scholar]
- 2. Arshavsky V. Y., Burns M. E. (2012) Photoreceptor signaling: supporting vision across a wide range of light intensities. J. Biol. Chem. 287, 1620–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daiger S. P., Bowne S. J., Sullivan L. S. (2007) Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 125, 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baehr W., Frederick J. M. (2009) Naturally occurring animal models with outer retina phenotypes. Vision Res. 49, 2636–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrari S., Di Iorio E., Barbaro V., Ponzin D., Sorrentino F. S., Parmeggiani F. (2011) Retinitis pigmentosa: genes and disease mechanisms. Curr. Genomics 12, 238–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo L. W., Ruoho A. E. (2008) The retinal cGMP phosphodiesterase γ-subunit - a chameleon. Curr. Protein Pept. Sci. 9, 611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Granovsky A. E., Natochin M., Artemyev N. O. (1997) The γ-subunit of rod cGMP-phosphodiesterase blocks the enzyme catalytic site. J. Biol. Chem. 272, 11686–11689 [DOI] [PubMed] [Google Scholar]
- 8. Barren B., Gakhar L., Muradov H., Boyd K. K., Ramaswamy S., Artemyev N. O. (2009) Structural basis of phosphodiesterase 6 inhibition by the C-terminal region of the γ-subunit. EMBO J. 28, 3613–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z., Artemyev N. O. (2010) Determinants for phosphodiesterase 6 inhibition by its γ-subunit. Biochemistry 49, 3862–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X. J., Skiba N. P., Cote R. H. (2010) Structural requirements of the photoreceptor phosphodiesterase γ-subunit for inhibition of rod PDE6 holoenzyme and for its activation by transducin. J. Biol. Chem. 285, 4455–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamazaki A., Bartucca F., Ting A., Bitensky M. W. (1982) Reciprocal effects of an inhibitory factor on catalytic activity and noncatalytic cGMP binding sites of rod phosphodiesterase. Proc. Natl. Acad. Sci. U.S.A. 79, 3702–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cote R. H., Bownds M. D., Arshavsky V. Y. (1994) cGMP binding sites on photoreceptor phosphodiesterase: role in feedback regulation of visual transduction. Proc. Natl. Acad. Sci. U.S.A. 91, 4845–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Artemyev N. O., Hamm H. E. (1992) Two-site high-affinity interaction between inhibitory and catalytic subunits of rod cyclic GMP phosphodiesterase. Biochem. J. 283, 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takemoto D. J., Hurt D., Oppert B., Cunnick J. (1992) Domain mapping of the retinal cyclic GMP phosphodiesterase γ-subunit. Function of the domains encoded by the three exons of the γ-subunit gene. Biochem. J. 281, 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mou H., Cote R. H. (2001) The catalytic and GAF domains of the rod cGMP phosphodiesterase (PDE6) heterodimer are regulated by distinct regions of its inhibitory γ-subunit. J. Biol. Chem. 276, 27527–27534 [DOI] [PubMed] [Google Scholar]
- 16. Guo L. W., Grant J. E., Hajipour A. R., Muradov H., Arbabian M., Artemyev N. O., Ruoho A. E. (2005) Asymmetric interaction between rod cyclic GMP phosphodiesterase γ-subunits and αβ-subunits. J. Biol. Chem. 280, 12585–12592 [DOI] [PubMed] [Google Scholar]
- 17. Guo L. W., Muradov H., Hajipour A. R., Sievert M. K., Artemyev N. O., Ruoho A. E. (2006) The inhibitory γ-subunit of the rod cGMP phosphodiesterase binds the catalytic subunits in an extended linear structure. J. Biol. Chem. 281, 15412–15422 [DOI] [PubMed] [Google Scholar]
- 18. Aravind L., Ponting C. P. (1997) The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 22, 458–459 [DOI] [PubMed] [Google Scholar]
- 19. Skiba N. P., Artemyev N. O., Hamm H. E. (1995) The carboxyl terminus of the gamma-subunit of rod cGMP phosphodiesterase contains distinct sites of interaction with the enzyme catalytic subunits and the α-subunit of transducin. J. Biol. Chem. 270, 13210–13215 [DOI] [PubMed] [Google Scholar]
- 20. Slepak V. Z., Artemyev N. O., Zhu Y., Dumke C. L., Sabacan L., Sondek J., Hamm H. E., Bownds M. D., Arshavsky V. Y. (1995) An effector site that stimulates G-protein GTPase in photoreceptors. J. Biol. Chem. 270, 14319–14324 [DOI] [PubMed] [Google Scholar]
- 21. Liu Y., Arshavsky V. Y., Ruoho A. E. (1996) Interaction sites of the COOH-terminal region of the γ-subunit of cGMP phosphodiesterase with the GTP-bound α-subunit of transducin. J. Biol. Chem. 271, 26900–26907 [DOI] [PubMed] [Google Scholar]
- 22. Tsang S. H., Burns M. E., Calvert P. D., Gouras P., Baylor D. A., Goff S. P., Arshavsky V. Y. (1998) Role for the target enzyme in deactivation of photoreceptor G protein in vivo. Science 282, 117–121 [DOI] [PubMed] [Google Scholar]
- 23. Slep K. C., Kercher M. A., He W., Cowan C. W., Wensel T. G., Sigler P. B. (2001) Structural determinants for regulation of phosphodiesterase by a G protein at 2.0 A. Nature 409, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 24. Granovsky A. E., Artemyev N. O. (2001) A conformational switch in the inhibitory gamma-subunit of PDE6 upon enzyme activation by transducin. Biochemistry 40, 13209–13215 [DOI] [PubMed] [Google Scholar]
- 25. Grant J. E., Guo L. W., Vestling M. M., Martemyanov K. A., Arshavsky V. Y., Ruoho A. E. (2006) The N terminus of GTP γ S-activated transducin α-subunit interacts with the C terminus of the cGMP phosphodiesterase γ-subunit. J. Biol. Chem. 281, 6194–6202 [DOI] [PubMed] [Google Scholar]
- 26. Guo L. W., Hajipour A. R., Ruoho A. E. (2010) Complementary interactions of the rod PDE6 inhibitory subunit with the catalytic subunits and transducin. J. Biol. Chem. 285, 15209–15219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morrison D. F., Cunnick J. M., Oppert B., Takemoto D. J. (1989) Interaction of the gamma-subunit of retinal rod outer segment phosphodiesterase with transducin. Use of synthetic peptides as functional probes. J. Biol. Chem. 264, 11671–11681 [PubMed] [Google Scholar]
- 28. Artemyev N. O., Rarick H. M., Mills J. S., Skiba N. P., Hamm H. E. (1992) Sites of interaction between rod G-protein α-subunit and cGMP-phosphodiesterase γ-subunit. Implications for the phosphodiesterase activation mechanism. J. Biol. Chem. 267, 25067–25072 [PubMed] [Google Scholar]
- 29. Artemyev N. O., Mills J. S., Thornburg K. R., Knapp D. R., Schey K. L., Hamm H. E. (1993) A site on transducin α-subunit of interaction with the polycationic region of cGMP phosphodiesterase inhibitory subunit. J. Biol. Chem. 268, 23611–23615 [PubMed] [Google Scholar]
- 30. Artemyev N. O. (1997) Binding of transducin to light-activated rhodopsin prevents transducin interaction with the rod cGMP phosphodiesterase γ-subunit. Biochemistry 36, 4188–4193 [DOI] [PubMed] [Google Scholar]
- 31. Muradov H., Boyd K. K., Artemyev N. O. (2010) Rod phosphodiesterase-6 PDE6A and PDE6B subunits are enzymatically equivalent. J. Biol. Chem. 285, 39828–39834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krispel C. M., Chen D., Melling N., Chen Y. J., Martemyanov K. A., Quillinan N., Arshavsky V. Y., Wensel T. G., Chen C. K., Burns M. E. (2006) RGS expression rate-limits recovery of rod photoresponses. Neuron 51, 409–416 [DOI] [PubMed] [Google Scholar]
- 33. Anderson G. R., Posokhova E., Martemyanov K. A. (2009) The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem. Biophys. 54, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He W., Cowan C. W., Wensel T. G. (1998) RGS9, a GTPase accelerator for phototransduction. Neuron 20, 95–102 [DOI] [PubMed] [Google Scholar]
- 35. Makino E. R., Handy J. W., Li T., Arshavsky V. Y. (1999) The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein β-subunit. Proc. Natl. Acad. Sci. U.S.A. 96, 1947–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skiba N. P., Hopp J. A., Arshavsky V. Y. (2000) The effector enzyme regulates the duration of G protein signaling in vertebrate photoreceptors by increasing the affinity between transducin and RGS protein. J. Biol. Chem. 275, 32716–32720 [DOI] [PubMed] [Google Scholar]
- 37. Hu G., Wensel T. G. (2002) R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9–1. Proc. Natl. Acad. Sci. U.S.A. 99, 9755–9760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arshavsky V. Y., Dumke C. L., Zhu Y., Artemyev N. O., Skiba N. P., Hamm H. E., Bownds M. D. (1994) Regulation of transducin GTPase activity in bovine rod outer segments. J. Biol. Chem. 269, 19882–19887 [PubMed] [Google Scholar]
- 39. Guo L. W., Ruoho A. E. (2011) N-terminal half of the cGMP phosphodiesterase γ-subunit contributes to stabilization of the GTPase-accelerating protein complex. J. Biol. Chem. 286, 15260–15267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woodruff M. L., Janisch K. M., Peshenko I. V., Dizhoor A. M., Tsang S. H., Fain G. L. (2008) Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J. Neurosci. 28, 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 [DOI] [PubMed] [Google Scholar]
- 42. Pentia D. C., Hosier S., Collupy R. A., Valeriani B. A., Cote R. H. (2005) Purification of PDE6 isozymes from mammalian retina. Methods Mol. Biol. 307, 125–140 [DOI] [PubMed] [Google Scholar]
- 43. Cote R. H. (2000) Kinetics and regulation of cGMP binding to noncatalytic binding sites on photoreceptor phosphodiesterase. Methods Enzymol. 315, 646–672 [DOI] [PubMed] [Google Scholar]
- 44. Mou H., Grazio H. J., 3rd, Cook T. A., Beavo J. A., Cote R. H. (1999) cGMP binding to noncatalytic sites on mammalian rod photoreceptor phosphodiesterase is regulated by binding of its γ- and δ-subunits. J. Biol. Chem. 274, 18813–18820 [DOI] [PubMed] [Google Scholar]
- 45. Hurley J. B., Stryer L. (1982) Purification and characterization of the γ regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J. Biol. Chem. 257, 11094–11099 [PubMed] [Google Scholar]
- 46. Cote R. H. (2005) Cyclic guanosine 5'-monophosphate binding to regulatory GAF domains of photoreceptor phosphodiesterase. Methods Mol. Biol. 307, 141–154 [DOI] [PubMed] [Google Scholar]
- 47. Cowan C. W., Wensel T. G., Arshavsky V. Y. (2000) Enzymology of GTPase acceleration in phototransduction. Methods Enzymol. 315, 524–538 [DOI] [PubMed] [Google Scholar]
- 48. Kleuss C., Pallast M., Brendel S., Rosenthal W., Schultz G. (1987) Resolution of transducin subunits by chromatography on blue Sepharose. J. Chromatogr. 407, 281–289 [DOI] [PubMed] [Google Scholar]
- 49. Wensel T. G., He F., Malinski J. A. (2005) Purification, reconstitution on lipid vesicles, and assays of PDE6 and its activator G protein, transducin. Methods Mol. Biol. 307, 289–313 [DOI] [PubMed] [Google Scholar]
- 50. Muradov K. G., Granovsky A. E., Schey K. L., Artemyev N. O. (2002) Direct interaction of the inhibitory γ-subunit of Rod cGMP phosphodiesterase (PDE6) with the PDE6 GAFa domains. Biochemistry 41, 3884–3890 [DOI] [PubMed] [Google Scholar]
- 51. Uversky V. N., Permyakov S. E., Zagranichny V. E., Rodionov I. L., Fink A. L., Cherskaya A. M., Wasserman L. A., Permyakov E. A. (2002) Effect of zinc and temperature on the conformation of the γ-subunit of retinal phosphodiesterase: a natively unfolded protein. J. Proteome Res. 1, 149–159 [DOI] [PubMed] [Google Scholar]
- 52. Matte S. L., Laue T. M., Cote R. H. (2012) Characterization of conformational changes and protein-protein interactions of rod photoreceptor phosphodiesterase (PDE6). J. Biol. Chem. 287, 20111–20121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wensel T. G., Stryer L. (1986) Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its γ-subunit and transducin. Proteins 1, 90–99 [DOI] [PubMed] [Google Scholar]
- 54. Deterre P., Bigay J., Robert M., Pfister C., Kühn H., Chabre M. (1986) Activation of retinal rod cyclic GMP-phosphodiesterase by transducin: characterization of the complex formed by phosphodiesterase inhibitor and transducin α-subunit. Proteins 1, 188–193 [DOI] [PubMed] [Google Scholar]
- 55. Otto-Bruc A., Antonny B., Vuong T. M., Chardin P., Chabre M. (1993) Interaction between the retinal cyclic GMP phosphodiesterase inhibitor and transducin. Kinetics and affinity studies. Biochemistry 32, 8636–8645 [DOI] [PubMed] [Google Scholar]
- 56. Pentia D. C., Hosier S., Cote R. H. (2006) The glutamic acid-rich protein-2 (GARP2) is a high affinity rod photoreceptor phosphodiesterase (PDE6)-binding protein that modulates its catalytic properties. J. Biol. Chem. 281, 5500–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wensel T. G. (2008) Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 48, 2052–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]