Background: The TGFβ receptor-interacting protein km23-1 plays an important role in TGFβ signal transduction in TGFβ-sensitive epithelial cells.
Results: The role of km23-1 in TGFβ activation of Ras/JNK/ERK, as well as TGFβ1 autoinduction, was determined.
Conclusion: km23-1 is required for TGFβ1 autoinduction through a Smad2-independent Ras/ERK/JNK pathway.
Significance: km23-1 functions as a critical adaptor coupling TGFβ receptors to Ras activation after TGFβ treatment.
Keywords: MAP kinases (MAPKs), Ras, Signal transduction, Signaling, Transforming growth factor beta (TGFbeta), MAPK, Ras, TGFbeta, dynein, km23-1
Abstract
We have previously elucidated the signaling events that are required for TGFβ1 autoinduction (Yue, J., and Mulder, K. M. (2000) J. Biol. Chem. 275, 30765–30773). Further, we have reported that the TGFβ receptor (TβR)-interacting protein km23-1 plays an important role in TGFβ signal transduction (Jin, Q., Ding, W., and Mulder, K. M. (2007) J. Biol. Chem. 282, 19122–19132). Here we examined the role of km23-1 in TGFβ1 autoinduction in TGFβ-sensitive epithelial cells. siRNA blockade of km23-1 reduced TGFβ1 mRNA expression, as well as DNA binding and transcriptional activation of the relevant activator protein-1 site in the human TGFβ1 promoter. Further, knockdown of km23-1 inhibited TGFβ-mediated activation of ERK and JNK, phosphorylation of c-Jun, and transactivation of the c-Jun promoter. Sucrose gradient analyses indicate that km23-1 was present in lipid rafts together with Ras and TβRII after TGFβ treatment. Immunoprecipitation/blot analyses revealed the formation of a TGFβ-inducible complex between Ras and km23-1 in vivo within minutes of TGFβ addition. Moreover, we demonstrate for the first time that km23-1 is required for Ras activation by TGFβ. Our results indicate that km23-1 is required for TGFβ1 autoinduction through Smad2-independent Ras/ERK/JNK pathways. More importantly, our findings demonstrate that km23-1 functions as a critical adaptor coupling TβR activation to activation of Ras effector pathways downstream.
Introduction
Ras family GTPases are key mediators in signaling pathways that convey extracellular signals from surface receptors to the interior of the cell. Consequently, they function as molecular switches in essential processes such as cell proliferation, differentiation, and survival (1–4). Activation of Ras is an essential step in the signaling of responses from a variety of growth factors, including TGFβ (1, 2). TGFβ can activate Ras, resulting in activation of ERK1/2, JNKs, and p38 MAPKs in different cell types (2, 5–10). We have reported that the rapid activation of Ras, ERK1/2, and JNKs by TGFβ in TGFβ-sensitive epithelial cells is required for specific TGFβ responses, including induction of its own expression (6, 8, 9, 11). Others have shown that TGFβ-stimulated ERK signaling regulates a subset of target genes, which are enriched for genes with defined roles in cell-matrix interaction, cell motility, and endocytosis (12, 13). In addition, TGFβ activation of JNK/p38 plays a very important role in TGFβ-induced epithelial-mesenchymal transition and apoptosis (12, 14). Although TGFβ activation of Ras was first reported in 1992 (15), the mechanisms underlying TGFβ activation of the Ras/MAPK pathways are not entirely understood.
The TGFβ family (TGFβ1, TGFβ2, and TGFβ3) comprises multifunctional cytokines that are differentially expressed and regulate diverse cellular processes, including cell proliferation, differentiation, apoptosis, fibrosis, wound repair, and inflammation in a wide range of target cells (10, 16, 17). TGFβ signals are transduced by a complex of TGFβ receptor II (TβRII)2 and receptor I (TβRI). The activated TβR complex phosphorylates Smad2 and Smad3, converting them into transcriptional regulators that can complex with Smad4. As mentioned above, TGFβ also uses non-Smad signaling pathways such as the ERK, JNK, and p38 MAPK pathways to convey its signals (12, 16). A major TGFβ response that is mediated primarily by non-Smad pathways is TGFβ autoinduction, an important mechanism for amplifying production of TGFβ, as well as its pleiotropic responses (18, 19).
TGFβ mediates its growth inhibitory effects in normal and transformed cells through an autocrine inhibitory loop. Because this normal growth check is often altered in human cancer cells, it is critical to understand the signaling mechanisms involved in this autoinductive process. Transcriptional autoinduction of TGFβ1 has been shown to be dependent upon activator protein-1 (AP-1) in various cell types (11, 19–21). In addition, although we have shown that TGFβ-activated Ras/JNK/ERK pathways are essential for TGFβ1 autoinduction, Smads only appeared to play an indirect role (11). Thus, although the TGFβ1 promoter complex contained JunD, Fra-2, c-Jun, and FosB, neither Smad3 nor Smad4 were detectable at the relevant TGFβ1-inducible promoter site (11). Further, because dominant-negative Smad3 was able to reduce TGFβ1 promoter transactivation, the role of Smad3 in this process was likely mediated by cross-talk with Ras/MAPKs, known to exist at several levels (2, 12, 22). Further adding to the complexity, the mechanisms underlying TGFβ1 autoregulation are distinct from those for TGFβ3, which were shown to require activation of JNK and p38, among other components (21). Overall, however, because of the critical role that TGFβ plays in human cancer development and progression, the signaling pathways mediating TGFβ autoregulation are important to explore. Any novel regulators of these pathways may represent new targets for therapeutic intervention, if they can be leveraged to block TGFβ production in human cancers that have lost the TGFβ autocrine inhibitory loop.
We have previously identified km23-1 as a TGFβ receptor-interacting protein (23), which is also known as km23 (24–28), mLC7–1 (23), Robl1 (29), DNLC2A (30), and DYNLRB1 (31). km23-1 interacts with and co-localizes in early endosomes with TβRII and Smad2 after TGFβ treatment (23, 32). Moreover, km23-1 undergoes rapid phosphorylation on serine residues after TβR activation, in keeping with the kinase specificity of the TβRs (23). Additional studies demonstrated that kinase-negative TβRs are required for km23-1 effects (23, 27). km23-1 was also required for both Smad2-dependent and Smad-independent TGFβ responses (23, 27, 32). In the current report, we describe a novel role for km23-1 in linking Ras activation by TGFβ to TGFβ autoinduction in TGFβ-sensitive epithelial cells.
MATERIALS AND METHODS
Reagents
The anti-FLAG M2 (F3165) and mouse IgG antibodies (Abs) were from Sigma-Aldrich. The anti-dynein intermediate chain (DIC) monoclonal Ab was from Chemicon (Temecula, CA). The TβRII Ab (SC-220), rabbit IgG, and protein A/G-agarose were from Santa Cruz Biotechnology (Santa Cruz, CA). TGFβ1 was purchased from R & D Systems (Minneapolis, MN). The dual-luciferase reporter assay system (E1960) was purchased from Promega (Madison, WI). The Ras activation assay kit (17-218) and anti-Ras Ab (05-516) were from Millipore. Phospho c-Jun (Ser73), Phospho-ERK1/2 (4370S), ERK1/2 (4780) phospho-SAPK/JNK, and SAPK/JNK Abs were from Cell Signaling.
Cell Culture
293T, HaCaT, and Mv1Lu (CCL-64) cells were purchased from American Type culture Collection (Manassas, VA), and were grown in DMEM supplemented with 10% FBS. The untransformed rat intestinal epithelial cells (IEC4-1) were routinely maintained in supplemented McCoy's 5A + insulin + glucose + 10% FBS medium as described previously (33). RKO human colon carcinoma cells (34) were routinely cultured in McCoy's 5A medium, supplemented with amino acids, pyruvate, antibiotics (streptomycin, penicillin), and 10% FBS. The cultures were routinely tested for mycoplasma using Hoechst 33258 staining.
siRNA Plasmid Preparation and Transfections
For transient transfections, the km23-1, km23-2, and NC siRNAs used were prepared and characterized as described previously (27, 32). The siRNA was designed in a region of km23-1 where the mink, rat, mouse, and human forms do not differ (27, 32). To generate the siRNA-resistant km23-1-FLAG construct (pFLAG-Δkm23-1), the pFLAG-km23-1 plasmid (23) was used as a template and mutated by site-directed mutagenesis PCR (Stratagene) as described previously (35). Compared with pFLAG-km23-1, there is no change in the amino acid sequence expressed by pFLAG-Δkm23-1.
For stable siRNA expression, the sense strand of the hairpin km23-1 siRNA, corresponding to nucleotides 251–271 of the human km23-1 coding region (5′-AAGACTATTTCCTGATTGTGA-3′), was inserted into the pRNATin-H1.2/hygro siRNA vector (GenScript, Piscataway, NJ) according to the manufacturer's instructions. A scrambled siRNA control (NC siRNA) was also provided by the company. These pRNATin-H1.2/hygro siRNA vectors were transfected into RKO cells using LipofectamineTM 2000 (Invitrogen) according to the manufacturer's protocol. The km23-1 siRNA, empty vector (EV), and NC siRNA RKO stable transfectants were established by selection of RKO human colon carcinoma cells on hygromycin B (200 μg/ml, 4 weeks) and cloned by limiting dilution. The km23-1 siRNA clone 1, km23-1 siRNA clone 5, EV, and NC siRNA stable transfectants were maintained in medium with 100 μg/ml of hygromycin B (20).
Luciferase Reporter Assays
The TGFβ1 phTG5-luc construct was kindly provided by Dr. S. J. Kim (36). The (−79/+170)-c-Jun reporter construct was kindly provided by Dr. X. F. Wang (37). Luciferase assays were performed as described previously using FuGENE 6 (Roche Applied Science; catalog number 11814443001) or Lipofectamine 2000 (Invitrogen) (21, 32). Renilla was used to normalize transfection efficiencies. 24 h after transfection, the cells were washed once with serum-free (SF) medium and incubated in SF medium for 1 h prior to TGFβ1 (5 ng/ml) treatment for an additional 18 h. The reporter assays were performed as described previously (20, 32).
Real Time PCR
HaCaT cells were transfected with EV, NC siRNA, km23-1 siRNA, or km23-2 siRNA and treated as for luciferase assays. RNA isolation and complementary DNA synthesis were performed as described previously (38). For real time PCR, experiments were conducted using an ABI-Prism 7700 Thermal Cycler and TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Relative expression was calculated for human km23-1 using primer/probe sets (Applied Biosystems). Primers and probes for endogenous control GAPDH and TGFβ1 were purchased from Applied Biosystems. For data analysis, raw Ct was first normalized to the housekeeping gene for each sample to obtain ΔCt. The normalized ΔCt was then calibrated to control cell samples to obtain ΔΔCt.
Electrophoretic Mobility Shift Assays
The cells were transfected with either NC siRNA or km23-1 siRNA. 48 h after transfection, the cells were cultured in SF medium for 1 h. 2 h after TGFβ1 (5 ng/ml) treatment, EMSAs were performed as described previously (20, 21). The oligonucleotides used for probe labeling were described previously (20).
Transient Transfections, IP/Blot, and Westerns
Transient Transfections, IP/blot, and Westerns were performed essentially as described previously (27, 32).
Ras Activity Assays
were carried out using a Ras activation assay kit (Millipore) according to the manufacturer's instructions. Briefly, active Ras was pulled down with purified GST-Raf-RBD agarose beads by incubating cell lysates with GST-Raf-RBD prebound to glutathione-Sepharose. Bound proteins were then subjected to 12% NuPAGE gel and blotted with an anti-Ras Ab.
Lipid Raft Fractionation
HaCaT cells were grown to 80% confluence in 100-mm dishes and were rinsed and incubated in SF medium for 1 h prior to treatment with vehicle or TGFβ1 (5 ng/ml) for 15 min. The cells were harvested for lipid raft fractionation analyses as described previously (39). Twelve 1-ml fractions were collected from the top of the tube, and aliquots of the first 10 fractions were analyzed by immunoblotting.
RESULTS
Blockade of Endogenous km23-1 Inhibits TGFβ Induction of TGFβ1 Gene Expression in HaCaT Cells
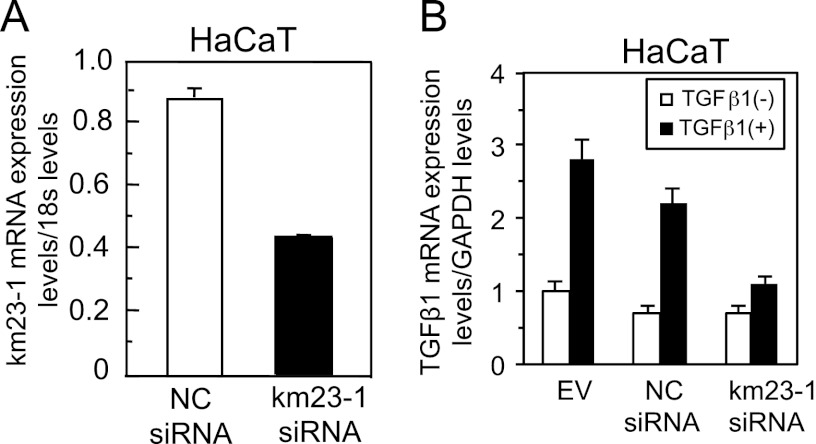
An important property of TGFβ is its ability to activate its own mRNA expression and thereby increase its own secretion (19). We have previously shown that Ras and ERK/JNK MAPK pathways are essential for TGFβ1 autoinduction (11). Furthermore, our previous results have shown that forced expression of km23-1 induces specific TGFβ responses, including an activation of JNK and a phosphorylation of c-Jun, suggesting that km23-1 might play an important role in the TGFβ/JNK pathway (27, 32). Thus, it is conceivable that the TβR-interacting km23-1 might be required for TGFβ1 production, which is an important biological response of TGFβ. HaCaT cells are seen as a human model for the response of TGFβ-sensitive epithelial cells to autocrine and paracrine TGFβ (40, 41). Accordingly, we performed real time RT-PCR analyses of TGFβ1 mRNA expression in human HaCaT cells after transiently transfecting with either EV, km23-1 siRNA, or NC siRNA. Knockdown efficiency of km23-1 was confirmed by real time RT-PCR analyses (Fig. 1A). More importantly, as shown in Fig. 1B, in the km23-1 siRNA-transfected cells, TGFβ autoinduction of TGFβ1 mRNA expression was significantly decreased. In contrast, in both EV and NC siRNA-transfected cells, TGFβ1 stimulated induction of TGFβ1 mRNA expression, confirming the presence of an active TGFβ1 auto-loop. Thus, our results indicate that the TβR-interacting protein km23-1 is required for TGFβ autoinduction of TGFβ1 mRNA expression.
FIGURE 1.
Blockade of endogenous km23-1 by siRNA inhibits TGFβ induction of TGFβ1 gene expression. A, km23-1 siRNA specifically knocks down endogenous km23-1. Real time RT-PCR analysis of km23-1 mRNA expression was performed as described previously (38). B, knockdown of km23-1 inhibits TGFβ induction of TGFβ1 gene expression. Real time RT-PCR analysis of TGFβ1 mRNA expression from HaCaT cells was performed as described under “Materials and Methods.” The data plotted are the means ± S.E. of triplicate samples from up to three independent experiments.
km23-1, but Not Smad2, Is Required TGFβ Induction of TGFβ1 Promoter Activity, Which Can Be Rescued by siRNA-resistant km23-1
Although we have previously demonstrated that TGFβ activation of Ras/MAPK pathways is critical for TGFβ induction of the proximal AP-1 site in the human TGFβ1 promoter (11), the role of km23-1 in the regulation of this promoter site has not been explored. Because our results here have shown that knockdown of km23-1 significantly inhibited TGFβ autoinduction of TGFβ1 mRNA expression, we determined whether blockade of km23-1 had any effect on TGFβ induction of TGFβ1 promoter activity. The IEC4-1 and Mv1Lu cells were used for these studies because they represent good models for TGFβ regulation in mammalian cells because of their high level of TGFβ sensitivity and their well characterized TGFβ pathways (6, 15, 42). In addition, the km23-1 siRNAs employed in these studies were previously shown to specifically knock down km23-1 expression in these cell lines (27, 32).
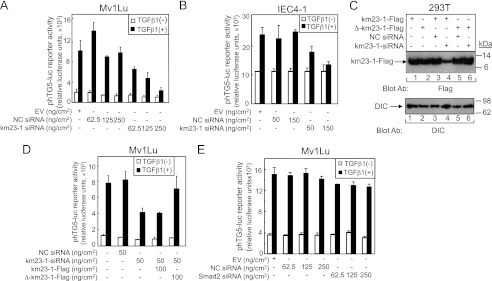
Here, we performed luciferase reporter assays after transiently transfecting Mv1Lu cells with phTG5-luc and the indicated amounts of either NC siRNA or km23-1 siRNA. The TGFβ1 promoter reporter phTG5-luc contains a 450-bp fragment encompassing the proximal AP-1 site of the human TGFβ1 promoter (11). As shown in Fig. 2A, in Mv1Lu cells, TGFβ induced a 5–7-fold increase in phTG5-luc activity when cells were transfected with EV and NC siRNAs, which is quite similar to that previously reported (11, 36). More importantly, the ability of TGFβ to activate the phTG5-luc reporter was reduced to only 3-fold when low and medium concentrations of km23-1 siRNAs (62.5 and 125 ng/cm2) were used. Transcriptional activity was further decreased to 2-fold after the high dose of km23-1 siRNA (250 ng/cm2) was applied. In contrast, although both basal and TGFβ-induced luciferase values were decreased when the concentration of NC siRNAs was increased to 125 ng/cm2, the fold induction by TGFβ was maintained at similar levels. Similarly, TGFβ induction of phTG5-luc reporter transactivation was inhibited in IEC4-1 cells transfected with km23-1 siRNAs, but there was no such effect in NC siRNA-transfected cells (Fig. 2B). Thus, our results demonstrate that km23-1 is required for TGFβ1 induction of phTG5-luc promoter reporter activity.
FIGURE 2.
km23-1, but not Smad2, is required for TGFβ induction of TGFβ1 promoter activation, which could be rescued by siRNA-resistant km23-1. A and B, km23-1 is required for TGFβ induction of TGFβ1 promoter activation in Mv1Lu cells (A) and in IEC4-1 cells (B). The cells were transfected with the indicated amounts of either NC siRNA or km23-1 siRNA along with the phTG5-luc reporter and were treated with TGFβ1 as described under “Materials and Methods.” The data plotted are the means ± S.E. of triplicate samples from three independent experiments. C, Western blot analysis to verify that expression of pFLAG-Δkm23-1 was not affected by km23-1 siRNA. 293T cells were transfected with the indicated plasmids, and Western blotting was performed with the indicated Abs. D, inhibition of TGFβ-dependent phTG5 promoter reporter activity by km23-1 knockdown could be rescued by siRNA-resistant km23-1. Mv1Lu cells were transfected with the indicated forms of either NC siRNA or km23-1-siRNA along with either pFLAG-km23-1 or pFLAG-Δkm23-1 and were treated with TGFβ1 as described under “Materials and Methods.” Luciferase reporter assays were performed as described under “Materials and Methods.” The data plotted are the means ± S.E. of triplicate samples from three independent experiments. E, blockade of Smad2 has no effect on TGFβ induction of TGFβ1 promoter activation. Mv1Lu cells were transfected with the indicated amounts of either NC siRNA or Smad2 siRNA along with the phTG5 reporter, and luciferase reporter assays were performed as described under “Materials and Methods.” The data plotted are the means ± S.E. of triplicate samples from three independent experiments.
To verify the specificity of the effects observed after gene depletion, rescue of the siRNA effect by expression of an siRNA-resistant form of the gene is often used (43). To perform such rescue experiments, we generated Δkm23-1-FLAG as an siRNA-resistant km23-1 and performed Western blot analyses after transiently transfecting 293T cells with km23-1-FLAG, siRNA-resistant Δkm23-1-FLAG (32), and either NC siRNA or km23-1 siRNA. As shown in Fig. 2C, both km23-1-FLAG (lane 1) and Δkm23-1-FLAG (lane 2) were expressed at similar levels in the absence of km23-1-siRNA. Further, as expected, NC siRNA had no effect on the expression of km23-1-FLAG (lane 3) or Δkm23-1-FLAG (lane 5). In contrast, km23-1 siRNA significantly reduced the expression of km23-1-FLAG (lane 4), but not the expression of Δkm23-1-FLAG (lane 6). Thus, our results demonstrate that expression of Δkm23-1-FLAG is resistant to km23-1 siRNA.
To confirm that km23-1 siRNA knockdown of TGFβ1 autoinduction was specifically mediated by the km23-1 siRNA, we performed rescue experiments using the phTG5-luc reporter after transiently transfecting Mv1Lu cells with the indicated forms of either NC siRNA or km23-1 siRNA, along with either km23-1-FLAG or siRNA-resistant Δkm23-1-FLAG. The results in Fig. 2D demonstrate that the inhibition of TGFβ1-dependent TGFβ1 transactivation by km23-1 knockdown could be rescued by the siRNA-resistant pCMV5-Δkm23-1-FLAG, but not by pCMV5-km23-1-FLAG. Therefore, the inhibition of TGFβ1 autoinduction was specifically mediated by the km23-1 siRNA and not by off target effects.
The Smad pathway is crucial to many aspects of the biological actions of TGFβ (16, 44). Although we have shown that the Ras/MAPKs are essential for TGFβ1 autoinduction, Smads3/4 appeared to play an indirect role because they were not present in the relevant TGFβ1 promoter complex (11). However, we had not examined the role of Smad2 in TGFβ autoinduction in our previous report. Because we have now shown that km23-1 is required for Smad2-depedent TGFβ signaling (32), here we examined whether Smad2 was required for TGFβ induction of the human TGFβ1 promoter. To this end, we performed luciferase reporter assays after transiently transfecting Mv1Lu cells with phTG5-luc and the indicated amount of either EV, NC siRNA, or Smad2 siRNA. The results in Fig. 2E demonstrate that blockade of Smad2 had no effect on TGFβ induction of the human TGFβ1 promoter. Thus, TGFβ regulation of the proximal AP-1 site in the human TGFβ1 promoter, which requires km23-1, occurs through Smad2-independent pathways. Collectively, our results demonstrate that km23-1 plays an important role in TGFβ1 autoinduction through Smad2-independent pathways.
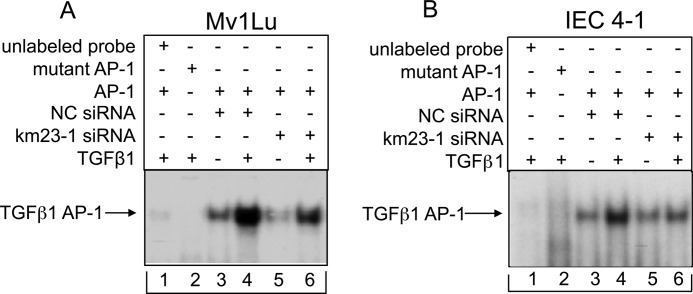
Blockade of Endogenous km23-1 Inhibits TGFβ1-inducible AP-1 Binding to the Proximal AP-1 Site in the Human TGFβ1 Promoter
Because blockade of km23-1 decreased TGFβ1 induction of phTG5-luc reporter activity, we examined whether blockade of km23-1 had any effect on AP-1 binding to the proximal AP-1 site in the human TGFβ1 promoter. Thus, we performed EMSAs using an oligonucleotide (−372 to −345), which spans the proximal AP-1 site in the TGFβ1 promoter as a probe. As shown in Fig. 3A, in the NC siRNA-transfected cells, exogenous TGFβ induced a significant increase in AP-1 binding activity (lane 3 versus lane 4). However, in the km23-1 siRNA-transfected cells, TGFβ-induced AP-1 binding activity was significantly decreased (lane 5 versus lane 6). The unlabeled and mutant AP-1 probes were used as the controls (lanes 1 and 2). We have also performed similar EMSAs in IEC4-1 cells. As shown in Fig. 3B, the binding to the proximal AP-1 site in TGFβ1 promoter was strongly induced by TGFβ in the NC siRNA-transfected cells (lane 3 versus lane 4). In contrast, the AP-1 binding activity induced by exogenous TGFβ was markedly reduced in the km23-1 siRNA transfected cells (lane 5 versus lane 6). This TGFβ-induced AP-1-DNA binding complex was completely blocked when unlabeled and mutant AP-1 probes were used as controls (lanes 1 and 2), demonstrating that the AP-1-DNA binding is specific. Thus, our results demonstrate that km23-1 is required for TGFβ-inducible AP-1-DNA complex formation at the TGFβ1 promoter.
FIGURE 3.
km23-1 depletion inhibits AP-1 binding to the proximal AP-1 site in the human TGFβ1 promoter after TGFβ1 stimulation. km23-1 knockdown inhibits AP-1 binding to the proximal AP-1 site in the human TGFβ1 promoter after TGFβ1 stimulation of Mv1Lu (A) and IEC4-1 (B) cells. The cells were transfected with siRNAs as described under “Materials and Methods,” followed by incubation in the absence or presence of TGFβ1 (5 ng/ml) for an additional 2 h. EMSAs were then performed as described under “Materials and Methods.” A 100-fold excess of unlabeled AP-1 oligonucleotide competes for TGFβ1-inducible AP-1-DNA binding (lane 1). The arrow indicates the TGFβ-inducible AP-1-DNA complex. The data are representative of three independent experiments.
The ERK Pathway Is Required for TGFβ Autoinduction and Can Be Blocked by km23-1 Depletion
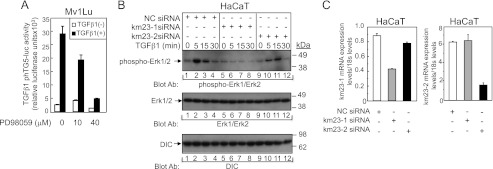
We have previously reported that TGFβ can induce AP-1 binding at the proximal AP-1 site of the TGFβ1 promoter to increase TGFβ1 expression through Ras/ERK/JNK pathways (11). To further confirm that the ERK pathway is required for TGFβ induction of TGFβ1 promoter activity, we employed PD98059 as a selective MEK inhibitor (Fig. 4A). As shown in Fig. 4A, PD98059 effectively suppressed TGFβ induction of TGFβ1 promoter activity in a dose-dependent manner, demonstrating that the MEK1/ERK pathway is indeed required for TGFβ induction of TGFβ1 promoter activity.
FIGURE 4.
The ERK pathway is required for TGFβ stimulation of TGFβ1 promoter activity, which can be inhibited by km23-1 depletion. A, the ERK pathway is required for TGFβ stimulation of TGFβ1 promoter activity. Mv1Lu cells were transfected with phTG5-Luc. 24 h after transfection, the cells were pretreated with the MEK inhibitor PD98059 at the concentrations indicated for 30 min and then incubated in the absence or presence of TGFβ1 for an additional 24 h. The cells were harvested, and luciferase assays were performed as described under “Materials and Methods.” B, blockade of km23-1 partially inhibits TGFβ activation of ERK in HaCaT cells. HaCaT cells were transiently transfected with NC, km23-1, or km23-2 siRNAs and were treated with TGFβ1 (5 ng/ml) for the indicated times. Western blotting was performed using the indicated Abs. C, km23-1 and km23-2 siRNAs selectively and specifically knockdown endogenous km23-1 and km23-2, respectively. The efficiency of km23-1 and km23-2 knockdown was confirmed by real time RT-PCR. The data are representative of three independent experiments.
Because MEK/ERK activation is upstream in the pathway for TGFβ-inducible regulation of TGFβ1 transcription and DNA binding, it was of interest to determine whether blockade of km23-1 affected TGFβ induction of ERK1/2 activity. Accordingly, we performed Western blot analyses to examine phospho-ERK1/2 expression induced by TGFβ in HaCaT cells after siRNA knockdown of km23-1. In addition, for comparison and to indicate specificity, we examined the effects of depletion of another isoform of km23, termed km23-2 (24, 38). As shown in Fig. 4B, in the NC siRNA-transfected cells, TGFβ induced a rapid increase in ERK1/2 activation (lane 2, top panel). The levels at 15–30 min after TGFβ treatment began to decrease (lanes 3 and 4, top panel). Similar results were obtained in the km23-2 siRNA-transfected cells, indicating that km23-2 was not absolutely essential for TGFβ activation of ERK1/2. In contrast, in the km23-1 siRNA-transfected cells, phospho-ERK levels were significantly decreased at all time points after TGFβ treatment (lanes 5–8, top panel). Total ERK1/2 expression was confirmed by Western blot analyses, as shown in the middle panel. The expression of DIC was used as a loading control. Knockdown efficiency of km23-1 and km23-2 in these cells was confirmed by real time RT-PCR (Fig. 4C). Thus, our results demonstrate that km23-1, but not km23-2, is specifically required for TGFβ activation of ERK in TGFβ-sensitive cells.
The JNK/Jun Pathway Is Required for TGFβ Stimulation of TGFβ1 Promoter Activity and Can Be Inhibited by km23-1 Depletion
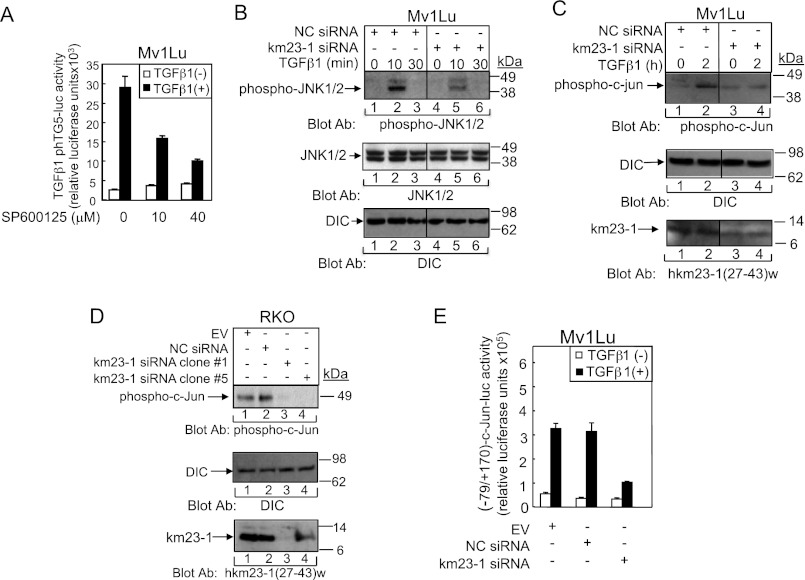
Our previous results have shown that overexpression of dominant-negative MAPK kinase 4 significantly inhibited TGFβ3 induction of TGFβ1 mRNA expression in IEC4-1 cells, suggesting that TGFβ activation of JNK is required for TGFβ autoinduction (11). To confirm whether the JNK pathway was required for TGFβ1 induction of TGFβ1 promoter activity in Mv1Lu cells, we employed the pharmacological JNK-selective inhibitor SP600125 (Fig. 5A). As shown in Fig. 5A, SP600125 effectively suppressed TGFβ1 induction of TGFβ1 promoter activity in a dose-dependent manner, demonstrating that JNK activity is also required for TGFβ1 induction of TGFβ1 promoter activity.
FIGURE 5.
The JNK/Jun pathway is required for TGFβ stimulation of TGFβ1 promoter activity, which can be inhibited by km23-1 depletion. A, the JNK pathway is required for TGFβ stimulation of TGFβ1 promoter activity. Studies were performed as for Fig. 4A, except that the cells were treated with the JNK inhibitor SP600125. B, blockade of km23-1 partially inhibits TGFβ activation of JNK in Mv1Lu cells. siRNA-transfected Mv1Lu cells, as described under “Materials and Methods,” were incubated in the absence or presence of TGFβ1 (5 ng/ml) for the indicated times. Western blotting was performed using the indicated Abs. Middle panel, DIC was used as a loading control. The bottom panel confirms knockdown of endogenous km23-1. The data are representative of three independent experiments. C, blockade of km23-1 decreases TGFβ induction of c-Jun phosphorylation in Mv1Lu cells. Studies were performed as for B except using Jun Abs. D, c-Jun phosphorylation was significantly suppressed in RKO cells stably transfected with km23-1 siRNA. The RKO cells and the indicated siRNA stable transfectant cells were grown and harvested for Western blotting using the indicated Abs as described under “Materials and Methods.” The bottom panel demonstrates stable knockdown of km23-1 in the two clones (clones 1 and 5). E, km23-1 is required for TGFβ induction of a Smad3/Jun-dependent promoter. Mv1Lu cells were transfected with the indicated constructs along with the (−79/+170)-c-Jun reporter, and luciferase reporter assays were performed. The data are representative of three independent experiments.
Next, we performed Western blot analyses to examine phospho-JNK1/2 expression levels induced by TGFβ in Mv1Lu cells after siRNA knockdown of km23-1. As expected, in the NC siRNA-transfected cells, TGFβ induced a rapid increase in phospho-JNK1/2 expression (Fig. 5B, top panel, lane 2). However, in the km23-1 siRNA-transfected cells, TGFβ induction of phospho-JNK1/2 was significantly decreased (lanes 5 and 6, top panel). Total JNK1/2 expression was confirmed by Western blot analyses, as shown in the middle panel. DIC was used as a loading control in the bottom panel as described previously (45). Similar results were also obtained in HaCaT cells (data not shown). Thus, km23-1 is required for TGFβ activation of JNK1/2 in TGFβ-sensitive epithelial cells.
Because JNKs are the main upstream kinases for Jun phosphorylation (46), we next tested whether depletion of km23-1 had any effect on c-Jun phosphorylation after TGFβ activation. As shown in Fig. 5C, in the NC siRNA-transfected cells, TGFβ induced a rapid increase in phospho-c-Jun expression (lane 2). In contrast, in the km23-1 siRNA-transfected cells, TGFβ induction of phospho-c-Jun was significantly decreased (lane 4). Thus, km23-1 is required for phosphorylation of c-Jun after TGFβ stimulation in Mv1Lu cells. Because this TGFβ-sensitive cell model employed transiently transfected km23-1 siRNAs, to provide further evidence of a km23-1 requirement for AP-1-dependent TGFβ autoinduction, we performed phosphoblotting for c-Jun in a model system stably expressing km23-1-siRNAs (Fig. 5D). The RKO cells used displayed constitutive phTG5-luc reporter transactivation and produced high levels of TGFβ1, making exogenous TGFβ unnecessary (20). As shown in Fig. 5D, the EV and NC siRNA stably transfected cells displayed constitutive phosphorylation of c-Jun (lanes 1 and 2). In contrast, in RKO cells stably transfected with km23-1 siRNA, the phosphorylation of c-Jun was significantly suppressed (lanes 3 and 4). Equal loading was confirmed using DIC, and stable km23-1 knockdown was confirmed using a km23-1-specific Ab (27) (bottom panel) as used in Fig. 5C. Thus, our results further confirm that km23-1 is required for TGFβ activation of AP-1-dependent events previously shown to be essential for TGFβ1 production.
Previous reports have suggested synergies among AP-1 components and Smad proteins for mediating various TGFβ-inducible responses (37). Although we have ruled out an effect of Smad2 in TGFβ1 autoinduction (Fig. 2E), we had previously found an indirect role for Smad3 in mediating TGFβ3 regulation of TGFβ1 expression, presumably through cross-talk among MAPKs and/or AP-1 components (11). For example, Smad3 has been shown to synergize with Jun family members in regulating c-Jun transcription (37). Because we showed that km23-1 is required for TGFβ activation of JNK/c-Jun, we chose to use a portion of the c-Jun promoter (−79/+170), previously shown to contain both AP-1 and Smad-binding element motifs that are regulated by TGFβ-inducible effects on Jun and Smad3 (37). As shown in Fig. 5E, in both the EV and NC siRNA-transfected cells, (−79/+170)-c-Jun luciferase activity was induced by TGFβ by ∼6- and 9-fold, respectively. In contrast, in the km23-1 siRNA-transfected cells, TGFβ induction of (−79/+170)-c-Jun reporter activity was significantly decreased compared with that in the NC siRNA-transfected cells (to levels of only 3-fold). Thus, km23-1 is required for TGFβ induction of a Smad3-/Jun-dependent promoter known to be involved in TGFβ1 autoinduction. More importantly, our results demonstrate that km23-1 depletion can block even TGFβ-inducible events requiring Smad3.
TGFβ Regulates Complex Formation between Ras, km23-1, and RII, and km23-1 Depletion Inhibits Ras Activation by TGFβ
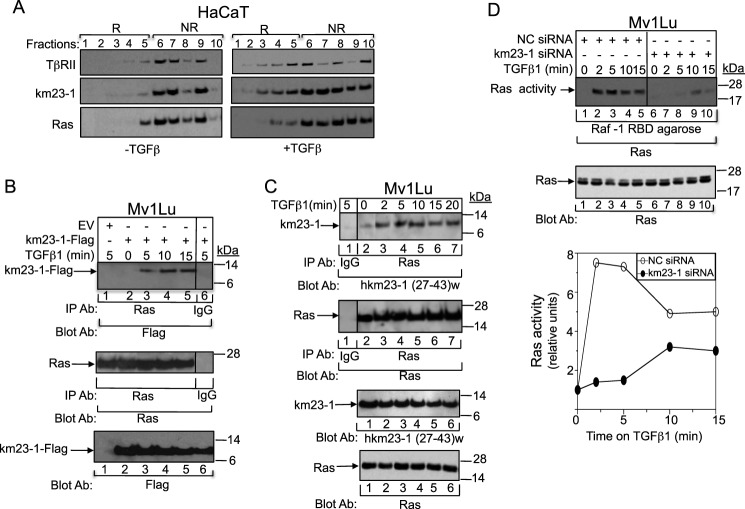
It is well documented that TβRs are endocytosed via both clathrin-coated vesicles and cholesterol-rich membrane microdomain lipid rafts/caveolae vesicles (47). In addition, a previous report has shown that lipid rafts are required for TGFβ-mediated MAPK activation (39). Here our results have suggested that the TβR-interacting protein km23-1 regulates both JNK and ERK pathways, suggesting that TGFβ-inducible events upstream of both of these cascades might also require km23-1. Because Ras is one such component and is known to be localized to lipid rafts (48), it was of interest to examine whether endogenous km23-1 might be co-localized with Ras in lipid rafts in the presence of TGFβ. Accordingly, we performed sucrose gradients to separate lipid raft from non-raft membrane compartments, followed by Western blotting with km23-1 antiserum, Ras, and TβRII. Immunoblotting for endogenous caveolin-1, a marker of membrane rafts, and endogenous EEA1, a marker of non-raft membrane compartments, was performed to identify the correct fractions corresponding to the raft versus non-raft compartments (marked as R or NR in Fig. 6A). Membrane rafts were concentrated in fractions 1–5, and non-raft membrane compartments were concentrated in fractions 6–10. As shown in Fig. 6A, in the absence of TGFβ (left panel), the majority of TβRII (top panel) was present in the non-raft fractions as expected (fractions 6–9). However, TGFβ treatment caused notable receptor redistribution by shifting TβRII to lipid rafts (fractions 1–5) (right panel), consistent with a previous report (39). In terms of km23-1 localization (middle panel), in the absence of TGFβ (left panel), the majority of km23-1 accumulated in non-raft membranes (fractions 6–9). However, as early as 15 min after TGFβ addition (right panel), a portion of km23-1 was present in lipid raft fractions (fractions 1–5). Similarly, in the absence of TGFβ (left panel), the majority of Ras was localized in nonlipid raft fractions (fractions 6–9), whereas TGFβ treatment caused a portion of Ras to shift to the lipid rafts. Thus, both endogenous km23-1 and Ras are present in raft membranes together with TβRII in the presence of TGFβ in TGFβ-sensitive cells.
FIGURE 6.
km23-1 is required for Ras activation by TGFβ, with TβRII, km23-1, and Ras being co-localized in lipid rafts after TGFβ treatment. A, TβRII, km23-1, and Ras are co-localized in lipid rafts after TGFβ treatment. HaCaT cells were incubated with SF medium for 30 min, followed by incubation in the absence (left panel) or presence of TGFβ (5 ng/ml) for 15 min (right panel). The cell lysates were subjected to sucrose density gradient centrifugation, and endogenous proteins from each sucrose fraction were analyzed by immunoblotting. R indicates lipid raft compartment, and NR indicates non-raft membrane compartment. The results shown are representative of two similar experiments. B, exogenous km23-1 interacts with endogenous Ras in a TGFβ-dependent manner. Mv1lu cells were transiently transfected with either EV (lane 1) or km23-1-FLAG (lanes 2–7) and were treated with TGFβ1 (5 ng/ml) for the indicated times. Top panel, cell lysates were subjected to IP/blot analyses using a Ras Ab or IgG (control) as the IP Ab and a FLAG Ab as the blotting Ab. The same membrane was reblotted with anti-Ras to show equal loading and expression of endogenous Ras (middle panel). Bottom panel, Western blot analysis to demonstrate equal loading and expression of km23-1-FLAG. C, endogenous km23-1 interacts with endogenous Ras in a TGFβ-dependent manner. Studies were performed as for B except that km23-1-FLAG was not transfected and a km23-1 specific Ab was used (27). The data are representative of three independent experiments. D, blockade of km23-1 resulted in an inhibition of Ras activation by TGFβ. Mv1Lu cells were transiently transfected with either NC siRNA or km23-1 siRNA and were treated with TGFβ1 (5 ng/ml) for the indicated times. Top and middle panels, Ras activity assays were performed as described under “Materials and Methods.” Bottom panel, plot of densitometry scans of results in top panel. The data are representative of three independent experiments.
Because km23-1 is co-localized with RII and endogenous Ras in the raft membrane fractions after TGFβ endocytosis, we examined whether km23-1 would interact with Ras after TGFβ treatment. Accordingly, we performed IP/blot analyses, using anti-Ras as the IP Ab and anti-FLAG as the blotting Ab, after transiently transfecting Mv1Lu cells with human km23-1-FLAG in the absence and presence of TGFβ. As shown in Fig. 6B, TGFβ stimulated a rapid interaction of km23-1 with Ras, occurring as early as 2 min after TGFβ treatment (lanes 3 and 6). Expression of EV only (lane 1) and the IgG control (lane 7) indicated that the interaction noted is specific for km23-1. Equal loading and expression of endogenous Ras was confirmed by reprobing with an anti-Ras Ab (middle panel). Equal expression of km23-1-FLAG was confirmed by Western blot analyses with an anti-FLAG Ab (bottom panel). Thus, our results demonstrate that km23-1 interacts with Ras in a TGFβ- and time-dependent manner in TGFβ-sensitive epithelial cells.
To ensure that the interaction was not the result of overexpression of km23-1, we examined whether endogenous km23-1 and endogenous Ras were present in the same complex after TGFβ stimulation. To assess this, we performed IP/blot analyses in the absence or presence of TGFβ without primary km23-1-FLAG transfection. As shown in Fig. 6C, there was minimal interaction between km23-1 and Ras in the absence of TGFβ (lane 1). However, TGFβ induced a rapid interaction of km23-1 with Ras at 2–20 min after TGFβ treatment (lanes 3–7, top panel). In contrast, the association between km23-1 and Ras was significantly decreased at 30 min after TGFβ addition (lane 8). The IgG control was negative (lane 2, top panel). Equal loading and expression of endogenous Ras and endogenous km23-1 were confirmed by Western blotting as shown in the middle panels. Collectively, our results indicate for the first time that TGFβ regulates the interaction of km23-1 with Ras in vivo in a time-dependent manner, suggesting that km23-1 plays an important role in TGFβ-dependent Ras/MAPK signaling events.
Because TGFβ regulated the interaction of km23-1 with Ras in vivo in a time-dependent manner, we determined whether blockade of km23-1 would have any effect on TGFβ-dependent Ras activation. Accordingly, we transiently transfected Mv1Lu cells with either km23-1 siRNA or NC siRNA and then performed Ras activation assays in the absence and presence of TGFβ. As expected for the NC siRNA-transfected cells, TGFβ rapidly stimulated Ras activation (Fig. 6D, lanes 1–5). However, in the km23-1 siRNA-transfected cells, Ras activation was decreased at all time points after TGFβ treatment (Fig. 6D, lanes 6–10), with respect to those for the NC siRNA. Equal loading and expression of total Ras was confirmed by Western blotting in the middle panel. The results were scanned by densitometry and are expressed graphically in the bottom panel. Thus, we show for the first time that km23-1 is required for Ras activation by TGFβ.
DISCUSSION
TGFβ1 has been reported to autoregulate its own mRNA expression, resulting in increased secretion of the peptide (18, 19). In TGFβ-responsive cells, such autoinduction can amplify the growth inhibitory effects of TGFβ in an autocrine fashion (2). However, in late stage solid tumors, which have lost growth inhibitory responsiveness to TGFβ, the TGFβ produced can result in enhanced tumor progression, largely mediated through the paracrine effects of TGFβ (2). Therefore, any therapeutic approach that can block TGFβ production should block the pro-oncogenic effects of TGFβ. Along these lines, our previous work in human colon carcinoma cells has shown that c-Fos is a critical target for blocking TGFβ1 secretion, the associated paracrine tumor-enhancing effects, and tumor progression in vivo (20, 49). In this report, we have shown that inhibition of km23-1 decreased both TGFβ induction of the human TGFβ1 promoter and TGFβ1 gene expression. Therefore, targeting km23-1 may represent one approach for blocking tumor progression by reducing TGFβ1 in the tumor microenvironment in vivo.
We have previously reported that the TGFβ receptor-interacting protein km23-1 plays an important role in TGFβ signal transduction in TGFβ-sensitive epithelial cells, including its requirement in Smad2-dependent signaling (27, 32). However, in these studies the TβRII-km23-1-Smad2 complexes were found in early endosomes and not in lipid raft compartments as we show here for km23-1 and Ras. Here we report that km23-1 is present in lipid rafts with Ras after TβRII endocytosis and that knockdown of km23-1 reduces TGFβ activation of Ras and of both ERK and JNK effector pathways downstream. Further, we demonstrate for the first time that blockade of Smad2 has no effect on TGFβ-inducible regulation of human TGFβ1 promoter transactivation in TGFβ-sensitive cells, indicating that Smad2 is not required for TGFβ1 production. This is consistent with previous work using fibroblasts from Smad2 and Smad3 knock-out mice demonstrating that TGFβ1 autoinduction was Smad3-dependent but did not involve Smad2 (50). Collectively, our results indicate for the first time that the TβR-interacting protein km23-1 is required for TGFβ1 autoinduction through Ras- and JNK/ERK-dependent pathways that do not involve Smad2.
Cooperative actions of MAPKs and Smad pathways have previously been implicated in mediating TGFβ responses (2, 11, 12). In particular, previous results have shown that many levels of cross-talk exist between JNK and Smad3 signaling. For example, JNK-mediated phosphorylation of Smad3 outside the SSXS motif enhances Smad3 nuclear translocation and transcriptional activity in response to TGFβ (51). In addition to this direct regulation of Smad3 by JNK activity, TGFβ-activated Smad3 can physically interact with and/or cooperate with Jun family transcription factors to activate transcription of TGFβ target genes (37, 52). Because we now show that blockade of km23-1 inhibits JNK activation by TGFβ, an inhibition of the phosphorylation, nuclear translocation, and subsequent AP-1-dependent effects of Smad3 would be expected. Therefore, although cross-talk between Smad3 and JNK/c-Jun activation appears to play a role in TGFβ autoinduction, km23-1 depletion also appears to inhibit these cooperative effects (Fig. 5, B–E).
km23-1 was originally described in TGFβ signaling by its ability to interact with the TβR complex in TGFβ-sensitive epithelial cells (23). Our previous results have shown that TGFβ rapidly regulates the interaction of km23-1 with TβRII (23). However, km23-1 also functions as a dynein light chain that can recruit signaling cargoes for intracellular transport (23, 32). In this regard, cytoplasmic dynein is a motor complex that transports membrane vesicles (i.e., endosomes, lysosomes) and diverse motor cargoes along microtubules to the minus ends (53). In addition to binding DIC at distinct regions, dynein light chains have been shown to directly interact with a number of proteins to exert diverse functions (53). Our results here have shown that TGFβ leads to the recruitment of Ras to km23-1 in a rapid, TGFβ-inducible manner and that km23-1 is essential for TGFβ activation of Ras. Thus, km23-1 appears to be functioning as a novel adaptor linking TβRs to Ras activation after TGFβ treatment.
Of particular interest with regard to the role of km23-1 as a Ras adaptor are recent reports of a structural homolog of km23-1 in bacteria (MglB) that interacts with a Ras-like small G protein (MglA) (54). km23-1 is actually part of an ancient superfamily that is widely represented in archaea and bacteria (55). This superfamily appears to be involved in regulating NTPase activity. By analogy to MglA/B, km23-1 would be expected to regulate the activity and biological functions of Ras family proteins. In contrast to km23-1, however, MglB functions as a GTPase-activating protein that regulates MglA in bacteria (54, 56, 57). Given the higher evolutionary level of km23-1, many of its functions would be expected to be far more complex than those of its bacterial counterparts, presumably involving additional positive and negative regulatory factors. Future studies will likely reveal other Ras family members, effectors, and biological processes that are regulated by km23-1.
In addition to the analogous role of km23-1 as a Ras-binding partner with respect to MglA-MglB, other TβR-interacting proteins have been described and shown to activate Ras/MAPK pathways downstream. For example, the TβRII-interacting protein Daxx has been shown to act as an adaptor linking activated TβRs to JNK activation by TGFβ (58). In addition, it has been reported that TNF receptor-associated protein 6 and TAK1 interact with TβRs and function as adaptors in the TGFβ activation of p38 and JNK (35, 59). Previous results have also shown that ShcA acts as a direct link between TGFβ stimulation and ERK signaling (60, 61). However, in contrast to our results here for km23-1, the adaptor protein ShcA was shown to associate specifically and more efficiently with TβRI than with TβRII (60). Because km23-1 preferentially associates with TβRII (23, 27), and our results herein have shown that km23-1 is required for TGFβ induction of ERK, km23-1 may function upstream of the ShcA-mediated activation of ERK. Overall, however, from the data presented here, km23-1 appears to be a novel linker between TβR activation and Ras signaling.
In summary, the TGFβ receptor-interacting protein km23-1 plays a novel role in Ras/MAPK signaling in TGFβ-sensitive epithelial cells via the following mechanism. In the absence of TGFβ, both Ras and km23-1 are present in non-raft membrane compartments. Once TβRs are activated and internalized, km23-1 acts as an adaptor linking TβRs to Ras. In membrane rafts, TGFβ activates Ras, followed by activation of both JNK and ERK. These cascades, in turn, stimulate Ras/MAPK-mediated TGFβ responses, such as AP-1-dependent transcriptional events. Here we have provided evidence of TGFβ-autoinducible regulation of the AP-1 site in the TGFβ1 promoter to induce TGFβ1 gene expression as an example of one TGFβ response that is regulated by this mechanism. Although Smad2 was not involved in this pathway, the indirect role of Smad3 was also abrogated by the inhibition of km23-1. Overall, then, using TGFβ1 autoinduction as a representative TGFβ-activated Ras/MAPK response, we have shown for the first time that inhibition of the TβR-interacting protein km23-1 can be used as a strategy to curtail Ras/MAPK/AP-1-mediated events induced by TGFβ.
This work was supported, in whole or in part, by National Institutes of Health Grants CA090765, CA092889, CA100239, and CA092889-08S1 (to K. M. M.).
- TβR
- TGFβ receptor
- AP-1
- activator protein-1
- Ab
- antibody
- IP
- immunoprecipitation
- SF
- serum-free
- DIC
- dynein intermediate chain
- EV
- empty vector
- NC
- negative control.
REFERENCES
- 1. Malumbres M., Barbacid M. (2003) RAS oncogenes. The first 30 years. Nat. Rev. Cancer 3, 459–465 [DOI] [PubMed] [Google Scholar]
- 2. Mulder K. M. (2000) Role of Ras and MAPKs in TGFβ signaling. Cytokine Growth Factor Rev. 11, 23–35 [DOI] [PubMed] [Google Scholar]
- 3. Young A., Lyons J., Miller A. L., Phan V. T., Alarcón I. R., McCormick F. (2009) Ras signaling and therapies. Adv. Cancer Res. 102, 1–17 [DOI] [PubMed] [Google Scholar]
- 4. Vigil D., Cherfils J., Rossman K. L., Der C. J. (2010) Ras superfamily GEFs and GAPs. Validated and tractable targets for cancer therapy? Nat. Rev. Cancer 10, 842–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 6. Hartsough M. T., Mulder K. M. (1995) Transforming growth factor β activation of p44 MAPK in proliferating cultures of epithelial cells. J. Biol. Chem. 270, 7117–7124 [DOI] [PubMed] [Google Scholar]
- 7. Hartsough M. T., Mulder K. M. (1997) Transforming growth factor-β signaling in epithelial cells. Pharmacol. Ther. 75, 21–41 [DOI] [PubMed] [Google Scholar]
- 8. Frey R. S., Mulder K. M. (1997) Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor β in the negative growth control of breast cancer cells. Cancer Res. 57, 628–633 [PubMed] [Google Scholar]
- 9. Frey R. S., Mulder K. M. (1997) TGFβ regulation of mitogen-activated protein kinases in human breast cancer cells. Cancer Lett. 117, 41–50 [DOI] [PubMed] [Google Scholar]
- 10. Kang J. S., Liu C., Derynck R. (2009) New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 19, 385–394 [DOI] [PubMed] [Google Scholar]
- 11. Yue J., Mulder K. M. (2000) Requirement of Ras/MAPK pathway activation by transforming growth factor β for transforming growth factor β 1 production in a Smad-dependent pathway. J. Biol. Chem. 275, 30765–30773 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y. E. (2009) Non-Smad pathways in TGF-β signaling. Cell Res. 19, 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu J., Lamouille S., Derynck R. (2009) TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahimi R. A., Leof E. B. (2007) TGF-β signaling. A tale of two responses. J. Cell Biochem. 102, 593–608 [DOI] [PubMed] [Google Scholar]
- 15. Mulder K. M., Morris S. L. (1992) Activation of p21ras by transforming growth factor β in epithelial cells. J. Biol. Chem. 267, 5029–5031 [PubMed] [Google Scholar]
- 16. Yue J., Mulder K. M. (2001) Transforming growth factor-β signal transduction in epithelial cells. Pharmacol. Ther. 91, 1–34 [DOI] [PubMed] [Google Scholar]
- 17. Padua D., Massagué J. (2009) Roles of TGFβ in metastasis. Cell Res. 19, 89–102 [DOI] [PubMed] [Google Scholar]
- 18. Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. (1988) Transforming growth factor β1 positively regulates its own expression in normal and transformed cells. J. Biol. Chem. 263, 7741–7746 [PubMed] [Google Scholar]
- 19. Kim S. J., Angel P., Lafyatis R., Hattori K., Kim K. Y., Sporn M. B., Karin M., Roberts A. B. (1990) Autoinduction of transforming growth factor β1 is mediated by the AP-1 complex. Mol. Cell Biol. 10, 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu G., Ding W., Liu X., Mulder K. M. (2006) c-Fos is required for TGFβ1 production and the associated paracrine migratory effects of human colon carcinoma cells. Mol. Carcinog. 45, 582–593 [DOI] [PubMed] [Google Scholar]
- 21. Liu G., Ding W., Neiman J., Mulder K. M. (2006) Requirement of Smad3 and CREB-1 in mediating transforming growth factor-β (TGF β) induction of TGF β3 secretion. J. Biol. Chem. 281, 29479–29490 [DOI] [PubMed] [Google Scholar]
- 22. Chapnick D. A., Warner L., Bernet J., Rao T., Liu X. (2011) Partners in crime. The TGFβ and MAPK pathways in cancer progression. Cell Biosci. 1, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang Q., Staub C. M., Gao G., Jin Q., Wang Z., Ding W., Aurigemma R. E., Mulder K. M. (2002) A novel transforming growth factor-β receptor-interacting protein that is also a light chain of the motor protein dynein. Mol. Biol. Cell 13, 4484–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ilangovan U., Ding W., Zhong Y., Wilson C. L., Groppe J. C., Trbovich J. T., Zúñiga J., Demeler B., Tang Q., Gao G., Mulder K. M., Hinck A. P. (2005) Structure and dynamics of the homodimeric dynein light chain km23. J. Mol. Biol. 352, 338–354 [DOI] [PubMed] [Google Scholar]
- 25. Kang H. C., Kim I. J., Kim K., Yoon H. J., Jang S. G., Park J. G. (2007) km23, a transforming growth factor-β signaling component, is infrequently mutated in human colorectal and gastric cancers. Cancer Genet. Cytogenet. 175, 173–174 [DOI] [PubMed] [Google Scholar]
- 26. Campbell I. G., Phillips W. A., Choong D. Y. (2006) Genetic and epigenetic analysis of the putative tumor suppressor km23 in primary ovarian, breast, and colorectal cancers. Clin. Cancer Res. 12, 3713–3715 [DOI] [PubMed] [Google Scholar]
- 27. Jin Q., Ding W., Staub C. M., Gao G., Tang Q., Mulder K. M. (2005) Requirement of km23 for TGFβ-mediated growth inhibition and induction of fibronectin expression. Cell Signal 17, 1363–1372 [DOI] [PubMed] [Google Scholar]
- 28. Ding W., Mulder K. M. (2004) km23. A novel TGFβ signaling target altered in ovarian cancer. Cancer Treat. Res. 119, 315–327 [DOI] [PubMed] [Google Scholar]
- 29. Nikulina K., Patel-King R. S., Takebe S., Pfister K. K., King S. M. (2004) The Roadblock light chains are ubiquitous components of cytoplasmic dynein that form homo- and heterodimers. Cell Motil. Cytoskeleton 57, 233–245 [DOI] [PubMed] [Google Scholar]
- 30. Jiang J., Yu L., Huang X., Chen X., Li D., Zhang Y., Tang L., Zhao S. (2001) Identification of two novel human dynein light chain genes, DNLC2A and DNLC2B, and their expression changes in hepatocellular carcinoma tissues from 68 Chinese patients. Gene 281, 103–113 [DOI] [PubMed] [Google Scholar]
- 31. Pfister K. K., Fisher E. M., Gibbons I. R., Hays T. S., Holzbaur E. L., McIntosh J. R., Porter M. E., Schroer T. A., Vaughan K. T., Witman G. B., King S. M., Vallee R. B. (2005) Cytoplasmic dynein nomenclature. J. Cell Biol. 171, 411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jin Q., Ding W., Mulder K. M. (2007) Requirement for the dynein light chain km23-1 in a Smad2-dependent transforming growth factor-β signaling pathway. J. Biol. Chem. 282, 19122–19132 [DOI] [PubMed] [Google Scholar]
- 33. Mulder K. M., Segarini P. R., Morris S. L., Ziman J. M., Choi H. G. (1993) Role of receptor complexes in resistance or sensitivity to growth inhibition by TGF β in intestinal epithelial cell clones. J. Cell Physiol. 154, 162–174 [DOI] [PubMed] [Google Scholar]
- 34. Mulder K. M., Brattain M. G. (1989). Growth factor expression and response in human colon carcinoma cell, in The Cell and Molecular Biology of Colon Cancer (Augenlicht L., ed) pp. 45–67, LRC Press, Boca Raton, FL [Google Scholar]
- 35. Lassus P., Rodriguez J., Lazebnik Y. (2002) Confirming specificity of RNAi in mammalian cells. Sci. STKE 2002, pl13. [DOI] [PubMed] [Google Scholar]
- 36. Kim S. J., Glick A., Sporn M. B., Roberts A. B. (1989) Characterization of the promoter region of the human transforming growth factor-β1 gene. J. Biol. Chem. 264, 402–408 [PubMed] [Google Scholar]
- 37. Wong C., Rougier-Chapman E. M., Frederick J. P., Datto M. B., Liberati N. T., Li J. M., Wang X. F. (1999) Smad3-Smad4 and AP-1 complexes synergize in transcriptional activation of the c-Jun promoter by transforming growth factor β. Mol. Cell Biol. 19, 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin Q., Gao G., Mulder K. M. (2009) Requirement of a dynein light chain in TGFβ/Smad3 signaling. J. Cell Physiol. 221, 707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zuo W., Chen Y. G. (2009) Specific activation of mitogen-activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Mol. Biol. Cell 20, 1020–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kato M., Ishizaki A., Hellman U., Wernstedt C., Kyogoku M., Miyazono K., Heldin C. H., Funa K. (1995) A human keratinocyte cell line produces two autocrine growth inhibitors, transforming growth factor-β and insulin-like growth factor binding protein-6, in a calcium- and cell density-dependent manner. J. Biol. Chem. 270, 12373–12379 [DOI] [PubMed] [Google Scholar]
- 41. Liu C., Xu P., Lamouille S., Xu J., Derynck R. (2009) TACE-mediated ectodomain shedding of the type I TGF-β receptor downregulates TGF-β signaling. Mol. Cell 35, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yue J., Sun B., Liu G., Mulder K. M. (2004) Requirement of TGF-β receptor-dependent activation of c-Jun N-terminal kinases (JNKs)/stress-activated protein kinases (SAPKs) for TGF-β up-regulation of the urokinase-type plasminogen activator receptor. J. Cell Physiol. 199, 284–292 [DOI] [PubMed] [Google Scholar]
- 43. Cullen B. R. (2006) Enhancing and confirming the specificity of RNAi experiments. Nat. Methods 3, 677–681 [DOI] [PubMed] [Google Scholar]
- 44. Derynck R., Zhang Y. (1996) Intracellular signalling. The mad way to do it. Curr. Biol. 6, 1226–1229 [DOI] [PubMed] [Google Scholar]
- 45. Pulipati N. R., Jin Q., Liu X., Sun B., Pandey M. K., Huber J. P., Ding W., Mulder K. M. (2011) Overexpression of the dynein light chain km23-1 in human ovarian carcinoma cells inhibits tumor formation in vivo and causes mitotic delay at prometaphase/metaphase. Int. J. Cancer 129, 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morton S., Davis R. J., McLaren A., Cohen P. (2003) A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 22, 3876–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le Roy C., Wrana J. L. (2005) Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112–126 [DOI] [PubMed] [Google Scholar]
- 48. Prior I. A., Harding A., Yan J., Sluimer J., Parton R. G., Hancock J. F. (2001) GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3, 368–375 [DOI] [PubMed] [Google Scholar]
- 49. Pandey M. K., Liu G., Cooper T. K., Mulder K. M. (2012) Knockdown of c-Fos suppresses the growth of human colon carcinoma cells in athymic mice. Int. J. Cancer 130, 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Piek E., Ju W. J., Heyer J., Escalante-Alcalde D., Stewart C. L., Weinstein M., Deng C., Kucherlapati R., Bottinger E. P., Roberts A. B. (2001) Functional characterization of transforming growth factor β signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 276, 19945–19953 [DOI] [PubMed] [Google Scholar]
- 51. Engel M. E., McDonnell M. A., Law B. K., Moses H. L. (1999) Interdependent SMAD and JNK signaling in transforming growth factor-β-mediated transcription. J. Biol. Chem. 274, 37413–37420 [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y., Feng X. H., Derynck R. (1998) Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature 394, 909–913 [DOI] [PubMed] [Google Scholar]
- 53. Kardon J. R., Vale R. D. (2009) Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 10, 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miertzschke M., Koerner C., Vetter I. R., Keilberg D., Hot E., Leonardy S., Søgaard-Andersen L., Wittinghofer A. (2011) Structural analysis of the Ras-like G protein MglA and its cognate GAP MglB and implications for bacterial polarity. EMBO J. 30, 4185–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koonin E. V., Aravind L. (2000) Dynein light chains of the Roadblock/LC7 group belong to an ancient protein superfamily implicated in NTPase regulation. Curr. Biol. 10, R774–R776 [DOI] [PubMed] [Google Scholar]
- 56. Zhang Y., Franco M., Ducret A., Mignot T. (2010) A bacterial Ras-like small GTP-binding protein and its cognate GAP establish a dynamic spatial polarity axis to control directed motility. PLoS Biol. 8, e1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leonardy S., Miertzschke M., Bulyha I., Sperling E., Wittinghofer A., Søgaard-Andersen L. (2010) Regulation of dynamic polarity switching in bacteria by a Ras-like G-protein and its cognate GAP. EMBO J. 29, 2276–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Perlman R., Schiemann W. P., Brooks M. W., Lodish H. F., Weinberg R. A. (2001) TGF-β-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell Biol. 3, 708–714 [DOI] [PubMed] [Google Scholar]
- 59. Yamashita M., Fatyol K., Jin C., Wang X., Liu Z., Zhang Y. E. (2008) TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol. Cell 31, 918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee M. K., Pardoux C., Hall M. C., Lee P. S., Warburton D., Qing J., Smith S. M., Derynck R. (2007) TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 26, 3957–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lin S., Yu L., Yang J., Liu Z., Karia B., Bishop A. J., Jackson J., Lozano G., Copland J. A., Mu X., Sun B., Sun L. Z. (2011) Mutant p53 disrupts role of ShcA protein in balancing Smad protein-dependent and -independent signaling activity of transforming growth factor-β (TGF-β). J. Biol. Chem. 286, 44023–44034 [DOI] [PMC free article] [PubMed] [Google Scholar]