Abstract
Recently we cloned and characterized the gene for the wheat transcription factor TaWRKY45 and showed that TaWRKY45 was upregulated in response to benzothiadiazole (BTH) and Fusarium head blight (FHB) and that its overexpression conferred enhanced resistance against F. graminearum. To characterize the functional role of TaWRKY45 in the disease resistance of wheat, in the present study we conducted expression analyses of TaWRKY45 with inoculations of powdery mildew and leaf rust and evaluated TaWRKY45-overexpressing wheat plants for resistance to these diseases. TaWRKY45 was upregulated in response to infections with Blumeria graminis, a causal fungus for powdery mildew, and Puccinia triticina, a causal fungus for leaf rust. Constitutive overexpression of the TaWRKY45 transgene conferred enhanced resistance against these two fungi on transgenic wheat plants grown under greenhouse conditions. However, the expression of two resistance-related genes, Pm3 and Lr34, was not induced by the inoculation with powdery mildew in TaWRKY45-overexpressing wheat plants. These results suggest that TaWRKY45 is involved in the defense responses for multiple fungal diseases in wheat but that resistance involving TaWRKY45 differs from at least Pm3 and/or Lr34-related resistance. Our present and previous studies indicate that TaWRKY45 may be potentially utilized to improve a wide range of disease resistance in wheat.
Keywords: leaf rust, multiple resistance, overexpression, powdery mildew, TaWRKY45, wheat
Introduction
Wheat is attacked by a large variety of pathogens, mostly of fungal origin. Powdery mildew, caused by the obligate biotrophic fungus, Blumeria graminis f. sp. Tritici, is one of the most consistently damaging diseases of wheat worldwide. It appears on susceptible cultivars from the seedling stage through head emergence and causes a decrease in the number and survival of tillers, the number of kernels per head, and the kernel weight (Everts and Leath 1992). Much effort has been devoted to the exploitation of genetic plant resistance to control this disease. To date, there have been 59 powdery mildew (Pm) resistance alleles identified and designated at 43 loci (Pm1-Pm43) (He et al. 2009), and Pm3 is the only wheat gene that has been cloned. Pm3 belongs to the largest class of R genes that encode nucleotide-binding site (NBS) and leucine-rich repeat (LRR) domains (Yahiaoui et al. 2004). Pm genes confer race-specific resistance to powdery mildew in wheat and have been extensively used in classical breeding (Bennett 1984, Hsam and Zeller 2002). Race-specific resistance usually gives complete protection against the pathogen at all growth stages, but the resistance is vulnerable to genetic changes in the pathogen and is therefore often of short-lived effectiveness.
Leaf rust, caused by the obligate parasitic fungus, Puccinia triticina ( recondita f. sp. tritici), is considered to be the most widespread and destructive disease of wheat. High levels of pathogenic variation and adaptability to diverse climatic conditions are hallmarks of P. triticina and contribute to its persistence as a major pathogen of wheat (Kolmer 2005). The pathogen interferes with wheat plants at all developmental stages, causing losses in yield and reduced grain quality. More than 60 leaf rust resistance genes (Lr) have been described in wheat (McIntosh et al. 2008). Lr34 is a race-nonspecific resistance gene located on chromosome 7DS, which encodes an ATP-binding cassette (ABC) transporter of the ABCG (formerly pleiotropic drug resistance, PDR) subfamily (Krattinger et al. 2009). Lr34 is also known to confer partial resistance against stem rust (Dyck 1987) and stripe rust (P. striiformis) (Singh 1992), as well as powdery mildew (Spielmeyer et al. 2005).
The regulation of gene expression, including both the temporal aspects and the magnitude of changes in gene expression, is one of the factors that determine susceptibility or resistance to pathogens in host plants. Among the several classes of DNA-binding transcription regulators, the WRKY transcription factors are the best characterized in the context of pathogen defense mechanisms (Eulgem et al. 2000). The WRKY family of proteins contains one or two highly conserved WRKY domains characterized by the hallmark hepta-peptide, WRKYGQK, at the N-terminal end and a novel zinc-finger like motif (Eulgem et al. 2000). The WRKY domain binds to the W box (C/TTGACC/T) in the promoter of target genes to modulate transcription (de Pater et al. 1996). The transcription of most of the 74 Arabidopsis WRKY genes is induced by infection with pathogens or by other defense-related signals, and the overexpression of several WRKYs in Arabidopsis enhances the resistance to various fungal and bacterial pathogens (Eulgem 2006). In rice, OsWRKY45 has been identified from a transcriptomic analysis of leaves from benzothiadiazole (BTH)-treated rice plants (Shimono et al. 2007). BTH treatment or OsWRKY45 overexpression enhances the resistance of rice against the blast fungus, Magnaporthe grisea, whereas the BTH-inducible resistance is compromised in OsWRKY45 RNA-interfered plants. Furthermore, the overexpression of OsWRKY45 has produced enhanced resistance to the bacterial diseases caused by Pseudomonas syringae (Qiu and Yu 2009) and Xanthomonas oryzae (Tao et al. 2009).
In our previous study, we had characterized TaWRKY45, a wheat ortholog of OsWRKY45, and demonstrated its role in Fusarium head blight (FHB) resistance (Bahrini et al. 2011). TaWRKY45 expression had been found to be induced by BTH treatment and inoculation with Fusarium graminearum, and transgenic wheat plants constitutively expressing wheat TaWRKY45 had shown increased type II resistance to FHB. Based on these findings, we presumed that TaWRKY45 could be used to enhance multiple disease resistance in wheat, particularly in the case of diseases caused by fungal pathogens. As a first step, we studied the expression of TaWRKY45 in wheat upon infection with Blumeria graminis, a causal fungus of powdery mildew, and Puccinia triticina, a causal fungus of leaf rust. We also evaluated the resistance of TaWRKY45-overexpressing wheat plants against powdery mildew and leaf rust, and we compared the expression patterns of two disease related genes, Pm3 and Lr34, against powdery mildew and leaf rust between transgenic and non-transgenic plants.
Materials and Methods
Plant materials
The wheat (Triticum aestivum L.) cultivar Bobwhite S9856 (Bw) derived from CIMMYT, which is susceptible to wheat powdery mildew and leaf rust, was used for the expression analysis and the resistance testing. Three cultivars, Norin 4 (N4), Norin 29 (N29) and Selkirk (Sk) were used for the resistance testing to powdery mildew: N4, highly susceptible; Sk, moderately resistant; N29, highly resistant. Furthermore, three cultivars used for the resistance testing to leaf rust: Norin 61 (N61), susceptible; Kitakami-komugi (Kt) and Hokushin (Hk), resistant. Transgenic Bobwhite lines with a full-length TaWRKY45-A cDNA driven by the CaMV 35S promoter were used for the evaluation of resistance (Bahrini et al. 2011). The BTH treatment was conducted according to our previous study (Bahrini et al. 2011).
Expression analysis by RT-PCR
Total RNA was extracted and the first-strand cDNA was synthesized using the methods of our previous study (Bahrini et al. 2011). For the amplification of TaWRKY45 transcripts, the following PCR conditions were used with the primer pair 5′-CCAAGCACTCAAAGGCCTAC-3′ and 5′-CTAAACCTACGACGCGCAAT-3′: 94°C for 2 min, 30 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, and a final extension of 72°C for 1 min. The TaWRKY45 transgene was amplified using the 5′-CCAAGCACTCAAAGGCCTAC-3′ and 5′-CGAGCTCGGATCCACTAGTAA-3′ primer pair with the following PCR conditions: 94°C for 2 min, 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min and a final extension at 72°C for 2 min. Pm3 was amplified using the primer pair 5′-GGAGGAGCAGCACAAGATTC-3′ and 5′-TAATGTGGTCTTGCCAAGGCC-3′ under following PCR conditions: 94°C for 2 min, 30 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, and a final extension of 72°C for 1 min. Lr34 was amplified using the 5′-TTGGGTTGCTTCTTGTTTCC-3′ and 5′-TGTCAAGAGGGCATTGAGTG-3′ primer pair. The PCR conditions were as follows: 94°C for 2 min, 32 cycles of 94°C for 30 s, 58°C for 1 min and 72°C for 30 s, and a final extension of 72°C for 1 min. The primers were designed using the Pm3 and Lr34 sequences available in the DDBJ/Genbank/EMBL databases under accession numbers AY325736 and FJ436983, respectively. The wheat actin gene was used as a control with the primer pair 5′-GCCGTGCTTTCCCTCTATG-3′ and 5′-GCTTCTCCTTGATGTCCCTTA-3′.
Amplified fragments of Pm3 and Lr34 cDNAs were cloned and sequenced to verify the integrity of the amplified sequences.
Fungus inoculation and disease assessment
The inoculation of powdery mildew and the incubation after inoculation were conducted according to Tosa and Sakai (1990). The primary culture of Blumeria graminis f. sp. tritici Th-1 strain was maintained on seedlings of the highly susceptible cultivar Norin 4 (N4).
The powdery mildew resistance test was conducted by blowing conidia on the primary leaves of 7-day-old wheat plants grown in seedling cases. For the test, we used TaWRKY45-overexpressing transgenic wheat plants, Bobwhite, N4, Selkirk (Sk, a moderately resistant cultivar), and Norin 29 (N29, a highly resistant cultivar). Host reactions were recorded 7 to 10 days after inoculation, when the susceptible control was heavily infected. The infection type (IT) of powdery mildew was scored on a 1–6 scale, as described by Xu et al. (2006). The scale values are interpreted as follows: 1, highly resistant (no visible disease symptom); 2, resistant (showing hypersensitive necrotic flecks); 3, moderately resistant (minute colonies with few conidia); 4, moderately susceptible (colonies with moderately developed hyphae and moderate conidial production); 5, susceptible (colonies with well-developed hyphae and abundant conidia but not coalesced colonies); and 6, highly susceptible (colonies with well-developed hyphae, abundant conidia, and coalesced colonies).
Inoculation of the leaf rust and incubation after inoculation were performed according to Yamada et al. (1960). The primary culture of Puccinia triticina (recondita f. sp. tritici) race 1B was maintained on seedlings of the highly susceptible cultivar Norin 61 (N61).
The inoculation of transgenic plants was conducted by shaking the primary culture on the leaves of two-week-old transgenic seedlings. The inoculated plants were placed in the dark for 20 h at room temperature and 100% relative humidity to allow the germination of spores, and the plants were then returned to the growth cabinet. N61, the susceptible cultivar, Kitakami-komugi (Kt) and Hokushin (Hk), the resistant cultivars, and non-transgenic Bobwhite were used as controls. Leaf rust symptoms were measured at 7 days after inoculation by recording the infection type on the first leaf, according to the 0 to 4 scale of Stakman et al. (1962), as follows: 0, no visible symptoms (immune); ;, hypersensitive necrotic or chlorotic flecks without uredia (highly resistant); 1, small uredia surrounded by a necrosis or chlorosis (resistant); 2, small to medium uredia surrounded by a necrotic border (moderately resistant); 3, medium to large uredia with or without chlorosis (susceptible); and 4, large uredia without chlorosis (highly susceptible).
Results
Expression profiling of TaWRKY45 and other genes related to powdery mildew resistance in the powdery mildew-inoculated plants
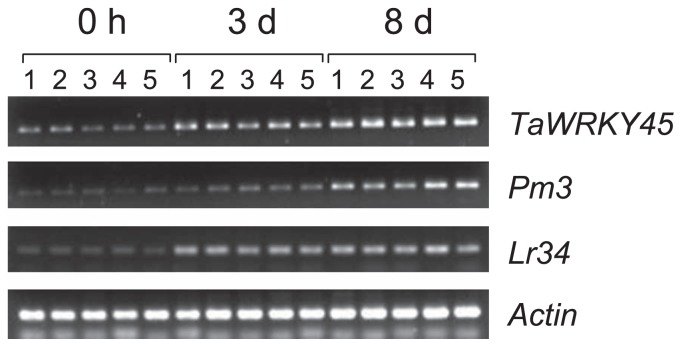
We examined the expression profile of TaWRKY45 in wheat seedlings (cv. Bobwhite) before and after powdery mildew inoculation. Based on the transcription profile by RT-PCR, the basal expression of TaWRKY45 was at a low level in the five replicates before inoculation (Fig. 1). TaWRKY45 transcripts accumulated 3 days after inoculation (dai) with B. graminis, and the expression level further increased at 8 dai. The time point of 3 dai corresponds to the appearance of microscopic colonies and at 8 dai, the powdery mildew symptoms were extensively developed in the Bobwhite cultivar (data not shown). These results suggested that the TaWRKY45 gene is induced in the leaves by powdery mildew infection and that this induction can be positively related to the defense mechanism of wheat against powdery mildew disease.
Fig. 1.
Transcription profiles of TaWRKY45, Pm3 and Lr34 genes in the leaves of Bobwhite before inoculation (0 h) and 3 days (3 d) and 8 days (8 d) after inoculation with B. graminis. Five plants at each time point were used as replicates, and plant number is indicated above the gel image.
The temporal expression patterns of Pm3 and Lr34 genes, the cloned genes conferring resistance to powdery mildew, were examined over the same timespan following powdery mildew inoculation. As shown in Fig. 1, the transcript level of Pm3 was very low in the seedlings before inoculation, but this level increased slightly at 3 dai and then further increased at 8 dai. Similarly, the expression of Lr34 was very low before powdery mildew inoculation and increased at 3 dai and 8 dai (Fig. 1).
Expression of TaWRKY45 after leaf rust inoculation
The expression of the TaWRKY45 gene was examined in leaves (cv. Bobwhite) before the inoculation of P. triticina and at different time points after the inoculation. TaWRKY45 transcripts showed a very low to non-detectable level before inoculation and at 3 h and 24 h after inoculation (Fig. 2). However, the level of the transcripts clearly increased in the inoculated seedlings at 7 dai, when macroscopic symptoms appeared on the leaves. Whereas, the transcript level of the control (non-inoculated) plants remained low. These results indicated that the TaWRKY45 gene was also induced in the leaves by leaf rust, although the response to leaf rust occurred later than the response to powdery mildew.
Fig. 2.
Transcription profiles of the TaWRKY45 gene in the leaves of Bobwhite after inoculation with P. triticina at 0 hours (0 h), 3 h, 24 h and 7 days (7 d) after inoculation. Control, non-inoculated seedlings; Inoculated, those seedlings inoculated with the fungus. Five plants at each time point were used as replicates, and plant number is indicated above the gel image.
Expression profiling of TaWRKY45 and other genes related to powdery mildew resistance in the BTH-treated plants
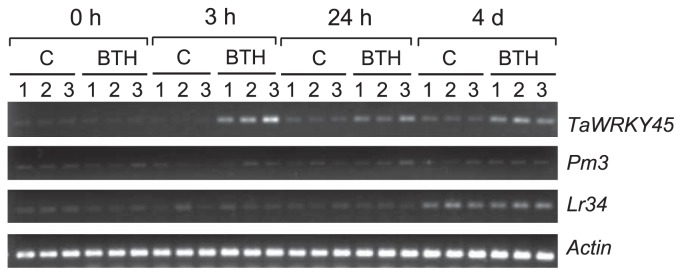
BTH is a functional analog of SA and one of the so-called plant activators. These activators induce the same characteristic set of systemic acquired resistance (SAR) genes. Prior research has indicated that BTH induces acquired resistance in wheat, including resistance to powdery mildew, leaf rust and leaf spot (Görlach et al. 1996). Moreover, we found that the expression of TaWRKY45 was induced in wheat leaves by BTH application (Bahrini et al. 2011). To determine whether BTH and TaWRKY45 are involved in Pm3- and/or Lr34-mediated resistance, we assessed the expression profiles of Pm3 and Lr34, along with TaWRKY45, after BTH application. As we had found previously (Bahrini et al. 2011), the TaWRKY45 transcripts were barely detectable before the BTH treatment of the leaves but accumulated rapidly within the first 3 h after treatment. The expression levels decreased slightly at 24 h but maintained a high level up to 4 days after BTH application, whereas in the control, the levels remained low (Fig. 3). In contrast to TaWRKY45, the expression level of Pm3 was not changed by the BTH treatment and remained at a very low level. Similarly, the expression of Lr34 was not affected by the BTH treatment.
Fig. 3.
Transcription profiles of TaWRKY45, Pm3 and Lr34 genes in leaves after BTH application at 0 hours (0 h), 3 h, 24 h and 4 days (4 d). C and BTH represent samples treated with the solvent only and with BTH, respectively. Three plants at each time point were used as replicates, and plant number is indicated above the gel image.
Evaluation of TaWRKY45-overexpressing wheat plants for resistance to powdery mildew
Wheat T1 lines overexpressing TaWRKY45 were used to test for resistance against powdery mildew. PCR and RT-PCR were performed at the seedling stage to check the inheritance and expression, respectively, of the transgene among T1-segregating progeny. As shown in Fig. 4A, 4B, the expression of the transgene was correlated with the introduced TaWRKY45 transgene.
Fig. 4.
Evaluation of TaWRKY45-overexpressing wheat plants for resistance to powdery mildew. (A and B) Results of genomic PCR (A) and RT-PCR (B) showing the segregation of the transgene inheritance and expression, respectively. (C) Primary leaves of transgenic wheat plants (lines 1, 3, 6, 7 and 10) and controls including non-transgenic Bobwhite (Bw), Norin 4 (N4), Selkirk (Sk) and Norin 29 (N29) at 7 dai with powdery mildew. The number above each leaf indicates the recorded infection type (IT). The number above each line name indicates the plant number. P indicates a plasmid control. The vertical bar on the left represents 1 cm scale.
The experiments to test for resistance against powdery mildew were conducted on the primary leaves of 7-day-old TaWRKY45-overexpressing plants. Non-transgenic Bobwhite, N4, Sk and N29 were also used as controls. The disease symptoms were evaluated at 7 to 10 dai using a 1-to-6 infection type (IT) scale (Xu et al. 2006; see the Materials and Methods). The visual symptoms of powdery mildew started to appear at 5 dai and became well developed by 7 dai. The primary leaves of the control cultivar Bobwhite showed well-developed hyphae and abundant conidia, but these symptoms were less severe than those observed for the highly susceptible cultivar, N4. The infection types of Bobwhite and N4 were recorded as IT5 (susceptible) and IT6 (highly susceptible), respectively (Fig. 4C). The two cultivars, Sk and N29, exhibited IT3 (moderate resistant) and IT1 (high resistant), respectively.
As shown in Fig. 4, the presence of the TaWRKY45 transgene, its expression (immediately before inoculation) and the resistance level against powdery mildew were well correlated in the segregated T1 generation. Four plants of line 1 were segregated for the presence of the transgene: plant 3 had the transgene, but the other three did not. The powdery mildew resistance of plant 3 was recorded as IT3 (moderately resistant), but the other plants (without the transgene) were classified as IT5 (susceptible). Conversely, all 4 of the plants of line 7 had the TaWRKY45 transgene and showed high resistance (IT1) to powdery mildew. In the highly resistant plants, we observed hypersensitive reactions, with the formation of chlorotic flecks and restriction of pathogen growth into small colonies with little production of conidia.
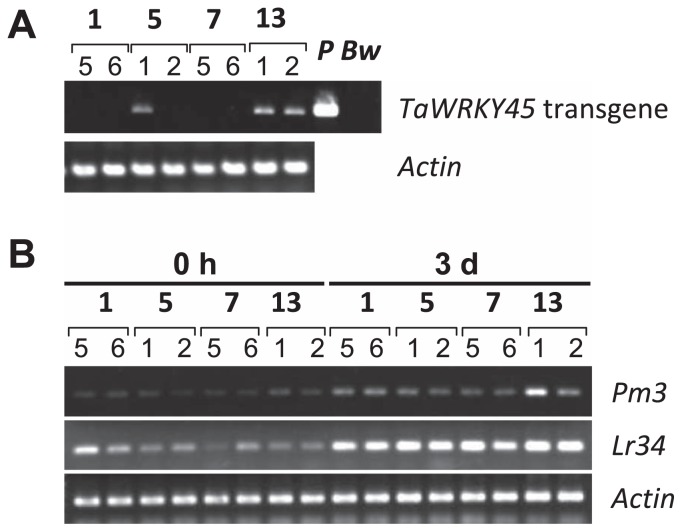
To determine if the powdery mildew resistance through the overexpression of TaWRKY45 observed in this study resulted from regulatory changes in the expression of Pm3 and/or Lr34, we assessed the expression of Pm3 and Lr34 in the segregated T1 generations and compared the results with those associated with TaWRKY45 transgene expression. As shown in Fig. 5, there was no difference between the transgenic wheat plants with and without the expression of the TaWRKY45 transgene (e.g., plants 1 and 2 of line 5). However, the expression levels of Pm3 and Lr34 were induced by the powdery mildew inoculation, as shown in Fig. 1.
Fig. 5.
Expression of the TaWRKY45 transgene and Pm3 and Lr34 in the T1 progeny of transgenic lines 1, 5, 7 and 13. The number below each line name indicates the plant number. P and Bw represent the plasmid construct of the transgene and Bobwhite as controls, respectively. (A) Transcription profile of the TaWRKY45 transgene in the T1 progenies of transgenic lines 1, 5, 7 and 13 before inoculation with powdery mildew. (B) Transcription profiles of the Pm3 and Lr34 genes in the leaves before (0 h) and 3 days (3 d) after inoculation with powdery mildew.
Evaluation of TaWRKY45-overexpressing wheat plants for resistance to leaf rust
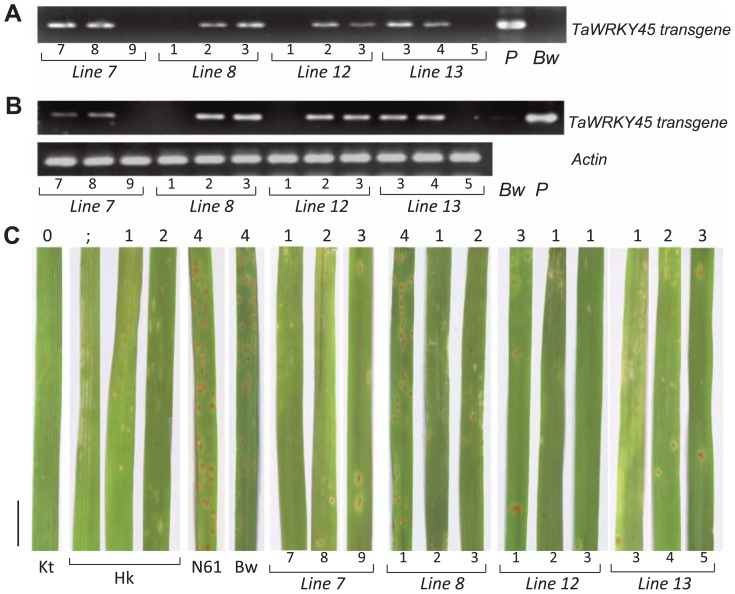
We evaluated the resistance to leaf rust of the same TaWRKY45-overexpressing lines that were used for the test of resistance against powdery mildew using the T1 generations. As shown in Fig. 6B, 6C, the presence of TaWRKY45 transgene and its expression immediately before inoculation were well correlated in the segregated T1 generation.
Fig. 6.
Evaluation of TaWRKY45-overexpressing wheat plants for resistance to leaf rust. (A and B) Results of genomic PCR (A) and RT-PCR (B) showing the segregation of the transgene inheritance and expression, respectively. (C) Leaves of transgenic plants (lines 7, 8, 12 and 13) and controls, including Kitakami-komugi (Kt), Hokushin (Hk), Norin 61 (N61) and non-transgenic Bobwhite (Bw), at 7 dai with leaf rust. The number above each leaf indicates the recorded infection type. The number above each line name indicates the plant number. P indicates a plasmid control. The vertical bar on the left represents 1 cm scale.
The symptoms of leaf rust were visible at 6 to 7 dai. The control cultivar (Bobwhite) showed large numbers of uredia, with an abundant production of spores, as was also observed for the highly susceptible cultivar, N61. They were recorded as IT4 (highly susceptible) (Fig. 6A). Kt did not show any symptoms and was classified as with IT0 (immune), whereas Hk showed reactions that varied from hypersensitive with chlorotic flecks (highly resistant, IT;) to the presence of small uredia with a necrotic border (moderately resistant, IT2).
As shown in Fig. 6, the presence of the TaWRKY45 transgene, its expression and the level of resistance against leaf rust were well correlated in the segregated T1 generation. Three plants of each transgenic line were segregated for the presence of the transgene: two plants had the transgene, and one did not. The plants with the transgene were showed resistance (IT1) or moderate resistance (IT2); however, plants without the transgene were classified as IT3 (susceptible) or IT4 (highly susceptible). In the resistant plants, we observed a hypersensitive reaction, with the formation of chlorotic flecks and a restriction of the pathogen growth to small colonies without conidial productions. This result suggests that the protection conferred by the TaWRKY45 transgene is a type of hypersensitive reaction.
Discussion
In plants, many WRKY proteins are involved in the defensive response against pathogen attack. We have previously cloned the gene for the WRKY45 transcription factor from wheat. This factor has been implicated in the resistance response pathway mediated by BTH treatment and in resistance against Fusarium head blight (FHB) (Bahrini et al. 2011). To analyze the involvement of TaWRKY45 in the basal defense against pathogens further, we analyzed its expression in response to the pathogenic fungi, Blumeria graminis and Puccinia triticina, the causal fungi of powdery mildew and leaf rust, respectively, and we then evaluated the transgenic wheat plants overexpressing TaWRKY45 for resistance to both fungal diseases.
We observed that the expression of TaWRKY45 progressively increased in the primary leaves at 3 days and 8 days after inoculation (dai) of the powdery mildew (Fig. 1). We also found that its expression was induced at 7 dai with the leaf rust fungus (Fig. 2). Usually, it is thought that an important reaction for resistance occurs in the early stage of infection, and a reaction occurred in the late stage of infection might be a stress response. Although we do not have enough data to classify the response of TaWRKY45 to both diseases, there is a possibility that the response and function of TaWRKY45 for both diseases are different. Nevertheless, we demonstrated that the overexpression of TaWRKY45 produced the significant resistances to both powdery mildew (Fig. 4) and leaf rust (Fig. 6). Furthermore, in our previous report (Bahrini et al. 2011) and in the present study, treatment with BTH induced the expression of TaWRKY45 (Fig. 3). These data indicate that TaWRKY45 is situated in the signaling pathway for the systemic acquired resistance induced by BTH and that the activation of TaWRKY45 by BTH results in resistance to powdery mildew and, possibly, to leaf rust, although the response of TaWRKY45 expression to leaf rust inoculation was comparatively late. This hypothesis is consistent with the findings of a previous study (Görlach et al. 1996), which had shown that BTH protects wheat plants systemically against powdery mildew and leaf rust infections through its effects on multiple steps in the life cycle of the pathogen.
Our results regarding the expression of Pm3 and Lr34 were similar to our findings for TaWRKY45. Pm3 and Lr34, which have been previously found to confer resistance to powdery mildew and/or leaf rust in wheat (Yahiaoui et al. 2004, Spielmeyer et al. 2005), were induced by powdery mildew inoculation (Fig. 1). This observation suggests that Pm3 and/or Lr34 are located downstream of the BTH-induced and TaWRKY45-mediated signaling pathway and that they confer resistance to powdery mildew and/or leaf rust through the regulation by TaWRKY45. However, we did not detect any induction of the expression of the Pm3 or Lr34 genes after the application of BTH (Fig. 3) or by the overexpression of TaWRKY45 (Fig. 4). These results indicate that TaWRKY45 functions in the defense response of wheat through a pathway independent of those involving for Pm3 and/or Lr34. As a next step, it will be very important to identify the genes regulated by TaWRKY45 that play a direct role in the resistance to these fungal diseases.
The results of this study and our previous study (Bahrini et al. 2011) reveal that the TaWRKY45 gene produces enhanced resistance to multiple fungal diseases: FHB (caused by F. graminearum), powdery mildew (caused by B. graminis) and leaf rust (caused by P. triticina). These results suggest that TaWRKY45 may provide a broader range of enhanced resistance that may include bacterial pathogens of wheat as well as fungal pathogens. Such broad-range effects have been demonstrated for OsWRKY45 in rice (Qiu and Yu 2009, Shimono et al. 2007, Tao et al. 2009). Wheat is affected by many diseases other than FHB, powdery mildew and leaf rust. These diseases include stem rust and other rusts caused by Puccinia species (fungal diseases), leaf blight caused by P. syringae (a bacterial disease), and several mosaics (viral diseases). Recently, Ug99, a race of stem rust (Puccinia graminis tritici), has become a serious threat to the global wheat production because of its wide range of virulence and the severe loss in yield that it causes (Singh et al. 2006). The evaluation of TaWRKY45-overexpressing wheat plants for resistance to a wide range of disease pathogens will be the next step in improving the disease resistance of wheat. In addition, the examination of allelic variations of TaWRKY45 to find a more active form will be useful in breeding new wheat cultivars by conventional methods.
Acknowledgments
We would like to thank Prof. Yukio Tosa for providing us with the powdery mildew strain, for his kindness in teaching us the experimental procedure used with powdery mildew and for his critical reading of our manuscript. We thank Mr. Hiroyuki Ito for his kindness in teaching us the experimental procedure used with leaf rust. We also thank Dr. Rie Kikuchi for her valuable suggestions and Mr. Masao Oshima for his technical assistance. We are grateful to the Vlaams Inter-universitair Instituut voor Biotechnologie (VIB) in Belgium and National Institute of Agrobiological Sciences (NIAS) genebank for furnishing the pB2GW7 expression vector from and the leaf rust strain, MAFF: 102012, respectively. I. B. was supported by a JIBIC Fellowship from the Tunisian Ministry of Higher Education, Scientific Research and Technology.
Literature Cited
- Bahrini I, Sugisawa M, Kikuchi R, Ogawa T, Kawahigashi H, Ban T, Handa H. Characterization of a wheat transcription factor, TaWRKY45, and its effect on Fusarium head blight resistance in transgenic wheat plants. Breed Sci. 2011;61:121–129. [Google Scholar]
- Bennett FGA. Resistance to powdery mildew in wheat: a review of its use in agriculture and breeding programmes. Plant Pathol. 1984;33:279–300. [Google Scholar]
- de Pater S, Greco V, Pham K, Memelink J, Kijne J. Characterization of a zinc-dependent transcriptional activator from Arabidopsis. Nucleic Acids Res. 1996;24:4624–4631. doi: 10.1093/nar/24.23.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PL. The association of a gene for leaf rust resistance with the chromosome 7D suppressor of stem rust resistance in common wheat. Genome. 1987;29:467–469. [Google Scholar]
- Eulgem T. Dissecting the WRKY web of plant defense regulators. PLoS Pathogens. 2006;2:e126. doi: 10.1371/journal.ppat.0020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Everts KL, Leath S. Effect of early season powdery mildew on development, survival, and yield contribution of tillers of winter wheat. Phytopathol. 1992;82:1273–1278. [Google Scholar]
- Görlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH, Oostendorp M, Staub T, Ward E, Kessmann H, Rayals J. Benzothiadizole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 1996;8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Chang Z, Yuan Z, Zhan H, Zhang X, Liu J. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor Appl Genet. 2009;118:1173–1180. doi: 10.1007/s00122-009-0971-z. [DOI] [PubMed] [Google Scholar]
- Hsam SLK, Zeller FJ. Breeding for powdery mildew resistance in common wheat (Triticum aestivum L.) In: Bélanger RR, Bushnell WR, Dick AJ, Carver TLW, editors. The Powdery Mildews: A Comprehensive Treatise. Am Phytopath Soc; St Paul, MN: 2002. pp. 219–238. [Google Scholar]
- Kolmer JA. Tracking wheat rust on a continental scale. Curr Opin Plant Biol. 2005;8:441–449. doi: 10.1016/j.pbi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science. 2009;323:1360–1363. doi: 10.1126/science.1166453. [DOI] [PubMed] [Google Scholar]
- McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers J, Morris C, Somers DJ, Appels R, Devos KM. Catalogue of gene symbols for wheat. KOMUGI Integrated Wheat Science Database. 2008 available at http://www.shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp.
- Qiu YP, Yu DQ. Overexpression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ Exp Bot. 2009;65:35–47. [Google Scholar]
- Shimono M, Sugano S, Nakayama A, Jiang C-J, Ono K, Toki S, Takatsuji H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–2076. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP. Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathol. 1992;82:835–838. [Google Scholar]
- Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, Njau P, Ward RW. Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 2006;2006;1(No. 054) [Google Scholar]
- Spielmeyer W, McIntosh RA, Kolmer J, Lagudah ES. Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor Appl Genet. 2005;111:731–735. doi: 10.1007/s00122-005-2058-9. [DOI] [PubMed] [Google Scholar]
- Stakman EC, Stewart DM, Loegering WQ. Identification of physiological races of Puccinia graminis var. tritici. U.S. Agric. Res. Serv. Bull. E-617 (rev.) 1962:53. [Google Scholar]
- Tao Z, Liu H, Qiu D, Zhou Y, Li X, Xu C, Wang S. A Pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 2009;151:936–948. doi: 10.1104/pp.109.145623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa Y, Sakai K. The genetics of resistance of hexaploid wheat to the wheatgrass powdery mildew fungus. Genome. 1990;33:225–230. [Google Scholar]
- Xu XY, Bai GH, Carver BF, Shaner GE, Hunger RM. Molecular characterization of a powdery mildew resistance gene in wheat cultivar Suwon92. Phytopathol. 2006;96:496–500. doi: 10.1094/PHYTO-96-0496. [DOI] [PubMed] [Google Scholar]
- Yahiaoui N, Srichumpa P, Dudler R, Keller B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 2004;37:528–538. doi: 10.1046/j.1365-313x.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Takahashi K, Takahashi H. On the physiologic races of wheat leaf rust, Puccinia recondite tritici, in Japan in 1952~’58. Bull Tohoku Natl Agric Exp Stn. 1960;20:42–69. [Google Scholar]