Abstract
Self-monitoring has traditionally been done using paper record (PR), which can be tedious and burdensome. A personal digital assistant (PDA) with dietary software may provide an alternative to a PR. The study aimed to describe the differences in dietary changes at 6 months between participants randomly assigned to use a PR or PDA for self-monitoring in a clinical trial of weight loss treatment. Self-monitoring adherence, change in weight and diet were assessed between 2006 and 2009. The sample (N=192) was 84% female and 78% White with a mean age of 49 years and body mass index of 34.0 kg/m2. At baseline, the groups did not differ in energy intake, % calories from fat and number of servings of the examined food groups. At 6 months, both groups had significant reductions in weight, energy intake and % calories from total fat and saturated fatty acids (Ps <0.001); no between-group differences were found. Compared to the PR group, the PDA group significantly increased consumption of fruit (P=0.02), vegetables (P=0.04), and decreased consumption of refined grains (P=0.02). Interactions between self-monitoring and the two groups were found in relation to changes in % calories from total fat (P=0.02), monounsaturated fatty acids (P=0.002) and trans-fatty acids (P=0.04). Frequent self-monitoring was significantly associated with total sugar (P=0.02) and added sugar (P=0.01) intake in both groups. Our findings suggest that the use of PDA for self-monitoring might improve self awareness of behavior and dietary changes.
Keywords: Adherence, Self-monitoring, Personal digital assistant (PDA), Paper records (PR), Dietary intake
Self-monitoring one's daily dietary intake has been recognized as an important component for successful outcomes in behavioral weight loss treatment (1, 2). It allows individuals to become aware of their eating behaviors, the amount of food consumed and situations that create obstacles to positive behavior changes. It also promotes corrective actions to help ensure that they meet their dietary goals (3). The most often-used method of self-monitoring continues to be the paper record (PR). However, the use of a PR and its associated work of looking up nutrient content of foods and calculating totals can become tedious and time consuming for the user (2).
Recent advancements in technology have provided a few alternative approaches to using a PR, e.g., use of an online diary or a personal digital assistant (PDA). A pilot study suggested that a PDA with dietary software program was a potential tool for facilitating self-monitoring food intake, possibly enhancing dietary goal attainment (4). Other researchers have used PDAs for collecting 24-hour energy intake (5) and keeping food records (6).
Several studies have reported that more frequent and consistent self-monitoring was significantly associated with greater weight loss and maintenance (1, 7-9) and also with successful achievement of dietary goals (10-12). However, little is known about the associations between dietary modifications and different approaches to self-monitoring. A case study reported the PDA-based dietary self-monitoring to be effective in reducing sodium intake among patients receiving in-center hemodialysis treatment (13). No differences in weight loss were observed in a study that compared use of a PDA with a PR; differences in dietary intake between these groups were not reported (14). In a pilot study, a significant increase in vegetables intake was found in the group that used the PDA compared to the control group, which received only printed nutrition materials (15). No previous studies have compared the differences in dietary changes with the use of a PDA and a PR. Because of the critical role that self-monitoring plays in successful weight loss and maintenance, easing the self-monitoring task is particularly important for overweight/obese individuals trying to lose weight. If use of a PDA for self-monitoring provides better self-awareness of behaviors, it may facilitate the dietitian's ability to support dietary improvements; the result may be improved outcomes with dietary or lifestyle changes. Hence, the study purpose was to describe and compare dietary changes between PR and PDA groups at six months in a behavioral weight loss treatment in overweight/obese adults. It was hypothesized that self-monitoring with a PDA would be more effective than self-monitoring with a PR in improving overall diet quality.
Methods
This was a secondary analysis of the 6-month evaluation from a 24-month randomized clinical trial that used a 3-group design to determine the effects of different methods of self-monitoring on weight loss and adherence to self-monitoring. As described in detail elsewhere (16), the study sample included overweight/obese adults aged 18 to 59 years old who had adequately completed a 5-day diary at screening. The mean body mass index was 34.1 kg/m2 and they completed, on average, 15 years of education. Individuals with conditions that required medical supervision of diet or exercise and those who participated in a weight-loss program in six months prior to recruitment were excluded. The study protocol was approved by the University of Pittsburgh Institutional Review Board; all participants provided written informed consent.
The study randomized 210 individuals to using either a standard PR, a PDA with dietary and exercise software, or a PDA with the same software plus a customized feedback program (PDA+FB). Since no differences were found in adherence to self-monitoring and the changes in dietary intake between the two PDA groups (PDA and PDA+FB) at six months, the two PDA groups were combined and analyses compared the changes in dietary intake between the two groups: PR users and PDA users (PDA and PDA+FB groups).
Intervention
All three treatment groups received the same standard behavioral intervention; the only difference was in the method of self-monitoring assigned to each group. The cognitive-behavioral intervention included 20 group sessions during the first 6 months. All participants were instructed to self-monitor diet daily over the study period and were trained in using their self-monitoring tool during the first two weeks of intervention. The PR group was instructed to record all the foods consumed with the corresponding number of energy and fat grams. They also calculated subtotals periodically throughout the day to compare intake values to their daily goals. They were provided with a reference booklet and were taught how to find information when food labels were unavailable. Participants in the PDA groups were provided with Palm Tungsten E2™ PDAs with dietary self-monitoring software (Dietmate Pro© and PICS, Version 1, 2003, Reston, VA) that tracked and provided values for energy, total fat grams, percent calories from saturated fat, carbohydrate, protein and fiber intake. The PDA also provided subtotals in relation to daily goals automatically after each dietary entry.
At each session, PR participants turned in their diaries and the PDA participants turned in their PDAs, The PDAs were downloaded to the study database; the interventionists then received printed reports that looked similar to the PR for their review. All groups received the interventionist's written feedback at the next session. The prescribed daily energy intake was between 1200 and 1800 calories depending on gender and baseline weight. The fat intake goal was 25% of the total daily calories for all subjects. The intervention emphasized restricting calories and replacing total fat intake, especially saturated fatty acids, with increased intake of fruit, vegetables and whole grain products.
Measures
At baseline and six months, dietary intake was measured in all groups with the two unannounced 24-hour dietary recalls (weekday and weekend day) guided by the Nutrition Data System Research software program (Nutrition Coordinating Center, University of Minnesota). Food group serving counts were used in the analysis. Adherence to self-monitoring was measured on a weekly basis and analyzed as a binary variable based on whether a participant completed daily recordings (adherent: self-monitored versus non-adherent: did not self-monitor). If the weekly record indicated that a participant consumed more than 50% of the weekly calorie goal, the participant was defined as adherent to self-monitoring for that week. For example, a participant with a daily calorie goal of 1200 (weekly goal = 8400 kcal) would be adherent to self-monitoring if the person recorded consuming ≥ 4200 calories for that week. Body weight was measured with a digital scale (Tanita Corporation of America, Inc., IL) with the person wearing light clothing and no shoes.
Statistical Analysis
Data are expressed as mean ± standard deviation or median (25th, 75th percentile) for continuous variables or as frequency counts (proportions) for categorical variables. Comparisons between the PR and PDA groups were examined using an independent samples t-test or a Mann-Whitney test for continuous variables and a chi-square test of independence for categorical variables. Analysis was also conducted as a single sample to assess the effect of different self-monitoring methods on dietary changes using the simple and multiple regression models. Possible interactions were explored between the adherence to self-monitoring and the treatment groups (PR vs. PDA) in relation to changes in dietary intake. A paired t-test or Wilcoxon's matched pairs test was also used for assessing changes within the groups. Statistical analyses were performed using SAS (version 9.2; SAS Institute Inc, Cary, NC).
Results and Discussion
Of 210 participants at baseline, 192 (91%) completed the 6-month assessment. No differences in age, gender, race or weight were noted between those who completed the assessment and those who did not. The parent study sample was predominantly female (84%) and White (78%) with a mean body mass index of 34.0 ± 4.5 kg/m2. We found no significant differences in baseline demographic, anthropometric characteristics or dietary intake between the PR and PDA group (Table 1).
Table 1. Percent change of weight and dietary intake by treatment groups (baseline n, PR=72, PDA=138; 6 mo n, PR=63, PDA=129).
| Measures | Group | Baseline | % Change | Pa |
|---|---|---|---|---|
| Weight, kg | PRc | 94.28 ±15.3 | −5.94 ± 5.9b | 0.4 |
| PDAd | 93.91 ±15.12 | −6.71 ± 6.9b | ||
|
| ||||
| Energy intake, kcal | PRc | 1970 (1533, 2483) | −343 (−836, 26)b | .20 |
| PDAd | 1972 (1698, 2456) | −532 (−966, −140)b | ||
|
| ||||
| % kcal total fat | PRc | 33.2±7.3 | −1.1 (−8.1, 3.7)b | .11 |
| PDAd | 34.1±7.1 | −4.2 (−9.8, 0.3)b | ||
|
| ||||
| % kcal SFAe | PRc | 11.3 (8.9, 13.4) | −0.1 (−4.5, 1.9)b | .08 |
| PDAd | 11.6 (9.4, 13.9) | −1.7 (−4.3, 0.5)b | ||
|
| ||||
| Fruit, # of servings | PRc | 1.1 (0.4, 2.0) | −0.05 (−1.0, 0.2) | .02 |
| PDAd | 0.6 (0.0, 1.7) | 0.06 (−0.5, 0.5) | ||
|
| ||||
| Vegetables, # of servings | PRc | 2.5 (1.6, 4.3) | −0.9 (−2.2, 0.6) | .04 |
| PDAd | 2.6 (1.6, 3.9) | 0.01 (−1.3, 1.1) | ||
|
| ||||
| Whole grains, # of servings | PRc | 0.9 (0.0, 1.6) | 0.0 (−0.6, 0.9) | .66 |
| PDAd | 0.9 (0.0, 1.8) | 0.1 (−0.7, 1.2) | ||
|
| ||||
| Refined grains, # of servings | PRc | 4.9±2.7 | −0.5 (2.7, 1.2) | .02 |
| PDAd | 5.1±2.6 | −1.5 (−3.2, 0.10) | ||
P for changes between the two groups, baseline to 6 months.
For P < .01 for changes within each group, baseline to 6 months.
PR= paper record.
PDA = personal digital assistant.
SFA = saturated fatty acids.
mean ± standard deviation (all such values) or median (25th tile, 75th tile) (all such values).
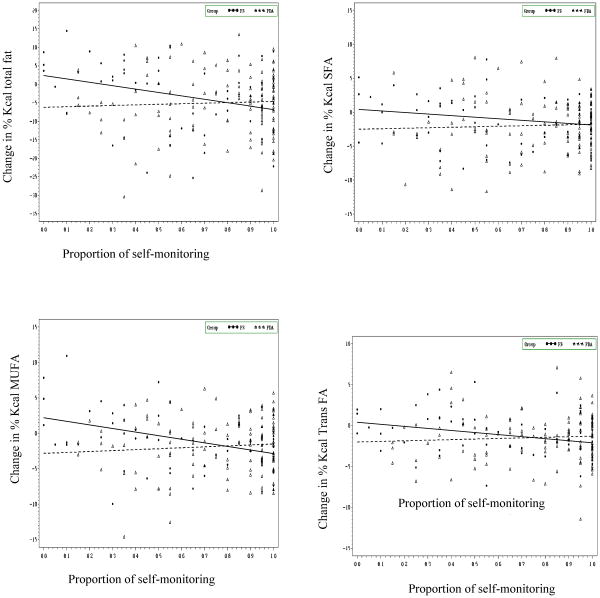
At six months, both groups had significant reductions in energy (P<0.001) and % calories from total fat (P<0.001) and saturated fat (P<0.001) which was paralleled by significant weight loss (P<0.001) with no differences between the groups. However, compared to the PR group, the PDA group significantly increased servings of fruit (P=0.02), and vegetables (P=0.04) consumed and decreased their servings of refined grains (P=0.02) (Table 1). A significant interaction between the groups and adherence to self-monitoring was observed in relation to total fat intake (β (s.e)= 10.7 (4.6); P= 0.02; R2=0.022). As described in Figure 1, there was a significant decrease in total fat intake with increasing adherence to self-monitoring in the PR group. However, when adherence to self-monitoring was low, there also was a significant reduction of total fat intake in the PDA group. Thus, these findings suggest that adherence to self-monitoring was less important to the PDA group than the PR group.
Figure 1.
Changes in dietary fat intake and self-monitoring by groups
Proportion of self-monitoring indicates the proportion of time that participants self-monitored. For example, a participant who had self-monitored 16 out of 20 sessions was 80% adherent to self-monitoring.
A similar trend in interaction between the groups and self-monitoring with saturated fat intake was found; however, this interaction was not significant (β(s.e)=3.1(2.1); P=0.14; R2=0.032). There also was an increase in mono-unsaturated fatty acid (% kcal) intake with increasing adherence to self-monitoring in the PDA group; however, this association was reversed in the PR group (β(s.e)= 6.4 (2.0); P<0.01; R2=0.075). Similarly, a significant interaction between the groups and self-monitoring adherence was found with change in trans fatty acid intake (β(s.e)= 3.2 (1.6); P=0.04; R2=0.022) again suggesting that those in the PDA group improved their diet more at a lower level of adherence to self-monitoring. No such interactions between self-monitoring adherence and groups were found in relation to total and added sugar intake, but a simple linear regression indicated a significant increase in the servings of total (β(s.e)= 28.7(12.7); P=0.02; R2=0.026) and added sugar (β(s.e)=30.3 (12.0); P=0.01; R2=0.033) intake with increasing adherence to self-monitoring in both groups.
The present study found that the PDA might provide some advantage over the use of PR when it is used for self-monitoring dietary intake. Participants in the PDA group increased their intake of fruit, vegetables, and whole grains and decreased their intake of refined grains when compared to the PR group. There also were significant associations between adherence to self-monitoring and changes in the intake of total fat, trans fatty acids and monounsaturated fatty acids. However, these associations were dependent on the method of self-monitoring suggesting that the PR group needed to be more adherent to self-monitoring to improve their diet quality. Consistent with the preliminary findings of others (4, 13, 15), this study adds to the evidence that a PDA might be a beneficial tool in helping individuals increase awareness of their eating behaviors and in the promotion of healthy behaviors.
These findings of improvement in overall diet quality in the PDA group compared to the PR group may be explained partly by the advantages of using a PDA. With the use of the PDA, individuals are no longer required to look up foods in a nutrient booklet and calculate the nutrient content of foods consumed; this likely reduces the burden of recording compared to the PR. The software program calculates the nutrient content and displays it next to the daily target for nutrient intake, thus providing immediate feedback to the individual. This feedback can assist individuals to make necessary changes in the next meal and align more closely with daily goals. With the PDA, individuals can preset a number of target nutrients (e.g., calories, sodium, calcium) to monitor their dietary intake. However, when using a PR, the greater the number of target nutrients, the longer it takes to adequately complete the self-monitoring exercise. Thus, the nutrient database in the PDA may reduce the recording time and moreover, the automatically provided nutrient content may improve adherence to self-monitoring and to dietary goals. Given that the PDA is portable and socially acceptable, individuals can record in any environment, which may reduce the uneasiness of self-monitoring dietary intake in public places.
Participants in the PR group were asked to record the type, amount, calories and fat of all foods consumed. Hence, they had complete control over the level of detail that was included in the diary. Moreover, they needed to tally their total calories and fat to determine whether they were at risk for exceeding their dietary limits. Thus, the PR group was required to put forth much more effort to learn about their food choices and how those choices affected their goals. In contrast, the PDA users needed to only enter the type and amount of food eaten and received feedback on calories and fat immediately and thus were able to learn much more about their food choices despite putting forth less effort than PR participants. This ability to learn more about food choices with less effort may have led to the improvements seen in the diet quality that occurred at a lower level of adherence in the PDA group as compared to the PR group.
Despite its potential to improve adherence to self-monitoring and diet quality, limitations to using a PDA should also be noted. Compared to the PR, it may take a longer time to learn to use a PDA. Some individuals, in particular those with lower literacy skills and the elderly may encounter difficulty in using the device. However, our participants ranged in age from 18 to 59, and age was never a factor in learning to use the PDA. Even participants who had never used a cell phone or computer previously were able to learn how to use the device. The interventionists assisted all participants until they felt comfortable in their ability to use the PDA and appeared to have the necessary skills to use the PDA.
Increase in total and added sugar intake with greater adherence to self-monitoring in both groups was not anticipated. The intervention emphasized reduction in total fat intake, especially saturated fatty acids as a means to meet the dietary goals. Since participants were not required to self-monitor either their total or added sugar, it is possible that the reduction in total fat was achieved by replacing some of the total fat with added sugar (17).
A limitation of the study is the inability to generalize these findings beyond the relatively homogenous study population, which was predominantly well-educated, White females who were employed full time. The strengths of this study include the use of data from a randomized clinical design to compare the use of PDAs to the conventional PR in improving weight loss and adherence to self-monitoring, the measure of adherence via the PR or PDA data, and the high retention of participants at six months (91%).
Conclusions
Overall, these results suggest that those who were adherent to self-monitoring were more successful in lowering their intake of total fat, saturated and trans-fatty acids. The PDA group had significantly greater intake of fruit, vegetables and reduced their intake of refined grains. Adherence to self-monitoring and changes in total fat, saturated and monounsaturated fatty acid intake were modified by the method used for self-monitoring. The use of a PDA for self-monitoring might help individuals become more aware of their eating behaviors, which in turn might improve long-term dietary changes.
Acknowledgments
Funding/Support: National Institutes of Health grants #R01-DK71817 and partial support by NIH K24 Award, NR010742. The conduct of the study was also supported by the Data Management Core of the Center for Research in Chronic Disorders NIH-NINR #P30-NR03924 and the General Clinical Research Center, NIH-NCRR-GCRC #5MOl-RR00056 and the Clinical Translational Research Center, NIH/NCRR/CTSA Grant UL1 RR024153 at the University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sushama D. Acharya, Email: sda9@pitt.edu, Graduate School of Public Health, University of Pittsburgh, Ph: 412 624 3081; Fax: 412 383 7293.
Okan U. Elci, Email: ouel@pitt.edu, Graduate School of Public Health, University of Pittsburgh, Ph: 412 624 2229; Fax: 412 383 7293.
Susan M. Sereika, Email: ssereika@pitt.edu, School of Nursing & Dept. of Biostatistics and Epidemiology, Graduate School of Public Health, University of Pittsburgh, Ph: 412 624 0799; Fax: 412 383 7293.
Mindi A. Styn, Email: mimst31@pitt.edu, School of Nursing & Dept. of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Ph: 412 624 0966; Fax: 412 383 7293.
Lora E. Burke, Email: lbu100@pitt.edu, School of Nursing & Dept. of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Ph: 412 624 2305; Fax: 412 383 7293.
References
- 1.Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behavr Ther. 1993;24:377–394. [Google Scholar]
- 2.Burke LE, Warziski M, Starrett T, et al. Self-monitoring dietary intake: Current and future practices. J Ren Nutr. 2005;15:281–290. doi: 10.1016/j.jrn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am. 2005;28:151–170. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Glanz K, Murphy S, Moylan J, Evensen MS, Curb JD. Improving dietary self-monitoring and adherence with hand-held computers: A pilot study. Ann Behav Med. 2004;27:S111. doi: 10.4278/0890-1171-20.3.165. [DOI] [PubMed] [Google Scholar]
- 5.Beasley J, Riley WT, Jean-Mary J. Accuracy of a PDA-based dietary assessment program. Nutrition. 2005;21:672–677. doi: 10.1016/j.nut.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 6.McClung HL, Sigrist LD, Smith TJ, et al. Monitoring energy intake: a hand-held personal digital assistant provides accuracy comparable to written records. J Am Diet Assoc. 2009;109:1241–1245. doi: 10.1016/j.jada.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Baker RC, Kirschenbaum DS. Weight control during the holidays: highly consistent self-monitoring as a potentially useful coping mechanism. Health Psychol. 1998;17:367–370. doi: 10.1037//0278-6133.17.4.367. [DOI] [PubMed] [Google Scholar]
- 8.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadden T, Berkowitz R, Womble L, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–21220. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 10.Acharya SD, Elci OU, Sereika SM, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009;3:151–160. doi: 10.2147/ppa.s5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helsel DL, Jakicic JM, Otto AD. Comparison of techniques for self-monitoring eating and exercise behaviors on weight loss in a correspondence-based intervention. J Am Diet Assoc. 2007;107:1807–1810. doi: 10.1016/j.jada.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Wanke KL, Daston C, Slonim A, et al. Adherence to the polyp prevention trial dietary intervention is associated with a behavioral pattern of adherence to nondietary trial requirements and general health recommendations. J Nutr. 2007;137:391–398. doi: 10.1093/jn/137.2.391. [DOI] [PubMed] [Google Scholar]
- 13.Sevick MA, Stone RA, Novak M, et al. A PDA-based dietary self-monitoring intervention to reduce sodium intake in an in-center hemodialysis patient. Patient Prefer Adherence. 2008;2:177–184. [PMC free article] [PubMed] [Google Scholar]
- 14.Yon BA, Johnson RK, Harvey-Berino J, Gold BC, Howard AB. Personal digital assistants are comparable to traditional diaries for dietary self-monitoring during a weight loss program. J Behav Med. 2007;30:165–175. doi: 10.1007/s10865-006-9092-1. [DOI] [PubMed] [Google Scholar]
- 15.Atienza AA, King AC, Oliveira BM, Ahn DK, Gardner CD. Using hand-held computer technologies to improve dietary. Am J prev Med. 2008;34:514–518. doi: 10.1016/j.amepre.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Burke LE, Styn MA, Glanz K, et al. SMART trial: A randomized clinical trial of self-monitoring in behavioral weight management -design and baseline findings. Contemp Clin Trials. 2009;30:540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loff B, Crammond B. Wanted: politicians to champion health (not obesity) Med J Aust. 192(7):397–379. doi: 10.5694/j.1326-5377.2010.tb03563.x. [DOI] [PubMed] [Google Scholar]