Abstract
A subset of neoplastic cells within human high-grade gliomas has features associated with stem cells. These cells may sustain glioma growth, and their stem-like properties may confer resistance to standard glioma treatments. Whether glioma stem cells derive from indigenous neural stem cells (NSC), or from tumor cells that have re-acquired stem cell-like properties, is unknown. However, signaling pathways that are tightly regulated and central to NSC biology, including the Ras/Raf/Erk pathway, are hyperactive and pathogenic in gliomagenesis. Furthermore, data in animal models suggests that, in some cases, tumors are initiated in the subventricular zone (SVZ), a stem/progenitor cell niche in the mature brain. We activated oncogenic K-ras in mouse glioneuronal precursor cells and adult SVZ cells using GFAP-Cre. GFAP-Cre/K-rasG12D mice showed a marked expansion of GFAP- and TUJ-1-expressing cell populations in the SVZ. In addition, mice developed intermediate grade, infiltrating glioma with 100% penetrance. Tumors were consistently located in the amygdalohippocampal region and nearby cortex, often in association with the lateral ventricle and expanded SVZ. Tumor cells expressed markers associated with neural progenitor cells, including Olig2, Bmi-1 and PDGFR-α. These data suggest that infiltrating tumor cells may arise from NSC transformed by activation of oncogenic K-ras in vivo.
Keywords: glioma, K-ras, subventricular zone, transgenic mouse model, neural stem cell
Introduction
High-grade gliomas are aggressive cancers that are generally refractory to standard treatment modalities. As such, they are among the most deadly of human neoplasms. Recent studies indicate that some neoplastic cells within human high-grade glioma have the capacity for self-renewal and multi-lineage differentiation, properties associated with normal neural stem cells (NSC) (1-4). It is hypothesized that these stem-like tumor cells are responsible for tumor growth and recurrence after therapy, making them attractive targets for novel glioma therapies (5-7). Furthermore, the ability of glioma stem cells (GSC) to differentiate along multiple lineages may underlie the histopathologic and clinical heterogeneity of glial neoplasms (2, 5). The mechanisms by which a fraction of glioma cells acquires stem-like properties are currently unknown. One hypothesis is that GSC arise from neoplastic transformation of endogenous NSC (5, 8-11). Another possibility is that transformed, lineage-committed precursor or fully differentiated cells reacquire stem-like properties (11, 12).
The best characterized stem cell population in the mature mammalian brain resides in the subventricular zone/rostral migratory stream (SVZ/RMS) (13-16). Evidence suggests that this area is composed of both stem cells and early lineage-restricted precursor cells (14, 15). GFAP-expressing, type B cells may represent the true stem cell in this population (14, 17). These cells give rise to GFAP-negative, type C or transit-amplifying cells (14, 15). In turn, type A cells arise from transit-amplifying cells and express markers of early neuronal differentiation (14, 15). In a similar fashion, the glioma stem cell hypothesis suggests that glioma cells are arranged hierarchically (5). GSC may divide symmetrically or asymmetrically, giving rise to two daughter GSC, to one GSC and one lineage-restricted tumor cell, or to two lineage restricted precursor cells. NSC and GSC may be studied in vitro, where they grow suspended in the culture medium in clusters known as “neurospheres” or “gliomaspheres”, respectively. The addition of serum and withdrawal of growth factors results in differentiation of neurosphere and gliomasphere cells into those that express neural, astrocytic and oligodendroglial phenotypes (2, 18).
Parallels between NSC and GSC extend beyond the capacities for self-renewal and multi-lineage differentiation. Many of the signaling pathways that regulate NSC proliferation, differentiation and migration are aberrantly regulated in glioma via mutation and/or overexpression of genes that encode for key effector or regulatory molecules (19). Evidence suggests that Ras/Raf/Erk signaling is one such pathway.
Through receptor tyrosine kinases, Ras signaling regulates important aspects of NSC biology. Type B cells have been shown to express PDGFR, while type C cells express EGFR (20, 21). Furthermore, intraventricular infusion of EGF or PDGF results in expansion of the SVZ and “glioma-like growths” (20, 21). Type C cells become highly invasive of surrounding brain and adopt a more glial phenotype in response to long-term, intraventricular infusion of EGF (20). These studies suggest the possibility that hyperactive Ras signaling in NSC could initiate gliomagenesis.
To test this hypothesis, we used the Cre-lox system to target a mutant and constitutively active form of K-ras to NSC in vivo. The GFAP-Cre mouse line, originally developed and characterized by Albee Messing's group, was used (22). Our data (not shown), using Rosa26 reporter (Rosa26r) mice and immunohistochemistry for Cre, are in accord with the findings of other groups, showing widespread recombinase activity and/or Cre expression in neurons, glia and a subset of GFAP-expressing SVZ precursors in the mature GFAP-Cre mouse brain (9, 22, 23). Activation of oncogenic K-ras with GFAP-Cre results in both expansion of the SVZ and development of multifocal, infiltrating, intermediate-grade glioma by two months of age. Proximity of the neoplasm to the expanded stem cell compartment and the expression by tumor cells of markers associated with NSC and GSC suggest that transformed stem/progenitor cells may give rise to the infiltrating tumor cells in vivo.
Results
Oncogenic K-ras activation results in marked expansion of the SVZ/RMS
GFAP-Cre transgenic mice were crossed with mice in which expression of oncogenic K-ras (K-rasG12D) is silenced by a stop signal flanked by loxP sequences. GFAP-Cre+/K-rasG12D progeny appear normal at birth. However, at approximately 4 weeks of age the mice develop largely benign, squamous papillary lesions. This is likely due to endogenous expression of GFAP in normal mouse skin, as demonstrated by Western blot analysis (data not shown). These lesions are slowly growing but attain sufficient size such that mice must be sacrificed by about 2.5 months of age (mean: 77.9 days, range: 44−127 days, n = 7).
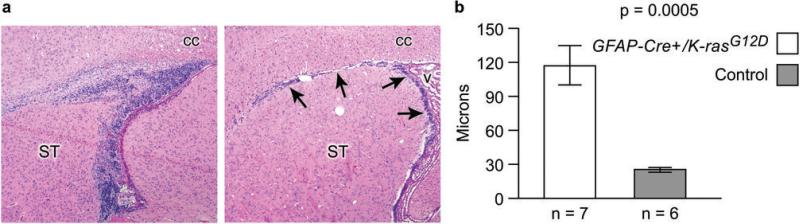
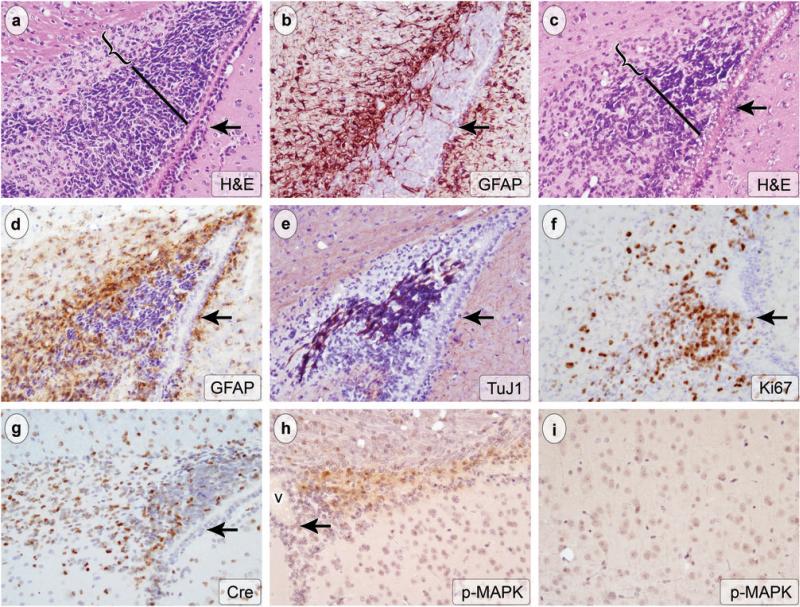
Examination of the brain at the time of euthanasia revealed no macroscopic abnormalities. Histologic examination, however, showed a marked expansion of the SVZ/RMS compared to age-matched controls (Figs. 1 and 2). In GFAP-Cre+/K-rasG12D mice, the mean width of the SVZ was increased more than four-fold compared to controls (Fig. 1, n = 7 experimental and 6 control animals per group, p = 0.0005). The expanded cell layer was composed of at least two cell types based on morphology and immunohistochemistry. The predominant cell type was located closest to the ependymal layer and had oval to angular, hyperchromatic nuclei and scant cytoplasm (bar in Fig. 2a, c). Apoptotic bodies and mitotic figures were abundant. These cells did not express GFAP (Fig. 2b, d). The second cell type formed a distinct layer in many cases and had round nuclei with open chromatin (bracket in Fig. 2a, c). These cells were strongly immunoreactive with antibody for GFAP (Fig. 2b, d). On the other hand, the GFAP-negative cells were immunoreactive for TUJ1, a marker of early neuronal differentiation (Fig. 2e). These data suggest that expression of oncogenic K-ras in the SVZ/RMS results in expansion of both GFAP-expressing type B cells as well as lineage restricted cells with neuronal differentiation. Ki67 immunohistochemistry showed a high proliferation index in the expanded cell layer, particularly in the GFAP−/TUJ1+ compartment (Fig. 2f). Cre expression was present largely in the GFAP-expressing compartment (Fig. 2g), and both cell types showed high levels of p-MAPK (Fig. 2h); these data confirm expression of the GFAP-Cre transgene and are consistent with activation of the Ras pathway in these cells. p-MAPK levels were low to undetectable in neurons and glia outside the SVZ/RMS (Fig. 2h, i).
Figure 1.
The adult stem/progenitor cell compartment was markedly expanded in GFAP-Cre/K-rasG12D compared to littermate control mice. The subventricular zone/rostral migratory (SVZ/RMS) stream is a stem/progenitor cell compartment in the mature brain and on parasagittal section consists of a thin layer of cells adjacent to the lateral ventricle and corpus callosum (arrows in a, right panel, original magnification 40x, v: ventricle, cc: corpus callosum, ST: striatum). In GFAP-Cre/K-rasG12D mice, both the vertical and horizontal limbs of the SVZ/RMS are increased in size (left panel in a, original magnification 40x). Compared to control mice, the thickness of the vertical limb of the SVZ, measured at the single widest point, was increased more than 4-fold in GFAP-Cre/K-rasG12D mice (b, n = 7 mutant and 6 control mice/group, p = 0.0005).
Figure 2.
The expanded stem/progenitor niche in GFAP-Cre-/K-rasG12D mice brains was composed of distinct subpopulations based on cytology and protein expression. Adjacent, representative, parasagittal sections from two animals (a-b and c-e) are shown, stained with H&E (a and c, original magnification 200x) and antibody for GFAP (c and d, original magnification 200x). An additional adjacent section stained with antibody to TUJ-1 is shown (e, original magnification 200x). Cells with angular, hyperchromatic nuclei populated the region closest to the ventricle (bars in a and c). A second population of cells with smaller, round nuclei and more open chromatin was also present (brackets in a and c). The cells with dark, angular nuclei were immunoreactive for TUJ1, a marker of immature neurons (e), and they did not express GFAP (b and d). Conversely, the smaller cells were immunoreactive for GFAP (b and d) and did not express TUJ1 (e). Both cell types were highly proliferative, as indicated by Ki67 immunohistochemistry (f, original magnification 200x). The cells expressed Cre protein (g, original magnification 200x) and high levels of p-MAPK (h, original magnification 200x, v: ventricle), consistent, respectively, with expression of the GFAP-Cre transgene and activation of the Ras pathway in these cells. Note the absence of significant p-MAPK labeling outside the expanded SVZ/RMS (i, h). p-MAPK signal is low to undetectable in cortex from the same animal shown in h (i, original magnification 200x). Arrow indicates the ependymal layer in all panels.
Activation of oncogenic K-ras results in multifocal, intermediate-grade glioma
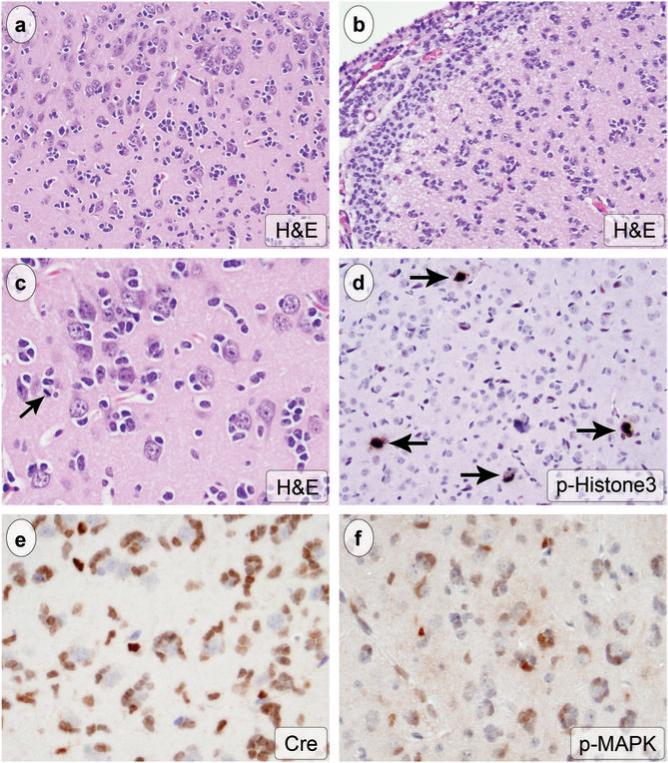
In addition to expansion of the stem/progenitor cell compartment, GFAP-Cre+/K-rasG12D mice developed bilateral, multifocal, infiltrating glioma with 100% penetrance. In many cases, the tumors involved the amygdalohippocampal region or nearby cortex. The neoplasms were characterized at low magnification by foci of hypercellularity on H&E stain (Figs. 3a-c, 4a). The infiltrating tumor cells had hyperchromatic, oval to angular nuclei and scant cytoplasm (Fig 3a-c). The tumor cells formed rings around overrun neurons (“satellitosis”, Fig. 3a, c) and accumulated beneath the pia mater (Fig. 3b), a histoarchitecture that is common in human glioma. Scattered mitotic figures were identified on H&E stain (arrow, Fig. 3c), and many more were highlighted with immunohistochemistry for phospho-histone3, a marker for cells in mitosis (arrows, Fig. 3d). The neoplastic cells showed strong immunoreactivity for Cre (Fig. 3e) and p-MAPK (Fig. 3f), indicating, respectively, expression of the GFAP-Cre transgene and activation of the Ras pathway. Ki67 immunohistochemistry labeled many neoplastic nuclei in tumor foci (Fig 4c). Many of the infiltrating cells were GFAP-immunoreactive (Fig 4e).
Figure 3.
GFAP-Cre+/K-rasG12D mice developed multifocal, intermediate grade, glioma. Tumors appeared on H&E at low power as foci of hypercellularity (a, original magnification 200x). Tumor cells infiltrated CNS parenchyma and formed secondary structures, including satellitosis of normal cortical neurons (a, c, original magnification 200x and 400x, respectively) and subpial accumulation (b, original magnification 200x). Individual tumor cells had dark, oval to angular nuclei and scant cytoplasm, and scattered mitotic figures were present (arrow in c). Considerably more mitotic figures were identified with immunohistochemistry for phospho-histone3 (arrows in d, original magnification 200x). The tumor cells expressed Cre (e, original magnification 400) and showed high levels of p-MAPK (f, original magnification 400x).
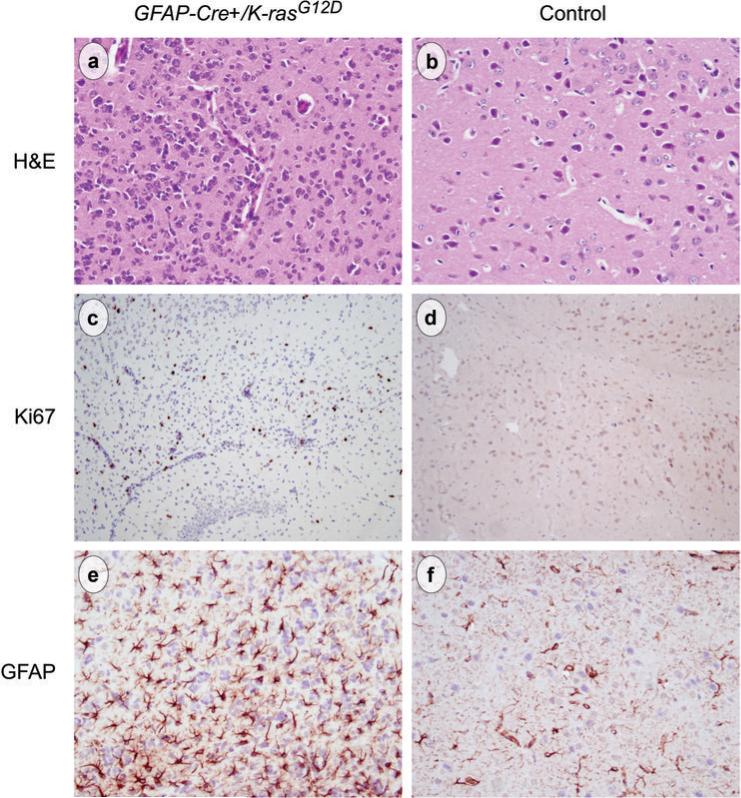
Figure 4.
Compared to sections from the same anatomic brain region in a littermate control mouse (b, d, f) tumor foci in mutant mice (a, c, e) showed an approximate 3-fold increase in cellularity (a vs b, original magnification 200x). Ki67 immunohistochemistry labeled many of the neoplastic cells but was largely negative in control brains (c vs d, original magnification 200x). Many of the tumor cells were immunoreactive for GFAP (e, original magnification 200x), which labels normal astrocytes in control brains (f, original magnification 200x).
An infiltrating growth pattern, hypercellularity and atypism define infiltrating glioma based on WHO criteria (24). These neoplasms may be further subdivided into lesions that lack significant mitotic activity (Grade II), those with increased cellularity and significant mitotic activity (Grade III) and those with even greater cellularity and mitotic activity accompanied by vascular proliferation and/or necrosis (Grade IV or glioblastoma multiforme). In GFAP-Cre+/K-rasG12D mice the cellularity was increased approximately 3-fold over the same area of the brain in a control mouse (compare panels a and b in Fig. 4). Mitotic activity was readily identified. Although there seemed to be increased vascularity in foci of tumor, the glomeruloid microvascular proliferation and necrosis characteristic of human grade IV tumors was not present. Therefore, the gliomas in GFAP-Cre+/K-rasG12D mice most closely resemble intermediate grade (grade 2−3) human glioma.
Infiltrating glioma cells in GFAP-Cre+/K-rasG12D mice express markers associated with “stemness”
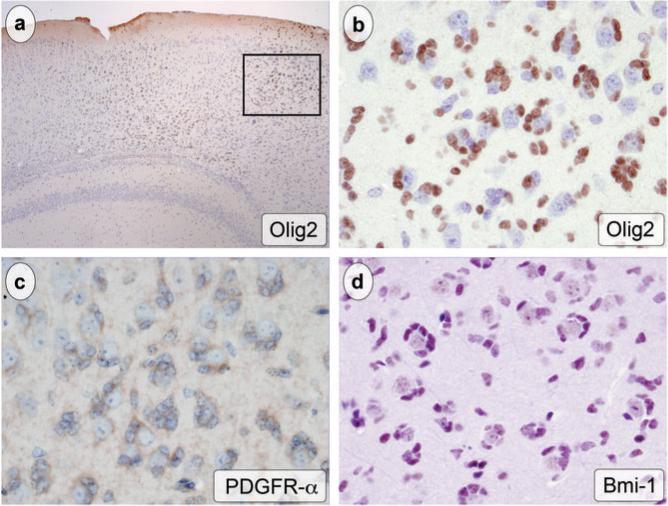
In human glioma specimens, a subset of neoplastic cells expresses markers that have been associated with stem cell biology. Olig2 is a bHLH transcription factor that has been linked to proliferation and lineage specification in NSC as well as the stem-like population of tumor cells in human high-grade gliomas (25). Immunohistochemical staining of tumor sections in GFAP-Cre+/K-rasG12D mice showed strong staining of tumor cell nuclei (Fig. 5a, b). Tumor cells were also immunoreactive for PDGFR-α, which is expressed by both normal neural stem cells and human glioma cells (Fig. 5c) (21, 26). Bmi-1 has been implicated in the self-renewal properties of both NSC and stem cells associated with brain tumors (27, 28). The majority of tumor cells in GFAP-Cre+/K-rasG12D mice showed strong nuclear expression of Bmi-1 (Fig. 5d). Examination of tumor cells in areas of subpial accumulation, a normally paucicellular region now composed almost exclusively of tumor cells, confirmed the co-expression of these stemness markers.
Figure 5.
The tumor cells expressed markers associated with NSC and GSC. Olig2 immunohistochemistry highlighted foci of tumor (a, original magnification 40x, boxed region at higher magnification in b). The brown, nuclear reaction product was seen in tumor cells encircling Olig2-negative neurons (b, original magnification 400x). The cytoplasm of atypical, neoplastic cells was marked brown using antibody to PDGFR-α (c, original magnification 400x). A Nova Red chromagen stains the infiltrating tumor cell nuclei with Bmi-1 immunohistochemistry (d, original magnification 400x).
Tumor foci are present in continuity with the ventricles and expanded SVZ
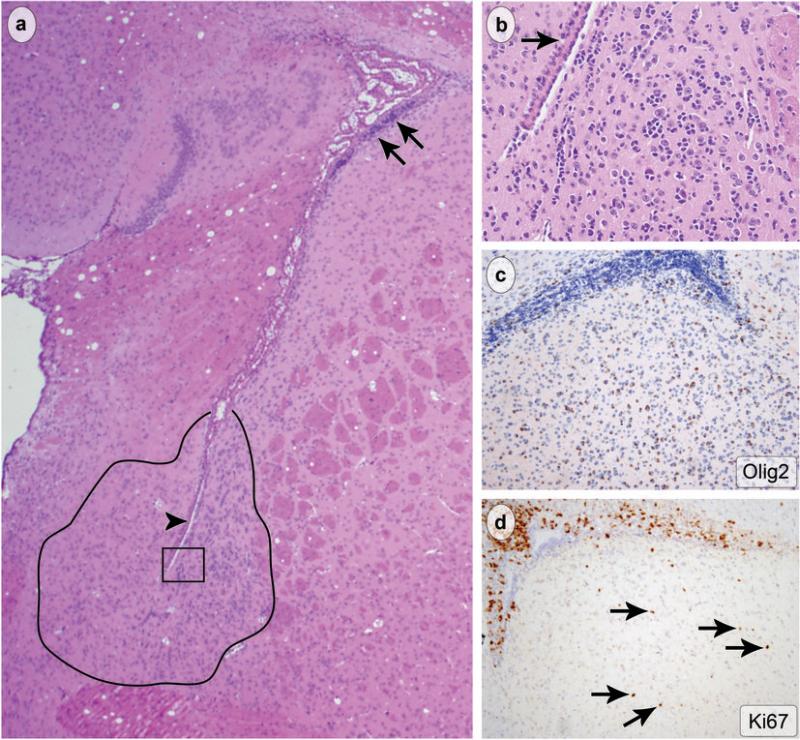
The amygdalohippocampal area and nearby structures were infiltrated by atypical, proliferating cells in nearly all (6/7) cases, and involvement of this region was bilateral in most. This suggests a common origin for the tumor cells. Indeed, portions of the amygdalohippocampal area and related structures are in direct contact with the SVZ of the lateral ventricle. Examination of other regions near the ventricles revealed small foci of atypical, infiltrating cells resembling those found in larger foci of tumor (Fig. 6a, b). Furthermore, in regions of frank SVZ expansion, cells labeling with Olig2 and Ki67 were observed in surrounding brain parenchyma, including the striatum and cortex (Fig 6c, d). These observations indicate that the tumors in GFAP-Cre+/K-rasG12D mice have a predilection for regions near the ventricles and that expression of oncogenic K-ras alters the migratory behavior of SVZ stem/progenitor cells.
Figure 6.
Tumor was present in close proximity to the ventricles and proliferating, Olig2-positive cells infiltrated regions adjacent to the SVZ/RMS. At low magnification hypercellular foci (tracing in a) were observed near the ventricle (arrowhead in a) and close to the expanded SVZ (arrows in a, original magnification 40x). At higher magnification (boxed area from a), the typical histoarchitecture and tumor cytology were observed (b, original magnification 200x). Olig2 (c, original magnification 100x) and Ki67 immunohistochemistry (d, original magnification 100x) showed aberrant migration of proliferating SVZ cells into adjacent CNS parenchyma (arrows in d).
Discussion
Unlike many human tumors, Ras mutation is not common in human glioma. However, increased Ras activity has been demonstrated in these tumors, and at least three likely mechanisms for this have been identified. First, the receptor tyrosine kinases epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) reside upstream of Ras and are often overexpressed in glioma (29). Furthermore, a constitutively active, mutant form of EGFR (EGFRvIII) is commonly expressed in human glioma (29). Neurofibromatosis type I is a neurocutaneous syndrome in which afflicted individuals are predisposed to glioma, often of the low-grade variety. This disorder is caused by mutations in the gene that encodes neurofibromin, a negative regulator of Ras activity (30). Finally, a recent report showed increased copies of Ras genes in over 40% of human glioma specimens (31). Thus the Ras pathway is central to human gliomagenesis. Emerging evidence suggests that the Ras signaling cascade also regulates NSC biology, including proliferation, differentiation and migration (20, 21). We simulated hyperactivity in the Ras pathway by activating oncogenic K-ras in the brain using a GFAP-Cre transgene. This results in marked expansion of the SVZ/RMS and multi-focal, intermediate grade glioma.
Our data (not shown) regarding the expression pattern of the GFAP-Cre transgene, in this particular strain of GFAP-Cre mice, are consistent with previous reports (9, 22, 23). In the adult brain, Cre protein was expressed by mature astrocytes and a subset of SVZ cells (data not shown). Although mature neurons did not express Cre, crosses with Rosa26r mice showed β-galactosidase activity, and thus recombination, in these cells as well. Two mechanisms could account for the expression of Cre in SVZ cells. First, a subset of these cells (type B cells) expresses GFAP in the mature mouse brain (14, 15, 17). Secondly, the transgene is expressed in radial glia during embryogenesis, as early as embryonic day 12.5 (9, 22). Fate-mapping studies indicate that these cells ultimately give rise to adult neurons, astrocytes and SVZ progenitor cells (23, 32-34). Since Cre-mediated recombination is irreversible, all of the progeny of radial glia would be expected to express oncogenic K-ras. Thus, while expression is not limited to the SZV in adult mice, GFAP-Cre mice may be used to target expression of oncogenes to adult stem cells in vivo.
β-galactosidase staining and Cre immunohistochemistry show the cellular compartments in which expression of oncogenic K-ras is possible, i.e., those cells in which Cre has removed the stop signal that silences expression of K-rasG12D. However, since the oncogene is targeted to the endogenous K-ras promoter, cell-specific mechanisms likely regulate the degree of expression in a given cell type. We used p-MAPK immunohistochemistry as a readout for activation of the Ras pathway in GFAP-Cre/K-rasG12D mice. The results clearly showed that the highest levels of p-MAPK are found in the SVZ/RMS and in the infiltrating tumor cells, with relatively little or no staining in neurons and mature astrocytes. Thus, despite the presence of recombination throughout the brain in multiple cell types, the greatest degree of Ras pathway activation was in the SVZ/RMS and the infiltrating tumor cells.
The gliomas that arise in GFAP-Cre/K-rasG12D mice were diffusely infiltrating and highly reminiscent of intermediate grade, human glioma. Satellitosis and subpial accumulation are patterns associated with, but not specific for, oligodendroglioma. However, the expression of GFAP and the cytologic features of the tumor cells on H&E more closely resembled human astrocytoma. The mice were not obviously symptomatic from these lesions and were sacrificed due to skin tumors at a mean age of 77.9 days. Therefore, it is unknown whether the gliomas would have progressed to a higher grade over time. The phenotype is highly penetrant, however, with 100% of mice developing lesions at a relatively young age.
Other mouse glioma models have been reported in which Ras signaling has been manipulated in the brain. Our results are consistent with Ding et al., who described lowand high-grade gliomas in mice using another Ras isoform (v12-H-ras) under direct control of the human GFAP promoter (35). Tumor development is associated with spontaneous acquisition of p53 mutations in astrocytes overexpressing this Ras isoform (36). Another study, in which GFAP-Cre mice were crossed with the K-rasG12D strain, failed to detect brain tumors in the bigenic, GFAP-Cre/K-rasG12D progeny (37). Similarly, targeting K-rasG12D to either nestin-expressing neural progenitor cells or GFAP-expressing cells using retroviruses failed to induce gliomas unless combined with constitutive activation of Akt or deletion of Arf (38, 39). Differences between these and the present study may reflect differing methodology and/or genetic background. Nevertheless, it is clear from the present data that activation of oncogenic K-ras using GFAP-Cre is sufficient for glioma formation in a mixed FVB/C57B/6 background.
In addition to fulfilling diagnostic criteria for glioma, the tumors in GFAP-Cre/K-rasG12D mice expressed proteins associated with NSC and GSC. The majority of tumor cells were Olig2-positive. In human glioma specimens, Olig2 is co-expressed with the majority of proliferating (Ki67-immunoreactive) cells and nearly all the CD133+ cells (25). Furthermore, Olig2 deletion abrogates the ability of Ink4a/Arf null, EGFRvIII-transformed neurospheres to form tumors when injected into the brains of host mice (25).
These observations and others have led to the suggestion that Olig2 represents a link between normal stem cells and the stem-like cells in glioma, and may be critical for human gliomagenesis (25). Many of the tumor cells (but not normal astrocytes or neurons) in our model also expressed cytoplasmic PDGFR-α. A subset of GFAP-expressing (Type B cells) in the normal SVZ expresses PDGFR-α (21). Furthermore, overexpression of PDGFR-α is a common finding in both low- and high-grade gliomas, and may facilitate tumor cell migration in these highly infiltrative neoplasms (26, 29). Finally, the tumor cells in GFAP-Cre/K-rasG12D mice express high levels of nuclear Bmi-1 protein. Bmi-1, a transcriptional repressor of the Ink4a/Arf locus, is required for the self-renewal capacity of neural and hematopoietic stem cells and is overexpressed in a variety of human cancers (27, 40, 41). The expression of NSC- and GSC-associated markers by infiltrating tumor cells in our model indicates their stem-like qualities and suggests that they may arise from K-ras-transformed endogenous neural stem cells.
Indeed, the SVZ/RMS in GFAP-Cre/K-rasG12D mice was markedly expanded and included non-overlapping GFAP-expressing and TUJ-1-expressing populations of cells. Expression of the GFAP-Cre transgene and activation of the Ras pathway in these cells was confirmed with immunohistochemistry for Cre and p-MAPK. Evidence suggests that the GFAP-expressing cells of the SVZ (Type B cells) constitute the true stem cell in this stem/progenitor cell niche, giving rise to transit-amplifying cells which, in turn produce TUJ-1-positive, immature neurons (Type A cells) (14). Our data suggest that expression of oncogenic K-ras in GFAP-expressing, Type A cells results in proliferation of this compartment, with both symmetric and asymmetric division, leading to expansion of both Type A cells and neuronal precursors, with loss of GFAP expression in the latter cells. The reason for the spatial segregation of the cell types is unclear, but may be related to a partially retained migration program on the part of the neuronal precursors. Foci of infiltrating, proliferating, atypical cells expressing stem cell markers in continuity or close proximity to the expanded SVZ/RMS suggests that some of the stem/progenitor cells acquire the capacity to infiltrate surrounding brain, perhaps facilitated by the expression of PDGFR-α.
The expansion of the SVZ/RMS in GFAP-Cre/K-rasG12D mice was similar to that reported with intraventricular infusion of EGF or PDGF in wild-type mice. Infusion of these upstream growth factors in the Ras signaling pathway resulted in reversible expansion of the SVZ and “glioma-like” growths in the vicinity of the ventricles (20, 21). Others have shown that p53 deletion results in focal hyperplasias of the SVZ and, following exposure to the mutagen ethylnitrosourea, high-grade gliomas develop in periventricular locations (10). Zhu and colleagues showed that GFAP-Cre-mediated loss of Nf1 in a germline homozygous p53 null mice resulted in high-grade gliomas; histologic examination of animals at early time points showed that, in the majority of animals with early tumors, the neoplasms were directly associated with the SVZ (9). Further deletion of Pten in the Nf1/p53 model accelerates the development of glioma which is preceded by ectopic migration of stem/progenitor cells from the SVZ (8). These data, as well as the results of our study, support the hypothesis that SVZ stem/progenitor cells can serve as cells of origin for glioma.
It is unknown in humans whether the GSC in gliomas derive from stem cells, lineage restricted precursor cells, or dedifferentiated astrocytes. It is possible that GSC arise through neoplastic transformation of cells at multiple and various stages along the spectrum of differentiation. If so, an important question is whether the differentiation status of the cell of origin determines the particular glioma phenotype in humans, and whether this has prognostic or therapeutic implications. Interestingly, a recent study showed differences in the clinical behavior of high-grade gliomas based on proximity to the SVZ, as evaluated by preoperative MRI. Tumors that were in contact with the SVZ upon initial presentation were more likely to be multifocal and were more likely to recur in locations that were non-contiguous with the primary lesion (42). On the other hand, in an orthotopic transplant model, Bachoo et al. reported that expression of constitutively active EGFR in Ink4a/Arf-deficient, mature astrocytes leads to apparent dedifferentiation, including loss of GFAP expression, upregulation of nestin, and reacquisition of neurosphere-forming ability (12). Furthermore, in a small number of animals, there was no difference in histopathology or survival when mice were transplanted with Ink4a/Arf-deficient, mutant EGFR-expressing NSC compared to mature astrocytes with the same genetic manipulations (12). However, Bruggeman and colleagues recently showed that tumors with more aggressive histology develop in mice transplanted with Bmi-1-deficient, Ink4a/Arf-null, mutant EGFR-expressing astrocytes when compared to tumors formed from NSC with the same genetic manipulations (43). Further work is required to resolve these issues.
In summary, our results show that the GFAP-Cre transgene can be used to target expression of oncogenic K-ras to glioneuronal precursor cells and adult NSC in vivo. Hyperactive Ras signaling alone is sufficient to produce gliomas that closely resemble human tumors. In addition, hyperactive Ras signaling in cells of the SVZ results in a marked expansion of this stem/progenitor cell compartment. Although other possibilities cannot be entirely excluded, the expression of “stemness” markers by tumor cells and the proximity of tumor to the expanded SVZ/RMS support the hypothesis that the SVZ/RMS is the source of infiltrating tumor cells in this model. A better understanding of the cell of origin in glioma, and whether its differentiation status affects the histopathologic and clinical behavior, may have implications for glioma classification, treatment and prognosis.
Methods and Materials
Generation of GFAP-Cre/KrasG12D mice
K-rasG12D mice were a gift from Dr. Tyler Jacks. The genetic manipulation in these mice targets oncogenic K-ras (K-rasG12D) to the endogenous K-ras locus (44). The allele has a stop codon flanked by loxP sites. Cre recombinase-mediated excision of the stop cassette allows expression of the oncogenic K-ras from the endogenous K-ras promoter. GFAP-Cre mice [FVB-Tg(GFAP-cre)25Mes/J, The Jackson Laboratory] were mated with KrasG12D mice (129/SvJac/C57BL/6) to generate GFAP-Cre/K-rasG12D and littermate control mice on a mixed 129/SvJac/C57BL/6/FBV background. GFAP-Cre mice were bred with Rosa26r mice on an FVB background for the purpose of tracing Cre-recombinase expression driven by the GFAP reporter (45). Mice were housed in the animal care facility at Vanderbilt University following the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All animal procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Genotyping of Transgenic Mice
Identification of the GFAP-Cre allele and KrasG12D recombined alleles were determined by PCR at three weeks of age by using oligonucleotide primers as described previously (44, 46).
β-galactosidase Histochemistry
Brains from GFAP-Cre/Rosa26r mice were fixed in 4% paraformaldehyde and stained for β-galactosidase expression using standard techniques. The tissues were dehydrated to 70% ethanol, embedded in paraffin, sectioned at 5μm, and counterstained with Neutral Fast Red.
Histology and Immunohistochemistry
Mice were anesthetized with Ketamine (120mg/kg) and Xylazine (10mg/kg) and terminally perfused transcardially with 30 ml of 4% paraformaldehyde. Brains were removed, fixed in 4% paraformaldehyde overnight, followed by fixation in 70% ethanol. A mid-sagittal cut separated the hemispheres. Additional parasagittal sections were generated from one hemisphere, and the opposite hemisphere was sectioned in the coronal plane. Tissue sections were embedded in paraffin, and sectioned at 5μm. The sections were stained with H&E or were immunostained using antibodies for Bmi-1 (05−637, Upstate, 1:200), Cre (PRB-106C, Covance, 1:125), GFAP (sc9065, Santa Cruz Biotechnology, 1:100), Ki67 (VP-K451, Vector Laboratories, 1:2000), Olig-2 (AB9610, Chemicon, 1:500) PDGFR-α (E2691, Spring Bioscience, ready-to-use), phosphorylated Histone H3Ser10 (06−570, Upstate, 1:2000) phosphorylated p-44/42 MAPKThr202/Tyr204 (4376S, Cell Signaling, 1:100), and TUJ1 (MMS-435P, Covance, 1:100).
Measurement of the SVZ
H&E-stained, parasagittal sections from GFAP-Cre/K-rasG12D and littermate controls were matched based on anatomic landmarks, including the size and configuration of the hippocampus. In this orientation, the SVZ/RMS has a nearly horizontal segment subjacent to the corpus callosum, and an oblique portion that connects to a nearly vertical section. The width of the SVZ at its widest point in the vertical section was measured perpendicular to the ependymal layer at 400X using an Olympus BX41 microscope, an Olympus DP70 camera and DP controller software with calibrated digital scalebar. Means were calculated and differences between groups evaluated with Student's t-test.
Acknowledgments
The authors thank Dr. Michael Cooper for helpful comments on the manuscript and Dr. Tyler Jacks for the K-rasG12D mice. We thank Ms. Jennifer Hofecker and Ms. Tracie Moss for expert assistance with tissue processing and sectioning and Ms. Jean McClure for help with preparation of the manuscript. The authors acknowledge the immunohistochemical services of the Vanderbilt University Immunohistochemistry Core Laboratory, and thank the Human Tissue Acquisition Laboratory, supported by grant no. P30 CA068485. This work was funded, in part, by a Vanderbilt Physician Scientist Development Award (TWA) and NIH/NINDS K08 NS062107-01A1 (TWA).
References
- 1.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004 Oct 1;64(19):7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 2.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002 Sep;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 3.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004 Nov 18;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 4.Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004 Dec 16;23(58):9392–400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005 Aug 25;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 6.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007 Oct 1;67(19):8980–4. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 7.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006 Dec 7;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 8.Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008 May 1;68(9):3286–94. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005 Aug;8(2):119–30. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006 Jan 25;26(4):1107–16. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008 Jun 26;58(6):832–46. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002 Apr;1(3):269–77. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 13.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006 Jan 20;494(3):415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 14.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999 Jun 11;97(6):703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 15.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997 Jul 1;17(13):5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage FH. Mammalian neural stem cells. Science. 2000 Feb 25;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 17.Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003 Apr 1;23(7):2824–32. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996 Apr 10;175(1):1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 19.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007 Oct;7(10):733–6. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 20.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002 Dec 19;36(6):1021–34. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 21.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006 Jul 20;51(2):187–99. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001 Oct;31(2):85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 23.Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, et al. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003 Mar 6;37(5):751–64. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 24.Louis DN, Ogaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumors of the central nervous system. IARC; Lyon: 2007. [Google Scholar]
- 25.Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007 Feb 15;53(4):503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995 Jan 17;60(2):168–73. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 27.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004 Aug 20;118(4):409–18. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002 Aug;2(8):616–26. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- 30.Yohay KH. The genetic and molecular pathogenesis of NF1 and NF2. Semin Pediatr Neurol. 2006 Mar;13(1):21–6. doi: 10.1016/j.spen.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Jeuken J, van den Broecke C, Gijsen S, Boots-Sprenger S, Wesseling P. RAS/RAF pathway activation in gliomas: the result of copy number gains rather than activating mutations. Acta Neuropathol. 2007 Aug;114(2):121–33. doi: 10.1007/s00401-007-0239-0. [DOI] [PubMed] [Google Scholar]
- 32.Bonfanti L, Peretto P. Radial glial origin of the adult neural stem cells in the subventricular zone. Prog Neurobiol. 2007 Sep;83(1):24–36. doi: 10.1016/j.pneurobio.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003 Jun;13(6):580–7. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 34.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, et al. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 2001 May 1;61(9):3826–36. [PubMed] [Google Scholar]
- 36.Shannon P, Sabha N, Lau N, Kamnasaran D, Gutmann DH, Guha A. Pathological and molecular progression of astrocytomas in a GFAP:12 V-Ha-Ras mouse astrocytoma model. Am J Pathol. 2005 Sep;167(3):859–67. doi: 10.1016/S0002-9440(10)62057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasgupta B, Li W, Perry A, Gutmann DH. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 2005 Jan 1;65(1):236–45. [PubMed] [Google Scholar]
- 38.Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002 Oct 1;62(19):5551–8. [PubMed] [Google Scholar]
- 39.Lyustikman Y, Momota H, Pao W, Holland EC. Constitutive activation of Raf-1 induces glioma formation in mice. Neoplasia. 2008 May;10(5):501–10. doi: 10.1593/neo.08206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004 Mar 18;428(6980):337–41. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 41.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005 Jun 15;19(12):1432–7. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007 Oct;9(4):424–9. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, et al. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell. 2007 Oct;12(4):328–41. doi: 10.1016/j.ccr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001 Dec 15;15(24):3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999 Jan;21(1):70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 46.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002 Feb;32(2):73–5. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]