Abstract
Photoperiod sensitivity is an important trait related to crop adaptation and ecological breeding in common buckwheat (Fagopyrum esculentum Moench). Although photoperiod sensitivity in this species is thought to be controlled by quantitative trait loci (QTLs), no genes or regions related to photoperiod sensitivity had been identified until now. Here, we identified QTLs controlling photoperiod sensitivity by QTL analysis in a segregating F4 population (n = 100) derived from a cross of two autogamous lines, 02AL113(Kyukei SC2)LH.self and C0408-0 RP. The F4 progenies were genotyped with three markers for photoperiod-sensitivity candidate genes, which were identified based on homology to photoperiod-sensitivity genes in Arabidopsis and 76 expressed sequence tag markers. Among the three photoperiod-sensitivity candidate genes (FeCCA1, FeELF3 and FeCOL3) identified in common buckwheat, FeELF3 was associated with photoperiod sensitivity. Two EST regions, Fest_L0606_4 and Fest_L0337_6, were associated with photoperiod sensitivity and explained 20.0% and 14.2% of the phenotypic variation, respectively. For both EST regions, the allele from 02AL113(Kyukei SC2)LH.self led to early flowering. An epistatic interaction was also confirmed between Fest_L0606_4 and Fest_L0337_6. These results demonstrate that photoperiod sensitivity in common buckwheat is controlled by a pathway consisting of photoperiod-sensitivity candidate genes as well as multiple gene action.
Keywords: common buckwheat, EST markers, photoperiod-sensitivity candidate genes, photoperiod sensitivity, QTL analysis
Introduction
Common buckwheat (Fagopyrum esculentum Moench, 2n = 16), a pseudocereal, has been widely cultivated in Asia, Europe and North America. In Japan, common buckwheat has been cultivated throughout most of the country, with cultivars classified into three ecotypes: summer, intermediate, and late-summer. The late-summer cultivars are cultivated in low-latitude regions where photoperiod is around 12 to 14 h during the cropping season, and their flowering is generally sensitive to photoperiod. The summer cultivars are cultivated in high-latitude regions where photoperiod is around 14.5 to 15.5 h during the cropping season (summer crop season), and they are generally insensitive to photo-period. Intermediate cultivars show moderate photoperiod sensitivity (Namai 1990). Common buckwheat is considered to be a short-day plant, with the threshold between flowering and non-flowering at a photoperiod of about 14.5 h (Nagatomo 1961). Under long-day conditions, (i.e., photo-period longer than 14.5 h), late-summer cultivars showed wide variation in flowering time, which resulted in low yield because of the increased incidence of malformed flowers and the decrease in ripening rate in the late-flowering individuals (Nagatomo 1961, Nakamura and Nakayama 1950, Sugawara 1958). The intermediate and summer cultivars are considered to have been derived from the late-summer cultivars through both natural and artificial selection (Matano and Ujihara 1979). Minami and Namai (1986a, 1986b) suggested that summer cultivars differentiated from late-summer cultivars through the selection of early-flowering plants under long-day conditions; this selection was part of the domestication of buckwheat for climatic adaptation to the northern part of Japan.
Iwata et al. (2005) reported that allelic richness (i.e., the degree of polymorphism of genetic markers) has decreased in Japanese common buckwheat varieties cultivated in high-latitude regions during selection for early flowering time, and they suggested that the decrease of allelic richness at neutral loci was caused by bottleneck effects caused by directional selection based on photoperiod sensitivity. Ohsawa (1997) reported that the heritability of flowering period was high (95.1%), suggesting that photoperiod sensitivity was a key feature of adaptation and differentiation in common buckwheat. Photoperiod-sensitivity genes, however, have not been identified in common buckwheat.
Minami (1985) suggested that photoperiod sensitivity in common buckwheat was controlled by a number of genes. In several crops, photoperiod sensitivity is considered to be inherited quantitatively (Putterill et al. 2004, Yano et al. 2001). Genetic regulation of flowering induced by photo-period has been studied in Arabidopsis and rice (Oryza sativa L.), and many genes related to photoperiod sensitivity have been detected as quantitative trait loci (QTLs) and isolated by positional cloning (Corbesier and Coupland 2005, Hayama and Coupland 2003). These genes compose the photoperiod-sensitivity pathway (Putterill et al. 2004), and have been confirmed among many species as orthologous genes (Hayama et al. 2002, 2003, Hayama and Coupland 2003, Kojima et al. 2002, Liu et al. 2001a, Nemoto et al. 2003, Yano et al. 2000). Moreover, photoperiod sensitivity in both long-day and short-day plants appears to be controlled by a common mechanism (Putterill et al. 2004). Based on this information, we hypothesized that photo-period sensitivity of common buckwheat would also be controlled by the action of multiple genes orthologous to those of the photoperiod-sensitivity pathways in other species.
To avoid the complex genetic patterns caused by the allog-amous (cross-pollinating) reproductive system of most common buckwheat, we used autogamous (self-pollinating) lines showing differences in photoperiod sensitivity for simplicity of analysis. We used expressed sequence tag (EST) markers derived from cDNAs of candidate photoperiod-sensitivity genes to detect photosensitivity QTLs most efficiently.
In the present study, QTLs related to photoperiod sensitivity in common buckwheat were identified in the following three steps: (1) evaluation of variation in photoperiod sensitivity between two autogamous parental lines, (2) linkage map development using EST markers, and (3) QTL analysis for photoperiod sensitivity.
Materials and Methods
Photoperiod sensitivity of parental lines
For detecting QTLs related to photoperiod sensitivity, we employed two parental lines, 02AL113(Kyukei SC2)LH.self (KYU) and C0408-0RP (CAN). KYU is an autogamous line bred at the National Agricultural Research Center for Kyushu Okinawa Region, and CAN is an autogamous line bred at Kade Research Ltd. (Canada). In general, differences in photoperiod sensitivity between these two lines was visible under photoperiods longer than 14.5 h. We used two photoperiod conditions, a natural long-day condition (day length of ~14.5 h) and an artificial long-day condition (15.5 h), to detect the difference in photoperiod sensitivity between KYU and CAN. For the natural long-day condition, 60 seeds each of KYU and CAN were sowed by 12 seeds per one planter (19 × 59 × 16 cm) and cultivated in growth cabinet without supplemental light at the University of Tsukuba. For the artificial long-day condition, 12 seeds each of KYU and CAN were sowed in a planter and cultivated in a growth cabinet with day length of 15.5 h. Metal halide lamps (MLBOC400C-U) (Mitsubishi Electric Corp., Tokyo, Japan) were used to control the day length at artificial long-day condition.
The investigation of photoperiod sensitivity was performed as follows. The dates of cotyledon development and of the first flower flowering were recorded for each individual. The number of days from the expansion of cotyledons to the first flower flowering was defined as days-to-flowering, which was used as a measure of the photoperiod sensitivity of each individual. The measurements were performed every day until 100 days after sowing. At 100 days after sowing, individuals that had not yet flowered were classified as ‘non-flowering’ and these were excluded from the following analyses. To evaluate differences in photoperiod sensitivity between the populations, we performed analysis of variance (ANOVA) and t tests based on the days-to-flowering under each photoperiod condition by using the program JMP 6.0 (SAS Institute Inc., NC, USA).
cDNA library construction and search for photoperiod-sensitivity candidate gene regions
We developed sets of 362 cDNA clones derived from inflorescences and 1920 cDNA clones derived from leaves. 362 cDNA clones derived from inflorescences was provided by the Niigata University of Pharmacy and Applied Life Sciences. To develop 1920 cDNA clones derived from leaves, we used ‘Miyazakizairai’ (late-summer cultivar) for plant material and sampled leaves six times at 4-h intervals from individuals that were either early flowering or non-flowering under the 15.5 h day-length condition because it is clarified that amount of photoperiod-sensitivity genes expression in Arabidopsis was changed over time. Total RNA was extracted by using RNeasy Plant Mini Kit (Qiagen Inc., CA, USA) following manufacturer’s instructions. cDNA library was directionally constructed (5′ SfiIA, 3′ SfiIB) in pDNR-LIB using the Creator SMART cDNA Library Construction Kit (Clontech Laboratories, Inc., CA, USA) and size-fractionated by using CHROMA SPIN+TE-1000 (Clontech Laboratories, Inc.). Libraries were manually arrayed in 96-well mi-crotiter plates. Glycerol stocks of overnight cultures were prepared in 96-well format. Plasmid DNAs were extracted and BigDye Terminator (Life Technologies Corp., NY, USA) cycle sequenced on ABI PRISM 3730xl Genetic Analyzer (Life Technologies Corp.) by using conventional procedures and the following primers: M13 forward primer (5′-GTAAAACGACGGCCAGT-3′) and M13 reverse primer (5′-AAACAGCTATGACCATGTTCA). Vector sequences and low quality regions were trimmed from EST sequences manually before removing overlapping regions by using BLASTN, and then 863 clones (139 inflorescence-derived clones, designated as the Fest_F group, and 724 leaf-derived clones, designated as the Fest_L group) were used for further analysis. To search for photoperiod-sensitivity candidate gene regions, a homology search against previously reported sequences to Arabidopsis was performed by using tBLASTX. Matches were considered to be significant when the smallest sum probability (p) was less than 0.0001 and bit scores were greater than 100.
Development of the mapping population
We developed a mapping population consisting of 100 F4 lines derived by single-seed descent from a cross between KYU as the female parent and CAN as the male parent. Because we observed stunted growth in the F5 generation, we used the F4 generation as the population for linkage mapping and QTL analysis.
Total DNA samples of both parental lines and the F4 lines were extracted by the following method. About 100 mg leaf tissue was mixed with 500 μl lysis buffer (0.3% sodium dodecyl sulfate, 20 mM Tris-HCl (pH 8.0 at 25°C), 5 mM EDTA, 400 mM NaCl), and incubated at 65°C for 10 min with 5 μl of 10 mg/ml Proteinase K (Wako Pure Chemical Ind., Ltd., Osaka, Japan) and 100 mg/ml RNase A (Qiagen Inc.). The DNA was purified with chloroform-octanol (24 : 1) and precipitated with isopropanol. The pellet was washed with 70% ethanol and resuspended in 100 μl Tris-EDTA buffer.
Marker development and linkage map construction
Primer sets corresponding to three candidate genes (identified in the homology search described above) and randomly selected 170 ESTs (78 ESTs from the Fest_F cDNA clones, 92 ESTs from the Fest_L cDNA clones) among 863 cDNA clones were designed by using PRIMER3 software (Supplemental Table 1). PCR was performed with candidate gene and EST primers and genomic DNA isolated from KYU and CAN as the template. The amplified products were then sequenced to check whether the target region had been amplified. PCR was performed under the following conditions: 30 ng of genomic DNA was amplified by PCR in a 30 μl final reaction volume containing 6 μM each of the forward and reverse primers, 6 mM each of deoxynucleotide triphosphate (dNTPs), 10× PCR buffer, and 0.75 units of Blend Taq DNA polymerase (TOYOBO Co., Ltd., Tokyo, Japan). PCR was performed by using the Mastercycler gradient PCR system (Eppendorf Scientific Inc., Hamburg, Germany). The PCR program consisted of a preliminary denaturation step of 2 min at 94°C; followed by 40 cycles of 30 s at 94°C (denaturation), 30 s at gradient temperatures (50 to 70°C) (annealing), and 2 min at 72°C (extension); with a final extension for 7 min at 72°C. PCR products were analyzed by electrophoresis in 8% acrylamide (29 : 1 acrylamide:bis-acrylamide) gels. For sequencing, the PCR products within a single band were purified by using the QIAquick PCR purification kit (Qiagen Inc.) and then sequenced by using the BigDye Terminator ver. 3.1 Cycle Sequencing Kit (Life Technologies Corp.) and an ABI PRISM 3100 Genetic Analyzer (Life Technologies Corp.).
Primer sets that produced large differences in amplicon length (detected on 8% acrylamide gels) between parental lines were developed as EST markers. In cases where no difference in amplicon length could be detected, we searched for single-nucleotide polymorphisms (SNPs) by sequencing, and developed cleaved amplified polymorphic sequence (CAPS) markers in regions where SNPs were found within restriction-enzyme recognition sites. When no SNP could be found within the recognition sequences of restriction enzymes, derived cleaved amplified polymorphic sequence (dCAPS) markers were developed instead. The SNP searches were performed by using Phred/Phrap/Consed software (Gordon and Green 1998). The selection of restriction enzymes to detect SNPs and design of mismatch primers for dCAPS was conducted with dCAPS Finder 2.0 (Neff et al. 2002; http://helix.wustl.edu/dcaps/dcaps.html).
To detect polymorphisms of CAPS and dCAPS markers, PCR products produced by using EST primers or dCAPS primers were digested with a corresponding restriction enzyme in 12 μl reaction volumes containing 10 μl of the PCR products, 6 units restriction enzyme, and 1.2 μl of the supplied 10× buffer. The reaction temperature was set to the recommended temperature for each restriction enzyme, and the reaction time was 3 h. PCR and electrophoresis conditions were the same as described above.
The candidate gene and EST markers were assayed in the F4 mapping population. A framework linkage map for QTL analysis was constructed with the software JOINMAP 4.0 (Van Ooijen 2006) by using genotype data for each marker. The fit to the Mendelian segregation ratio for each marker was tested with the Chi-square test (P < 0.05). Markers were assigned to linkage groups with a logarithm of odds (LOD) threshold of 3.0.
QTL analysis
The F4 population was cultivated in a growth cabinet and scored for photoperiod sensitivity under a 15.5 h photoperiod, as described above. QTL analysis was performed by using the software MAPQTL version 5 (Van Ooijen 2005). Interval mapping analysis was performed (Lander and Botstein 1989) to locate preliminary QTL positions on the map. Interval mapping was used to select markers significantly associated with the trait to constitute an initial set of cofactors. A backward-elimination procedure was applied to the initial set of cofactors. Only significant markers (P < 0.02) were used as cofactors in the multiple QTL method (MQM) (Jansen 1993, Jansen and Stam 1994) analysis for QTL detection. After the selection of cofactors, MQM analysis was performed. A 1000-permutation test was applied to each data set to establish the LOD value to be used for determining the significance (P < 0.05) of identified QTLs (Churchill and Doerge 1994). The phenotypic variance explained by a single QTL was calculated as the square of the partial correlation coefficient (r2) with the observed variable, adjusted for cofactors. When candidate gene markers could not be mapped, the association between each of candidate gene markers and photoperiod sensitivity was analyzed with ANOVA. To evaluate digenic interactions between pairs of QTLs, we performed two-way ANOVA for two-locus QTL genotypes, as represented by the genotypes of markers nearest QTLs These ANOVA were calculated by using the program JMP 6.0.
Results
Natural variation in photoperiod sensitivity between autogamous lines
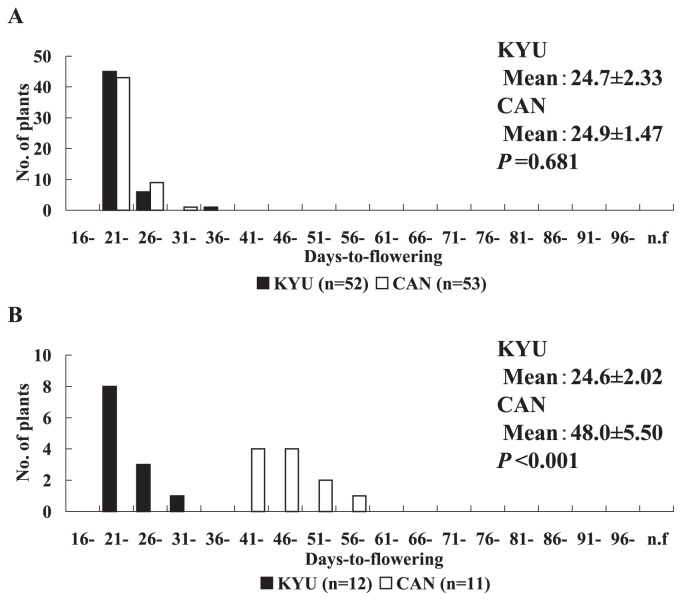
The frequency distributions of days-to-flowering of KYU and CAN are shown in Fig. 1. Under the natural long-day condition, the days-to-flowering distributions of the KYU (range 22–38 days) and CAN (21–32 days) populations were similar (Fig. 1A), and the means (24.7 and 24.9 for KYU and CAN, respectively) were not significantly different (P = 0.681) (Fig. 1A). On the other hand, under the artificial long-day condition with 15.5 h day length, the mean days-to-flowering in KYU (mean 24.6; range 24–31 days) was significantly shorter (P < 0.0001) than that of CAN (mean 48.0; range 41–59 days) (Fig. 1B).
Fig. 1.
Variation in days-to-flowering between the parental lines. (A) Variation in days-to-flowering of parental lines under natural long-day condition (~14.5 h day length). (B) Variation in days-to-flowering of parental lines under artificial long-day condition (15.5 h day length). KYU, 02AL113(Kyukei SC2)LH.self., CAN, C0408-0 RP; P value in each figure showed significance of difference by t-test.
Search for photoperiod-sensitivity candidate gene regions
In tBLASTX searches against the reference sequences of proteins in Arabidopsis, three Fest_L EST regions (Fest_L0268, Fest_L0327 and Fest_L0352) showed high homology with photoperiod-sensitivity genes in Arabidopsis. Fest_L0268 showed high homology with CONSTANS-LIKE 3 (COL3), CONSTANS-LIKE 1 (COL1) and CONSTANS-LIKE 2 (COL2); Fest_L0327, with CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY); and Fest_L0352, with EARLY FLOWERING 3 (ELF3) (Table 1). Among the sequences with homology to multiple genes, Fest_L0268 showed the highest homology with COL3, and Fest_L0327 showed the highest homology with CCA1. We considered these three EST regions as candidate genes related to photoperiod sensitivity in common buckwheat, and designated them FeCOL3, FeCCA1, and FeELF3, respectively. None of the Fest_F regions showed high homology to photoperiod genes in Arabidopsis.
Table 1.
Confirmed sequences showing high homology with photoperiod-sensitivity genes in Arabidopsis
| EST region | Gene product | Gene ID | Bit score | E-value |
|---|---|---|---|---|
| Fest_L0268 | COL3 (CONSTANS-LIKE 3); protein binding/transcription factor/zinc ion binding | 817016 COL3 | 124 | 4.00E-29 |
| COL1 (constans-like 1); transcription factor/zinc ion binding | 831442 COL1 | 112 | 2.00E-25 | |
| COL2 (constans-like 2); transcription factor/zinc ion binding | 821298 COL2 | 112 | 2.00E-25 | |
| Fest_L0327 | CCA1 (CIRCADIAN CLOCK ASSOCIATED 1); DNA binding/transcription activator/transcription factor/transcription repressor | 819296 CCA1 | 142 | 4.00E-34 |
| LHY (LATE ELONGATED HYPOCOTYL); DNA binding/transcription factor | 839341 LHY | 137 | 5.00E-33 | |
| Fest_L0352 | ELF3 (EARLY FLOWERING 3); protein C-terminus binding/transcription factor | 817134 ELF3 | 104 | 1.00E-22 |
Development of linkage map using EST markers
We developed primers for the three candidate genes (FeCOL3, FeCCA1 and FeELF3) and randomly selected 170 ESTs (78 from the Fest_F group, 92 from the Fest_L group) from among the original 863 EST regions (Supplemental Tables 1 and 2 showed results of BLASTX for randomly selected 170 EST regions). Among these, we confirmed polymorphism between KYU and CAN for the three candidate gene and 115 ESTs regions. Sixteen of the 115 polymorphic EST regions included large indels, whereas the three candidate gene and 99 ESTs regions included small indels and SNPs. The 16 EST regions containing large indels were used as length markers; in addition, we developed three candidate gene and 60 SNP-based markers (39 CAPS and 21 dCAPS markers) from among the 99 ESTs markers. Information on the three candidate gene markers and 76 EST markers is shown in Table 2.
Table 2.
Summary information for the three candidate genes and 76 EST markers
| Maker name | Type of marker | SNP region | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Product length (bp) | Annealing temp. (°C) | Restriction enzyme |
|---|---|---|---|---|---|---|---|
| FeCOL3 | CAPS | intron | TCAAGACTCAGCTGGTGAACG | GGACGGATCAGAAAACTCGTC | 713 | 62 | BclI |
| FeCCA1 | CAPS | exon | TCGAGCCAAGCAGTTACTACG | CCACAAGAGGAACGGTCAAC | 901 | 62 | Hpy188I |
| FeELF3b | CAPS | intron | TTGGGAGTTCTGGGGATGAG | TGCCTTGCTCTTTTCCTGGT | 1280 | 62 | DraI |
|
| |||||||
| Fest_F0005b | EST | – | CAAGCCAACAAGCTGGAGAA | AATGGGAGAATGCTTAGTTGCTTAC | 810 | 62 | – |
| Fest_F0035 | EST | – | CAGCTAAGGCAGACGGTTGA | TCATTGCCAGATCTCATTGGA | 148 | 58 | – |
| Fest_F0049 | EST | – | GTGAAATTATGTTTTCTTTTAGAGG | TTCTTGTCTATGAGCACTGAAA | 123 | 54 | – |
| Fest_F0052a | EST | – | GGACGGACATTGGCTGATTAC | TGACCAACCACACACAAAACAC | 163 | 58 | – |
| Fest_F0056a | EST | – | AAGGATCACAAGAAACCGGAA | CAAGCCAAGTATCCATGACAAAC | 266 | 62 | – |
| Fest_F0057 | EST | – | TCTTTGTTGTGTCCAAGATTGCTG | CCAAATCGTAATCATAAGCGTTCC | 314 | 54 | – |
| Fest_F0078 | EST | – | AAAACCCCTCTTTTCTCCCC | TGGCCTTGATTCCACCTTCT | 217 | 62 | – |
| Fest_F0090 | EST | – | CAGCAGTTCATGCATCTGGTAA | CGATGATGATGATGGTGGAGA | 348 | 58 | – |
| Fest_F0095 | EST | – | ATTACTGCCACCTCATCAGACC | AGATCGCTCAGTATCGTGCC | 699 | 62 | – |
| Fest_F0110 | EST | – | GAGGAGGAACAAGACGCACA | TCAAGCCAGTGACATACCACAA | 1354 | 62 | – |
| Fest_F0117 | EST | – | GAGTCCTTTTCCAAGCCCATC | TGGAGAGGTCAATTCCAGCC | 702 | 58 | – |
| Fest_F0119b | EST | – | GGGAGGATTCATTTCTACAGCA | CCAATGTCAGCAGCTTAAGAGAG | 183 | 58 | – |
| Fest_F0129 | EST | – | TGGACGAGCTTTCATAGACG | TCTTCTGAAACATAAATCACAAATG | 161 | 54 | – |
| Fest_F0138b | EST | – | CACATACCAACCAGAACTCAATACA | GCCCGAGTATCGTTTGCTCTC | 184 | 58 | – |
| Fest_L0080 | EST | – | CTTCAGTTTTCCAGTTCCTTCCA | CAGCGCAATGTTTCCCTTC | 787 | 54 | – |
| Fest_L0433 | EST | – | GAAAGAGTTCAAACAAGAGC | AACACCACTCTTCAAAGTTC | 1128 | 58 | – |
|
| |||||||
| Fest_F0031_1 | CAPS | exon | CAAAGCACGTCCAAACAACA | GGATAATGGCGGTGTCAAAA | 788 | 58 | Hpy188I |
| Fest_F0053_2 | CAPS | exon | GCGTTATTTCCCGACTGATG | GATGCACATAGACATGGCTGG | 212 | 62 | BsuRI |
| Fest_F0076_9a | CAPS | exon | TTCTGTTAGTTAAAATGAAAATACA | ATAGCAACGGACACAAATAA | 613 | 54 | HindIII |
| Fest_F0077_2 | CAPS | exon | TTCGGGAGAAATCACAAATACG | TCGAAAGGATTGTTTGCAGTTG | 498 | 62 | ApaLI |
| Fest_F0081_7 | CAPS | exon | CATTACAACACACGCATCGG | AGCAAGGCAAGCTCTTCTGG | 369 | 58 | BshNI |
| Fest_F0083_8 | CAPS | intron | CATGCGCAAACTCCCTTTC | CGTACCACGATCAATTTACAGATCA | 2010 | 54 | MspI |
| Fest_F0087_2 | CAPS | exon | CAGACCTCAAATGTTCCACCAG | GAAGAGGCAAAGGAGGCAAG | 520 | 62 | TaqI |
| Fest_F0100_15a | CAPS | intron | TCCAATCGCGTTGACAGAA | GAAAATGCCGTCCCACAAG | 431 | 58 | AvaII |
| Fest_F0102_1a | CAPS | exon | GGGCAGACTCGGTGCTATTC | GGTGAAGGGATTGTGGCTGT | 291 | 62 | HinfI |
| Fest_F0139_3 | CAPS | exon | ATTCTGGTGTTCACTGCTCTTA | GAGGCCATTGATCGTGATTC | 357 | 62 | TaqI |
| Fest_L0030_1 | CAPS | intron | AAAGAAGGAAAAGGCTCCCC | GATGCAACTATCCATGCCCC | 1135 | 58 | BsuRI |
| Fest_L0041_2 | CAPS | exon | GTCCACAGGAGGAAAGGCAC | CGTGAAACACCAAATTACGACC | 527 | 62 | TaqI |
| Fest_L0046_2 | CAPS | exon | CATGTTCTCAAGCAGCACAAAA | GGATACAGGGTTGGTGGAGG | 373 | 62 | HinfI |
| Fest_L0064_2 | CAPS | exon | CGGAGAATGGCTTCCAAGAG | GAGAAGTGGCATCTGGCTGG | 659 | 66 | TaqI |
| Fest_L0101_1 | CAPS | intron | GGCATCCATGTTTAGCTCTGG | ACCTCAAGGGCACGGTTCT | 392 | 62 | HinfI |
| Fest_L0124_7 | CAPS | exon | CCACCTGGTCCTTCGTCTTC | GAAACGCCACCAACCATACC | 376 | 66 | MspI |
| Fest_L0130_2 | CAPS | exon | CTGAACCAACACAAACGATCAA | TGGTTTGGAACAAGGAAGTCG | 1386 | 62 | HinfI |
| Fest_L0136_5 | CAPS | exon | AAGCAGCACCTTCACAGCAA | CGAAGAAGCTGGGGTCGTAG | 431 | 66 | BsuRI |
| Fest_L0169_3 | CAPS | exon | AACCTCGCATTTTCAGTCCC | ACAGCAATACTCCGGGCTTC | 629 | 60 | MspI |
| Fest_L0186_3 | CAPS | exon | CCACACACACCAAAAGCACC | CACAGAACTAGGACGACTCCTGAA | 492 | 62 | TaqI |
| Fest_L0211_5b | CAPS | exon | GGGAATCGGAGTTGGTTACTCTC | CTGGTGGCTTTGGATGGTTT | 525 | 62 | TaqI |
| Fest_L0219_2 | CAPS | exon | GCTTGTAGATGGGGTGTTGG | CGTTTCGGCAGAGTTTCATC | 717 | 62 | StyI |
| Fest_L0266_5 | CAPS | exon | TCTCTCATCCCAAAACCCAA | TAATCCAACGGCTCAAACCA | 391 | 60 | HhaI |
| Fest_L0292_1 | CAPS | exon | ACCCGTCCCAGTCAAAGAAA | AAAGTCATAAACCCTGATGCCC | 479 | 60 | BsoBI |
| Fest_L0377_4 | CAPS | exon | CGTTTTCATGGTGTCGTTCC | CGGAGAAACGGGTAAGGTGT | 576 | 62 | EcoRV |
| Fest_L0426_12 | CAPS | intron | ATCGATTTCGAGGGGTTTTG | TATTGAGGAGGCCCATTCTG | 700 | 62 | MseI |
| Fest_L0432_1 | CAPS | exon | CAAAGCAGGCAGAGAAAGCA | GCAGATGCAAAATCAACTTCCC | 559 | 62 | AvaII |
| Fest_L0480_1 | CAPS | intron | TGCTGCAACTCACGAGATTAAC | TGGATGCCAGTTCAAAGGTC | 597 | 62 | BglII |
| Fest_L0490_2 | CAPS | exon | GAGCAGCGGGTTGTCTTCT | TAGCGTCTCCAAACTGTCCG | 808 | 62 | HhaI |
| Fest_L0494_6 | CAPS | exon | TCCACAATCTCTCCTTCCTCC | AGCACCAACAAACGCAAAAC | 603 | 62 | TaqI |
| Fest_L0506_1 | CAPS | intron | CGTCGTCATTCTCGCTCTCC | GCATTCATCATTGGGGCTGT | 870 | 62 | DraI |
| Fest_L0542_3a | CAPS | exon | GCGAATTCGGTTTCACAAAA | TACCCTCGCCGACTACAACA | 427 | 62 | KpnI |
| Fest_L0543_1 | CAPS | exon | TTGATTCCTGGGCAACAGAG | ACTTGACAGCAATGCAAAACG | 488 | 60 | MspI |
| Fest_L0556_6 | CAPS | exon | CCCTAATAACCCCGAAACCC | AAAGCCTTCTCAGCAGTGTCC | 481 | 62 | MspI |
| Fest_L0578_2 | CAPS | exon | GGCCCGTCAAAACCAAAA | CCCTGAACCTCAATTGCGAC | 375 | 62 | SacI |
| Fest_L0606_4 | CAPS | exon | TGACAAGATGAAGGAGCTGGA | AGCAGGAGAGCCTGTTGATTT | 308 | 62 | TaqI |
| Fest_L0609_1a | CAPS | exon | TGCCTCTAACTTGTTCCAATTCC | TCGTCGACATCATCTTCCGT | 227 | 62 | AseI |
| Fest_L0706_2 | CAPS | exon | GGGGAGTGAGGAAACCAACA | ACAGAAACCCAATCAATCCACC | 514 | 62 | BstXI |
| Fest_L0712_10 | CAPS | exon | TAACAACCCGCTCTCTTCCC | ATCCATGACACGTCTCGCTC | 592 | 62 | HinfI |
|
| |||||||
| Fest_F0032_2 | dCAPS | intron | ACGCATTCAAATTCAATGCA | GAAGAAGCTGGCGAAGAAGA | 154 | 52 | EcoT22I |
| Fest_F0034_1 | dCAPS | intron | TACTTGATCCAAATCCTTAATATTAGGATC | CGGGATTCTTTGGTGGTTT | 151 | 58 | BamHI |
| Fest_F0043_1 | dCAPS | exon | TACCGGCTTCCCATTGTCAA | TTCCGGAAAGAGGAATCACC | 180 | 62 | HindII |
| Fest_F0050_3 | dCAPS | exon | GTGTCCTCGAAAAGTCCACC | GCCCTTAGGGAGATCAGGAA | 182 | 60 | StyI |
| Fest_F0075_2 | dCAPS | exon | AGAAGTGAGGATGACTTTAA | ATTTCCCATTGGCTTTTT | 158 | 52 | DraI |
| Fest_F0079_1 | dCAPS | exon | TCGATTTTTACCTTCACCCGCCATGGTCAA | CAGTTTAACGGCAAACATACCCTTTT | 138 | 56 | HindII |
| Fest_F0080_4 | dCAPS | exon | TGAGGAGGTGAGGCTTACCG | TGTTTTGTGGGTTTTTTGACTG | 158 | 58 | HhaI |
| Fest_F0085_1a | dCAPS | exon | GACCAAAGAAACAAATTTTA | TTGGTTGGTCATTAATCTAT | 151 | 52 | DraI |
| Fest_F0101_1 | dCAPS | exon | AGGATAAAAAGAAGAAGAAGAGTAAAGATT | CCTGCGACATGCATAATTAAA | 176 | 58 | HinfI |
| Fest_F0108_1 | dCAPS | exon | GAAGTAGTTGTAGACAGAGT | TAGTTTGGTAATAATCTAGC | 224 | 50 | HinfI |
| Fest_F0112_3 | dCAPS | exon | TACTAATACAAACTTAATTT | CTTATGATAGGTGCTAAT | 198 | 42 | DraI |
| Fest_F0127_1 | dCAPS | exon | CGCCGTCGCCGCCGCTCAGGAGTCTCCGTC | TCCAATCGCTCAGAAGAGGAATCCGAAGG | 139 | 62 | HindII |
| Fest_F0133_1 | dCAPS | exon | AAGTGTCCTTGATCTTCAAG | AGCTCAACAACTCTTCACTT | 175 | 50 | HindIII |
| Fest_F0134_1 | dCAPS | exon | CAAGAACCTCCAAAGTAAGTTCTAATATGC | GCTTACAATTGCAACTTTACAACGA | 168 | 58 | EcoT22I |
| Fest_L0053_3 | dCAPS | exon | AACTACAGTACTGGTGGGTC | CTCATCACCCTTCTTCTTC | 164 | 54 | MspI |
| Fest_L0129_12a | dCAPS | exon | GCGCAGCTTGCTATTGCTCC | CCCAAAGATCCCAATCCCTC | 177 | 56 | MspI |
| Fest_L0242_1 | dCAPS | exon | CGCTATGCCGCCTTACTGCG | GCCATGTCGCGGAAGAAGGTC | 167 | 54 | HhaI |
| Fest_L0329_3 | dCAPS | intron | CATCCATTCTTGATTTCAAG | TACACTCAGGAGTCAGGAAC | 171 | 56 | ApoI |
| Fest_L0337_6 | dCAPS | exon | ATCTTCAGGCCTAATGTTAA | AATCAGCAAACTCTCTAAGC | 289 | 54 | HindII |
| Fest_L0472_1 | dCAPS | exon | GGATGAGGTGGGGCCAGGTGCCAGAGCTGT | TTCAAGGGAGAAATGGAGGCCATGA | 123 | 56 | BstXI |
| Fest_L0582_7 | dCAPS | exon | GAAAACATGTTCTTAGATAT | AGAAGGATAGCTTAAATG | 154 | 50 | EcoRV |
Marker showed segregation ratio that was significantly different from the expected Mendelian ratio.
Marker could not be mapped (Fig. 2).
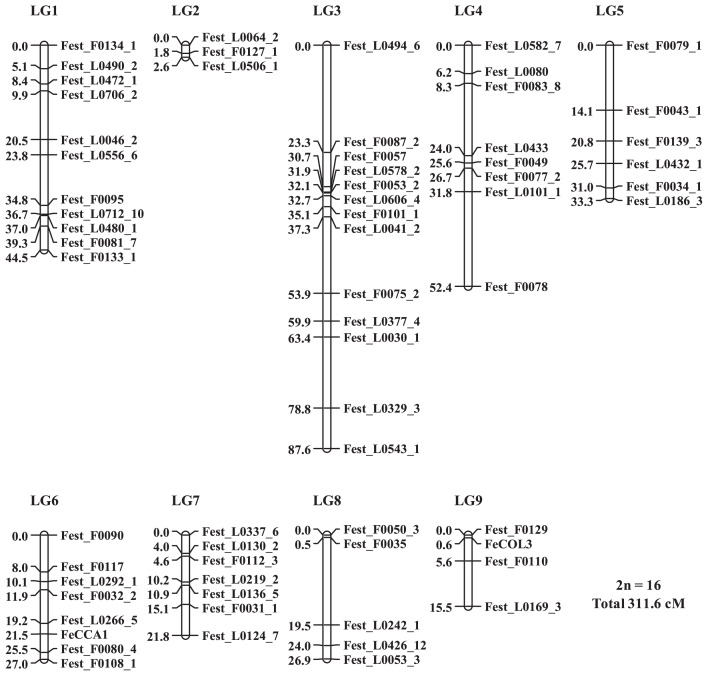
Through the linkage mapping of these markers, the positions of 63 EST markers were estimated. Nine other EST markers showed departure from Mendelian segregation ratios, and four EST markers could not be mapped (Table 2 and Fig. 2). The map consisted of 9 linkage groups (LG) ranging from 2.6 cM (LG2) to 87.6 cM (LG3) and covered 311.6 cM in total. The segregation ratios for the three candidate gene markers were not significantly different from the expected Mendelian ratios. FeCOL3 and FeCCA1 markers were mapped to LG9 and LG6, respectively, but FeELF3 could not be mapped (Table 2 and Fig. 2).
Fig. 2.
Linkage map of common buckwheat.
QTL analysis for photoperiod sensitivity
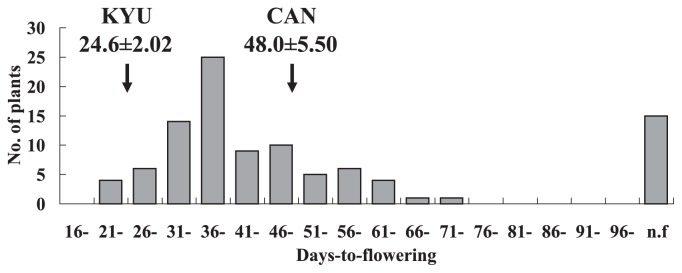
The segregation of photoperiod sensitivity in the F4 progenies was observed as a wide but unimodal distribution. Transgressive segregation in the direction of late flowering was observed (Fig. 3).
Fig. 3.
Distribution of days-to-flowering in F4 progeny (n = 100) of a cross of 02AL113(Kyukei SC2)LH.self (KYU) × C0408-0 RP (CAN). Arrows indicate means ± SD for each parent. n.f., non-flowering.
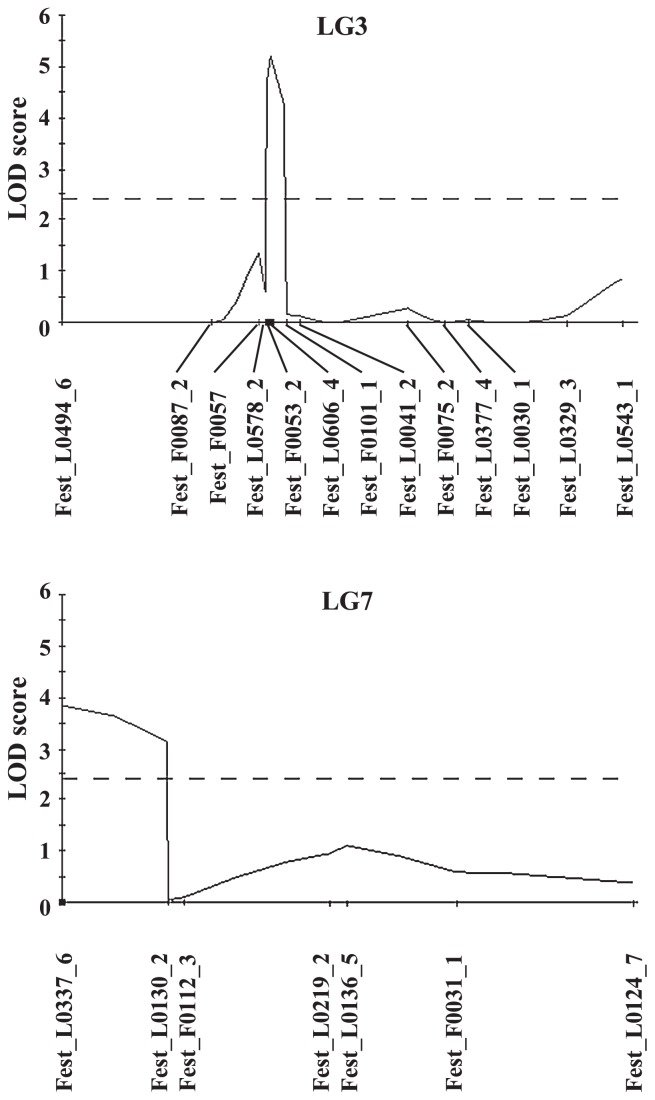
When we performed interval mapping, two regions were associated with photoperiod sensitivity: one in LG3 (25.3 to 50.3 cM) and one in LG7 (0 to 15.1 cM) (LOD score >2.4, P < 0.05). The results of cofactor analysis indicated a significant difference (P < 0.02) at eight markers (Fest_L0490_2 and Fest_L0472_1 in LG1, Fest_L0606_4 in LG3, Fest_F0077_2 and Fest_L0101_1 in LG4, Fest_L0337_6 in LG7 and Fest_L0242_1 and Fest_L0053_3 in LG8). In the MQM analysis, two QTLs (Fest_L0606_4 and Fest_L0337_6 in LG3 and 7, respectively) showed relatively high LOD values (LOD score >2.4, P < 0.05) (Table 3 and Fig. 4). For each of these two QTLs, the KYU alleles had negative additive effects on days-to-flowering (−8.2 days and −6.8 days, for Fest_L0606_4 and Fest_L0337_6, respectively) and explained about 20.0% and 14.2% of the phenotypic variation in the F4 progenies, respectively.
Table 3.
Quantitative trait loci (QTLs) detected for photoperiod sensitivity in the 02AL113(Kyukei SC2)LH.self (KYU) × C0408-0 RP (CAN) F4 population by multiple QTL analysis
| LG | Position (cM) | Locus | LOD | Additive effect | PVE (%) |
|---|---|---|---|---|---|
| 3 | 34.0 | Fest_L0606_4 | 5.2 | −8.2 | 20.0 |
| 7 | 0.0 | Fest_L0337_6 | 3.8 | −6.8 | 14.2 |
| 6 | 21.5 | FeCCA1 | 0.13 | 1.2 | 0.4 |
| 9 | 0.6 | FeCOL3 | 0.11 | −1.1 | 0.4 |
| U | – | FeELF3 | – | – | – |
U, unknown genome location
Fig. 4.
LOD score for QTLs detected in multiple QTL analysis. The QTL likelihood maps for each linkage group were obtained by using the MQM procedure of MapQTL, fixing one marker cofactor per putative QTL. Selected markers used as cofactors in analysis are indicated by black rectangles along the x-axes. The linkage group number is indicated at the top of each graph. Horizontal dashed lines indicate the 2.4 (P < 0.05) LOD score threshold.
In the candidate gene markers, FeCCA1 and FeCOL3 showed low LOD values (LOD score 0.13 and 0.11, respectively) as a result of the MQM analysis (Table 3). FeELF3 could not be mapped. Thus, we searched for associations between FeELF3 and photoperiod sensitivity by using the ANOVA. Table 4 shows the result of the ANOVA for association between the FeELF3 and photoperiod sensitivity. It was shown that FeELF3 was significantly related to photo-period sensitivity (P = 0.028). For FeELF3 markers, CAN allele showed smaller average of days-to-flowering than KYU allele, the difference was 8.6 days while KYU allele had early average of days-to-flowering (the difference was 15.3 and 12.5 days, for Fest_L0606_4 and Fest_L0337_6, respectively) in the two QTL’s.
Table 4.
Quantitative trait loci (QTLs) detected for photoperiod sensitivity in the 02AL113(Kyukei SC2)LH.self (KYU) × C0408-0 RP (CAN) F4 population by ANOVA. KYU and CAN indicate average of days-to-flowering for the 02AL113(Kyukei SC2)LH.self allele and homozygosity for the C0408-0 RP allele, respectively.
| LG | Position (cM) | Locus | F value | P value | Average of days- to-flowering | |
|---|---|---|---|---|---|---|
|
| ||||||
| KYU | CAN | |||||
| U | – | FeELF3 | 3.72 | 0.028 | 51.5 | 42.9 |
| 3 | 34.0 | Fest_L0606_4 | 10.40 | <0.001 | 39.3 | 54.6 |
| 7 | 0.0 | Fest_L0337_6 | 6.17 | 0.003 | 40.6 | 53.1 |
U, unknown genome location
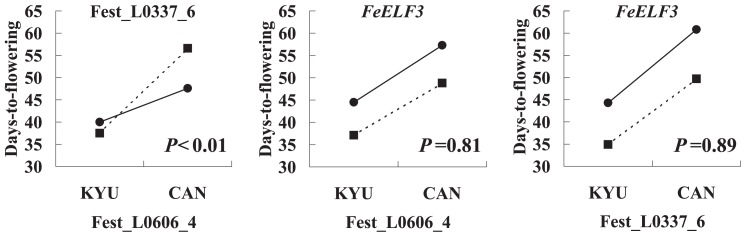
To test for the existence of QTL interactions, genotype data for Fest_L0606_4, Fest_L0337_6 and FeELF3 were used in a two-way ANOVA to test the phenotypic difference among the four homozygous genotype classes (Fig. 5). A significant interaction was detected between Fest_L0606_4 and Fest_L0337_6 (P < 0.01). On the other hand, interactions between Fest_L0606_4 and FeELF3 and between Fest_L0337_6 and FeELF3, were not significant (P = 0.81 and P = 0.89, respectively).
Fig. 5.
Mean values for days-to-flowering in different combinations of genotype classes for Fest_L0606_4 and Fest_L0337_6. The x-axis indicates the genotype at Fest_L0606-4 and Fest_L0337_6: KYU and CAN indicate homozygosity for the 02AL113(Kyukei SC2)LH.self allele and homozygosity for the C0408-0 RP allele, respectively. The genotype of Fest_L0337_6 and FeELF3 are indicated by circles (homozygous for KYU allele) or squares (homozygous for CAN allele). P value in each figure showed results of significance for interaction by two way ANOVA.
Discussion
A number of studies have been conducted for investigating photoperiod sensitivity in common buckwheat. Minami (1985) suggested that photoperiod sensitivity of common buckwheat is controlled by the action of multiple genes. However, neither genes nor candidate regions related to photoperiod sensitivity had been identified until now. In the present study, a difference in days-to-flowering between the KYU and CAN populations was expressed under a 15.5 h photoperiod, but no difference was observed under the 14.5 h photoperiod. These results showed that the difference in days-to-flowering between KYU and CAN observed under the 15.5 h photoperiod was caused by a difference of photo-period sensitivity: the KYU population had weaker photo-period sensitivity than the CAN population. The KYU line was bred by interspecific hybridization among F. homotropicum (an autogamous species) and F. esculentum cv. Botansoba which is cultivated in high-latitude regions (Matsui 2006); similarly, the CAN line was also bred by crossing F. homotropicum and F. esculentum which is cultivated in high-latitude regions (Wang and Campbell 1998). Thus, we assumed that both lines had been selected under long-day conditions. This may be why the difference in photoperiod sensitivity between KYU and CAN was not evident unless an artificially long day length (15.5 h) was applied.
In Arabidopsis, the photoperiod-sensitivity pathway consists of photoreceptors, the circadian clocks, and floral promoters (Putterill et al. 2004). In many species with photo-period sensitivity, such as rice, wheat (Triticum aestivum), and morning glory (Pharbitis nil), genes orthologous to photoperiod-sensitivity genes found in Arabidopsis were confirmed to be related to heading date or flowering time (Hayama et al. 2002, 2003, Kojima et al. 2002, Liu et al. 2001a, Nemoto et al. 2003, Yano et al. 2000). In the present study, we found three such candidate gene regions (FeCOL3, FeCCA1 and FeELF3) among 863 cDNA clones. In Arabidopsis, CCA1 and ELF3 are genes that connect the circadian clock to the photoperiod-sensitivity pathway (Covington et al. 2001, Hicks et al. 2001, Liu et al. 2001b, Wang et al. 1997, Wang and Tobin 1998), and COL3 is from a CO-related gene family that connects floral promoters to the photoperiod-sensitivity pathway (Putterill et al. 1995). Here, we found that the FeELF3 candidate gene region in common buckwheat was strongly associated with photoperiod sensitivity. Although the candidate genes identified in the present study would only be part of the total group of genes included in a photoperiod-sensitivity pathway, our report is the first in which candidate genes identified based on their role in photo-period sensitivity in another species were associated with photoperiod sensitivity in common buckwheat. The results indicate that it would be efficient to study the photoperiod-sensitivity pathway by using other candidate genes and analyzing their effects on photoperiod sensitivity. Although mutations in intron region were reported to be associated with gene expression (Fiume et al. 2004, Fu et al. 2005, Isshiki et al. 1998, Samadder et al. 2008), it was not clear whether FeELF3 marker based on SNP in an intron region directly associated with photoperiod sensitivity right now. If SNP’s associated with photoperiod sensitivity in direct will be detected in the future, the allelic variations in FeELF3 gene region will become more informative information.
In the MQM analysis, we detected two QTLs, Fest_L0606_4 and Fest_L0337_6, which explained about 20.0% and 14.2% the variation observed in the F4 progenies, respectively. In the present study, we constructed a linkage map for QTL analysis consisting of 9 linkage groups containing 63 ESTs and two candidate gene regions and covering 311.6 cM in total. This map was the first linkage map to include ESTs and candidate gene regions thought to be related to photoperiod sensitivity in common buckwheat. Unfortunately, we assumed that the genome coverage of this map is not so high because Konishi and Ohnishi (2006) constructed a linkage map of buckwheat covering about 900 cM by using AFLP and SSR markers. Thus, result of MQM analysis at unmapped FeELF3 candidate gene region was not shown. If the genome coverage of this map will be higher, other QTLs included FeELF3 candidate gene region might be detected.
The genetic analysis of quantitative traits with molecular markers makes it possible to evaluate epistatic interactions between individual loci. A digenic interaction between the QTLs detected in the MQM analysis was observed in this study. The additive effect of the KYU allele of Fest_L0337_6 was estimated to be −6.8 days. However, the observed two-locus interaction between Fest_L0606_4 and Fest_L0337_6 suggests that the effects of Fest_L0337_6 are changed depending on the allele present at Fest_L0606_4. In Arabidopsis and rice, interactions between genes related to photoperiod sensitivity have been reported (Putterill et al. 2004, Yano et al. 1997). These results suggested that photo-period sensitivity in common buckwheat was controlled by both main and interaction effects of the QTLs detected. Thus, it is necessary to analyze both the main effects and the interactions between QTLs to fully understand photoperiod sensitivity.
In summary, our study is the first report that photoperiodsensitivity in common buckwheat is controlled by the action of multiple genes, including interaction effects between these genes. The photoperiod sensitivity was controlled by at least three loci, including candidate genes identified as being part of the photoperiod-sensitivity pathway in Arabidopsis. These findings will serve as a stepping-stone to future studies on the role of photoperiod sensitivity in local adaptation and ecological breeding in common buckwheat.
Supplementary Material
Acknowledgments
We thank Dr. J. Aii at the Niigata University of Pharmacy and Applied Life Sciences for providing the Fest_F cDNA clones. We also thank Dr. C. Campbell at the Kade Research Ltd. for providing seeds of the C0408-0RP. This study was partly supported by the Japanese Society for the Promotion of Science via a Grant-in-Aid for Scientific Research (B) (16380003).
Literature Cited
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Coupland G. Photoperiodic flowering of Arabidopsis: integrating genetic and physiological approaches to characterization of the floral stimulus. Plant Cell Environ. 2005;28:54–66. [Google Scholar]
- Covington MF, Panda S, Liu LX, Strayer AC, Wagner RD, Kay AS. ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell. 2001;13:1305–1315. doi: 10.1105/tpc.13.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume E, Christou P, Giani S, Breviario D. Introns are key regulatory elements of rice tubulin expression. Planta. 2004;218:693–703. doi: 10.1007/s00425-003-1150-0. [DOI] [PubMed] [Google Scholar]
- Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Gen Genomics. 2005;273:54–65. doi: 10.1007/s00438-004-1095-4. [DOI] [PubMed] [Google Scholar]
- Gordon DAC, Green P. Consed: A graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- Hayama R, Izawa T, Shimamoto K. Isolation of rice genes possibly involved in the photoperiodic control of flowering by a fluorescent differential display method. Plant Cell Physiol. 2002;43:494–504. doi: 10.1093/pcp/pcf059. [DOI] [PubMed] [Google Scholar]
- Hayama R, Yokoi S, Yano M, Shimamoto K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature. 2003;422:719–722. doi: 10.1038/nature01549. [DOI] [PubMed] [Google Scholar]
- Hayama R, Coupland G. Shedding light on the circadian clock and the photoperiodic control of flowering. Curr Opin Plant Biol. 2003;6:13–19. doi: 10.1016/s1369-5266(02)00011-0. [DOI] [PubMed] [Google Scholar]
- Hicks KA, Albertson MT, Wagner RD. EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell. 2001;13:1281–1292. doi: 10.1105/tpc.13.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki M, Morino K, Hakajima M, Okagaki RJ, Wessler SR, Izawa T, Shimamoto K. A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5′ splice site of the first intron. Plant J. 1998;15:133–138. doi: 10.1046/j.1365-313x.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Iwata H, Imon K, Tsumura Y, Ohsawa R. Genetic diversity of common buckwheat varieties in Japan based on microsatellite markers. Genome. 2005;48:367–377. doi: 10.1139/g04-121. [DOI] [PubMed] [Google Scholar]
- Jansen RC. Interval mapping of multiple quantitative trait loci. Genetics. 1993;135:205–211. doi: 10.1093/genetics/135.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RC, Stam P. High resolution of quantitative traits into multiple loci via interval mapping. Genetics. 1994;136:1447–1455. doi: 10.1093/genetics/136.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002;43:1096–1105. doi: 10.1093/pcp/pcf156. [DOI] [PubMed] [Google Scholar]
- Konishi T, Ohnishi O. A linkage map for common buckwheat based on microsatellite and AFLP markers. Fagopyrum. 2006;23:1–6. [Google Scholar]
- Lander ES, Botstein D. Mapping Mendelian factors underlying quantitative traits by using RFLP linkage maps. Genetics. 1989;121:1447–1455. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu J, McIntosh L, Kende H, Zeevaart JAD. Isolation of a CONSTANS ortholog from Pharbitis nil and its role in flowering. Plant Physiol. 2001a;125:1821–1830. doi: 10.1104/pp.125.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Covington FM, Fankhauser C, Chory J, Wagner RD. ELF3 encodes a circadian clock–regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell. 2001b;13:1293–1304. doi: 10.1105/tpc.13.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matano T, Ujihara A. Agroecological classification and geographical distribution of the common buckwheat, Fagopyrum esculentum M. in the East Asia. JARQ. 1979;13:157–162. [Google Scholar]
- Matsui K. A genetic and breeding study for the production of self-compatible buckwheat cultivars (Self-compatible buckwheat cultivars) Bulletin of the National Agricultural Research Center for Kyushu Okinawa Region. 2006;47:1–42. [Google Scholar]
- Minami H. Doctoral thesis. University of Tsukuba; 1985. Ecological-genetic studies of ecological differentiation in common buckwheat; p. 62. [Google Scholar]
- Minami H, Namai H. Potential genetic variation of flowering time in late-summer type cultivar of buckwheat (Fagopyrum esculentum Moench) in Kyushu region [Japan] Jpn J Breed. 1986a;36:67–74. [Google Scholar]
- Minami H, Namai H. Populational change in flowering time caused by different harvesting date observed in the late-summer type cultivar Miyazakizairai of buckwheat (Fagopyrum esculentum) Jpn J Breed. 1986b;36:155–162. [Google Scholar]
- Nagatomo T. Studies on physiology of reproduction and some cases of inheritance in buckwheat. Research Report of Plant Breeding Laboratory in University of Miyazaki. 1961;1:1–213. [Google Scholar]
- Nakamura M, Nakayama H. On the enervative sterility in buckwheat. Japan Jour Crop Sci. 1950;19:122–125. [Google Scholar]
- Namai H. Pollination biology and reproductive ecology for improving genetics and breeding of common buckwheat, Fagopyrum esculentum (1) Fagopyrum. 1990;10:23–46. [Google Scholar]
- Neff MM, Turk E, Kalishman M. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 2002;18:613–615. doi: 10.1016/s0168-9525(02)02820-2. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Kisaka M, Fuse T, Yano M, Ogihara Y. Characterization and functional analysis of three wheat genes with homology to the CONSTANS flowering time gene in transgenic rice. Plant J. 2003;36:82–93. doi: 10.1046/j.1365-313x.2003.01859.x. [DOI] [PubMed] [Google Scholar]
- Ohsawa R. Evaluation of a Japanese germplasm collection of common buckwheat using a multivariate approach. Proceedings of the 8th SABRAO Congress; 1997. pp. 107–108. [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Putterill J, Laurie R, Macknight R. It’s time to flower: the genetic control of flowering time. Bioessays. 2004;26:363–373. doi: 10.1002/bies.20021. [DOI] [PubMed] [Google Scholar]
- Samadder P, Sivamani E, Lu J, Li X, Qu R. Transcriptional and post-transcriptional enhancement of gene expression by the 5′ UTR intron of rice rubi3 gene in transgenic rice cells. Mol. Genet Genomics. 2008;279:429–439. doi: 10.1007/s00438-008-0323-8. [DOI] [PubMed] [Google Scholar]
- Sugawara K. On the injury of buckwheat pistil, Retardation of pistil growth as influenced by day-length. Japan Jour Crop Sci. 1958;26:269–270. [Google Scholar]
- Van Ooijen JW. Map-QTL_5: software for the mapping quantitative trait loci in mapping populations. Kyazma BV; Wageningen: 2005. [Google Scholar]
- Van Ooijen JW. JoinMap, Software for the Calculation of Genetic Linkage Maps. Kyazma BV; Wageningen The Netherlands: 2006. Version 4. [Google Scholar]
- Wang YJ, Campbell C. Interspecific hybridization in buckwheat among Fagopyrum esculentum, F. homotropicum and F. tataricum. Proc 7th Int Symp Buckwheat; Winnipeg, Canada. 1998. pp. 1–12. [Google Scholar]
- Wang ZY, Kenigsbuch D, Sun L, Harel E, Ong SM, Tobin ME. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9:491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Tobin ME. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- Yano M, Harushuma Y, Nagamura Y, Kurata N, Minobe Y, Sasaki T. Identification of quantitative trait loci controlling heading date in rice using a high-density linkage map. Theor Appl Genet. 1997;95:1025–1032. [Google Scholar]
- Yano M, Katayose Y, Ashikan M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell. 2000;12:2473–2483. doi: 10.1105/tpc.12.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Kojima S, Takahashi Y, Lin H, Sasaki T. Genetic control of flowering time in rice, a short-day plant. Plant Physiol. 2001;127:1425–1429. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.